Abstract
Fabric phase sorptive extraction (FPSE) has gained notable attention and interest both in batch and automatic mode utilizing advanced sol-gel derived microextraction sorbents and the hydrophobic/hydrophilic properties of fabric substrates. Recently, the innovative on-line fabric disk sorptive extraction (FDSE) has opened new opportunities in the field of automatic sample preparation (preconcentration/separation). A novel sol-gel sorbent based on caprolactone-dimethylsiloxane-caprolactone block polymer comprised of a non-polar dimethylsiloxane and hydrophilic caprolactone as a coating on hydrophobic polyester fabric substrate and its evaluation in an automatic FDSE system coupled with flame atomic absorption spectrometry (FAAS), is presented for the first time. The capabilities of the proposed flow injection system were assessed for trace Cu(II), Ni(II), Zn(II), Pb(II), and Cd(II) determination in urine samples. The method was based on the on-line formation of target analytes with ammonium pyrrolidine dithiocarbamate (APDC) and their retention onto the surface of the fabric disk medium. Methyl isobutyl ketone (MIBK) was used to elute metal–APDC complexes directly into the nebulizer-burner system of FAAS. For 90 s of preconcentration time, enhancement factors of 250, 130, 185, and 36 and detection limits (3 s) of 0.15, 0.41, 1.62, and 0.49 μg L−1 were obtained for Cu(II), Ni(II), Pb(II), and Cd(II), respectively. For 30 s of preconcentration time, an enhancement factor of 49 and a detection limit of 0.12 μg L−1 was achieved for Zn(II) determination. The precision, expressed as relative standard deviation (RSD), was lower than 3.5% for all metals. The accuracy of the proposed method was sufficient and evaluated by analyzing certified reference materials and biological samples.
1. Introduction
Since the discovery of solid phase extraction (SPE), there has been significant progress in the field of sample preparation, mostly focused on automation, miniaturization as well as on the simplification of the overall analytical procedure. During on-line SPE, sample contamination and analyte loss are avoided, leading to the improved reproducibility and sensitivity of the method. The time of analysis is also shorter, thanks to automatic sampling and reagent manipulation. Moreover, on-line SPE can be considered as an environmentally friendly technique as it fulfills the green analytical chemistry criteria [1,2]. During the last years, much research and intensive work has been done regarding the development and characterization of new, advanced sorbent materials. The improved selectivity or specificity towards target analytes, the higher sorption capacity, the enhanced physicochemical or mechanical stability, as well as the limited sample and solvent consumption [3,4] are the main objectives for the development of such materials. Sorbents employed in SPE can be broadly classified into inorganic based (silica gel, modified silica, alumina, titania, zirconia, etc.) and organic based (natural and synthetic polymer) sorbents. Inorganic based sorbents, like bonded silica (C8, C18, etc.), are widely used for trace analyte enrichment but they cannot efficiently trap the most polar compounds as they suffer from some inherent limitations such as poor selectivity and narrow range of pH stability [5]. On the other hand, polymer based sorbents are advantageous against inorganic ones and open new doors in the field of sample preparation. Some of their advantages include (a) chemical stability over the whole pH range; (b) the problem pertaining to high active sites found on silica and other oxides can be avoided; (c) the absence of silanol groups reduces non-specific analyte interactions; (d) their high degree of hydrophobicity provides larger analyte retention capacity; (e) their chemical stability and mechanical properties (spherical shape) provide excellent flow characteristics; (f) their high selectivity and ability to regenerate; and (g) their ability to withstand drying out without affecting the analytical performance characteristics, make them one of the most frequently used analytical tools for the isolation and enrichment of a wide range of analytes found in complex matrices [6].
Generally, polymer based sorbents can be made by polymerizing a monomer such as styrene, acrylamide, and methacrylic acid, etc., by cross linking with another olefinic compound called the cross-linker; e.g., divinylbenzene or ethyleneglycoldimethacrylate. Various functionalized polymeric sorbents like Oasis (Waters, Milford, MA, USA), Strata-X (Phenomenex, Torrance, CA, USA), Bond Elut Plexa PCX (Agilent Technologies, Santa Clara, CA, USA), Nobias PA-1 (Hitachi High Technologies, Tokyo, Japan), LiChrolut EN (Merck, Darmstadt, Germany), Hypersep SCX (Thermo Fisher Scientific, Paisley, UK), etc., have been introduced for the retention of various categories of organic compounds in chromatographic separations [7] as well as for metal determination in on-line automatic SPE systems: Hypersep SCX for Ag(I), Cr(III), Fe(III), Cd(II), Pb(II), Cu(II), Co(II) and Ni(II) determination [8,9], Bond Elut Plexa PCX for Cd (II), Pb(II) and Cu(II) [10,11], Oasis-HLB for Pb(II), Cd(II), Cr(VI), Cu(II), Ni(II), Hg(II) [12,13,14], Nobias PA-1 for Cd(II), Cr(VI), Cu(II), Pb(II) and V(V), δ56Fe, δ66Zn and δ114Cd stable isotopes [15,16,17] and the copolymer of Strata-X sorbent for the quantification of Cd(II), Pb(II), Cu(II) and Cr(VI) [18].
A successful approach to the preparation of inorganic polymers and organic-inorganic hybrid materials is being presented by the sol-gel technology which has received increasing attention for applications in various scientific fields (e.g., separation techniques, chemical catalysis, biotechnology and drug delivery). Sol-gel technology has demonstrated numerous advantages, one of which is the compatibility of the technique with polymers and the polymerization processes. This feature allows the synthesis of materials as sorbents, for sample in preparation techniques, in the presence of organic molecules by employing mild reaction conditions. In addition, the possibility to generate thermally-and chemically-stable materials with controlled morphology, surface properties and pore structures, by carefully modifying the synthesis conditions, is another interesting challenge in the field of sample preparation [19,20].
The fabric phase sorptive extraction (FPSE) technique invented by Kabir and Furton [21] exploits the inherent advantages of sol-gel technology and uniquely integrates solid phase microextraction (equilibrium driven extraction) as well as solid phase microextraction (exhaustive extraction) into a single sample preparation technology platform. As such, FPSE has gained considerable attention and diversified applications both in batch and automatic mode, in the field of sample preparation. This new alternative technique enables the determination of a plethora of analytes by using sorbent materials consisting of a sol-gel polymer or copolymer coating covalently bonded to a fabric substrate. The substrate is not inert, so its contribution to the overall polarity of the FPSE medium is inevitable. The FPSE technique simplifies the sample preparation procedure and has been already successfully applied in different fields for the determination of various analytes, in batch mode, showing good analytical performance characteristics and low pre-analytical steps [3,22,23].
Recently, an innovative technique called fabric disk sorptive extraction (FDSE) has been presented by Anthemidis et al. [24]. The FDSE is based on the use of sol-gel coated fabric disks packed in a series and fixed appropriately into an on-line microcolumn. FPSE media with various sol-gel coatings and fabric substrates (polyester, cellulose, glass wool etc.) can be used as fabric disks, in order to manufacture a variety of microcolumns with different geometrical characteristics. Advantages arising from the FDSE technique in on-line SPE systems include: (a) elaboration of new sorbent materials; (b) easy incorporation of the microcolumns in automatic systems; (c) easy permeation of the incoming flow through the pores of the fabric substrate due to limited backpressure; and (d) high reliability of sorbents resulting in a large number of sorption/elution cycles (over 500). Till now, the FDSE technique has been only applied for the determination of Pb(II) and Cd(II) by flame atomic absorption spectrometry (FAAS) in environmental water samples, showing good extraction sensitivity, and excellent reproducibility.
In this work, a novel sol-gel sorbent based on a caprolactone-dimethylsiloxane-caprolactone block polymer has been prepared and used as a sol-gel coating (PCL–DMS–CL) for fabric substrate and evaluated for the first time using the FDSE technique. The developed FDSE system coupled with FAAS was evaluated for the determination of essential, Cu(II), Ni(II), Zn(II) and toxic, Pb(II), Cd(II), metals providing improved sensitivity and flexibility. The accuracy of the developed FI-FDSE-FAAS method was sufficient and evaluated by the analysis of certified reference materials and biological samples.
2. Materials and Methods
2.1. Instrumentation
A 5100 PC Perkin-Elmer flame atomic absorption spectrometer (Perkin-Elmer, Norwalk, CT, USA) equipped with single element hollow cathode lamps (Perkin-Elmer Lumina HCL) was used for metal determination. The monochromator spectral bandpass (slit) was set at 0.7 nm for Cu(II), Zn(II), Pb(II) and Cd(II) and at 0.2 nm for Ni(II) and the wavelength was set at 324.8, 213.9, 232.0, 283.3, and 228.8 nm for Cu(II), Zn(II), Ni(II), Pb(II) and Cd(II), respectively. The flame was slightly leaner, than the manufacturer recommended, to compensate for the effect of the methyl isobutyl ketone (MIBK), which serves as additional fuel. The air and acetylene flow rates were set at 10.0 and 1.0 L min−1, respectively, resulting in a 5.8 mL min−1 nebulizer free uptake. A FIAS-400 flow injection system (Perkin-Elmer) consisted of two peristaltic pumps P1, P2, and a 5-port, 2-position injection valve (IV) was used for the automatic handling of the FDSE procedure, in combination with FAAS. The FAAS and FIAS-400 flow system was controlled by a personal computer running the AA Lab Benchtop version 7.2 software. A “T” type mixing device before the inlet of the nebulizer was adopted as a flow compensation (FC) unit between the elution flow rate (4.8 mL min−1) and the free pneumatic nebulizer aspiration (5.8 mL min−1). A displacement bottle (Tecator, Hoganas, Sweden, http://www.foss.dk) was employed for the delivery of the extractant solvent, MIBK.
The FDSE preconcentration microcolumn was manufactured by using and modifying a polyethylene body of an insulin syringe 1 mL (BD Microfine plus, USA) that was cut to be 2.0 cm long and packed with the sol-gel coated fabric disks in a row. The sol-gel PCL–DMS–CL fabric medium was cut in 50 disks (i.d. 4.6 mm) and firmly packed into the FDSE microcolumn, without the need of glass wool or frits at the two ends, as it was reported elsewhere [24]. The effective length of the sorbent material was 8.4 mm and the amount was 72 mg. This specific configuration of the FDSE microcolumn offers noteworthy advantages including: (1) limited backpressure thanks to easy permeation through the pores of the fabric substrate; (2) no channel formation resulting in high extraction efficiency; and (3) application of higher loading flow rates for higher enhancement factors. The performance characteristics of the FDSE microcolumn were unaffected for over 600 sorption/elution cycles.
2.2. Reagents and Samples
Ultra pure water was used throughout the study, produced by a Milli-Q system (Merck, Darmstadt, Germany). All chemicals were of analytical grade and purchased by Merck (Darmstadt, Germany, http://www.merck.de). Standard solutions of Cu(II), Ni(II), Zn(II), Pb(II) and Cd(II) were prepared by stepwise dilution of 1000 mg L−1 stock standard solutions (Titrisol, Merck) to the required μg L−1 levels, in 0.01 mol L−1 HNO3, just before use. The chelating agent 0.05% m/v ammonium pyrrolidine dithiocarbamate (APDC) (Merck, Darmstadt, Germany) was prepared daily by dissolving the appropriate amount in the ultra pure water. The eluent, MIBK was saturated with ultra pure water, without any other purification.
The substrate for the FDSE medium, 100% polyester fabric, was purchased from Jo-Ann Fabric (Miami, FL, USA).
The accuracy of the developed method was estimated by analyzing two certified reference materials (CRMs): BCR 278-R (Community Bureau of Reference Brussels, Belgium) containing trace elements in mussel tissue and Seronorm™ Trace Elements Urine Level-1, containing trace elements in urine. In addition, a urine mixture (800 mL) provided by young healthy persons was analyzed by the proposed method, after a wet-digestion procedure. Mussel tissue and urine samples were digested using concentrated HNO3. The digestion procedure was carried out at 130–140 °C into a stainless-steel pressurized bomb according to the manufacturer’s recommendations. After cooling the system, the digests were properly diluted in ultra pure water and the resulting solutions were used for the analysis.
2.3. Preparation of Sol-Gel Poly(caprolactone-dimethylsiloxane-caprolactone) Coating on Polyester Substrate
The sol-gel coating procedure on the fabric substrate involves the following steps: (i) surface treatment of the polyester substrate; (ii) design and preparation of the sol solution incorporating all the necessary sol-gel reaction ingredients; (iii) conditioning and ageing of the sol-gel coated fabric medium; (iv) post-coating cleaning of the fabric medium [25].
A comprehensive and step-by-step procedure for substrate surface treatment, design, and preparation of the sol solution, sol-gel coating on the substrate surface, conditioning and ageing of the sol-gel sorbent and the cleaning of the sol-gel sorbent coated FPSE media have been described elsewhere [25]. Briefly, a 15 cm × 10 cm piece of polyester fabric was prepared for the sol-gel coating. The sol solution was prepared by dissolving 10 g of poly(caprolactone-dimethylsiloxane-caprolactone) block copolymer into 20 mL of methylene chloride/acetone mixed solvent system (50:50 v/v). Subsequently, 10 mL methyl trimethoxysilane and 4 mL trifluoroacetic acid (containing 5% water) were added to the mixture. In order to obtain a homogeneous solution, the solution was vortexed for 3 min, followed by centrifugation for 5 min. The clear, supernatant sol solution was then transferred into a 60–mL amber glass reaction vessel. The clean, treated polyester fabric was then gently immersed into the solution. The sol-gel coating process continued for a period of 4 h. After the completion of the coating process, the sol-gel sorbent coated polyester fabric was removed from the reaction vessel and was air dried for 30 min. Subsequently, the sol-gel sorbent coated FPSE medium was conditioned and aged in a home-made conditioning device built inside a gas chromatography oven under a continuous helium gas flow at 50 °C for 24 h. The conditioned FPSE medium was then rinsed with methylene chloride and methanol, respectively. The cleaned FPSE medium was then air dried for 30 min. Finally, the clean and dried FPSE medium was stored in an air-tight glass container until its use in fabric disk sorptive extraction.
2.4. FI-FDSE-FAAS Analytical Procedure
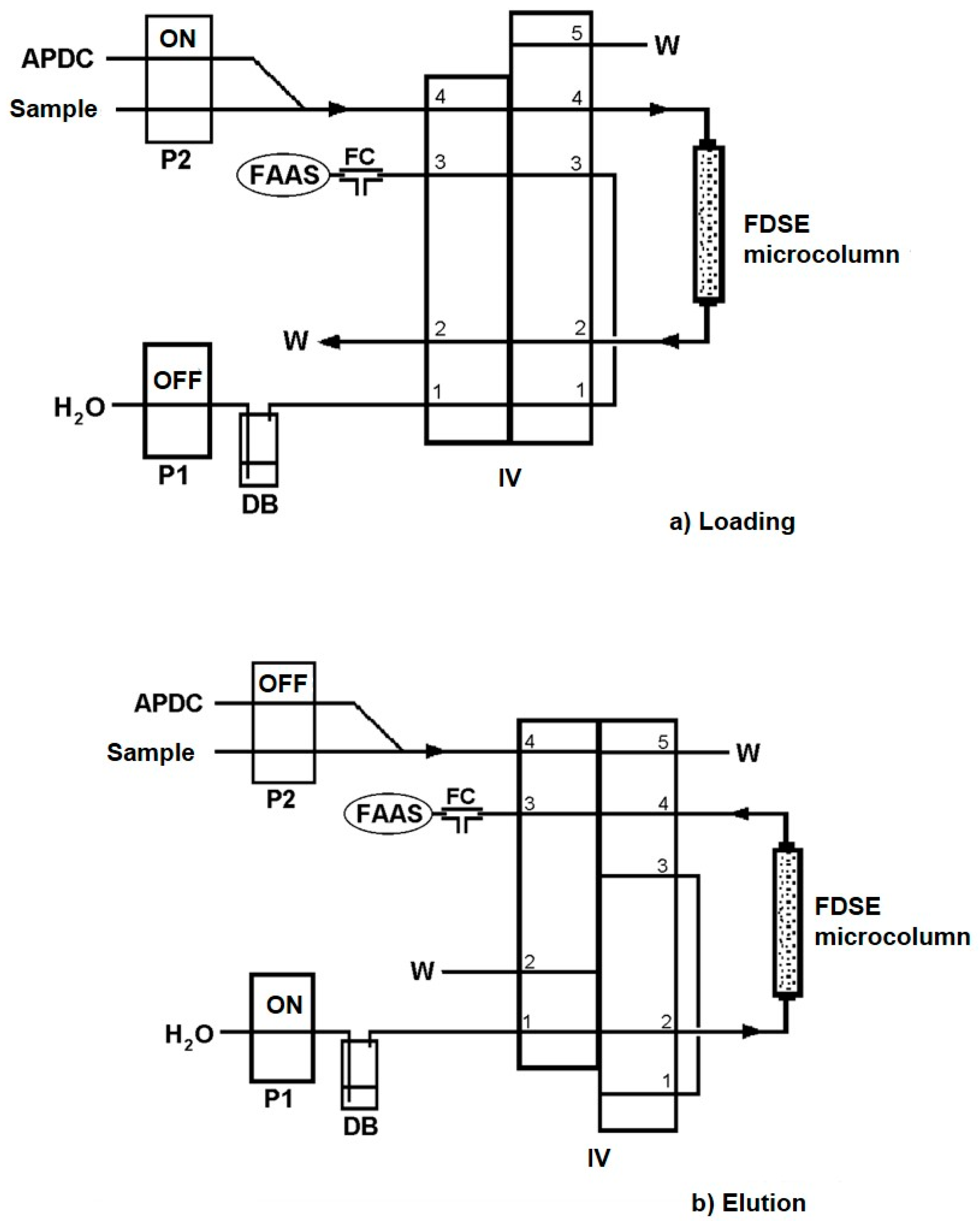
The developed automatic method for metal determination includes two main operation steps, which are given in detail in Table 1. The on-line flow injection manifold is schematically illustrated in Figure 1. In the loading step, (Table 1, Step 1, Figure 1a), the injection valve (IV) was in the “Load” position and pump P2 in operation for the sample and chelating agent, APDC, solution delivery through the FDSE microcolumn at a fixed flow rate for a loading time of 90 s for Cu(II), Ni(II), Pb(II), Cd(II) and 30 s for Zn(II). The on-line formed metal-APDC complexes were adsorbed onto the surface of the FDSE sorbent material. Meanwhile, pump P1 was inactive and the pneumatic FAAS nebulizer aspirated air through the FC unit. During the elution step, (Table 1, Step 2, Figure 1b), the IV was in the “Elute” position and pump P1 was activated for the propulsion of the extractant solvent, MIBK, through the microcolumn for 20 s at a flow rate of 4.8 mL min−1, in order to transfer the eluted complexes directly to the FAAS nebulizer for measurement and quantification. Meanwhile, pump P2 was inactivated to avoid the additional consumption of the sample. The FDSE microcolumn was arranged on the IV in such a way that it enabled an elution process in a reverse direction than that of the loading one, keeping the dispersion of the eluted analytes as low as possible. Five replicates per assay were performed in all instances. The recorded signal of the absorbance was sharp and the peak height was proportional to metal concentration in the sample.

Table 1.
Main operation sequences of the FI-FDSE-FAAS method for metal determination.
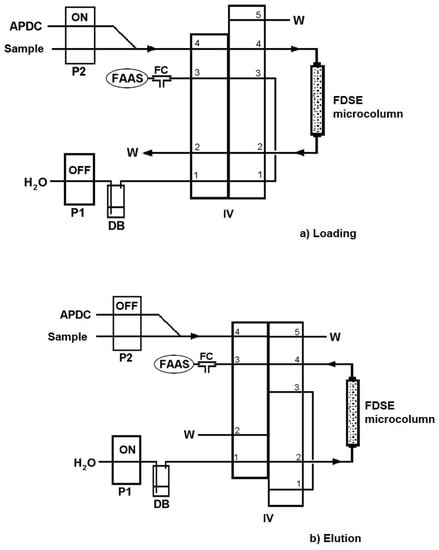
Figure 1.
Schematic diagram of the on-line FI-FDSE system for metal determination by FAAS. (a) Loading/preconcentration and (b) Elution/measurement steps. APDC, 0.05% (m/v) APDC aqueous solution; FC, flow compensation unit; P1, P2, peristaltic pumps; IV, injection valve; DB, displacement bottle for MIBK delivery; W, waste.
3. Results and Discussion
3.1. Surface Morphology and Properties of Sol-Gel Sorbent FDSE Medium
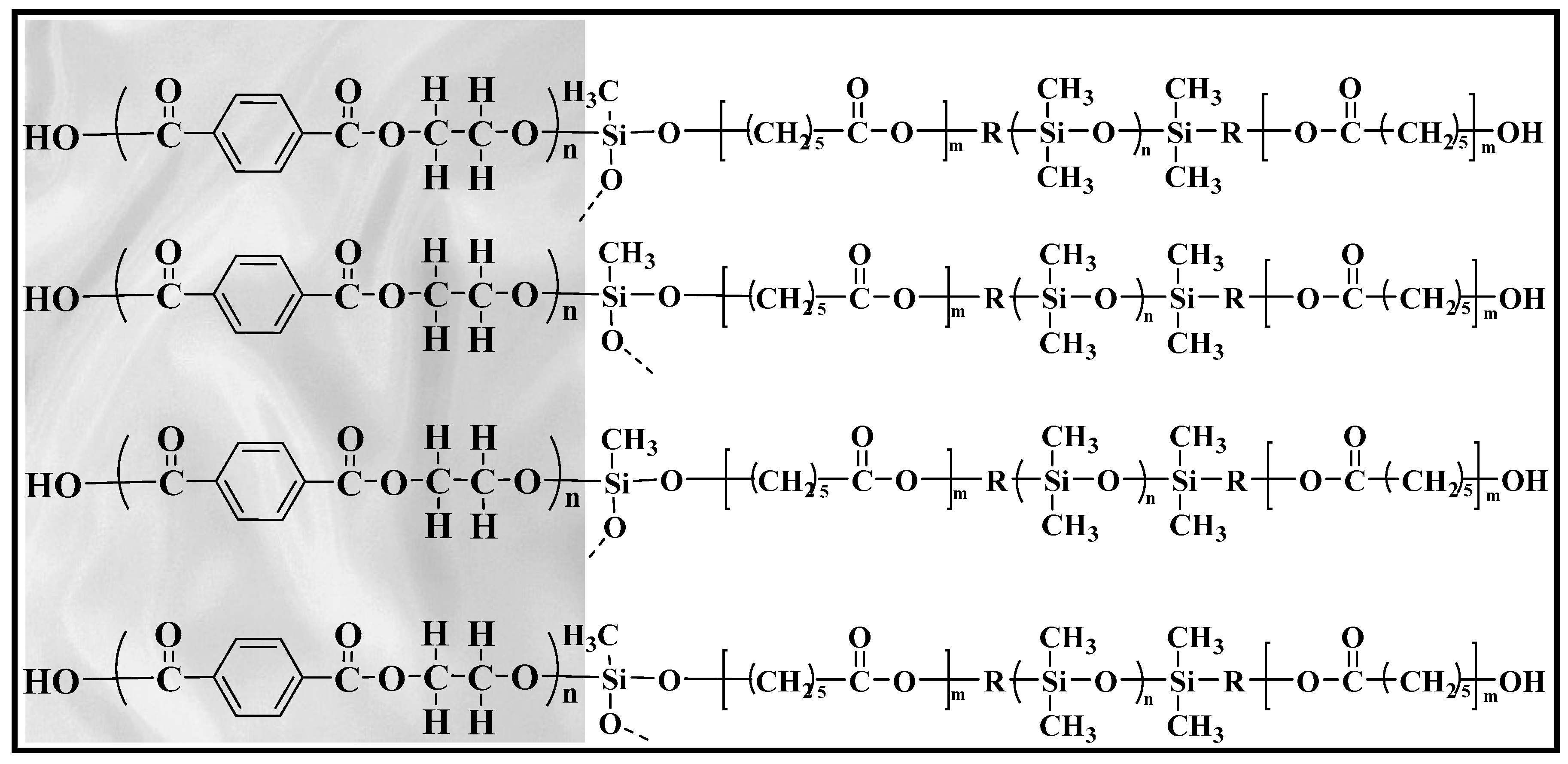
The fabric sorbent extraction medium used in the fabric disk construction was a hydrophobic polyester fabric substrate coated with a medium polar sol-gel poly(caprolactone-dimethylsiloxane-caprolactone), PCL–DMS–CL, with a sorbent loading of 4.35 mg cm−2. Polydimethylsiloxane (PDMS) is the most frequently used material in sample preparation techniques thanks to its inherent versatility, high thermal stability, and application potential for a wide range of analytes. PDMS is a non-polar polymer presenting high affinity for the extraction of non-polar compounds and has been used as coating for different extraction devices [26,27]. On the other hand, polycaprolactone (PCL), a cyclic ester possessing a seven-membered ring, is a hydrophilic semi-crystalline polymer with an exceptional blend-compatibility with other polymers. Copolymers of PCL, block or random, can be formed using monomers like DMS [28]. In this study, the efficient incorporation of PCL into the polymeric structure of DMS in a solution under extraordinarily mild thermal conditions has led to the development of a brand new sol-gel coating for fabric substrate. The sol-gel coating procedure results in the well-maintained porosity of the polyester fabric, allowing fast and easy permeation of the sample solution through its surface. The analyte extraction kinetic is accelerated and thus its extraction equilibrium time becomes shorter. The hybrid nature of the sol-gel developed network along with the strong covalent bond between the coating and the fabric substrate, result in a robust sorbent material able to be exposed in several organic solvents with limited risk of analyte loss. A schematic representation of sol-gel poly(caprolactone-dimethylsiloxane-caprolactone) coated polyester fabric is presented in Figure 2.
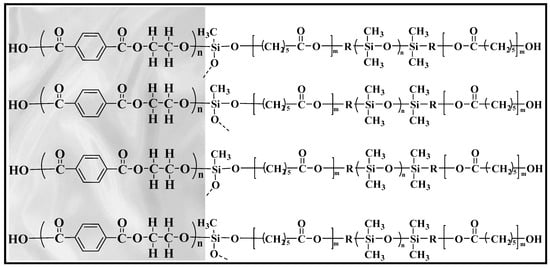
Figure 2.
Schematic representation of sol-gel poly(caprolactone-dimethylsiloxane-caprolactone) coating, chemically bonded to the polyester fabric substrate.
3.2. Study of Preconcentration Conditions
Chemical and hydrodynamic parameters affecting the sensitivity of the proposed FI-FDSE-FAAS method were optimized using the univariate methodology. Standard aqueous solutions of Cu(II) at 10.0 μg L−1, Ni (II) at 20.0 μg L−1, Zn(II) at 5.0 μg L−1, Pb(II) at 50.0 μg L−1 and Cd(II) at 10.0 μg L−1 were employed throughout the study using the manifold depicted in Figure 1.
3.2.1. Retention of the Analytes onto the Microcolumn
Several chelating agents like dithiocarbamates (DTCs), are broadly used for the preconcentration/separation of heavy metal ions due to their strong binding and complexing ability with them. Among them, APDC has been found to be more suitable for atomic absorption especially at low pH values than other dithiocarbamates. It is worth mentioning that APDC forms stable complexes with a large number of metals and for this reason it is widely used in extraction procedures [18,29]. APDC is a highly polar chelating agent with logKow < 1.10. However, after complexing with metal ions, electron rich centers become overall electronically charge neutral and consequently, the complex turns into a relatively hydrophobic compound. As such, a hydrophobic surface capable of offering London dispersion type of intermolecular interactions could provide high affinity to selectively extract and efficiently preconcentrate metal-APDC complexes. In this work, a hydrophobic polyester fabric substrate coated with a mixed polymer of non-polar dimethylsiloxane and caprolactone was adopted as an efficient extraction medium. Hence, a solution of 0.05% m/v APDC in water, was used throughout the study as it was proved adequate for the complexation of all analytes.
3.2.2. pH Studies
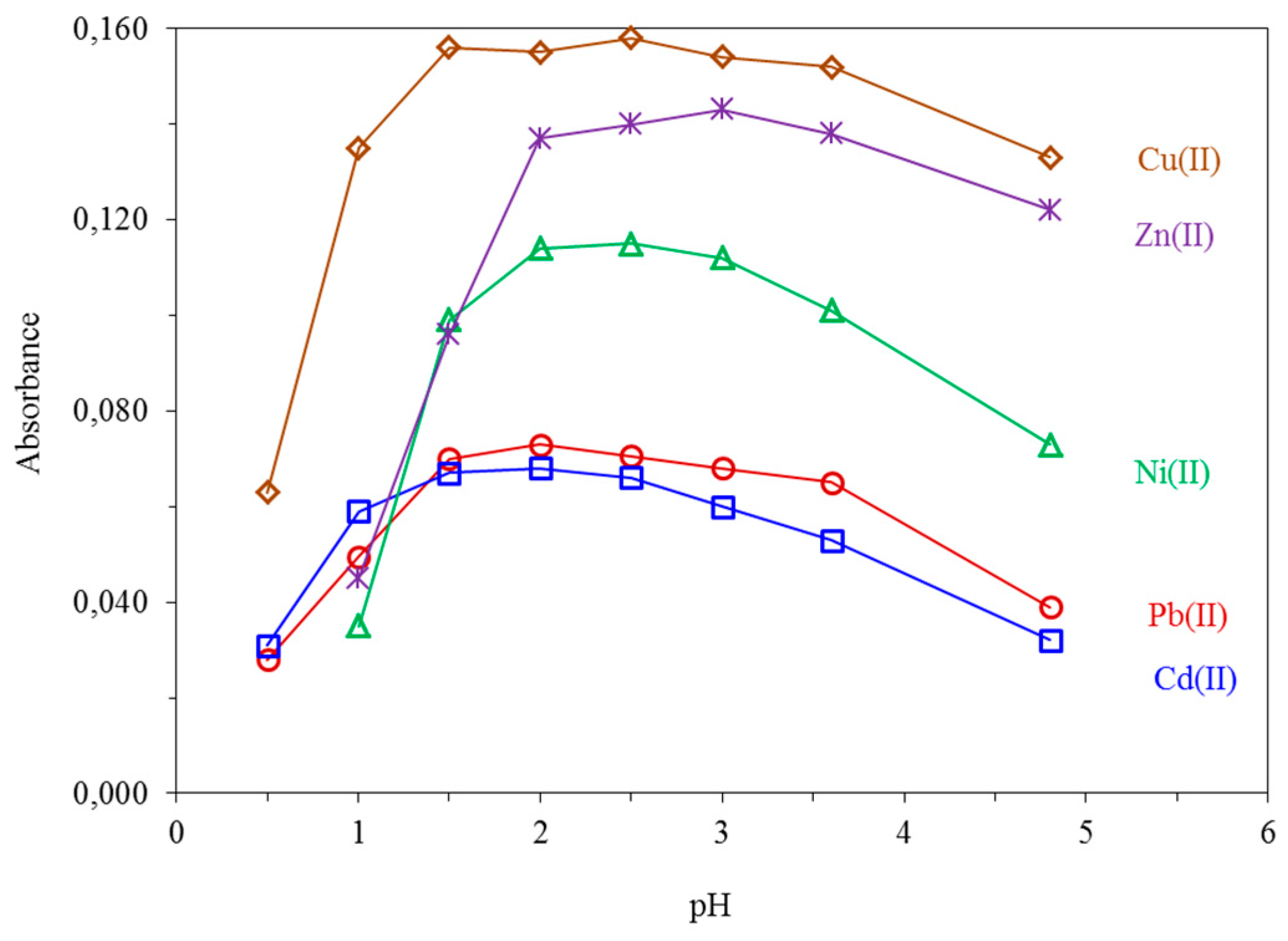
The pH value of the sample solution strongly affects the complex formation and its retention onto the sorbent surface during the loading step. The effect of pH on the absorbance was studied for pH values between 0.5 and 4.8 by adjusting it with dilute HNO3. The maximum absorption signal was obtained within the pH range of 1.5–3.6 for Cu(II) and Pb(II), 1.5–3.0 for Ni(II), 2.0–3.6 for Zn(II), and 1.5–2.5 for Cd(II), respectively, as shown in Figure 3. Finally, a pH value between 2.0–2.5 was employed for all analytes throughout the study. The adopted pH value facilitates the direct use of the aqueous samples after common acid preservation at pH ≈ 2.0.
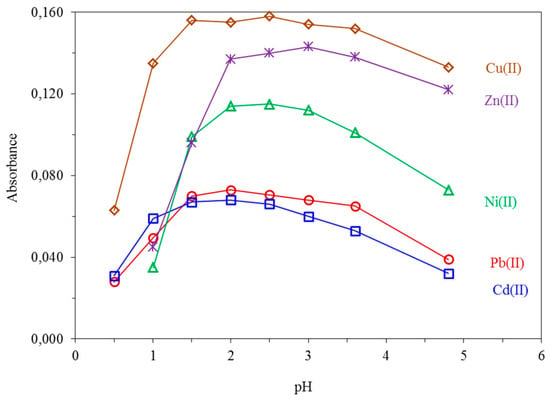
Figure 3.
Effect of pH on the absorbance of 10.0 μg L−1 Cu(II), 20.0 μg L−1 Ni(II), 5.0 μg L−1 Zn(II), 50.0 μg L−1 Pb(II) and 10.0 μg L−1 Cd(II). All other parameters as given in Table 1.
3.2.3. Effect of Loading Flow Rate
The efficiency of the on-line, time-based solid phase extraction system is significantly affected by the total loading flow rate defining the preconcentrated sample amount as well as the linear velocity of the solution into the microcolumn and thus, the sensitivity of the method. The sample and APDC solutions were pumped by the peristaltic pump (P2, Figure 1) while the ratio of their flow rates was fixed at ca. 11.5. The effect of the loading flow rate (sample + APDC flow rate) on the absorbance was studied in the range of 5.5–12.5 mL min−1. The absorbance was increased almost linearly within the examined range for an appropriate loading time for all analytes, proving that the kinetic of the complex formation was fast and the contact time for all metals was adequate. This is an important advantage, because various loading flow rates can be used with proportional sensitivity. Finally, for higher sensitivity, a loading flow rate of 12.5 mL min−1 was adopted for all metals.
3.2.4. Selection of Eluent and Elution Flow Rate
Out of the many chelator-solvent pairs that have been suggested in the literature, the combination of APDC and MIBK is the most widely used for metal preconcentration, either with liquid–liquid extraction or SPE [29]. MIBK exhibits ideal combustion properties for FAAS as it contributes to the rising of the flame temperature and a decrease of the extractant viscosity. This feature leads to better atomization conditions as well as better elution of the retained complexes. In the present work, MIBK was chosen as an eluent (extractant solvent), producing the higher and sharper signals than the other organic solvents. Considering the elution flow rate, it should be compatible with the FAAS nebulizer’s free uptake. The elution flow rate was studied in the range of 2.4–6.4 mL min−1. As it was shown, maximum absorbance was recorded at flow rates between 4.0–4.8 mL min−1. For higher and lower flow rates, the analytical signal was decreased possibly due to the insufficient elution and the analyte dispersion into the eluent segment during its transportation towards the FAAS nebulizer. A MIBK flow rate of 4.8 mL min−1 was used for further experiments.
3.2.5. Effect of Preconcentration Time
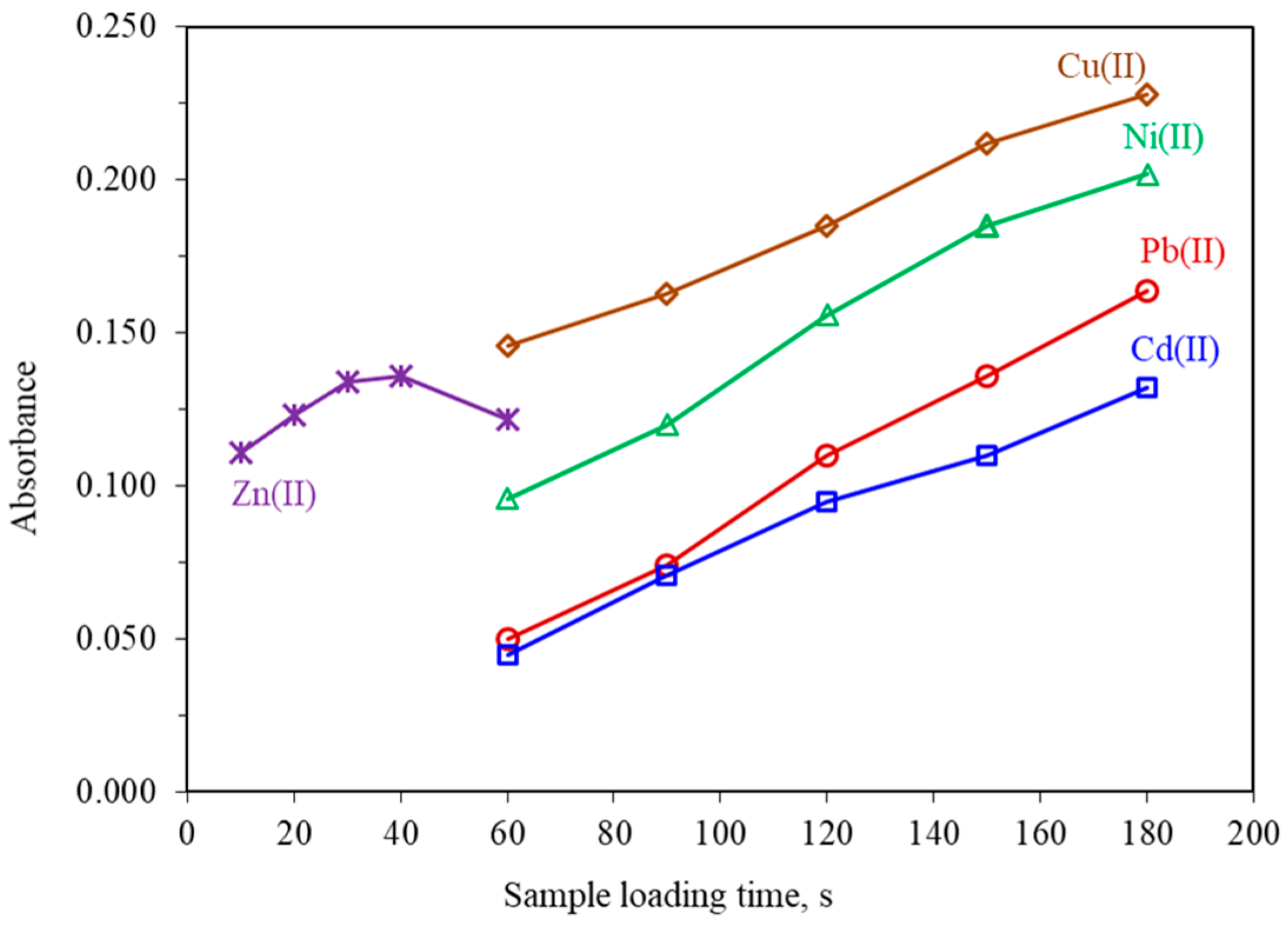
In on-line time-dependent SPE methods, the amount of preconcentrated analyte is mainly affected by the loading time, which is also termed as the preconcentration time. Generally, the higher loading time the higher the analytical signals. The influence of the loading time on the absorbance of all metals was examined from 60 to 180 s for Cu(II), Ni(II), Pb(II) and Cd(II) and from 10 to 60 s for Zn(II). The obtained results given in Figure 4, showed that the absorbance increased almost linearly up to 180 s for Cu(II), Ni(II), Pb(II), and Cd(II) and up to 30 s for Zn(II). It must be mentioned that in the case of Zn(II), for loading times higher than 30 s, the recorded signals were slightly decreased, probably due to the partial leaching of the Zn(II) complex. Consequently, a loading time of 30 s for Zn(II) and 90 s for the other metals were selected as a compromise between sensitivity, sample consumption, and sampling frequency.
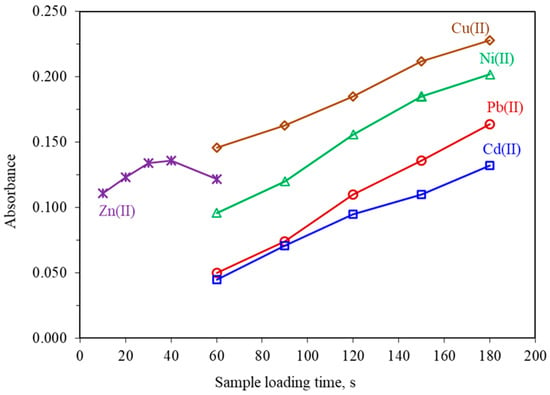
Figure 4.
Effect of loading time on the absorbance of 10.0 μg L−1 Cu(II), 20.0 μg L−1 Ni(II), 5.0 μg L−1 Zn(II), 50.0 μg L−1 Pb(II) and 10.0 μg L−1 Cd(II). All other parameters as given in Table 1.
3.3. Test for Interferences
The determination of trace metal ions can be affected by the matrix components. The interferences of ions usually found in biological fluids such as urine as well as some toxic metals were investigated in the frame of their complexation and retention onto the fabric medium. The recovery of 5.0 μg L−1 Cu(II), 10.0 μg L−1 Ni(II), 40.0 μg L−1 Pb(II), 10.0 μg L−1 Cd(II) and 5.0 μg L−1 Zn(II), was examined using the proposed on-line preconcentration system under the optimized conditions, considering as a criterion for an interference the deviation of the percentage recovery more than ± 5%. Alkaline and alkaline-earth metals, like Na(I) and K(I) were tolerated at least up to 2000 mg L−1, while Mg(II) and Ca(II) at least up to 500 mg L−1. Some common anions like Cl−, NO3−, HCO3−, SO4−2 were tested and found that they were tolerated at concentrations at least up to 1500 mg L−1. Experimental results proved that Al(III), Cr(III), Cr(IV), Fe(III), and Mn(II) can be tolerated up to 5.0 mg L−1 while Co(II) and Hg(II) up to 0.5 mg L−1 for all metals’ determination. As FAAS is a single element analyzer, for the determination of each analyte, the presence of the other four metals was examined, showing a toleration for each metal at least up to 1.0 mg L−1.
3.4. Analytical Performance Characteristics
The effectiveness of the proposed method in the context of analytical performance characteristics was estimated under the optimal conditions and summarized in Table 2. For 90 s preconcentration time, the sampling frequency was 33 h−1 for Cu(II), Ni(II), Pb(II), and Cd(II) and 72 h−1 for Zn(II). The detection limit was calculated by 3 s criterion, 3 s/S, where s is the standard deviation of the blank measurements (n = 11) and S is the slope of each calibration curve. The detection limits were determined to be 0.15 for Cu(II), 0.41 for Ni(II), 0.12 for Zn(II), 1.62 for Pb(II), and 0.49 μg L−1 for Cd(II). The precision of the method expressed as the relative standard deviation, (RSD) was calculated between 2.2% and 3.5%. The enhancement factors were calculated as the ratio of the slopes of the calibration curves obtained using the proposed preconcentration method and direct aspiration without preconcentration. For Cu(II), Ni(II), Zn(II), Pb(II), and Cd(II) the enhancement factors ranged from 36 to 250.

Table 2.
Analytical performance characteristics of the on-line FI-FDSE-FAAS method for metal determination.
The figures of merit of the proposed FI-FDSE-FAAS method and other selected on-line SPE methods coupled to FAAS [8,10,12,14,15,18,24,30,31,32,33,34,35,36,37,38,39,40,41,42,43], are compiled in Table S1 (Supplementary Materials). The proposed method features good sensitivity and precision with better or similar detection limits than earlier works for Cu(II) [8,15,31,32,34], Ni(II) [8,38,39,40], Zn(II) [41,42,43], Pb(ΙΙ) [8,24], and Cd(II) [24] assays, using a shorter preconcentration time.
4. Applications
The accuracy of the developed FI-FDSE-FAAS method was estimated by analyzing the CRMs BCR 278-R and Seronorm™ Trace Elements Urine Level-1. The trueness of the analytical method was demonstrated by the student t-test in order to find if there are statistically significant differences versus the certified values for each target analyte. The texp values were calculated by the following equation: , where is the sample mean value, is the certified value, s is the standard deviation of the sample values and n is the replicate determinations (n = 3). In this case, there are two degrees of freedom (n − 1), and therefore t value obtained from the table of the student t-distribution, tcrit, is 4.303 at the 95% confidence level. The analytical values and texp values for Cu(II), Ni(II), Zn(II), Pb(II), and Cd(II), in the above CRMs are presented in Table 3. Since all texp values are lower than tcrit, no statistically significant differences were found at the 95% probability level, indicating the applicability of the FI-FDSE-FAAS method in such types of samples.

Table 3.
Applications of the developed FI-FDSE-FAAS method in CRMs for metal determination.
The method was also applied for the determination of Cu(II), Ni(II), Zn(II), Pb(II) and Cd(II) in urine samples collected by young healthy persons following the procedure described in Section 2.2. The recovery was estimated by adding known amounts of analyte standards in the examined samples. Results are presented in Table 4. Recoveries were satisfactory and ranged from 95.0 to 102.0 proving good analytical performance of the proposed method in the analysis of urine samples.

Table 4.
Analytical results of essential and toxic metal determination in urine samples using the FI-FDSE-FAAS method.
5. Conclusions
A novel automatic flow injection fabric disk sorptive extraction platform for essential and toxic metal enrichment for flame atomic absorption spectrometric determination was developed. In the proposed method, a sorbent packing material consisted of polyester fabric disks coated with sol-gel poly (caprolactone–dimethylsiloxane–caprolactone) was demonstrated for the first time. The method proved to be simple, rapid, inexpensive, and reliable for Cu(II), Ni(II), Zn(II), Pb(II), and Cd(II) determination in biological and urine samples with good performance characteristics. The extraction efficiency of the FDSE microcolumn was found unaffected even after applying more than 600 sorption/elution analytical cycles, demonstrating high chemical and mechanical stability. In addition, high sample loading flow rates can be applied resulting in improved preconcentration factors. Future research should be focused on studying the on-line FDSE potentials and its analytical performance for the determination of organic compounds in various matrices.
Supplementary Materials
The following are available online at http://www.mdpi.com/2297-8739/5/3/34/s1, Table S1: Comparative analytical performance characteristics of the proposed FI-FDSE-FAAS method with other selected on–line SPE-FAAS methods for metal determination.
Author Contributions
Conceptualization, V.K.; Data curation, V.K. and A.A.; Investigation, V.K.; Resources, A.K. and K.G.F.; Supervision, A.A.; Validation, V.K.; Writing, original draft, V.K.; Writing, review & editing, V.S., A.K., K.G.F. and A.A.
Funding
This research has been financially supported by the General Secretariat for Research and Technology (GSRT) and the Hellenic Foundation for Research and Innovation (HFRI) (Scholarship Code: 340).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Miró, M.; Hansen, E.H. On-line sample processing involving microextraction techniques as a front-end to atomic spectrometric detection for trace metal assays: A review. Anal. Chim. Acta 2013, 782, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Locatelli, M.; Ulusoy, H. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Masqué, N.; Marcé, R.M.; Borrull, F. New polymeric and other types of sorbents for solid-phase extraction of polar organic micropollutants from environmental water. Trends Anal. Chem. 1998, 17, 384–394. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Marć, M.; Szczepańska, N.; Namieśnik, J. New Polymeric Materials for Solid Phase Extraction. Crit. Rev. Anal. Chem. 2017, 47, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gilart, N.; Borrull, F.; Fontanals, N.; Marcé, R.M. Selective materials for solid-phase extraction in environmental analysis. Trends Environ. Anal. Chem. 2014, 1, 8–18. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Giakisikli, G.; Mitani, C. Flow injection dual-syringe sorbent extraction platform for metal determination in environmental matrices utilizing a new strong cation exchange sorbent micro-cartridge and flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2012, 92, 1276–1288. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Giakisikli, G.; Zachariadis, G. The HyperSep SCX micro-cartridge for on-line flow injection inductively coupled plasma atomic emission spectrometric determination of trace elements in biological and environmental samples. Anal. Methods 2011, 3, 2108–2114. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Xidia, S.; Giakisikli, G. Study of bond Elut Plexa PCX cation exchange resin in flow injection column preconcentration system for metal determination by flame atomic absorption spectrometry. Talanta 2012, 97, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sixto, A.; Fiedoruk-Pogrebniak, M.; Rosende, M.; Cocovi-Solberg, D.; Knochen, M.; Miró, M. A mesofluidic platform integrating restricted access-like sorptive microextraction as a front end to ICP-AES for the determination of trace level concentrations of lead and cadmium as contaminants in honey. J. Anal. At. Spectrom. 2016, 31, 473–481. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Maloumidou, T. Flow injection online solid phase extraction system using Oasis-HLBTM micro-cartridge for chromium(vi) and copper determination by flame atomic absorption spectrometry. Anal. Methods 2011, 3, 1392–1398. [Google Scholar] [CrossRef]
- Portugal, L.A.; Laglera, L.M.; Anthemidis, A.N.; Ferreira, S.L.; Miró, M. Pressure-driven mesofluidic platform integrating automated on-chip renewable micro-solid-phase extraction for ultrasensitive determination of waterborne inorganic mercury. Talanta 2013, 110, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.N.; Giakisikli, G.; Xidia, S.; Miró, M. On-line sorptive preconcentration platform incorporating a readily exchangeable Oasis HLB extraction micro-cartridge for trace cadmium and lead determination by flow injection-flame atomic absorption spectrometry. Microchem. J. 2011, 98, 66–71. [Google Scholar] [CrossRef]
- Giakisikli, G.; Zachariadis, P.; Kila, I.; Teshima, N.; Anthemidis, A. Flow Injection Solid Phase Extraction for Trace Metal Determination Using a Chelating Resin and Flame Atomic Absorption Spectrometry Detection. Anal. Lett. 2015, 49, 929–942. [Google Scholar] [CrossRef]
- Giakisikli, G.; Ayala Quezada, A.; Tanaka, J.; Anthemidis, A.N.; Murakami, H.; Teshima, N.; Sakai, T. Automatic On-line Solid-phase Extraction—Electrothermal Atomic Absorption Spectrometry Exploiting Sequential Injection Analysis for Trace Vanadium, Cadmium and Lead Determination in Human Urine Samples. Anal. Sci. 2015, 31, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Conway, T.M.; Rosenberg, A.D.; Adkins, J.F.; John, S.G. A new method for precise determination of iron, zinc and cadmium stable isotope ratios in seawater by double-spike mass spectrometry. Anal. Chim. Acta 2013, 793, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kazantzi, V.; Giakisikli, G.; Anthemidis, A. Reversed phase StrataTM-X resin as sorbent for automatic on-line solid phase extraction atomic absorption spectrometric determination of trace metals: Comparison of polymeric-based sorbent materials. Int. J. Environ. Anal. Chem. 2017, 97, 508–519. [Google Scholar] [CrossRef]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Ayazi, Z. Application of nanocomposite-based sorbents in microextraction techniques: A review. Analyst 2017, 142, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent 14,216,121, 17 March 2014. [Google Scholar]
- Kazantzi, V.; Anthemidis, A. Fabric Sol-gel Phase Sorptive Extraction Technique: A Review. Separations 2017, 4, 20. [Google Scholar] [CrossRef]
- Heena; Kaur, R.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Determination of cobalt(II), nickel(II) and palladium(II) Ions via fabric phase sorptive extraction in combination with high-performance liquid chromatography-UV detection. Sep. Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 958, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fontanals, N.; Marcé, R.M.; Borrull, F. New materials in sorptive extraction techniques for polar compounds. J. Chromatogr. A 2007, 1152, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998; pp. 345–347. [Google Scholar]
- Sivrikaya, S.; Imamoglu, M.; Yıldız, S.Z.; Kara, D. Novel Functionalized Silica Gel for On-line Preconcentration of Cadmium(II), Copper(II), and Cobalt(II) with Determination by Flame Atomic Absorption Spectrometry. Anal. Lett. 2016, 49, 943–957. [Google Scholar] [CrossRef]
- Chamjangali, M.A.; Bagherian, G.; Mokhlesian, A.; Bahramian, B. Synthesis and application of chloromethylated polystyrene modified with 1-phenyl-1,2-propanedione-2-oxime thiosemicarbazone (PPDOT) as a new sorbent for the on-line preconcentration and determination of copper in water, soil, and food samples by FAAS. J. Hazard. Mater. 2011, 192, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Saçmaci, Ş.; Şahan, S.; Şahin, U.; Kartal, Ş.; Ülgen, A. On-line solid-phase separation/preconcentration for the determination of copper in urine by flame atomic absorption spectrometry. Mater. Sci. Eng. C 2014, 44, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Song, H.; Chen, M.L. Dithizone immobilized silica gel on-line preconcentration of trace copper with detection by flame atomic absorption spectrometry. Talanta 2011, 85, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Çetin, T.; Ülgen, A.; Tokalıoğlu, Ş. On-line Solid Phase Extraction of Copper in Water Samples with Flow Injection Flame Atomic Absorption Spectrometry. Clean Soil Air Water 2011, 39, 244–249. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S.; Trzewik, B.; Grzybek, P.; Zaitz, M.M.; Gawin, M.; Mrowiec, H. Cu(II)-imprinted styrene-divinylbenzene beads as a new sorbent for flow injection-flame atomic absorption determination of copper. Microchem. J. 2009, 93, 87–92. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, H.; Zhao, S.; Yan, H.; Han, D. On-line solid phase extraction coupled to flame atomic absorption spectrometry for the determination of trace copper and zinc in environmental and biological samples. Int. J. Environ. Anal. Chem. 2008, 88, 689–699. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Zachariadis, G.A.; Stratis, J.A. On-line preconcentration and determination of nickel and zinc in natural water samples by flow injection—Flame atomic absorption spectrometry using ptfe-turnings for column packing. Int. J. Environ. Anal. Chem. 2010, 90, 127–136. [Google Scholar] [CrossRef]
- Lemos, V.A.; Novaes, C.G.; Lima Ada, S.; Vieira, D.R. Flow injection preconcentration system using a new functionalized resin for determination of cadmium and nickel in tobacco samples. J. Hazard. Mater. 2008, 155, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wutke, N.B.; Diniz, K.M.; Corazza, M.Z.; de Oliveira, F.M.; Ribeiro, E.S.; da Fonseca, B.T.; Segatelli, M.G.; Teixeira Tarley, C.R. Preconcentration of nickel(II) by a mini-flow system with a novel ternary oxide solid phase and flame atomic absorption spectrometry. Anal. Lett. 2016, 49, 723–736. [Google Scholar] [CrossRef]
- Escudero, L.A.; Blanchet, A.J.; Sombra, L.L.; Salonia, J.A.; Gasquez, J.A. Determination of the total and extractable fraction of Ni in lake sediments and natural waters of San Luis (Argentina) by FAAS using a simple solid phase extraction system. Microchem. J. 2014, 116, 92–97. [Google Scholar] [CrossRef]
- Yilmaz, S.; Tokalioĝlu, Ş.; Şahan, S.; Ülgen, A.; Şahan, A.; Soykan, C. On-line preconcentration/determination of zinc from water, Biological and food samples using synthesized chelating resin and flame atomic absorption spectrometry. J. Trace Elem. Med. Biol. 2013, 27, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.N.; Mosquetta, R.; Carasek, E.; Coelho, N.M.M. Determination of Zn(II) in alcohol fuel by flame atomic absorption spectrometry after on-line preconcentration using a solid phase extraction system. J. Anal. Chem. 2012, 67, 448–454. [Google Scholar] [CrossRef]
- Peixoto, R.R.A.; Macarovscha, G.T.; Cadore, S. On-line Preconcentration and Determination of Zinc Using Zincon and Flame Atomic Absorption Spectrometry. Food Anal. Methods 2012, 5, 814–820. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).