Abstract
A previously synthesized and functionalized magnetic glycidyl methacrylate-based nanocomposite, mPGMT-deta, was tested as a sorbent for Re(VII) oxoanions in Mo(VI)-containing solutions. The effect of pH on the removal efficiency and the separation factor was examined in the range of 2 to 9. A maximum separation factor (βRe/Mo) of 8.85 was observed at pH 6. The nature of rhenium oxoanions binding to the active sites of mPGMT-deta was analyzed using density functional theory (DFT). The calculations indicated that the formation of MoO42−//hedetaH22+ adduct is electrostatically favored at pH 6, while the inclusion of solvation effects makes the formation of ReO4−//hedetaH22+ adduct thermodynamically more favorable. Solvation played a dominant role in determining the selectivity of oxoanion sorption to the nanocomposite. The adsorption isotherm, kinetics, and thermodynamics of Re(VII) onto mPGMT-deta were determined. The equilibrium data were best-fitted using the Langmuir adsorption model (R2 = 0.999), with a maximum sorption capacity for Re(VII) of 0.43 mmol/g. The uptake kinetics of the sorption process obeyed the pseudo-second-order model, with the influence of diffusion and external mass transfer. Based on the thermodynamic parameters, Re(VII) sorption was spontaneous and endothermic.
1. Introduction
Rhenium (Re), a rare and critical metal with exceptional physical and chemical properties (such as a high melting point), frequently occurs in industrial wastewater from urban mining and mine drainage [1]. Re is used as a catalyst [2,3], a heat-resistant superalloy for the production of aerospace and electronics [4], and in radiomedical diagnosis [5]. Due to its unique performance and limited availability, replacing Re in industrial applications is highly demanding. Restricted natural reserves of Re pose serious obstacles in ensuring its long-term availability. Consequently, there has been considerable interest in the separation and purification of Re-rich ores and end-of-life devices [6,7]. Rhenium is not found in independent mineral ores either in rocks or in soils; instead, it is predominantly associated with molybdenum (Mo), which is often found in pegmatitic rocks, and sometimes occurs with other base metals such as copper and zinc [8,9]. The challenging separation of Mo and Re is also a consequence of their similar chemical behavior, such as similar ionic radii and anionic species [5,10]. In particular, the extremely acidic conditions in wastewater and various coexisting metal ions pose a major challenge for the removal of Re from wastewater [11]. Therefore, the development of advanced materials capable of enhancing the efficiency of rhenium separation and purification from secondary sources is essential to ensure a sustainable supply and to minimize environmental impact [12].
The separation and recovery of Re from Mo-Re solution have been discussed in the literature using various techniques, such as membrane techniques [13], chemical precipitation [14], ion exchange [15], solvent extraction [16] or adsorption [12,17]. Among these, adsorption stands out as an effective method for the separation of rhenium due to its simplicity, cost-effectiveness, and high selectivity [18,19]. The adsorbents investigated for rhenium removal from aqueous solutions include metal oxide composites [20], metal–organic frameworks [21], graphene oxides [22], ion exchange resins [23], macroporous polymers [24], and modified biomaterials [25].
Owing to the presence of reactive epoxy groups that change the surface chemistry and the ability to react with different nucleophiles, glycidyl methacrylate (GMA)-based macroporous copolymers have been applied in diverse fields, such as enzyme supports, catalysis, sorbents for the removal of organic compounds, and heavy and precious metals [24,26]. Another advantage of these materials is their adaptable porous structure, which can be finely tuned by varying polymerization conditions. The advantages of non-magnetic and magnetic GMA-based sorbents and their versatility and efficiency in environmental protection have been described in the literature [27].
In light of the continuous improvement of appropriate methodologies aimed at achieving a deeper understanding of the material structure, computational and theoretical modeling plays a key role. Whether employed independently or alongside experimental techniques, computational methods provide valuable insights for explaining and predicting material structures. Density functional theory (DFT) has become an essential tool for modelling across various scientific fields, such as material science [28], biotechnology [29], pharmacy [30], catalysis [31], solid-state physics [32], etc. DFT has greatly enhanced the use of computational materials science in the development and design of polymer materials [33]. Typically, quantum mechanical calculations, recognized for their robustness and cost-efficiency, can be employed to gain a deeper understanding of adsorption parameters such as preferred adsorption sites, adsorption energy, and the structural behavior of adsorbents prior to and following the metal ion removal process [34]. With numerous studies conducted in recent years, the application of computational material science, especially DFT calculations, sets a new framework in the field of polymer science.
In this study, a previously synthesized magnetic sorbent based on GMA and trimethylolpropane trimethacrylate (TMPTMA) and post-functionalized with diethylene triamine, mPGMT-deta, was tested as a potential sorbent for the separation of Re(VII) oxoanions from aqueous molybdenum (Mo) and rhenium (Re) aqueous solutions. The effects of the pH on the removal efficiency and separation of Re(VII) from Mo(VI) were investigated. A deeper understanding of the mPGMT-deta structure and the strength of the interactions at the molecular level was gained by quantum–mechanical calculations coupled with FTIR and XPS analyses. The possible mechanisms of Re(VII) oxoanion sorption are discussed, with a detailed investigation of the interaction energies and preferred binding sites of the metal ions. Furthermore, the kinetic, isotherm, and thermodynamic parameters of the sorption of Re(VII) onto mPGMT-deta were studied.
2. Materials and Methods
2.1. Chemicals
All chemicals used for the nanocomposite synthesis were analytical-grade products and were used as received. Molybdenum and rhenium stock solutions were prepared by dissolving reagent-grade (NH4)6Mo7O24·4H2O and NaReO4 (Sigma-Aldrich, Hamburg, Germany) in deionized water (Milli-Q Millipore,18 MΩ/cm conductivity). pH adjustments were performed with 1 mol/dm3 HCl and 1 mol/dm3 NaOH solutions.
2.2. Instrumentation and Characterization
The FTIR spectra were recorded in the attenuated total reflectance (ATR) mode using a Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA) in the wavenumber range of 400–4000 cm−1 with a resolution of 4 cm−1. The point of zero charge (pHpzc) for mPGMT-deta was determined using the drift equilibrium method [35]. pH measurements were performed in duplicate using an Orion Star™ A211 pH meter (Thermo Scientific, Waltham, MA, USA).
The Re(VII) and Mo(VI) concentrations were determined using inductively coupled plasma optical emission spectrometry (ICP-OES) (Perkin Elmer, Plasma 400 ICP, Waltham, MA, USA). XPS analysis was carried out on a SPECS customized UHV surface analysis system containing a sputter ion gun, a PHOIBOS 100 spectrometer (SPECS Surface Nano Analysis GmbH, Berlin, Germany) for energy analysis, a dual-anode Al/Ag monochromatic source, and an electron flood gun. XPS spectra were taken using monochromatic Al Ka line (photon energy of 1486.74 eV) in FAT 40 mode with an energy step of 0.5 eV and dwell time of 0.2 s (survey spectra), i.e., in FAT 20 mode with energy step of 0.1 eV and dwell time of 1 s (high-resolution spectra).
2.3. Sorption Experiments
Macroporous mPGMT-deta, synthesized in the shape of regular spherical particles (diameter 0.15–0.30 mm) via free-radical polymerization of GMA and TMPTMA in the presence of 10% magnetite and amino-functionalized with diethylenetriamine [36] was used in sorption experiments.
2.3.1. Separation of Re(VII) and Mo(VI)
Separation of Re(VII) from Mo(VI)-containing aqueous solutions was examined as a function of pH (1–9) in batch static experiments, at 298 K. Sorption experiments were carried out by contacting 0.1 g mPGMT-deta with 10 cm3 of mixed Re(VII) (0.005 mol/dm3) and Mo(VI) (0.01 mol/dm3) aqueous solution at adjusted pH for 24 h. After magnetic decantation and filtration, the concentrations of Re(VII) and Mo(VI) retained in the supernatant liquid were measured using ICP-OES. The sorption capacity, removal efficiency, distribution, and separation factors were calculated using Equations (1)–(4) [37].
where Qt is the sorption capacity (mol/g); E is the removal efficiency (%); D is the distribution factor; βRe/Mo is the separation factor; Ci, Ct, and Ce are the initial concentration of metal ions in the solution, concentrations in aqueous solution at time t (min), and equilibrium concentration (mol/dm3), respectively; V is the volume of the aqueous phase (dm3); and m is the mass of the sorbent beads (g).
2.3.2. Adsorption Isotherm Experiments
The adsorption equilibrium data were collected in the range of initial Re(VII) concentrations of 0.001–0.015 mol/dm3. The experiments were performed by contacting 0.1 g of mPGMT-deta with 10 cm3 of a Re(VII) solution at pH = 6 and 298 K for 24 h. Langmuir, Freundlich, Dubinin–Radushkevich, and Fowler–Guggenheim adsorption models [38] were used to describe the Re(VII) ion sorption onto mPGMT-deta.
2.3.3. Kinetic Experiments
The sorption kinetics of Re(VII) ions onto mPGMT-deta were examined at time intervals of 0–360 min, at 298 K, pH = 6. In each experiment, 0.3 g of mPGMT-deta was placed in contact with 30 cm3 of the Re(VII) solution (Ci = 0.005 mol/dm3). At predetermined time intervals (5, 15, 30, 60, 90, 180, 240, and 360 min), 0.1 cm3 aliquots were collected, diluted, and analyzed by ICP-OES. The experimental data were fitted to the linear forms of the pseudo-first-order, pseudo-second-order, Elovich, liquid-film diffusion, intra-particle diffusion, and Bangham models [27].
2.3.4. Thermodynamic Experiments
The effect of temperature on Re(VII) ion sorption onto mPGMT-deta was examined through a thermodynamic study conducted over a temperature range of 298–343 K. For this, mPGMT-deta (0.1 g) was placed in contact with 10 cm3 of Re(VII) solution (Ci = 0.005 mol/dm3) at pH = 6 for 24 h. The concentrations of Re(VII) retained in the supernatant were measured by the ICP-OES. The changes in the Gibbs free energy (ΔG°, kJ/mol), enthalpy change (ΔH°, kJ/mol), and entropy change (ΔS°, J/molK) were calculated according to Equations (5)–(7) [39].
where Ka represents a distribution constant at temperature T, Ca (mol/dm3) and Ce (mol/dm3) are the Re(VII) concentrations sorbed onto mPGMT-deta at equilibrium and the concentration of Re(VII) in solution at equilibrium, respectively, R (8.314 J/mol K) is the universal gas constant, and T (K) is the absolute temperature.
2.4. Desorption Experiment
Desorption of Re(VII) from the mPGMT-deta was conducted in a batch system. An amount of 0.1 g of mPGMT-deta was contacted with 10 cm3 of 0.005 mol/dm3 Re(VII) solution at pH = 6 and 298 K, for 360 min. The solid residue was separated by magnetic decantation, washed with distilled water, and dried. The Re(VII)-loaded mPGMT-deta was subsequently mixed with 20 cm3 of a desorption agent for 360 min at room temperature. After magnetic decantation and filtration, the concentration of Re(VII) in the supernatant was determined using ICP-OES. Desorption experiments were carried out using four different desorbing agents: 1 mol/dm3 HNO3, 1 mol/dm3 NaOH, 3 mol/dm3 NaCl, and a mixture of 1 mol/dm3 NaCl/0.1 mol/dm3 HCl. The desorption capacity and efficiency were calculated using Equations (8) and (9), respectively [40].
where Qd (mol/g) is the desorption capacity; Ed (%) is the desorption efficiency; Cd (mol/dm3) is the concentration of Re(VII) in the solution after desorption; Vd (dm3) is the volume of the desorption agent; and m (g) is the mass of the mPGMT-deta after sorption.
2.5. Theoretical Calculations
To better understand the molecular-level interactions involved in the sorption of the oxoanions Re(VII) and Mo(VI), a combination of molecular docking and quantum–mechanical calculations was employed. In the docking study, the ReO4− and MoO42− ions were treated as rigid target species, whereas two flexible ligands, representing the monomeric and dimeric forms of GMA modified with deta, were analyzed for their conformational flexibility. The geometries of both the target and ligands were optimized using the APFD/def2-TZVP level of theory. At the same computational level, natural population analysis (NPA) was performed to determine atomic charges, which were subsequently used in the docking simulations. Target and ligand structures were prepared using AutoDock Version 4.2.6, with docking parameters generated via AutoDockTools [41]. The docking grid box was configured to encompass both target and ligand, and the Lamarckian genetic algorithm was applied for the docking search, with 100 runs per ligand. Visualization and analysis of docking outcomes were conducted using Discovery Studio (BIOVIA) software [42].
Quantum mechanical calculations were performed using the Gaussian09 program (Gaussian, Inc., Wallingford, CT, USA) to explain the sorption mechanism at the molecular level. Sorption modeling was conducted using the DFT method (APFD method) and def2-TZVP basis set, to evaluate the change in Gibbs free energy (ΔG) associated with the binding of Re(VII) and Mo(VI) oxo species (MoO42− and ReO4−) to the hedetaH22+ species, which serve as simplified representations of polymer sorption centers. The solvation energies calculated using the SMD method were considered. Interaction energies were calculated while considering the basis set superposition errors (BSSEs) [43]. The initial geometries of MoO42−, ReO4−, and hedetaH22+ species, as well as the MoO42−//hedetaH22+ and ReO4−//hedetaH22+ adducts, were optimized, and the parameters obtained by optimization were used to evaluate ΔG for the adduct formation process. In these processes, oxo species form adducts with hedetaH22+ species through electrostatic interactions driven by the attraction between the negatively charged oxo species and the positively charged hedetaH22+ species. The solvation energies (ΔE(s)) were calculated using Equation (10).
The Gibbs energy changes in the adduct formation process in an aqueous solution (ΔG(aq)) were estimated using Equation (11).
The Gibbs free energy change in the gas phase (ΔG(g)) represents a thermodynamic potential commonly used to assess the spontaneity and energy efficiency of chemical processes. The expression for ΔG(g) is given according to Equation (12).
where ΔU is the internal energy change in the system, ΔH is the enthalpy change in the system, ΔS is the entropy change in the system, T is the temperature of the system, V is the volume of the system, and Δp is the pressure change in the system. The enthalpy change in the system (ΔH) was determined using Equation (13).
where ΔEe is the electronic energy change, ΔEt is the thermal energy change, and VΔp is the work performed during the process.
3. Results and Discussion
Macroporous mPGMT-deta, with specific surface area, Ss,Hg of 68 m2/g, pore diameter which corresponds to half of pore volume, DV/2, of 105 nm, and ligand concentration Clig = 1.64 mmol/g [36] was used for Re(VII) separation from Mo-containing aqueous solutions.
3.1. The pH Influence on Separation of Re(VII) and Mo(VI)
As reported in the literature, pH is a crucial parameter that influences the sorption of metal ions from aqueous solutions. The initial pH of the aqueous solution affects both the solubility of Re and Mo ions, that is, their speciation in aqueous solution, as well as the functional groups present on the surface of the sorbent [27,44,45]. To determine the optimal pH for the separation of Re(VII) from Mo(VI), the influence of the initial solution pH on the removal efficiency, final solution pH, and the separation factor was examined using mPGMT-deta in the pH range of 2 to 9.
As can be seen in Figure 1a, the maximum E for Re(VII) and Mo(VI) of >90% was observed at pH = 2. With a further increase in the pH, the removal efficiency decreased. During the experiments, a moderate increase in solution pH was observed after sorption (Figure 1a), particularly in the acidic range. Similar pH shift profiles after sorption have also been reported in the literature [46,47]. Although the maximum E of Re(VII) and Mo(VI) ions using mPGMT-deta was achieved at pH = 2, the βRe/Mo at this pH reached a minimum. As shown in Figure 1b, a maximal βRe/Mo value of 8.85 was observed at pH = 6.
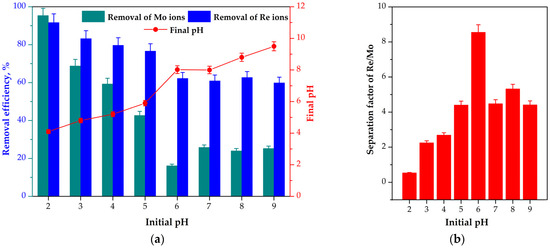
Figure 1.
Effect of initial pH on the (a) Re(VII) and Mo(VI) removal efficiency and final pH; (b) separation of Re(VII) and Mo(VI) by mPGMT-deta. Vertical error bars represent 95% confidence intervals.
To understand the effect of pH on the sorption behavior and change in pH, the pHpzc values of the mPGMT-deta and the speciation of Mo(VI) and Re(VII) as a function of solution pH were taken into account. In contrast to Re(VII), which remains stable as the perrhenate anion, ReO4−, across a wide pH range of 1–13 [48], Mo(VI) exhibits various anionic mononuclear and polynuclear forms, depending on the pH value [49]. The pHpzc value of 7.2 (Figure S1) indicates the basicity of the sorbent. The positively charged mPGMT-deta surface at pH < pHpzc electrostatically attracts negatively charged ions and causes a high removal efficiency of the tested metal ions. During the interaction with negatively charged Mo(VI) and Re(VII) anions, partial deprotonation of positively charged groups may occur, resulting in the release of H+ ions from the sorbent surface, which leads to an increase in solution pH. In contrast, due to electrostatic repulsion, the negatively charged mPGMT-deta surface does not promote the sorption of Re(VII) and Mo(VI) anions, which reduces both the sorption efficiency of Re(VII) and Mo(VI) and the extent of pH change at pH > pHpzc.
3.2. Sorption Mechanism
3.2.1. Theoretical Analysis of Physisorption-Driven Binding Energies
To gain insight into the molecular-level mechanisms behind the separation of Re(VII) from Mo(VI)-containing solutions, a molecular docking study was conducted. The aim was to determine which of the investigated ions (ReO4− or MoO42−) exhibits a stronger affinity for binding to the mPGMT-deta copolymer. This study also explored which functional groups of the deta-functionalized GMA monomer are primarily responsible for ion binding and whether the ions preferentially interact with a single monomeric unit (mono-GMAdeta) or with functional groups from two adjacent monomer units (bi-GMAdeta).
The molecular docking results (Figure 2) indicate that the ReO4− ion shows a stronger affinity for binding to a single monomeric unit (mono-GMAdeta), with a binding energy of −6.44 kcal/mol, compared to its interaction with functional groups from two adjacent monomer units (bi-GMAdeta), which has a binding energy of −5.27 kcal/mol. In the most stable orientation on the mono-GMAdeta/ ReO4− complex, ReO4− ion forms eight conventional N–H···O hydrogen bonds (with bond lengths of 1.68, 1.93, 2.07, 2.27, 2.42, 2.58, 2.80, and 3.03 Å) and an average length of 2.35 Å. The slightly weaker interaction observed in the bi-GMAdeta/ReO4− complex is attributed to a reduced number of classical hydrogen bonds—specifically, seven N–H···O hydrogen bonds (with bond lengths of 1.85, 2.01, 2.15, 2.19, 2.52, 2.55, and 2.58 Å) and one weaker C–H···O bond measuring 2.26 Å. The decomposition of the binding free energies (Table S1) indicates that the electrostatic component is the most significant contributor to the overall binding affinity in both systems, with values around −10 kcal/mol. In contrast, the main destabilizing factor is the torsional free energy, which is nearly twice as high in the bi-GMAdeta/ReO4− complex (approximately −7.4 kcal/mol) compared to the mono-GMAdeta/ ReO4− complex (around −3.6 kcal/mol). This increased torsional penalty explains why the ReO4− ion shows a preference for binding to the monomeric (mono-GMAdeta) unit.
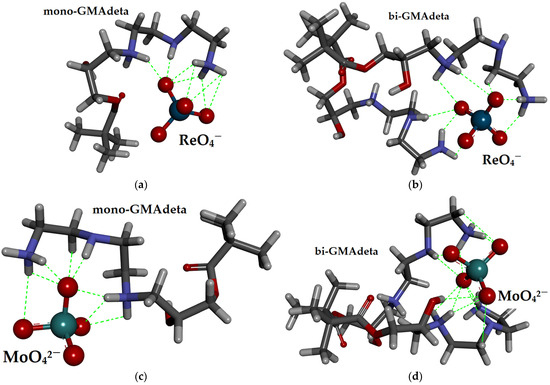
Figure 2.
Molecular docking results illustrating hydrogen bonds (shown as green dashed lines) responsible for the binding of (a) ReO4− ions to mono-GMAdeta; (b) ReO4− ions to bi-GMAdeta; (c) MoO42− ions to mono-GMAdeta; (d) MoO42− ions to bi-GMAdeta structures.
The MoO42− ion also demonstrates a stronger binding affinity to the monomeric unit (mono-GMAdeta/ MoO42− complex), with a binding energy of −16.23 kcal/mol. In its most stable configuration, this complex forms seven N–H···O hydrogen bonds (with bond lengths of 1.57, 1.67, 1.90, 2.02, 2.20, 2.25, and 2.40 Å; averaging 2.00 Å), along with one weaker C–H···O interaction measuring 3.28 Å. In comparison, the most stable orientation of the bi-GMAdeta/MoO42− system features one O–H···O hydrogen bond (with bond length of 2.36 Å), nine N–H···O hydrogen bonds (with bond lengths of 1.78, 2.07, 2.14, 2.15, 2.20, 2.30, 2.31, 2.44, and 2.50 Å), and two weaker C–H···O bonds (with bond lengths of 2.77 and 2.88 Å). Despite the higher number of hydrogen bonds in the bi-GMAdeta/MoO42− system, its overall binding energy is lower (−12.16 kcal/mol), primarily due to less favorable hydrogen bond geometry. This is reflected in the longer average N–H···O bond length of 2.26 Å, compared to 2.00 Å in the monomer system. The presence of three N–H···O bonds shorter than 2.0 Å in the mono-GMAdeta/ MoO42− system, indicative of strong hydrogen bonding, contrasts with only one such bond in the bi-GMAdeta/MoO42− system.
Decomposition of binding free energies (Table S1) confirms that the electrostatic contribution in the mono-GMAdeta/MoO42− system is significantly greater (approximately −24 kcal/mol) than in the bi-GMAdeta/MoO42− system (around −16 kcal/mol). While the torsional free energy, a main destabilizing factor, is higher in the mono-GMAdeta/MoO42− system (+7.4 kcal/mol) than in the bi-GMAdeta/MoO42− system (+3.6 kcal/mol), this difference is insufficient to offset the much larger electrostatic advantage observed in the monomeric complex.
Although the absolute ΔG values generated by AutoDock 4.2 may lack precision, the program is generally reliable for comparing the relative binding affinities of ligands within the same series targeting a specific receptor. Therefore, the conclusion that both ions exhibit a stronger affinity for the mono-GMAdeta unit can be considered trustworthy, even if the specific binding free energy values are not quantitatively accurate. AutoDock 4.2 provides only a rough, heuristic estimate of the binding free energy (ΔG), with key limitations in solvation and entropy modeling [50]. It uses an implicit solvent model based on the solvent-accessible surface area (SASA) and atom-type desolvation parameters, lacking explicit water molecules and rigorous electrostatics. As a result, it overestimates polar and ionic interactions and cannot reliably model key solvent effects. Entropy is simplified, considering only the torsional components. These factors cause high cumulative errors, making the absolute ΔG estimates unreliable. This explanation helps clarify why molecular docking predicts that the MoO42− ion has a higher binding affinity for GMAdeta units than the ReO4− ion—an outcome that contradicts experimental observations.
To obtain more accurate results, quantum–mechanical (QM) calculations were carried out using the SMD method, a computational approach that provides improved estimates of solvation free energies by incorporating a more detailed representation of solute–solvent interactions, particularly the cavity, dispersion, and solvent structure contributions. QM calculations were performed on dimer systems containing a chemical species that represents the model of the sorption center of the polymer, while the second component of the dimer represents the oxo species of Re and Mo. Sorption is modeled on the principles of physisorption based on the electrostatic attraction between the absorption center and oxo species.
A part of the nanocomposite composed of the deta fragment and the fragment formed by the opening of the epoxy ring was used as the absorption center (Scheme 1). For simplicity, hedeta designation for the adsorption center was introduced.
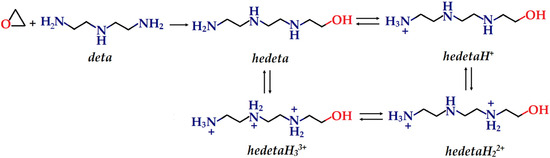
Scheme 1.
Opening of the epoxy ring by amine (deta), with the formation of a hedeta species.
If the pKa values of deta are taken into account (pKa,1 = 4.42, pKa,2 = 9.21, and pKa,3 = 10.02), the distribution of deta species in the solution as a function of pH is obtained [51]. Based on the distribution of particular deta species, one can conclude that detaH22+ ions appear in the solution up to pH 6, with a maximum distribution (≈100%). Given this conclusion, it can be anticipated that the hedetaH22+ ion is the predominant species at the pH value where the selectivity is highest.
Before presenting the results of the calculations, it would be useful to state the distributions of the Re(VI) and Mo(VI) species in the solution as a function of pH 6. In a wide range of pH values (from 1 to 13), perrhenate (ReO4−) dominates in a solution of rhenium (VII) species. Therefore, it is expected that the perrhenate ion (ReO4−) is the only one present in the solution at pH 6, while MoO42− is the predominant ionic species of Mo(VI). To gain insight into the sorption selectivity at the molecular level, QM calculations were carried out on model systems corresponding to ReO4−//hedetaH22+ and MoO42−//hedetaH22+ adducts (Figure 3).
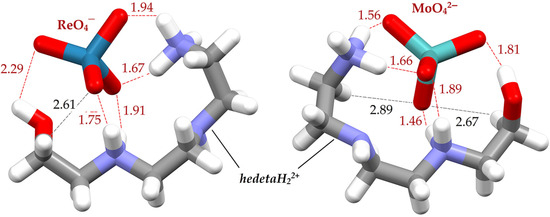
Figure 3.
The geometries of adducts of the adsorption centers of nanocomposite (hedetaH22+) with the perrhenate ReO4− and molybdate MoO42− anions.
The results of QM calculations have shown that the interaction of the MoO42− ion with hedetaH22+ species (energy of −423.91 kcal/mol) is stronger than the interaction of ReO4− ions with hedetaH22+ species (energy of −224.86 kcal/mol), because the hedetaH22+ species exerts a stronger electrostatic attraction toward the more negatively charged oxoanion, specifically the MoO42− ion in this case. Beyond the more favorable electrostatic interactions, the stronger binding of MoO42− ion can also be attributed to the higher number of hydrogen bonds formed with the hedetaH22+ species (seven hydrogen bonds in total). In this adduct, the MoO42− ion acts as a hydrogen-bond acceptor. These interactions include one conventional O–H···O hydrogen bond (length of 1.81 Å), four N–H···O hydrogen bonds (lengths of 1.46, 1.56, 1.66, and 1.89 Å), and two weak C–H···O hydrogen bonds (lengths of 2.67 and 2.89 Å). In contrast, the ReO4− ion forms only six hydrogen bonds, which are generally longer than those observed in the MoO42−//hedetaH22+ adduct. Within the ReO4−//hedetaH22+ adduct, there is one conventional O–H···O hydrogen bond measuring 2.29 Å, four N–H···O hydrogen bonds with lengths of 1.67, 1.75, 1.91, and 1.94 Å, and one weak C–H···O interaction at 2.61 Å.
Theoretical models cannot explain the results of the sorption measurements of Re(VII) and Mo(VI) oxo species to mPGMT-deta (Figure 1), indicating that electrostatics is not a decisive factor for adsorption selectivity at pH 6. To gain deeper insight into the adduct formation process, Gibbs free energy values were calculated. The outcomes of these calculations are summarized in Table 1 and Table S2.

Table 1.
Calculated thermodynamic parameters (in kcal/mol) for the thermo-chemical analysis of the formation process of the MoO42−//hedetaH22+ and ReO4−//hedetaH22+ adducts.
The interaction energies discussed above correspond to the electronic component of the energy (ΔEe). When the thermal energy contribution (ΔEt), pressure–volume work (VΔp), and entropy term (TΔS, with T = 298 K) are included, the Gibbs free energy in the gas phase (ΔG(g)) is obtained. Since the electronic energy typically represents the largest portion of ΔG(g) (Table 1), it becomes evident that the calculated ΔG(g) values alone cannot fully account for the experimentally observed sorption selectivity. However, when the solvation energy is incorporated into the Gibbs free energy, yielding ΔG(aq), the observed selectivity can be explained. Computational results indicate that the solvation of the MoO42−//hedetaH22+ adduct (ΔE(s) = 385.97 kcal/mol) is less favorable in solution compared to the ReO4−//hedetaH22+ adduct (ΔE(s) = 182.09 kcal/mol). Specifically, the calculations suggest that solvated individual ions are energetically more favorable than solvated adducts (Table 1). By adding the solvation energy values associated with adduct formation to the gas-phase Gibbs free energy values (ΔG(g)), the Gibbs free energy in aqueous solution (ΔG(aq)) was obtained. Based on the calculated ΔG(aq) (Table 1), the formation of ReO4−//hedetaH22+ adduct (ΔG(aq) = −26.09 kcal/mol) is more thermodynamically favorable than the formation of MoO42−//hedetaH22+ adduct (ΔG(aq) = −23.12 kcal/mol). This finding aligns well with the experimentally observed sorption selectivity at pH 6 (Figure 1).
3.2.2. FTIR and XPS Analysis
FTIR spectroscopy was conducted to enable a more comprehensive understanding of the adsorption mechanism. The spectra of mPGMT-deta before and after Re(VII) sorption (labeled mPGMT-deta/Re) are presented in Figure 4a.
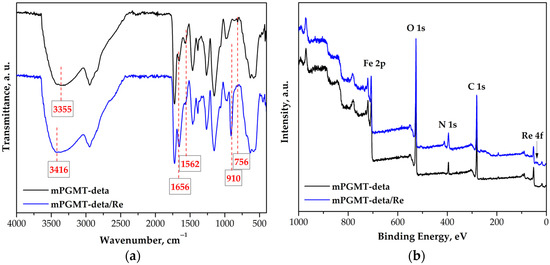
Figure 4.
(a) FTIR-ATR spectra; (b) wide-scan XPS spectra of mPGMT-deta before and after Re(VII) sorption.
The FTIR-ATR spectrum of mPGMT-deta showed broad absorption bands at 3500–3040 cm−1, with a maximum at 3355 cm−1 (overlapping N–H and O–H stretching vibrations), a band at 756 cm−1 (N–H wagging vibrations), and bands at 1656 cm−1 and 1562 cm−1 (N–H bending vibrations) of the primary and secondary amine groups, respectively, indicating successful amino-functionalization [35,52].
The N–H bending vibrations of the secondary amines disappeared from the FTIR-ATR spectrum recorded after Re(VII) sorption. Furthermore, the band corresponding to the N–H bending vibrations of primary amines exhibited an increase in intensity. In addition, a change in the intensity and position of the broadband in the region 3700–3040 cm−1 after Re(VII) ion sorption was observed. This indicates the binding of Re(VII) ions to the amino groups in amino-functionalized nanocomposites [53]. According to the literature, the binding of metal ions changes the type of hybridization in nitrogen, causing a weakening of the N-H bond [54]. The main changes in the ATR-FTIR spectra after Re(VII) ion sorption were observed at 1000–700 cm−1. In the mPGMT-deta/Re spectrum, a new band appeared at 910 cm−1, corresponding to the Re–O stretching vibrations, confirming that the Re ions were successfully sorbed onto the mPGMT-deta surface [52,55].
To complement the insights into the sorption mechanism obtained from FTIR analysis and to gain a deeper understanding of the surface chemistry and oxidation states after Re adsorption, XPS analysis in the binding energy range of 0–1000 eV was carried out. The XPS wide-scan spectra of mPGMT-deta before and after Re(VII) sorption are depicted in Figure 4b. As expected, C 1s, O 1s, N 1s, and Fe 2p were identified in the XPS spectrum of the mPGMT-deta surface before the Re sorption. After sorption, the presence of a new peak corresponding to Re 4f confirmed that the rhenium ions interacted with the active sites on the mPGMT-deta sorbent.
As shown in Figure 5, the HRES Re 4f spectrum displays Re 4f7/2 and Re 4f5/2 doublets with binding energy maxima at 45.7 eV and 47.1 eV, respectively. The photoelectron lines obtained by deconvolution of Re 4f indicate the existence of two oxidation states of rhenium, Re(VII) and Re(VI), in agreement with the literature data [56]. This result provides evidence for the partial reduction in Re(VII) to Re(VI) on the surface of the mPGMT-deta sorbent. Although chemically altered, rhenium in its new oxidation state can still bind non-covalently to the amino-functionalized nanocomposite.
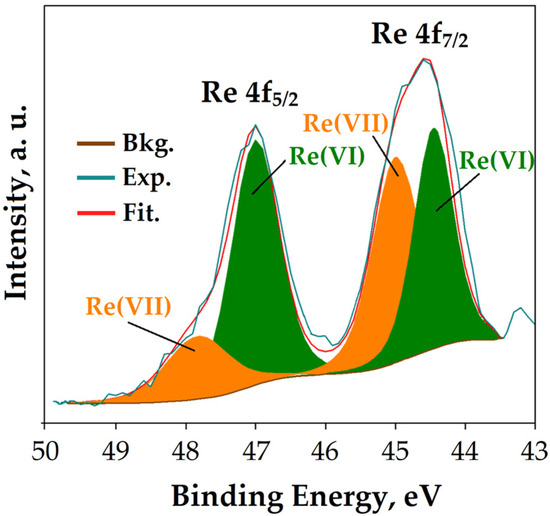
Figure 5.
HRES Re 4f spectrum after Re(VII) sorption onto mPGMT-deta.
The HRES N 1s spectrum before Re(VII) sorption (Figure 6a) shows two peaks with a maximum at 399.2 eV and 400.4 eV, which correspond to the photoelectron line of nitrogen within the non-protonated primary and secondary amino groups, respectively [26]. After the sorption of the Re ions, the HRES N1s spectrum (Figure 6b) was deconvoluted into four peaks.
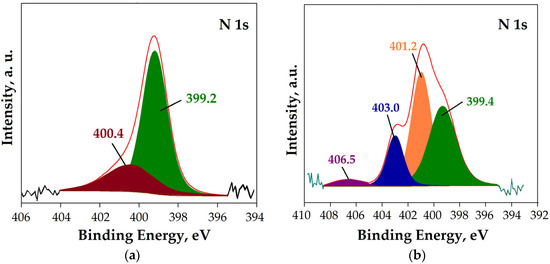
Figure 6.
HRES N 1s core-level spectrum for mPGMT-deta (a) before; (b) after Re(VII) sorption.
The peak corresponding to non-protonated amino groups shifted to a higher binding energy (399.4 eV). The two new peaks centered at 401.2 eV and 403.0 eV represent the protonated amine groups and N-O bond, respectively, indicating that amino groups are involved in the interaction with Re ions [35,57]. Additionally, the peak with the lower intensity at 406.3 eV corresponds to the photoelectron line of nitrogen within the N=O bond [57]. Further evidence supporting the formation of nitroso compounds through the oxidation of secondary amines is an increase in the intensity of the peak at 1656 cm−1 in the FTIR spectrum after the sorption of Re(VII) as a consequence of overlapping N–H and N=O stretching vibrations (Figure 4a). This observation aligns with the known characteristics of nitroso compounds, which exhibit a strong absorption band in the 1500–1660 cm−1 region, corresponding to N=O stretching vibrations [58].
3.3. Adsorption Isotherm Study
Adsorption isotherms are crucial for understanding the mechanism and capacity of sorbates to bind to the active sites on the sorbent surface. In the present study, four adsorption isotherm models were applied using linear regression to analyze the equilibrium data of perrhenate sorption onto mPGMT-deta. The linear equation forms of the Langmuir, Freundlich, Dubinin–Radushkevich, and Fowler–Guggenheim models are presented in Table S3. Graphical representations of the obtained results are shown in Figure S2, while the calculated isotherm parameters, along with the corresponding determination coefficients (R2), are summarized in Table 2.

Table 2.
Adsorption isotherm parameters for Re(VII) sorption onto mPGMT-deta.
Based on the obtained R2 values, the adsorption isotherm models fitted the equilibrium data in the following order: Langmuir > Fowler–Guggenheim > Freundlich > Dubinin–Radushkevich. The Langmuir model was found to best represent the equilibrium data, with R2 = 0.999 and maximum sorption capacity of 0.43 mmol/g, indicating monolayer sorption at specific homogeneous sites on the mPGMT-deta particle surface. The dimensionless equilibrium parameter, RL, is an important characteristic of the Langmuir isotherm that determines the shape of the isotherm and indicates whether the sorption is irreversible (RL = 0), favored (0 < RL< 1), unfavorable (RL > 1), or linear (RL = 1) [59]. The RL values, obtained in the range of 0.05–0.36, show that Re(VII) ion sorption on mPGMT-deta is a favored process.
Based on the Dubinin–Radushkevich isotherm, the sorption of Re(VII) onto mPGMT-deta followed a physisorption mechanism, as evidenced by the mean adsorption energy (E) value of 6.93 kJ/mol, which is below the commonly accepted threshold of 8 kJ/mol for physisorption [60]. The Fowler–Guggenheim model considers the sorbate species interactions on the sorbent surface. The interaction energy (W) between the sorbed species, with a value of −7.66 kJ/mol, indicates an endothermic sorption process (W < 0 kJ/mol), likely due to repulsive interactions between the sorbed Re species on the mPGMT-deta surface [61]. According to the Freundlich isotherm, the adsorption intensity (1/n) was 0.34, suggesting favorable Re(VII) sorption by the investigated sorbent [59], which aligns with the RL results obtained from the Langmuir isotherm.
3.4. Kinetic Study
The time-dependent behavior of Re(VII) sorption by mPGMT-deta (Figure 7a) shows rapid sorption in the first 30 min. Subsequently, the sorption rate slowly decreased and reached a maximum in 180 min, followed by equilibrium with no significant change in sorption capacity due to the saturation of the active centers on the mPGMT-deta sorbent.
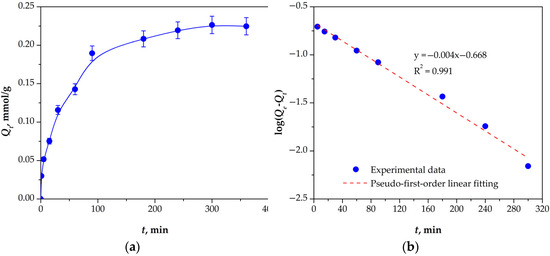
Figure 7.
(a) Time-dependent sorption plot; (b) pseudo-first-order plot for Re(VII) sorption onto mPGMT-deta. Vertical error bars represent 95% confidence intervals.
To gain insight into the kinetics of Re(VII) sorption by mPGMT-deta, the experimental data were analyzed using three surface-reaction-based kinetic models. The linear equation forms of the pseudo-first-order, pseudo-second-order, and Elovich models are listed in Table S4. The kinetic parameters calculated using these models are presented in Table 3. Based on the R2 values, both the pseudo-first-order (0.991) (Figure 7b) and pseudo-second-order (0.992) (Figure S3) kinetic models exhibited similar correlations with the experimental data. Furthermore, the calculated Qecalc values of pseudo-first-order (0.21 mmol/g) and pseudo-second-order (0.26 mmol/g) align well with the experimental Qe value of 0.23 mmol/g. On the other hand, the Elovich model (Figure S3) showed the lowest R2 value (0.966) compared to the pseudo-first-order and pseudo-second-order kinetic models, indicating that surface chemisorption is unlikely to be the dominant mechanism. The obtained kinetic analysis results, together with the low sorption energy value obtained from the Dubinin–Radushkevich isotherm, indicate that the sorption of Re(VII) on mPGMT-deta is predominantly governed by physisorption.

Table 3.
Surface-reaction-based kinetic parameters for Re(VII) sorption onto mPGMT-deta.
To determine whether the rate of Re(VII) sorption on the mPGMT-deta nanocomposite is controlled by diffusion through the surface film or pores, the experimental results were further analyzed using linear regression of the intra-particle diffusion, Bangham models, and Boyd film diffusion (Table S4). The values of the diffusion-based kinetic parameters are shown in Table 4, while the fitting results are depicted in Figure 8 and Figure S3.

Table 4.
Diffusion-based kinetic parameters for Re(VII) sorption onto mPGMT-deta.
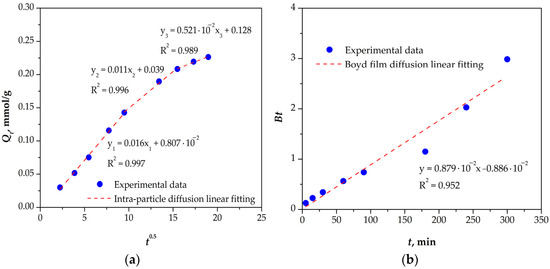
Figure 8.
(a) Intra-particle diffusion plot; (b) Boyd film diffusion plot for Re(VII) sorption onto mPGMT-deta.
The intra-particle diffusion plot (Figure 8a) displays three distinct linear segments, indicating that multiple mechanisms contribute to the sorption process. The first segment exhibited a high correlation coefficient (R2 = 0.997) and passed nearly through the origin, implying that film diffusion or external surface sorption was negligible and that intra-particle diffusion was the dominant mechanism at this stage. The second linear segment did not pass through the origin, suggesting that the sorption of Re(VII) onto mPGMT-deta was governed by both liquid film and intra-particle diffusion [26]. The third linear region corresponds to the final equilibrium stage, marked by the lowest diffusion rate (kid,3 = 5.21 ·10−3 mmol/g min0,5), during which Re(VII) ions slowly transfer from the macropores into the micropores of the mPGMT-deta.
Application of the Bangham model (Figure S4) to the Re(VII) sorption data yielded a linear relationship with a high correlation coefficient (R2 = 0.994), suggesting that pore-diffusion significantly contributes to the sorption mechanism. This is consistent with the Boyd film diffusion plot shown in Figure 8b, which is nearly linear over the entire range (R2 = 0.952) and passes close to the origin (intercept = 0.886 × 10−2), indicating that the sorption of Re(VII) onto mPGMT-deta is primarily controlled by an intra-particle diffusion mechanism, with minor influence of film diffusion.
3.5. Thermodynamic Study
A thermodynamic study of Re(VII) sorption onto mPGMT-deta was performed in the temperature range 298–343 K. Based on Equations (5)–(7), the thermodynamic parameters (ΔG°, ∆H°, and ΔS°) were calculated from the slope and intercept of the linear plot of lnKa versus 1/T, as shown in Figure S4. The thermodynamic data obtained are listed in Table 5.

Table 5.
The thermodynamic parameters for Re(VII) onto mPGMT-deta.
The negative ΔG° values for the Re(VII) sorption indicate that the process is thermodynamically favorable and spontaneous. The increase in the absolute value of ΔG° with increasing temperature suggested a stronger affinity for Re(VII) sorption at higher temperatures. Additionally, a positive value of ∆H° indicated an endothermic process.
3.6. Desorption Study
Regeneration of sorbents represents a vital aspect of rhenium recovery, particularly due to its low natural abundance and significant economic importance. Effective desorption of Re(VII) from saturated sorbent materials contributes to reducing operational costs and supports the long-term sustainability and efficiency of the extraction process. Based on the procedure described in Section 2.4, desorption experiments were performed by using 1 mol/dm3 HNO3, 1 mol/dm3 NaOH, 3 mol/dm3 NaCl, and a mixture of 1 mol/dm3 NaCl/0.1 mol/dm3 HCl, and the results are depicted in Figure 9. As can be seen, the efficiency of the used desorption agents decreased in the following order: NaOH > HNO3 > NaCl > NaCl/HCl. All tested desorption agents achieved desorption efficiencies above 75%. Among them, 1 mol/dm3 NaOH exhibited the highest desorption efficiency, reaching approximately 95% for the removal of Re(VII) from the loaded sorbent. On the other hand, the NaCl/HCl mixture was the least effective, with a desorption efficiency of around 75%.
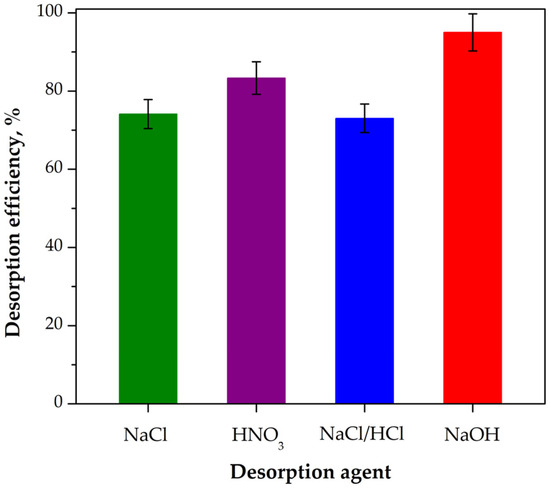
Figure 9.
Regeneration of Re(VII) from mPGMT-deta sorbent by different desorption agents. Vertical error bars represent 95% confidence intervals.
3.7. Comparison Study
Numerous studies have been conducted to achieve the efficient separation of Re(VII) from Mo-containing solutions, evaluating the performance of various sorbents based on their maximum adsorption capacity (Qₘ) and separation factor (βRe/Mo). Several previously published findings were compared with the results presented in this study (Qₘ = 80.1 mg/g (0.43 mmol/g), βRe/Mo = 8.85 at pH 6) and summarized in Table 6. However, differences in experimental conditions, particularly the initial concentration ratio of Re(VII) and Mo(VI) in the aqueous solution, complicate the direct comparison of the reported data. Although the separation factor of the mPGMT-deta sorbent is not particularly high compared to that of some of the listed sorbents, certain findings from this study, especially the operation at pH 6, are noteworthy. In contrast, most sorbents reported in Table 6 achieve high separation factors under highly acidic or basic conditions, which often result in increased waste and secondary pollution, making the process less environmentally and economically viable.

Table 6.
Comparison of the maximal sorption capacity and separation factor with various sorbent materials reported in previous studies.
4. Conclusions
In this study, an amino-functionalized magnetic polymeric sorbent, mPGMT-deta, was evaluated for its ability to separate Re(VII) oxoanions from aqueous solutions containing molybdenum (Mo) and rhenium (Re). The effect of pH on the removal efficiency and separation factor was analyzed over the range of 2–9. The highest removal efficiency, exceeding 90% for both Re(VII) and Mo(VI), was observed at pH 2, whereas an optimal separation factor of 8.85 was recorded at pH 6.
To gain a deeper understanding of the structural and interaction properties of the sorbent, FTIR and XPS analyses were complemented with quantum mechanical (QM) calculations. The QM results indicated that the formation of the MoO42−//hedetaH22+ adduct is electrostatically favored at pH 6, whereas incorporating solvation effects makes the formation of the ReO4−//hedetaH22+ adduct thermodynamically more favorable. XPS analysis revealed the presence of two oxidation states of rhenium and the partial reduction in Re(VII) to Re(VI) on the mPGMT-deta surface.
The comparable correlation coefficients for the pseudo-first-order and pseudo-second-order models, along with the low R2 value for the Elovich model, suggest that surface chemisorption is unlikely to be the dominant sorption mechanism. The Langmuir model provided the best fit for the equilibrium data, indicating monolayer sorption at homogeneous sites on the mPGMT-deta particle surface, with a maximum sorption capacity of 0.43 mmol/g. Additionally, the low sorption energy value (6.93 kJ/mol) derived from the Dubinin–Radushkevich model indicated that Re(VII) sorption is primarily governed by physisorption. Thermodynamic parameters, including the standard Gibbs free energy, enthalpy, and entropy changes, demonstrated that Re(VII) sorption was favorable, endothermic, and spontaneous. The increase in the standard Gibbs free energy with increasing temperature suggested an enhanced affinity for Re(VII) sorption at higher temperatures. Desorption tests using NaOH, HNO3, NaCl, and a NaCl/HCl mixture achieved efficiencies exceeding 75%. Among them, 1 mol/dm3 NaOH emerged as the most effective desorption agent, reaching an efficiency of 95%. This study showed that the obtained mPGMT-deta could be an economical sorbent for efficient removal and separation of Re(VII) from Mo-containing solutions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/separations12080206/s1, Table S1: Molecular docking results for the most stable orientation on four model systems, with the types of interactions responsible for ion binding, as well as estimated free energy decomposition; Table S2: Calculated thermodynamic parameters (in kcal/mol) for the formation process of the MoO42−//hedetaH22+ and ReO4−//hedetaH22+ adducts; Table S3: Equations for adsorption isotherm models; Table S4: Equations for kinetic sorption models; Figure S1: pHpzc plot for mPGMT-deta; Figure S2: Linear plots of isotherm models of the following: (a) Langmuir; (b) Freundlich; (c) Dubinin–Radushkevich; (d) Fowler–Guggenheim for sorption of Re(VII) onto mPGMT-deta; Figure S3: Linear plots of kinetic models of the following: (a) pseudo-second-order; (b) Elovich for sorption of Re(VII) onto mPGMT-deta; Figure S4: Linear plot of Bangham kinetic model for Re(VII) sorption onto mPGMT-deta; and Figure S5: Thermodynamic plot for the Re(VII) sorption onto mPGMT-deta.
Author Contributions
Conceptualization, B.M. and A.N.; methodology, B.M. and A.N.; software, B.M., G.J. and T.T.; carrying out measurements, T.T. and P.S.; data curation, B.M., G.J. and P.S.; writing—original draft preparation, B.M., A.N. and T.T.; writing—review and editing, G.J., P.S. and A.O.; visualization, B.M. and G.J.; supervision, A.N. and A.O.; funding acquisition, A.N. and A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contracts No. 451-03-136/2025-03/200026 and 451-03-136/2025-03/200135) and the European Regional Development Fund—Operational Programme Science and Education for Smart Growth (BG05M2OP001-1.001-0008).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The research presented in this study aligns with Goal 6 (Ensure availability and sustainable management of water and sanitation for all) of the Agenda 2030–United Nations Sustainable Development Goals (SDGs).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yagi, R.; Okabe, T.H. Review: Rhenium and Its Smelting and Recycling Technologies. Int. Mater. Rev. 2024, 69, 142–177. [Google Scholar] [CrossRef]
- Dilworth, J.R. Rhenium Chemistry—Then and Now. Coord. Chem. Rev. 2021, 436, 213822. [Google Scholar] [CrossRef]
- Chatterjee, T.; Ravikanth, M. Rhenium Complexes of Porphyrinoids. Coord. Chem. Rev. 2020, 422, 213480. [Google Scholar] [CrossRef]
- Tan, Z.H.; Wang, X.G.; Ye, L.H.; Hou, G.C.; Li, R.; Yang, Y.H.; Liu, J.L.; Liu, J.D.; Yang, L.; Wang, B.; et al. Effects of Rhenium on the Microstructure and Creep Properties of Novel Nickle-Based Single Crystal Superalloys. Mater. Sci. Eng. A 2019, 761, 138042. [Google Scholar] [CrossRef]
- Zhang, M.; Du, J.; Dong, Z.; Qi, W.; Zhao, L. Recovery and Separation of Mo(VI) and Re(VII) from Mo-Re Bearing Solution by Gallic Acid-Modified Cellulose Microspheres. Sep. Purif. Technol. 2022, 281, 119879. [Google Scholar] [CrossRef]
- Jing, H.; Zhang, Q.; Hu, Z.; Jiang, H.; Gao, B.; Zhang, T.; Yin, Y. Advances in Enrichment and Purification Technology of Ammonium Perrhenate. Separations 2025, 12, 89. [Google Scholar] [CrossRef]
- Shen, L.; Tesfaye, F.; Li, X.; Lindberg, D.; Taskinen, P. Review of Rhenium Extraction and Recycling Technologies from Primary and Secondary Resources. Miner. Eng. 2021, 161, 106719. [Google Scholar] [CrossRef]
- Lin, S.; Mao, J.; Xiong, J.; Tong, Y.; Lu, X.; Zhou, T.; Wu, X. Toward a Mechanistic Understanding of Rhenium(VII) Adsorption Behavior onto Aminated Polymeric Adsorbents: Batch Experiments, Spectroscopic Analyses, and Theoretical Computations. Chemosphere 2023, 345, 140485. [Google Scholar] [CrossRef]
- Guagliardi, I.; Rovella, N.; Apollaro, C.; Bloise, A.; Rosa, R.D.; Scarciglia, F.; Buttafuoco, G. Modelling Seasonal Variations of Natural Radioactivity in Soils: A Case Study in Southern Italy. J. Earth Syst. Sci. 2016, 125, 1569–1578. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, H.; Lou, Z.; Shan, W.; Xing, Z.; Deng, G.; Wu, D.; Fang, D.; Biswas, B.K. Selective Adsorption of Molybdenum(VI) from Mo–Re Bearing Effluent by Chemically Modified Astringent Persimmon. J. Hazard. Mater. 2011, 186, 1855–1861. [Google Scholar] [CrossRef]
- Cai, X.; Kong, L.; Hu, X.; Peng, X. Recovery of Re(VII) from Strongly Acidic Wastewater Using Sulphide: Acceleration by UV Irradiation and the Underlying Mechanism. J. Hazard. Mater. 2021, 416, 126233. [Google Scholar] [CrossRef]
- Huo, T.; Chen, Z.; Ou, X.; Wei, X.; Sun, Y.; Liu, C.; Li, H.; Chen, Z.; Zhu, J.; Lu, S.; et al. Fabricating Magnetic Thermo-Sensitive Imprinted Polymers with Enhanced Adsorption and Recognition Performance of Rhenium. React. Funct. Polym. 2023, 184, 105512. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Liu, Y.; Sarkar, A.K.; Bediako, J.K.; Kim, H.Y.; Yun, Y. Super-Stable, Highly Efficient, and Recyclable Fibrous Metal–Organic Framework Membranes for Precious Metal Recovery from Strong Acidic Solutions. Small 2019, 15, 1805242. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Wang, Y.; Liu, B.; Huang, Y.; Su, S.; Sun, H.; Yang, S. Rhenium Extraction from Dilute Solution by Precipitation Flotation and Oxidative Volatilization Techniques. J. Environ. Chem. Eng. 2023, 11, 111457. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Han, G.; Wang, M.; Huang, Y.; Su, S.; Xue, Y.; Wang, Y. Clean Separation and Purification for Strategic Metals of Molybdenum and Rhenium from Minerals and Waste Alloy Scraps–A Review. Resour. Conserv. Recycl. 2022, 181, 106232. [Google Scholar] [CrossRef]
- Olea, F.; Valenzuela, M.; Zurob, E.; Parraguez, B.; Abejón, R.; Cabezas, R.; Merlet, G.; Tapia, R.; Romero, J.; Quijada-Maldonado, E. Hydrophobic Eutectic Solvents for the Selective Solvent Extraction of Molybdenum (VI) and Rhenium (VII) from a Synthetic Pregnant Leach Solution. J. Mol. Liq. 2023, 385, 122415. [Google Scholar] [CrossRef]
- Weng, H.; Zhang, P.; Guo, Z.; Chen, G.; Shen, W.; Chen, J.; Zhao, X.; Lin, M. Efficient and Ultrafast Adsorption of Rhenium by Functionalized Hierarchically Mesoporous Silica: A Combined Strategy of Topological Construction and Chemical Modification. ACS Appl. Mater. Interfaces 2021, 13, 8249–8262. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Mohammad, R.M.; Alghamdi, H.M.; Elgarahy, A.M. Hybrid Adsorbents for Pollutants Removal: A Comprehensive Review of Chitosan, Glycidyl Methacrylate and Their Composites. J. Mol. Liq. 2025, 426, 127262. [Google Scholar] [CrossRef]
- Bao, W.; Yu, T.; Liu, Y.; Sun, Z.; Yuan, L.; Mei, L.; Shi, W.; Zhang, Z. Cutting-Edge Characterization Techniques to Decipher Adsorption Mechanisms of Radionuclides and Heavy Metals. Coord. Chem. Rev. 2025, 539, 216748. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, K.; Tan, X.; Wang, X.; Alsaedi, A.; Hayat, T.; Chen, C. Interaction Mechanism of Re(VII) with Zirconium Dioxide Nanoparticles Archored onto Reduced Graphene Oxides. ACS Sustain. Chem. Eng. 2017, 5, 2163–2171. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhou, Y.; Fang, F.; Li, X. Tailored Metal-Organic Frameworks Facilitate the Simultaneously High-Efficient Sorption of UO22+ and ReO4− in Water. Sci. Total Environ. 2021, 799, 149468. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-W.; Li, Z.-J.; Wu, Q.-Y.; Zheng, L.-R.; Zhou, L.-M.; Chai, Z.-F.; Wang, X.-L.; Shi, W.-Q. Simultaneous Elimination of Cationic Uranium(vi) and Anionic Rhenium(vii) by Graphene Oxide–Poly(Ethyleneimine) Macrostructures: A Batch, XPS, EXAFS, and DFT Combined Study. Environ. Sci. Nano 2018, 5, 2077–2087. [Google Scholar] [CrossRef]
- Hou, Y.; Fu, Z.; Luo, J.; Liu, X.; Sun, H.; Li, G. Selective Separation of Rhenium from Oxygen-Pressure Leach Solution of Molybdenite Concentrate Using Modified D201 Resin: Experiments and Theoretical Calculations. J. Mol. Liq. 2024, 408, 125371. [Google Scholar] [CrossRef]
- Marković, B.M.; Vuković, Z.M.; Spasojević, V.V.; Kusigerski, V.B.; Pavlović, V.B.; Onjia, A.E.; Nastasović, A.B. Selective Magnetic GMA Based Potential Sorbents for Molybdenum and Rhenium Sorption. J. Alloys Compd. 2017, 705, 38–50. [Google Scholar] [CrossRef]
- Muhammad, A.; Yang, Q.; Kanwal, A.; Zhao, J.; Nawaz, M.; Ren, H.; Yang, P. Highly Selective Adsorption of Rhenium by Amyloid-like Protein Material. Sci. China Technol. Sci. 2024, 67, 1417–1430. [Google Scholar] [CrossRef]
- Tadić, T.; Marković, B.; Vuković, Z.; Stefanov, P.; Maksin, D.; Nastasović, A.; Onjia, A. Fast Gold Recovery from Aqueous Solutions and Assessment of Antimicrobial Activities of Novel Gold Composite. Metals 2023, 13, 1864. [Google Scholar] [CrossRef]
- Nastasović, A.; Marković, B.; Suručić, L.; Onjia, A. Methacrylate-Based Polymeric Sorbents for Recovery of Metals from Aqueous Solutions. Metals 2022, 12, 814. [Google Scholar] [CrossRef]
- Schleder, G.R.; Padilha, A.C.M.; Acosta, C.M.; Costa, M.; Fazzio, A. From DFT to Machine Learning: Recent Approaches to Materials Science–a Review. J. Phys. Mater. 2019, 2, 032001. [Google Scholar] [CrossRef]
- Mamand, D.M.; Awla, A.H.; Kak Anwer, T.M.; Qadr, H.M. Quantum Chemical Study of Heterocyclic Organic Compounds on the Corrosion Inhibition. Chim. Tech. Acta 2022, 9, 20229203. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M. Periodic DFT Calculations—Review of Applications in the Pharmaceutical Sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K. Density Functional Theory (DFT) Calculations and Catalysis. Catalysts 2021, 11, 454. [Google Scholar] [CrossRef]
- Hasnip, P.J.; Refson, K.; Probert, M.I.J.; Yates, J.R.; Clark, S.J.; Pickard, C.J. Density Functional Theory in the Solid State. Phil. Trans. R. Soc. A. 2014, 372, 20130270. [Google Scholar] [CrossRef]
- Adekoya, O.C.; Adekoya, G.J.; Sadiku, E.R.; Hamam, Y.; Ray, S.S. Application of DFT Calculations in Designing Polymer-Based Drug Delivery Systems: An Overview. Pharmaceutics 2022, 14, 1972. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Pandith, A.H.; Qureashi, A.; Bashir, A.; Manzoor, T. The Emerging Role of Quantum Computations in Elucidating Adsorption Mechanism of Heavy Metal Ions: A Review. Chem. Pap. 2022, 76, 3351–3370. [Google Scholar] [CrossRef]
- Suručić, L.; Janjić, G.; Marković, B.; Tadić, T.; Vuković, Z.; Nastasović, A.; Onjia, A. Speciation of Hexavalent Chromium in Aqueous Solutions Using a Magnetic Silica-Coated Amino-Modified Glycidyl Methacrylate Polymer Nanocomposite. Materials 2023, 16, 2233. [Google Scholar] [CrossRef]
- Markovic, B.; Spasojevic, V.; Dapcevic, A.; Vukovic, Z.; Pavlovic, V.; Randjelovic, D.; Nastasovic, A. Characterization of Glycidyl Methacrylate Based Magnetic Nanocomposites. Hem. Ind. 2019, 73, 25–35. [Google Scholar] [CrossRef]
- Li, W.; Dong, X.; Zhu, L.; Tang, H. Highly Selective Separation of Re(VII) from Mo(VI) by Using Biomaterial-Based Ionic Gel Adsorbents: Extractive Adsorption Enrichment of Re and Surface Blocking of Mo. Chem. Eng. J. 2020, 387, 124078. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Zhao, Q.; Li, C.; Huang, J.; Hu, X.; Li, J.; Li, M. Application of a Novel Bifunctionalized Magnetic Biochar to Remove Cr(VI) from Wastewater: Performance and Mechanism. Separations 2023, 10, 358. [Google Scholar] [CrossRef]
- Xu, B.; Yin, X.; Ning, S.; Zhong, Y.; Wang, X.; Fujita, T.; Hamza, M.F.; Wei, Y. Study of the Adsorption and Separation Behavior of Scandium and Zirconium by Trialkyl Phosphine Oxide-Modified Resins in Sulfuric and Hydrochloric Acid Media. Toxics 2024, 12, 350. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Systèmes, D. Free Download: BIOVIA Discovery Studio Visualizer. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 29 July 2025).
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Cheng, Q.; Luo, E.; Gan, L.; Luo, W.; Luo, X.; Wan, S.; Li, S. Construction of Quaternary Ammonium Nitroxy-Hybrid Magnetic Mesoporous Silica Adsorbent System and Its Selective Adsorption Research of Re(Ⅶ)/Cu(Ⅱ). Sep. Purif. Technol. 2025, 354, 129181. [Google Scholar] [CrossRef]
- Shi, T.; Ma, L.; Xi, X.; Nie, Z. Preparation of Functional Polytertiary Amine Macroporous Resin and Its Adsorption and Separation Properties for Tungsten and Molybdenum. Sep. Purif. Technol. 2024, 332, 125759. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, G.; Mo, W.; Zhang, C.; Huang, H.; Li, S.; Gao, M.; Lu, X.; Zhang, B.; Zhu, H. Enhanced Phosphate Sequestration by Fe(iii) Modified Biochar Derived from Coconut Shell. RSC Adv. 2019, 9, 10425–10436. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, N.; Bian, S.; Li, J.; Xu, S.; Zhang, Y. Enhancing the Adsorption Capability of Areca Leaf Biochar for Methylene Blue by K2FeO4 -Catalyzed Oxidative Pyrolysis at Low Temperature. RSC Adv. 2019, 9, 42343–42350. [Google Scholar] [CrossRef]
- Fan, L.; Li, W.; Dai, Z.; Zhou, M.; Qiu, Y. Efficient Separation of Re (VII) and Mo (VI) by Extraction Using E-1006–Ammonium Sulfate Aqueous Two-Phase System. Separations 2024, 11, 142. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Liu, B.; Han, G.; Du, Y.; Su, S. Adsorption Behaviors of Strategic W/Mo/Re from Wastewaters by Novel Magnetic Ferrite Nanoparticles: Adsorption Mechanism Underlying Selective Separation. J. Hazard. Mater. 2022, 424, 127675. [Google Scholar] [CrossRef]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, X.; Zhao, O.; Liu, S.; Liu, C.; Wang, J.; Huang, J. The Evolution of Self-Assemblies in the Mixed System of Oleic Acid–Diethylenetriamine Based on the Transformation of Electrostatic Interactions and Hydrogen Bonds. Soft Matter 2014, 10, 8023–8030. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Dong, Y.; Chen, L.; Jiang, F.; Tang, H.; Feng, D. Roles of Nitrogen- and Sulphur-Containing Groups in Copper Ion Adsorption by a Modified Chitosan Carboxymethyl Starch Polymer. Separations 2024, 11, 283. [Google Scholar] [CrossRef]
- Su, H.; Sun, J.; Li, D.; Wei, J. Local Hydrogen Bonding Environment Induces the Deprotonation of Surface Hydroxyl for Continuing Ammonia Decomposition. Phys. Chem. Chem. Phys. 2024, 26, 16871–16882. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Elkhooly, T.; Reicha, F. The Effect of Different Solvents for Chitosan Solubilization on The Crystal Growth of in Situ Prepared Hydroxyapatite. Egypt. J. Phys. 2017, 45, 29–38. [Google Scholar] [CrossRef]
- Chong, X.; Bao, W.; Wang, Y.; Sun, Z.; Chen, L.; Chen, Q.; Zhang, Z. Preparation of Magnetic Fe3O4 Composite Cu-MOFs and Their Removal of Perrhenate Ions. J. Solid State Chem. 2025, 348, 125390. [Google Scholar] [CrossRef]
- Kołczyk-Siedlecka, K.; Socha, R.P.; Yang, X.; Eckert, K.; Wojnicki, M. Study on Kinetics and Mechanism of Re(VII) Ion Adsorption and Desorption Using Commercially Available Activated Carbon and Solutions Containing Se(VI) as an Impurity. Hydrometallurgy 2023, 215, 105973. [Google Scholar] [CrossRef]
- Wang, C.; Sun, L.; Wang, Q.; Wang, Y.; Cao, Y.; Wang, X.; Chen, P.; Sun, W. Adsorption Mechanism and Flotation Behavior of Ammonium Salt of N-Nitroso-N-Phenylhydroxyamine on Malachite Mineral. Appl. Surf. Sci. 2022, 583, 152489. [Google Scholar] [CrossRef]
- Góbi, S.; Crandall, P.B.; Maksyutenko, P.; Förstel, M.; Kaiser, R.I. Accessing the Nitromethane (CH3NO2) Potential Energy Surface in Methanol (CH3OH)–Nitrogen Monoxide (NO) Ices Exposed to Ionizing Radiation: An FTIR and PI-ReTOF-MS Investigation. J. Phys. Chem. A 2018, 122, 2329–2343. [Google Scholar] [CrossRef]
- Melaku, A.; Alemayehu, E.; Worku, A.; Lennartz, B. Synthesis of Magnetic Iron Oxide Heat-Activated Termite Mound Composite for Adsorption of Basic Blue 41 Dye from Textile Wastewater: Characterization and Box–Behnken Optimization. Separations 2025, 12, 117. [Google Scholar] [CrossRef]
- Mahajan, T.; Paikaray, S.; Mahajan, P. Applicability of the Equilibrium Adsorption Isotherms and the Statistical Tools on to Them: A Case Study for the Adsorption of Fluoride onto Mg-Fe-CO3 LDH. J. Phys. Conf. Ser. 2023, 2603, 012056. [Google Scholar] [CrossRef]
- Jemutai-Kimosop, S.; Okello, V.A.; Shikuku, V.O.; Orata, F.; Getenga, Z.M. Synthesis of Mesoporous Akaganeite Functionalized Maize Cob Biochar for Adsorptive Abatement of Carbamazepine: Kinetics, Isotherms, and Thermodynamics. Clean. Mater. 2022, 5, 100104. [Google Scholar] [CrossRef]
- Fathi, M.B.; Nasiri, M. Synthesis and Characterization of Modified Resins and Their Selective Sorption towards Rhenium from Binary (Re and Mo) Solutions. Iran. J. Chem. Chem. Eng. 2023, 42, 1471–1477. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Liao, Y.; Liu, F.; Li, H.; Jiang, Q.; Huang, B.; Wang, Y.; Xiao, L.; Liu, H.; et al. Rhenium Recovery from Roasting Leachate of Molybdenum Concentrate by N-Methylimidazole Functionalized Anion Exchange Resin. J. Radioanal. Nucl. Chem. 2023, 332, 747–760. [Google Scholar] [CrossRef]
- Shan, W.; Wang, D.; Zhang, Z.; Lou, Z.; Xiong, Y.; Fan, Y. Synthesis of Schiff Base-Functionalized Silica for Effective Adsorption of Re(VII) from Aqueous Solution. J. Taiwan Inst. Chem. Eng. 2019, 100, 277–284. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Li, Q.; Zhang, Z.; Zhang, L.; Liu, X. Simultaneous Speciation of Inorganic Rhenium and Molybdenum in the Industrial Wastewater by Amino-Functionalized Nano-SiO2. J. Taiwan Inst. Chem. Eng. 2015, 55, 126–132. [Google Scholar] [CrossRef]
- Cyganowski, P.; Cierlik, A.; Leśniewicz, A.; Pohl, P.; Jermakowicz-Bartkowiak, D. Separation of Re(VII) from Mo(VI) by Anion Exchange Resins Synthesized Using Microwave Heat. Hydrometallurgy 2019, 185, 12–22. [Google Scholar] [CrossRef]
- Lou, Z.; Wan, L.; Guo, C.; Zhang, S.; Shan, W.; Xiong, Y. Quasi-Complete Separation Re(VII) from Mo(VI) onto Magnetic Modified Cross-Linked Chitosan Crab Shells Gel by Using Kinetics Methods. Ind. Eng. Chem. Res. 2015, 54, 1333–1341. [Google Scholar] [CrossRef]
- Jia, M.; Cui, H.; Jin, W.; Zhu, L.; Liu, Y.; Chen, J. Adsorption and Separation of Rhenium(VII) Using N-methylimidazolium Functionalized Strong Basic Anion Exchange Resin. J. Chem. Technol. Biotechnol. 2013, 88, 437–443. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).