Simultaneous Determination of Polycyclic Aromatic Hydrocarbons and Anthraquinone in Yerba Mate by Modified MSPD Method and GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Yerba Mate Samples and Application of the Proposed Method
2.3. Instrumentation
2.4. GC-MS Analysis
2.5. Sample Preparation Evaluation
2.6. Established Sample Preparation Procedure
2.7. Method Validation
2.8. Statistical Analysis
3. Results and Discussion
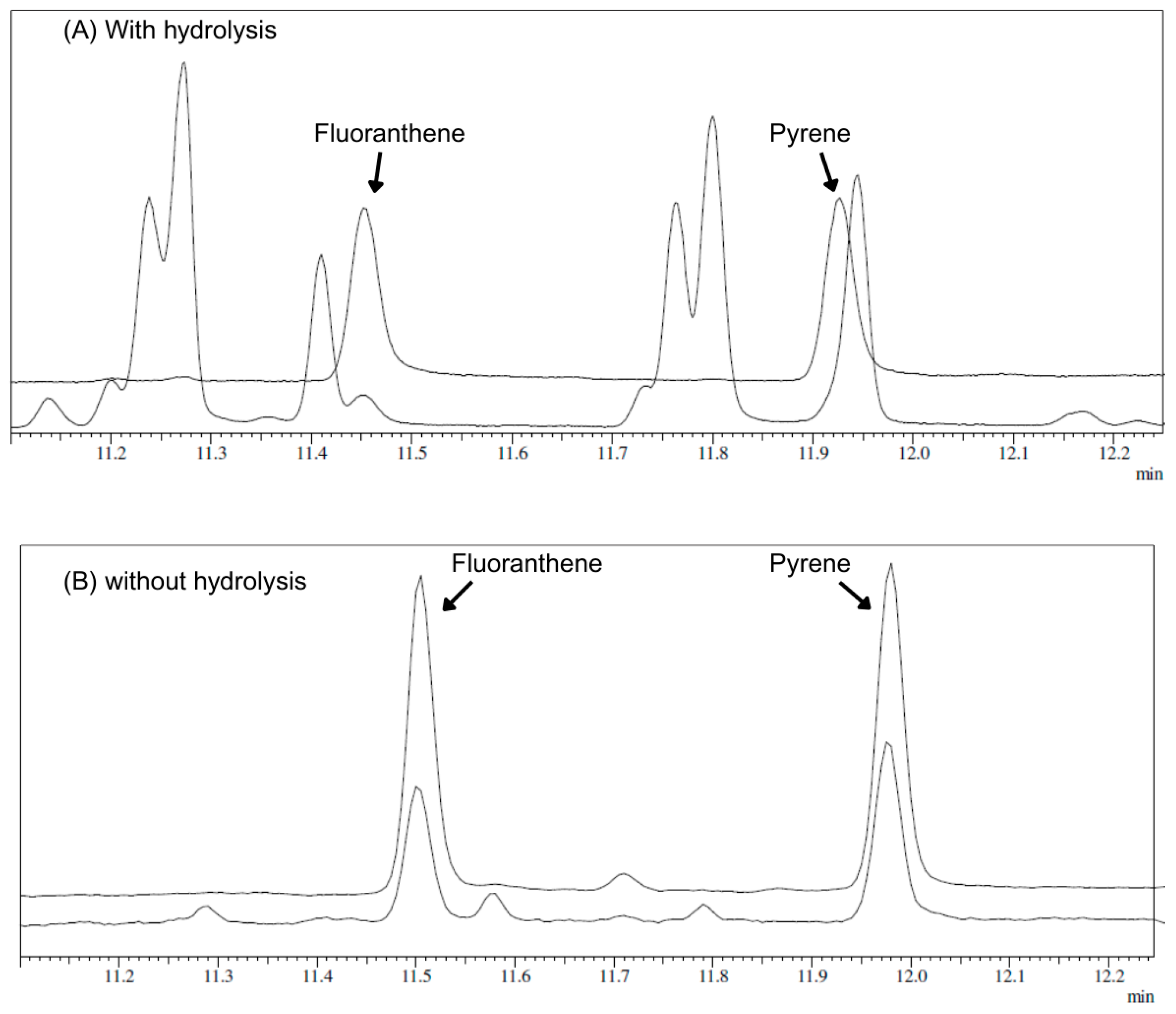
3.1. Sample Preparation Method
3.2. Validation of the Method
3.3. Application of the Validated Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croge, C.P.; Cuquel, F.L.; Pintro, P.T.M. Yerba mate: Cultivation systems, processing and chemical composition. a review. Sci. Agric. 2020, 78, 1–11. [Google Scholar] [CrossRef]
- Song, Y.; Cao, J.; Cao, F.; Su, E. A systematic review on the yerba mate (Ilex paraguariensis A. St. Hil.). J. Food Compos. Anal. 2025, 142, 107466. [Google Scholar] [CrossRef]
- Fernandez, M.d.L.A.; Boiteux, J.; Santiano, F.; Fontana, C.L.; Silva, M.F.; Espino, M. Therapeutic product based on yerba mate extract and eutectic system by one step ultrasound approach. Sustain. Chem. Pharm. 2023, 32, 101008. [Google Scholar] [CrossRef]
- Gerber, T.; Nunes, A.; Moreira, B.R.; Maraschin, M. Yerba mate (Ilex paraguariensis A. St.-Hil.) for new therapeutic and nutraceutical interventions: A review of patents issued in the last 20 years (2000–2020). Phyther. Res. 2023, 37, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Baerley, V.R.; Itavo, C.C.B.F.; Itavo, L.C.V.; Nazário, C.E.D.; Gomes, M.d.N.B.; Difante, G.d.S.; dos Santos, G.T.; de Melo, G.K.A.; Gurgel, A.L.C.; Soares, É.S.d.M.; et al. Antioxidant action of yerba mate on carcass and meat characteristics and fatty acid profile in meat and fat of lambs finished in tropical pastures. Trop. Anim. Health Prod. 2023, 55, 109. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos, A.C.; Frazzon, J.; Noreña, C.P.Z. Phenolic Compounds Present in Yerba Mate Potentially Increase Human Health: A Critical Review; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- de Aguiar, N.S.; Gabira, M.M.; Duarte, M.M.; Tomasi, J.d.C.; Hansel, F.A.; Lavoranti, O.J.; Deschamps, C.; Helm, C.V.; Wendling, I. How shading levels affect bioactive compounds in leaves of yerba mate clones. Biochem. Syst. Ecol. 2024, 113, 104796. [Google Scholar] [CrossRef]
- Hegel, P.; Granone, L.; Hrnčič, M.K.; Pereda, S.; Kotnik, P.; Knez, Z. Alkaloid-rich vs. antioxidant-rich yerba mate (Ilex paraguariensis) extracts: Exploiting the selectivity of supercritical CO2 using hydrated ethanol as co-solvent. J. Supercrit. Fluids 2021, 172, 105200. [Google Scholar] [CrossRef]
- Schenk, M.; Ferrario, M.; Schmalko, M.; Rivero, R.; Taravini, I.; Guerrero, S. Development of extracts obtained from yerba mate leaves with different industrial processing steps: Antimicrobial capacity, antioxidant properties, and induced damage. J. Food Process. Preserv. 2021, 45, e15482. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Chanaj-Kaczmarek, J.; Cielecka-Piontek, J. Yerba Mate—A Long but Current History. Nutrients 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Agents classified by the IARC monographs. Igarss 2018, 116, 1–512. [Google Scholar]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Mokoena, L.; Tshilongo, J. A Review on Recent Developments in the Extraction and Identification of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Water 2024, 16, 2520. [Google Scholar] [CrossRef]
- Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; Wang, W.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA J. 2012, 10, 6–10. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EU) 2015/1933 of 27 October 2015. Off. J. Eur. Union 2015, 58, 11–14. [Google Scholar]
- Valduga, A.T.; Gonçalves, I.L.; Puton, B.M.S.; Hennig, B.d.L.; de Brito, E.S. Anthraquinone as emerging contaminant: Technological, toxicological, regulatory and analytical aspects. Toxicol Res. 2024, 40, 11–21. [Google Scholar] [CrossRef]
- Londoño, V.A.G.; Pok, P.S.; Resnik, S. Polycyclic Aromatic Hydrocarbons (PAHs) in ‘Yerba Mate’ (Ilex paraguariensis) Fractions. Polycycl. Aromat. Compd. 2020, 40, 1381–1389. [Google Scholar] [CrossRef]
- INMETRO. Orientação Sobre Validação de Métodos Analíticos: Documento de Caráter Orientativo (DOQ-CGCRE-008); Coordenação Geral de Acreditação: Duque de Caxias, Brazil, 2020; 30p. Available online: http://www.inmetro.gov.br/Sidoq/Arquivos/Cgcre/DOQ/DOQ-Cgcre-8_08.pdf (accessed on 29 July 2025).
- Sante (European Commission). Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. Document SANTE/11312/2021. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 29 July 2025).
- Paris, A.; Ledauphin, J.; Poinot, P.; Gaillard, J.-L. Polycyclic aromatic hydrocarbons in fruits and vegetables: Origin, analysis, and occurrence. Environ. Pollut. 2018, 234, 96–106. [Google Scholar] [CrossRef]
- Parigoridi, I.-E.; Tsoumani, E.; Akrida-Demertzi, K.; Demertzis, P.G. Evaluation of three extraction methods for the isolation of PAHs from recycled paperboard materials intended for food contact applications. Eur. Food Res. Technol. 2023, 249, 665–673. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Mujuru, M.; Ambushe, A.A. Comparison and optimization of extraction methods for the determination of polycyclic aromatic hydrocarbons in sediment from blood river in Limpopo province, South Africa. In Proceedings of the 39th Johannesburg International Conference on “Chemical, Biological and Environmental Engineering” (JCBEE-23), Johannesburg, South Africa, 16–17 November 2023; Available online: https://www.researchgate.net/profile/Tumelo-Mogashane/publication/376396943_Comparison_and_Optimization_of_Extraction_Methods_for_The_Determination_of_Polycyclic_Aromatic_Hydrocarbons_in_Sediment_from_Blood_River_in_Limpopo_Province_South_Africa/links/6576bd49ea5f7f02055dbb8b/Comparison-and-Optimization-of-Extraction-Methods-for-The-Determination-of-Polycyclic-Aromatic-Hydrocarbons-in-Sediment-from-Blood-River-in-Limpopo-Province-South-Africa.pdf (accessed on 2 August 2025).
- Santos, P.M.; Sánchez, M.d.N.; Pavón, J.L.P.; Cordero, B.M.; Fernández, R.V. Liquid-liquid extraction-programmed temperature vaporizer-gas chromatography-mass spectrometry for the determination of polycyclic aromatic hydrocarbons in saliva samples. Application to the occupational exposure of firefighters. Talanta 2018, 192, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Hoppe, K.; Drzewiecka, K. Determination of Polycyclic Aromatic Hydrocarbon Content in Garden Herbal Plants Using Liquid Chromatographic Analysis (HPLC-FL). Plants 2023, 12, 551. [Google Scholar] [CrossRef]
- Górka, A.; Baran, D.; Słowik-Borowiec, M. Assessment of heavy metals, PAHs, and pesticide levels in yerba mate on the European market. Environ. Sci. Pollut. Res. 2025, 32, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Zanoelo, M.; Oldoni, T.L.C.; Bilck, A.P.; da Cunha, M.A.A.; Pereira, E.A. Determination of Polycyclic Aromatic Hydrocarbons in Mate Tea Microencapsulated with Maltodextrin and Lasiodiplodan ((1→6) β–D-Glucan). Polycycl. Aromat. Compd. 2023, 43, 9338–9346. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M. Levels and risk assessment of polycyclic aromatic hydrocarbons, acrylamide and 5-hydroxymethylfurfural in yerba mate and its infusions. Food Control 2023, 152, 109860. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Lee, S.-H.; Kim, Y.-Y.; Shin, H.-S. Polycyclic aromatic hydrocarbon risk assessment and analytical methods using quechers pretreatment for the evaluation of herbal medicine ingredients in Korea. Foods 2021, 10, 2200. [Google Scholar] [CrossRef]
- Knøfler, I.H.; Andersson, K.E.; Becker, R.L.; Christiansen, S.; Nielsen, N.J.; Christensen, J.H. Is Fucus a suitable biomonitoring organism for polycyclic aromatic hydrocarbon contamination? A study from the Faroe Islands. Environ. Sci. Pollut. Res. 2024, 31, 26699–26712. [Google Scholar] [CrossRef]
- Słowik-Borowiec, M.; Szpyrka, E.; Książek-Trela, P.; Podbielska, M. Simultaneous Determination of Multi-Class Pesticide Residues and PAHs in Plant Material and Soil Samples Using the Optimized QuEChERS Method and Tandem Mass Spectrometry Analysis. Molecules 2022, 27, 2140. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, G.; Liu, P.; Pan, R.; Liu, X.; Lu, C. Determination of 16 Polycyclic Aromatic Hydrocarbons in Tea by Simultaneous Dispersive Solid-Phase Extraction and Liquid–Liquid Extraction Coupled with gas Chromatography–Tandem Mass Spectrometry. Food Anal. Methods 2016, 9, 2374–2384. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, X.; Gui, J.; Wan, Y.; Chen, J.; Tan, T.; Liu, F.; Guo, L. Ultrasonic Solvent Extraction Followed by Dispersive Solid Phase Extraction (d-SPE) Cleanup for the Simultaneous Determination of Five Anthraquinones in Polygonum multiflorum by UHPLC-PDA. Foods 2022, 11, 386. [Google Scholar] [CrossRef]
- Zitka, O.; Babula, P.; Sochor, J.; Kummerova, M.; Krystofova, O.; Adam, V.; Havel, L.; Beklova, M.; Hubalek, J.; Kizek, R. Determination of eight polycyclic aromatic hydrocarbons and in pea plants (Pisum sativum L.) Extracts by high performance liquid chromatography with electrochemical detection. Int. J. Electrochem. Sci. 2012, 7, 908–927. [Google Scholar] [CrossRef]
- Jiries, A.; Hussain, H.; Lintelmann, J. Determination of Polycyclic Aromatic hydrocarbons in wastewater, sediments, sludge and plants in Karak province, Jordan. Water Air Soil Pollut. 2000, 121, 217–228. [Google Scholar] [CrossRef]
- Panzl, M.V.; Almeida, J.M.S.; Pedrozo-Peñafiel, M.; Menchaca, D.; Aucélio, R.Q.; Rodríguez-Haralambides, A. Evaluation of Polycyclic Aromatic Hydrocarbons in Dried Leaves of Yerba Mate (Ilex paraguariensis) and Their Extraction into Infusions. Polycycl. Aromat. Compd. 2023, 43, 1575–1589. [Google Scholar] [CrossRef]
- Rozentale, I.; Yan Lun, A.; Zacs, D.; Bartkevics, V. The occurrence of polycyclic aromatic hydrocarbons in dried herbs and spices. Food Control 2018, 83, 45–53. [Google Scholar] [CrossRef]
- Ianiri, G.; Fratianni, A.; Avino, P.; Panfili, G. Determination of Polycyclic Aromatic Hydrocarbons from Atmospheric Deposition in Malva sylvestris Leaves Using Gas Chromatography with Mass Spectrometry (GC-MS). Atmosphere 2024, 15, 1402. [Google Scholar] [CrossRef]
- Wang, P.; Jin, B.; Lian, C.; Guo, K.; Ma, C. Comparative Analysis of Polycyclic Aromatic Hydrocarbons and Halogenated Polycyclic Aromatic Hydrocarbons in Different Parts of Perilla frutescens (L.) Britt. Molecules 2022, 27, 3133. [Google Scholar] [CrossRef]
- Concha-Graña, E.; Muniategui-Lorenzo, S.; De Nicola, F.; Aboal, J.R.; Rey-Asensio, A.I.; Giordano, S.; Reski, R.; López-Mahía, P.; Prada-Rodríguez, D. Matrix solid phase dispersion method for determination of polycyclic aromatic hydrocarbons in moss. J. Chromatogr. A 2015, 1406, 19–26. [Google Scholar] [CrossRef]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. A 1989, 475, 353–361. [Google Scholar] [CrossRef] [PubMed]
- de Melo, A.P.Z.; Hoff, R.B.; Molognoni, L.; Kleemann, C.R.; de Oliveira, T.; de Oliveira, L.V.A.; Daguer, H.; Barreto, P.L.M. Determination of Polycyclic Aromatic Hydrocarbons in Seafood by PLE-LC-APCI-MS/MS and Preliminary Risk Assessment of the Northeast Brazil Oil Spill. Food Anal. Methods 2022, 15, 1826–1842. [Google Scholar] [CrossRef]
- Kemmerich, M.; Demarco, M.; Bernardi, G.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Balls-in-tube matrix solid phase dispersion (BiT-MSPD): An innovative and simplified technique for multiresidue determination of pesticides in fruit samples. J. Chromatogr. A 2020, 1612, 460640. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Grześkowiak, T.; Ligor, M.; Frankowski, R. Achievements and Challenges of Matrix Solid-Phase Dispersion Usage in the Extraction of Plants and Food Samples. Processes 2024, 12, 1146. [Google Scholar] [CrossRef]
- El-Deen, A.K. An Overview of Recent Advances and Applications of Matrix Solid-Phase Dispersion. Sep. Purif. Rev. 2024, 53, 100–117. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. A review on the recent progress in matrix solid phase dispersion. Molecules 2018, 23, 2767. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Dawidowicz, A.L. Can matrix solid phase dispersion (MSPD) be more simplified? Application of solventless MSPD sample preparation method for GC-MS and GC-FID analysis of plant essential oil components. Talanta 2016, 151, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Lynam, K. Polycyclic Aromatic Hydrocarbon (PAH) Analysis in Fish by GC/MS Using Agilent Bond Elut QuEChERS dSPE Sample Preparation and a High Efficiency DB-5 ms Ultra Inert GC Application Note. Agilent Food Testing and Hydrocarbon Processing; Publication No. 5990-6668; Agilent Technologies, Inc.: Wilmington, DE, USA, 2012; Available online: https://www.agilent.com/cs/library/applications/5990-6668EN.pdf (accessed on 1 August 2025).
- Vieira, M.A.; Maraschin, M.; Rovaris, Â.A.; Amboni, R.D.d.M.C.; Pagliosa, C.M.; Xavier, J.J.M.; Amante, E.R. Occurrence of polycyclic aromatic hydrocarbons throughout the processing stages of erva-mate (Ilex paraguariensis). Food Addit. Contam. Part A 2010, 27, 776–782. [Google Scholar] [CrossRef]


| Compounds | Abbreviation | CAS | Boiling Point (°C) | log Kow | Molecular Mass (Da) | Carcinogenicity |
|---|---|---|---|---|---|---|
| Acenaphthene | Acp | 83-32-9 | 96 | 3.98 | 154.2 | 3 |
| Acenaphthylene | Acy | 208-96-8 | 275 | 4.07 | 152.2 | 3 |
| Anthracene | Ant | 0120-12-7 | 342 | 4.45 | 178.2 | 3 |
| Anthraquinone | AQ | 84-65-1 | 379 | 3.39 | 208.2 | 2B |
| Benz[a]anthracene | BaA | 56-55-3 | 438 | 5.61 | 228.3 | 2B |
| Benzo[a]pyrene | BaP | 50-32-8 | 495 | 6.06 | 252.3 | 1 |
| Benzo[b]fluoranthene | BbF | 205-99-2 | 481 | 6.04 | 252.3 | 2B |
| Benzo[g,h,i]perylene | BghiP | 191-24-2 | 550 | 6.5 | 276.3 | 3 |
| Benzo[k]fluoranthene | BkF | 207-08-9 | 480 | 6.06 | 252.3 | 2B |
| Chrysene | Chr | 0218-01-09 | 448 | 5.9 | 228.3 | 2B |
| Dibenzo[a,h]anthracene | DBahA | 53-70-3 | 524 | 6.84 | 278.3 | 2A |
| Fluoranthene | Fa | 206-44-0 | 375 | 4.9 | 202.3 | 3 |
| Fluorene | Flr | 86-73-7 | 295 | 4.18 | 166.2 | 3 |
| Indeno[1,2,3-c,d]pyrene | Ind | 193-39-5 | 536 | 6.58 | 276.3 | 2B |
| Naphthalene | Nph | 91-20-3 | 218 | 3.29 | 128.2 | 2B |
| Phenanthrene | Phe | 85-01-8 | 340 | 4.45 | 178.2 | 3 |
| Pyrene | Pyr | 129-00-0 | 393 | 4.88 | 202.3 | 3 |
| Compounds | r2 | Linear Range (µg L−1) | Method LOD (µg kg−1) | Method LOQ (µg kg−1) | Repeatability Recovery (RSDr), % | Intermediate Precision Recovery (RSDpi), % | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spike Level (µg kg−1) | Spike Level (µg kg−1) | |||||||||
| 12 | 60 | 240 | 12 | 60 | 240 | |||||
| Acp | 0.9998 | 0.5–50 | 1.8 | 6 | 93 (13) | 94 (4) | 101 (3) | 85 (19) | 82 (10) | 87 (1) |
| Acy | 0.9999 | 0.5–50 | 1.8 | 6 | 110 (9) | 97 (2) | 100 (3) | 77 (14) | 83 (3) | 89 (2) |
| Ant | 0.9994 | 0.5–50 | 1.8 | 6 | 89 (19) | 98 (4) | 104 (2) | 79 (18) | 77 (5) | 77 (2) |
| AQ | 0.9995 | 1–50 | 3.6 | 12 | 95 (25) | 120 (7) | 107 (8) | 70 (59) | 74 (19) | 82 (10) |
| BaA | 0.9998 | 0.5–50 | 1.8 | 6 | 106 (20) | 96 (6) | 108 (4) | 97 (10) | 88 (5) | 88 (5) |
| BaP | 0.9991 | 0.5–50 | 1.8 | 6 | 105 (9) | 89 (9) | 105 (6) | 119 (8) | 84 (9) | 91 (6) |
| BbF | 0.9991 | 0.5–50 | 1.8 | 6 | 114 (10) | 98 (4) | 103 (4) | 120 (2) | 93 (2) | 90 (5) |
| BghiP | 0.9998 | 0.5–50 | 1.8 | 6 | 80 (16) | 89 (2) | 98 (3) | 116 (9) | 86 (3) | 94 (3) |
| BkF | 0.9996 | 0.5–50 | 1.8 | 6 | 116 (6) | 99 (4) | 108 (4) | 78 (17) | 87 (4) | 96 (8) |
| Chr | 0.9995 | 0.5–50 | 1.8 | 6 | 108 (14) | 85 (11) | 102 (3) | 95 (14) | 76 (3) | 77 (4) |
| DBahA | 0.9988 | 0.5–50 | 1.8 | 6 | 110 (12) | 89 (8) | 98 (3) | 117 (9) | 106 (10) | 111 (9) |
| Fa | 0.9998 | 0.5–50 | 1.8 | 6 | 110 (17) | 95 (5) | 97 (4) | 94 (16) | 80 (4) | 85 (5) |
| Flr | 0.9993 | 0.5–50 | 1.8 | 6 | 119 (1) | 96 (3) | 101 (4) | 78 (16) | 77 (6) | 84 (3) |
| Ind | 0.9996 | 0.5–50 | 1.8 | 6 | 118 (14) | 98 (6) | 105 (3) | 99 (20) | 94 (6) | 106 (6) |
| Nph | 0.9999 | 0.5–50 | 1.8 | 6 | 78 (12) | 97 (4) | 107 (2) | 103 (12) | 78 (7) | 82 (3) |
| Phe | 0.9998 | 0.5–50 | 1.8 | 6 | 101 (18) | 104 (5) | 107 (3) | 79 (16) | 70 (3) | 76 (3) |
| Pyr | 0.9997 | 0.5–50 | 1.8 | 6 | 120 (19) | 97 (3) | 101 (1) | 98 (16) | 74 (8) | 84 (2) |
| Sorbents or Salts | Chlorophyll a (664 nm) | Chlorophyll b (647 nm) | Total Phenols (765 nm) |
|---|---|---|---|
| C18 | 0.500 ± 0.013 b | 0.223 ± 0.008 a | 0.629 ± 0.037 ab |
| CaCl2 | 0.493 ± 0.017 b | 0.261 ± 0.032 a | 0.667 ± 0.123 ab |
| Florisil | 0.530 ± 0.016 ab | 0.251 ± 0.020 a | 0.736 ± 0.073 ab |
| PSA | 0.149 ± 0.024 c | 0.107 ± 0.023 b | 0.405 ± 0.060 b |
| Silica | 0.610 ± 0.023 a | 0.311 ± 0.018 a | 1.041 ± 0.166 a |
| Diatomaceous earth | 0.481 ± 0.014 b | 0.225 ± 0.019 a | 0.467 ± 0.037 b |
| Calcined diatomaceous earth | 0.502 ± 0.007 b | 0.243 ± 0.016 a | 0.394 ± 0.029 b |
| Compounds | Concentration in Commercial Yerba Mate Samples (µg kg−1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EV02 | EV03 | EV04 | EV05 | EV06 | EV07 | EV08 | EV09 | EV10 | EV12 | EV14 | EV16 | EV18 | EV20 | |
| Acy | n.d. | n.d. | <LOQ | <LOQ | <LOQ | <LOQ | 23.64 | n.d. | 38.33 | 16.81 | n.d. | <LOQ | n.d. | 16.17 |
| Ant | n.d. | n.d. | <LOQ | n.d. | n.d. | n.d. | 13.73 | n.d. | 12.47 | <LOQ | n.d. | n.d. | <LOQ | 9.99 |
| AQ | n.d. | 34.54 | <LOQ | <LOQ | n.d. | n.d. | 21.76 | n.d. | <LOQ | n.d. | n.d. | n.d. | 36.61 | 21.42 |
| BaA | n.d. | <LOQ | <LOQ | n.d. | n.d. | n.d. | <LOQ | n.d. | <LOQ | n.d. | n.d. | n.d. | <LOQ | <LOQ |
| BaP | <LOQ | <LOQ | <LOQ | <LOQ | n.d. | 7.02 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | n.d. | <LOQ | <LOQ |
| BbF | <LOQ | 12.96 | 6.96 | <LOQ | <LOQ | 7.36 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BghiP | n.d. | n.d. | n.d. | n.d. | n.d. | 11.42 | <LOQ | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. |
| BkF | n.d. | <LOQ | n.d. | n.d. | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Chr | n.d. | n.d. | <LOQ | n.d. | n.d. | <LOQ | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | <LOQ | <LOQ |
| Fa | n.d. | 8.30 | <LOQ | 11.54 | n.d. | 32.06 | 30.75 | n.d. | 29.46 | 10.11 | n.d. | n.d. | <LOQ | 16.82 |
| Flr | n.d. | n.d. | n.d. | n.d. | n.d. | 31.36 | n.d. | n.d. | 14.48 | n.d. | <LOQ | n.d. | n.d. | n.d. |
| Nph | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 8.61 | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. | <LOQ |
| Phe | n.d. | 30.90 | 18.42 | 11.74 | n.d. | 18.79 | 81.31 | n.d. | 53.60 | 19.79 | n.d. | n.d. | 17.47 | 63.79 |
| Pyr | n.d. | <LOQ | <LOQ | 12.71 | n.d. | 36.66 | 23.44 | n.d. | 21.94 | <LOQ | n.d. | n.d. | n.d. | 9.28 |
| ∑PAHs | 0 | 86.70 | 25.37 | 35.99 | 0 | 144.67 | 203.24 | 0 | 170.28 | 46.70 | 0 | 0 | 54.08 | 137.48 |
| Compounds | Concentration Found in Yerba Mate Samples at Different Stages of Processing (µg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| YM03 | YM04 | YM05 | YM06 | YM07 | YM08 | YM09 | YM10 | YM11 | |
| Ant | n.d. | n.d. | <LOQ | n.d. | n.d. | 8.35 | 6.68 | n.d. | n.d. |
| AQ | 28.95 | n.d. | n.d. | n.d. | n.d. | 20.13 | 18.56 | n.d. | n.d. |
| BaA | <LOQ | n.d. | 3.42 | n.d. | n.d. | <LOQ | 6.71 | 9.07 | n.d. |
| BaP | 14.98 | 11.99 | 11.23 | <LOQ | 10.23 | 9.82 | 14.10 | 49.94 | 13.51 |
| BbF | 7.26 | <LOQ | 11.20 | n.d. | n.d. | <LOQ | 11.50 | 41.92 | 8.96 |
| BghiP | n.d. | 2.57 | n.d. | n.d. | 1.15 | n.d. | n.d. | 31.64 | 0.49 |
| BkF | 16.23 | <LOQ | 10.28 | <LOQ | <LOQ | <LOQ | 12.66 | 46.50 | 13.95 |
| Chr | <LOQ | n.d. | 11.27 | n.d. | n.d. | <LOQ | 16.87 | 15.21 | n.d. |
| Fa | 40.10 | 52.87 | 49.46 | 67.27 | 70.08 | 33.59 | 57.88 | n.d. | n.d. |
| Ind | <LOQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 23.35 | n.d. |
| Nph | n.d. | n.d. | <LOQ | n.d. | n.d. | n.d. | <LOQ | <LOQ | 7.09 |
| Phe | 26.89 | 12.76 | 91.14 | 49.47 | 47.24 | 76.53 | 113.16 | n.d. | n.d. |
| Pyr | 48.24 | 79.37 | 59.58 | 92.53 | 99.66 | 44.21 | 65.25 | n.d. | n.d. |
| ∑PAHs | 182.64 | 159.56 | 247.57 | 209.27 | 228.36 | 192.64 | 323.36 | 217.63 | 44.00 |
| ∑4PAHs | 22.24 | 11.99 | 37.12 | 0.00 | 10.23 | 9.82 | 49.18 | 116.14 | 22.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, D.M.; da Silva, J.D.; de Souza, I.F.; Prates, G.A.B.; Dutra, V.A.; Prestes, O.D.; Zanella, R. Simultaneous Determination of Polycyclic Aromatic Hydrocarbons and Anthraquinone in Yerba Mate by Modified MSPD Method and GC-MS. Separations 2025, 12, 240. https://doi.org/10.3390/separations12090240
Hoffmann DM, da Silva JD, de Souza IF, Prates GAB, Dutra VA, Prestes OD, Zanella R. Simultaneous Determination of Polycyclic Aromatic Hydrocarbons and Anthraquinone in Yerba Mate by Modified MSPD Method and GC-MS. Separations. 2025; 12(9):240. https://doi.org/10.3390/separations12090240
Chicago/Turabian StyleHoffmann, Dylan M., José D. da Silva, Igor F. de Souza, Gabriel A. B. Prates, Vagner A. Dutra, Osmar D. Prestes, and Renato Zanella. 2025. "Simultaneous Determination of Polycyclic Aromatic Hydrocarbons and Anthraquinone in Yerba Mate by Modified MSPD Method and GC-MS" Separations 12, no. 9: 240. https://doi.org/10.3390/separations12090240
APA StyleHoffmann, D. M., da Silva, J. D., de Souza, I. F., Prates, G. A. B., Dutra, V. A., Prestes, O. D., & Zanella, R. (2025). Simultaneous Determination of Polycyclic Aromatic Hydrocarbons and Anthraquinone in Yerba Mate by Modified MSPD Method and GC-MS. Separations, 12(9), 240. https://doi.org/10.3390/separations12090240











