A Low-Cost Approach Using Diatomaceous Earth Biosorbent as Alternative SPME Coating for the Determination of PAHs in Water Samples by GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumental and Chromatographic Conditions
2.3. Preparation of Diatomaceous Earth Fibers
2.4. Optimization of SPME Procedure
2.5. Comparison of the Extraction Efficiencies Using Diatomaceous Earth and Commercial Fibers
2.6. Analytical Figures of Merit of the Method Developed
3. Results and Discussion
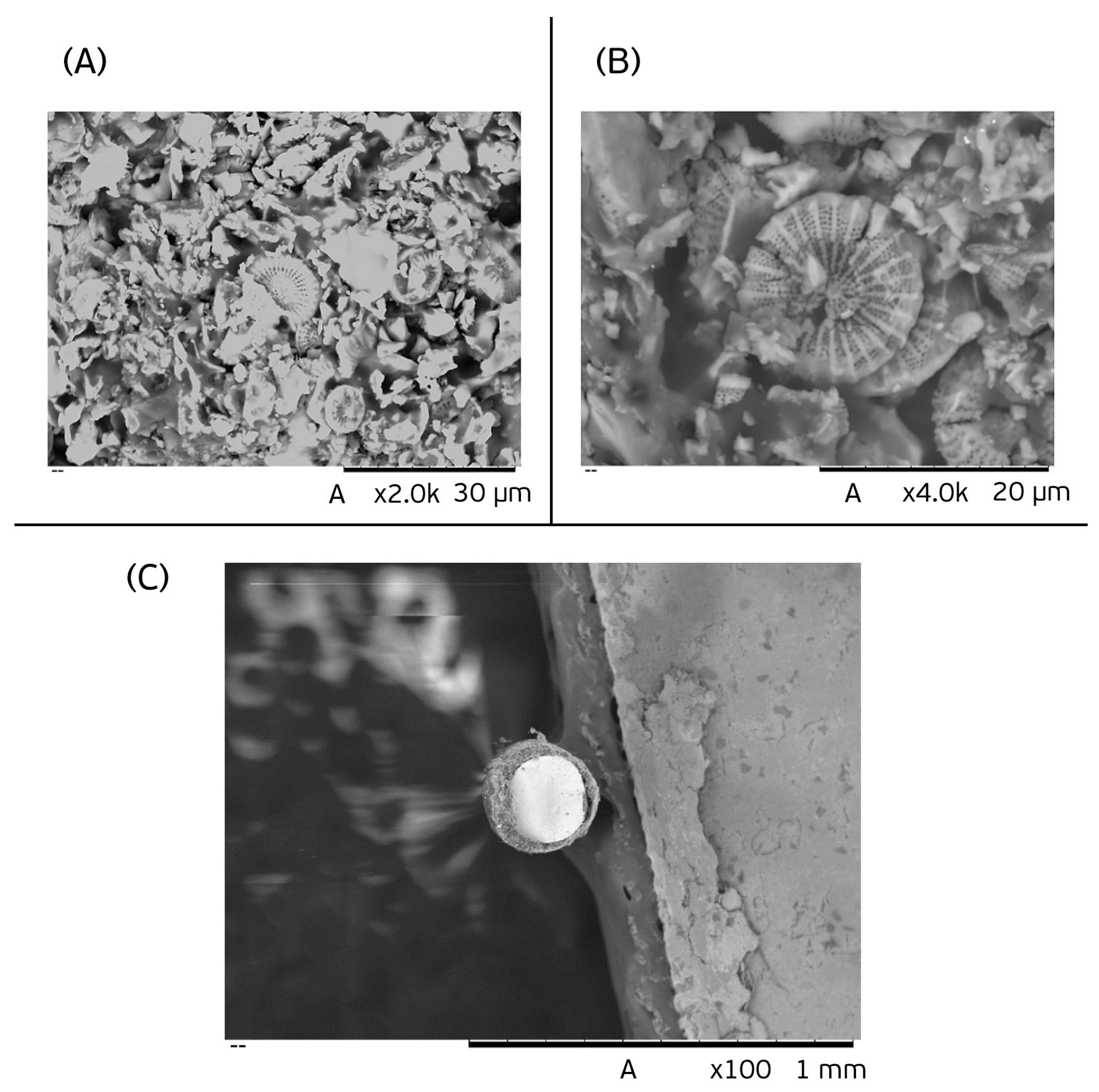
3.1. Characterization of the Diatomaceous Fiber
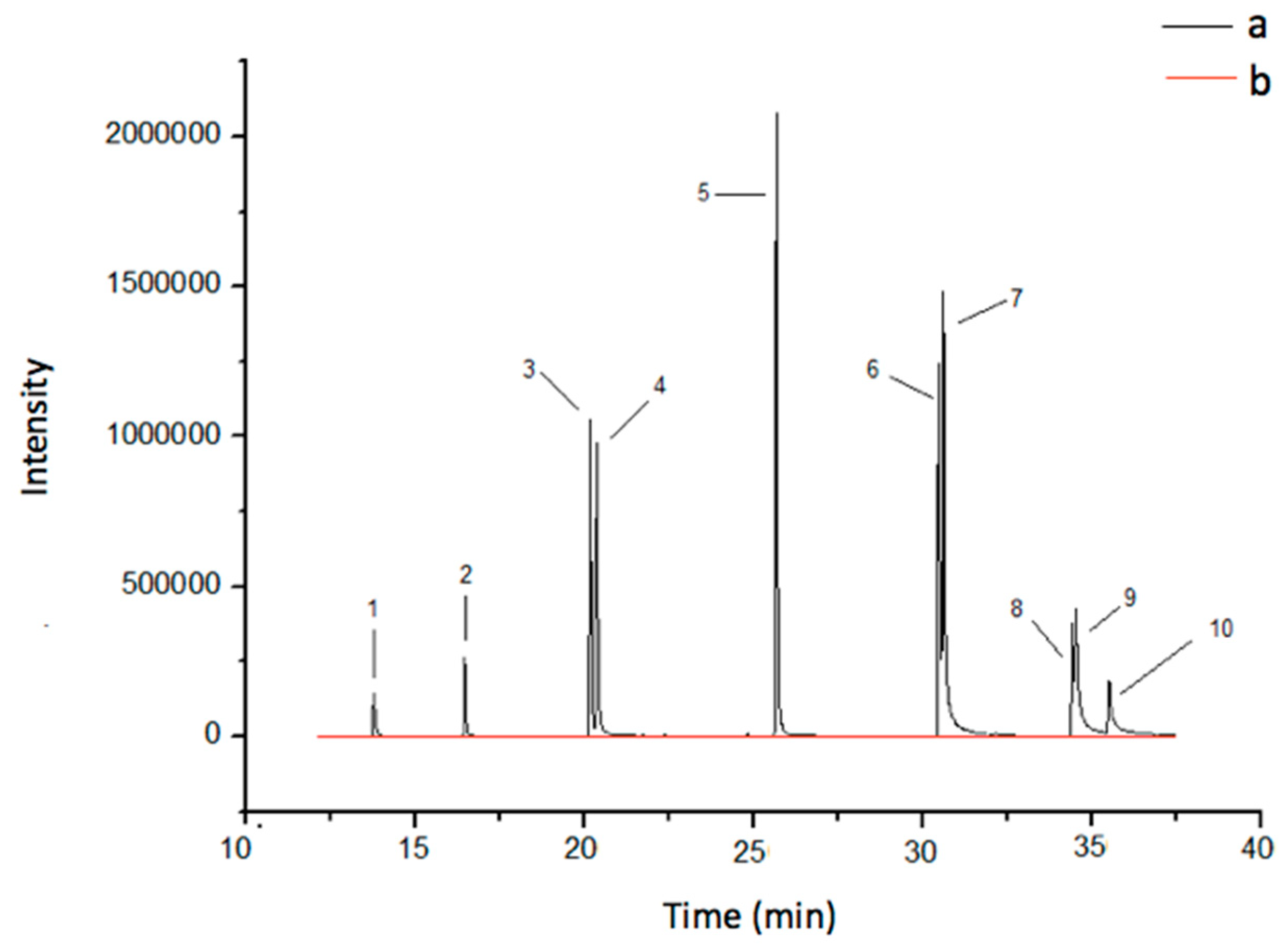
3.2. Optimization of DI-SPME Extraction Procedure
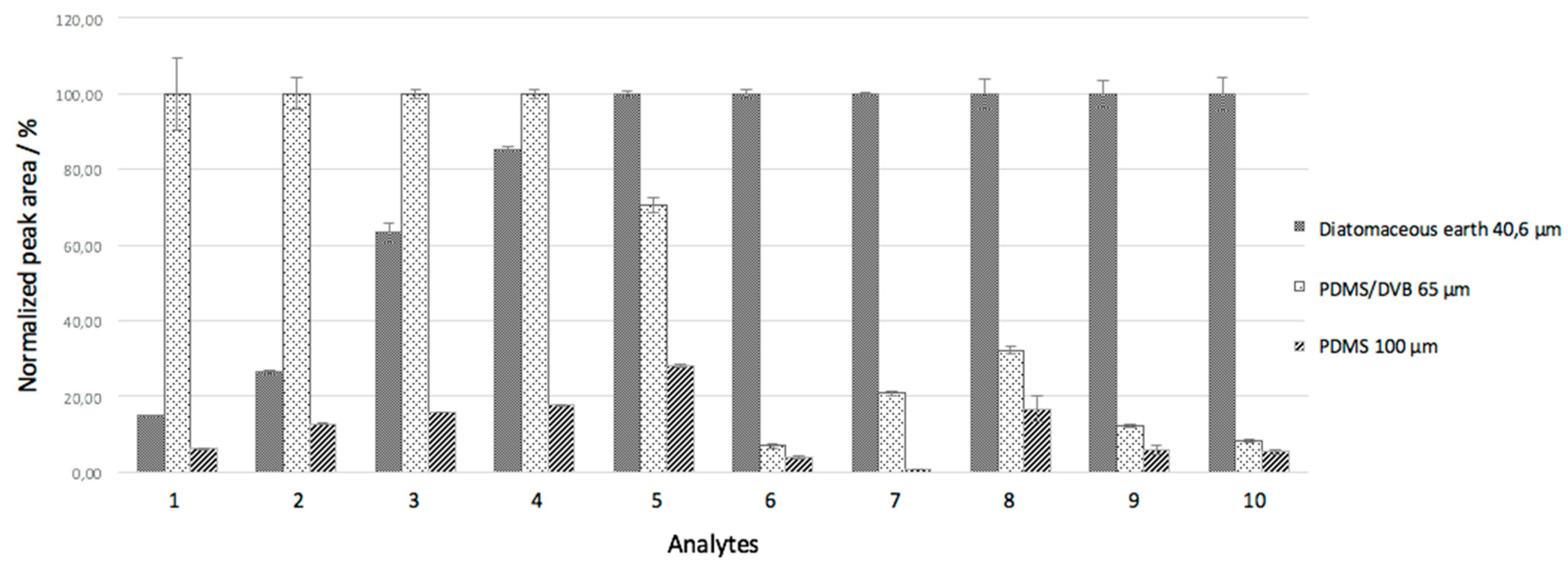
3.3. Comparison between the Extraction Efficiencies of the Biosorbent and Commercial Coatings
3.4. Validation Parameters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benson, R.; Conerly, O.D.; Sander, W.; Batt, A.L.; Boone, J.S.; Furlong, E.T.; Glassmeyer, S.T.; Kolpin, D.W.; Mash, H.E.; Shenck, K.M.; et al. Human health screening and public health significance of contaminants of emerging concern detected in public water supplies. Sci. Total Environ. 2017, 579, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; He, Y.; Jakel, M.; Reinhard, M.; Gin, K.Y. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Heleno, F.F.; Lima, A.C. Evaluation of analytical methods for BTEX analysis in water using extraction by headspace (HS) and solid phase microextraction (SPME). Quim. Nova 2010, 33, 329–336. [Google Scholar] [CrossRef]
- Hong, W.F.; Jia, H.; Li, Y.F. Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicol. Environ. Saf. 2016, 128, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Slezakva, K.; Castro, D.; Delerue-Matos, C. Impact of vehicular traffic emissions on particulate-bound PAHs: Levels and associated health risks. Atmos. Res. 2013, 127, 141–147. [Google Scholar] [CrossRef]
- Cristale, J.; Silva, F.S.; Zocolo, G.J.; Marchi, M.R.R. Influence of sugarcane burning on indoor/outdoor PAH air pollution in Brazil. Environ. Pollut. 2012, 169, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.D.; Chang, M.B. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ. 2017, 31, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Siritham, C.; Thammakhet-Buranacha, C. A preconcentrator-separator two-in-one online system for polycyclic aromatic hydrocarbons analysis. Talanta 2017, 15, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, R.; Bai, S.; Wang, C.; Wang, Z. A solid phase microextraction fiber coated with graphene-poly9ethylene glycol) composite for the extraction of volatile aromatic compounds from water samples. Talanta 2014, 119, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Laopongsit, W.; Srzednicki, G.; Craske, J. Preliminary study of solid phase micro-extraction (SPME) as a method for detecting insect infestation in wheat grain. J. Stored Prod. Res. 2014, 59, 88–95. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Evolution of solid-phase microextraction technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef]
- Dias, A.N.; Simão, V.; Merib, J.; Carasek, E. Cork as a new (green) coating for solid-phase microextraction: Determination of polycyclic aromatic hydrocarbons in water samples by gas chromatography-mass spectrometry. Anal. Chim. Acta 2013, 772, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Carasek, E.; Merib, J. Membrane-based microextraction techniques in analytical chemistry: A review. Anal. Chim. Acta 2015, 23, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Chem. Ind. Press: Beijing, China, 2009. [Google Scholar]
- Tsao, Y.U.; Wang, Y.C.; Wu, S.F.; Ding, W.H. Microwave-assisted headspace solid-phase microextraction for the rapid determination of organophosphate esters in aqueous samples by gas chromatography-mass spectrometry. Talanta 2011, 84, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Merib, J.; Dias, A.N.; Stolberg, J.; Budziak, D.; Carasek, E. A low-cost biosorbent-based coating for the highly sensitive determination of organochlorine pesticides by solid-phase microextraction and gas chromatography-electron capture detection. J. Chromatogr. A 2017, 1525, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Suterio, N.G.; do Carmo, S.N.; Budziak, D.; Merib, J.; Carasek, E. Use of a Natural Sorbent as Alternative Solid-Phase Microextraction Coating for the Determination of Polycyclic Aromatic Hydrocarbons in Water Samples by Gas Chromatography-Mass Spectrometry. J. Braz. Chem. Soc. 2018, 29. [Google Scholar] [CrossRef]
- Silveira, C.B.; Goulart, M.R. Methodologies for the reuse of the diatomaceous earth residue, from filtration and clarification of beer. Quim. Nova 2011, 34, 625–629. [Google Scholar]
- Souza, G.P.; Filgueira, M. Characterization of natural diatomaceous composite material. Ceramica 2003, 49, 40–43. [Google Scholar] [CrossRef]
- Othmer, K. Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 1993; p. 108. [Google Scholar]
- Menezes, H.C.; Paulo, B.P.; Paiva, M.J.N.; Barcelos, S.M.R.; Macedo, D.F.D.; Cardeal, Z.L. Determination of polycyclic aromatic hydrocarbons in artisanal cachaça by DI-CF-SPME–GC/MS. Microchem. J. 2015, 118, 272–277. [Google Scholar] [CrossRef]
- Aguinaga, N.; Campillo, N.; Vinas, P.; Hernández-Córdoba, M. Determination of 16 polycyclic aromatic hydrocarbons in milk and related products using solid-phase microextraction coupled to gas chromatography–mass spectrometry. Anal. Chim. Acta 2007, 23, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Sánchez, V.H.; Marqués, S.; Molina, L. Insights in the regulation of the degradation of PAHs in Novosphingobium sp. HR1a and utilization of this regulatory system as a tool for the detection of PAHs. Sci. Total Environ. 2017, 590, 381–393. [Google Scholar] [CrossRef] [PubMed]


| Analytes | m/z |
|---|---|
| acenaphthylene | 152, 153, 151 |
| fluorene | 166, 165, 167 |
| phenanthrene | 178, 176, 179 |
| anthracene | 178, 179, 176 |
| pyrene | 202, 203, 200 |
| benzo[a]anthracene | 228, 226, 229 |
| chrysene | 228, 226, 229 |
| benzo[b]fluoranthene | 252, 250, 126 |
| benzo[k]fluoranthene | 252, 250, 126 |
| benzo[a]pyrene | 252, 250, 126 |
| Analyte | LOD (µg L−1) | LOQ (µg L−1) | Linear Range (µg L−1) | Linear Equation | R |
|---|---|---|---|---|---|
| Acenaphthylene | 0.16 | 0.49 | 0.49–25 | y = 66,956x – 30,445 | 0.9890 |
| Fluorene | 0.17 | 0.50 | 0.50–25 | y = 87,809x – 54,517 | 0.9911 |
| Phenanthrene | 0.14 | 0.42 | 0.42–25 | y = 326,565x – 245,240 | 0.9777 |
| Anthracene | 0.11 | 0.33 | 0.33–25 | y = 364,057x – 339,574 | 0.9598 |
| Pyrene | 0.15 | 0.50 | 0.50–25 | y = 979,497x – 935,649 | 0.9914 |
| benzo[a]anthracene | 0.03 | 0.10 | 0.10–25 | y = 506,040x – 544,597 | 0.9832 |
| Chrysene | 0.14 | 0.42 | 0.42–25 | y = 691,902x – 796,526 | 0.9592 |
| benzo[b]fluoranthene | 0.06 | 0.17 | 0.17–25 | y = 158,587x – 50,481 | 0.9990 |
| benzo[k]fluoranthene | 0.11 | 0.33 | 0.33–25 | y = 431,634x – 806,517 | 0.9848 |
| benzo[a]pyrene | 0.15 | 0.46 | 0.46–25 | y = 295,450x – 567,387 | 0.9667 |
| Analyte | Spiked Concentration (µg L−1) | Relative Recovery (%) (n = 3) | RSD, Intra-Day (%) (n = 3) | RSD, Inter-Day (%) (n = 3) |
|---|---|---|---|---|
| acenaphthylene | 0.5 | 100 | 5 | 10 |
| fluorene | 0.5 | 83 | 15 | 10 |
| phenanthrene | 0.5 | 97 | 10 | 13 |
| anthracene | 0.5 | 93 | 13 | 3 |
| pyrene | 0.5 | 92 | 2 | 6 |
| benzo[a]anthracene | 0.5 | 94 | 2 | 6 |
| chrysene | 0.5 | 96 | 2 | 6 |
| benzo[b]fluoranthene | 0.5 | 90 | 15 | 17 |
| benzo[k]fluoranthene | 0.5 | 97 | 15 | 17 |
| benzo[a]pyrene | 0.5 | 93 | 7 | 17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinert, N.P.; Vieira, C.M.S.; Da Silveira, C.B.; Budziak, D.; Carasek, E. A Low-Cost Approach Using Diatomaceous Earth Biosorbent as Alternative SPME Coating for the Determination of PAHs in Water Samples by GC-MS. Separations 2018, 5, 55. https://doi.org/10.3390/separations5040055
Reinert NP, Vieira CMS, Da Silveira CB, Budziak D, Carasek E. A Low-Cost Approach Using Diatomaceous Earth Biosorbent as Alternative SPME Coating for the Determination of PAHs in Water Samples by GC-MS. Separations. 2018; 5(4):55. https://doi.org/10.3390/separations5040055
Chicago/Turabian StyleReinert, Naysla Paulo, Camila M. S. Vieira, Cristian Berto Da Silveira, Dilma Budziak, and Eduardo Carasek. 2018. "A Low-Cost Approach Using Diatomaceous Earth Biosorbent as Alternative SPME Coating for the Determination of PAHs in Water Samples by GC-MS" Separations 5, no. 4: 55. https://doi.org/10.3390/separations5040055
APA StyleReinert, N. P., Vieira, C. M. S., Da Silveira, C. B., Budziak, D., & Carasek, E. (2018). A Low-Cost Approach Using Diatomaceous Earth Biosorbent as Alternative SPME Coating for the Determination of PAHs in Water Samples by GC-MS. Separations, 5(4), 55. https://doi.org/10.3390/separations5040055





