Preparation of EDTA-2Na-Fe3O4-Activated Carbon Composite and Its Adsorption Performance for Typical Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Carbon Composite Materials
2.3. Optimization of Experimental Design by Response Surface Method
2.4. Characterization of Activated Carbon Composites
2.5. Single-Factor Adsorption Experiment
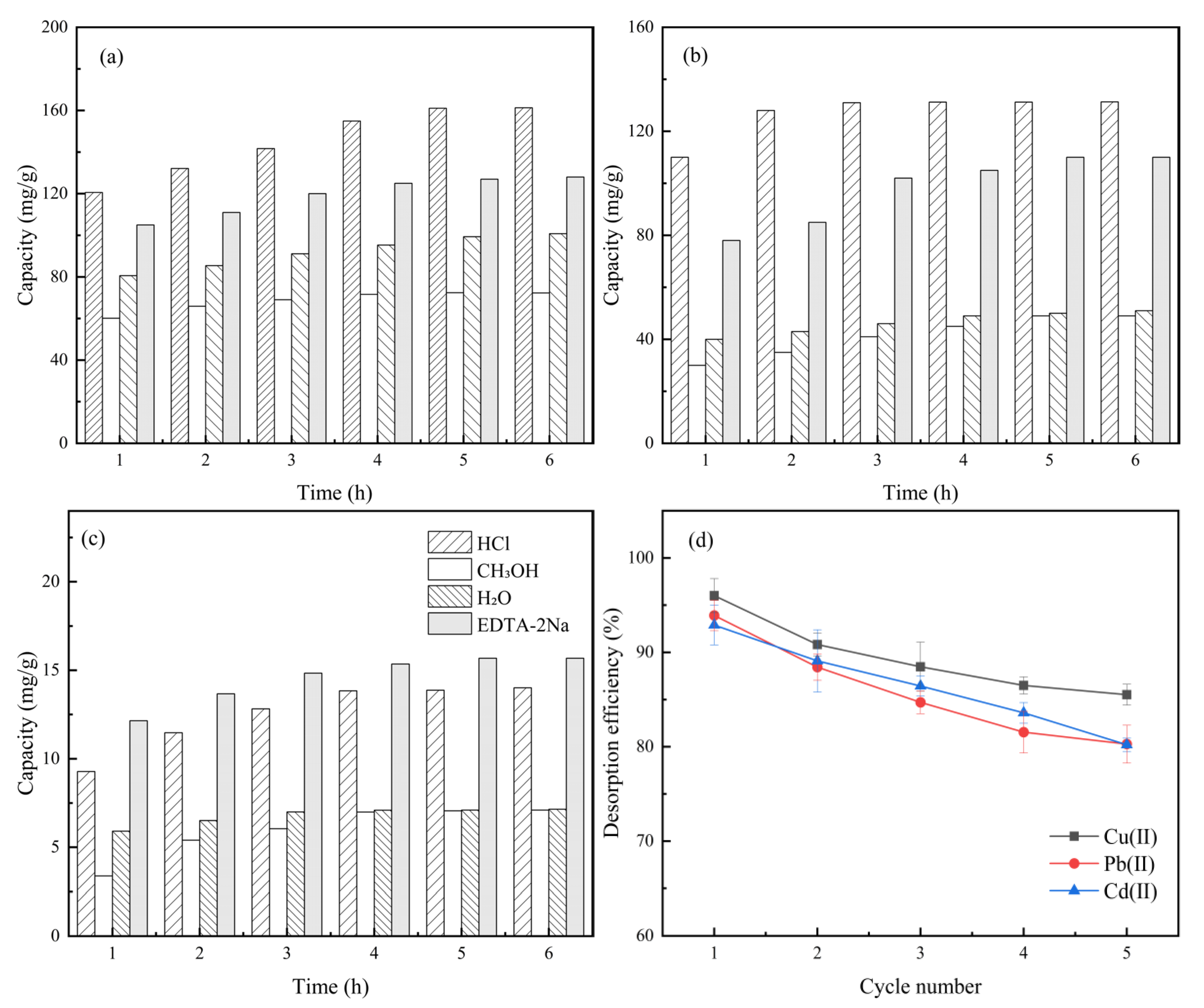
2.6. Regeneration Experiment
2.7. Research on Adsorption Mechanism
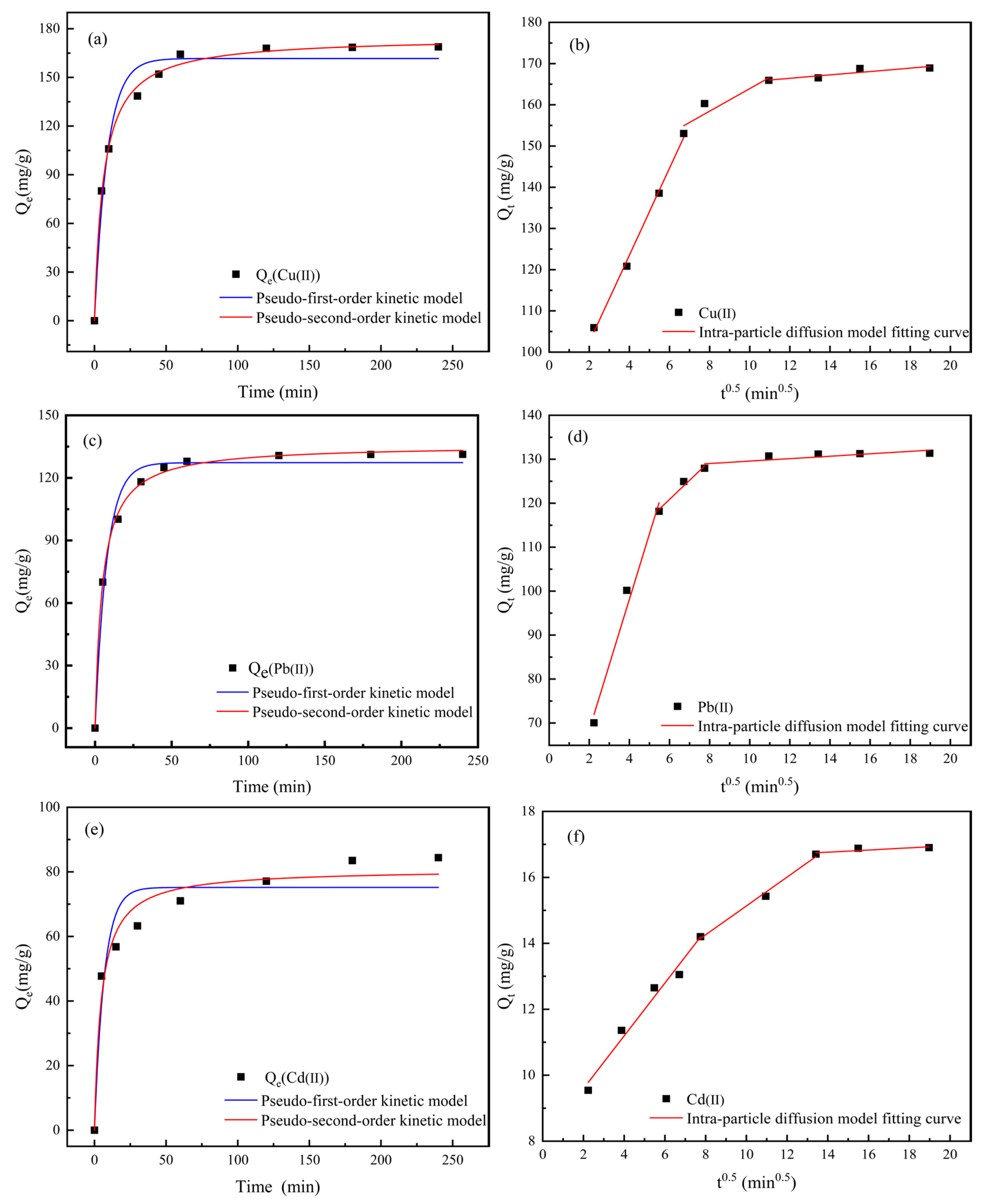
2.7.1. Research on Adsorption Kinetics
- 1.
- Pseudo-First-Order Model
- 2.
- Pseudo-Second-Order Model
- 3.
- Intraparticle Diffusion Model
2.7.2. Research on Adsorption Isotherms
- 4.
- Langmuir isothermal adsorption model:
- 5.
- Freundlich isothermal adsorption model:
3. Results and Discussion
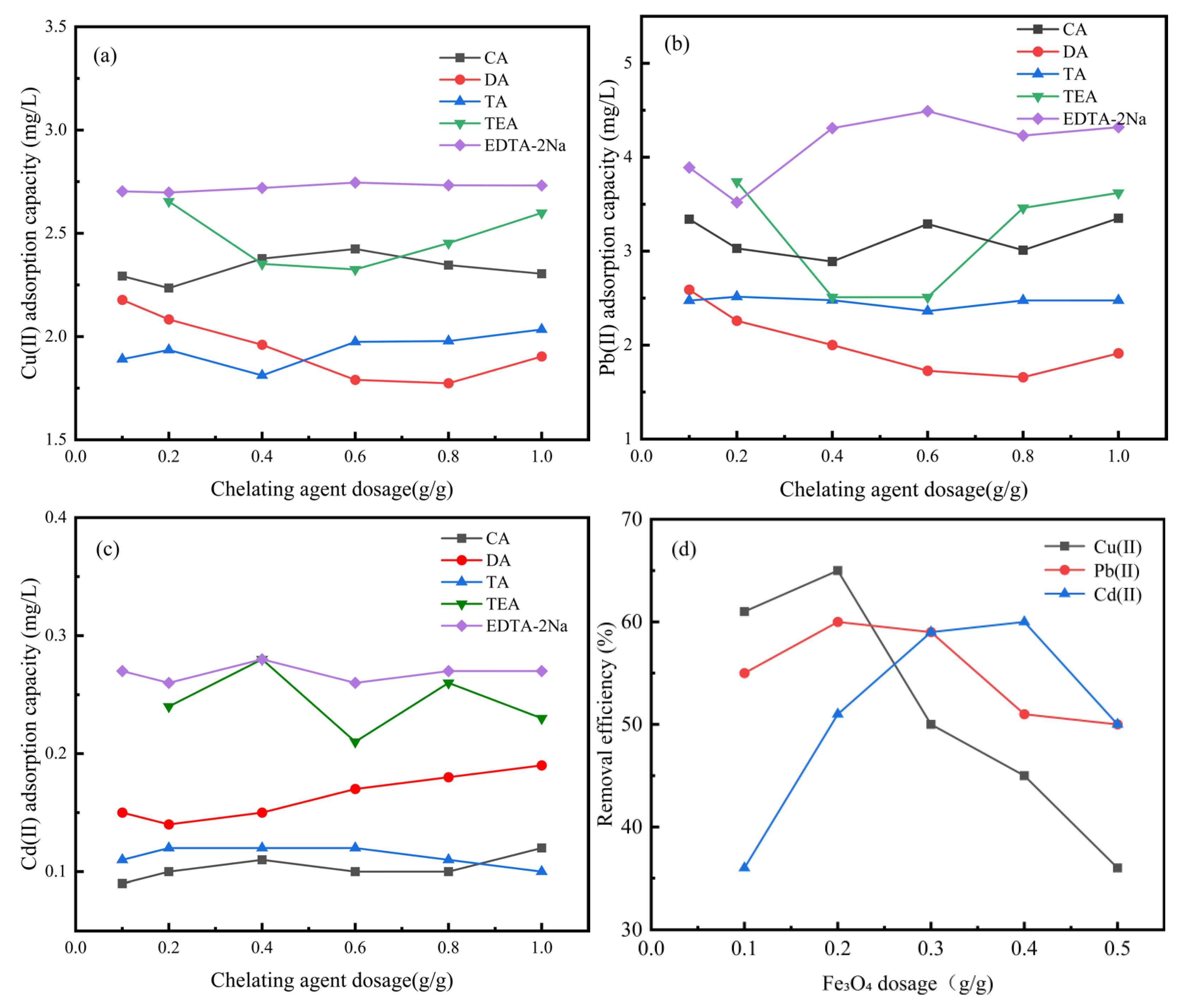
3.1. Selection of Chelating Agents and Fe3O4 Range
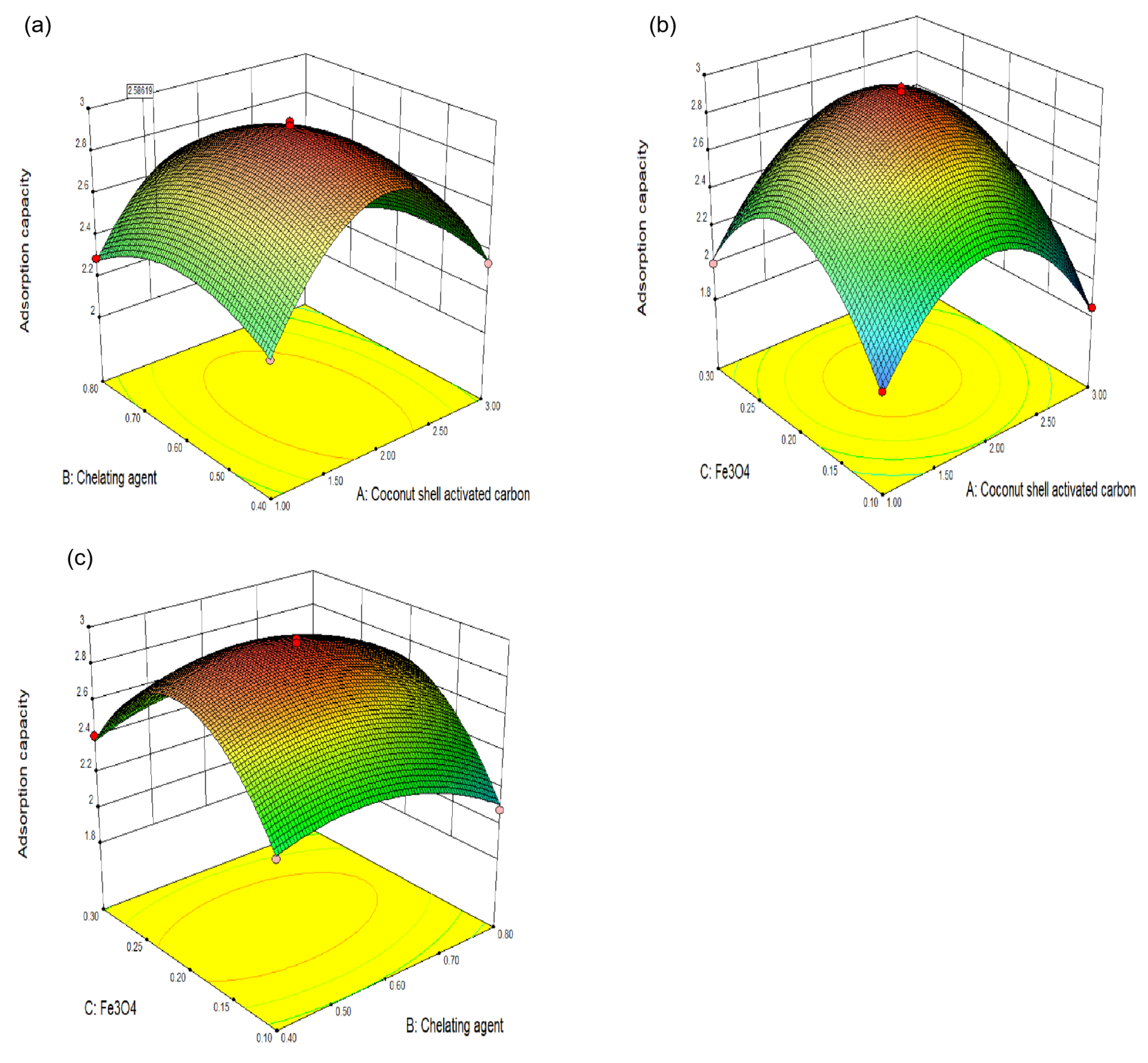
3.2. An Analysis of the Experimental Results of Response Surface Optimization
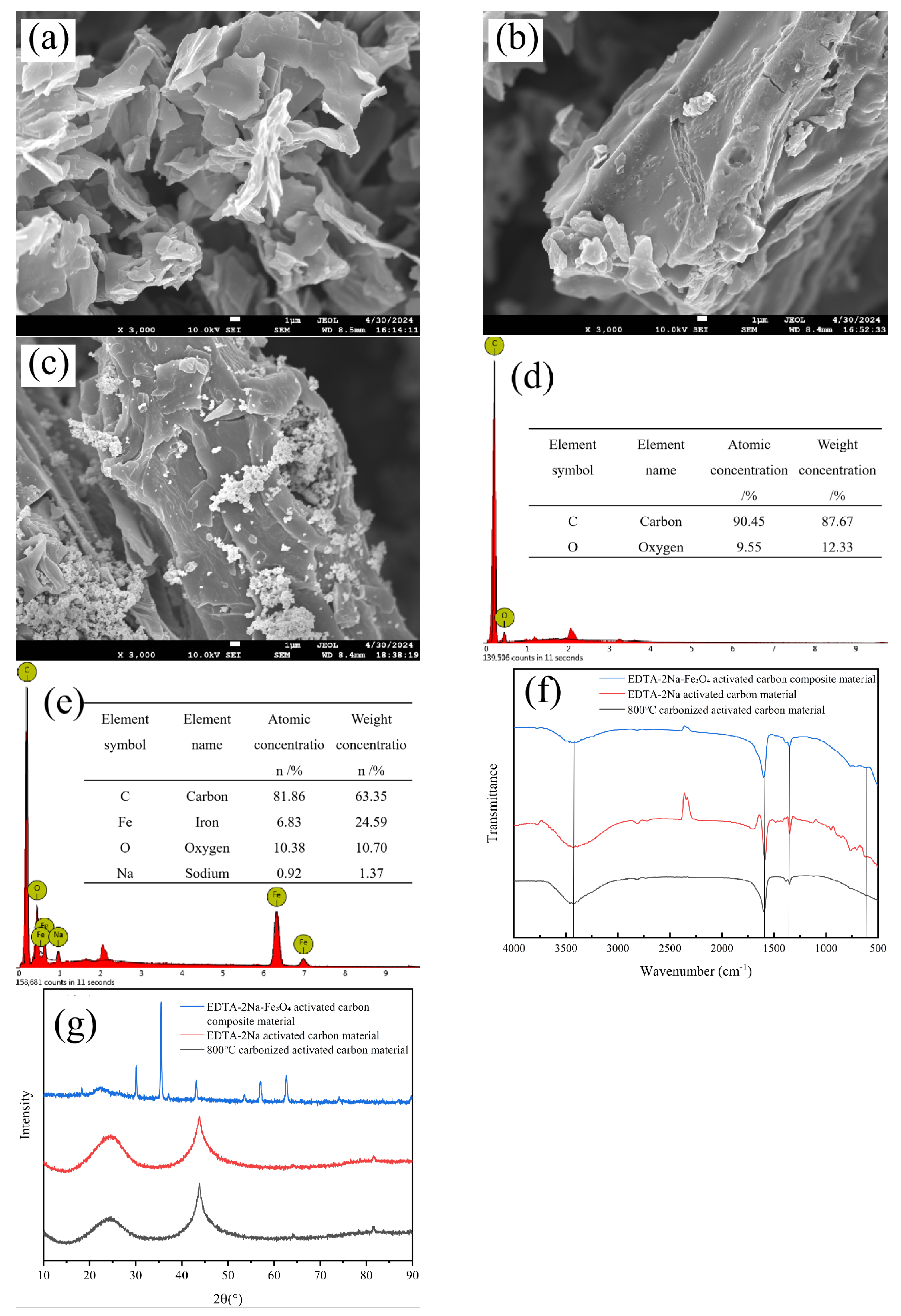
3.3. Material Characterization
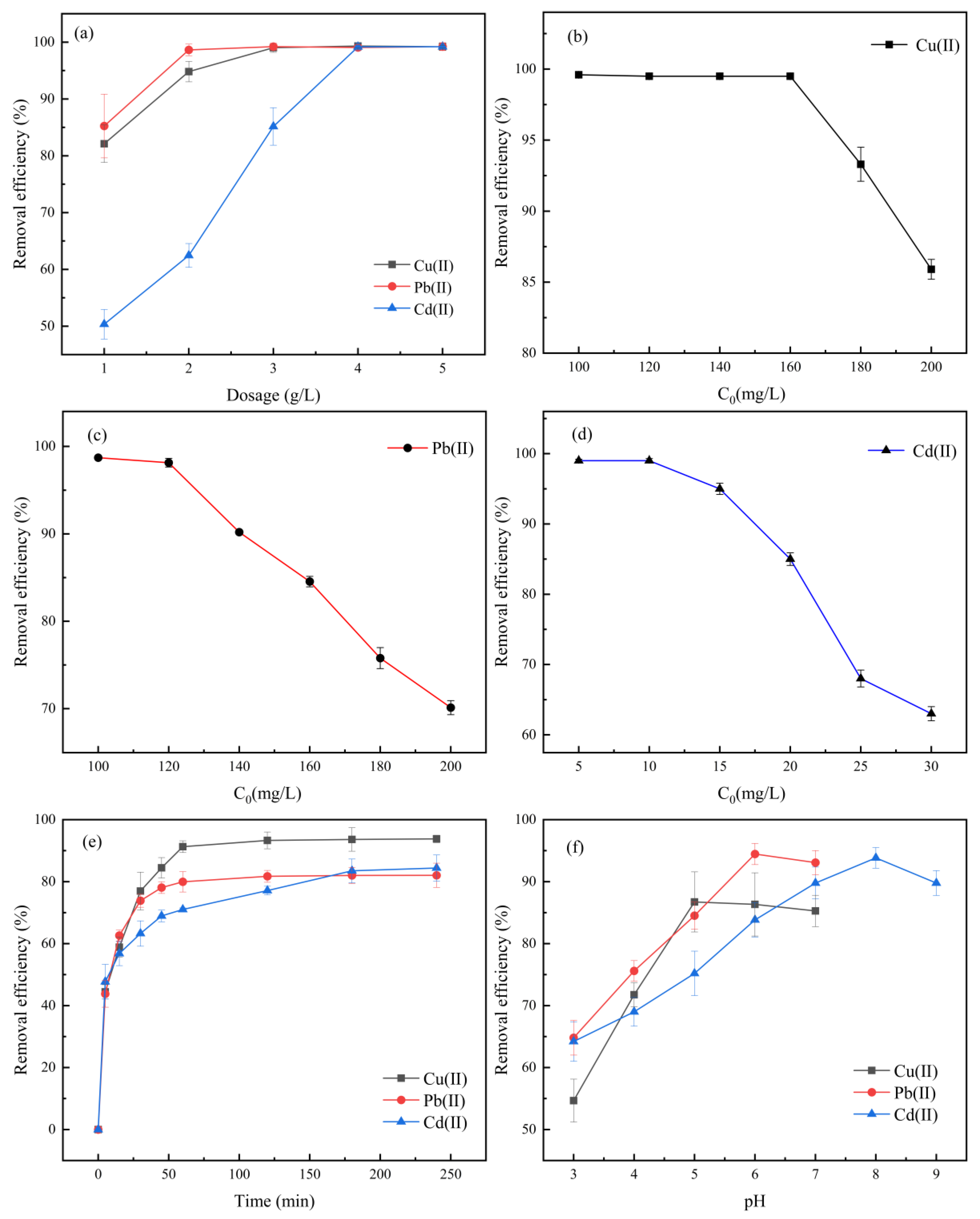
3.4. Single-Factor Adsorption Experimental Analysis
- (1)
- The economic dosing thresholds of Cu2+, Pb2+, and Cd2+ are 3 g/L, 2 g/L, and 4 g/L, respectively. At this time, the adsorption capacity per unit mass and the removal rate reach equilibrium. Excessive addition leads to no significant change in the adsorption efficiency due to the particle aggregation effect, indicating that excessive addition has a limited effect on increasing the adsorption capacity.
- (2)
- The adsorption capacity increases with the increase in the concentration gradient. When the initial concentration reaches 180 mg/L (Cu2+), 160 mg/L (Pb2+), and 20 mg/L (Cd2+), the adsorption capacity approaches the maximum value. Further increasing the initial concentration will lead to a stabilization of the adsorption capacity, while the removal rate decreases due to the saturation of the adsorption sites. Therefore, the optimal initial concentrations are selected as Cu2+ 180 mg/L, Pb2+ 160 mg/L, and Cd2+ 20 mg/L. At this time, the adsorption capacity is close to the maximum value.
- (3)
- The removal rate of the adsorption process increased rapidly within the initial 60 min (Cu2+, Pb2+, and Cd2+ reached more than 85% of the equilibrium adsorption capacity, respectively), and then the adsorption rate slowed down significantly, basically reaching equilibrium after 240 min. From an economic perspective, the comprehensive benefit of adsorption efficiency and time cost is optimal at 60 min. Therefore, the adsorption time for subsequent experiments is uniformly set at 60 min.
- (4)
- At a low pH (3.0–5.0), H+ competes with heavy metal ions for adsorption sites, resulting in a relatively weak adsorption capacity. When the pH rises to 5.0–6.0, the deprotonation of surface functional groups enhances electrostatic attraction, and the adsorption capacity increases significantly. When pH further increases (>6.0), the adsorption capacity of Cu2+ and Pb2+ decreases due to the formation of hydroxide precipitates (Cu(OH)2 and Pb(OH)2) or hydroxyl complexes. Cd2+ begins to form Cd(OH)2 precipitates and Cd(OH)3− and other complexes when pH>8, resulting in a decrease in adsorption capacity.
3.5. Regeneration Experiment Analysis
3.6. Analysis of Adsorption Mechanism
Adsorption Kinetics Analysis
3.7. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; He, M.; Teng, W.; Li, F.; Qiu, X.; Li, K.; Wang, H. Ordered mesoporous materials for water pollution treatment: Adsorption and catalysis. Green Energy Environ. 2024, 9, 1239–1256. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, C.; Yan, Z.; Jiang, H.; Xu, H. Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere 2018, 211, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, H.; Regupathi, I.; Pillai, M.G.; Miranda, L.R. Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Colloids Surf. B Biointerfaces 2009, 70, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Parsadoust, F.; Shirvani, M.; Shariatmadari, H.; Dinari, M. Effects of GLDA, MGDA, and EDTA chelating ligands on Pb sorption by montmorillonite. Geoderma 2020, 366, 114229. [Google Scholar] [CrossRef]
- Cui, X.; Martin, D.C. Fuzzy gold electrodes for lowering impedance and improving adhesion with electrodeposited conducting polymer films. Sens. Actuators A Phys. 2003, 103, 384–394. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Zhang, B.; Xu, L.; Hu, X. Equilibrium and kinetics studies on the absorption of Cu (II) from the aqueous phase using a β-cyclodextrin-based adsorbent. Carbohydr. Polym. 2012, 88, 609–617. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Chen, R.; Chai, L.; Li, Q.; Shi, Y.; Wang, Y.; Mohammad, A. Preparation and characterization of magnetic Fe3O4/CNT nanoparticles by RPO method to enhance the efficient removal of Cr(VI). Curr. Top. Microbiol. Immunol. 2013, 20, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, S.D.; Kamani, H.; Jaafari, J.; Mahvi, A.H. Experimental design and response surface modeling for optimization of fluoroquinolone removal from aqueous solution by NaOH-modified rice husk. Desalination Water Treat. 2016, 57, 16456–16465. [Google Scholar] [CrossRef]
- He, D.; Du, J.; Liu, P.; Liu, X.; Chen, X.; Li, W.; Zhang, K.; Ma, F. Influence of EDTA-2Na on the hydroxyapatite coating deposited by hydrothermal-electrochemical method on Ti6Al4V surface. Surf. Coat. Technol. 2019, 365, 242–247. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Z.; Shi, S.; Zhang, S.; Yang, S.; Zhang, X.; Gao, P.; Zhang, Y. Preparation of Cellulose-Grafted Acrylic Acid Stabilized Jujube Branch Biochar-Supported Nano Zero-Valent Iron Composite for Cr (VI) Removal from Water. Nanomaterials 2025, 15, 441. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liang, H.; Yang, L.; Yang, X.; Yang, C.; Hu, G.; Zhao, T. Porous biochar derived from waste distiller’s grains for hexavalent chromium removal: Adsorption performance and mechanism. J. Environ. Chem. Eng. 2023, 11, 110137. [Google Scholar] [CrossRef]
- Nguyen, T.H.H.; Pham, T.D.; Truong, T.T. Adsorption characteristics and mechanisms of individual and a quinary mixture of heavy metal ions on novel CoFe2O4-BiFeO3 nanosorbents in water. Inorg. Chem. Commun. 2024, 170, 113177. [Google Scholar] [CrossRef]
- Lv, Y.; Wen, W.; Han, S.; Li, K.; Fu, Z.; Mu, F.; Luo, M. Preparation, Characterization, and Mechanism of SMS Titanium–Manganese Nanocomposite for Antimony Removal from Water. Separations 2025, 12, 38. [Google Scholar] [CrossRef]
- Kamenická, B.; Weidlich, T.; Švancara, I. Voltammetric determination of flufenamic acid and adsorption studies with biochar in the absence/presence of cetyltrimethylammonium bromide. Talanta 2023, 266, 125073. [Google Scholar] [CrossRef] [PubMed]

| Value Level | Influence Factor | ||
|---|---|---|---|
| A—Coconut Shell Activated Carbon (g) | B—Chelating Agent (g) | C—Fe3O4 (g) | |
| −1 | 1 | 0.4 | 0.1 |
| 0 | 2 | 0.6 | 0.2 |
| 1 | 3 | 0.8 | 0.3 |
| Source | Sum of Squares | df | Mean Squares | F Value | Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 2.5458 | 9 | 0.2829 | 233.6379 | <0.0001 | significant |
| A | 0.0078 | 1 | 0.0078 | 6.4528 | 0.0387 | |
| B | 0.0351 | 1 | 0.0351 | 29.0015 | 0.0010 | |
| C | 0.0392 | 1 | 0.0392 | 32.3776 | 0.0007 | |
| AB | 0.0121 | 1 | 0.0121 | 9.9941 | 0.0159 | |
| AC | 0.0006 | 1 | 0.0006 | 0.5162 | 0.4957 | |
| BC | 0.0056 | 1 | 0.0056 | 4.6460 | 0.0681 | |
| A2 | 1.1059 | 1 | 1.1059 | 913.4451 | <0.0001 | |
| B2 | 0.1181 | 1 | 0.1181 | 97.5718 | <0.0001 | |
| C2 | 1.0109 | 1 | 1.0109 | 835.0008 | <0.0001 | |
| Residual | 0.0085 | 7 | 0.0012 | |||
| Lack of Fit | 0.0045 | 3 | 0.0015 | 1.4917 | 0.3447 | not significant |
| Pure Error | 0.0040 | 4 | 0.0010 | |||
| Cor Total | 2.5543 | 16 |
| Std. Dev. | 0.034 | R-Squared | 0.9964 |
|---|---|---|---|
| Mean | 2.39 | Adj R-Squared | 0.9929 |
| C.V.% | 1.41 | Pred R-Squared | 0.9783 |
| PRESS | 0.055 | Adeq Precision | 44.978 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg/g) | R2 | K2 (g/(mg·min)) | Qe (mg/g) | R2 | |
| Cu | 0.012 | 168.82 | 0.9241 | 0.00591 | 169.49 | 0.9986 |
| Pb | 0.018 | 131.2 | 0.9852 | 0.00525 | 131.58 | 0.9984 |
| Cd | 0.016 | 16.88 | 0.9124 | 0.059019 | 16.95 | 0.9522 |
| Intraparticle Diffusion Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 (g/mg/min0.5) | C1 (mg/g) | R12 | K2 (g/mg/min0.5) | C2 (mg/g) | R22 | K3 (g/mg/min0.5) | C3 (mg/g) | R32 | |
| Cu | 10.55071 | 81.3309 | 0.99679 | 2.73737 | 136.57061 | 0.87643 | 0.41067 | 161.51277 | 0.81805 |
| Pb | 14.85224 | 38.76026 | 0.98086 | 4.35142 | 94.7643 | 0.87643 | 0.27721 | 126.81259 | 0.8659 |
| Cd | 0.80018 | 7.99424 | 0.97641 | 0.43794 | 10.75559 | 0.99222 | 0.03254 | 16.30941 | 0.89743 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | KF (mg/g) | 1/n | R2 | |
| Cu | 181.84 | 0.00207 | 0.96619 | 3.10221 | 0.76574 | 0.99959 |
| Pb | 162.31 | 0.00825 | 0.93661 | 12.87275 | 0.45627 | 0.90977 |
| Cd | 20.36 | 0.04187 | 0.97062 | 2.40471 | 0.62008 | 0.94158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Han, S.; Wen, W.; Bai, X.; Sun, Q.; Chen, L.; Zhang, H.; Mu, F.; Luo, M. Preparation of EDTA-2Na-Fe3O4-Activated Carbon Composite and Its Adsorption Performance for Typical Heavy Metals. Separations 2025, 12, 205. https://doi.org/10.3390/separations12080205
Lv Y, Han S, Wen W, Bai X, Sun Q, Chen L, Zhang H, Mu F, Luo M. Preparation of EDTA-2Na-Fe3O4-Activated Carbon Composite and Its Adsorption Performance for Typical Heavy Metals. Separations. 2025; 12(8):205. https://doi.org/10.3390/separations12080205
Chicago/Turabian StyleLv, Yannan, Shenrui Han, Wenqing Wen, Xinzhu Bai, Qiao Sun, Li Chen, Haonan Zhang, Fansong Mu, and Meng Luo. 2025. "Preparation of EDTA-2Na-Fe3O4-Activated Carbon Composite and Its Adsorption Performance for Typical Heavy Metals" Separations 12, no. 8: 205. https://doi.org/10.3390/separations12080205
APA StyleLv, Y., Han, S., Wen, W., Bai, X., Sun, Q., Chen, L., Zhang, H., Mu, F., & Luo, M. (2025). Preparation of EDTA-2Na-Fe3O4-Activated Carbon Composite and Its Adsorption Performance for Typical Heavy Metals. Separations, 12(8), 205. https://doi.org/10.3390/separations12080205









