Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Total Element Concentration in Toothpaste by ICP-MS
2.2.2. Particle Size by Dynamic Light Scattering and Zeta Potential by Laser Doppler Velocimetry
2.2.3. Evaluation of Particle Size and Morphology by Scanning Transmission Electron Microscopy in Combination with Energy-Dispersive X-ray Spectroscopy
2.2.4. Particle Size and Concentration by AF4 Coupled to Multiple Detectors
2.2.5. Particle Size by spICP-MS
2.2.6. Particle Size and Concentration by Hydrodynamic Chromatography Coupled to ICP-MS (HDC-ICP-MS)
3. Results and Discussion
3.1. Pre-Characterisation of the Particles Present in the Toothpaste
3.2. Sample Preparation for AF4
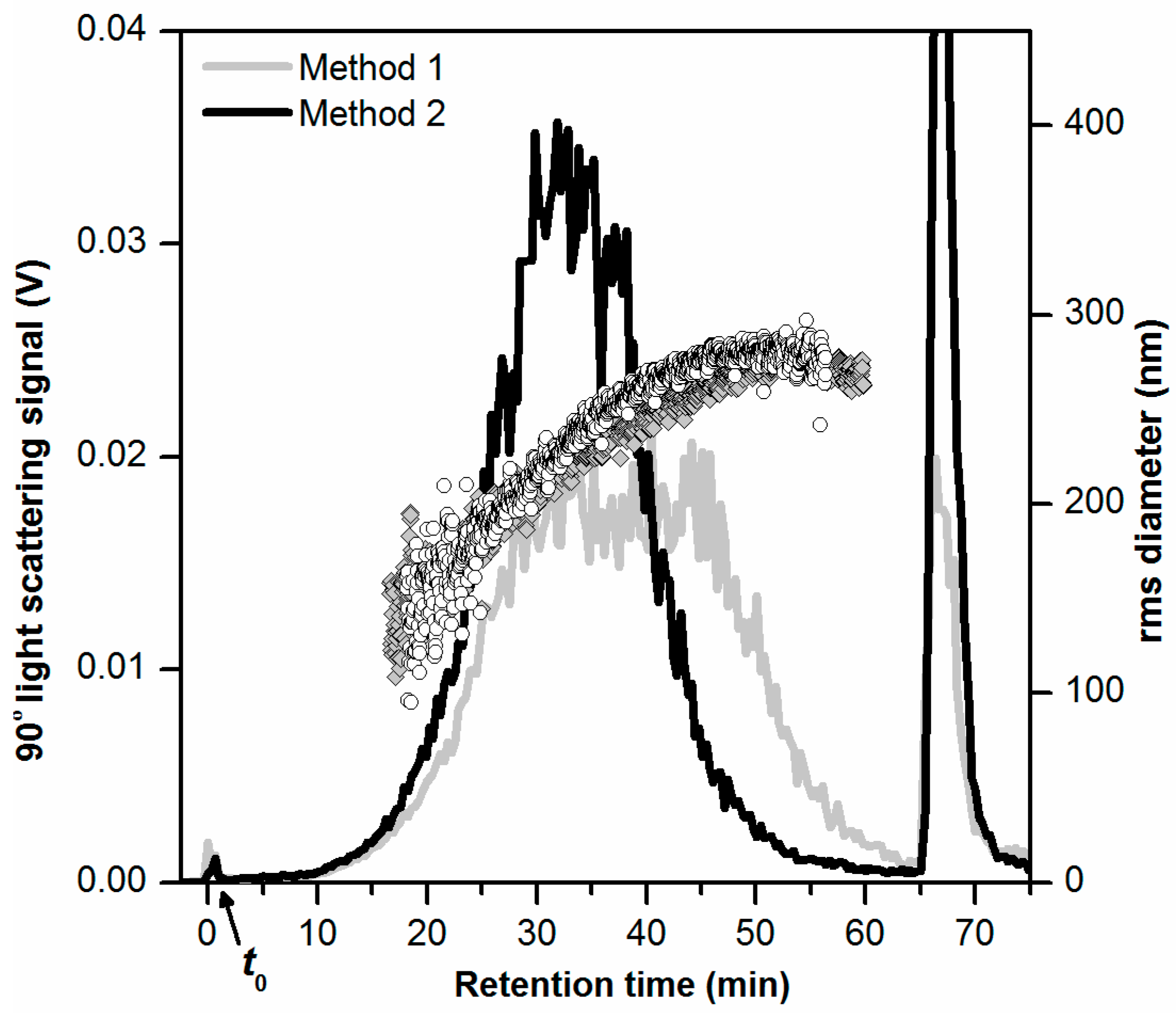
3.3. Detection Methods
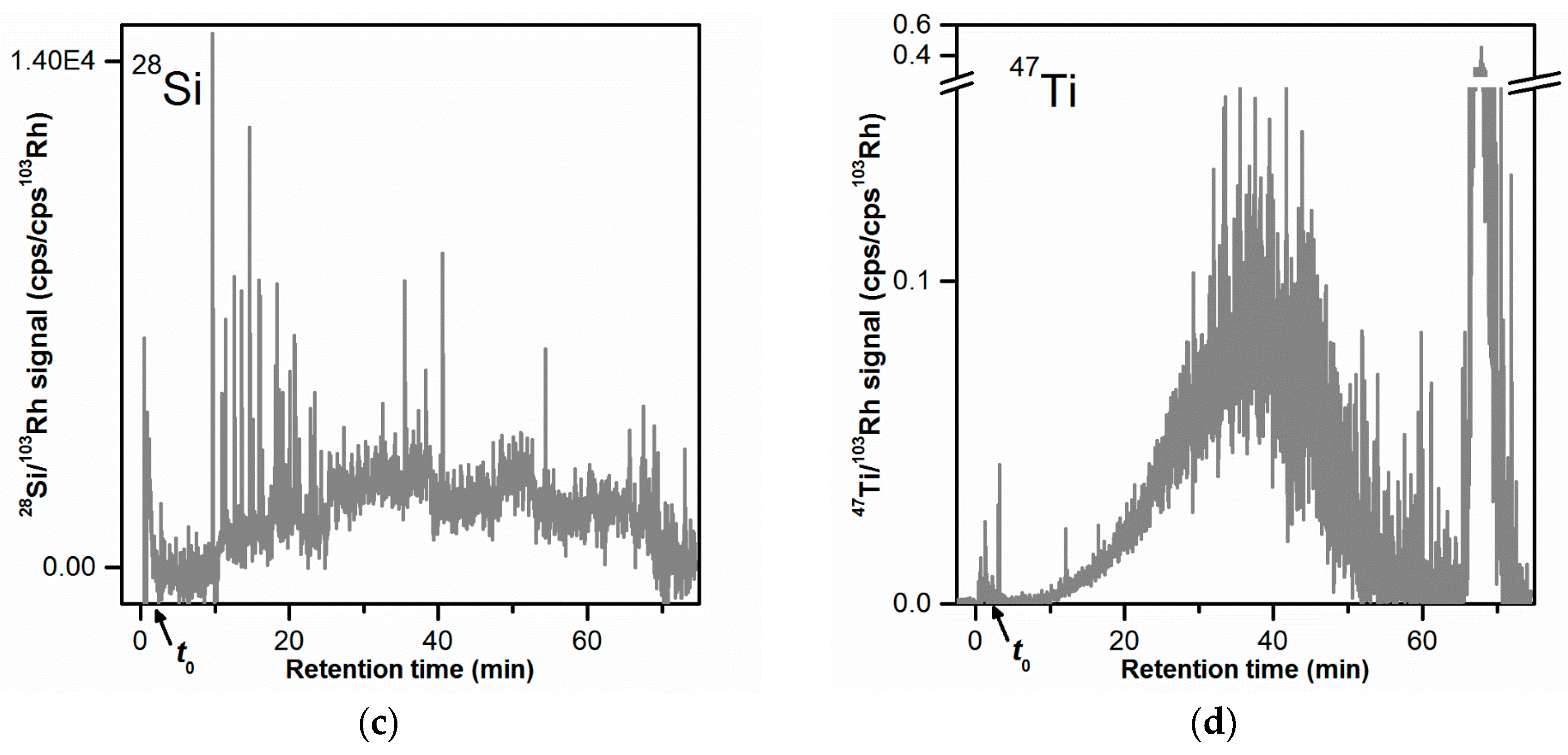
3.4. Carrier Liquid Composition
3.5. Membrane Composition
3.6. Injected Mass
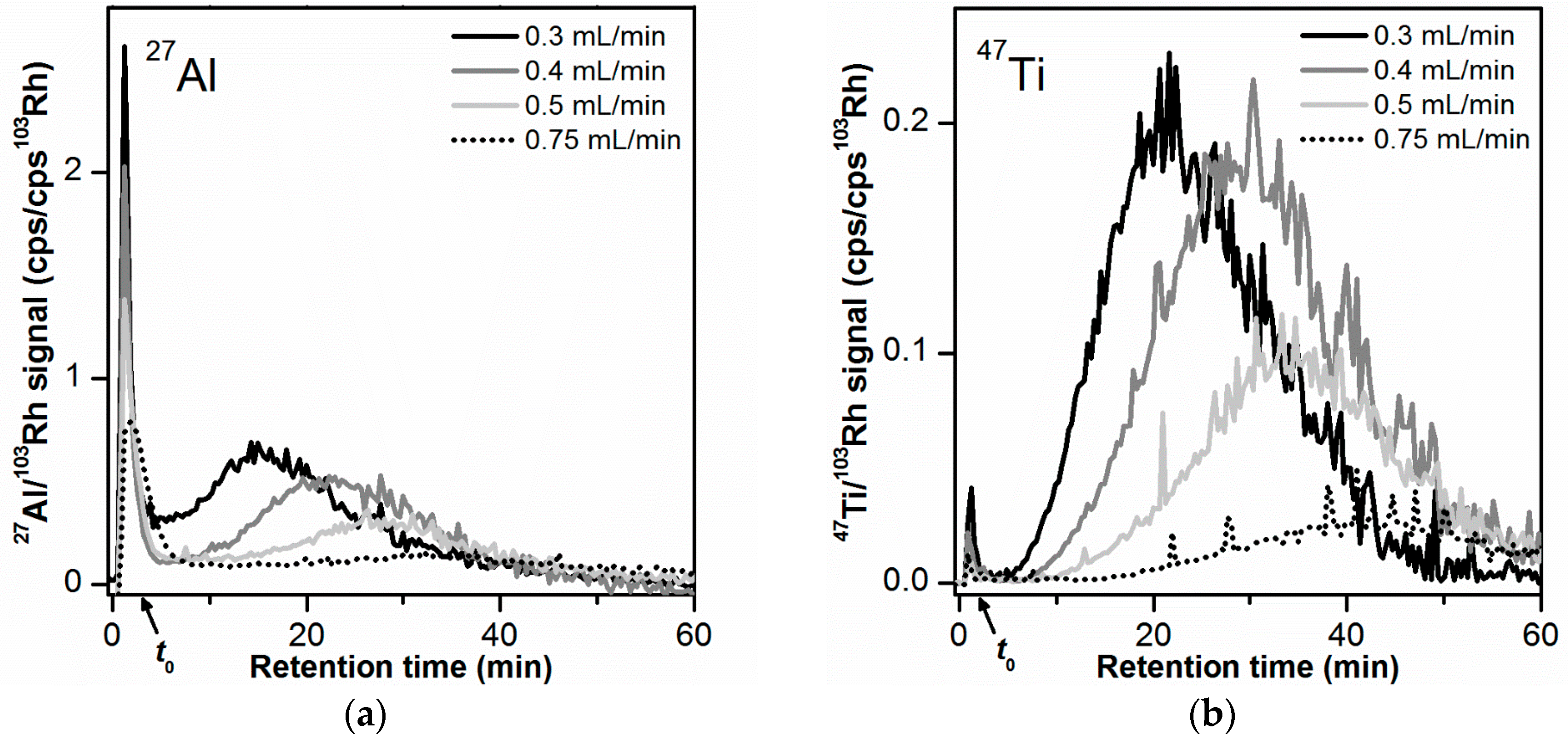
3.7. Cross Flow Rate Optimization
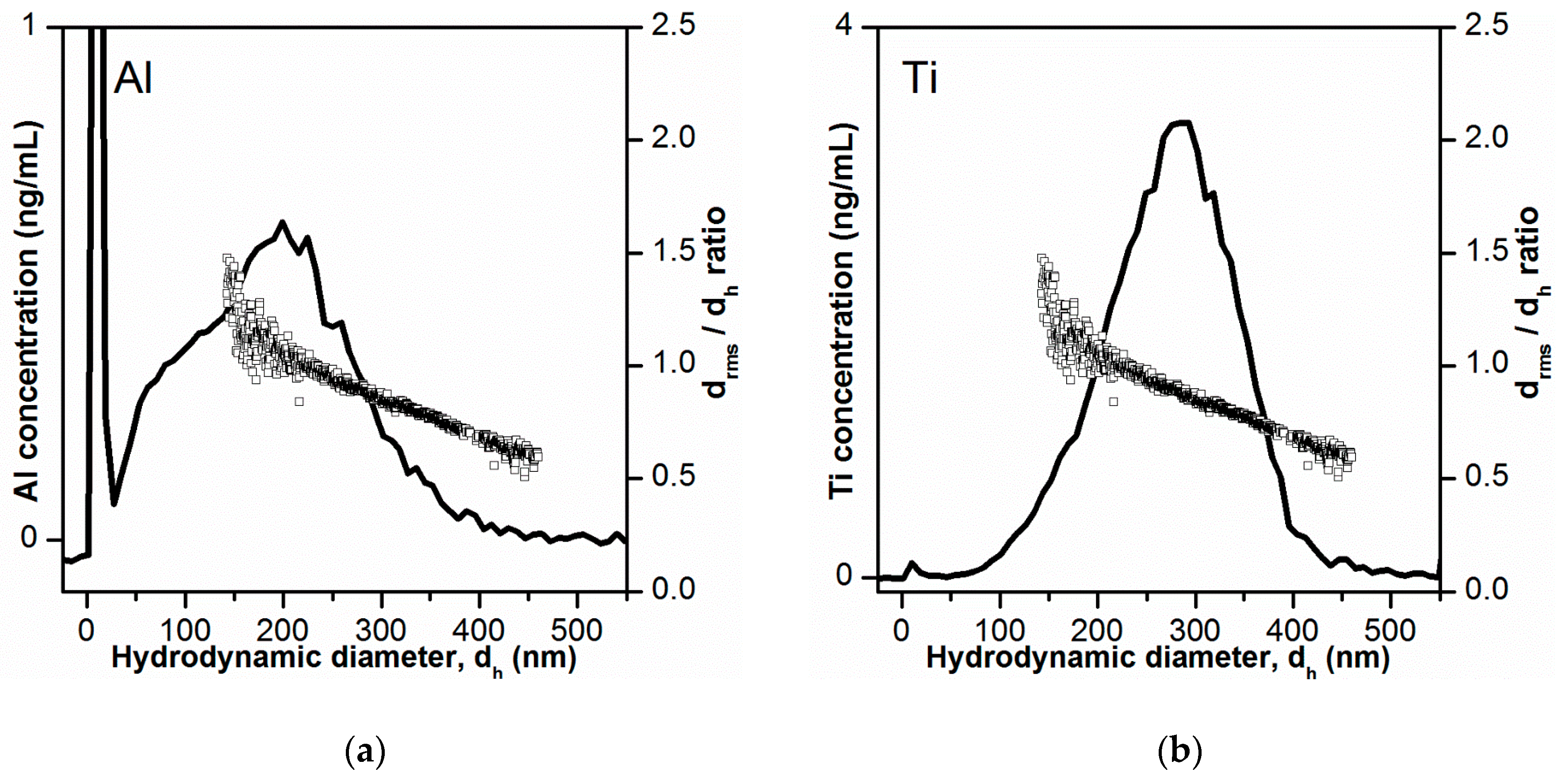
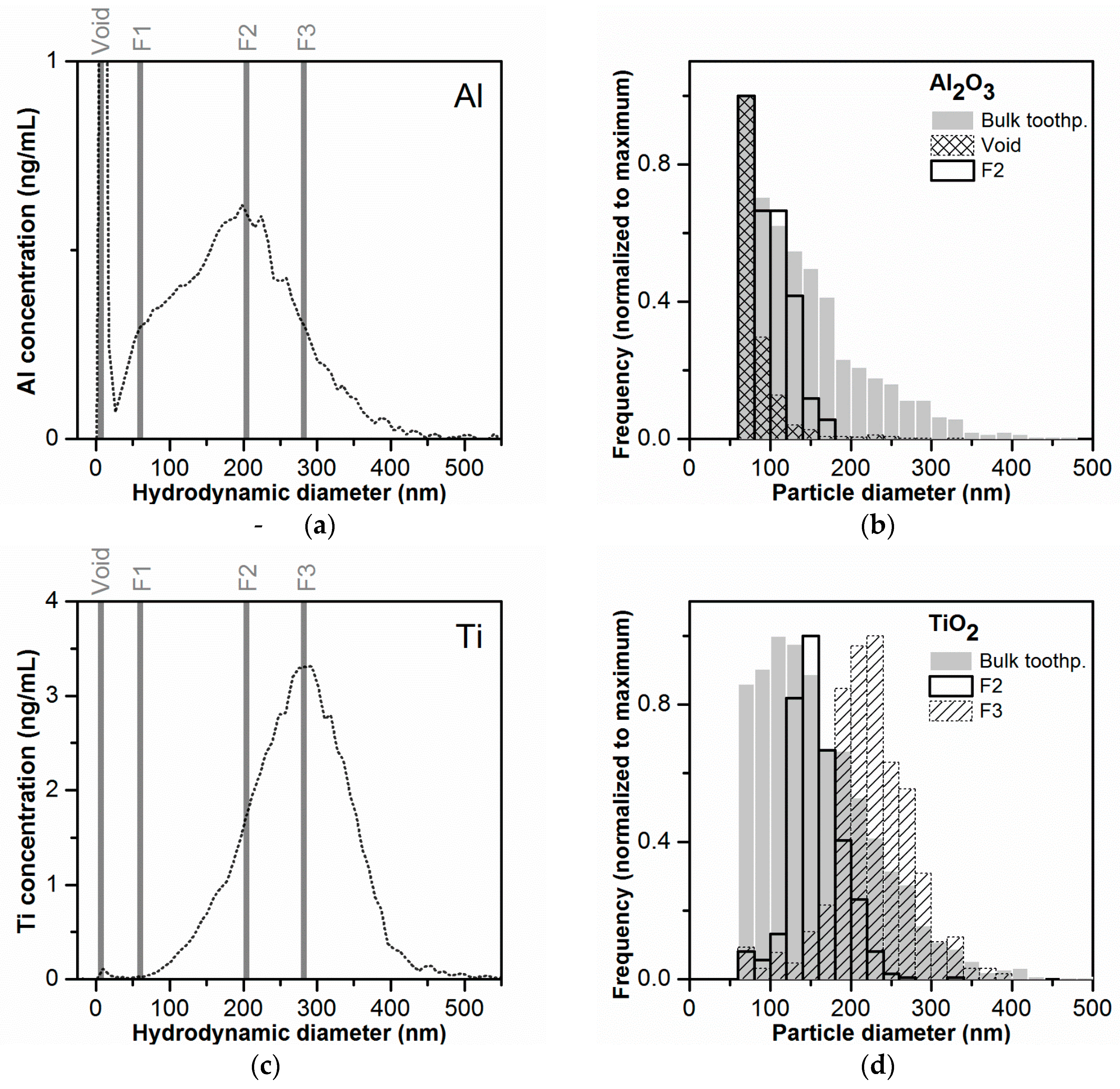
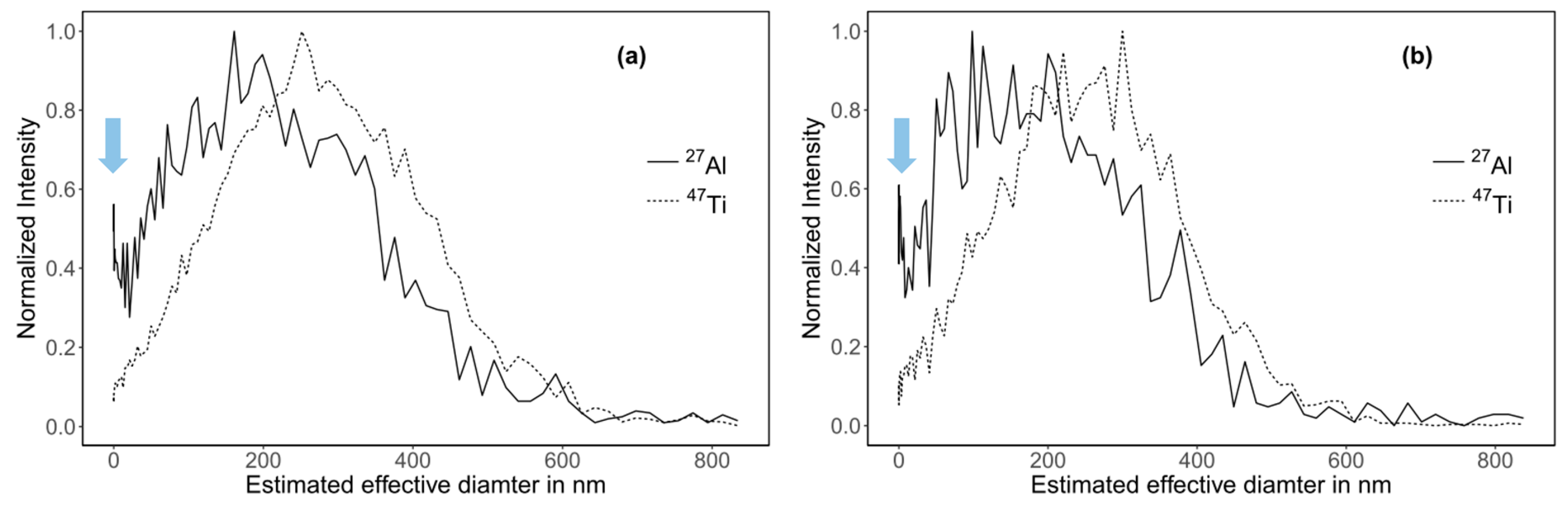
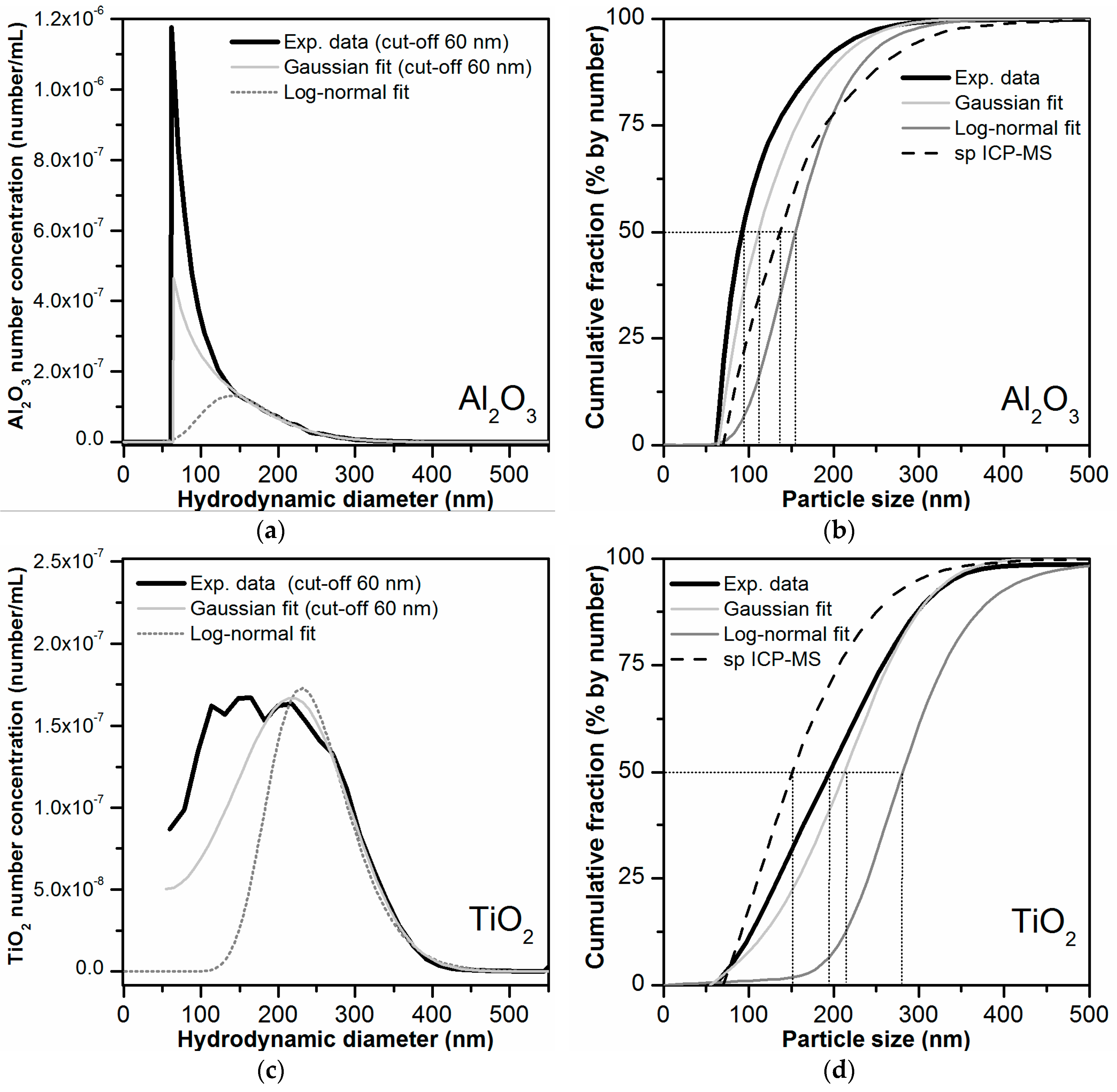
3.8. Determination of Particle Size Distribution
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, L 342, 1–59.
- The European Commission Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Off. J. Eur. Union 2011, L 275, 38–40.
- Linsinger, T.P.J.; Roebben, G.; Gilliland, D.; Calzolai, L.; Rossi, F.; Gibson, N.; Klein, C. Requirements on Measurements for the Implementation of the European Commission Definition of the Term “Nanomaterial”; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Rauscher, H.; Roebben, G.; Amenta, V.; Boix Sanfeliu, A.; Calzolai, L.; Emons, H.; Gaillard, C.; Gibson, N.; Linsinger, T.; Mech, A.; et al. Towards a Review of the EC Recommendation for a Definition of the Term “Nanomaterial”—Part 1: Compilation of Information Concerning the Experience with the Definition; Rauscher, H., Roebben, G., Eds.; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- von der Kammer, F.; Legros, S.; Hofmann, T.; Larsen, E.H.; Loeschner, K. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. TrAC Trends Anal. Chem. 2011, 30, 425–436. [Google Scholar] [CrossRef]
- Mattarozzi, M.; Suman, M.; Cascio, C.; Calestani, D.; Weigel, S.; Undas, A.; Peters, R. Analytical approaches for the characterization and quantification of nanoparticles in food and beverages. Anal. Bioanal. Chem. 2017, 409, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef] [PubMed]
- Heroult, J.; Nischwitz, V.; Bartczak, D.; Goenaga-Infante, H. The potential of asymmetric flow field-flow fractionation hyphenated to multiple detectors for the quantification and size estimation of silica nanoparticles in a food matrix. Anal. Bioanal. Chem. 2014, 406, 3919–3927. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Legros, S.; Loeschner, K.; Liu, J.; Navratilova, J.; Grombe, R.; Linsinger, T.P.J.; Larsen, E.H.; von der Kammer, F.; Hofmann, T. First steps towards a generic sample preparation scheme for inorganic engineered nanoparticles in a complex matrix for detection, characterization, and quantification by asymmetric flow-field flow fractionation coupled to multi-angle light scattering. J. Anal. At. Spectrom. 2015, 30, 1286–1296. [Google Scholar] [CrossRef]
- Contado, C.; Pagnoni, A. TiO2 in commercial sunscreen lotion: Flow field-flow fractionation and ICP-AES together for size analysis. Anal. Chem. 2008, 80, 7594–7608. [Google Scholar] [CrossRef] [PubMed]
- Nischwitz, V.; Goenaga-Infante, H. Improved sample preparation and quality control for the characterisation of titanium dioxide nanoparticles in sunscreens using flow field flow fractionation on-line with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2012, 27, 1084–1092. [Google Scholar] [CrossRef]
- Müller, D.; Cattaneo, S.; Meier, F.; Welz, R.; de Vries, T.; Portugal-Cohen, M.; Antonio, D.C.; Cascio, C.; Calzolai, L.; Gilliland, D.; et al. Inverse supercritical fluid extraction as a sample preparation method for the analysis of the nanoparticle content in sunscreen agents. J. Chromatogr. A 2016, 1440, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Sabbioni, E.; Micetic, I.; Alimonti, A.; Petrucci, F. Size and metal composition characterization of nano- and microparticles in tattoo inks by a combination of analytical techniques. J. Anal. At. Spectrom. 2017, 32, 616–628. [Google Scholar] [CrossRef]
- Loeschner, K.; Navratilova, J.; Købler, C.; Mølhave, K.; Wagner, S.; von der Kammer, F.; Larsen, E.H. Detection and characterization of silver nanoparticles in chicken meat by asymmetric flow field flow fractionation with detection by conventional or single particle ICP-MS. Anal. Bioanal. Chem. 2013, 405, 8185–8195. [Google Scholar] [CrossRef] [PubMed]
- Storehagen, S.; Ose, N.; Midha, S. Dentifrices and Mouthwashes Ingredients and Their Use; University of Oslo: Oslo, Norway, 2003. [Google Scholar]
- NanoDefine Project Development of Methods and Standards Supporting the Implementation of the Commission Recommendation for a Definition of Nanomaterial (FP7-NMP-2013-LARGE-7, no. 604347). Available online: http://www.nanodefine.eu (accessed on 6 May 2018).
- Loeschner, K.; Navratilova, J.; Legros, S.; Wagner, S.; Grombe, R.; Snell, J.; von der Kammer, F.; Larsen, E.H. Optimization and evaluation of asymmetric flow field-flow fractionation of silver nanoparticles. J. Chromatogr. A 2013, 1272, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Quantitative Chemical Analysis; WH Freeman: New York, NY, USA, 2010; ISBN 9781464135385. [Google Scholar]
- Park, J.; Lakes, R.S. Biomaterials: An Introduction, 3rd ed.; Springer-Verlag: New York, NY, USA, 2007; ISBN 9780387378794. [Google Scholar]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Rakcheev, D.; Philippe, A.; Schaumann, G.E. Hydrodynamic chromatography coupled with single particle-inductively coupled plasma Mass Spectrometry for investigating nanoparticles agglomerates. Anal. Chem. 2013, 85, 10643–10647. [Google Scholar] [CrossRef] [PubMed]
- UniChrom. Available online: http://www.unichrom.com (accessed on 26 November 2018).
- Koeber, R.; Kestens, V.; Bienert, R.; Müller, P. NanoDefine Technical Report D1.6—Characterization Dossier for Reference Materials. Available online: http://www.nanodefine.eu/publications/reports/NanoDefine_TechnicalReport_D1.6.pdf (accessed on 6 May 2018).
- Wiilknitz, P. Cleaning Power and Abrasivity of European Toothpastes. Adv. Dent. Res. 1997, 11, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Boyer, I.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Alumina and Aluminum Hydroxide as Used in Cosmetics. Int. J. Toxicol. 2016, 35, 16S–33S. [Google Scholar] [CrossRef] [PubMed]
- European Comission Comission Decision of February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. Off. J. Eur. Union 2006, L 97, 1–528.
- Fruijtier-Pölloth, C. The safety of nanostructured synthetic amorphous silica (SAS) as a food additive (E 551) (The preparation of this manuscript was financially supported by the Association of Amorphous Silica Producers). Arch. Toxicol. 2016, 90, 2885–2916. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A. (Ed.) Kirk-Othmer Chemical Technology of Cosmetics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- The European Parliament and the Council of the European Union Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2012, L 83, 1–295.
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VI. Update. J. Colloid Interface Sci. 2014, 426, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Nogi, K.; Naito, M. (Eds.) Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780444563361. [Google Scholar]
- Nelson, R.D. Dispersing Powders in Liquids. In Handbook of Powder Technology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 7, ISBN 9780444430045. [Google Scholar]
- Xu, Z.; Ducker, W.; Israelachvili, J. Forces between Crystalline Alumina (Sapphire) Surfaces in Aqueous Sodium Dodecyl Sulfate Surfactant Solutions. Langmuir 1996, 12, 2263–2270. [Google Scholar] [CrossRef]
- Palla, B.J. Mixed surfactant systems to control dispersion stability in severe environments for enhancing chemical mechanical polishing (CMP) of metal surfaces. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2000. [Google Scholar]
- Imae, T.; Muto, K.; Ikeda, S. The pH dependence of dispersion of TiO2 particles in aqueous surfactant solutions. Colloid Polym. Sci. 1991, 269, 43–48. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, H.; Li, X.-F.; Wang, Z.-F.; Lin, F. Stability of TiO2 and Al2O3 Nanofluids. Chin. Phys. Lett. 2011, 28, 86601. [Google Scholar] [CrossRef]
- Veronovski, N.; Andreozzi, P.; La Mesa, C.; Sfiligoj-Smole, M. Stable TiO2 dispersions for nanocoating preparation. Surf. Coatings Technol. 2010, 204, 1445–1451. [Google Scholar] [CrossRef]
- Schimpf, M.; Caldwell, K.; Giddings, J.C. (Eds.) Field-Flow Fractionation Handbook; John Wiley & Sons, Inc.: New York, NY, USA, 2000; ISBN 0-471-18430-6. [Google Scholar]
- Siripinyanond, A.; Barnes, R.M. Flow field-flow fractionation-inductively coupled plasma mass spectrometry of chemical mechanical polishing slurries. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1885–1896. [Google Scholar] [CrossRef]
- Aureli, F.; D’Amato, M.; Raggi, A.; Cubadda, F. Quantitative characterization of silica nanoparticles by asymmetric flow field flow fractionation coupled with online multiangle light scattering and ICP-MS/MS detection. J. Anal. At. Spectrom. 2015, 30, 1266–1273. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; Peters, R.J.B.; van Bemmel, M.E.M.; Rivera, Z.E.H.; Wagner, S.; von der Kammer, F.; Tromp, P.C.; Hofmann, T.; Weigel, S. Physicochemical characterization of titanium dioxide pigments using various techniques for size determination and asymmetric flow field flow fractionation hyphenated with inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 6679–6691. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, N.; Losert, S.; Adlhart, C.; Lattuada, M.; Ulrich, A. Membrane–particle interactions in an asymmetric flow field flow fractionation channel studied with titanium dioxide nanoparticles. J. Chromatogr. A 2014, 1334, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Barahona, F.; Geiss, O.; Urbán, P.; Ojea-Jimenez, I.; Gilliland, D.; Barrero-Moreno, J. Simultaneous Determination of Size and Quantification of Silica Nanoparticles by Asymmetric Flow Field-Flow Fractionation Coupled to ICPMS Using Silica Nanoparticles Standards. Anal. Chem. 2015, 87, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- von der Kammer, F.; Baborowski, M.; Friese, K. Field-flow fractionation coupled to multi-angle laser light scattering detectors: Applicability and analytical benefits for the analysis of environmental colloids. Anal. Chim. Acta 2005, 552, 166–174. [Google Scholar] [CrossRef]
- Striegel, A.M.; Brewer, A.K. Hydrodynamic Chromatography. Annu. Rev. Anal. Chem. 2012, 5, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, G.; Kraak, J.C.; Poppe, H. Dispersion in packed-column hydrodynamic chromatography. J. Chromatogr. A 1993, 634, 149–159. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Tiede, D.; Tear, S.P.; David, H.; Lewis, J. A robust size-characterisation methodology for studying nanoparticle behaviour in ‘real’ environmental samples, using hydrodynamic chromatography coupled to ICP-MS. J. Anal. At. Spectrom. 2009, 24, 964. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Wang, X.; Gore, D.; Tiede, D.; Baxter, M.; David, H.; Tear, S.P.; Lewis, J. Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J. Anal. At. Spectrom. 2010, 25, 1149. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Evaluation of Hydrodynamic Chromatography Coupled with UV-Visible, Fluorescence and Inductively Coupled Plasma Mass Spectrometry Detectors for Sizing and Quantifying Colloids in Environmental Media. PLoS ONE 2014, 9, e90559. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Gangloff, M.; Rakcheev, D.; Schaumann, G.E. Evaluation of hydrodynamic chromatography coupled with inductively coupled plasma mass spectrometry detector for analysis of colloids in environmental media—Effects of colloid composition, coating and shape. Anal. Methods 2014, 6, 8722–8728. [Google Scholar] [CrossRef]
- Holdich, R.G. Fundamentals of Particle Technology; Midland Information Technology and Publishing: Nottingham, UK, 2002; ISBN 9780954388102. [Google Scholar]
- Gigault, J.; El Hadri, H.; Reynaud, S.; Deniau, E.; Grassl, B. Asymmetrical flow field flow fractionation methods to characterize submicron particles: application to carbon-based aggregates and nanoplastics. Anal. Bioanal. Chem. 2017, 409, 6761–6769. [Google Scholar] [CrossRef] [PubMed]

| Step | Duration (min) | Mode | Cross Flow Rate (mL/min) |
|---|---|---|---|
| 1 | 2 | Elution | - |
| 2 | 2 | Elution | Usually same as step 6; final method: 0.5 mL/min |
| 3 | 1 | Focus | - |
| 4 | 5 | Focus + injection | - |
| 5 | 10 | Focus | - |
| 6 | 65 | Elution | Tested: 0.3–0.75 mL/min; final method: 0.5 mL/min |
| 7 | 5 | Elution | - |
| 8 | 5 | Elution + injection | - |
| m/z | Cross Flow Rate (mL/min) | tr, Main Peak (min) | w, Main Peak (min) | RS | Rec. Void Peak (%) | Rec. Main Peak (%) | Rec. Release Peak (%) | Rec. Total (%) |
|---|---|---|---|---|---|---|---|---|
| 27Al | 0.3 | 16.7 ± 0.8 | 16.5 ± 1.0 | 1.8 ± 0.2 | 3 ± 0 | 16 ± 1 | 1 ± 1 | 20 ± 1 |
| 0.4 | 22.2 ± 1.6 | 17.8 ± 0.3 | 2.2 ± 0.2 | 3 ± 0 | 10 ± 3 | 2 ± 1 | 15 ± 3 | |
| 0.5 | 27.0 ± 0.0 | 19.0 ± 1.4 | 2.5 ± 0.2 | 3 ± 0 | 10 ± 1 | 6 ± 2 | 18 ± 2 | |
| 0.75 | 29.1 ± 3.4 | 35.2 ± 6.4 | 1.3 ± 0.5 | 3 ± 1 | 4 ± 2 | 9 ± 10 | 16 ± 13 | |
| 47Ti | 0.3 | 23.1 ± 0.1 | 17.0 ± 0.2 | 2.5 ± 0.1 | 1 ± 0 | 88 ± 8 | 4 ± 1 | 93 ± 8 |
| 0.4 | 29.7 ± 0.7 | 18.7 ± 0.8 | 3.0 ± 0.0 | 0 ± 0 | 73 ± 22 | 11 ± 3 | 85 ± 19 | |
| 0.5 | 34.8 ± 0.1 | 20.9 ± 0.4 | 3.1 ± 0.1 | 0 ± 0 | 50 ± 1 | 22 ± 4 | 72 ± 2 | |
| 0.75 | 42.3 ± 0.8 | 28.1 ± 2.1 | 2.9 ± 0.3 | 0 ± 0 | 16 ± 3 | 50 ± 10 | 66 ± 13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, M.; Uusimäki, T.; Philippe, A.; Loeschner, K. Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste. Separations 2018, 5, 56. https://doi.org/10.3390/separations5040056
Correia M, Uusimäki T, Philippe A, Loeschner K. Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste. Separations. 2018; 5(4):56. https://doi.org/10.3390/separations5040056
Chicago/Turabian StyleCorreia, Manuel, Toni Uusimäki, Allan Philippe, and Katrin Loeschner. 2018. "Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste" Separations 5, no. 4: 56. https://doi.org/10.3390/separations5040056
APA StyleCorreia, M., Uusimäki, T., Philippe, A., & Loeschner, K. (2018). Challenges in Determining the Size Distribution of Nanoparticles in Consumer Products by Asymmetric Flow Field-Flow Fractionation Coupled to Inductively Coupled Plasma-Mass Spectrometry: The Example of Al2O3, TiO2, and SiO2 Nanoparticles in Toothpaste. Separations, 5(4), 56. https://doi.org/10.3390/separations5040056





