Ion-Exchange Membrane Permselectivity: Experimental Evaluation of Concentration Dependence, Ionic Species Selectivity, and Temperature Response
Abstract
1. Introduction
2. Experimental System and Materials
2.1. Experimental System
2.2. Solutions
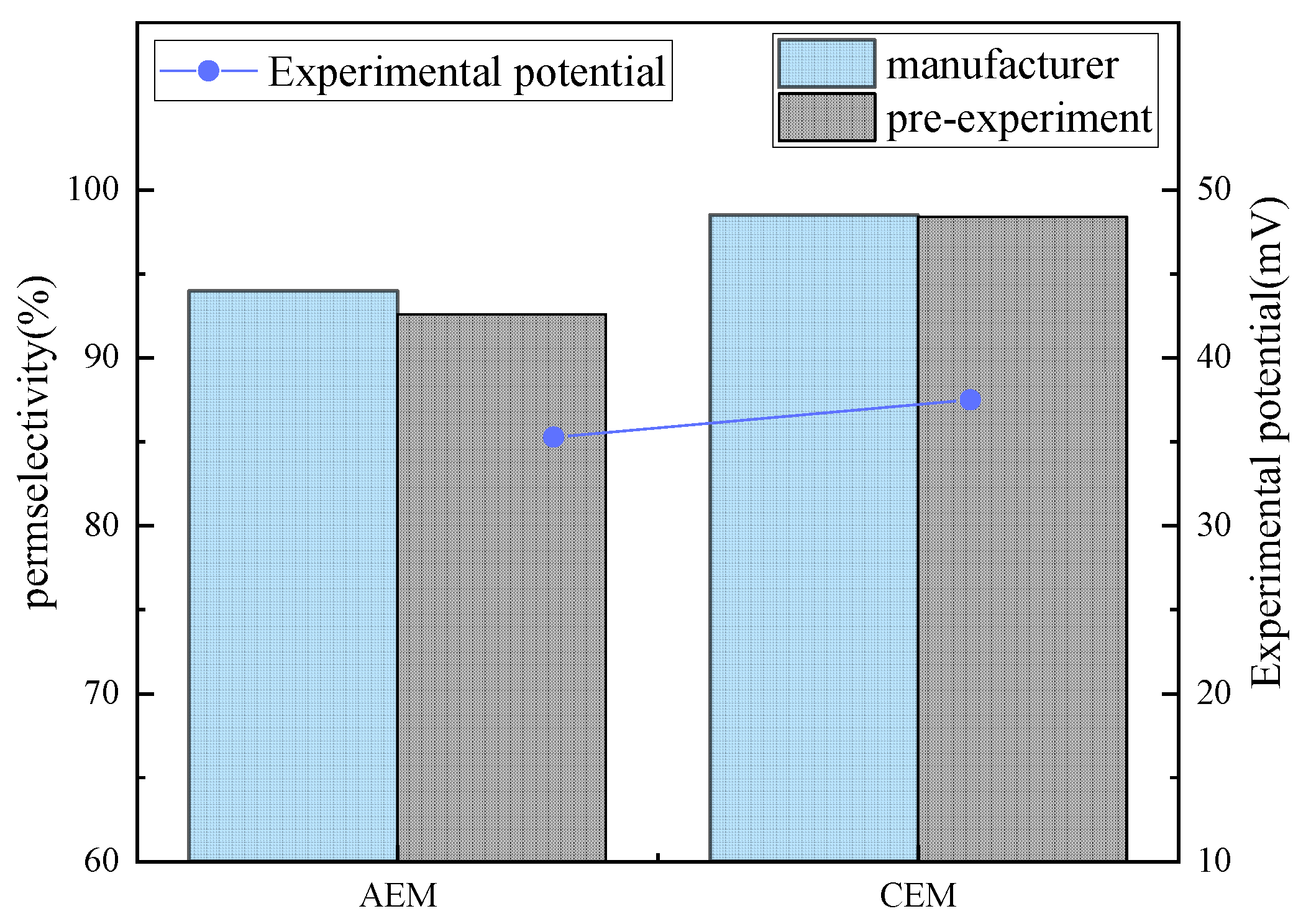
2.3. Pre-Experimental Result
3. Results and Analysis
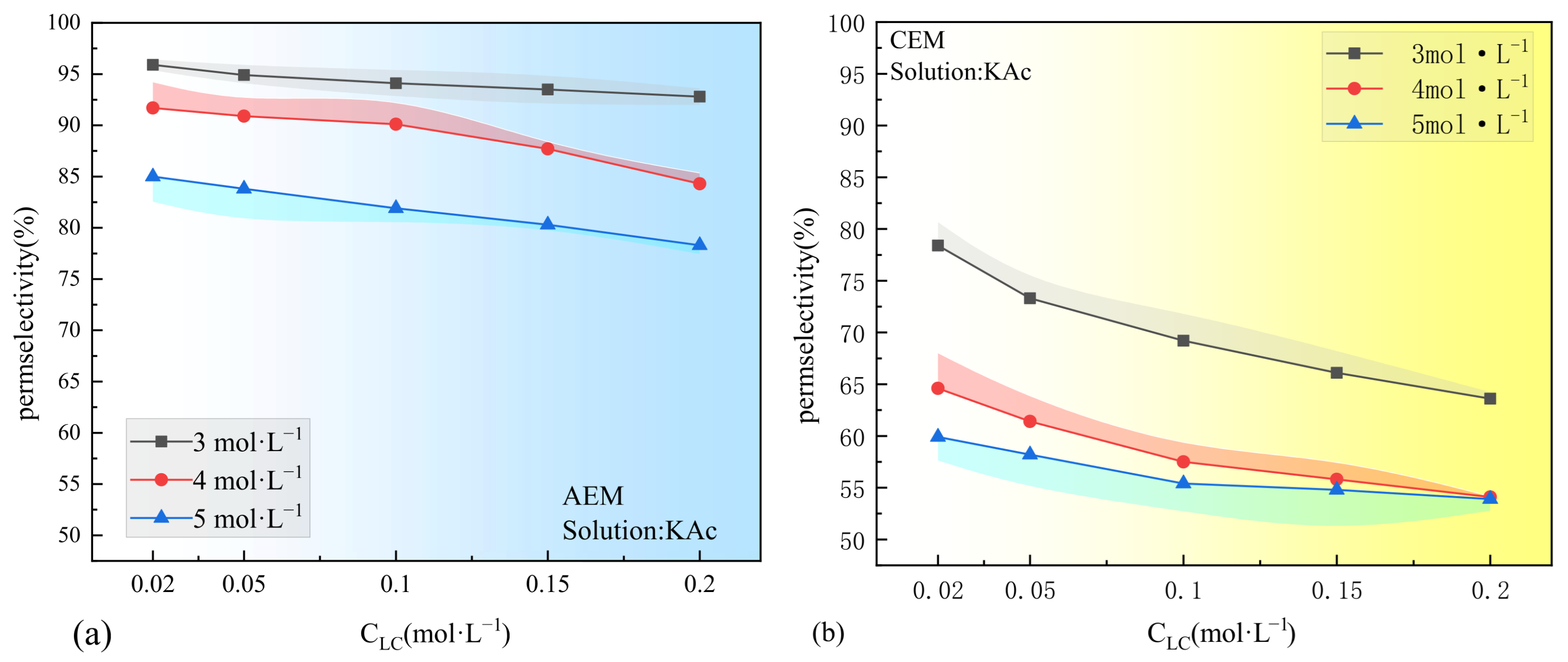
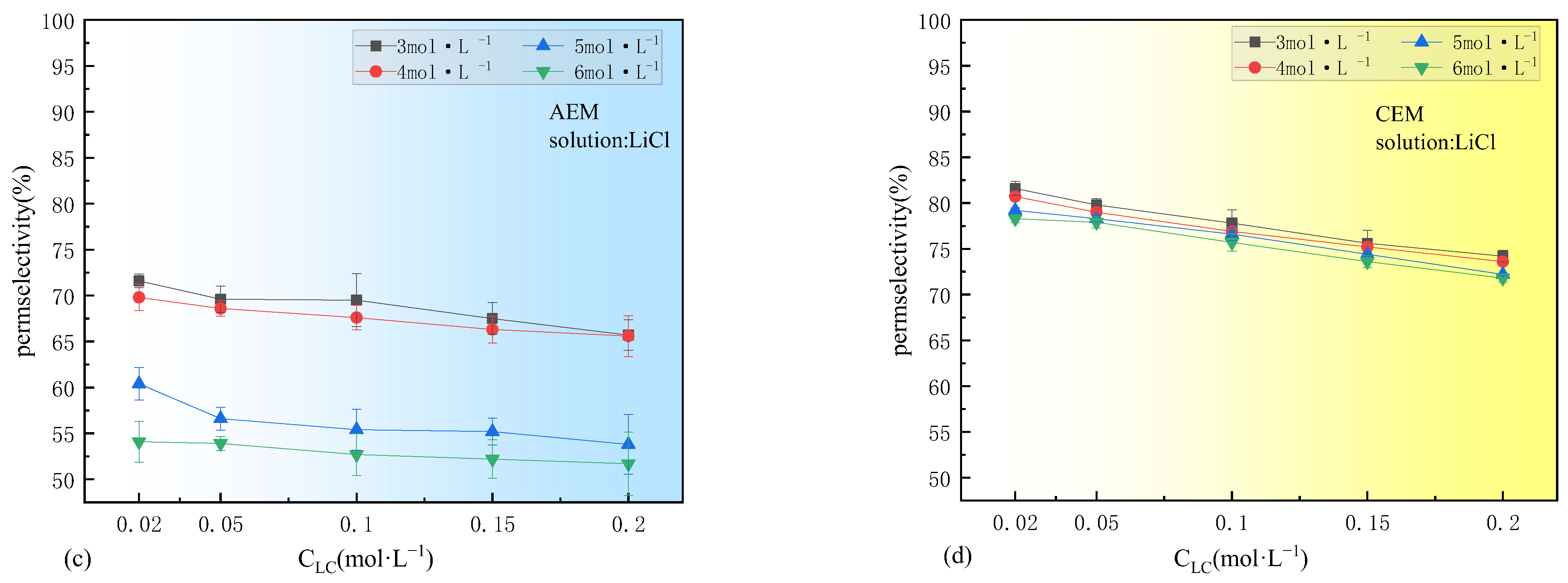
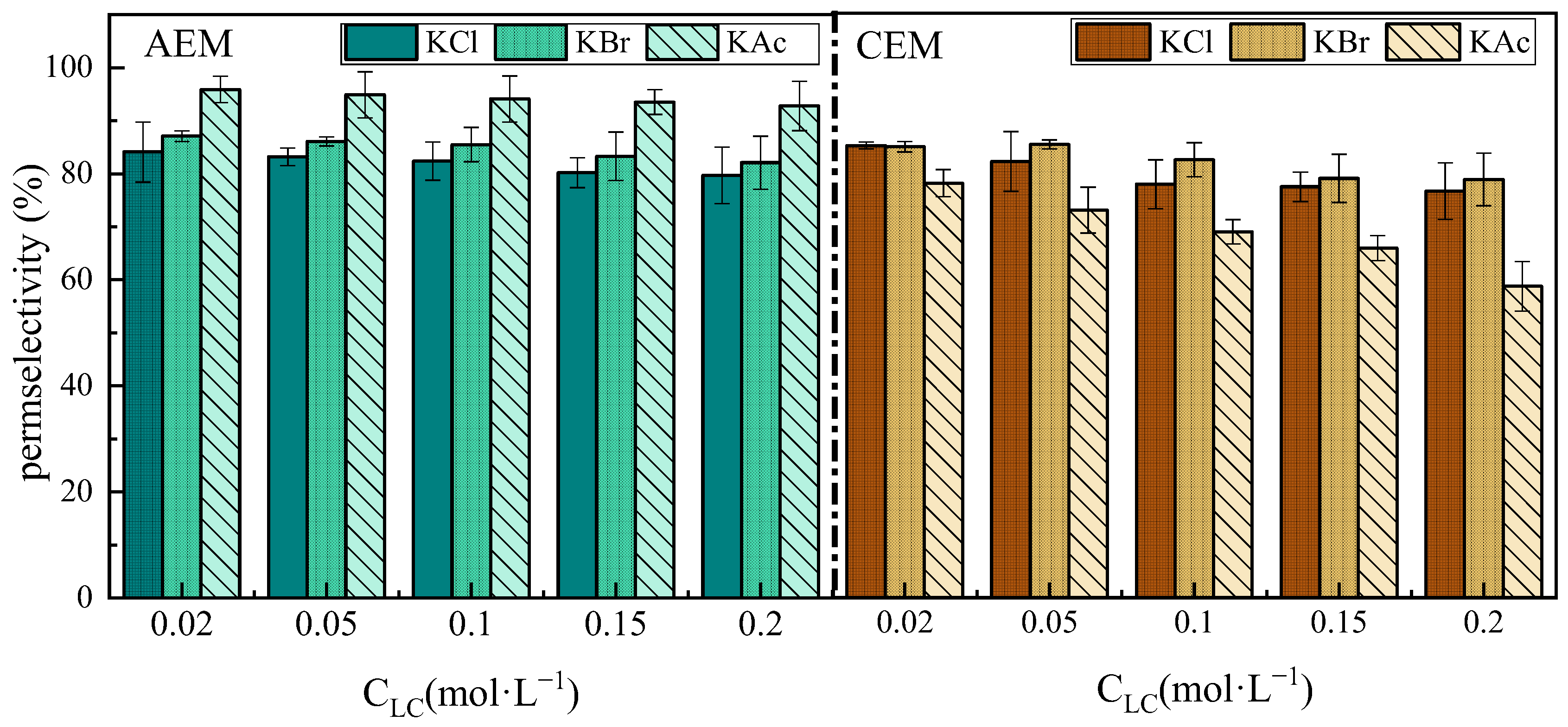
3.1. Concentration Dependence of Permselectivity
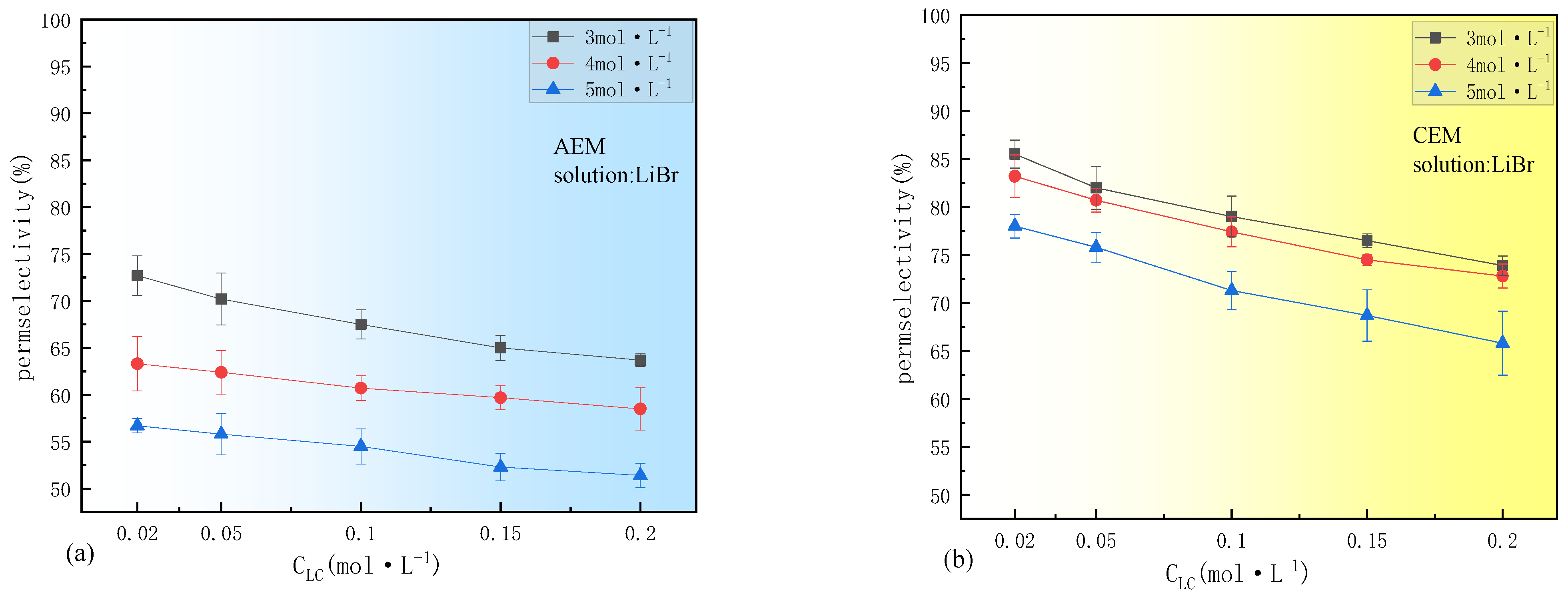
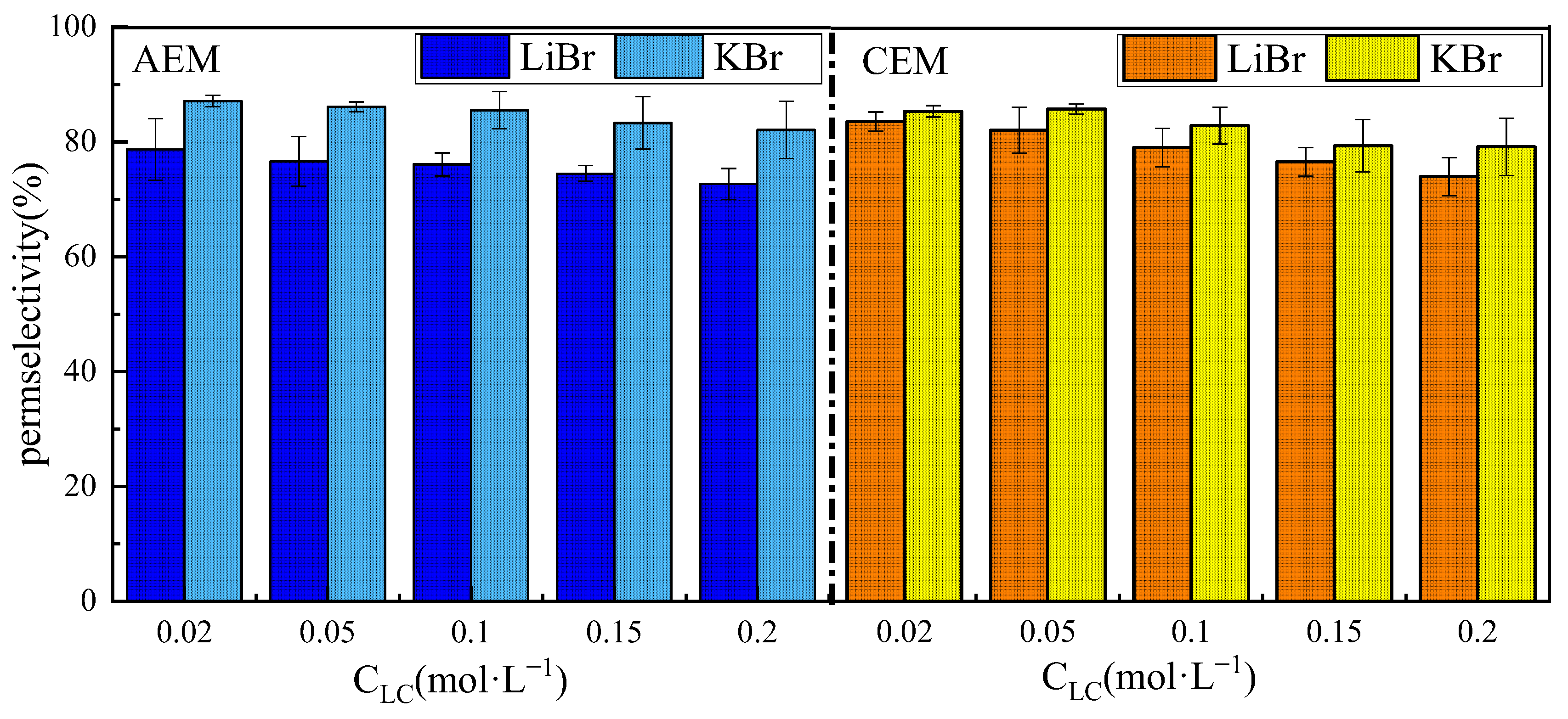
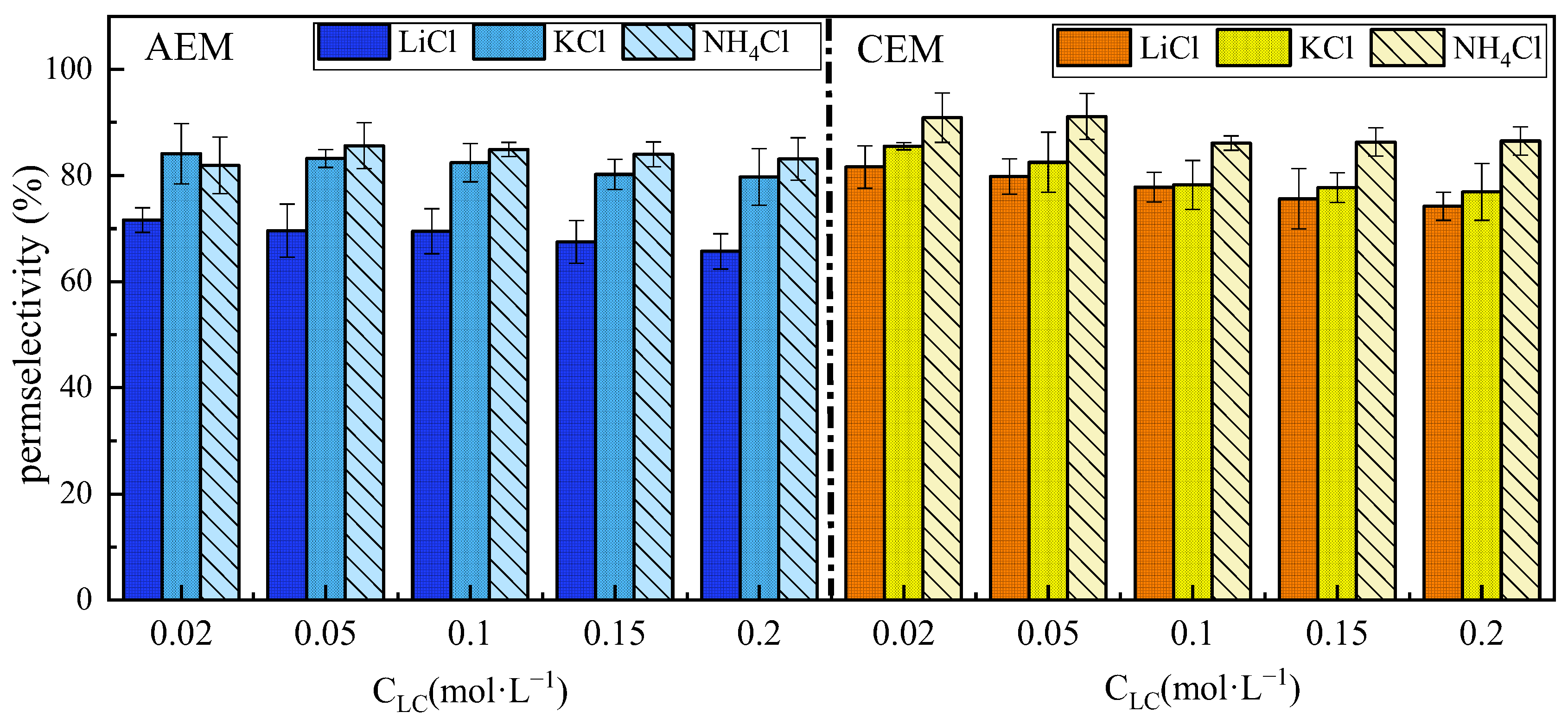
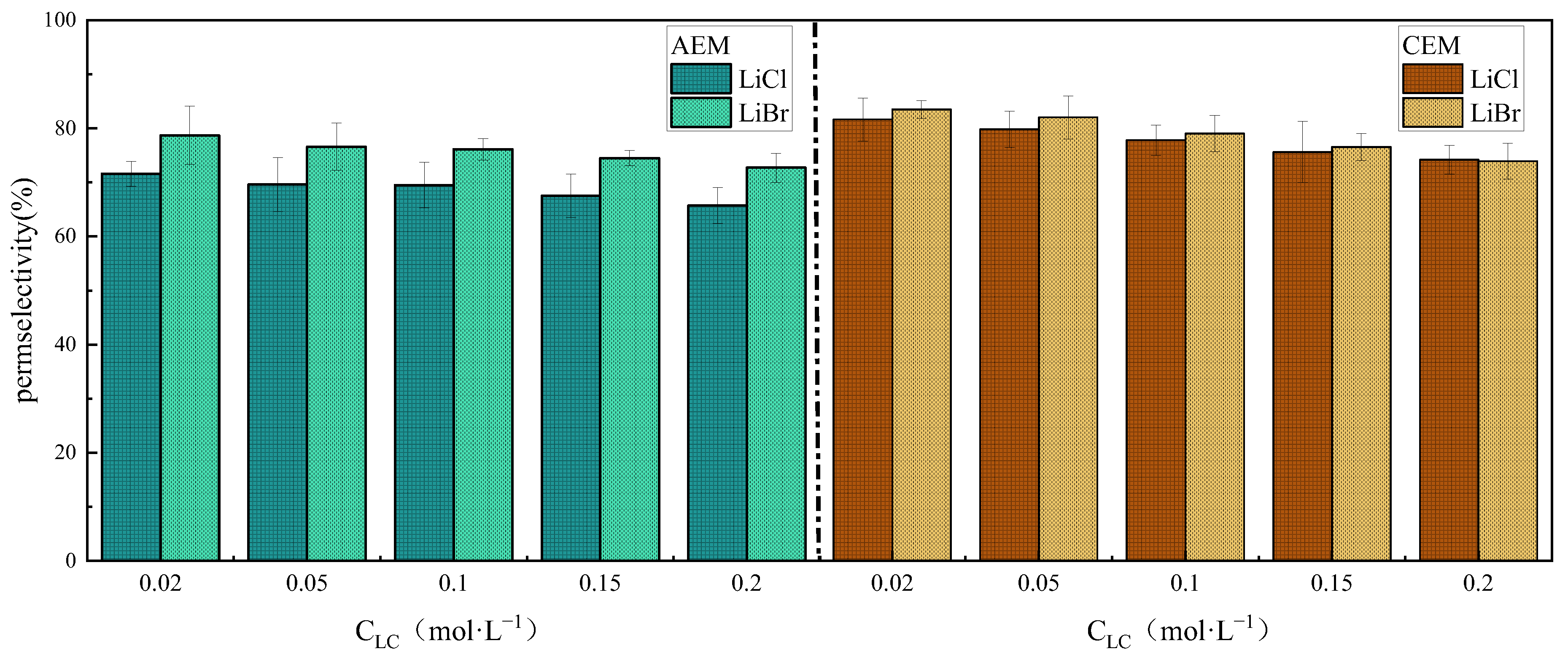
3.2. Ion Species Dependence of Permselectivity
3.2.1. The Effect of Cations
3.2.2. The Effect of Anions
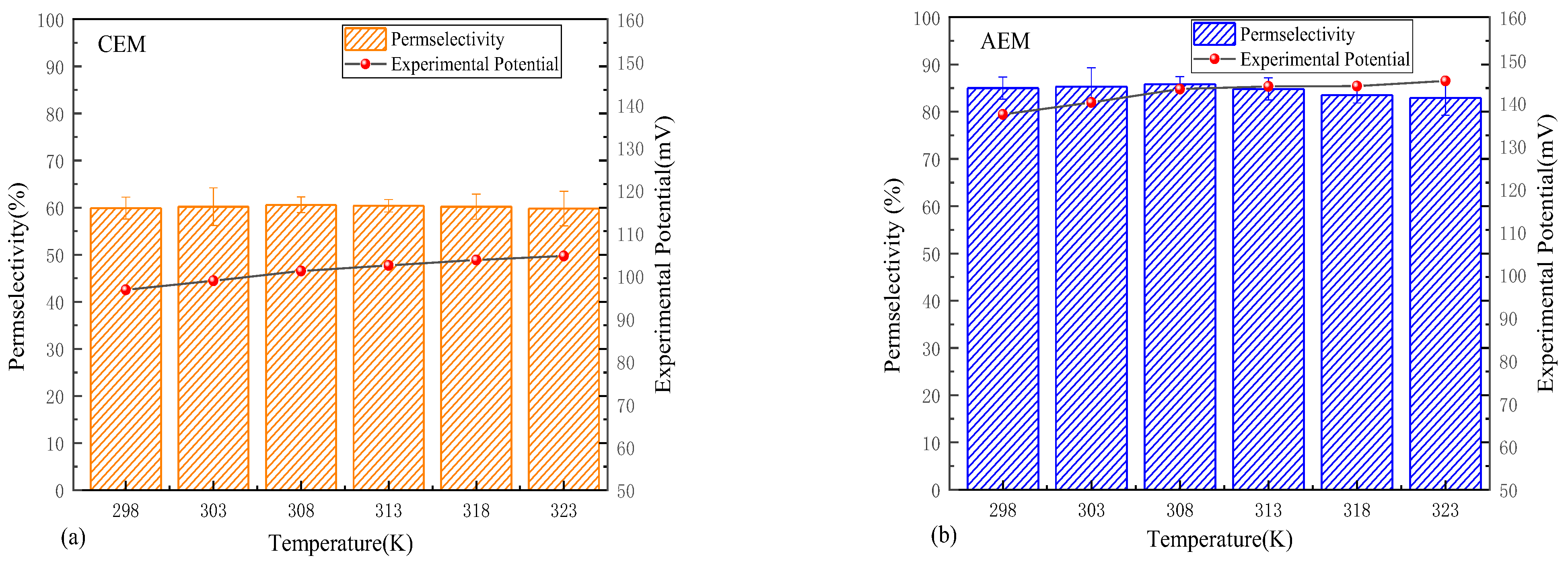
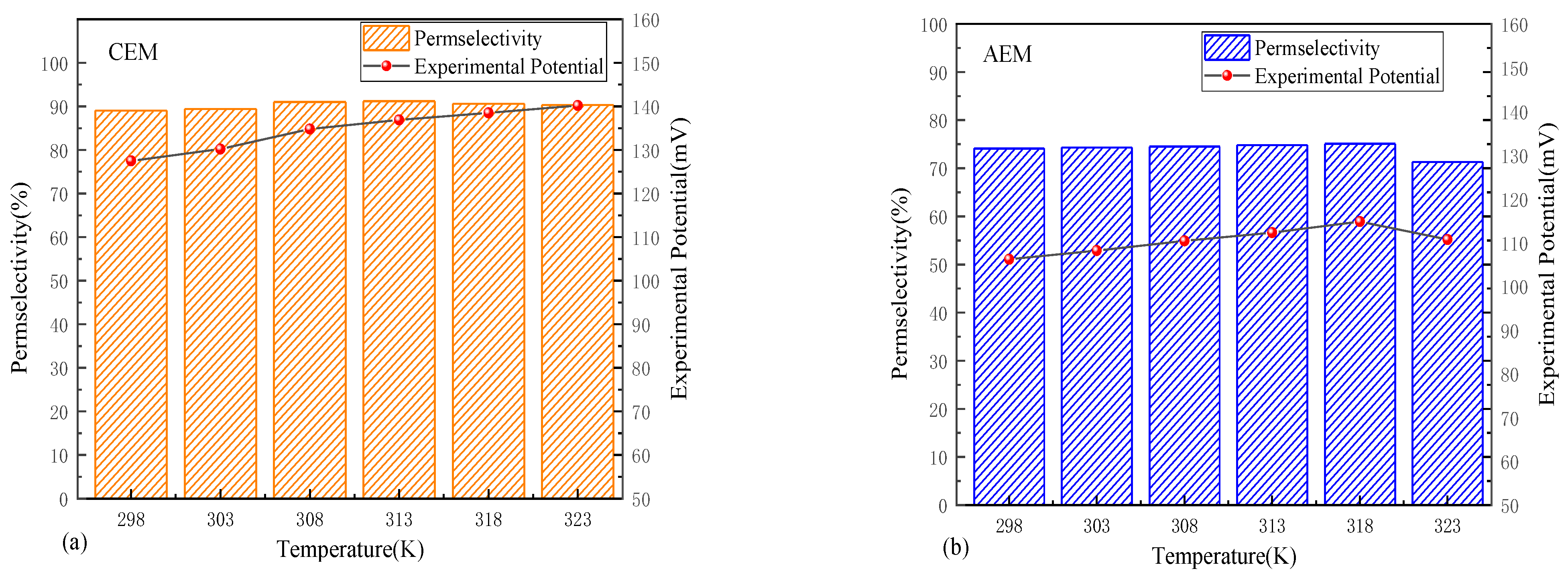
3.3. Temperature Dependence of Permselectivity
4. Conclusions
- (1)
- The permselectivity of IEMs significantly decreases with the increase in concentration of either the dilute solutions (0.02–0.2 M) or the concentrated solutions (3–5 M). This decline is attributed to the weakening of the Donnan effects and water transport. If ions with large hydration radii and low mobility aggregate in the membrane boundary layer on the concentrated solution side, their corresponding oppositely charged ions are significantly affected by the increase in the concentrated solution;
- (2)
- The effect of ion species on permselectivity is closely related to the hydration radius and hydration energy. Ions with lower hydration energy can more effectively remove surrounding water molecules, resulting in less transmembrane transport resistance and higher permselectivity. Ions with a higher charge density exhibit poorer permselectivity due to tighter binding with membrane fixed groups during ionic transport. Ions with low mobility or diffusion coefficients are strongly affected by concentration polarization, which impairs their selective permeability and that of their counterions;
- (3)
- Under moderate solution temperature conditions, the thermal effects on permselectivity are minimal, with ion thermodynamic properties predominantly determining performance. The permselectivity increases slightly with a rise in temperature. However, when the temperature exceeds 318 K, membrane deterioration becomes dominant, significantly reducing the permselectivity of IEMs. Therefore, an optimal operating temperature range exists.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclature | |||
| Symbols | Abbreviations | ||
| C | Concentration, mol⋅L−1 | AEMs | Anion-exchange membranes |
| F | Faraday constant, 96,485 C⋅mol−1 | AGDD | Air-gap diffusion distillation |
| M | Molarity, mol⋅L−1 | ARS | Absorption refrigeration system |
| R | Gas constant, 8.314 J⋅mol−1⋅K−1 | CEMs | Cation-exchange membranes |
| T | Kelvin temperature, K | ED | Electrodialysis |
| z | Ion valence | FCD | Fixed charge density |
| ΔEexp | Measured membrane potential | HC | High concentration |
| ΔEth | Theoretical membrane potential | IEMs | Ion-exchange membranes |
| γ | Activity coefficient | KAc | Potassium acetate |
| LC | Low concentration | ||
| PMMA | Polymethyl methacrylate | ||
| RED | Reverse electrodialysis | ||
| REDHE | Reverse-electrodialysis heat engine | ||
| SGE | Salinity gradient energy | ||
References
- Fan, H.; Yip, N.Y. Elucidating conductivity-permselectivity tradeoffs in electrodialysis and reverse electrodialysis by structure-property analysis of ion-exchange membranes. J. Membr. Sci. 2019, 573, 668–681. [Google Scholar] [CrossRef]
- Xi, J.; Ming, H.; Liu, S.; Shen, X.; Geng, C.; Gao, W.; Meng, J.; Gao, Y.; Zhao, Z.; Lv, J.; et al. Effect of anion-exchange membrane type for FCDI performance at different concentrations. Environ. Technol. 2022, 44, 3585–3591. [Google Scholar] [CrossRef]
- Wenten, I.G.; Bazant, M.Z.; Khoiruddin, K. Mitigating electrodialysis membrane fouling in seawater desalination. Sep. Purif. Technol. 2024, 345, 127228. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Zhang, T.; Sun, S. Study of the Seawater Desalination Performance by Electrodialysis. Membranes 2022, 12, 767. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Hulme, A.M.; Davey, C.; Tyrrel, S.; Pidou, M.; McAdam, E. Transitioning from electrodialysis to reverse electrodialysis stack design for energy generation from high concentration salinity gradients. Energy Convers. Manag. 2021, 244, 114493. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.W.; Sun, D.X.; Lv, Y.; Liu, M.; Zhu, X. Enhancement of power density and hydrogen productivity of the reverse electrodialysis process by optimizing the temperature gradient between the working solutions. Chem. Eng. J. 2024, 498, 155385. [Google Scholar] [CrossRef]
- Gubari, M.Q.; Zwain, H.M.; Alekseeva, N.V.; Baziyani, G.I. Features of feed concentration and temperature effects on membranes operation in electrodialysis systems–a review. J. Phys. Conf. Ser. 2021, 1973, 012178. [Google Scholar] [CrossRef]
- Geise, G.M.; Cassady, H.J.; Paul, D.R.; Logan, B.E.; Hickner, M.A. Specific ion effects on membrane potential and the permselectivity of ion exchange membranes. Phys. Chem. Chem. Phys. 2014, 16, 21673–21681. [Google Scholar] [CrossRef]
- Dlugolecki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Dong, F.; Xu, S.; Wu, X.; Jin, D.; Wang, P.; Wu, D.; Leng, Q. Cross-linked poly (vinyl alcohol)/sulfosuccinic acid (PVA/SSA) as cation exchange membranes for reverse electrodialysis. Sep. Purif. Technol. 2021, 267, 118629. [Google Scholar] [CrossRef]
- Daniilidis, A.; Vermaas, D.A.; Herber, R.; Nijmeijer, K. Experimentally obtainable energy from mixing river water, seawater or brines with reverse electrodialysis. Renew. Energy 2014, 64, 123–131. [Google Scholar] [CrossRef]
- Jin, D.; Xi, R.; Xu, S.; Wang, P.; Wu, X. Numerical simulation of salinity gradient power generation using reverse electrodialysis. Desalination 2021, 512, 115132. [Google Scholar] [CrossRef]
- Tedesco, M.; Brauns, E.; Cipollina, A.; Micale, G.; Modica, P.; Russo, G.; Helsen, J. Reverse Electrodialysis with saline waters and concentrated brines: A laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. [Google Scholar] [CrossRef]
- Zlotorowicz, A.; Strand, R.V.; Burheim, O.S.; Wilhelmsen, Ø.; Kjelstrup, S. The permselectivity and Water Transference Number of Ion Exchange Membranes in Reverse Electrodialysis. J. Membr. Sci. 2017, 523, 402–408. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Coronell, O. Modeling and validation of concentration dependence of ion exchange membrane permselectivity: Significance of convection and Manning’s counter-ion condensation theory. J. Membr. Sci. 2020, 620, 118411. [Google Scholar] [CrossRef]
- Zimmermann, P.; Splberg, S.B.; Tekinalp, Ö.; Lamb, J.J.; Wilhelmsen, Ø.; Deng, L.; Burheim, O.S. Heat to Hydrogen by RED—Reviewing Membranes and Salts for the RED Heat Engine Concept. Membranes 2021, 12, 48. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.V.M.; Biesheuvel, P.M. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.V.M.; Biesheuvel, P.M. Nernst-Planck transport theory for (reverse) electrodialysis: III. Optimal membrane thickness for enhanced process performance. J. Membr. Sci. 2018, 565, 480–487. [Google Scholar] [CrossRef]
- Jovan, K.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Ion Diffusion Coefficients in Ion Exchange Membranes: Significance of Counterion Condensation. Macromolecules 2018, 51, 5519–5529. [Google Scholar] [CrossRef]
- Kamcev, J.; Sujanani, R.; Jang, E.S.; Yan, N.; Moe, N.; Paul, D.R.; Freeman, B.D. Salt concentration dependence of ionic conductivity in ion exchange membranes. J. Membr. Sci. 2018, 547, 123–133. [Google Scholar] [CrossRef]
- Zhang, B.; Hong, J.G.; Xie, S.; Xia, S.; Chen, Y. An Integrative Modeling and Experimental Study on the Ionic Resistance of Ion-Exchange Membranes. J. Membr. Sci. 2016, 524, 362–369. [Google Scholar] [CrossRef]
- Giacalone, F.; Olkis, C.; Santori, G.; Cipollina, A.; Brandani, S.; Micale, G. Novel solutions for closed-loop reverse electrodialysis: Thermodynamic characterisation and perspective analysis. Energy 2019, 166, 674–689. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Xu, S.; Gong, Y.; Yang, S.; Jin, D. Performance of a Reverse Electrodialysis Cell Working with Potassium Acetate Methanol Water Solution. Energy 2021, 232, 120944. [Google Scholar] [CrossRef]
- Wu, X.; Ren, Y.; Zhang, Y.; Xu, S.; Yang, S. Hydrogen production from water electrolysis driven by the membrane voltage of a closed-loop reverse electrodialysis system integrating air-gap diffusion distillation technology. Energy Convers. Manag. 2022, 268, 115974. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, D.; Guo, H.; Zhang, J.; Tao, S.; Chen, R.; Chen, L.; Gong, M. Experimental study and prospect analysis of LiBr-H2O reverse electrodialysis heat engine. Appl. Energy 2023, 350, 121791. [Google Scholar] [CrossRef]
- Tamburini, A.; Tedesco, M.; Cipollina, A.; Micale, G.; Ciofalo, M.; Papapetrou, M.; Van Baak, W.; Piacentino, A. Reverse electrodialysis heat engine for sustainable power production. Appl. Energy 2017, 206, 1334–1353. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, H.; Geise, G. Specific co-ion sorption and diffusion properties influence membrane permselectivity. J. Membr. Sci. 2018, 563, 492–504. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Flotron, S.; Zhu, S.; Call, D.F.; Coronell, O. Junction Potentials Bias Measurements of Ion Exchange Membrane Permselectivity. Environ. Sci. Technol. 2018, 52, 4929–4936. [Google Scholar] [CrossRef]
- Hamer, W.J.; Wu, Y.C. Osmotic Coefficients and Mean Activity Coefficients of Uni-univalent Electrolytes in Water at 25 °C. J. Phys. Chem. Ref. Data 1972, 1, 1047–1100. [Google Scholar] [CrossRef]
- Bauer, B.; Strathmann, H.; Effenberger, F. Anion-exchange membranes with improved alkaline stability. Desalination 1990, 79, 125–144. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 6.—The standard partial molar volumes of aqueous ions at 298.15 K. J. Chem. Soc. Faraday Trans. 1993, 89, 713–718. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Elsevier: Heidelberg, Germany, 2004. [Google Scholar]
- Skolotneva, E.; Tsygurina, K.; Mareev, S.; Melnikova, E.; Pismenskaya, N.; Nikonenko, V. High Diffusion Permeability of Anion-Exchange Membranes for Ammonium Chloride: Experiment and Modeling. Int. J. Mol. Sci. 2022, 23, 5782. [Google Scholar] [CrossRef]
- Pan, L.Q.; Zheng, Q.N.; Feng, Q.H.; Shen, Y.-B.; Hu, W.-Y.; Cao, C.-F.; Zhang, G.-D.; Gao, J.-F.; Song, P.; Shi, Y.-Q.; et al. Hydrophobic silicone modified membranes for efficient oil/water separation: Synthesis, fabrication and application. Sep. Purif. Technol. 2025, 353, 128485. [Google Scholar] [CrossRef]
- Le, X.T. Permselectivity and microstructure of anion exchange membranes. J. Colloid Interface Sci. 2008, 325, 215–222. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Manzanares, J.A.; Nikonenko, V.V.; Lebedev, K.; Lovtsov, E. Space charge effect on competitive ion transport through ion-exchange membranes. Desalination 2002, 147, 387–392. [Google Scholar] [CrossRef]
- Karimi, L.; Ghassemi, A. How Operational Parameters and Membrane Characteristics Affect the Performance of Electrodialysis Reversal Desalination Systems: The State of the Art. J. Membr. Sci. Res. 2016, 2, 111–117. [Google Scholar]
- Zelovich, T.; Vogt-Maranto, L.; Simari, C. Non-Monotonic Temperature Dependence of Hydroxide Ion Diffusion in Anion Exchange Membranes. Chem. Mater. 2022, 34, 2133–2145. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Xu, H.; Shen, Q.; Sun, Y.; Liu, F.; Zhang, Y. Temperature dependence of water and salt transport in concentration gradient batteries: Insight from membrane properties. J. Membr. Sci. 2025, 713, 123382. [Google Scholar] [CrossRef]
- Jia, Y.W.; Chen, G.Q.; Kentish, S.E. Investigating the effect of temperature and concentration on the performance of reverse electrodialysis systems. Desalination 2024, 592, 118184. [Google Scholar] [CrossRef]

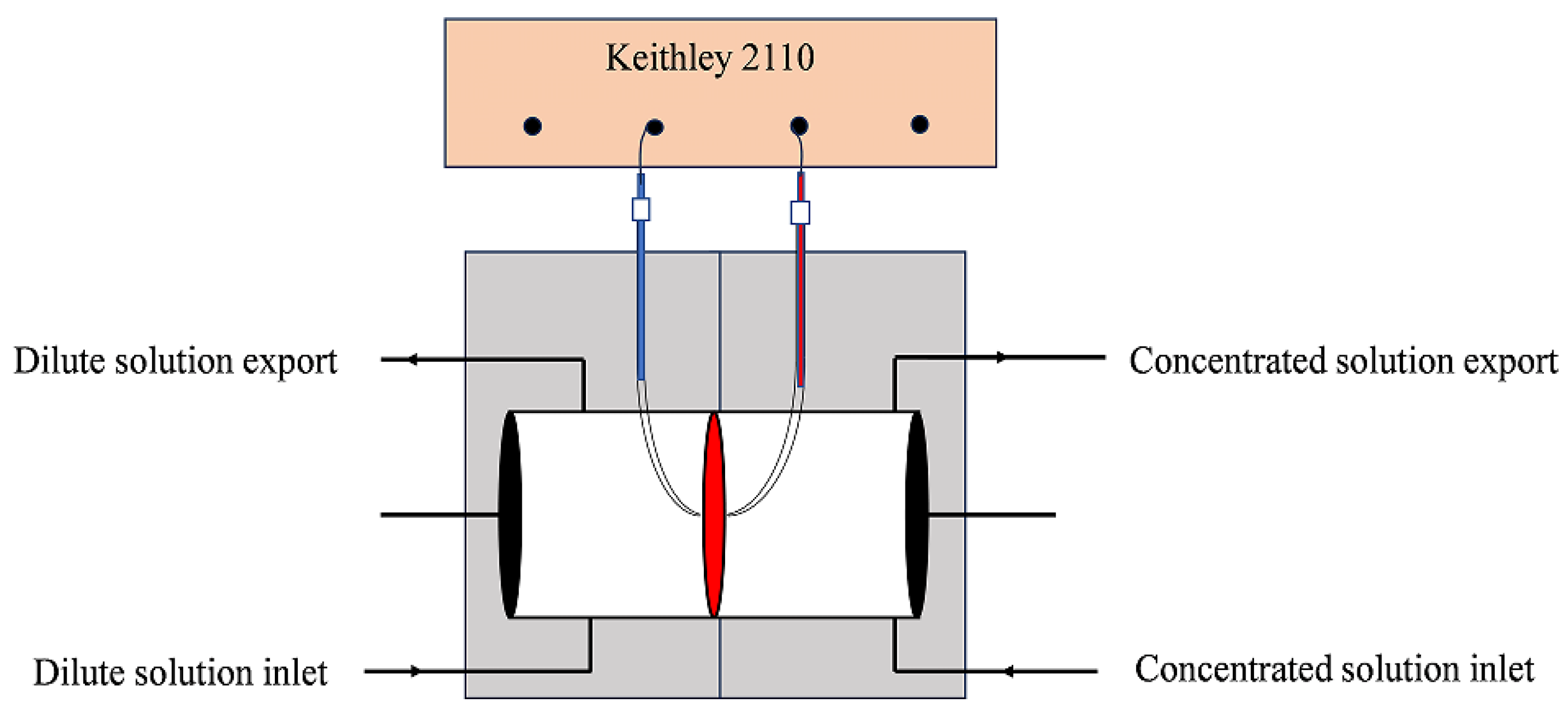
| Equipment | Model | Range | Accuracy |
|---|---|---|---|
| HC & LC peristaltic pumps (Longer Precision Pump Co., Ltd., Baoding, China) | BT300-2 J | 0–300 rpm | 0.1 rpm |
| Electronic balance (Changshu Shuangjie Testing Instrument Factory, Changshu, China) | JJ-1023BC | 0–1020 g | ±0.001 g |
| Magnetic stirrer (ASONE CORPORATION, Osaka, Japan) | RS-6DN | – | – |
| Conductivity meter (METTLER TOLEDO International Inc., Zurich, Switzerland) | Mettler 5 Easy Plus | 0~500 mS·cm−1 | ±0.5% |
| Digital multimeter (Keithley Instruments Inc., Cleveland, OH, USA) | Keithley 2110 | 1 μV–750 V | 120 ppm |
| Thermostatic water tank (JULABO Labortechnik GmbH, Seelbach, Germany) | Julabo Vivo iTherm-B5 | 20–95 °C | ±0.05 °C |
| Reference electrode (Shanghai Yueci Electronic Technology Co., Ltd., Shanghai, China) | R303, Ag/AgCl | - | ±2.5 mV |
| Parameter | Value |
|---|---|
| Length | 101.50 mm |
| Width | 80.43 mm |
| Height | 102.12 mm |
| Luggin capillary–membrane distance | 1 mm |
| Flow rate | 1.38 cm/s |
| Membrane Type | δ/μm | SD/% | AR/Ω·cm2 | IEC/meq·g−1 |
|---|---|---|---|---|
| AEM-Type 10 | 125 | 23 | 1.7 | 1.5 |
| CEM-Type 10 | 135 | 21 | 2.0 | 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Zhu, X.; Wu, X.; Guan, H. Ion-Exchange Membrane Permselectivity: Experimental Evaluation of Concentration Dependence, Ionic Species Selectivity, and Temperature Response. Separations 2025, 12, 207. https://doi.org/10.3390/separations12080207
Lv J, Zhu X, Wu X, Guan H. Ion-Exchange Membrane Permselectivity: Experimental Evaluation of Concentration Dependence, Ionic Species Selectivity, and Temperature Response. Separations. 2025; 12(8):207. https://doi.org/10.3390/separations12080207
Chicago/Turabian StyleLv, Junyi, Xiaojing Zhu, Xi Wu, and Hongfei Guan. 2025. "Ion-Exchange Membrane Permselectivity: Experimental Evaluation of Concentration Dependence, Ionic Species Selectivity, and Temperature Response" Separations 12, no. 8: 207. https://doi.org/10.3390/separations12080207
APA StyleLv, J., Zhu, X., Wu, X., & Guan, H. (2025). Ion-Exchange Membrane Permselectivity: Experimental Evaluation of Concentration Dependence, Ionic Species Selectivity, and Temperature Response. Separations, 12(8), 207. https://doi.org/10.3390/separations12080207







