Abstract
In cases of suspected arson, a body may be intentionally burnt to cause loss of life, dispose of remains, or conceal identification. A primary focus of a fire investigation, particularly involving human remains, is to establish the cause of the fire; this often includes the forensic analysis of fire debris for the detection of ignitable liquid residues (ILRs). Commercial containers for the collection of fire debris evidence include metal cans, glass jars, and polymer/nylon bags of limited size. This presents a complication in cases where the fire debris consists of an intact, or partially intact, human cadaver. This study proposed the use of a body bag as an alternative sampling container. A method was developed and tested for the collection and analysis of ILRs from burnt porcine remains contained within a body bag using dynamic headspace sampling (using an Easy-VOC™ hand-held manually operated grab-sampler and stainless steel sorbent tubes containing Tenax TA) followed by thermal desorption comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry (TD-GC×GC-TOFMS). The results demonstrated that a body bag containing remains burnt with gasoline tested positive for the presence of gasoline, while blank body bag controls and a body bag containing remains burnt without gasoline tested negative. The proposed method permits the collection of headspace samples from burnt remains before the remains are removed from the crime scene, limiting the potential for contamination and the loss of volatiles during transit and storage.
Keywords:
forensic chemistry; fire debris analysis; fire debris packaging; burnt remains; ignitable liquid residues (ILRs); volatile organic compounds (VOCs); dynamic headspace sampling; thermal desorption; comprehensive two-dimensional gas chromatography (GC×GC); time-of-flight mass spectrometry (TOFMS) 1. Introduction
The detection and identification of ignitable liquid residues (ILRs) from fire debris can be a significant challenge for fire investigators and forensic practitioners. Detection of ILRs is important in cases of arson whereby a structure, vehicle, or other property has been intentionally destroyed, often through the application of accelerants [1]. Arson can also lead to the loss of life, whether accidentally or intentionally, to dispose of the remains, destroy evidence, or conceal the victim’s identity. When a fire investigation involves a fatality, the body or remains must be recovered to determine the cause and manner of death [2]. Additionally, detection of ILRs from the body or tissues may assist in understanding the circumstances surrounding death.
The identification of ignitable liquids requires the extraction of highly volatile organic compounds (VOCs) from fire debris collected at a scene [3,4]. A range of containers may be used to collect fire debris depending on the volume of debris and state of recovery [5,6,7]. These typically include metal cans [8], glass jars (e.g., Mason jars) [2,9], and polymer/nylon bags [6,10,11,12,13] of varying size, though, generally, not large enough for the collection of burnt human remains. While detection of ILRs from human remains can be conducted through the analysis of postmortem blood samples [14,15,16] or other postmortem tissues excised during the autopsy [2], contamination or a loss of volatiles may occur during these processes. Nevertheless, the recovery of victim remains from a fire scene usually involves the use of a body bag to transport the remains to the morgue so that an autopsy can be performed; therefore, this study introduces the use of a body bag as an integral addition to the fire debris analysts’ packaging arsenal. The body bag not only provides a packaging container large enough for the collection of burnt victim remains, but also permits sample collection at the scene, reducing the potential for contamination and the loss of volatiles.
The extraction of VOCs from fire debris can be undertaken using a range of sample collection techniques. Common methods include direct headspace sampling [2,17], activated charcoal strips [1,3,18], dynamic headspace sampling [19,20,21], and solid-phase microextraction (SPME) [4,8,22,23]. For trace analysis, methods that concentrate the sample, such as dynamic headspace sampling and SPME, are preferred for the analysis of ILRs. Sample collection is followed by analysis using gas chromatography-mass spectrometry (GC-MS) as outlined in ASTM E1618-14 [24]. However, fire debris typically generates a highly complex mixture of VOCs, and GC-MS can produce unresolved chromatograms whereby trace levels of ignitable liquids may be masked by background pyrolysis products. Additionally, human remains produce a range of natural VOCs when burnt [25] that must be distinguished from the VOCs present in gasoline or other ignitable liquids, further confounding the characterization of accelerant use at a fire scene. The use of comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry (GC×GC-TOFMS) has been shown to alleviate such issues through increased peak capacity, improved resolution, enhanced sensitivity, and structured chromatograms, allowing for the rapid characterization of ignitable liquids present [26].
This study proposes a new method involving collection of VOCs directly from the body bag using dynamic headspace sampling, combined with analysis by GC×GC-TOFMS. As in previous studies [2,25], pig carcasses were used as analogues for human remains. Due to its prevalence in arson cases [7], gasoline was chosen as the accelerant of interest in this study. The benefit of this technique is the ability to sample directly from the body bag at the crime scene, and prior to transport and storage, to reduce the loss of key compounds for characterization of ILRs. Sample collection directly onto a sorbent tube allows the tube to be sealed and stored safely for several weeks prior to the analysis being conducted. This method has the potential to significantly increase the likelihood of detecting and identifying ILRs from victims of arson.
2. Materials and Methods
2.1. Experimental Design
Due to the legal and ethical regulations associated with the acquisition and burning of human cadavers, pig carcasses were selected as a substitute for human remains in this study. Two adult, domestic pig carcasses (Sus scrofa domesticus L.), weighing approximately 50–60 kg each, were purchased, postmortem, by way of excess stock from Hawkesbury Valley Meat Processors (licensed abattoir in Sydney, NSW, Australia). In accordance with the guidelines of the Australian code for the care and use of animals for scientific purposes [27], animal ethics approval was not required for this study since the pig carcasses used herein were: (1) acquired postmortem; and (2) not killed specifically for the purposes of this research.
To mimic a clothed human cadaver, each pig carcass was clothed in a white 100% cotton t-shirt and a pair of black polyester briefs (Kmart, Sydney, NSW, Australia) prior to burning. The experimental burns were performed outdoors by trained fire and rescue personnel at the Fire & Rescue NSW Fire Investigation Research Unit (Londonderry, NSW, Australia) within 24 h of death. Each pig carcass was burned independently on top of a 2 m × 2 m stainless steel tray, which was used to contain any fluid leakage from the pigs, as well as to prevent the fires from spreading out of control. The pig carcasses were placed on their sides for burning, and leaf litter and brush were placed underneath and on top of each pig carcass to assist in sustaining the fire.
The first experimental burn was conducted in the absence of any ignitable liquids, with a propane gas torch used to ignite the brush surrounding the pig carcass. The second experimental burn was conducted in the presence of ~720 mL unleaded 91-octane gasoline (purchased locally). A hand-held butane gas lighter was used to ignite the gasoline immediately after it was poured onto the pig carcass and surrounding brush. A small sample of the gasoline (~30 mL) was retained as a reference sample. The temperature of each experimental burn was measured and recorded using a DT85 data-logger and five Type N thermocouples (20 m × 3 mm; TC Measurement & Control, Melbourne, VIC, Australia) placed above the upper and lower torso regions and underneath the hind legs, torso, and head of each pig carcass. Each fire was extinguished with water after 20 min of burning.
2.2. Sample Collection
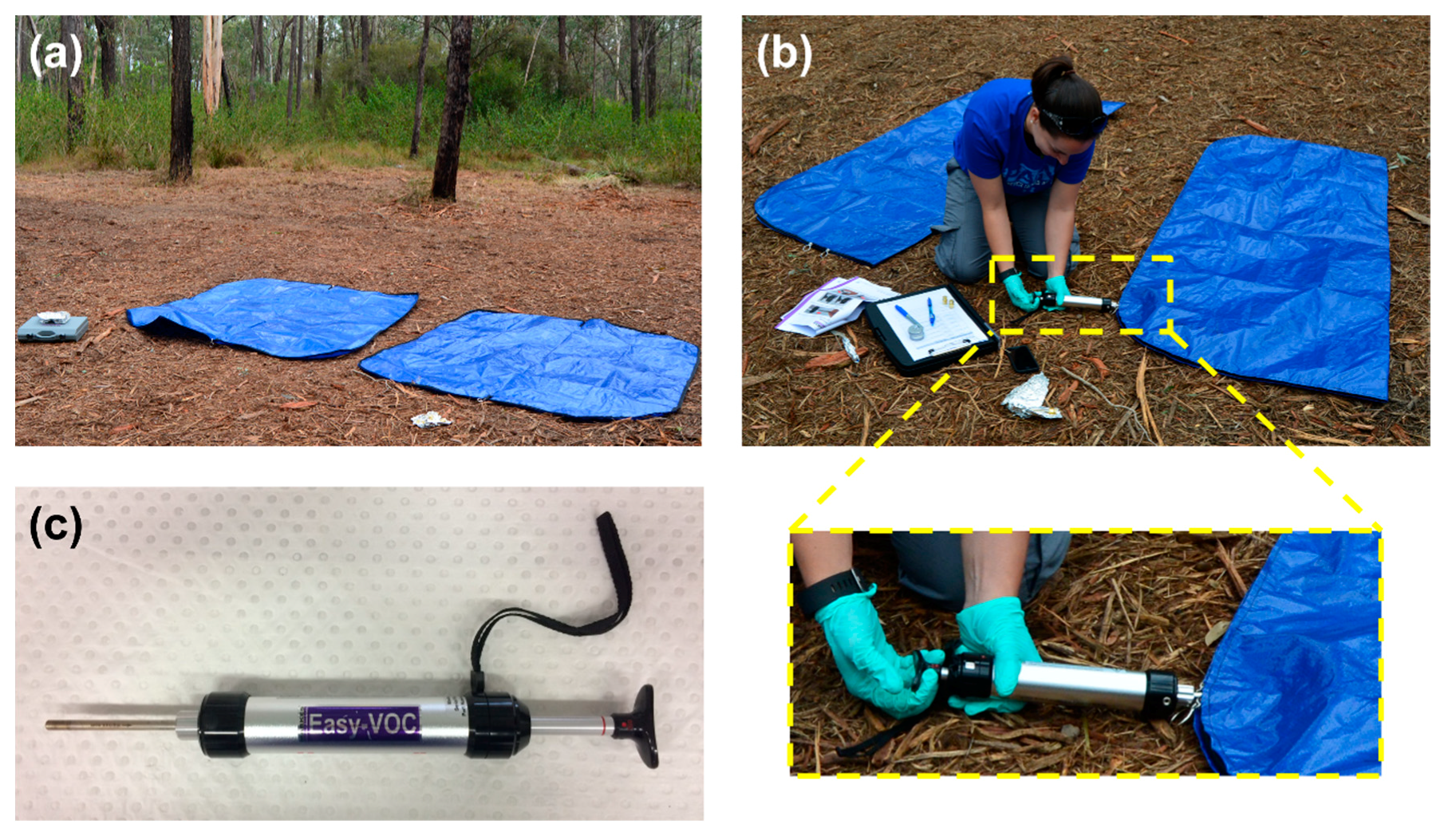
Prior to conducting the experimental burns, control headspace samples were collected in triplicate, from two brand-new polyethylene tarpaulin body bags (175 cm × 70 cm; Pro-Pac Packaging Limited, Wetherill Park, NSW, Australia) for the purpose of measuring the background VOC profile produced by the body bags and surrounding environment. Each body bag was left open to the outdoor environment (Figure 1a) for 15 min, before being zipped closed and sampled through a small opening in the body bag closure (Figure 1b). The headspace within each body bag was sampled dynamically onto a stainless steel sorbent tube containing Tenax TA (35/60 mesh; Markes International Ltd., Llantrisant, RCT, UK) using an Easy-VOC™ grab-sampler (Figure 1c) (Markes international Ltd.) in 5 × 100 mL headspace aliquots, sampled successively (total headspace volume sampled = 500 mL).
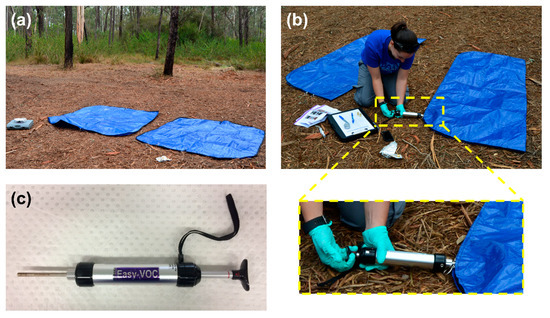
Figure 1.
Collection of control headspace samples from empty body bags: (a) body bags open to environment prior to sample collection; (b) headspace sample collection through small opening in body bag closure; and (c) Easy-VOC™ grab-sampler with sorbent tube attached.
After burning, each pig carcass was left to cool for 30 min before being placed into one of the polyethylene tarpaulin body bags. The headspace within the body bag was permitted to accumulate for 15 min. A total of five replicate experimental headspace samples were collected from each pig carcass using the procedure previously described for the control samples. For the purposes of consistency, all experimental headspace samples were collected from the corner of the body bag nearest to the head. The headspace within each body bag was allowed to re-accumulate for 5 min between samples.
Following sample collection, each sorbent tube was sealed with brass long-term storage caps, wrapped in aluminum foil, and placed in a glass air-tight container for transportation to the laboratory in accordance with the U.S. Environmental Protection Agency Method TO-17 [28]. Sorbent tubes were stored at 4 °C in this condition in the laboratory, until GC×GC-TOFMS analysis was performed. Adhering to the guidelines of the Australian code for the care and use of animals for scientific purposes [27], the two pig carcasses burned in this study were utilized in a subsequent study investigating the influence of fire modification on the odor of decomposition [29], in an attempt to reduce the number of pig carcasses utilized in the authors’ research studies.
2.3. TD-GC×GC-TOFMS Analysis
Sorbent tube thermal desorption (TD) was achieved via a UNITY 2 Thermal Desorber and Series 2 ULTRA multitube autosampler (Markes International Ltd.), followed by sample analysis on a Pegasus® 4D GC×GC-TOFMS system (LECO, Castle Hill, NSW, Australia). The TD unit and autosampler were coupled to the GC×GC-TOFMS system via a 1 m uncoated fused silica transfer line (Markes International Ltd.) maintained at 140 °C, and connected to the first dimension (1D) GC column inside the 1D GC oven using an Ultimate Union Kit (Agilent Technologies, Mulgrave, NSW, Australia). Markes Maverick control software for Unity 2 (version 4.1.29; Markes International Ltd.) was used to control the TD unit and autosampler, and ChromaTOF® software (version 4.51.6.0; LECO) was used for GC×GC-TOFMS control.
Prior to first use, all freshly packed sorbent tubes were initially conditioned under a 50 mL/min flow of high purity helium (BOC, Sydney, NSW, Australia) for 120 min at 320 °C, followed by 30 min at 335 °C, according to the manufacturer’s recommendations. Thermal desorption of the sorbent tubes was operated under double split flow conditions (i.e., split flow during both tube and trap desorption steps). Three different tube desorption/trap desorption split flow conditions were tested using high purity helium (BOC): (1) 20 mL/min/20 mL/min (split ratio = 15.4:1); (2) 20 mL/min/50 mL/min (split ratio = 36.4:1); (3) 50 mL/min/100 mL/min (split ratio = 102.0:1). For all split flow conditions tested, tube desorption was performed at a temperature of 320 °C for 15 min with collection onto a Tenax TA/Carbograph 1TD general purpose cold trap maintained at −10 °C, followed by trap desorption at 320 °C for 3 min. Split flow conditions were evaluated using the samples collected from the pig carcass burnt in the presence of gasoline. Optimal desorption conditions were determined to be a double split flow of 50 mL/min during tube desorption, and 100 mL/min during trap desorption, in order to reduce overloading observed from the gasoline trace (see Section 3.2). Thermal desorption of the reference gasoline sample (i.e., 0.2 μL 91-octane gasoline spiked directly onto sorbent tube) was performed under a double split flow of 100 mL/min during tube desorption, and 100 mL/min during trap desorption (split ratio = 153.0:1), to further reduce overloading of the gasoline trace.
GC×GC-TOFMS analysis was performed according to a previously published method designed to achieve a near-theoretical maximum in peak capacity gain for the forensic analysis of ignitable liquids [26]. Briefly, the column configuration consisted of a 60 m × 0.25 mm inner diameter (i.d.), 0.50 μm film thickness (df) Rxi®-1ms column in the 1D and a 1.1 m × 0.25 mm i.d., 0.5 μm df Stabilwax® column in the second dimension (2D) (Restek Corporation, Bellefonte, PA, USA). The 1D and 2D columns were connected directly before the modulator using a SilTite™ µ-Union (SGE Analytical Science, Wetherill Park, NSW, Australia). A constant flow of 2.0 mL/min of high purity helium (BOC) was used as the carrier gas. The 1D oven was held at 40 °C for 3 min, and then ramped at 3.5 °C/min to a final temperature of 255 °C, which was held for a further 5 min (total GC×GC-TOFMS runtime = 69.43 min). Relative to the 1D oven, the 2D oven and modulator were programmed to have +5 °C and +20 °C offsets, respectively. The modulation period was 2 s with a 0.5 s hot pulse time and 0.5 s cooling time between stages. Mass spectra were acquired at a rate of 400 spectra/s for m/z 35–400. The MS transfer line and ion source were maintained at 240 °C and 225 °C, respectively. Electron ionization was achieved at 70 eV with the detector operating at a 200 V offset above the optimized detector voltage.
2.4. Data Processing
Data processing and chromatographic visualization were achieved using ChromaTOF® software (version 4.51.6.0; LECO). To assist with chromatographic visualization of trace components, a color scale of 0–10% of the normalized signal intensity was utilized. The baseline of each chromatogram was smoothed automatically, with an 80% offset. Peak searching was performed using an expected peak width of 8 s in the 1D and 0.1 s in the 2D, with a signal-to-noise ratio (S/N) cut-off of 150 for base peaks and 20 for subpeaks. Subpeaks were combined with each other, and their corresponding base peak when a mass spectral match of 65% or greater was detected. A forward search of the 2011 National Institute of Standards and Technology (NIST) mass spectral library database was used to tentatively identify peaks with a minimum similarity match of 80% or greater.
Chromatographic alignment was carried out using the Statistical Compare software feature within ChromaTOF® (LECO). Samples were input into Statistical Compare and separated into four classes: (1) reference gasoline (n = 3); (2) body bag control (n = 6); (3) pig burnt without gasoline (n = 3); and (4) pig burnt with gasoline (n = 3). During chromatographic alignment, maximum retention time deviations permitted between samples were restricted to 2 s (i.e., one modulation period) in the 1D and 0 s in the 2D. Peak re-searching was performed during chromatographic alignment using a lower S/N cut-off of 20 to search for peaks not found during the initial peak finding step. Peak alignment required a minimum similarity match of 60% or greater. Analytes that did not meet this mass spectral match threshold, and that were not detected in at least three samples across the four classes, were removed from the final compound list. Statistical Compare was used to calculate the ratio of between-class variance to within-class variance (i.e., the Fisher ratio) for each analyte using analyte peak areas (calculated using unique mass). Analytes with Fisher ratios above a critical value (Fcrit—computed in Microsoft Excel using the F-distribution) were considered to be class-distinguishing analytes (i.e., analytes that statistically differed in abundance between the defined classes) [30,31,32]. Analytes with a Fisher ratio above the Fcrit threshold (i.e., Fcrit = 3.59) were exported as a *.csv file and imported into Microsoft Excel for the manual removal of chromatographic artefacts (e.g., column or sorbent bleed) and further processing.
Principal component analysis (PCA) was applied to the analytes of interest (i.e., only those analytes that were retained after Fisher ratio filtering) using The Unscrambler® X (version 10.3.31813.89; CAMO Software, Oslo, Norway). Prior to PCA, data pre-processing steps performed in The Unscrambler® X included mean centering, variance scaling, and unit vector normalization [33]. Following PCA, the dataset was evaluated and confirmed to contain no outlying points using the Hotelling’s T2 95% confidence limit.
3. Results and Discussion
3.1. Experimental Burn Conditions and Observations
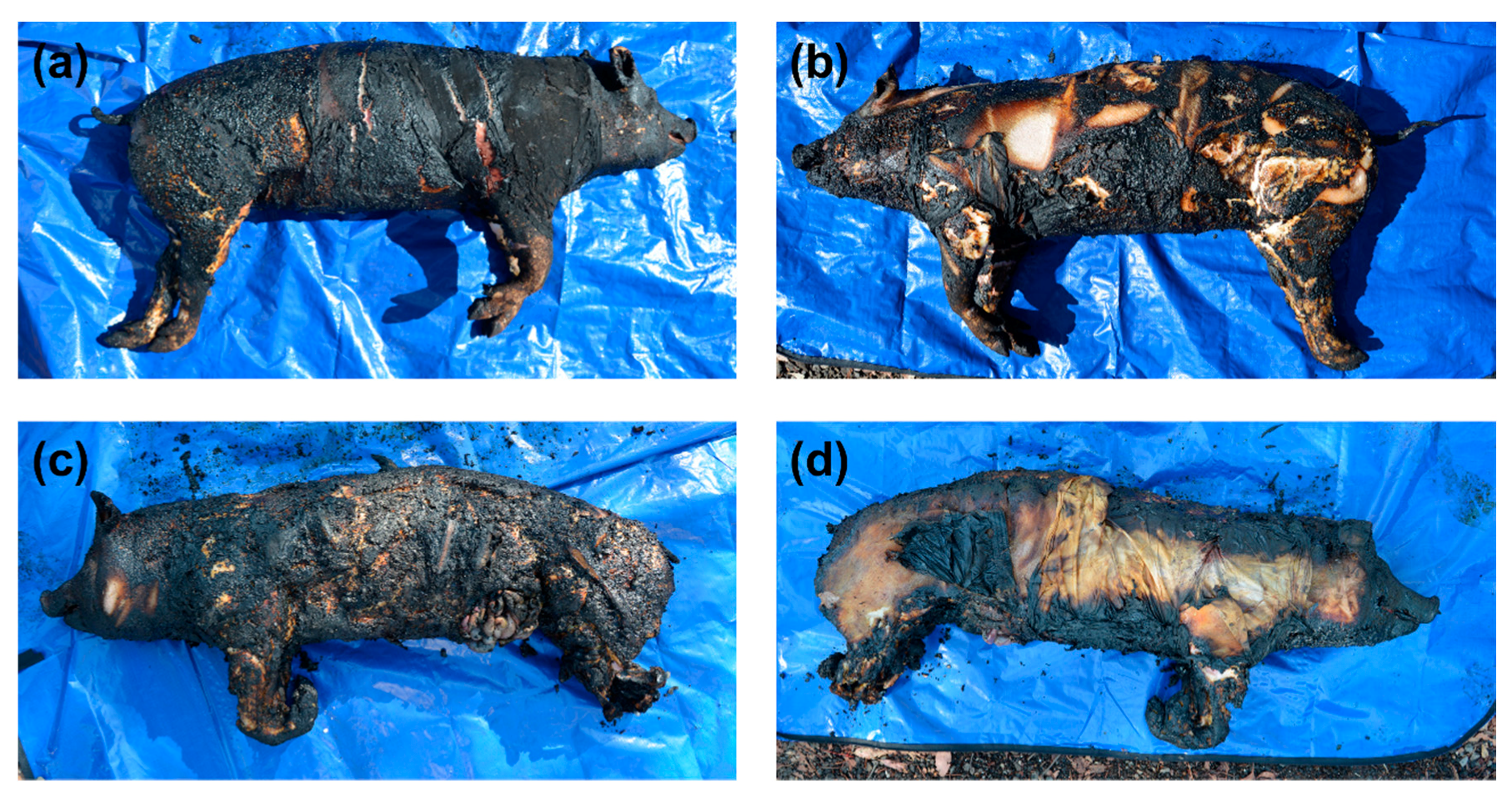
The thermocouples placed underneath the pig carcass burnt without gasoline recorded fire temperatures ranging from 91.8 to 832.2 °C, while the thermocouple used to measure the temperature of the fire directly above the lower torso measured a maximum temperature of 648 °C (note that the thermocouple placed above the upper torso failed to record a reading during the fire—the reason for the malfunction is unknown). The overall level of burning observed varied (Figure 2a,b), with the topside presenting the most consistent degree of burning. On the Crow–Glassman scale (CGS) [34], the level of fire damage to the head, neck, and torso regions of the remains was categorized as level 2, while the degree of fire damage to the limbs, which remained intact, was categorized as level 1. The polyester briefs were entirely consumed during the fire, while a small portion of the 100% cotton t-shirt was found charred underneath the upper torso of the pig carcass.
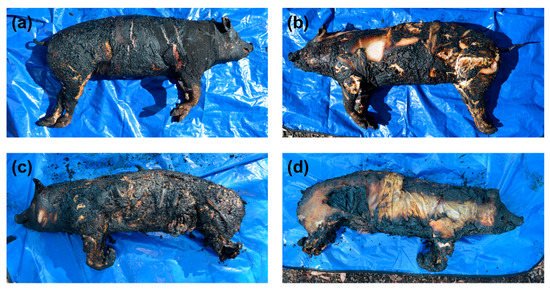
Figure 2.
Photographs of the (a) topside and (b) underside of the pig carcass burnt without gasoline and the (c) topside and (d) underside of the pig carcass burnt with gasoline.
Lower overall temperatures were recorded for the fire conducted with gasoline, with fire temperatures ranging from 250.0 to 451.8 °C underneath the pig carcass, and 536.7–669.2 °C above the pig carcass. Fire damage was largely observed to be limited to the topside of the pig carcass (Figure 2c), with the exception of the head and limbs, which showed extensive fire damage on both sides (Figure 2c,d). After burning, a tear in the abdominal region resulting in intestinal herniation (i.e., exposure of the visceral organs—Figure 2c) was observed. Both the anterior and posterior limbs were very delicate following burning (Figure 2c,d), with the front limbs displaying cadaveric spasm, and elements of the hind legs disarticulated and unrecoverable after the fire. Overall, the level of fire damage was categorized as CGS level 2 for the head and limbs, as well as the neck, torso, and posterior regions on the topside of the remains. Fire damage to the neck, torso, and posterior regions on the underside of the remains were categorized as CGS level 1. A small portion of the polyester briefs was recovered intact and unburnt underneath the remains, along with the entire right side of the 100% cotton t-shirt, which showed extensive charring along the edges.
Depending upon the resting position of the remains during burning, and the combustibility of the substrate upon which the remains are burned (e.g., a non-combustible floor), fire modification is often limited to a single side of the remains (i.e., the side which is not in contact with the substrate) and clothing shielded from the fire (i.e., located between the remains and the substrate) may also be recoverable. In this study, the pig carcass burnt without gasoline was burnt on top of a pile of brush considerably larger than that used in the second burn for the pig carcass burnt with gasoline, where the pile of brush was concentrated more on top of the pig carcass, as opposed to underneath. As a result, the pig carcass burnt without gasoline exhibited burns on both sides of the remains with very little clothing recoverable, while the pig carcass burnt with gasoline exhibited burns predominantly limited to one side of the remains with larger pieces of unburnt clothing intact and recoverable as previously described. This information is important to consider when conducting experimental burns, with the desired burn pattern dependent upon the scenario being simulated, also bearing in mind the large variability that occurs in every fire due to the uncontrollable variables of combustion. Given this variability, it is imperative that experimental burns, such as those conducted herein, are replicated, to provide a statistically significant dataset.
This information is also important to consider when collecting fire debris for the analysis of ILRs in cases of suspected arson. Following burning, any recoverable clothing and/or unburnt substrate below the remains, where ignitable liquids may pool and be absorbed, should be considered for collection and subsequent analysis of ILRs. However, in cases where clothing and substrate are unrecoverable, or are known to produce volatiles that interfere with ILR detection and identification, (e.g., carpet and carpet padding [35]), the intact or partially intact remains may present the only source of fire debris available for analysis and, as such, is the focus of this study.
3.2. Sample Collection Method
The collection of headspace samples was achieved using an Easy-VOC™ hand-held manually operated grab-sampler from Markes International Ltd. (Figure 1c). The Easy-VOC™ device permits precise volumes of air, sampled in 50 or 100 mL aliquots, to be sampled (and thus pre-concentrated) directly onto a sorbent tube. If required, larger volumes of air can be sampled by drawing a series of aliquots onto the same sorbent tube in rapid succession, increasing sensitivity for trace-level target compounds. Since the Easy-VOC™ device does not require electrical power or batteries, it is an ideal choice for collecting samples in the field.
In this study, five 100 mL aliquots of headspace were sampled successively onto each sorbent tube (total headspace volume sampled = 500 mL), and a total of five replicate headspace samples were collected from each set of remains. The first three replicates collected from the pig carcass burnt with gasoline were used to evaluate the split ratio (i.e., tube and trap desorption split flow conditions for thermal desorption) for optimal detection of the gasoline trace. The final two replicates collected were analyzed with the optimal split ratio, to provide a confirmation of results and to provide replicates for multivariate data analysis (see Section 3.4).
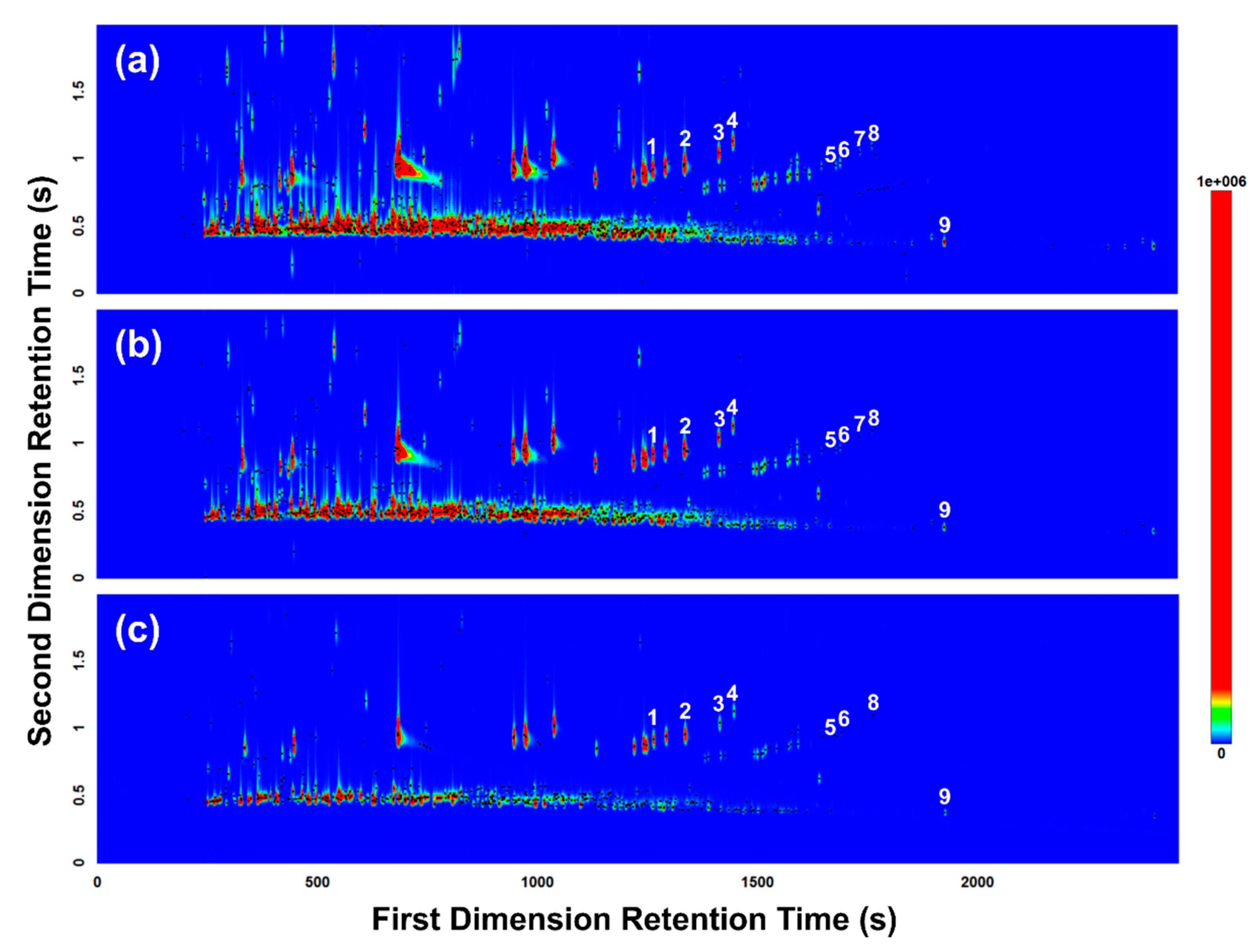
A split ratio of 15.4:1 was tested first (i.e., tube desorption/trap desorption split flow = 20 mL/min/20 mL/min), resulting in overloaded and co-eluting peaks (Figure 3a). To reduce overloading, the split ratio was increased to 36.4:1 (i.e., tube desorption/trap desorption split flow = 20 mL/min/50 mL/min), but overloading was still observed for some of the more concentrated volatiles (Figure 3b). The split ratio was further increased to 102.0:1 (i.e., tube desorption/trap desorption split flow = 50 mL/min/100 mL/min), producing sharp, focused peaks with minimal overloading observed (Figure 3c). As the split ratio was increased to reduce overloading of the more concentrated volatiles, the number of compounds detected overall inadvertently decreased, due to the loss of trace volatiles. This can be observed in Figure 3, with the loss of peak markers particularly in the latter half of the contour plots (e.g., loss of the C5-alkyl benzenes [26] in Figure 3b,c). The increased split ratio and subsequent loss of volatiles detected, however, did not significantly affect the overall detection of the targeted gasoline compounds (see ASTM E1618-14—Table 3 [24]) used to positively identify the presence of gasoline in this study (see Section 3.3), with the exception of the loss of 5-methylindane. The loss of 5-methylindane was accepted, and the split ratio of 102.0:1 was chosen as optimal, in favor of the sharp, focused peaks produced, which provided stronger overall mass spectral matches for tentative identification of peaks and was more amenable to subsequent multivariate data analysis (see Section 3.4).
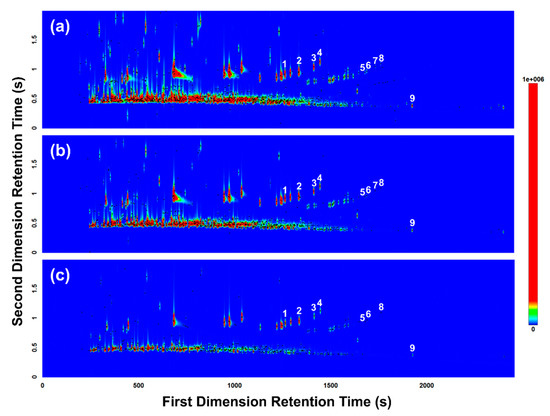
Figure 3.
GC×GC-TOFMS total ion current (TIC) contour plots of headspace samples collected from the pig carcass burnt with gasoline and analyzed with three different tube desorption/trap desorption split flow conditions: (a) 20 mL/min/20 mL/min (split ratio = 15.4:1); (b) 20 mL/min/50 mL/min (split ratio = 36.4:1); (c) 50 mL/min/100 mL/min (split ratio = 102.0:1). Peak markers (represented by black dots) highlight each VOC detected. Numerals denote ASTM E1618-14 [24] gasoline target compounds detected: (1) 1,3,5-trimethylbenzene; (2) 1,2,4-trimethylbenzene; (3) 1,2,3-trimethylbenzene; (4) indane; (5) 1,2,4,5-tetramethylbenzene; (6) 1,2,3,5-tetramethylbenzene; (7) 5-methylindane; (8) 4-methylindane; and (9) dodecane.
Given the overloading observed at the lower split ratios tested, it stands to reason that a smaller volume, and thus fewer aliquots, of headspace could have been sampled. Headspace sampling in this study lasted approximately 5 min per sample/sorbent tube (i.e., ~1 min on average per aliquot of headspace sampled). Accounting for initial headspace accumulation (15 min) and re-accumulation between samples (5 min), the entire sample collection procedure (for five replicates) took ~60 min. Sampling a lower volume of headspace via the collection of fewer aliquots per sample may be desirable for a more rapid sample collection procedure; a lower overall sample volume may also reduce or eliminate the need for headspace re-accumulation between samples further reducing the timeline for sample collection. It is noted that the final three replicate samples collected in this trial were analyzed in order of collection to see if the detected signal decreased over time—i.e., diminishing odor due to removal of headspace from body bag with each sample collected. However, no diminishing signal was observed.
Although a smaller volume of headspace could likely have been sampled in this trial, given the high concentration of gasoline detected following burning, this may not always be the case. Ignitable liquids are consumed or otherwise weathered at an unpredictable rate during the course of a fire and firefighting efforts and, as a result, often leave behind only trace quantities or residues of the original ignitable liquid (hence, ILR) [36]. Given the variability that occurs in every fire, the authors recommend sampling larger volumes of headspace (e.g., 500 mL) and collecting multiple samples if possible (e.g., 4–5). Replicate samples can be used to determine the optimal split ratio, starting with a low to mid-level split ratio, such as 15.4:1, and then increasing or decreasing the split ratio as needed. Additional replicates can be run at the optimal split ratio for sample confirmation or for use with multivariate data analysis, as performed herein. Future studies should, however, consider testing smaller volumes of headspace in addition to varying or entirely removing the headspace re-accumulation step between samples.
3.3. Sample Analysis
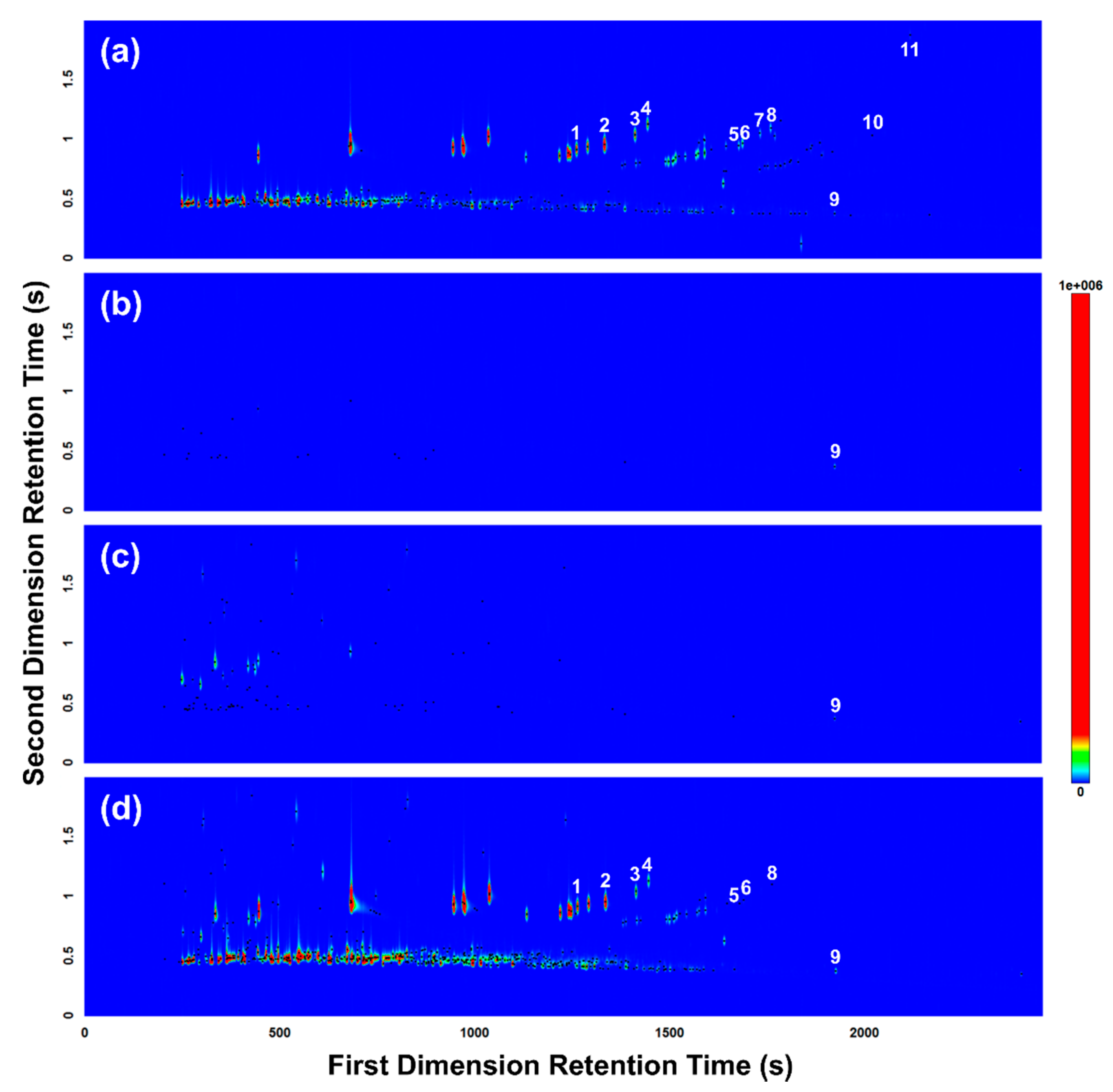
The detection and identification of gasoline (or gasoline-interfering contaminants) was facilitated through target compound analysis and comparison with a reference gasoline sample. Target compound analysis was performed using the list of 15 key specific compounds outlined in ASTM E1618-14 [24] for the characterization of gasoline. The gasoline target compounds included (1) 1,3,5-trimethylbenzene; (2) 1,2,4-trimethylbenzene; (3) 1,2,3-trimethylbenzene; (4) indane; (5) 1,2,4,5-tetramethylbenzene; (6) 1,2,3,5-tetramethylbenzene; (7) 5-methylindane; (8) 4-methylindane; (9) dodecane; (10) 4,7-dimethylindane; (11) 2-methylnaphthalene; (12) 1-methylnaphthalene; (13) ethylnaphthalenes (mixed); (14) 1,3-dimethylnaphthalene; and (15) 2,3-dimethylnaphthalene.
In total, 11 of the 15 gasoline target compounds were detected in the reference gasoline sample (i.e., unleaded 91-octane gasoline) as shown and labelled in Figure 4a. Each of the two empty body bags analyzed (in triplicate) as controls tested negative for the presence of gasoline and were established to be free of gasoline-interfering contaminants, with the exception of dodecane (Figure 4b). Overall, very few volatiles were detected in the body bag control samples, and those that were detected were present at trace levels. Each of the three replicate samples collected from the body bag containing the pig burnt without gasoline tested negative for the presence of gasoline (Figure 4c). Several background pyrolysis products were detected, but these compounds did not match the gasoline target compounds with the exception of dodecane, which was detected in all samples analyzed in this trial. Each of the three replicate samples collected from the body bag containing the pig burnt with gasoline tentatively tested positive for the presence of gasoline, with 8 gasoline target compounds detected (Figure 4d), matching 8 of the 11 gasoline target compounds detected in the reference gasoline sample (Figure 4a). All gasoline target compounds detected in the experimental samples were established to be well resolved from the background pyrolysis products produced from the burnt pig carcasses (Figure 4d).
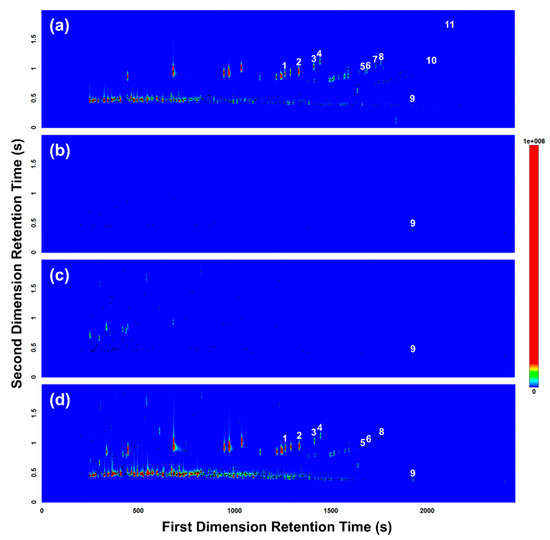
Figure 4.
GC×GC-TOFMS TIC contour plots of headspace samples collected and analyzed from (a) reference gasoline; (b) body bag control; (c) pig burnt without gasoline; and (d) pig burnt with gasoline. Peak markers (represented by black dots) highlight each VOC detected. Numerals denote ASTM E1618-14 [24] gasoline target compounds detected: (1) 1,3,5-trimethylbenzene; (2) 1,2,4-trimethylbenzene; (3) 1,2,3-trimethylbenzene; (4) indane; (5) 1,2,4,5-tetramethylbenzene; (6) 1,2,3,5-tetramethylbenzene; (7) 5-methylindane; (8) 4-methylindane; (9) dodecane; (10) 4,7-dimethylindane; and (11) 1-methylnaphthalene.
Following sample collection, the two burnt pig carcasses were utilized in a subsequent study investigating the influence of fire modification on the odor of decomposition [29], as previously described. This study [29] showed that when the remains were left to decompose naturally in an Australian autumn environment, the combustion and pyrolysis products diminished over time, while the gasoline signature was lost entirely by Day 9 (note that no sampling occurred between Day 1 and Day 9 in the referenced study). A more recent study conducted by the authors, further investigating the influence of fire modification on the odor of decomposition during the Australian winter (with more frequent sampling conducted following burning), found that the gasoline signature could be lost within as little as 24 h (data not yet published). This work demonstrates the importance of sampling the remains as soon as possible after discovery, to limit the potential loss of the ignitable liquid signature due to continued weathering and possible microbial degradation, or due to masking by more prevalent decomposition VOCs.
3.4. Multivariate Data Analysis
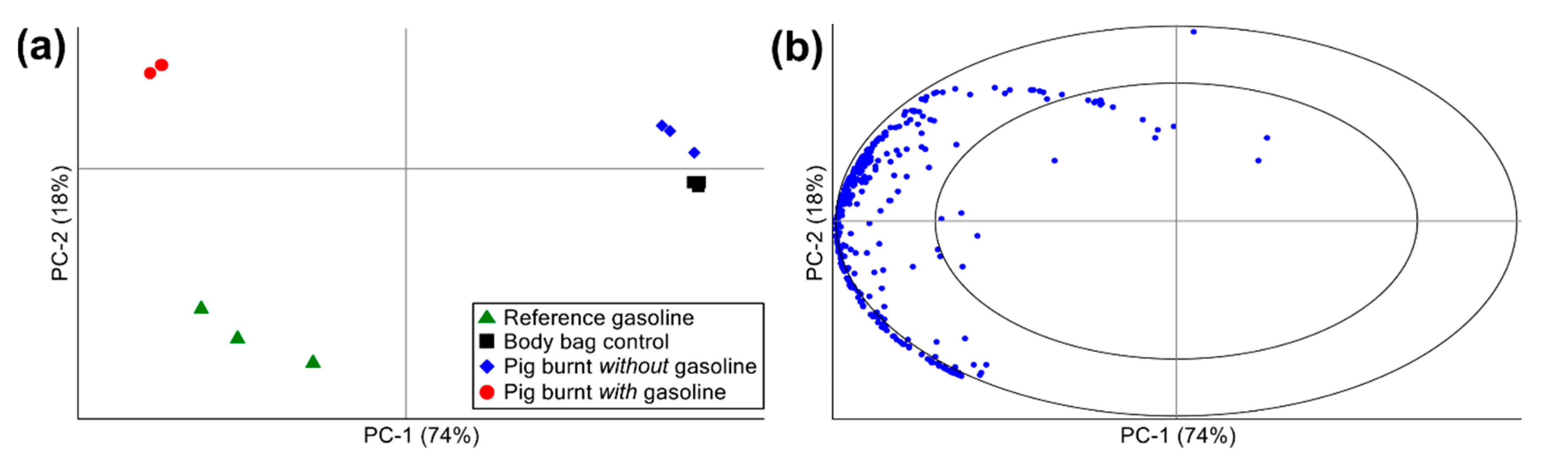
PCA, a multivariate statistical methodology, was performed on the semi-quantitative data collected in this study as a rapid, objective, and automated/computerized pattern matching technique used to verify the chromatographic results discussed above. Mean centering, variance scaling, and unit vector normalization pre-processing steps were applied to the dataset prior to performing PCA. Figure 5 displays the PCA scores and correlation loadings plots generated using the pre-processed GC×GC-TOFMS peak area data for all analytes of interest detected across the four sample classes: (1) reference gasoline; (2) body bag control; (3) pig burnt without gasoline; and (4) pig burnt with gasoline.
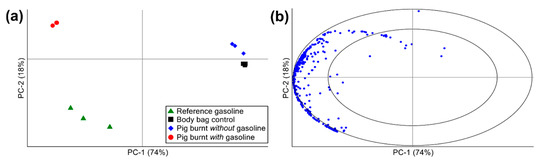
Figure 5.
PCA (a) scores and (b) correlation loadings plots calculated using pre-processed GC×GC-TOFMS peak area data for all analytes of interest detected across the four sample classes: (1) reference gasoline (n = 3); (2) body bag control (n = 6); (3) pig burnt without gasoline (n = 3); and (4) pig burnt with gasoline (n = 3).
Overall, 92% of the explained variation within the dataset was captured by the first two principal components (i.e., PC-1 = 74% and PC-2 = 18%). The reference gasoline and volatile profile of the pig carcass burnt with gasoline grouped together on the left side of the scores plot, and were both readily differentiated from the body bag controls and the pig carcass burnt without gasoline horizontally along PC-1 (Figure 5a), thus accounting for the largest explained variation within the dataset. The majority of the analytes of interest detected were responsible for this differentiation as observed in the correlation loadings plot (Figure 5b), whereby all but three of the analytes of interest detected grouped on the left side of the plot. These analytes included predominantly alkanes, cycloalkanes, alkenes, and aromatics (e.g., alkylbenzenes, alkylnaphthalenes, and indanes), the majority of which fell within the radius between the ellipses of the correlation loadings plot and, thus, were shown to have high discriminatory power (i.e., explaining greater than 50% of the variance within the dataset, with the inner ellipse representing 50% of the explained variance within the dataset and the outer ellipse (i.e., the unit circle) indicating 100% explained variance). The three analytes appearing on the right side of the correlation loadings plot (tentatively identified as furan, carbon disulfide, and allyl nonyl ester oxalic acid) were detected at low levels across most of the samples (with the exception of the reference gasoline), and are attributed to background VOCs produced by the body bags and/or surrounding sampling environment.
Differentiation along PC-2, accounting for only 18% of the variation in dataset, included differentiation between the body bag controls and the pig burnt without gasoline, as well as differentiation between the reference gasoline and the pig burnt with gasoline (Figure 5a). Overall, the differentiation observed along PC-2 between the reference gasoline and pig burnt with gasoline is small compared to the differentiation observed between these samples and the body bag controls and pig burnt without gasoline along PC-1. This minor differentiation observed between the profiles of the reference gasoline and pig burnt with gasoline is accounted for by the presence of combustion and pyrolysis products from the pig carcass (e.g., aldehydes, ketones, alcohols, and sulfur-containing compounds) and the consummation/weathering of the gasoline signature (e.g., missing gasoline target compounds). Nevertheless, the grouping and overall similarity observed between the reference gasoline and pig burnt with gasoline in Figure 5a supports the chromatographic results above for the positive detection of gasoline in the headspace samples collected from the pig carcass burnt with gasoline. The differentiation between the body bag controls and both the reference gasoline and pig burnt with gasoline also supports the chromatographic evidence that the body bag is free of gasoline-interfering contaminants.
4. Conclusions
This study demonstrated that dynamic headspace sampling of a body bag, followed by TD-GC×GC-TOFMS analysis, is an integral addition to the fire debris analysts’ sample collection and analysis arsenal, allowing for the detection of ILRs in cases of suspected arson involving human remains. This method permits the collection of headspace samples before the remains are removed from the crime scene, limiting the potential for contamination and the loss of volatiles due to potential microbial degradation or further weathering. Overall, the body bag was established to be free of gasoline-interfering contaminants, demonstrating its potential as an alternative sample collection medium for fire debris analysis in cases involving burnt victim remains. However, it is important to note that body bags are not considered vapor-tight and, therefore, should not be used for long-term storage of fire debris evidence. It is recommended that headspace samples be collected immediately after headspace accumulation is complete at the scene of collection, in order to prevent extensive vapor loss and the potential for cross-contamination. Future studies should consider not only sampling different volumes of headspace, but also varying headspace re-accumulation periods, volumes, and types of ignitable liquids, burn times (e.g., allowing fire to extinguish itself without intervention), and time passed before sample collection (e.g., allow time for weathering and/or microbial degradation to occur to reflect a scenario where remains are not discovered immediately after burning).
Author Contributions
Conceptualization, K.D.N. and S.L.F.; Methodology, K.D.N.; Sample analysis, K.D.N.; Data analysis, K.D.N.; Resources, S.L.F.; Writing—Original Draft Preparation, K.D.N. and S.L.F.; Writing—Review & Editing, K.D.N. and S.L.F.; Supervision, S.L.F.
Funding
This research received no external funding.
Acknowledgments
The authors wish to thank Morgan Cook and the Fire & Rescue NSW Fire Investigation & Research Unit for their assistance with carrying out the fires conducted for this trial. The authors also wish to thank all UTS research group members, extended contacts, and laboratory technical staff that contributed to the execution of field work and sample collection/analysis throughout this trial: LaTara Rust, Vitor Taranto, Baree Chilcote, Nicole Cattarossi, Darshil Patel, Katelynn Perrault, Mohammed Shareef, Robert Chatterton, Greg Dalsanto, Ronald Shimmon, and R. Verena Taudte. This work was supported financially by the University of Technology Sydney.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sinkov, N.A.; Sandercock, P.M.L.; Harynuk, J.J. Chemometric classification of casework arson samples based on gasoline content. Forensic Sci. Int. 2014, 235, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pahor, K.; Olson, G.; Forbes, S.L. Post-mortem detection of gasoline residues in lung tissue and heart blood of fire victims. Int. J. Leg. Med. 2013, 127, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Frysinger, G.S.; Gaines, R.B. Forensic analysis of ignitable liquids in fire debris by comprehensive two-dimensional gas chromatography. J. Forensic Sci. 2002, 47, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Fettig, I.; Krüger, S.; Deubel, J.H.; Werrel, M.; Raspe, T.; Piechotta, C. Evaluation of a headspace solid-phase microextraction method for the analysis of ignitable liquids in fire debris. J. Forensic Sci. 2014, 59, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Pert, A.D.; Baron, M.G.; Birkett, J.W. Review of analytical techniques for arson residues. J. Forensic Sci. 2006, 51, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Sigman, M. Performance testing of commercial containers for collection and storage of fire debris evidence. J. Forensic Sci. 2007, 52, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.M.L. Fire investigation and ignitable liquid residue analysis—A review: 2001–2007. Forensic Sci. Int. 2008, 176, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.A.; Edmiston, P.L. Preferential extraction of hydrocarbons from fire debris samples by solid phase microextraction. J. Forensic Sci. 2003, 48, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Sinkov, N.A.; Johnston, B.M.; Sandercock, P.M.L.; Harynuk, J.J. Automated optimization and construction of chemometric models based on highly variable raw chromatographic data. Anal. Chim. Acta 2011, 697, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Borusiewicz, R. Comparison of new Ampac bags and FireDebrisPAK® bags as packaging for fire debris analysis. J. Forensic Sci. 2012, 57, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Borusiewicz, R.; Kowalski, R. Volatile organic compounds in polyethylene bags—A forensic perspective. Forensic Sci. Int. 2016, 266, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Grutters, M.M.P.; Dogger, J.; Hendrikse, J.N. Performance testing of the new AMPAC fire debris bag against three other commercial fire debris bags. J. Forensic Sci. 2012, 57, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Belchior, F.; Andrews, S.P. Evaluation of cross-contamination of nylon bags with heavy-loaded gasoline fire debris and with automotive paint thinner. J. Forensic Sci. 2016, 61, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Schuberth, J. Post-mortem test for low-boiling arson residues of gasoline by gas chromatography-ion-trap mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1994, 662, 113–117. [Google Scholar] [CrossRef]
- Schuberth, J. A full evaporation headspace technique with capillary GC and ITD: A means for quantitating volatile organic compounds in biological samples. J. Chromatogr. Sci. 1996, 34, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, M.; Kashimura, S.; Hara, K.; Hieda, Y.; Kageura, M. The utility of volatile hydrocarbon analysis in cases of carbon monoxide poisoning. Int. J. Leg. Med. 1996, 109, 75–79. [Google Scholar] [CrossRef]
- ASTM E1388-17 Standard practice for static headspace sampling of vapors from fire debris samples. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM E1412-16 Standard practice for separation of ignitable liquid residues from fire debris samples by passive headspace concentration with activated charcoal. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2016.
- Borusiewicz, R.; Zieba-Palus, J. Comparison of the effectiveness of Tenax TA and Carbotrap 300 in concentration of flammable liquids compounds. J. Forensic Sci. 2007, 52, 70–74. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1413-13 Standard practice for separation of ignitable liquid residues from fire debris samples by dynamic headspace concentration. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2013.
- Nichols, J.E.; Harries, M.E.; Lovestead, T.M.; Bruno, T.J. Analysis of arson fire debris by low temperature dynamic headspace adsorption porous layer open tubular columns. J. Chromatogr. A 2014, 1334, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kaneko, T.; Suzuki, S. A solid-phase microextraction method for the detection of ignitable liquids in fire debris. J. Forensic Sci. 2008, 53, 668–676. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2154-15a Standard practice for separation and concentration of ignitable liquid residues from fire debris samples by passive headspace concentration with solid phase microextraction (SPME). In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM E1618-14 Standard test method for ignitable liquid residues in extracts from fire debris samples by gas chromatography-mass spectrometry. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2014.
- DeHaan, J.D.; Taormina, E.I.; Brien, D.J. Detection and characterization of volatile organic compounds from burned human and animal remains in fire debris. Sci. Justice 2016, 57, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Nizio, K.D.; Cochran, J.W.; Forbes, S.L. Achieving a near-theoretical maximum in peak capacity gain for the forensic analysis of ignitable liquids using GC×GC-TOFMS. Separations 2016, 3, 26. [Google Scholar] [CrossRef]
- Australian Code for the Care and Use of Animals for Scientific Purposes. 2013. Available online: https://www.nhmrc.gov.au/guidelines-publications/ea28 (accessed on 26 May 2018).
- U.S. Environmental Protection Agency (EPA). Compendium Method TO-17: Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes; EPA: Washington, DC, USA, 1999; pp. 1–53. [Google Scholar]
- Nizio, K.D.; Forbes, S.L. Preliminary investigation of the influence of fire modification on the odour of decomposition using GC×GC-TOFMS. Chromatogr. Today 2017, 10, 32–39. [Google Scholar]
- Pierce, K.M.; Hoggard, J.C.; Hope, J.L.; Rainey, P.M.; Hoofnagle, A.N.; Jack, R.M.; Wright, B.W.; Synovec, R.E.; Hospital, C.; Point, S.; et al. Fisher ratio method applied to third-order separation data to identify significant chemical components of metabolite extracts. Anal. Chem. 2006, 78, 5068–5075. [Google Scholar] [CrossRef] [PubMed]
- Brokl, M.; Bishop, L.; Wright, C.G.; Liu, C.; McAdam, K.; Focant, J.-F. Multivariate analysis of mainstream tobacco smoke particulate phase by headspace solid-phase micro extraction coupled with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1370, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Nizio, K.D.; Perrault, K.A.; Troobnikoff, A.N.; Ueland, M.; Shoma, S.; Iredell, J.R.; Middleton, P.G.; Forbes, S.L. In vitro volatile organic compound profiling using GC×GC-TOFMS to differentiate bacteria associated with lung infections: A proof-of-concept study. J. Breath Res. 2016, 10, 026008. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Goodpaster, J.V. Comparing the effects of weathering and microbial degradation on gasoline using principal components analysis. J. Forensic Sci. 2012, 57, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Glassman, D.M.; Crow, R.M. Standardization model for describing the extent of burn injury to human remains. J. Forensic Sci. 1996, 41, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, W. Volatiles from carpet: A source of frequent misinterpretation arson analysis. J. Chromatogr. A 1994, 674, 329–333. [Google Scholar] [CrossRef]
- Sandercock, P.M.L.; Du Pasquier, E. Chemical fingerprinting of gasoline: 2. Comparison of unevaporated and evaporated automotive gasoline samples. Forensic Sci. Int. 2004, 140, 43–59. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).