1. Heavy Metals in the Environment
Metals are natural constituents of soil with a great adsorption capacity. Some metals are essential elements for the existence of all known life forms (e.g., Fe
2+, Zn
2+, Mn
2+, Co
2+, etc.), since they perform several functions in biological systems. However, some metals are toxic even at minimal concentrations and can cause chromosomal mutations (beryllium), reduction in growth rate (antimony), cell lysis (silver) or deactivation of enzymes (arsenic), for example. Other examples of metals that are considered to be toxic to humans, as well as to the environment, are nickel, chromium, copper, zinc, mercury, lead, and cadmium [
1,
2]. Natural processes which introduce heavy metals into the environment are volcanic eruptions, erosions, comets, weathering of minerals, ocean evaporation, and combustion.
The widespread use and dissemination of metals has increased rapidly during the 20th century. Their impact on the environment is a matter of rising concern because of their persistence and non-degradable nature. In nature they are mostly present in insoluble forms, which are not available for uptake, but heavy metals arising from anthropogenic sources have a high bioavailability due to their mobile and soluble reactive forms [
3]. Main anthropogenic sources of heavy metals in the environment encompass alloy production, mining, leather tanning, explosive manufacturing, battery production, atmospheric deposition, coating, biosolids, improper stacking of industrial solid waste, photographic materials, pesticides, phosphate fertilizers, printing pigments, textiles, sewage, irrigation, smelting, steel and electroplating industries, dyes, and wood preservation. While anthropogenic sources increasingly give rise to permanent pollution, natural sources are usually a seasonal phenomenon influenced by weather, which generally does not generate pollution [
4].
Many heavy metals are capable of entering the food chain, where they cause serious damage. The toxicity of each metal and their potential harmful effects depends on the amount, route of admission, and duration of exposure [
5]. A major source of heavy metals in the soil is mining activities and subsequent ore processing. During these processes, metals are mobilized by biological or chemical leaching, and pass to the soil and nearby water sources. As a result of leaching, heavy metals migrate to the lower soil layers and cause destruction and alteration of the ecosystem, including accumulation of pollutants and a loss of biodiversity. The subsequent recovery could take several decades [
6].
2. Techniques for Heavy Metals Removal
Removal of pollutants in the form of heavy metals has become an urgent requirement in recent decades. Technologies routinely used for heavy metal removal from the environment include chemical precipitation, reverse osmosis, ion-exchange, ultrafiltration, and electro-dialysis. They are, unfortunately, often inefficient and extremely expensive. Moreover, they can generate toxic compounds, which are seen as unfavorable and uneconomical [
7]. There is, therefore, an acute need for the development of cheap, highly efficient and selective alternatives that can alleviate concentrations of heavy metals to environmentally accepted levels. One promising alternative being developed is bioremediation [
8]. Bioremediation is a technique used for heavy metal removal from the environment that utilizes inherent biological mechanisms to eliminate or reduce amounts of toxic contaminants using microorganisms, plants or their products to restore contaminated environments to their original state [
9]. It is a cost-effective and environmentally friendly technique compared to conventional techniques, which are ineffective especially at low metal concentrations [
1].
Bioremediation is not a new concept. This approach has been used since around 600 B.C. by ancient Romans to treat the wastewater. The Romans built intricate networks of sewers for collecting wastewater that underwent subsequent biological treatment. Although this phenomenon was vaguely understood, sewage systems were planned, designed and constructed for centuries. In order to prevent system backup and overload, collection vats and lagoons were provided. These were sites of microorganism-mediated biodegradation of organic waste. What the Romans saw as a way of self-purification took place as a function of retention time via the action of microbial activity. Although their cleaning processes were not as developed as those of today, they were nonetheless efficient. Since 1972, this technique has been applied as a means of cleaning polluted ecosystems [
10].
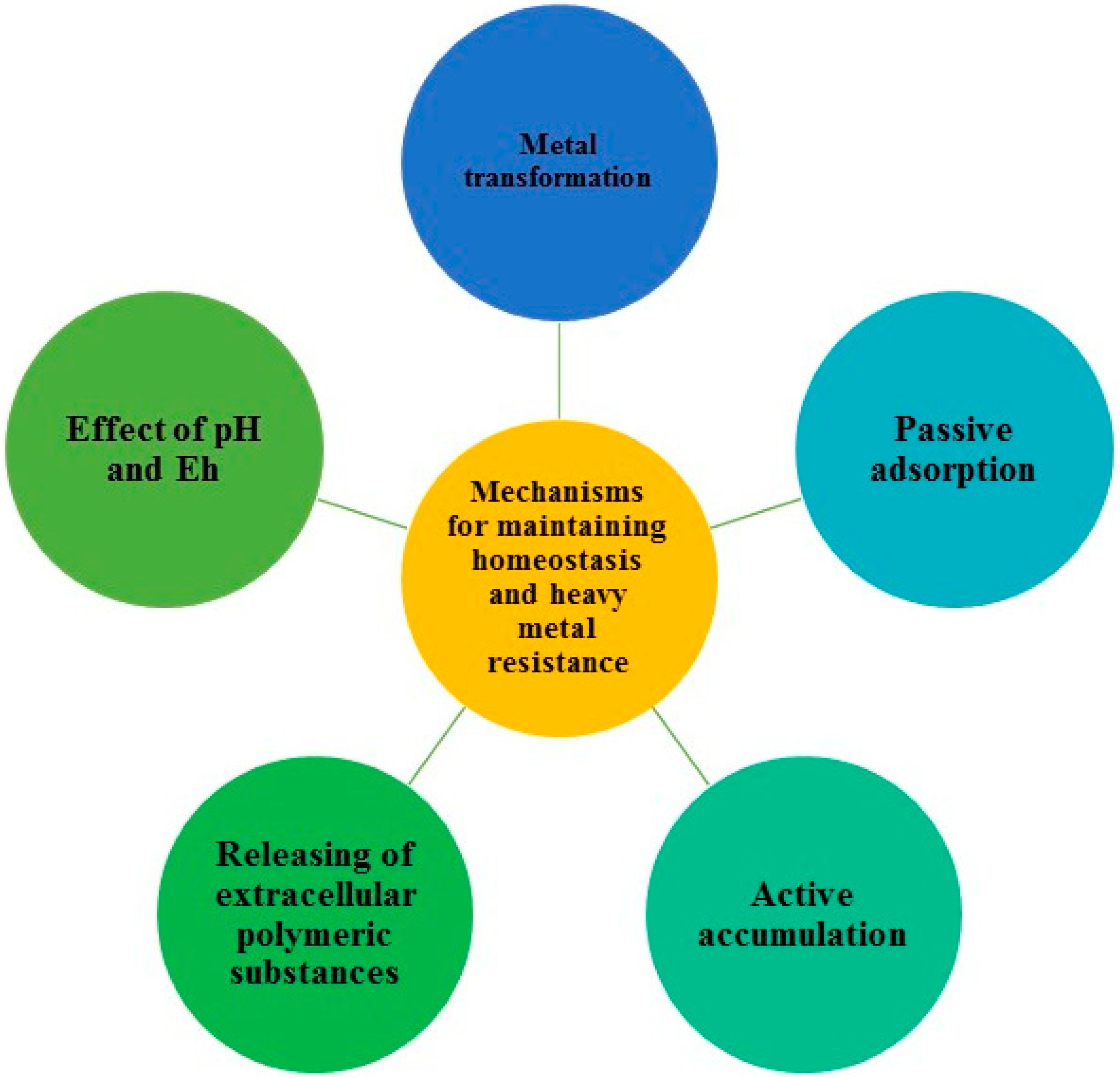
Microbial remediation is defined as the use of microorganisms to achieve the absorption, oxidation, precipitation and reduction of heavy metals in the soil or water solution [
11]. Microorganisms are the unique owners of various metabolic pathways that utilize toxic compounds as a source of energy for cell processes through fermentation, respiration and co-metabolism. They have evolved mechanisms for maintaining homeostasis and resistance to heavy metals in order to survive in toxic environments [
12]. These mechanisms include:
metal transformation either by alkylation or by redox processes, in which the mobility and toxicity of transformed metal differ from those in its original state;
metabolism-independent (passive) adsorption of the metal on the cell surface via electrostatic interactions with functional groups (e.g., carboxylic, amine and phosphoryl groups),which is influenced by bacterial surface properties, metal speciation and chemistry;
metabolism-dependent (active) intracellular accumulation of toxicants by living cells;
release of extracellular polymeric substances (EPS) consisting of polysaccharides, proteins, DNA and RNA, which change the mobility of metals by binding;
participating in soil carbon cycling, which influences the amount of organic matter that subsequently affects mobility of metals in soil; and,
affecting pH and Eh (redox potential) values of the soil [
13].
These mechanisms (
Figure 1) working together could lead to an extraordinarily resistant bacterium. However, cell machinery is often not complete with respect to these mechanisms. Hence, mixed bacterial cultures have great relevance, since bacteria with incomplete resistance mechanisms can complement each other, thereby enhancing overall resistance [
10].
2.1. Microbial Remediation
Microorganisms have an excellent ability to remove heavy metals. They are important components in biogeochemical cycles and possess various biological mechanisms allowing them to transform soluble and insoluble forms of xenobiotics, such as pesticides and heavy metals, to less toxic or non-toxic forms [
14]. Due to their small size they have a high surface-volume ratio, which enables them to have a large contact area with the components of the surrounding soil that contain heavy metals [
3].
2.1.1. Biosorption
Despite the fact that the ability of living microbes to adsorb metals from aqueous solution has been examined since 18th century, living or non-living microorganisms have only been used as adsorbents for the removal of toxic materials from aqueous solutions during the past three decades [
15]. Biosorption, the process defined as sorption and/or complexation of dissolved metals based on the chemical activity of microbial biomass or by materials derived from biological sources, provides the base for new biosorption technology for metal removal and recovery [
16]. The first component that comes into contact with metal is the cell wall of bacteria. This is where the metal ion can be deposited, as well as within the structure of cell wall. The presence of functional groups in the cell wall, including carboxyl, phosphonate, amine and hydroxyl groups, plays a vital role in biosorption [
17]. In general, gram-positive microorganisms have a greater sorption capacity due to their thick layer of peptidoglycan, which contains a large number of sorption sites [
18]. Biosorption involves the removal of heavy metals to non-living biomass by the means of passive binding from an aqueous solution; thus, the process is not metabolically dependent. In contrast to biosorption, bioaccumulation is an active process based on living cells, in which removal of metals requires metabolic activity of living organisms [
19]. The advantages of using biosorption together with bioaccumulation are the potential for in situ application, secondary pollution is not produced as a result of bioremediation and cost effectiveness. There are a number of factors that influence biosorption, e.g., pH value, temperature, biosorbent dosage, ionic strength, biosorbent size and initial solute concentration [
15].
2.1.2. Bioaccumulation
Essential metals, which are required by organisms, are actively taken up by the specific uptake systems into the cell. However, non-essential metals may be improperly identified and mistaken for essential metals, and hence also taken up [
20]. The term bioaccumulation can be defined as the uptake of toxic pollutants only by living cells. The toxicant is actively transported into the cell across the cell membrane, where it is accumulated intracellularly [
17]. Bioaccumulation is dependent on intrinsic structural and biochemical properties, genetic and physiological adaptations, environmental modification of metal, and the metal’s availability and toxicity [
21]. Metal accumulation is influenced by the surface characteristics of the microorganism, but metals can also lead to alterations in the properties of the surface, such as charge changes [
22]. Cell density may also influence the metal accumulation process; accumulation decreases with an increase of biomass concentration due to the electrostatic interactions of the functional groups of the bacterial cell wall. Higher concentration of cells in suspension causes their linkage, thus lowering the amount of active sites available for binding of metals [
23]. Temperature also influences accumulation, since chemical since reaction rates increase at higher temperatures. However, high temperatures can be lethal for living cells due to the destructive effect on bacterial cell membranes [
24]. The main differences between biosorption and bioaccumulation are listed in the
Table 1.
3. Streptomycetes as a Largest Group of Actinobacteria
The filamentous bacteria, Actinomycetes, are free-living, saprophytic organisms widely distributed in water, soil and colonized plant surfaces. This phylum encompasses 6 classes, 19 orders, 50 families, and 221 genera, but new taxa continue to be discovered. Actinomycetes involve phenotypically diverse organisms with a wide variety of morphologies ranging from cocci to highly differentiated mycelia and spores, which could be advantageous for long-distance dispersal [
25]. Of 22,500 biologically active compounds obtained from microbes, 45% are produced solely by actinomycetes [
26]. The genus
Streptomyces, the most abundant genus of soil bacteria and actinomycetes, is known for producing a large amount of bioactive secondary metabolites, including antibiotics, antiviral and anticancer drugs, immunomodulators, insecticides and herbicides. They are aerobic microorganisms with high G+C content (75%) and large genomes in comparison with the other microorganisms [
27]. They produce about 50% of all known antibiotics produced by microorganisms and they are a resource for 75% of medically useful antibiotics [
4]. Streptomycetes also play a fundamental role in recycling carbon in polymeric macromolecules [
28].
In order to survive in an environment contaminated by metals, streptomycetes produce a wide range of metal ion chelators, such as siderophores, to provide protection from the negative effects of heavy metals or specific metal uptake for specialized metabolic processes [
29]. Streptomycetes also produce extracellular polymeric substances (EPS) which are, together with siderophores, of interest in removing heavy metals from the environment. While EPS obtained from bacteria have been intensively studied, understanding of their production by bacteria of the
Streptomyces genus is still incomplete [
26].
• siderophores
Iron is an essential element for proper growth and functioning of almost all living microorganisms because of its role in enzymatic processes, electron transfer, oxygen metabolism and DNA/RNA synthesis [
30]. However, because of its low bioavailability in nature, bacteria have developed the specific uptake mechanism-organic compounds siderophores. The primary function of these low-molecular weight compounds (200–2000 Da) is to chelate Fe(III) from different habitats and make it available for plant and microbial cells [
31]. Despite their preference for iron, they also form complexes with others metals that are of environmental concern (e.g., cadmium, nickel, cobalt, zinc and lead) with various affinities. Siderophore-metal complexes are unable to enter the bacterial cell, thereby reducing free metal concentrations in the environment. Microorganisms are able to produce a wide range of siderophores, which can be categorized into the catecholate, carboxylate or hydroxamate families according to the characteristic functional groups [
32,
33]. In recent years, siderophores have gained higher attention because of their potential applications in various areas of environmental research, bioremediation and chelation of heavy metals [
32]. Siderophores are extremely effective in mobilizing and solubilizing a wide range of metals in nature. Due to their strong affinity or selectivity for concrete metal (other than iron), they have become a useful tool in the environmentally friendly and cost-effective technique of bioremediation. They also play an important role in metal mobilization from metal contaminated soils or mine waste material [
32]. It has been stated that the presence of toxic heavy metals in the environment induces the production of some siderophores, suggesting that these chelators may have a role in bacterial heavy metal tolerance. By the means of binding heavy metal to the siderophore extracellularly, free heavy metals concentrations and their toxicity are reduced [
34,
35]. The advantages and the importance of siderophores in the bioremediation applications are obvious, but there are still many questions which remain to be answered.
• extracellular polymeric substances
EPS are high molecular weight biopolymers produced by both eukaryotic and prokaryotic organisms living in natural and artificial environments [
36] in order to help in cell-to-cell aggregation, adhesion to substratum, protection from desiccation or resistance to harmful exogenous materials. In addition to these characteristics, EPS serve as biosorbing agents by accumulating nutrients, and also play a crucial role in biosorption of heavy metals [
37]. As a tool for the removal of heavy metals from the environment, microbial biosorbents have been studied extensively. EPS produced by many bacteria (as well as microalgae and fungi) are of particular relevance because of their ability to bind metal ions from solution. The use of these biopolymers appears to be a more effective, economical and safer alternative to chemical methods already used, because they exhibit great metal-binding properties with different degrees of affinity and specificity [
38]. EPS are mixtures of proteins, polysaccharides, phospholipids, nucleic acids, etc. [
39], and due to their anionic nature form complexes with metal cations resulting in metal ions immobilized within their matrices. EPS-mediated biosorption occurs by the electrostatic interaction between negatively charged biopolymeric molecules of EPS and cationic metal ligands outside the cell, leading to formation of a stable complex [
40]. However, the enzymatic activities in this biopolymer assist detoxification of these metals by transformation with subsequent precipitation. Bacteria could be embedded in EPS or EPS can be loosely attached to the bacterial cell surface. EPS surrounding the bacteria are able to chelate some metals and bind them to the cell surface as a result [
41]. EPS could be result from a variety of microbial processes, such as shedding of cell surface material, active secretion, cell lysis or adsorption from the environment [
42]. Production of EPS depends on many factors, such as phase of bacterial growth, microbial species, presence of nutrients and environmental conditions [
43]. Production of EPS is enhanced in the presence of stressful conditions, including due to heavy metals [
37].
Important characteristics with a significant role in the removal of metal ions by actinomycetes are their enormous specific surface, extensive intracellular space and their ability to produce harvesting beads in liquid media. Resistance to high concentrations of heavy metals is an equally important characteristic present in many strains, but the mechanisms involved are not yet well understood [
10].
Generally,
Actinobacteria represent a major group of bacteria existing in high abundance in soils. An essential ecological role played by
Actinobacteria is shown by their ability to remove xenobiotic compounds, such as heavy metals and pesticides, among others. Because of their metabolic many-sidedness,
Actinobacteria have received significant global interest for several biotechnological applications [
44].
3.1. Streptomycetes as a Tool for Heavy Metal Remediation
Streptomycetes, a major component of soil, represent a suitable agent for bioremediation of metals with future biotechnological applications, due to their metabolic diversity, ability to form spores under unfavorable environmental conditions, mycelial formation and relatively rapid colonization of substrates. Biosorption capability and metal resistance may also be widespread among actinomycetes growing in polluted areas [
45].
Actinomycete genera derived from highly heavy metal-contaminated areas could be multi-metal resistant. Resistance could be a result of continuous exposure to heavy metals present in the environment, which can be advantageous for bioremediation [
46]. The following sections identify research findings relating to streptomycetes and their heavy metal bioremediation abilities.
3.1.1. Chromium Removal
Chromium is a naturally occurring element which can exist in several oxidation states, with hexavalent Cr(VI) and trivalent Cr(III) the most common forms. While Cr(III) is an essential nutrient needed for normal fat and sugar metabolism, Cr(VI) is a thousand times more toxic, more mobile and more soluble than Cr(III) [
47]. Bacteria, including streptomycetes, have been shown to possess Cr(VI)-reducing ability [
48].
Polti et al. [
49] performed an experiment with
Streptomyces MC1 in minimal medium (MM) and minimal glycerol medium (MMY) in the presence of 50 mg/L of Cr(VI), and demonstrated the ability of
Streptomyces MC1 to reduce Cr(VI) to Cr(III) in the presence of glycerol up to 96%. After 70 days of incubation, the bacterial strain was capable of accumulation of 8% of chromium as Cr(III) in MMY medium. Specific uptake of chromium after 7 days of incubation in MM and MMY medium was 1.48 mg/g and 1.56 mg/g wet biomass in MM and MMY, respectively. After 70 days, the uptake increased to 3.54 and 2.32 mg/g wet biomass in MM and MMY, respectively.
In work from 2009 [
50] and 2010 [
51], Polti et al. demonstrated the ability of the same streptomycete strain to reduce Cr(VI) to Cr(III) under different cultivation conditions and observed 45% reduction after 3 days in MM with glucose. In an experiment in 2007 [
45], they examined 41 actinomycetes isolated from El Cadillal (EC, uncontaminated area, control), copper filter plant (CFP) and sugar cane plant (SCP) for their ability to remove Cr(VI). They observed relatively high removal of more than 40% for strains from SCP, 10–45% for strains from CFP and very low (2–26%) removal ability for strains from EC. Results seem to indicate the presence of differences in actinomycete removal activity between isolates obtained from non-contaminated and contaminated soil.
Research on chromium biosorption processes performed by Sahmoune et al. [
52] with dead biomass of
Streptomyces rimosus showed the best adsorption capacity—64 mg/g biomass—at pH values in the range 4–8. The highest specific Cr(VI) removal in the case of Polti et al. [
45] was 75.5 mg/g of cells.
Benimeli et al. [
53] carried out an investigation of the Cr(VI)-reducing activity in soil with four Cr-resistant
Streptomyces strains (MC1, M3, C55, R22) in sterile and non-sterile soil. In both types of soil, they observed reduction of Cr(VI) concentration (94% and 86% reduction in sterile and non-sterile soil, respectively) after 21 days of growth without any prior treatment.
El Baz et al. [
54] obtained 59 actinobacteria strains belonging to
Streptomyces and
Amycolatopsis genera from soil samples from abandoned mining areas near Marrakech (Morocco), of which 27 were screened for heavy metal bioaccumulation ability. In their testing, they detected a wide range of lead accumulation ability on lead-Duxbury agar (13–615 mg/g cells for
Streptomyces sp. BN7 and
Streptomyces sp. BN3, respectively), but no capability to accumulate Cr.
In another study conducted by El-Gendy et al. [
2], 69 actinomycetes isolated from polluted sites in Egypt were evaluated for metal-removing potential in single, binary and ternary metal systems. In a single metal system, all the isolates tested showed appreciable Cr(VI) removal, ranging from 9.1% to 59.4%. In a binary metal system consisting of Ni(II) and Cr(VI), Cr(VI) elimination ranged from 2 to 68.1%, whereas in a Zn(II) + Cr(VI) system, the value was 6.1–86.2%. In a ternary metal system with Ni(II) and Zn(II), Cr(VI) biosorption efficiency was 1.9–77.6%. It was observed that the presence of Zn(II) intensified the removal of Cr(VI) by dead biomass in a bimetallic system, but Cr(VI) suppressed Zn(II) removal efficiency inversely.
A report by Rho and Kim [
55] also demonstrates the order of adsorption potential of heavy metals for
Streptomyces viridochromogenes in single and mixed metal systems: Zn(II) > Cu(II) > Pb(II) > Cd(II), whereas for
Streptomyces chromofuscus K101 the order was Zn(II) > Pb(II) > Fe(II) ≥ Cu(II) ≥ Cd(II) [
56].
3.1.2. Nickel Removal
Nickel belongs among trace elements necessary for normal growth and development of organisms, including bacteria, but in excess it causes oxidative stress with subsequent disruption of cell membrane and enzyme inhibition [
57]. In
Streptomyces, nickel-containing enzymes (nickel-containing superoxide dismutase) have been discovered and this metal had been identified as a regulator of specific gene expression [
58]. Streptomycetes living in contaminated sites are prone to develop resistance mechanisms allowing them survive under conditions of environmental stress.
One selected study, which focused on the biosorption of nickel ions from aqueous solution, was carried out by Long et al. in 2018 [
59]. In their work, they investigated non-living strain
Streptomyces roseorubens SY for its ability to adsorb Ni(II) onto its surface. Under optimum conditions, they revealed the high sorption capacity of this streptomycete—208.39 mg/g—showing this microorganism to be a highly efficient biosorbent of nickel.
Dried biomass of
Streptomyces coelicolor A3(2) was used in an experiment by Öztürk et al. [
60], who studied the biosorption of Ni(II) as a function of concentration, pH and temperature. At pH 8.0, temperature of 25 °C and initial nickel concentration of 250 mg/L, they observed nickel ion uptake as high as 7.3% compared to that reported earlier in the literature.
In 1989, Abbas and Edwards [
28] carried out a study assessing the effects of eight heavy metals, including nickel, on a number of streptomycete species and found that nickel gave inhibition rates in the range 28–42%. The most nickel-tolerant streptomycete appeared to be
Streptomyces thermovulgaris, whereas
Streptomyces thermonitrificans was the most sensitive strain. In general, the most tolerant species observed (for six of eight metals tested) was
Streptomyces canarius, whereas the thermophile
S. thermonitrificans was the most sensitive (for seven metals out of eight).
El-Gendy and El-Bondkly [
2], on the other hand, performed a heavy metal (Ni(II), Cr(VI), Zn(II)) remediation experiment using
Nocardiopsis sp. MORSY1948 and
Nocardia sp. MORSY2014 actinomycetes biomass, for which results are summarized in
Table 2.
In a single metal system, at pH 6 and temperature 30 °C, they achieved efficiency of nickel removal 60.1% for the strain
Nocardiopsis sp. MORSY1948 and 50.5% removal in the case of
Nocardia sp. MORSY2014. In binary metal systems, nickel biosorption capacity in the presence of Zn(II) for
Nocardiopsis sp. MORSY1948 was enhanced by nearly 20% compared to the single metal system. In the system composed of Ni(II) and Cr(VI), the nickel removal ability of this strain was estimated to be nearly 80%. In the binary metal system consisting of Zn(II) + Ni(II) and Ni(II) + Cr(II), they noted enhancement of nickel biosorption in the presence of zinc for
Nocardia sp. MORSY2014 was similar to that of the strain
Nocardiopsis sp. MORSY1948. In a ternary mixture composed of zinc, nickel and chromium, the biosorption of Ni (II) was 74% and 62.92% for the bacteria
Nocardiopsis sp. MORSY1948 and
Nocardia sp. MORSY2014, respectively. They also observed higher heavy metals removal efficiency with dead biomass in comparison with living cells, which agrees with the conclusions of Daboor et al. [
56] and Simeonova et al. [
61], who performed experiments with dead biomass of
Streptomyces chromofuscus K101 and
Streptomyces fradiae, respectively.
Fourteen years earlier, in 2002, Rho and Kim [
55] also performed an experiment with mixed metal systems and noted the highest Ni(II) biosorption capacity for strain
S. viridochromogenes, which was also the most effective heavy metal adsorbent in the mixed metal reaction consisting of cadmium, copper, zinc, nickel and lead. This strain, among all the strains tested, appeared to be the most effective in nickel adsorption in freeze-dried form and in the form of cell wall suspension prepared from freeze-dried mycelium. In the case of using the cell wall as the adsorption agent, the sorption efficiency was 38.7 mg/g wall, whereas the biosorption capacity value in the case of freeze-dried biomass was only 29.9 mg/g.
3.1.3. Zinc Removal
Zinc is an essential transition metal with an irreplaceable role in catalytic and structural function of proteins. Bacteria incorporate it into 5–6% of all their proteins, which are involved in, for example, DNA replication, pH regulation and glycolysis. After iron, zinc is the second most important metal ion in living organisms, explaining the motivation for detailed investigation of microorganisms coping with elevated levels of this element [
62].
Li et al. [
63] carried out an experiment to describe the zinc biosorption characteristics of live and dead biomass of
Streptomyces ciscaucasicus strain CCNNWHX 72–14. Initially, they tested conventional parameters to set ideal values. They determined the optimum biosorption conditions: pH 5.0, agitation of 90 rpm and 2 g/L of biosorbent dose, which were also the biosorption experimental conditions. At these conditions, they observed higher zinc sorption capacity for dead biomass than for living biomass (54 mg/g and 42.75 mg/g for dead and living biomass, respectively). This observation might be explained by their FT-IR (fourier-transform infrared spectroscopy) analysis, which revealed that more functional groups are involved in the sorption by dead biomass than by live
S. ciscaucasicus strain CCNNWHX 72–14 biomass. They also performed a competitive zinc biosorption experiment with Cu(II), Ni(I) and Cd(II), which disclosed a decreasing tendency of zinc biosorption in the presence of different groups of competing ions. In a solution containing all four metal ions, in comparison with solution containing only zinc, the value of zinc removal efficiency by live biomass decreased from 72.5% to 29.7%, while for dead biomass the decrease was from 86.2% to 31.2%. This decrease in biosorption efficiency was caused by restricted metal binding sites on the biosorbent. They also noted a greater extent of decrease in the dead biomass zinc removal ratio compared to live biomass in the presence of all competing ions. This competitive biosorption experiment allowed them to set the order of competing metal ions as Cu(II) > Cd(II) > Ni(I).
Investigation into competing metal ions zinc biosorption was also performed by Daboor and colleagues in 2014 [
56]. They studied the metal adsorbing activity of dead
S. chromofuscus K101 in solution with zinc, lead, and iron ions. In their experiment, in contrast to Li et al. [
63], they observed a significant decrease in adsorption of all metals, but no significant change in zinc adsorption in single and mixed ions systems (0.80 mg/g and 0.79 mg/g dry weight biomass, respectively), which could be explained by the fact that zinc occupied other metals’ binding sites. The order of metal uptake in a single ion system compared to a mixed ions system was not altered; the order of metal adsorption was led by zinc and followed by lead and iron (0.80 mg/g, 0.72 mg/g, 0.56 mg/g for single metal ion system and 0.79 mg/g, 0.61 mg/g, 0.34 mg/g for mixed metal ions system, respectively).
In the literature, several pieces of research on zinc biosorption by different strains of streptomycetes were reported. In one illustrative example, analysis carried out by Mameri et al. [
64] used the non-living
Streptomyces rimosus strain, which showed the capability to take up 30 mg/g of zinc under optimal conditions, which were different from those set by Li [
63]. Mameri’s optimal pH value was higher (at 7.5) and the stirring speed was also increased (to 250 rpm). Nevertheless, after additional chemical treatment of the biomass by 1 M NaOH, biosorption capacity increased to 80 mg/g biomass.
Another study led by Lin et al. [
65] reveals zinc cell wall adsorption followed by intracellular accumulation by the newly discovered streptomycete strain,
Streptomyces zinciresistens. They observed Zn(II) accumulation capacity of this strain of 26.8 mg/g living cells. On the contrary, El Baz and colleagues [
54] detected no zinc accumulation capacity in any of 27 selected strains of actinobacteria isolated from abandoned mining areas in Morocco, probably due to the medium used. It was shown that medium composition could modulate the availability of metal and subsequently its toxicity [
45].
Data obtained from experiments undertaken by Sedlakova-Kadukova et al. [
66] revealed high biosorption and bioaccumulation ability of a novel, extremely Zn-tolerant
Streptomyces K11 strain isolated from a highly alkaline aluminum brown mud disposal site. Their study of biosorption on non-living biomass revealed that this process is mainly chemically controlled, with a maximum biosorption capacity of 49.03 mg/g of streptomycete biomass. On the other hand, the results of bioaccumulation into the living biomass of
Streptomyces K11 indicated very high bioaccumulation capacity of 287.6 mg/g, which clearly reflected the extremely high zinc tolerance of this strain (9807 mg/L). Overall, the mean extracellular uptake of Zn(II) slightly exceeded the intracellular uptake (43% vs. 36%).
3.1.4. Copper Removal
Cu(II) represents one of the most stable divalent metals and shows high affinity for metalloproteins [
67]. In microbial cells, copper is used as a catalyzer for electron transfer reactions in some metalloenzymes such as cytochrome oxidase. Cu-binding proteins constitute less than 0.3% of bacterial annotated genome. However, copper intracellular levels must be strictly controlled due to the toxicity of this metal [
68].
Öztürk [
60] performed a copper biosorption experiment with
S. coelicolor A3 (2) strain for the first time. At the optimal conditions of pH 5 and temperature 25 °C they achieved a maximal adsorption ability of the bacteria of 50.9% at initial metal concentration of 32.2 mg/L within 5 min. The minimal copper uptake value obtained was 21.8% at an initial concentration 221 mg/L.
Albarracin et al. [
44] demonstrated copper cell accumulation by the
S. coelicolor strain. This strain was capable of reduction of copper in the supernatant by 71.2% from the initial metal concentration of 39 mg/L after six days of incubation. Intracellular deposition of copper was proved by pellet acid digestion.
A study from 2008 conducted by Simeonova et al. [
61] exploited glutaraldehyde crosslinked dead biomass of
S. fradiae to adsorb different heavy metal ions. They performed a batch sorption study with Cu(II), Ni(II), Zn(II) and Pb(II), in which the best sorption result obtained for copper was 16 mg/g biomass. According to the findings, the metal adsorption order they set was: copper > zinc > nickel > lead. They also investigated fixed–bed copper biosorption in varying bed heights of the biosorbent, flow rate and initial copper concentration. The most effective adsorption was observed at bed height of 30 cm and flow rate of 0.5 cm
3/min (6.12 mg/g biomass). The most suitable initial concentration of copper ions, along with bed height of 30 cm and flow rate 0.5 cm
3/min, appeared to be 0.7 g/L, at which the biosorption value was maximal (17.32 mg/g biomass).
In a competitive biosorption study performed by Lin et al. [
65], copper, chromium and nickel were used as competitive ions for zinc and cadmium biosorption in the
S. zinciresistens strain. Their results showed that copper played an important role in competition with zinc and cadmium and their adsorption in the presence of Cu(II) was lower. Also, the removal efficiency of Zn(II) and Cd(II) in the presence of all five metal ions in solution decreased from 77.38% to 4% and from 76.53% to 5.03% in the case of zinc and cadmium, respectively.
El Baz et al. [
54], in an investigation of zinc, also found no copper bioaccumulation ability in any of 27 selected strains of actinobacteria isolated from abandoned mining areas in Morocco.
3.1.5. Lead Removal
Lead belongs to the group of very toxic metals that are harmful to all living organisms even at trace amounts. In microorganisms, lead can cause diverse damage, including denaturation of proteins and nucleic acid, and inhibition of transcription and other enzymatic activities [
57]. In a batch system, Kirova et al. [
69] investigated the biosorption potential of
S. fradiae pretreated with NaOH for Pb(II) removal from solution. The maximum biosorption capacity reached in their experiment, of 138.88 mg/g, is comparable with the result obtained by Selatnia et al. [
70] (135 mg/g), who performed a biosorption experiment using NaOH-pretreated dead
S. rimosus. Kirova et al. [
69] also focused on interference of co-present ions of copper and zinc on lead biosorption. They concluded that at the highest lead concentration (200 mg/L), Cu(II) and Zn(II) ions caused 27.22% and 24.88% decreases in Pb(II) uptake, respectively. This comparable effect of Cu(II) and Zn(II) on lead biosorption could be explained by the similarities in their molecular mass (63.57 and 65.38), ionic radii (73 and 74 pm) and electronegativity (1.90 and 1.65 Pauling) [
71].
Sanjenbam et al. [
72] studied metal sorption of lead by
Streptomyces VITSVK9 sp. isolated from marine sediment in India. The aim of their work was to investigate the influence of different biosorbent dosage, initial metal ion concentration and pH values on biosorption of this metal. In their experiment, they achieved 83.23% Pb-sorption efficiency of this streptomycete at biomass dosage of 3 g/L. They also observed increased biosorbent binding capacity with increasing metal ion concentration; however, beyond the maximum, a further increase in concentration of metal ions contributed to the decrease of biosorption. They identified neutral pH as optimal for lead biosorption. Under these conditions, they reached maximum lead adsorption of 150 mg/g.
On the basis of a preliminary heavy metal resistance experiment, the most resistant microorganism isolated from wastewater treatment plant,
Streptomyces sp. WW1, was screened for bioremediation potential in a paper published by Aburas [
14]. He investigated adsorption ability of bacteria for a variety of heavy metals, observing 32% removal activity and 25% for Zn(II), which were the lowest removal capacities. In contrast, the maximum elimination percentage was recorded for Cr(III), of 99.5%.
Daboor et al. [
56] described heavy metal uptake efficiency of
S. chromofuscus K101 in single metal and mixed metal ion systems (iron, lead and zinc). In the single metal system, lead adsorption reached a moderate adsorption value among the metals tested of 0.72 µg/mg, similar to that of the mixed metal suspension of 0.61 µg/mg. Furthermore, the overall metal uptake order was the same for the single metal and mixed metal systems: zinc > lead > iron. The important result of their research is that metal ion biosorption capacity decreases with the presence of competitive ions in the solution in the case of all ions, including lead.
Biosorption in mixed metal systems was also the focus of Rho and Kim’s [
55] investigation. In a mixture of cadmium, copper, zinc, lead and nickel, they observed the highest biosorption by
S. viridochromogenes for lead. Lead was also the most prone to adsorb on cell wall preparations prepared from freeze-dried mycelium and freeze-dried streptomycetes (385 mg/g and 163 mg/g, respectively). However, the situation was different in a single metal system, in which zinc was found to be the most probable ion able to bind on streptomycete biomass (123 mg/g for zinc in comparison with 113.9 mg/g for lead). The experiment showed the suitability of
S. viridichromogenes as a successful lead bioremediation agent.