Abstract
The rapid pace of global industrialization and population growth has intensified freshwater scarcity and water pollution, necessitating urgent solutions. Adsorption technology, favored for its cost-effectiveness, simplicity, and scalability, has emerged as a promising approach. Hydrogels, particularly cellulose-based hydrogels (CBHs), have gained significant attention as green adsorbents due to their biodegradability, non-toxicity, low cost, and exceptional adsorption capacity. This paper reviews recent advancements in CBHs for sustainable wastewater treatment, focusing on synthesis techniques, performance, and mechanisms for removing heavy metals, dyes, and micropollutants. Updated applications and their outcomes are also discussed. Despite their advantages, CBHs face challenges such as limited mechanical strength, practical production difficulties, insufficient reuse studies, and separation inefficiencies. This review addresses these issues and explores future prospects for their practical implementation. The findings provide valuable insights into advancing CBHs in sustainable and efficient water treatment solutions.
1. Introduction
Extreme climate change, such as global warming and drought, as well as emerging pollutants, have been threatening water security [1,2,3,4,5]. Compounding this issue is the ongoing rise in the global population and levels of industrialization, resulting in an unabating demand for potable water supplies [6,7,8,9,10]. Moreover, the issue of water scarcity is becoming increasingly pronounced due to the contamination of water bodies by wastewater emanating from various industrial sectors, including textiles, smelting, chemicals, pharmaceuticals, and food production [11,12,13,14,15]. It is imperative to treat wastewater prior to its discharge. In order to meet the requirements for the discharge of treated wastewater, a variety of efficient water and wastewater treatment technologies have been developed to remove pollutants from water [16,17,18,19,20]. These include the development of new membranes [21,22,23,24,25,26,27], disinfection technologies [28,29,30,31,32,33], advanced oxidation processes [34,35,36,37,38], and the use of high-performance adsorbents in the context of adsorption treatment [39,40,41,42,43,44]. Among the technologies mentioned above, adsorption is one of the most widely used for the sustainable and effective removal of pollutants from water.
Compared to other water treatment technologies, adsorption is economical, efficient, selective, and easy to operate with low energy requirements [45,46,47,48]. In the industrial context, conventional adsorbents encompass derivatives of activated carbon, graphene, carbon nanotubes, and analogous materials. Furthermore, adsorbent materials encompass polymer-based systems (for instance, polyacrylonitrile derivatives [49,50,51,52,53,54]) and porous materials (e.g., metal–organic frameworks [55,56,57,58,59,60,61] and zeolites [62,63,64,65]). Among these, porous materials are frequently utilized as functional components in the construction of composite adsorbents—a consequence of their elevated specific surface area and tunable pore structure. However, these industrial adsorbents typically exhibit certain substantial drawbacks, including the requirement of energy-intensive production processes (e.g., pyrolysis), the complexity of regeneration, and the overall high costs of production [66,67,68,69,70]. Consequently, there is a growing interest in the development of alternative adsorbents utilizing a range of innovative materials that have demonstrated enhanced adsorptive removal of pollutants. In this regard, hydrogels are relatively new in the development of alternative adsorbents with the built-in ability to capture a wide range of pollutants from water [71,72,73,74,75,76].
Hydrogels are three-dimensional networks of polymer chains that can absorb and retain large amounts of water without being water soluble [77,78,79]. They are widely used in biomedical and environmental applications due to their bioactivity, biocompatibility, and high-water content. In addition, they are highly porous, hydrophilic, non-toxic, and highly functional, making them an ideal alternative to green adsorbents [80,81]. Hydrogels can be classified according to their composition, cross-linking mechanism, and swelling behavior [82,83,84,85]. In the field of hydrogel preparation, petroleum-based materials such as N-isopropylacrylamide, hydroxyethyl methacrylate, and acrylic acid are frequently utilized due to their efficacy in contaminant removal from water. However, the utilization of synthetic polymer hydrogels is accompanied by significant environmental concerns, as well as the challenges posed by their cost and limited accessibility. Consequently, there is an increasing inclination towards utilizing natural materials in the fabrication of hydrogels [1,75,86,87]. Among the natural polymers utilized in the development of hydrogels, cellulose stands as the most prevalent polysaccharide (a significant constituent of plant cell walls) and is widely regarded as the optimal choice for hydrogel-based polymers due to its unique properties such as low cost, easy availability, biodegradability, high biocompatibility, non-toxicity, renewability, and reusability [45,88]. Nevertheless, inherent limitations of natural polymers result in certain disadvantages of hydrogels based on natural materials, including inadequate mechanical strength [89,90]. Fortunately, the employment of diverse strategies has led to the enhancement of natural polymer hydrogels in terms of mechanical strength. For instance, dual and interpenetrating network hydrogels have been shown to be an effective strategy for enhancing the mechanical strength of natural polymer-based hydrogels [1,91,92].
In the domain of water and wastewater treatment, cellulose-based hydrogels (CBHs) have been shown to possess significant potential as highly adsorbent materials for the removal of micropollutants, heavy metals, and dyes from contaminated water [93,94,95,96,97]. In recent years, driven by the goal of a circular economy, researchers have expanded the application dimensions of CBHs. This has enabled not only enhanced adsorption capacity and selectivity of target pollutants, but also efficient recovery and enrichment of strategic metal resources (e.g., uranium and lithium) in wastewater through the design of specific functional groups (e.g., phosphoric acid moiety and amidoxime moiety) [98]. For instance, in one report, phosphorylated CNF (PHO-CNF) was utilized with an exceptionally high adsorption capacity (1550 mg/g) and selectivity for U(VI) [99]. This can be attributed to the high surface area, anionic charge, and the high affinity of the phosphate group for U(VI) of PHO-CNF. In addition, it has been demonstrated that the charge–radius ratio of the phosphate group to U(VI) and the hydrolysis of U(VI) also contribute to the high selectivity. Huang et al. generated nanostructured poly-amidoxime (PAO) structural domains in situ in the CNF matrix, which resulted in high adsorption and selectivity for U [100]. This functionality-oriented molecule was designed to provide the high surface area of PHO-CNF and the high affinity of PHO-CNF for U(VI). This functionally oriented molecular design provides an innovative solution for integrated water treatment–resource recovery technologies.
In light of the aforementioned points, the present review offers a comprehensive overview of recent advancements in the development of sustainable and efficient CBHs for wastewater treatment. The paper’s central theme is advanced preparation techniques and performance optimization methods for CBHs, as well as their recent applications in the removal of pollutants from wastewater. The discussion highlights their advantages, limitations, and future prospects. A critical analysis of various examples was undertaken, and it can be concluded that CBHs are one of the effective adsorbents for the removal of heavy metals, dyes, and micropollutants. It is hoped that this paper will provide insights into new strategies for developing high-performance CBHs for water treatment.
Although previous reviews have established a methodological framework for CBHs from synthesis to performance evaluation (e.g., Le et al.) [77], focusing on the application of CBHs in the field of heavy metal removal (e.g., Kushwaha et al. and Persano et al.) [45,88], their analyses have still concentrated on only one aspect of CBHs, particularly neglecting dye adsorption and micropollutant remediation. This paper offers a distinctive integration of existing research, highlighting advances in the removal mechanism of CBHs and their potential for micropollutant removal. Through critical analysis of various examples, it is concluded that CBHs will emerge as a multifunctional remediation platform, transcending the conventional role of adsorbents.
2. Cellulose-Based Hydrogels for Sustainable and Efficient Water Treatment
2.1. Basic Properties and Chemical Structure of Cellulose
Cellulose, the most abundant natural polymer on Earth, is a long-chain polysaccharide consisting of β-D-glucose units linked together by β-1,4-glycosidic bonds [101,102]. This unique molecular architecture renders cellulose exceptional physicochemical stability and utility through water insolubility [103]. The long chains of cellulose form two distinct regions: highly ordered crystalline and less-structured amorphous through a dual structural arrangement. The former further enhances stability and mechanical strength, while the latter brings reactivity as well as biological properties [101,104]. Predominantly sourced from plant cell walls, cellulose can also be biosynthesized by microbial species. There are ongoing efforts to utilize agricultural waste as the primary cellulose resource [105].
Cellulose can also be converted into cellulose derivatives (CD), such as cellulose nanocrystals (CNC), cellulose nanofibrils (CNF), bacterial nanocellulose (BNC), and other cellulose derivatives, through chemical or mechanical pre-treatments, in response to the needs of different wastewater treatments [106,107,108]. Specifically, the chemical modification of cellulose by esterification [109], grafting, etherification, oxidation [110], and cross-linking is a common procedure, yielding the desired CDs. In addition to these methods, treatments involving acids, alkalis, and a combination of organic and inorganic reagents can also be employed to achieve the desired result [106,111,112]. It has been demonstrated that through the process of modification, it is possible to introduce additional functional groups into cellulose, thereby increasing the number of hydrogel adsorption sites. In addition, this process enables the regulation of the pore structure of cellulose, resulting in an enhancement of the specific surface area of the hydrogel. Furthermore, it has been shown that modified cellulose can be further optimized to enhance its performance advantages, and it can be endowed with responsiveness to specific stimuli. It is evident that modified hydrogels have the capacity to be utilized in the immobilization of enzymes or catalysts, enhancing operational stability, recyclability, and catalytic efficacy in continuous processes.
2.2. Cellulose-Based Hydrogels for Water and Wastewater Treatment
CBHs have been demonstrated to be both sustainable and effective in the removal of a variety of water pollutants, including metals, dyes, and micropollutants, while enabling desalination and atmospheric water harvesting [74]. The hydrophilic nature of CBHs facilitates water uptake and transport. This, in conjunction with their high porosity, enables efficient water uptake and the effective adsorption of pollutants [113]. In addition, hydrogels prepared with cellulose have been shown to exhibit excellent stability and mechanical strength, and can be reused for water treatment [77]. Another fascinating feature of CBH is their biodegradability, which allows them to be degraded by microorganisms in water, air, or soil [88]. This has the effect of reducing the environmental impact of residual materials after water treatment and increasing the sustainability of the whole process.
The preparation of CBHs can be categorized into three distinct methodologies: physical cross-linking, chemical cross-linking, and self-assembly synthesis. In physical cross-linking methods, cellulose chains are cross-linked using physical means such as temperature, pressure, or radiation, and the hydrogels obtained consist of non-covalent cross-links and are, therefore, reversible networks [114]. These non-covalent forces can be van der Waals forces, hydrogen bonding, hydrophobic, or electronic interactions [45]. Chemical cross-linking, which involves the use of chemical cross-linking agents to covalently link cellulose chains, has been shown to outperform physical cross-linking [115]. The reason for this is that chemical cross-linking produces irreversible networks with higher stability, adsorption, and mechanical properties. Self-assembled 3D networks, on the other hand, rely on cellulose’s inherent capacity to form ordered structures, a process that involves intermolecular forces such as van der Waals forces, hydrogen bonding, and electrostatic interactions [116,117,118,119].
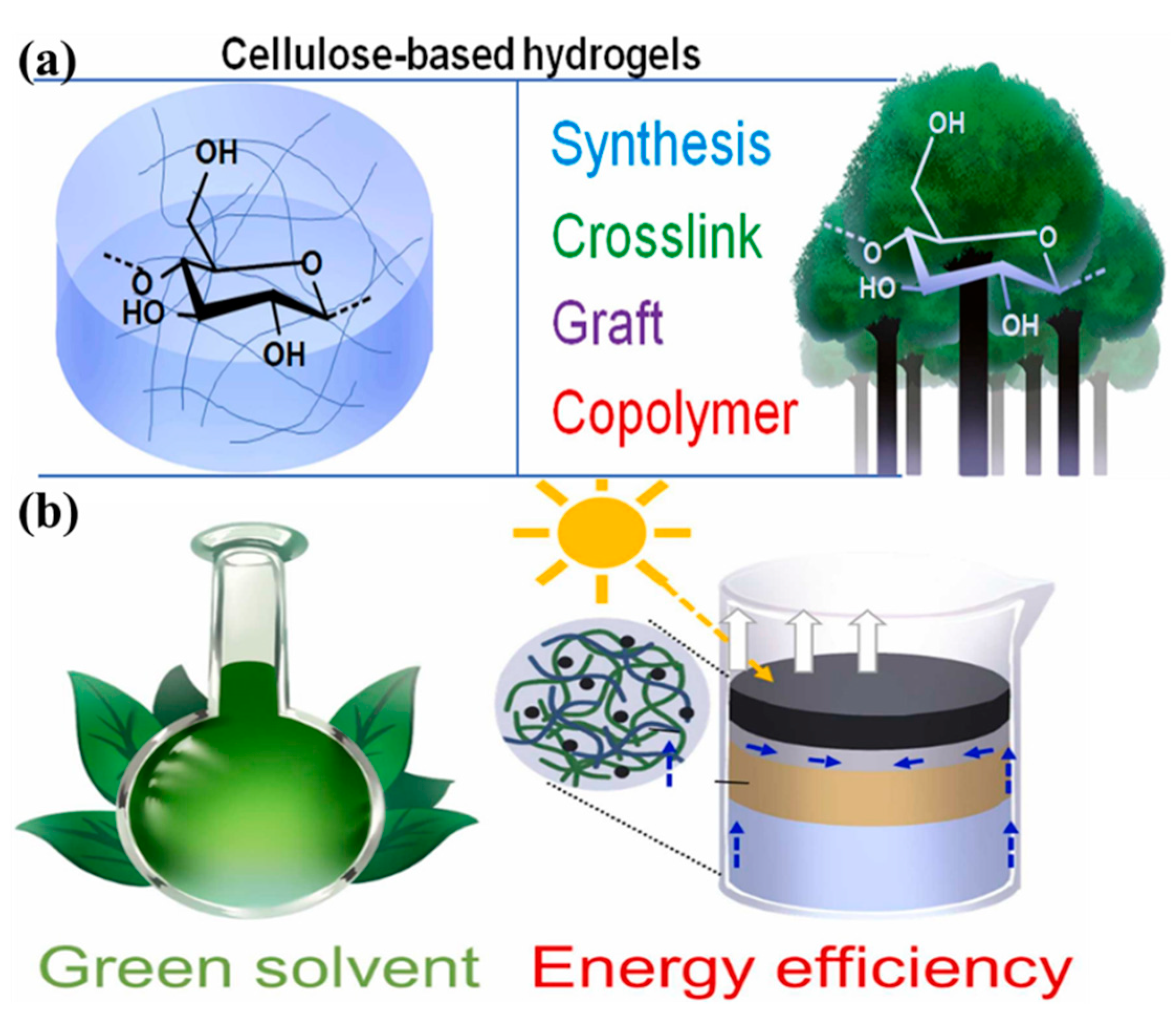
CBHs performance is tunable via cross-linking, co-mingling, or functionalization, enhancing contaminant selectivity, structural stability, reusability, and operational efficiency in aqueous remediation [120]. Precise control over factors governing cellulose hydrogel characteristics (e.g., cross-linking density and crystallinity ratio) enables tailored engineering of functionality and stability to meet application-specific performance benchmarks in biomedical, environmental, and materials engineering systems [101,121]. The initial factor to be considered is the selection of a solvent to enhance the solubility of cellulose, which consequently impacts the structure and properties of the resulting hydrogel [122,123]. Presently, green solvents, such as deep eutectic solvents, are more prevalent in research, as they are capable of effectively dissolving cellulose while maintaining its excellent biocompatibility and biodegradability [101,124,125]. The properties of CBHs are influenced by many factors, including pH, temperature, and swelling kinetics. For instance, the pH of the medium affects the ionization state of cellulose, thereby influencing its structural integrity and interactions within the hydrogel matrix. Conversely, elevated temperatures have been observed to promote cellulose solubilization, as well as cross-linking reactions, resulting in the rapid formation of stable hydrogel networks [126]. Furthermore, the cross-linking method employed is pivotal in determining the structural and functional properties of CBHs. Given the considerable effect that the cross-linking method has on the material’s properties and its suitability for a specific application, the chosen method must be consistent with the intended use of the hydrogel [127]. As illustrated in Figure 1, the synthesis of CBHs is depicted, along with a schematic representation of a green and energy-efficient production process.
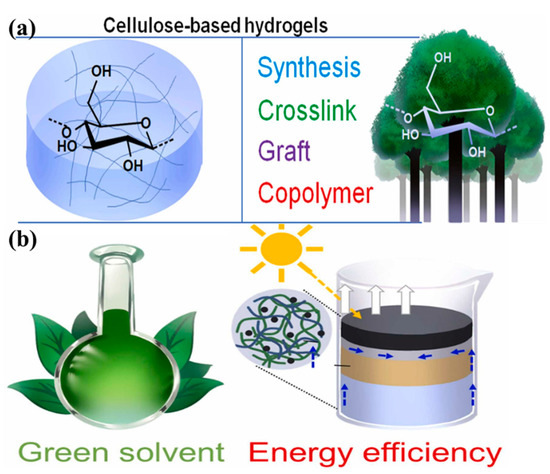
Figure 1.
Synthesis of cellulose-based hydrogels: (a) chemical or physical cross-linking and copolymerization; (b) schematic representation of the production of cellulose-based hydrogels using green solvents and energy-saving techniques. Reproduced from [77], Copyright (2023), with permission from Elsevier.
3. Sustainable Synthesis and Processing of Cellulose-Based Hydrogels
In the context of sustainable water treatment, the synthesis methods employed have a significant impact on the stability, regeneration, and reusability of the internal structure of hydrogels. In the context of efficient water treatment, the synthesis methods employed result in variations in the composition and nature of reactive functional groups present on the surface of the hydrogel, as well as in the specific surface area of the porous structure. These variations, in turn, have a significant impact on the adsorption capacity, hydrophilicity, and the rate of water treatment of the hydrogel. In light of these considerations, the factors influencing the sustainability and efficiency of hydrogels during the synthesis and processing of CBHs are becoming increasingly significant in materials research [96]. A significant challenge in synthesizing CBHs pertains to their solubility in common solvents. While certain cellulose derivatives exhibit water solubility, natural cellulose demonstrates virtually no solubility in most prevalent organic and inorganic solvents. Consequently, the selection of an appropriate solvent system for the dissolution of cellulose is a prerequisite for the synthesis of hydrogels. Commonly employed solvents include alkali/urea (or thiourea), LiCl/dimethylacetamide, N-methyl-morpholine-N-oxide, and ionic liquids [113].
The presence of hydrophilic functional groups (e.g., hydroxyl groups (-OH)) on the cellulose chains allows them to form stable 3D network structures through physical cross-linking (e.g., electrostatic interactions) and chemical cross-linking (covalent interactions using cross-linking agents) [113]. These processes are essential for retaining a large amount of water in the interstitial spaces and, thus, forming hydrogels. The primary methodologies employed for the preparation of CBHs encompass physical cross-linking and chemical cross-linking. Each method possesses distinct advantages and application areas. Through judicious selection and combination of these methods, the structure and performance of hydrogels can be tailored to meet the specific requirements of wastewater treatment. Table 1 provides a concise overview of several manufacturing methodologies and their respective characteristics.

Table 1.
Fabrication methods and their characteristics.
Table 1.
Fabrication methods and their characteristics.
| Methods | Characteristic | References |
|---|---|---|
| Physical cross-linking by hydrogen bonding, ionic interactions, hydrophobic interactions, π–π interactions, and van der Waals forces | Low toxicity, high porosity, higher adsorption opportunities, low sensitivity to pH, and easy regeneration (it is a reversible process), but weak mechanical strength and stability | [113] |
| Chemical cross-linking using cross-linking agents | High mechanical strength and stability, easy to handle, and common; however, toxicity exists and has a large impact on biocompatibility | [128] |
| IPN or semi-IPN | High strength, toughness, and self-healing; complex preparation and existing compatibility issues between different polymer networks | [129] |
| Use of radiation to initiate free radical reactions that promote cross-linking | Fast response, excellent transparency, homogeneity, and mechanical properties, particularly suitable for 3D printing technology; high requirements for control of radiation source, dose, and exposure time | [127] |
3.1. Physical Cross-Linking
The physical cross-linking process renders CBHs reversible since cellulose chains are assembled in a three-dimensional structure formed by intertwining intra- and intermolecular hydrogen bonding, ionic bonding, hydrophobic interactions, and non-permanent forces (e.g., electrostatic forces). In contradistinction to chemical cross-linking, physical cross-linking does not utilize toxic cross-linking agents. Consequently, the resulting hydrogels are more biocompatible and possess low toxicity, which constitutes their primary advantage [130,131,132,133,134]. Furthermore, the presence of a weak interaction force between polymer chains facilitates the formation of physically crosslinked hydrogels with greater ease and expediency. Furthermore, this cross-linking renders the hydrogels more biodegradable, as the weak interactions within the polymer network are more susceptible to degradation than covalent bonds [135]. The aforementioned properties extend the application areas of physically crosslinked CBHs. Conversely, the absence of strong chemical bonds (covalent bonds) within the hydrogel network typically results in physically crosslinked hydrogels that are prone to rapid dissolution and reduced stability [136]. The advent of the dual network hydrogel process represents a significant advancement in addressing the limitations of physically crosslinked hydrogels [137].
In conclusion, despite the poor stability and mechanical properties of CBHs developed through physical cross-linking processes, they are still widely used for adsorption water treatment because of their many attractive advantages, including high porosity, higher chance of adsorption of contaminants, low sensitivity to pH, easy reversible regeneration, and no reduction of the final adsorption capacity due to the potential reaction with cross-linking agents [113,138].
3.1.1. Repeated Freeze–Thaw Cycles
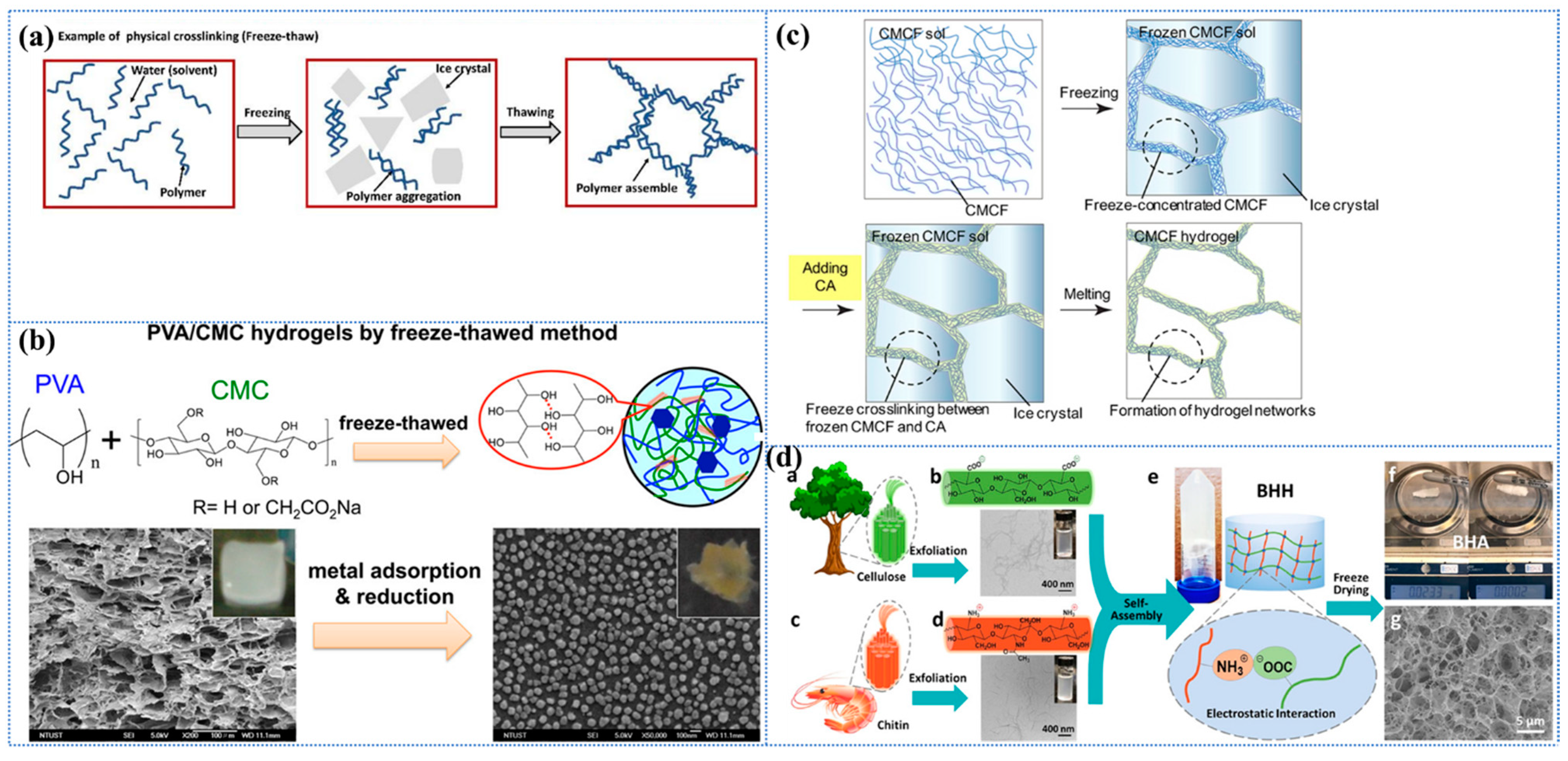
One of the most widely utilized physical cross-linking methodologies employed in the fabrication of sustainable and efficient cellulose-based hydrogel adsorbents is the cryogenic method, which is also designated as the repeated freeze–thaw cycle process [139,140]. The freeze–thaw process has been shown to promote the interaction of hydrogen bonds between cellulose molecules through repeated freeze–thaw cycles. This results in a reduction in mechanical strength, yet it ensures excellent biocompatibility and tunability [141,142]. During these cycles, when the polymer solution is subjected to low temperatures for the purpose of crystallization, the large amount of solvent or low molecular solutes present within it serves to increase the concentration of the polymer by minimizing the chain space in the polymer and forcing the chains to align and combine into a connected network structure of insoluble polymers, which ultimately leads to phase separation and the formation of a hydrogel (as shown in Figure 2a) [113,136]. Furthermore, the process of freeze–thaw cycling has been demonstrated to result in the formation of a porous structure within the hydrogel, thereby increasing the specific surface area. This phenomenon is primarily attributable to the space left by the ice crystals that melt during the thawing phase [113]. The degree of gelation and the mechanical properties of freeze–thaw hydrogels are contingent on pH, polymer concentration, freezing temperature, freeze–thaw time, and number of freeze–thaw cycles.
In a study conducted by Wang et al. [143], a polyvinyl alcohol (PVA)/carboxymethyl cellulose (CMC) composite hydrogel with a high degree of cross-linking was developed for the adsorption of heavy metal ions through repeated freeze–thaw cycles (Figure 2b). Specifically, an insoluble hydrogel was produced by freezing a mixed aqueous solution of PVA and CMC in varying proportions at −20 °C and thawing it at room temperature, repeating the process five times. The findings demonstrated that the proportion of PVA to CMC exerted a substantial influence on the physical characteristics and swelling of the hydrogels. An increase in the CMC content resulted in a decrease in the gel fraction but an increase in the degree of swelling. The swelling degree of pure PVA hydrogel was 416%, while the hydrogel with a 1:2 ratio of PVA/CMC reached 1437%. This phenomenon can be attributed to the enhanced hydrophilicity of CMC, which is accompanied by a reduced amount of PVA. The reduced amount of PVA leads to a lower physical cross-linking density of the hydrogel, thereby facilitating the penetration of water molecules and consequently resulting in an increased swelling capacity. In addition, the hydrogel exhibited excellent adsorption and reduction capabilities, particularly towards Ag+. Unfortunately, their study did not evaluate the recycling performance of this hydrogel further to verify its adsorption sustainability, but the report still opens up the idea of designing CBH with tunable performance. In a separate report, Graphene Oxide (GO) and Attapulgite (ATP) were innovatively introduced into the three-dimensional network structure of bacterial cellulose (BC) PVA by freeze–thaw cycling, and BC/PVA/GO/ATP composite hydrogels were constructed by using the strong interacting hydrogen bonding, which resulted in the increase in the specific surface area of the hydrogel and the improvement in the thermal stability [144]. In contrast, this paper also investigated the reusability and sustainability of the material, which remained above 83.67% after four cycles. The introduction of GO and ATP has been demonstrated to enhance mechanical properties, optimize the pore structure, and form a more compact porous structure. Furthermore, the study has opened up the possibility of conferring multifunctionality to hydrogels. However, the dispersive nature of GO and ATP has also been shown to present significant challenges during preparation; moreover, the study overlooked the impact of these substances on biocompatibility.
In recent years, freeze cross-linking, a novel physical cross-linking method for the synthesis of products with high water content, high compressive strength, and high compression recovery, was developed [145]. Hydrogels were prepared by adding aqueous citric acid (CA) into frozen carboxymethylcellulose nanofiber (CMCF) sols, followed by the thawing of the sols (as illustrated in Figure 2c). This cross-linking method is simple and does not require synthetic reagents, which makes it highly practical and sustainable. The resulting hydrogels are characterized by their high level of biodegradability and are considered non-toxic and environmentally friendly. Furthermore, the freeze cross-linking method facilitates the production of more versatile CMCF hydrogels through the mixing of the CMCF solution with other powder materials (e.g., bentonite) before freezing, and allows for moulding [145]. The powder adsorbent material can be immobilized in this way and, subsequently, easily and efficiently removed from the medium without the use of adhesives, which causes a reduction in the specific surface area of the adsorbent. It is imperative to exercise meticulous control over the freezing rate in this novel cross-linking technique, where slower rates may result in abnormally large pores and faster rates may result in weaker mechanical structures.
3.1.2. Ionotropic Gelation
Ionotropic Gelation (IG) is a notable physical cross-linking hydrogel technology that forms a network structure by allowing electrostatic interactions to produce ionic cross-linking between two ionic substances [146,147,148], at least one of which should be a polymer (e.g., cellulose), under specific conditions [113,149]. In a study, SA/HPC hydrogel beads were synthesized by IG of sodium alginate (SA) and hydroxypropyl cellulose (HPC) solutions at different ratios (0:50, 75:25, and 100:0) [150]. The adsorption properties of the prepared ionically cross-linked hydrogels were found to be largely influenced by the concentration of the two ionic substances from which they were composed, as indicated by adsorption tests. The 75:25 ratio exhibited a higher adsorption capacity of 47.72 mg/g and a 95.45% adsorption percentage of Pb2+. The ionic conductivity, biocompatibility, low toxicity, and reversible crosslinking are all important features of ionic crosslinked CBHs. However, in the context of ionic crosslinked hydrogels, the size of the cations [151], their valence [152], hydration, and the nature of the interaction are pivotal in determining their gel behavior [153].
It is evident that, despite the capacity of CBHs produced by the IG method to furnish a mobile space for free ions, this concomitantly signifies a diminution in thermal and chemical stability, which is deleterious to the long-term utilization of hydrogels. The conductivity and mechanical properties of hydrogels have been demonstrated to be negatively correlated in extant studies, a factor that may also limit their application areas. Additionally, there remains a challenge in accurately characterizing the ion–polymer interactions present within ionic crosslinked networks, which in turn hinders the development of tailored properties.
3.1.3. Self-Assembling
In many cases, adsorption wastewater treatment prefers hydrogels without chemical cross-linking agents to avoid the toxicity of the cross-linking agent. In this sense, self-assembled CBHs hydrogels without the use of foreign cross-linkers have attracted much attention. Self-assembled CBHs are formed when cellulose and its derivatives are used as building blocks to self-assemble to form nanofibers or microfibers through noncovalent interactions (van der Waals forces, electrostatic interactions, hydrogen bonding, and π–π stacking interactions); then, these fibers are entwined to form a strong three-dimensional network structure [154,155,156]. For instance, TEMPO (2,2,6,6-tetramethylpiperidin-1-yloxy)-oxidized cellulose nanofibers (TOCN) and cationic guar gum (CGG) form a hydrogel instantaneously upon contact with each other, and this cross-linking is wholly spontaneous without the assistance of any cross-linking agent [154]. The TOCN/CGG hydrogel is notable for its porous structure and abundance of active sites, which confer upon it a remarkable potential for the removal of heavy metal ions and organic dyes from wastewater. This hydrogel system is entirely biomass-based and does not include any toxic chemicals [157]. Furthermore, the coating of wrapped filter papers in a layer-by-layer deposition process results in hydrogel-coated oil/water separation materials [113,158]. The mechanical properties of self-assembled CBH can be optimized by varying the concentration of the constituent monomers. Li et al. [159] conducted further research on TOCN/CGG self-assembled hydrogels following Cu2+ adsorption, which demonstrated sensitive responsiveness to load weight due to their flexibility and conductivity, which can be utilized as electrodes with optimal performance for supercapacitors, providing a good example for sustainable all-polysaccharide hydrogel management and the utilization of aquatic heavy metal ions. Moreover, the coordination of complex self-assembled structures and various functions is imperative to expand the application areas of CBHs.
To further investigate the gelation behavior of the self-assembly technique, ref. [160] used different concentrations and combinations of negatively charged TOCN on the surface and partially deacetylated chitin nanofibers (PDChNF) with positively charged surfaces to produce CBH by a simple electrostatic force-induced self-assembly gelation process (Figure 2d). The freeze–dried aerogels demonstrated remarkably high levels of adsorption efficiency, with the capacity to adsorb As3+ (217 mg·g−1) and methylene blue (MB) (531 mg·g⁻1) [160]. Subsequent studies demonstrated that the process of self-assembled CBH formation is concentration-independent; however, higher concentrations are conducive to the retention of greater quantities of water. The yield of self-assembly is found to be contingent on the mass ratio of PDChNF/TOCN, which increases with increasing mass ratio in the early stage. The findings indicate that the optimal mass ratio is 5/5, at which point the yield can reach 99% [160]. However, it is observed that the net charge of PDChNF is not equal to zero at this point, which can be attributed to the larger size and aspect ratio of TOCN; thus, a higher charge of PDChNF is required to maintain gelation. Due to the strong electrostatic attraction between the components, the self-assembly preparation is shown to be significantly faster than other physical and chemical cross-linking techniques (with a completion time of under one minute). In addition to being environmentally friendly and time-efficient, the self-assembly technique requires a very low concentration of nanofibers (0.01 wt%), the constituent of the hydrogel, compared to other conventional methods. In summary, the generation of CBHs by electrostatic force-induced self-assembly is a novel and simple method, and the adsorption process is sustainable yet efficient.
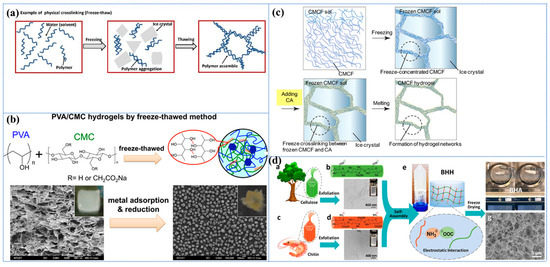
Figure 2.
(a) Schematic representation of a physically cross-linked hydrogel prepared by freeze–thawing. Reproduced from [113]. (b) The schematic of the PVA/CMC hydrogel, prepared by the freeze–thawing process, is presented, along with its cross-sectional image following the adsorption of heavy metals. Reproduced from [143], Copyright (2016), with permission from American Chemical Society. (c) Schematic representation of the CMCF-F hydrogel, prepared by the freeze cross-linking method. Reproduced from [145], Copyright (2020), with permission from American Chemical Society. (d) (a,b) TEMPO and mechanical exfoliation to produce TOCNF; (c,d) NaOH and mechanical exfoliation to produce PDChNF; (e) electrostatic-force-induced self-assembly gelation; (f) digital images illustrate that the ultralight BHA was captured by a marker pen due to static electricity; (g) SEM image of BHA exhibits a highly porous structure. Reproduced from [160], Copyright (2019), with permission from American Chemical Society.
Figure 2.
(a) Schematic representation of a physically cross-linked hydrogel prepared by freeze–thawing. Reproduced from [113]. (b) The schematic of the PVA/CMC hydrogel, prepared by the freeze–thawing process, is presented, along with its cross-sectional image following the adsorption of heavy metals. Reproduced from [143], Copyright (2016), with permission from American Chemical Society. (c) Schematic representation of the CMCF-F hydrogel, prepared by the freeze cross-linking method. Reproduced from [145], Copyright (2020), with permission from American Chemical Society. (d) (a,b) TEMPO and mechanical exfoliation to produce TOCNF; (c,d) NaOH and mechanical exfoliation to produce PDChNF; (e) electrostatic-force-induced self-assembly gelation; (f) digital images illustrate that the ultralight BHA was captured by a marker pen due to static electricity; (g) SEM image of BHA exhibits a highly porous structure. Reproduced from [160], Copyright (2019), with permission from American Chemical Society.
3.2. Chemical Cross-Linking
Chemical cross-linking involves the introduction of a cross-linking agent that reacts with the hydroxyl groups on the cellulose molecular chain to form strong covalent bonds, which results in tighter bonding and the formation of an irreversible hydrogel network with higher stability, adsorption, and mechanical properties than physical cross-linking [161,162,163]. Chemically cross-linked hydrogels are characterized by their resistance to melting or decomposition, even under heat treatment, and their insolubility in water until the covalent bonds are broken, thus justifying the term ’permanent system’ [128,164]. The process of chemical cross-linking can be initiated through various mechanisms, including esterification, free radical polymerization, click chemistry, and Michael addition, among others. Cross-linking agents are available from both synthetic and natural sources, and the most commonly used cross-linking agents are citric acid [165], succinic acid, epichlorohydrin [166], polyethylene glycol diacrylate, divinyl sulfone, and glutaraldehyde [167,168].
However, the utilization of cross-linking agents may also engender certain issues, as the pore size of the polymer chains is diminished, and the increased crystallinity can exert a not insignificant negative influence on the adsorption capacity and swelling rate. Furthermore, cross-linkers and higher cross-linking densities have been observed to bind to some of the binding sites of the adsorbent, thereby reducing the adsorption capacity. Therefore, it is essential to strike a balance between the desired mechanical strength and the maximization of adsorption capacity [138].
Despite the numerous enhancements in performance characteristics exhibited by chemically crosslinked CBHs, the selection of crosslinking agents and the concentrations employed must be approached with the utmost seriousness. An excessive or inappropriate utilization of crosslinking agents has the potential to induce toxicity, thereby constituting a potential hazard to biological organisms [101]. For instance, certain isocyanates are recognized for their cytotoxic effects. Consequently, there is an urgent need to develop cross-linking techniques that can enhance mechanical properties while ensuring biocompatibility.
The development of chemically crosslinked hydrogels is currently focused on the identification or synthesis of new, low-toxicity, environmentally friendly crosslinking agents, as well as the optimization of crosslinking conditions and routes to obtain the desired properties without compromising their safety.
3.2.1. Free Radical Polymerization Crosslinking
A significant number of CBHs are synthesized by a free radical polymerization process, which is a well-characterized three-step process including initiation, propagation of the polymer chain, and termination [138]. The initiation step involves the use of a special chemical (e.g., potassium persulfate (KPS), tetramethylene diamine (TEMED), or ammonium persulfate (APS)) as an initiator, which decomposes to produce free radicals in the presence of light, pressure, temperature, redox reactions, or radiation. The polymer chains begin to form and propagate by initiating a reaction. During the process of propagation, the polymer chains undergo an extension phase, at which point the incorporated crosslinker reacts with the growing polymer chains to form a three-dimensional polymer network structure. The termination of polymerization is brought about by a combination or disproportionation reaction [113]. In comparison to alternative types of polymerization processes, free radicals are utilized extensively due to their numerous advantages, including a faster synthesis process, less demanding reaction conditions, ease of implementation, wider temperature range, and lower cost [136].
Acrylic acid (AA)-grafted cellulose-based nanohydrogels (Poly-X-CNF-g-AA) were prepared by free radical polymerization induced at 90 °C in the presence of 50 mg of APS as an initiator and 20 mg of N, N′-methylene-bis-(acrylamide) (MBA) as a cross-linking agent in 24 mL of cellulose-based sol with grafted AA chains on the surface (Figure 3a) [169]. The material was immersed in an Al3+ solution to facilitate further cross-linking, thereby forming a dual network hydrogel. In their study, Ning et al. [170] synthesized HEC-co-p(AA-AM)/TA hydrogels by grafting AA and acrylamide (AM) onto hydroxyethyl cellulose (HEC) and then modifying them with tannic acid (TA). The resulting hydrogels exhibited excellent MB adsorption properties (3438.27 mg/g) and high reusability. This phenomenon can be ascribed to the incorporation of TA-like modifiers within the CBHs, a process that facilitated the attainment of a uniform pore structure, thereby enhancing the adsorption performance [170]. Zhao et al. [171] prepared a modified cellulose-based hydrogel by an innovative method of simultaneous crosslinking and grafting of AM and AA (Figure 3b). During the process, APS was added to an aqueous solution of microcrystalline cellulose (MCC) to generate hydroxyl radicals. Subsequently, a mixed solution of AA, AM, and MBA was added gradually, and stirring continued at 50 °C for 2 h until complete polymerization. The hypothesis that acrylate and acrylamide monomers can be concurrently and efficiently crosslinked and grafted onto the initial cellulose material was substantiated. This pathway facilitates the synthesis of more complex, compact, and stable network structures, thereby enhancing strength and toughness. In addition, their ability to simultaneously increase the number of different kinds and amounts of active groups rapidly, simply, and effectively makes this hydrogel simultaneously exhibit remarkable adsorption capacity and high reusability for Cu2+, Pb2+, and Cd2+, which is a great advantage of this innovative approach. However, this polymerization pathway is contingent on elevated temperatures and extended reaction times, in addition to homogeneous mixing.
One of the most promising methods for preparing hydrogels is photoinduced polymerization, whose most common cross-linking mechanism is also free radical cross-linking. In comparison with alternative conventional techniques, this method possesses numerous advantageous characteristics, including low cost, simplicity, non-pollution, fast curing, low energy consumption, and low reaction temperature. During the reaction process, when the photosensitizer is exposed to light, it generates free radicals and triggers a polymerization reaction to form a crosslinked polymer network. This crosslinking technique is characterized by its gentleness, efficiency, and environmental friendliness [161]. Lamkhao et al. [172] successfully synthesized a highly porous photocatalyst/hydrogel composite by novel photoinitiated AM polymerization using MBA as a cross-linker and zinc oxide (ZnO) as a photosensitizer. This provides a direction for the development of composite hydrogels with the synergistic effect of both the adsorption of hydrogels and the photocatalytic ability of photocatalysts to improve the pollutant removal efficiency.
The enzyme type and its catalytic pathway can be selected to determine the mechanism of enzyme-induced cross-linking, which can also be free radicals. The most significant advantages of this method include mild cross-linking conditions, ease of handling, rapid gelation, excellent biodegradability, clean cross-linking systems, and the ability to cross-link in situ [173]. This approach avoids the toxicity of chemical cross-linking agents and improves mechanical strength. However, the most significant disadvantages of enzyme cross-linking methods are the high cost; susceptibility to allergic reactions; and, in some cases, low efficiency.
It is evident that the application of numerous methods on CBHs, including photoinitiation and enzymatic crosslinking, has not been fully developed due to certain limitations. However, as scientific and technological progress continues, the development of non-toxic, environmentally friendly cross-linking agents and gentle, energy-saving cross-linking methods is poised to become a central focus in research endeavors concerning CBHs.
3.2.2. Schiff Base Reaction
The Schiff base reaction involves the cross-linking of aldehyde and amine groups to the polymer chain via a condensation reaction to form dynamic covalent imine bonds [168,174]. This process enables chemical cross-linking without the use of additional cross-linking agents and can be used as a natural cross-linking mechanism while giving hydrogels their unique dynamic reversibility. This reaction is characterized by its reversibility, rapid synthesis, high chemical selectivity, and non-toxicity of the products. Hydrogels obtained using this method are self-healing, biocompatible, and sensitive to pH changes, allowing the preparation of multifunctional hydrogels with smart responses [175]. Remarkably, this inherent self-repairing capability does not necessitate an external stimulus; rather, it functions optimally within mild conditions [176]. Furthermore, the gelation time, mechanical strength, and self-repairing properties of the hydrogels can be tuned by modifying the reaction conditions [177]. The propensity of cellulose to undergo the Schiff base reaction is attributable to the prevalence of hydroxyl groups within its molecular structure.
Sethi et al. [178] covalently crosslinked dialdehyde carboxymethyl cellulose (DCMC) with gelatin using Schiff base reaction to form a novel hydrogel (DCMC-cl-G) without using any foreign cross-linking agent. This novel hydrogel was then used for the adsorptive removal of rhodamine B (RhB) and methyl violet (MV). It was demonstrated that the absence of any foreign cross-linking agent during the Schiff base reaction can result in the formation of biopolymer-based hydrogels that exhibit enhanced stability, and superior adsorption capacity (with maximum percentages of RhB and MV of 96.5% and 90%, respectively), biodegradability (82.67% degradation in 12 weeks), and ease of reuse [178]. The study demonstrated that the Schiff alkali reaction is not inferior to chemical cross-linking methods using cross-linking agents for the preparation of hydrogels, especially biopolymer-based hydrogels with excellent properties. In a separate report, the aldehyde groups of bifunctional hairy nanofibrillar cellulose (BHNC) and the amine groups of aminated dendritic fibrous nanostructured (colloidal) silica particles (DFNS-NH2) were employed for the formation of hybrid hydrogel nanocomposites by chemical cross-linking via the Schiff base reaction (Figure 3c) [179]. The absence of the need for an additional cross-linker in this synthesis is attributable to the functionality of the amine groups on the surface of DFNS as active sites for the Schiff base reaction with the aldehyde groups of BHNC. Furthermore, this novel material possesses the capacity for enhanced and adaptable adsorption capacity through the modulation of functional groups on DFNS-BHNC or the alteration of the mass ratio of DFNS-BHNC, constituting a significant advantage [179]. These adsorbent materials are exclusively prepared from biodegradable and non-toxic organic and inorganic materials in green solvents through a green cross-linking process and also possess a wide range of functional groups that can be used as potential adsorbents for a wide range of pollutants.
In comparison with conventional chemical cross-linking techniques, which rely heavily on cross-linkers, hydrogels derived from the Schiff base reaction are not limited by their toxicity and low biocompatibility. The covalent bonds obtained from the Schiff base reaction are closely related to the structure of the hydrogel network, which in turn affects the properties of the hydrogel such as porosity and solubility [180]. The inherent properties of CBHs, including self-healing, mechanical, and swelling properties, can be readily tuned by modulating the number of reactants and the strength of the Schiff base reaction. In addition, within specific environments, the Schiff base structure may lack sufficient stability and may be susceptible to hydrolysis [181]. At present, the focus of the Schiff base reaction is on the traditional condensation reaction, with relatively little research being carried out on novel synthetic methods (e.g., microwave-assisted synthesis and ionic liquid catalysis). Moreover, the potential of the Schiff base reaction for application in many fields has not yet been fully explored, and its stability and degradation still need to be optimized.
3.2.3. Click Chemistry
Click chemistry is a method of synthesizing macromolecules based on carbon-heteroatom bonding. It is a simple and rapid process that can provide a mild and efficient pathway to modify cellulose, endowing it with specific structures, properties, and functions [161,182,183,184]. Representative reactions of click chemistry include copper-catalyzed azide-alkyne cycloaddition (CuAAC) and thiol-ene/one-click reactions. However, the former is comparatively less reported than the latter, suggesting potential for further development.
The thiol-ene/yne reaction is typically conducted under mild aqueous conditions, exhibiting high conversion and selectivity without the necessity of toxic metal catalysts. Wang et al. [182] utilized a novel and straightforward cellulose-based hydrogel fabrication method to selectively separate anionic and cationic dyes. The synthesis of cellulose methacrylate (CM) was initiated and, subsequently, CM organic gels were prepared by the self-crosslinking of vinyl groups. These organic gels were then converted into CM-MA hydrogels and CM-CH hydrogels through the chemical grafting of 3-mercaptopropionic acid (MA) and Cysteamine hydrochloride (CH) via thiol-ene click chemistry, a process that occurred under UV irradiation, thereby generating additional active binding sites.
Liu et al. [185] discovered that the amino–anhydride reaction adheres to the principles of the click reaction, and that it can be expeditiously executed, resulting in the formation of hydrogels in water, which is auspicious for a broad spectrum of applications. In their experiments, they initially copolymerized maleic anhydride (MA) and AA to form PAM, then added a certain amount of glycidyl-trimethyl-ammonium chloride 90% (GTMAC) to cellulose solution to obtain QC, and finally prepared a new quaternary ammonium-based semi-IPN-gel adsorbent by a simple amino–anhydride click reaction by mixing PAM, QC, and poly-(allylamine hydrochloride) (PAH) (Figure 3d). In addition, the optimal conditions for the preparation of hydrogels were investigated through a series of one-way experiments (involving a 50:1 molar ratio of AA to MA, a 10:1 molar ratio of PAM to PAH at 6% mass concentration, and a 15% mass concentration of QC). However, it should be noted that one-factor experiments may overlook the potential interactions between multiple factors. Furthermore, the employment of single-factor experiments has the potential to elucidate the distinct effects of various factors on the performance of hydrogels. This approach can facilitate the identification of the most economical and efficient preparation conditions, thereby ensuring the stability and reproducibility of the material’s performance in subsequent studies. This, in turn, will provide a theoretical foundation for future in-depth research. The present study provides a comprehensive analysis of hydrogels made by this particular reaction from a number of perspectives and, thus, complements reports on the preparation of hydrogels based on the reaction of amino–anhydride in water. However, the authors do not report much on the reusability of this method, which is a significant shortcoming.
The Diels–Alder (DA) reaction of thermally reversible cycloaddition between conjugated diene and pro-diene (e.g., substituted olefin) compounds can also be categorized as a click reaction that can be carried out in aqueous environments under mild conditions without the need for catalysts or initiators [177]. This demonstrates the advantages of high efficiency, selectivity, and the absence of by-product formation [186]. The utilization of water as a solvent in DA reactions has become a fundamental aspect of research, primarily due to the hydrophobic effect, which results in a substantial increase in the reaction rate [187]. Moreover, the sustainable advantages associated with its green properties have contributed to its emergence as a predominant approach in contemporary research. Kramer et al. [187] used cellulose nanofibers as substrates to synthesize cellulose-based nanohydrogels via the DA click reaction of furan/maleimide in water. However, the extant literature suggests that the CBHs obtained through the DA reaction require specific chemical modification, which increases the complexity and cost of the synthesis, especially for the application of the hydrogels obtained from this reaction to water treatment. Consequently, there are too few reports in this area.
3.2.4. Radiation Crosslinking
Synthesis of CBHs by radiation treatment using different types of radiation (e.g., gamma rays, electron beams, microwave, and UV radiation) is also an alternative method of inducing cross-linking in polymer chains without the use of chemical cross-linkers, initiators, or catalysts [127]. This approach has the advantage of avoiding potential toxicity problems and can be carried out at ambient or below-ambient temperatures [188,189]. The density of chemical bonds is contingent on the irradiation dose and duration that are utilized to initiate the reaction kinetics. In comparison with chemically induced cross-linking, this method is a wholly environmentally benign synthetic process involving zero waste generation and offers significant advantages in the formation and sterilization of single-step CBHs [138]. Radiation cross-linking is a process that has been demonstrated to result in the production of hydrogels that exhibit excellent transparency, homogeneity, and mechanical properties, making them particularly suitable for use in 3D printing technology [101].
It has been demonstrated that gamma rays can induce cross-linking, thereby forming highly cross-linked and stable hydrogels. This process has been shown to enhance mechanical strength and swelling capacity, thus improving the adsorption efficiency of pollutants [190]. In addition, it has been reported that the rational incorporation of AA further enhances the adsorption properties by providing additional functional groups while improving the adsorption efficiency and selectivity of the hydrogel [191]. Sutradhar et al. also identified research gaps regarding γ-radiation-induced hydrogels, including the optimal radiation dose and competitive adsorption relationships in the presence of multiple contaminants. In light of the aforementioned studies and to address the extant research gap, they initially synthesized γ-irradiated carboxymethyl cellulose (CMC)/AA hydrogels with the capacity to enhance the adsorption efficiency and capacity of MB dyes (Figure 3e) [192]. Subsequent exploration of the synthesis protocol, radiation dose, adsorption efficiency, environmental friendliness, competitive adsorption behavior, and the effectiveness of MB dye removal was undertaken in-depth, providing unique insights for wastewater treatment applications. In the synthesis process, CMC/AA hydrogels were obtained by incorporating AA at different concentrations (7.5% to 15.0%) into the CMC slurry; followed by neutralization with KOH; and then by γ-irradiation of the mixtures using a Co-60 source at doses of 1, 2, 3, 5, 8, and 10 kGy [192]. It has been demonstrated that when properly grafted AA (at 5–7.5%) is utilized, it exerts a disruptive effect on the crystal structure of CMC, thereby enhancing the thermal stability of the hydrogel and increasing the adsorption capacity. However, further cross-linking resulted in reduced pore size and a more rigid structure, thus reducing dye penetration. However, further evaluation of the overall effectiveness of hydrogels synthesized by γ-radiation polymerization for environmental applications is still required. In a separate study, Masry et al. [193] pioneered a special study on the synthesis of hydroxyethyl cellulose (HEC)/AA/CX (Cyanex 471X) hydrogels using gamma radiation and systematically investigated the effect of gamma radiation on the HEC properties, gel fractions, swelling properties, and metal adsorption capacity. It is noteworthy that Nattawan et al. [194] successfully inserted cellulose purified from bagasse into hydrogel composites by the gamma irradiation technique, which also demonstrated notable adsorption capacity and adsorption efficiency.
Microwave (MW) irradiation-assisted synthesis has been utilized for hydrogel preparation, a method that is more environmentally friendly than traditional heating modes, such as heat conduction and convection mechanisms [195,196,197]. This method has garnered significant attention due to its ability to substantially reduce the time and energy consumption of the cross-linking process without compromising the physical and chemical properties of the final product. Additionally, it is characterized by its faster, more selective, and more homogeneous heating characteristics. Furthermore, the resulting hydrogels exhibit a high degree of water retention and biocompatibility [198]. However, the effect of the MW-assisted cross-linking reaction on the physicochemical properties of the resulting hydrogels has not been explored in more detail. To address this issue, Santoso et al. [195] evaluated the physicochemical properties of epichlorohydrin (ECH)-crosslinked cellulose hydrogels prepared by the MW method, including the pore morphology, chemical composition, crystallinity, thermal stability, and water absorption capacity (Figure 3f). In summary, the cross-linking agent ECH was incorporated into the cellulose solution and the MW-assisted cross-linking process was conducted using a domestic microwave oven. Concurrently, a thermostatic water bath was regulated to facilitate the cross-linking of the cellulose polymer chains. The study demonstrated that MW irradiation has great potential for the rapid, cost-effective, and energy-efficient preparation of CBHs. However, further studies are required to ascertain the effect of MW irradiation power and duty cycle on the degree of cross-linking, which in turn affects the mechanical properties and the porous structure of the resulting hydrogel materials. Such studies will provide an important basis for the development of high-performance porous hydrogels. In a separate study, a novel CAB hydrogel bead was developed for the adsorption of chlorpyrifos (CP) in water [199]. This was achieved by preparing the bead dropwise from an aqueous solution of cellulose acetate (CA) via microwave irradiation of a crosslinking polyethylene glycol (PEG) crosslinker. The cross-linking process induced by microwave irradiation ensured the integrity of the network, facilitated by the H-bonding of the CA and PEG units of the hydrogel unit.
In conclusion, the radiation-crosslinked cellulose-based hydrogel technology is a green preparation method with significant potential. However, it should be noted that cellulose and its derivatives are susceptible to degradation during the radiation process, which may result in a reduction in their mechanical strength.
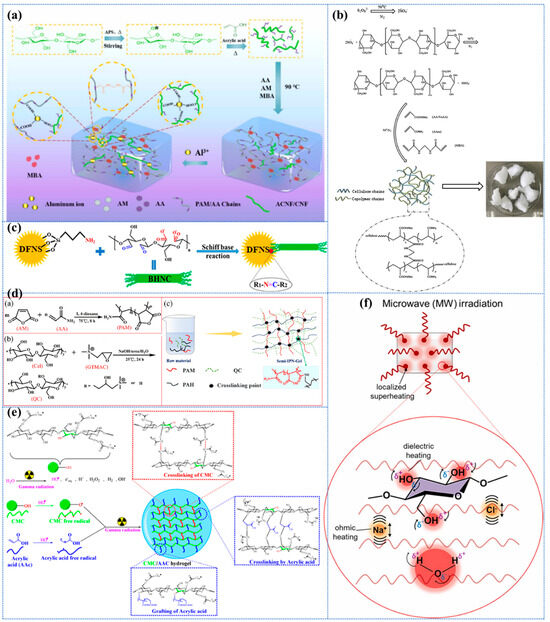
Figure 3.
(a) Schematic diagram of Poly-X-CNF-g-AA composite double cross-linked hydrogel. Reproduced from [169], Copyright (2023), with permission from Elsevier. (b) Program for the synthesis of biosorbent (MCC-g-(AA-co-AM)). Reproduced from [171], Copyright (2019), with permission from Elsevier. (c) Cross-linking between BHNC and DFNS-NH2 by Schiff base reaction. Reproduced from [179], Copyright (2020), with permission from American Chemical Society. (d) Synthesis process: (a) PAM; (b) QC; and (c) semi-IPN-gel. Reproduced from [185], Copyright (2022), with permission from Elsevier. (e) Possible grafting and cross-linking reaction mechanisms in CMC/AA hydrogels. Reproduced from [192]. (f) Schematic of MW irradiation on a cellulose gel solution. Reproduced from [195], Copyright (2022), with permission from Elsevier.
Figure 3.
(a) Schematic diagram of Poly-X-CNF-g-AA composite double cross-linked hydrogel. Reproduced from [169], Copyright (2023), with permission from Elsevier. (b) Program for the synthesis of biosorbent (MCC-g-(AA-co-AM)). Reproduced from [171], Copyright (2019), with permission from Elsevier. (c) Cross-linking between BHNC and DFNS-NH2 by Schiff base reaction. Reproduced from [179], Copyright (2020), with permission from American Chemical Society. (d) Synthesis process: (a) PAM; (b) QC; and (c) semi-IPN-gel. Reproduced from [185], Copyright (2022), with permission from Elsevier. (e) Possible grafting and cross-linking reaction mechanisms in CMC/AA hydrogels. Reproduced from [192]. (f) Schematic of MW irradiation on a cellulose gel solution. Reproduced from [195], Copyright (2022), with permission from Elsevier.
3.3. Composite Hydrogels
The numerous branches and intricate chemistry inherent in the cellulose structure give rise to relative interactions between molecules in hydrogels prepared from pure cellulose that are insufficient to ensure satisfactory mechanical strength. However, the combination of cellulose and other types of polymers or nanoparticles has been shown to produce cellulose-based composite hydrogels with a range of functionalities.
3.3.1. Nano-Reinforced Cellulose-Based Hydrogels
Nanocellulose Composite Hydrogels
In recent years, nanocellulose is often investigated for the preparation of environmentally friendly hydrogel composites, which can effectively enhance the adsorption capacity of hydrogels due to the presence of carboxyl groups in its structure [200,201,202,203,204,205]. Nanocellulose-based hydrogels exhibit a multitude of characteristic properties, including but not limited to good porosity, swelling capacity, adsorption and mechanical properties, self-healing, and smart responsiveness. However, it is also limited by the poor dispersion of nanocellulose in nonpolar solvents, leading to agglomeration as well as insufficient interfacial adhesion with their hydrophobic substrates. Consequently, recent studies on nanocellulose-based hydrogels have concentrated on their physical incorporation as fillers into synthetic and natural polymer networks for reinforcing composite hydrogels [206,207,208,209,210,211]. These nanocellulose composite hydrogels can be prepared by a number of methods, including homogenization, freeze–thawing, free radical polymerization, or UV/ion-mediated cross-linking [212]. It has been demonstrated that the controlled incorporation of modest quantities of nanocellulose into hydrogels can enhance their physical properties and prevent agglomeration. For instance, Roa et al. [200] reported that a nanocomposite hydrogel formed by free radical polymerization of 1%wt CNF with [2-(acryloyl-oxy)-ethyl]-trimethylammonium chloride (ClAETA) exhibited a maximum adsorption capacity of 1379.0 mg/g for methyl orange (MO) and concluded that the incorporation of CNF improved the MO adsorption with time. A one-way experiment revealed that the solubility of the hydrogel increased with the increase in CNF concentration and the thermal stability was improved, which might be due to the increase in the number of carboxyl groups in the hydrogel. In order to conduct further investigation into the potential of low-cost biodegradable hydrogels for the efficient removal of heavy metals from water, Alves et al. [213] prepared composite hydrogels containing different nanocellulose contents using trisodium citrate as a cross-linker. The findings demonstrated that increasing the nanocellulose content resulted in enhanced swelling capacity, increased thermal stability, elevated adsorption capacity for Cu2+, and enhanced biodegradability.
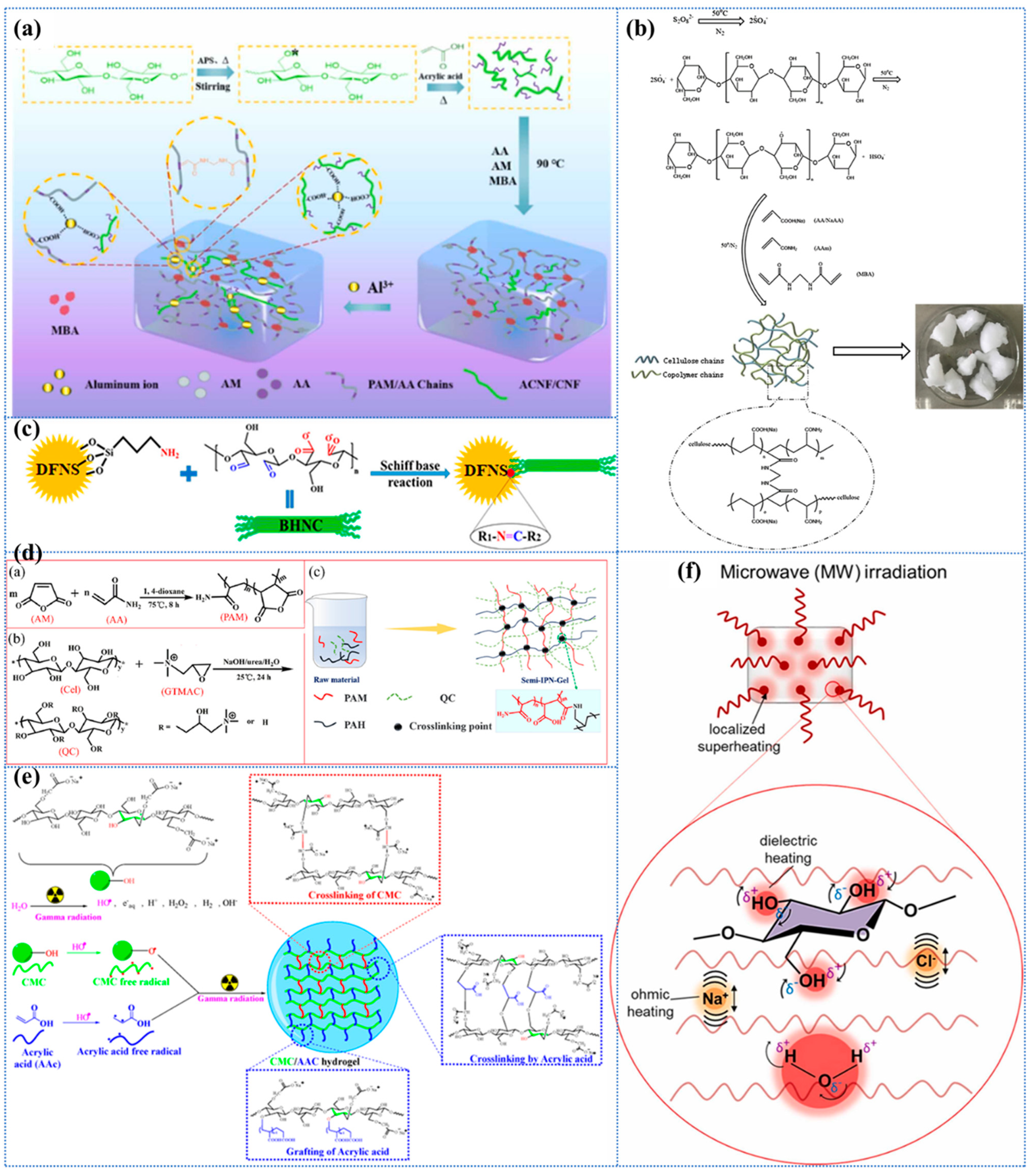
The utilization of nanocellulose-based hydrogels as carriers for immobilizing nanocomposites, such as ZnO/AgBr, has been demonstrated to enhance the removal efficiency of heavy metals through a combination of synergistic adsorption and photocatalytic effects. In a recent study, Zhang et al. [214] reported a novel nanocellulose/CDs hydrogel (NCH) consisting of cellulose nanocellulose nanofibrils (CN), carbon dots (CDs), and ZnO/AgBr nanocomposites. In this study, the carbon dots enhanced the amino-induced Cr6+ adsorption and accelerated the photocatalytic effect, resulting in an adsorption capacity of up to 315 mg/g. Zhang et al. [215] developed a pioneering CCNL composite hydrogel by first grafting CDs onto cellulose nanofibrils (CNs) and then adding glutaraldehyde cross-linking agent to a homogeneous mixture of chitosan (CS), CNs-CDs, and laminar double hydroxide (LDH) in a constitutive solution (Figure 4a).
The surface modification of nanocellulose is of paramount importance for the creation of covalent cross-linking sites using grafts (e.g., polyacrylamide, polyvinyl alcohol, carboxyl, or aldehyde groups) to enhance its interaction with the matrix [212]. For instance, Rodrigues et al. [216] synthesized chitosan-g-poly-(acrylic acid) gels containing cellulose nanoscale whiskers for the adsorption of Pb2+ and Cu2+ in wastewater.
Cellulose-Based Hydrogels Reinforced with Inorganic Nanomaterials
It has been established that the adsorptive properties of pure CBHs are frequently inadequate [217]. Consequently, the amalgamation of natural polymers with inorganic materials has been postulated to engineer composites with superior properties [218]. Moreover, the incorporation of inorganic materials has been shown to confer specific functionalities, including conductive, catalytic, adsorptive, and potentially antimicrobial properties, while concomitantly enhancing mechanical and thermal stability properties due to the synergistic effect of the cellulose matrix and the inorganic materials. Inorganic nanoparticles offer significant advantages over conventional micron-sized inorganic fillers due to their large surface area and stronger interaction with the matrix. It has been demonstrated that cellulose complexes with nanoparticles are effective in preventing agglomeration, ensuring colloidal safety and contributing to the simple separation of magnetic components post-treatment [106]. MoS2 is an inorganic nanomaterial that exhibits distinctive optical and electronic properties, making it a subject of considerable interest. When MoS2 is composited with other materials, it has been observed to confer several advantageous properties to adsorbents, including accelerated adsorption kinetics, enhanced adsorption efficiency, and improved metal selectivity [219]. In a recent report, an amphiphilic and surface-active MoS2/cellulose acetate fiber sponge has been successfully used to purify oily water by easily and selectively removing targeted oil droplets [220].
Metals and metal nanoparticles (including ZnO, TiO2, Ag, and Fe3O4) exhibit a variety of beneficial properties, such as high specific surface area, a wide range of catalytic capabilities, unique electromagnetic and optical behaviors due to their small size, and enhanced reactivity [221,222,223]. Hydrogels loaded with metals and metal nanoparticles have been shown to possess enhanced adsorption capacity for a range of contaminants, including dyes, heavy metals, and pharmaceuticals. In addition, these hydrogels have been observed to exhibit superior crystallinity and thermal stability. Moreover, the integration of metal nanoparticles facilitates the development of responsive materials, wherein variations in metal properties can be employed to stimulate responsive applications. In certain instances, metal ions have been observed to induce gelation—functioning as cross-linking agents for cellulose—which can result in the formation of metal-based methyl groups through complexation, which may lead to a sustained bactericidal effect [221]. It is important to note that the dispersion of metals and metal nanoparticles in the hydrogel matrix is a significant factor in determining the catalytic efficiency, as aggregation of nanoparticles will substantially reduce the specific surface area and the corresponding available catalytic sites. TiO2 is regarded as a highly effective inorganic nanomaterial in the synthesis of composite hydrogels. It possesses the distinctive capacity to degrade organic dye molecules through a process known as photocatalysis, concurrently enhancing the porosity, specific surface area, and pore volume of the composites [1,224]. In a report, TiO2 cross-linked montmorillonite nanosheets (MMTNS) were introduced into CMC-CS hydrogels to obtain a high surface area, available anion adsorption sites, and macropores that would be favorable for MB dye penetration (Figure 4b) [225]. The experimental results demonstrated that 97% of the dye was removed within 360 min, which was attributed to the strong ion exchange and electrostatic interactions between the MB molecules and the hydrogel. In recent years, different polycrystalline species of iron oxyhydroxides include α, β, γ, and δ [226]. Among them, β-FeOOH nanoparticles exhibit photoactivity and are also considered effective photocatalysts for dye degradation due to their synergistic effect with the photo-Fenton reaction. In addition, uniform distribution of β-FeOOH using hydrogels not only solves the problem of adsorption saturation and non-regeneration, but also enhances its adsorption and separation properties, significantly reduces the aggregation of the photocatalysts, and exposes more active sites, which facilitate the adsorption and surface photocatalytic reactions [40,227]. Yang et al. [228] prepared cellulose–chitosan composite hydrogels by co-dissolution and regeneration process using 60 wt% LiBr aqueous solution as solvent (as shown in Figure 4c). The resultant hydrogel was then subjected to immersion in a FeCl3 solution, resulting in the synthesis of β-FeOOH nanoparticles through in situ synthesis. It was demonstrated that an enhancement in the loading of β-FeOOH resulted in an augmentation in the rate of adsorption and photocatalytic degradation of MO. This phenomenon was ascribed to the sustained formation of hydroxyl radicals and holes.
It is widely accepted that nanoscale hydroxyapatite (HA) is a low-cost, environmentally friendly, biodegradable, and easily synthesized biomaterial. It has a high adsorption capacity for divalent heavy metal ions and is stable under very high oxidizing and reducing conditions [218]. The formation of cellulose/HA composite hydrogels involved the incorporation of HA into a cellulose solution, followed by sonication, addition to epichlorohydrin (ECH), homogeneous mixing, and subsequent oven baking.
Inorganic nanomaterials pose significant challenges in terms of their ability to form composites, primarily due to the persistent issues of dispersion and interfacial compatibility.
Organic Nanomaterials Reinforcing Cellulose-Based Hydrogels
Graphene and its derivatives (GO, reduced GO, and graphitic carbon nitride (G-C3N4)) have emerged as a new generation of organic nano-enhanced water treatment materials [229,230]. Graphene, a material with a wide range of fascinating properties, including remarkably high mechanical strength, thermal stability, transmittance, and electrical conductivity, has attracted significant attention in various fields of research [231,232]. Notably, the functionalization of graphene can result in a remarkably diverse array of multifunctional active adsorption sites [1]. For instance, the further amine functionalization of (TEMPO) cellulose oxide nanofibrils (TCNF)/GO hydrogels with polyethyleneimine (PEI) by Nan et al. resulted in a significant increase in surface energy, mechanical strength, and adsorption capacity [233]. This increase was attributed to an increase in the number of available amine-active adsorption sites, leading to strong hydrogen bonding. Moreover, the CMC/polyacrylic acid (PAA) hydrogels were found to be significantly enhanced with GO, which resulted in an improvement in the adsorption of cationic MB dye molecules [234]. This is attributable to a substantial reduction in the pore size and an increase in the surface area of the hydrogels by the addition of GO, as well as an improvement in the anionic charge of the hydrogels. Moreover, in addition to electrostatic interactions, it was found that hydrogen bonding and π–π stacking interactions contributed to the trapping of MB molecules onto the hydrogels. GO nanosheets, with their two-dimensional structure, possess a higher specific surface area and enhanced dispersibility. The strong covalent interactions that exist between GO nanosheets and polymer chains are particularly advantageous for the formation of composite hydrogels [235]. For instance, vinyl-triethoxy-silane (VTES) was utilized as a chemical cross-linking agent for CS- and CMC-based cross-linked nanocomposite hydrogels (NCH) following the vinyl functionalization of GO nanosheets [235].
g-C3N4, an additional two-dimensional graphene derivative, also exhibits excellent physicochemical properties due to the advantageous properties of -NH2 [236,237]. In practice, however, the adsorption capacity of g-C3N4 for organic dyes is reduced due to self-aggregation and difficulties in recovery. It was found that by adding them as reinforcement to cellulose, the aggregation of g-C3N4 was effectively suppressed, which improved reusability and simplified the separation process [238]. Moreover, for pure CMC hydrogels, it is not possible to maintain a stable structure in an aqueous environment since CMC is a water-soluble cellulose ether [239]. In their study, Chen et al. [238] combined the advantages and disadvantages of the two aforementioned materials to develop a novel CMC hydrogel enhancement, to enhance the mechanical, thermal, and adsorption properties of the hydrogel through the synergistic effect obtained by chemically cross-linking nanoscale g-C3N4. The result was a promotion in its practical application. Specifically, bagasse cellulose (SBC) and carboxymethyl cellulose (CMC) were dissolved and stirred to obtain a mixed cellulose solution (SBC/CMC); then, g-C3N4 powder was added to the solution and stirred; finally, ECH was added and crosslinked for 10 h in 60 °C water bath to obtain a g-C3N4 @SBC/CMC hydrogel (Figure 4d) [238]. The study indicates that g-C3N4 has excellent compatibility with cellulose substrates and can be uniformly dispersed in a reticulated structure without aggregation. Adsorption experiments demonstrate that the hydrogel exhibits a remarkably high selective adsorption of cationic dyes, which features intelligent separation of specific dyes and facilitates the recovery of valuable chemicals. The primary interactions between the cationic dyes and the composites are found to be synergistic interactions arising from π–π coupling, and electrostatic and hydrogen bonding. Significant electrostatic interactions with cationic MB are highly probable, with a maximum adsorption capacity of up to 362.3 mg/L, because the Tri-S-Triazine structure of the nitrogen lone pair of electrons in g-C3N4 is negatively charged. Reproducibility studies further demonstrated remarkable advantages, exhibiting only a marginal decline in adsorption capacity after seven cycles, thereby attesting to the dyes’ exceptional durability. However, at elevated ionic strengths, a decline in adsorption capacity is observed, attributable to the competition between sodium ions and cationic MB for adsorption sites.
Metal–organic frameworks (MOFs), also referred to as porous coordination polymers, are formed through the binding of organic ligands to metal-containing nodes. These frameworks possess a variety of advantageous characteristics, including tunable multifunctional, small-sized, and microporous structures, as well as high activity, which makes them particularly well-suited for use as reinforcing additives in hydrogels [240,241]. A substantial body of research has demonstrated that the in situ growth of MOFs on the hydrogel surface enhances the adsorption properties of hydrogels [242]. Typically, MOFs are in powder form with high specific surface area and adsorption capacity, but they face difficulties in recovery in practical water treatment due to their fine particles and light texture [243,244]. The selection of appropriate substrates, such as bio-organic macromolecular cellulose derivatives, has been demonstrated to regulate the growth of MOFs, thereby facilitating the recovery of functional MOFs. In recent years, cellulose has been regarded as an optimal support body for the immobilization of guest materials, with numerous examples of MOF/cellulose composites having been documented [245]. Nonetheless, the direct loading of MOFs onto cellulose substrates typically results in an inhomogeneous distribution and aggregation of MOFs particles. In order to address this issue, Cui et al. [245] developed a UiO-66/PDA/BC aerogel, in which UiO-66 nanoparticles were uniform in size, simple to use, and separated by coating a polydopamine (PDA) layer on a BC substrate to facilitate the subsequent uniform coating of UiO-66 nanoparticles. The in situ growth of MOFs must be carried out under restrictive synthesis conditions to ensure the stability of the hydrogel during processing. In order to overcome this limitation, Zhao et al. [246] prepared sodium alginate (SA)/CS/CNF/Cu2+ hydrogels (SCC-Cu) by using the semi-solvated acidified sol–gel transition method with an internal gelation method and prepared copper-based MOF composites (SCC-CuMOF) by using an in situ growth method (Figure 4e). CNF fulfils a dual role in this process, functioning both as a cross-linking agent between SA and CS, and as a filler between the dual networks, which contributes to an effective enhancement of the hydrogel’s strength. In addition, the presence of Cu2+ provides additional active sites, thereby facilitating enhanced loading of the MOF.
Covalent organic frameworks (COFs) have been identified as having significant potential for the removal of heavy metals due to their low crystalline density, high adsorption capacity, large surface area, tunable porosity, porous structure, good stability, and recyclability [247,248]. In a manner analogous to the issue of MOFs, the most common approach for COF-based composites is to mix the COF with the substrate, with hydrogels being a particularly suitable choice. It is important to note that achieving homogeneous dispersion of COF particles in hydrophilic matrices (e.g., CMC) by direct mixing is challenging [249]. However, the rather heterogeneous incorporation of COF can potentially lead to degradation of the mechanical properties of the hydrogel. Therefore, if COFs are coated on the surface of the hydrogel, they can be dispersed relatively homogeneously, while at the same time reinforcing the hydrogel [249]. In recent years, there has been a paucity of reports on the enhancement of CBHs by COFs, and the studies are relatively novel. For instance, Li et al. [250] utilized an in situ spray gel-assisted biosynthesis strategy for the first time, which led to the generation of continuous BCCOF-SO3H multilayered composite hydrogels by spraying COFs at different concentrations during the growth of BC hydrogels (Figure 4f). The composites exhibited a substantial number of hydroxyl and sulfonic acid groups, and through ion-exchange and ligand interactions, they provided a substantial number of active adsorption sites. These sites were found to have extremely high adsorption capacity, thus providing a novel method for the preparation of COF composites. In a separate study, Zhao et al. [249] prepared a novel COF@CNF@CMC composite hydrogel bead (C-CCHB) for the removal of Ni2+ from an aqueous solution. Specifically, COF was first synthesized using 2,2′-bipyridine-5,5′-formaldehyde (BP) and 1,3,5-tris(4-aminophenyl)-benzene (TAPB) as monomers and dimethyl-sulfoxide (DMSO) as a solvent. Subsequently, COF was grafted onto CNF by the Schiff base reaction, forming COF@CNF with an imine bond. Finally, COF@CNF and CMC were mixed and extruded into FeCl3 to obtain C-CCHB.
3.3.2. Multi-Network Composite Cellulose-Based Hydrogels
Dual-Network Cellulose-Based Hydrogels
In contrast to conventional chemically cross-linked hydrogels, dual-network (DN) hydrogels, which consist of dual polymer networks with different properties, have been shown to be an effective means of enhancing the mechanical properties, fracture toughness, and brittleness of polymer hydrogels [251,252]. Indeed, they have been demonstrated to be comparable to rubber and biological tissues, and they have attracted considerable attention in recent years for a variety of potential applications. However, multistep methods for constructing DN hydrogels have many limitations, including a polymerization step that is both cumbersome and time-consuming, the need for molds, the inability to control the shape of the hydrogel in situ, and the need to use toxic initiators [253]. Pure CBHs exhibit suboptimal mechanical properties, with their single rigid network being susceptible to fracture. This can impede their capacity for swelling and water absorption, which may hinder their application. However, the introduction of a secondary network into the cellulose-based matrix can significantly enhance their functionality. For instance, Li et al. [254] utilized a CS-cellulose composite as the primary network of a rigid hydrogel to enhance stability, while polyacrylamide can be employed as the secondary network of a flexible hydrogel to improve the mechanical properties. This results in a dual-network hydrogel with superior adsorption properties, stability, and non-toxicity. During the process, N-methyl morpholine-N-oxide (NMMO) was utilized to dissolve the cellulose, while Deep Eutectic Solvent (DES) was employed as a co-solvent to prevent thermal decomposition of the cellulose, thereby enhancing its solubility. It has been demonstrated that the incorporation of two-dimensional nanosheets, such as GO and its derivatives, can significantly enhance the crystallization process of crystalline polymers, which underscores the pivotal role these nanosheets play in the effective construction of polymer-based DN hydrogels [255]. For instance, Liu et al. [255] impregnated GO sheets into SA/nano-fibrillated cellulose (NFC) to successfully prepare SA/NFC/GO bi-network hydrogels by a simple one-step freeze–drying process. In this instance, the presence of GO sheets promoted the crystallization of NFC chains along GO sheets based on heterogeneous nucleation, which served as a physical cross-linking point for NFC chains (Figure 5a). This simple cross-linking method may provide new insights into the preparation of inorganic particle-induced cellulose-based DN hydrogels, which could replace the clumsy freeze–thaw method.
In order to enhance the mechanical properties of natural polymer hydrogels, whilst simultaneously increasing their adsorption capacity and recyclability, Cai et al. [256] proposed the utilization of magnetization-modified montmorillonite (MTT-Fe3O4) for the preparation of DN hydrogels with CS/CMC/SA substrates, which exhibited mechanical properties that were twice those of the individual networks. The utilization of cryo-gelation facilitates the concentration of polymers within unfrozen microphases, thereby producing dense polymer walls, which contribute to the acquisition of elasticity, compressibility, and shape memory properties by hydrogels [257]. However, there is a paucity of exploratory studies on the preparation of hydrogels using a combination of cryo-gelation and dual-network structures. In order to provide a complementary framework to the aforementioned research, Li et al. [258] prepared novel microfibrillar cellulose/polyethyleneimine (MFC/PEI-CD) hydrogels that exhibited superb elasticity, high mechanical strength, and a macro-porous structure through the synergistic action of the cryo-gel and bi-network. Specifically, MFC is pre-crosslinked with bis(vinyl-sulfonyl)-methane (BVSM) to form the first MFC network. Subsequently, a second network between PEI and glutaraldehyde crosslinker was formed within the below-freezing MFC network (Figure 5b). This innovative approach, which integrates cryo-gelation with dual networks, offers a novel methodology for the fabrication of adsorbent hydrogels, with the objective of facilitating the dynamic removal of heavy metal contaminants.
IPNs and Semi-IPNs Cellulose-Based Hydrogels
Another potential method of enhancing the properties of hydrogels is to construct an interpenetrating polymer network (IPN). IPNs are polymeric materials formed by intertwining two or more crosslinked networks that can be synthesized simultaneously or sequentially and crosslinked individually [259,260]. One network in the IPN structure is a tightly crosslinked rigid polyelectrolyte, while the other network is a loosely crosslinked flexible neutral polymer interpenetrating into the first network [261]. The distinction between a semi-IPN and a full IPN is that, in the former, only one component is cross-linked, while in the latter, all components are cross-linked [259,262]. IPNs offer numerous advantages, including low preparation cost, excellent mechanical properties, biodegradability, and ease of diffusion [263]. Hydrogels derived from IPNs and semi-IPNs have found widespread application in the domain of wastewater treatment, particularly in the context of organic dyes, due to their exceptional physicochemical and biological characteristics [264,265].
To investigate the effects of different types of nanocellulose with different properties on IPN networks, Yue et al. prepared IPN-structured hydrogels with covalently crosslinked polyacrylamide (PAM) networks and chelated crosslinked SA networks in the presence of Ca2+ [261]. They then incorporated three types of nanocellulose (CNCs, BCs, and TOCNs) into SA-PAM matrices as multifunctional crosslinking agents. The results demonstrated that the BCs were more effective in improving the mechanical properties of the hydrogels, while the TOCNs were the most effective in increasing the adsorption capacity. In a separate study, Li et al. successfully prepared NFC-based hydrogels with IPN structures by crosslinking radical polymerization (see Figure 5c) [266]. The second network was formed by the polymerization of 2-dimethylaminoethyl methacrylate monomer (DMAEMA) and MBA, triggered by the thermal decomposition of APS. Tert-butyl acrylate-co-2-hydroxyethyl methacrylate (HEMA) or MBA is susceptible to free radical polymerization and has been shown to significantly improve polymer interpenetration. It is often used as an important component in IPNs. Bai et al. [267] prepared PVA/CNC-based hydrogels by using in situ photo-crosslinking and freeze–thaw cycling techniques with the addition of HEMA or MBA as the crosslinking agent (Figure 5d). The formation of IPN was facilitated by the strong interactions between PVA and CNC, which were further enhanced by the bond network created by HEMA or MBA photo-crosslinking.
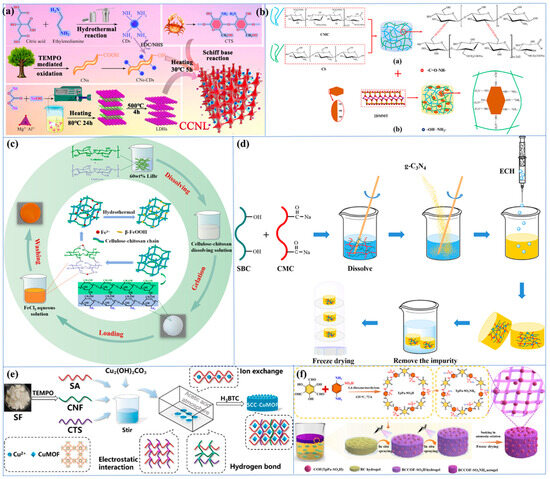
Figure 4.
(a) Preparatory process for CCNL. Reproduced from [215], Copyright (2024), with permission from Elsevier. (b) Schematic representation of the formation mechanism: (a) CMC/CS hydrogel formed by amidation and chain intertwining; (b) TiO2@MMTNS/CMC/CS hydrogel formed by hydrogen bonding, amidation, and chain intertwining. Reproduced from [225], Copyright (2020), with permission from Elsevier. (c) Schematic diagram of the preparation process of cellulose–chitosan/β-FeOOH composite hydrogels. Reproduced from [228], Copyright (2024), with permission from Elsevier. (d) Schematic diagram of the preparation of g-C3N4@SBC/CMC nanocomposite hydrogels. Reproduced from [238], Copyright (2022), with permission from Elsevier. (e) Schematic of the preparation of SCC-CuMOF. Reproduced from [246], Copyright (2023), with permission from Elsevier. (f) Schematic representation of the preparation of BCCOF-SO3 and its freeze–dried aerogel. Reproduced from [250], Copyright (2025), with permission from Elsevier.
Figure 4.
(a) Preparatory process for CCNL. Reproduced from [215], Copyright (2024), with permission from Elsevier. (b) Schematic representation of the formation mechanism: (a) CMC/CS hydrogel formed by amidation and chain intertwining; (b) TiO2@MMTNS/CMC/CS hydrogel formed by hydrogen bonding, amidation, and chain intertwining. Reproduced from [225], Copyright (2020), with permission from Elsevier. (c) Schematic diagram of the preparation process of cellulose–chitosan/β-FeOOH composite hydrogels. Reproduced from [228], Copyright (2024), with permission from Elsevier. (d) Schematic diagram of the preparation of g-C3N4@SBC/CMC nanocomposite hydrogels. Reproduced from [238], Copyright (2022), with permission from Elsevier. (e) Schematic of the preparation of SCC-CuMOF. Reproduced from [246], Copyright (2023), with permission from Elsevier. (f) Schematic representation of the preparation of BCCOF-SO3 and its freeze–dried aerogel. Reproduced from [250], Copyright (2025), with permission from Elsevier.
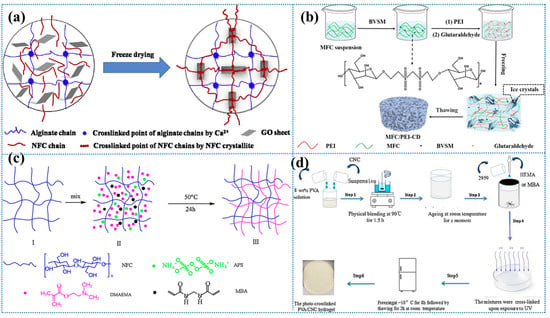
Figure 5.
(a) Schematic representation of the role of GO in DN hydrogel preparation. Reproduced from [255], Copyright (2021), with permission from Elsevier. (b) Schematic diagram for the synthesis of the MFC/PEI-CD. Reproduced from [258], Copyright (2023), with permission from Elsevier. (c) Nanocellulose/Poly-(2-dimethylaminoethyl methacrylate) interpenetrating polymer network hydrogels. Reproduced from [266], Copyright (2018), with permission from Elsevier. (d) Preparation processes of photo-crosslinked PVA/CNC/poly-HEMA hydrogels and photo-crosslinked PVA/CNC/poly-MBA hydrogels. Reproduced from [267], Copyright (2018), with permission from Elsevier.
Figure 5.
(a) Schematic representation of the role of GO in DN hydrogel preparation. Reproduced from [255], Copyright (2021), with permission from Elsevier. (b) Schematic diagram for the synthesis of the MFC/PEI-CD. Reproduced from [258], Copyright (2023), with permission from Elsevier. (c) Nanocellulose/Poly-(2-dimethylaminoethyl methacrylate) interpenetrating polymer network hydrogels. Reproduced from [266], Copyright (2018), with permission from Elsevier. (d) Preparation processes of photo-crosslinked PVA/CNC/poly-HEMA hydrogels and photo-crosslinked PVA/CNC/poly-MBA hydrogels. Reproduced from [267], Copyright (2018), with permission from Elsevier.
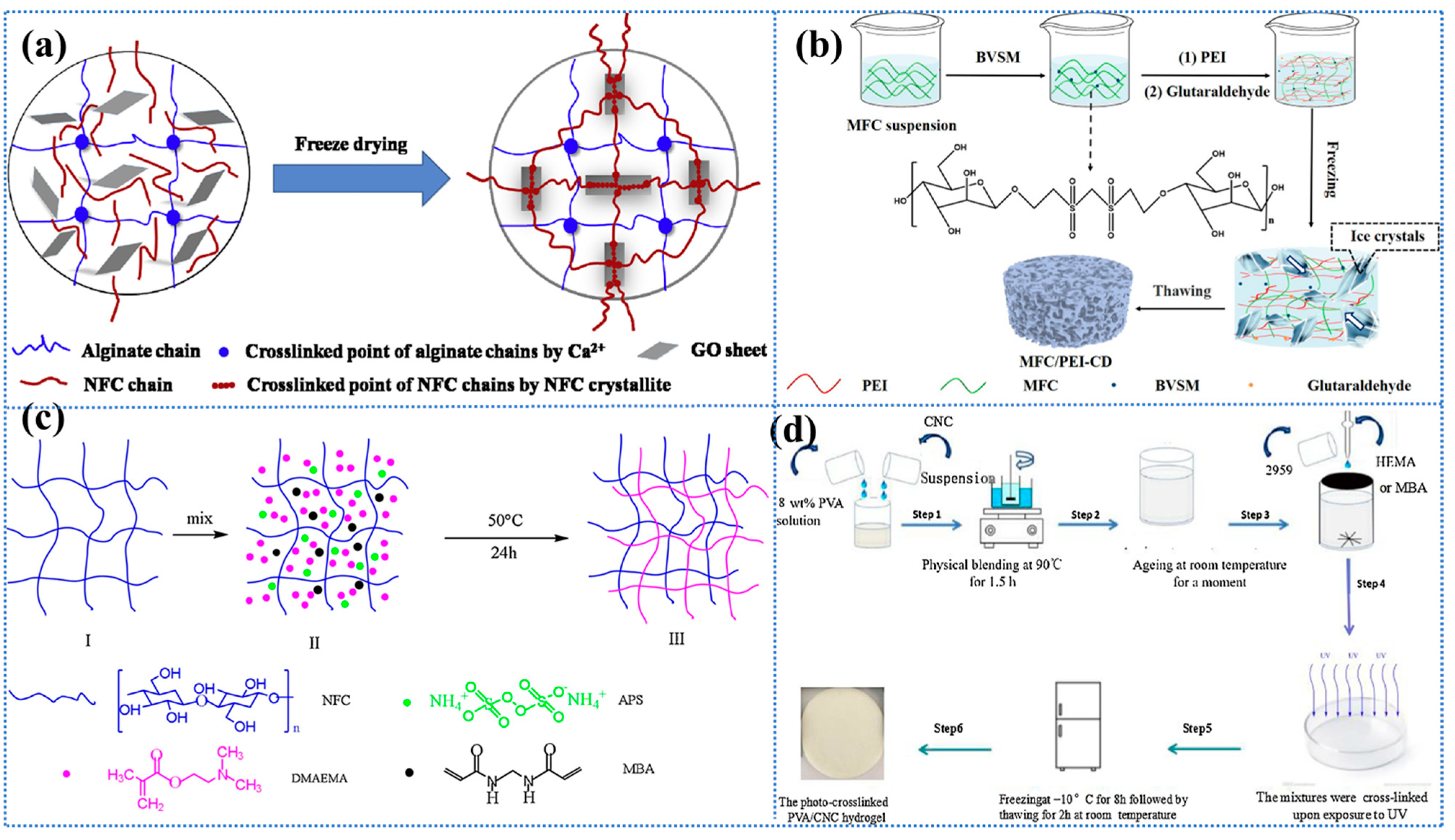
4. Cellulose-Based Hydrogels for Heavy Metal, Dye, and Micropollutant Removal Applications
4.1. Heavy Metal Adsorption
Heavy metal ions tend to accumulate in living organisms and pose a serious threat to human health due to their toxicity, high permeability, and lack of degradability [88,268]. Common heavy metal ions include radioactive ions (e.g., UO22+) [269] and carcinogenic (e.g., As3+) [270], bioinorganic (e.g., Cu2+), and toxic metals (e.g., Cd2+, Cr6+, Pb2+, and Hg2+) [271,272]. In recent years, research interest has been attracted by hydrogels based on cellulose and its derivatives due to their potential application as heavy metal absorbents, thanks to the porous structure, numerous functional groups, and high adsorption capacity [273]. In the context of heavy metal adsorption on CBHs, the predominant mechanisms encompass complexation, ion exchange, reduction, and electrostatic interactions; occasionally, these mechanisms are employed in conjunction, which has been documented in extant studies [45]. Figure 6a shows the various mechanisms of metal adsorption. Moreover, as illustrated in Table 2, recent comparative analyses of CBHs for the removal of heavy metals by adsorption are summarized.
The present study explores the potential for enhancing the efficiency and sustainability of CBHs in the adsorption of heavy metal ions, a subject that has recently garnered significant research interest. To remove heavy metals more efficiently by the adsorption process, it is necessary to specifically adapt the surface chemistry of the cellulose-based hydrogel adsorbent to the specified metal species. For instance, the incorporation of functionalized phosphonic acid moieties as chelating groups has led to a significant enhancement in the adsorption efficiency of CBHs, through the exploitation of a synergistic coordination effect [274]. Specifically, a novel CS/cellulose phosphonate (MCCP) composite hydrogel (CS/MCCP) was prepared by two steps of phosphorylation and Mannich reaction (Figure 6b). This exhibited high selectivity, excellent adsorption capacity, and an ultra-fast adsorption rate for Pb2+ and Cu2+ (the maximum adsorption amounts were 211.42 and 74.29 mg/g, respectively). It can be hypothesized that the observed phenomenon is attributable to the modification of the MCCP surface with a significant number of phosphate groups (PO₄3⁻), which undergoes preferential coordination with high valence and small radius Pb2⁺ and Cu2⁺ ions, exhibiting lower affinity for low-charge-density ions. Furthermore, the Mannich reaction, which was utilized in this study, forms a three-dimensional interpenetrating network between CS and cellulose, thereby introducing multifunctional groups that facilitate synergistic interactions and significantly increase the adsorption-specific surface area. It can be deduced from these findings that the adsorption selectivity can be enhanced by the introduction of selective coordination groups and the construction of special spatial structures. As demonstrated in Figure 6d, the adsorption behavior in a mixed solution of several metals is investigated, thereby illustrating the high selectivity of the adsorbent. In order to develop fast, highly selective, and highly adsorbable CBHs, Zhao et al. identified a significant potential for nanomaterials (e.g., MOFs) to make breakthrough advances in adsorption properties. The introduction of MOF into the hydrogel was accomplished via the in situ growth method, resulting in the preparation of SCC-CuMOF composites [246]. These composites exhibited the capacity for efficient and rapid removal of Pb2+ with selectivity, achieving a maximum adsorption capacity of up to 531.38 mg/g. The underlying adsorption mechanism is depicted in Figure 6e. This phenomenon can be attributed to the selective adsorption of target molecules by MOF, facilitated by pore size screening and size exclusion effects. Additionally, the organic ligands of MOF can enhance specific interactions with contaminants through the modification of functional groups on the hydrogel, thereby improving selectivity. Furthermore, the percentage decreased from 93.0% to 73.9% after five cycles, which still offers a clear sustainability advantage. The employment of microorganisms, such as BC, to spontaneously generate natural hydrogels and form coatings constitutes an efficacious approach to surface modification in order to solve the problems of low gel strength and limited adsorption properties that always exist in the original CMC hydrogels. In the study by Zeng et al. [275], the physical encapsulation of bamboo shoot particles (BSP) was achieved through the construction of a composite CMC/fish gelatin (FGL) hydrogel. This approach exploited the synergistic effect between the matrix of BSP and CMC, resulting in the generation of additional adsorption sites within the composite hydrogel. Consequently, this enhanced the adsorption efficiency in the simultaneous removal of Cd2+, Hg2+, and Pb2+. The composite hydrogels primarily relied on electrostatic interactions and coordination between oxygen and metals for the simultaneous removal of heavy metals (Figure 6f) [275].
The development of smart, responsive hydrogels represents a significant research trend in the field of heavy metal adsorbents. For instance, the incorporation of magnetite into hydrogels can enhance their efficacy in the removal of contaminants from aqueous systems, while concurrently ensuring that the integrity and mechanical strength of the hydrogel are maintained. Furthermore, the incorporation of magnetite into the hydrogel structure endows it with magnetically responsive properties, facilitating expeditious separation and retrieval within a magnetic field environment. Abdul et al. [276] utilized a straightforward technique to incorporate magnetite within hydrogel beads, thereby enhancing the material’s thermal stability. Li et al. [277] prepared a green and efficient adsorbable magnetic hydrogel showing good Cu2+ adsorption (adsorption capacity of 15.95 mg/g) by freeze–melting method (Figure 6g). Furthermore, the adsorption rate of the magnetic hydrogel remained above 80% after four desorption and adsorption cycles.
To enhance the adsorption properties and application range of CBHs, researchers have developed a variety of functionalized hydrogels. The monitoring of heavy metal UO22+ through visual adsorption can be achieved by exploiting the optically responsive properties of cellulose, particularly HPC. Zhang et al. [269] developed a structurally colored hydrogel with high selective adsorption (maximal adsorption capacity of 572.3 mg/g) by embedding HPC with copolymers of AM and AA to enable visual detection of low concentrations of UO22+. Furthermore, CDs have been shown to function as heavy metal sensors, with their luminescent properties being enhanced through doping processes [278]. This sensitivity to various metal ions suggests a potential for fluorescent detection materials to emerge as a promising field. In the study, the researchers doped CDs in the prepared self-repairing fluorescent BCS hydrogels to achieve optimal detection and adsorption of Ag (Figure 6h) [279]. The maximum adsorption of silver ions on the hydrogel was as high as 407 mg/g, and the linear fluorescence response was in the range of 0–75 μM, with a detection limit of 3.798 μM. The maximum adsorption capacity of Pb2+ and Cu2+ was determined to be 1250 and 1111 mg/g, respectively. Figure 6i shows a mechanistic diagram of hydrogel adsorption and detection of Ag+. This multifunctional hydrogel combines self-repairing, adsorption, and detection properties and is the first report of the direct determination and rapid adsorption of Ag+ using a fluorescent hydrogel, which is bound to be an important trend for future research.
In recent years, a considerable body of research has reported the advantages of nanocellulose-based hydrogels for sustainable wastewater treatment when compared with conventional CBHs [271]. However, the challenges of agglomeration that these hydrogels face in the commercial context must also be considered.
Figure 6.
(a) A Schematic of adsorption mechanisms for metal adsorption. Reproduced from [45], Copyright (2018), with permission from Elsevier. (b) Synthetic route for CS/MCCP; (c) SEM image of CS/MCCP; (d) removal of six common metals by CS/MCCP. Reproduced from [274], Copyright (2024), with permission from Elsevier. (e) Possible adsorption mechanism of Pb2+ in SCC-CuMOF. Reproduced from [246], Copyright (2023), with permission from Elsevier. (f) Possible mechanisms for the simultaneous removal of Cd2+, Hg2+, and Pb2+ by composite CMC/FGL@BSP hydrogels. Reproduced from [275], Copyright (2024), with permission from Elsevier. (g) Schematic illustration of preparation of magnetic hydrogel for detection and Cu2+ removal. Reproduced from [277], Copyright (2022), with permission from Elsevier. (h) Schematic of the preparation of the self-healing BCS hydrogel; (i) mechanism diagram of hydrogel (a) adsorption and (b) detection of Ag+. Reproduced from [279], Copyright (2022), with permission from Elsevier.
Figure 6.
(a) A Schematic of adsorption mechanisms for metal adsorption. Reproduced from [45], Copyright (2018), with permission from Elsevier. (b) Synthetic route for CS/MCCP; (c) SEM image of CS/MCCP; (d) removal of six common metals by CS/MCCP. Reproduced from [274], Copyright (2024), with permission from Elsevier. (e) Possible adsorption mechanism of Pb2+ in SCC-CuMOF. Reproduced from [246], Copyright (2023), with permission from Elsevier. (f) Possible mechanisms for the simultaneous removal of Cd2+, Hg2+, and Pb2+ by composite CMC/FGL@BSP hydrogels. Reproduced from [275], Copyright (2024), with permission from Elsevier. (g) Schematic illustration of preparation of magnetic hydrogel for detection and Cu2+ removal. Reproduced from [277], Copyright (2022), with permission from Elsevier. (h) Schematic of the preparation of the self-healing BCS hydrogel; (i) mechanism diagram of hydrogel (a) adsorption and (b) detection of Ag+. Reproduced from [279], Copyright (2022), with permission from Elsevier.
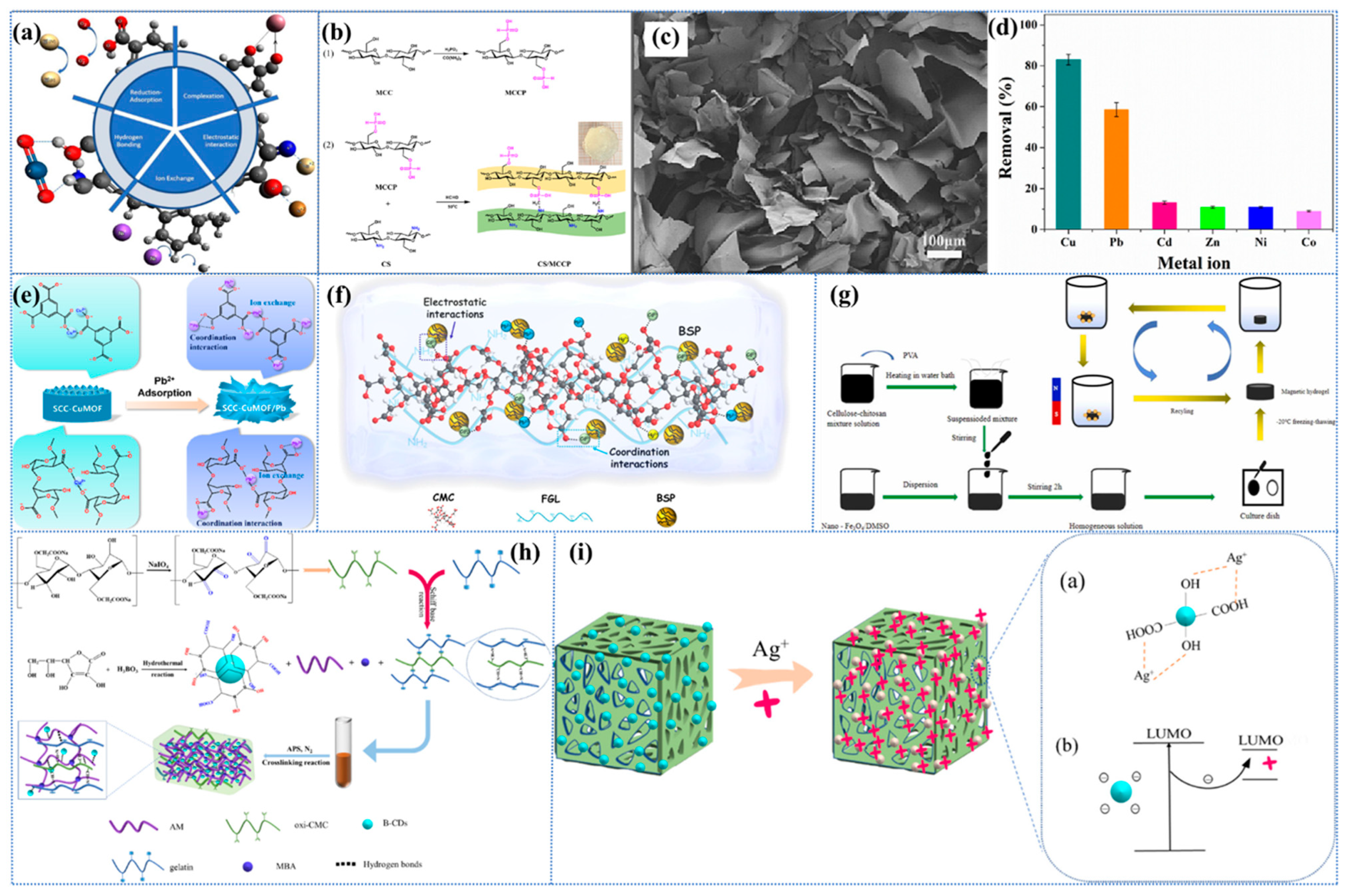

Table 2.
Sustainable and efficient cellulose-based hydrogels for adsorption of heavy metals.
Table 2.
Sustainable and efficient cellulose-based hydrogels for adsorption of heavy metals.
| Cellulose-Based Hydrogels | Heavy Metals | Adsorption Capacity (mg/g) | Cyclic Performance | Reference |
|---|---|---|---|---|
| CMC/FGL@BSP | Cd2+, Hg2+, and Pb2+ | 147.7, 88.62, and 163.89, respectively. | [275] | |
| SCC-CuMOF | Pb2+ | 531.38 | from 93.0% to 73.9% after five cycles | [246] |
| SA/CNF | Pb2+ | 544.66 | Maintained above 81 per cent after 5 cycles | [280] |
| κ-CG/Cellulose | Pb2+ | 486 ± 28.5 | More than 79% after eight cycles | [281] |
| G50 | UO22+ | 572.3 | [269] | |
| CMC/CS/SA(PSCA) | Cr6+, Ni2+, and Cu2+ | More than 750 | [282] | |
| CS/MCCP | Pb2+ and Cu2+ | 211.42 and 74.29, respectively. | [274] | |
| Cellulose (37%)–chitosan (63%) | Cu2+ | 94.3 | [283] | |
| Cellulose/chitosan/PVA/nano-Fe3O4 | Cu2+ | 15.95 | above 80% after four cycles | [277] |
| BCS | Ag+, Pb2+, and Cu2+ | 407, 1250, and 1111, respectively. | regenerated 7 times without much loss of adsorption properties | [279] |
| Cellulose-g-poly (acrylic acid)/poly (vinyl alcohol) | Cu2+ | 142.7 | [284] | |
| CMC-Al beads | Pb2+, Ni2+, and Co2+ | 550, 620, and 760, respectively. | [285] |
4.2. Dye Removal
Dyes represent a further form of pollution that has been extensively utilized in numerous industries, including cosmetics, textiles, plastics, pharmaceuticals, paints, and papers. The classification of dyes can be divided into three categories, namely, (i) anionic or acidic/reactive dyes (due to sulphate groups), (ii) cationic or basic dyes (due to amine groups), and (iii) non-ionic dyes [212,286]. A substantial body of research has demonstrated the efficacy of adsorbents derived from CBHs in the removal of dyes (Table 3). The type of dye, the aqueous environment in which the dye is located, and the functionalization of the adsorbent are the key variables affecting the adsorption of dyes [287]. It is of particular interest to note that Wang et al. modified CM by branching with CH (to provide cationic amino group) or MA (to provide anionic carboxyl group), which resulted in very high selective adsorption of the anionic dye Methylene Blue (MLB, 706.65 mg g−1) and cationic dye Methylene Blue (MB, 934.63 mg g−1), respectively [182]. This phenomenon is mainly attributed to the electrostatic interactions between the surface charge of the material and the charge of the dye molecules, and this synergistic effect is further enhanced by the sieving effect produced by the adsorbent pore size. Therefore, the high affinity of the modified material for the dye can greatly improve its adsorption capacity. For instance, the maximum adsorption capacity of the CMC/GO/ZnO composite hydrogel modified with ZnO increased from 172.41 mg/g to 303.03 mg/g for Basic fuchsin (BF) dye relative to the original CMC/GO hydrogel [288]. As illustrated in Figure 7a, the preparation of CMC/GO/ZnO composite hydrogels and their interaction with BF dye is demonstrated. These findings demonstrate the significant potential of functionalized cellulose hydrogels in selective anion or cation removal.
It has been demonstrated that nanocellulose-based hydrogels have the capacity to remove dyes with high efficiency, thus indicating their potential to become a significant raw material in the production of high-performance adsorbents [212]. It has been demonstrated that both virgin and functionalized CNF and CNC exhibit rapid and robust adsorption of dyes [289]. The surface modification of nanocellulose has been shown to contribute to the development of functional nanocellulose-based hydrogels, which exhibit enhanced adsorption properties. For instance, hydrogels are subject to chemical modification through the introduction of negatively charged groups (anionic sulphonates), thereby enhancing their capacity to adsorb cationic dyes, such as MG [290]. In comparison with the unmodified spherical nanocellulose (SNC) hydrogel, the hydrogel grafted with 2-acrylamido-2-methylpropanesulfonic acid (AMPSA), which provides an anionic sulphonate functional group, has an anionic character. Consequently, the maximum adsorption capacity of the cationic dye malachite green (MG) increases from 49.045 to 357.143 mg/g. It is evident that the graft-modified hydrogel under scrutiny in this study demonstrates remarkable selective adsorption properties, in addition to exhibiting high levels of reusability (Figure 7b) [290]. The negative electrical properties conferred by the sulfonate group on the hydrogel surface generate strong electrostatic attraction with positively charged MG molecules, which greatly improves the adsorption capacity and also brings about remarkable selectivity. In addition, AMPSA grafting increased the cross-linking density of the hydrogel, forming more sulfonate sites while shortening the MG diffusion path.
The development of multifunctional adsorbents for the simultaneous remediation of cationic and anionic dyes has become imperative in the face of complex water environments where single-purpose adsorbents (which can only remove anionic or cationic dyes) are often difficult to cope with. For instance, CS/CMC-NCH hydrogels crosslinked with cationic monomers (diallyl-dimethylammonium chloride (DADMAC) and anionic monomers (AMPSA)) exhibited remarkably high adsorption capacity for both MB and MO (655.98 and 404.52 mg/g, respectively) and excellent regeneration capacity (no loss of adsorption efficiency during the initial 17 cycles) (Figure 7c) [235]. Based on the amazing synergistic adsorption capacity between biopolymers (e.g., CMC and Sodium dextran Sulfate (DS)) and pristine or modified inorganic additives (e.g., Ag-Zeolite (AgZ)), a bifunctional adsorbent of CMC-DS/AgZ nanocomposite hydrogel beads was first developed for the effective adsorption of Basic Red 46 (BR46) and MB cationic dyes, both individually and simultaneously (Figure 7d) [291]. This report demonstrates that the doping of AgZ can efficiently remove two cationic dyes, BR46 and MB (single and binary systems) (344.82 and 454.55 mg/g, respectively), with strong bacteriostatic effects on bacteria [291]. This is a pioneering step in the development of new materials integrating adsorption–disinfection combination processes, thus opening up new research directions for water treatment. This finding paves the way for novel research endeavors in the field of water treatment.
However, the adsorption capacity of CBHs is inevitably limited when adsorption equilibrium is reached. A possible solution is to develop hydrogels with dual functions (i.e., photocatalytic degradation and adsorption) to further enhance the dye removal performance by adding different kinds of catalytically active nanoparticles. For instance, TiO2 nanoparticles are one of the most effective photocatalysts, and it has been found that through the unique structure brought about by the doping of TiO2 and the synergistic effect of the presence of TiO2, the hydrogel has a good removal ability of MB and prevents secondary contamination in wastewater treatment [292]. A report indicated that the dispersion of TiO2 within the hydrogel structure was directly proportional to the efficiency of MB degradation [293]. This phenomenon can be attributed to the creation of numerous active sites within the hydrogel, which enhances light absorption and promotes the generation of holes and electrons. Furthermore, the high transparency of the hydrogel is conducive to the photodegradation process of the dye.
In order to obtain an efficient and reusable dye adsorbent, magnetic Fe3O4 particles were loaded onto the hydrogel by chemical co-precipitation in situ synthesis, which allowed for easy separation and recovery of the modified cellulose-based hydrogel [294]. Xu et al. [295] promoted adsorption by adding magnetic nanoparticles to induce magnetic effects and increase the surface area, and found that the presence of Fe3O4 particles in the low- and medium-concentration ranges increased the adsorption capacity of the hydrogels. Conversely, at elevated concentrations, interactions between magnetic particles or excessive cross-linking have been observed to reduce the effective adsorption sites and, consequently, the amount of adsorption.
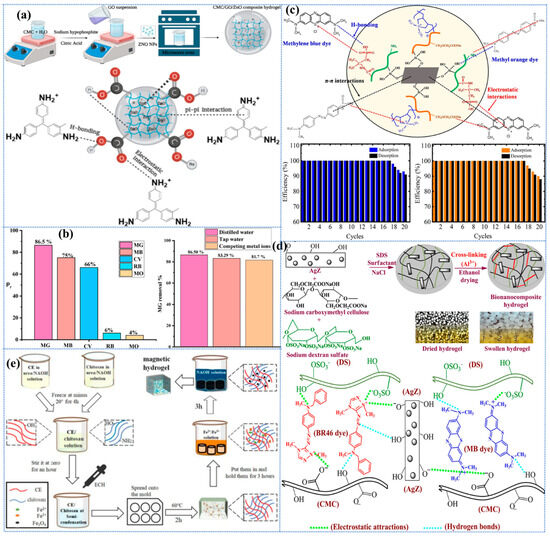
Figure 7.
(a) The preparation of CMC/GO/ZnO composite hydrogels and their interaction with BF dye. Reproduced from [288], Copyright (2024), with permission from Elsevier. (b) (left) Removal of MG, MB, CV, MO, and RB (Time = 60 min); (right) Competitive removal of MG in distilled water, tap water and in presence of metal ions. Reproduced from [290], Copyright (2023), with permission from Elsevier. (c) Mechanism of adsorption of MB and MO dyes onto CS/CMC-NCH and reusability of adsorption onto CS/CMC-NCH. Reproduced from [235], Copyright (2021), with permission from Elsevier. (d) Schematic representation of the fabrication of CMC-DS-AgZ composite hydrogel (top) and the mechanism of adsorption of BR46 and MB onto CMC-DS-AgZ (bottom). Reproduced from [291], Copyright (2024), with permission from Elsevier. (e) Schematic representation of the synthesis of magnetic cellulose/CS composite hydrogels. Reproduced from [295], Copyright (2024), with permission from Elsevier.
Figure 7.
(a) The preparation of CMC/GO/ZnO composite hydrogels and their interaction with BF dye. Reproduced from [288], Copyright (2024), with permission from Elsevier. (b) (left) Removal of MG, MB, CV, MO, and RB (Time = 60 min); (right) Competitive removal of MG in distilled water, tap water and in presence of metal ions. Reproduced from [290], Copyright (2023), with permission from Elsevier. (c) Mechanism of adsorption of MB and MO dyes onto CS/CMC-NCH and reusability of adsorption onto CS/CMC-NCH. Reproduced from [235], Copyright (2021), with permission from Elsevier. (d) Schematic representation of the fabrication of CMC-DS-AgZ composite hydrogel (top) and the mechanism of adsorption of BR46 and MB onto CMC-DS-AgZ (bottom). Reproduced from [291], Copyright (2024), with permission from Elsevier. (e) Schematic representation of the synthesis of magnetic cellulose/CS composite hydrogels. Reproduced from [295], Copyright (2024), with permission from Elsevier.

Table 3.
Sustainable and efficient cellulose-based hydrogels for adsorption of dyes.
Table 3.
Sustainable and efficient cellulose-based hydrogels for adsorption of dyes.
| Cellulose-Based Hydrogels | Dyes | Qe (mg/g) | Optimal Conditions | Cycles | Reference | ||
|---|---|---|---|---|---|---|---|
| pH | T (°C) | Dose (g) | |||||
| CMC-g-(PSPMA) | MB | 1675 | 6 | 0.05 | 5 | [296] | |
| Cellulose/MTM | MB | 277 | 7 | 25 | [297] | ||
| CA/CNC | MB | 676.7 | 7 | 25 | 0.02 | [82] | |
| HEC-Co-P(AA-AM)/TA | MB | 3438.27 | 8 | 25 | 0.2 | 5 | [170] |
| CMC/GO/ZnO | BF | 303.03 | 6 | 0.01 | 5 | [288] | |
| TiO2@MMTNS/CMC/CS | MB | 283.97 | 8 | 60 | 0.2 | 5 | [225] |
| CS/CMC-NCH | MB MO | 655.98 404.52 | 3 | 25 | 0.6 | 20 | [235] |
| g-C3N4@SBC/CMC | MB | 362.3 | 7 | 25 | 1 | 7 | [238] |
| CS/CMC-PEG | CR MB | 1053.88 331.72 | 4 11 | 30 | 0.6 0.8 | [298] | |
| CGC/NaAlg | MB CR | 400.504 11.45 | 6 2 | 25 | 0.2 | 6 | [299] |
| SNC-g-poly-(AMPSA)-cl-MBAm | MG | 357.143 | 6 | 30 | 8 | [290] | |
| CM-MA CM-CH | MB MLB | 934.63 706.64 | 25 | 5 | [182] | ||
| CS/CMC-NCH | MB MO | 655.98 404.52 | 8 2 | 45 | 0.4 0.6 | 20 | [235] |
| CMC-DS-AgZ | BR46 MB | 344.82 454.55 | 7 | 1 | 5 | [291] | |
4.3. Micropollutant Removal
Micropollutants, otherwise known as trace organic compounds, represent a group of emerging contaminants, and some common examples of micropollutants include pesticides, pharmaceuticals, hormones, personal care products, surfactants, disinfectants, disinfection by-products, and perfluorinated compounds [300,301]. Despite their typical presence in the trace concentration range (ng/L or μg/L), these substances pose a potential risk due to their persistence and bioaccumulation. As illustrated in Table 4, some comparative analyses have been conducted on the application of CBHs in the removal of micropollutants.
Levofloxacin (LEV) is a class of antibiotic drugs whose residues not only contribute to bacterial resistance and affect aquatic organisms, but also pose a potential threat to human health through the food chain [302]. In their study, Zhang et al. [303] utilized the advantageous properties of β-cyclodextrins, cellulose nanofibrils (CNs), and CDs to develop a pioneering βCCH for the enhanced simultaneous adsorption and fluorescence detection of LEV. The synergy of CNs and CDs resulted in an unprecedented maximum adsorption capacity of 1376.9 mg/g, while the limit of detection was reduced to 0.09 μg/L. This innovative approach offers a new avenue for the development of advanced antibiotic detection and adsorbent materials that are both stable and cost-effective. As illustrated in Figure 8a, the synthetic route is delineated, in conjunction with the adsorption and detection mechanism of βCCH. Tetracycline (TC) is the most extensively utilized antibiotic owing to its high efficacy and low cost. Nonetheless, its recurrent use can precipitate grave health complications. Based on the ability of wood-derived cellulose nanocrystals (WCNs) to provide a backbone for accelerated network structures to enhance TC adsorption and the ability of CDs to act as fluorescent probes for TC and to provide adsorption sites, Luo et al. [304] prepared a non-toxic FMIH with highly efficient adsorption performance (maximum adsorption capacity of 544.4 mg/g) and sensitive detection of TC. As illustrated in Figure 8b, the preparation route for the FMIH is delineated.
Diclofenac (DCF), a widely utilized anti-inflammatory pharmaceutical agent on a global scale, poses a significant challenge in the context of wastewater treatment. Tie et al. [305] constructed nanocellulose fine-tuned PAA hydrogels via a double crosslinked network, thereby enhancing the maximum adsorption capacity for DCF to 559.8 mg g⁻1. Fluoride is among the most prevalent contaminants, and it is imperative to note that exceeding acceptable levels can result in irreparable health implications. Sinha et al. [306] synthesized novel CMC-g-AMPS/Fe/Al hydrogels impregnated with AC for efficient F removal and improved mechanical properties (Figure 8c). These exhibited excellent F− adsorption efficiency because Fe, Al, -COOH, and hydroxyl groups are the main functional parts constituting the polymer network. Ingestion of ibuprofen by humans and animals results in incomplete metabolism, with the release of water-soluble metabolites that are more toxic than the parent molecule [307]. In order to ensure that the surface structure of Alg/AC composite hydrogel beads can recover its initial hydrogel state after adsorption, so as to maintain its effectiveness in the adsorption of ibuprofen, Lee et al. [308] prepared alginate (Alg)/Activated carbon (AC)/CMC composite hydrogel beads by doping with CMC as a swelling agent, thus improving its reusability and showing a high recovery rate (>93%) after 10 cycles.
Bisphenol A (BPA) is an emerging micropollutant that is characterized by low-dose, high toxicity, and a significant biological risk due to its ability to disrupt the hormonal composition of living organisms [309]. In the study by Ouyang et al. [310], SCZC was prepared using a combination of SA, CNFs, CDs, and ZIF-8 for the purpose of BPA adsorption and detection. The function of CDs was to enhance the amino-induced adsorption of BPA and act as a fluorescent sensor for qualitative and quantitative detection. In addition, ZIF-8 provided additional adsorption sites for BPA (maximum adsorption capacity of 1696 mg/g). As illustrated schematically in Figure 8d, the mechanism of adsorption and detection of BPA on SCZC hydrogels is demonstrated.
Microfibers (MFs) represent the predominant form of microplastics in ecosystems, thereby constituting a substantial environmental concern. The existing wastewater treatment infrastructure is inadequate in addressing this issue, as it is incapable of effectively removing microfibers. In light of this, Rodrigues et al. [311] evaluated the excellent performance of bacterial cellulose hydrogel (BCH) as a bio-flocculant for the removal of MFs, achieving a flocculation rate of 93.6% under optimal conditions. As illustrated in Figure 8e, this comprehensive framework encompasses the entire process of MFs, from their generation to their eventual removal, while also elucidating the factors that influence this process. Their present study establishes a novel paradigm for research on the use of BCH as a sustainable and effective bio-flocculant for the removal of MFs.
Figure 8.
(a) Synthesis route of βCCH-5 (left) and schematic representation of the adsorption and detection mechanism (right). Reproduced from [303], Copyright (2024), with permission from Elsevier. (b) Preparatory route for FMIH. Reproduced from [304], Copyright (2022), with permission from Elsevier. (c) Schematic of the preparation of CMC-g-AMPS/Fe/Al/AC composite hydrogels. Reproduced from [306], Copyright (2020), with permission from Elsevier. (d) Schematic diagram of the mechanism of adsorption and detection of BPA by SCZC. Reproduced from [310], Copyright (2024), with permission from Elsevier. (e) Sources of MFs, along with the factors influencing the BC hydrogel synthesis process and the flocculation process for MFs removal. Reproduced from [311].
Figure 8.
(a) Synthesis route of βCCH-5 (left) and schematic representation of the adsorption and detection mechanism (right). Reproduced from [303], Copyright (2024), with permission from Elsevier. (b) Preparatory route for FMIH. Reproduced from [304], Copyright (2022), with permission from Elsevier. (c) Schematic of the preparation of CMC-g-AMPS/Fe/Al/AC composite hydrogels. Reproduced from [306], Copyright (2020), with permission from Elsevier. (d) Schematic diagram of the mechanism of adsorption and detection of BPA by SCZC. Reproduced from [310], Copyright (2024), with permission from Elsevier. (e) Sources of MFs, along with the factors influencing the BC hydrogel synthesis process and the flocculation process for MFs removal. Reproduced from [311].

Table 4.
Sustainable and efficient cellulose-based hydrogels for adsorption of micropollutants.
Table 4.
Sustainable and efficient cellulose-based hydrogels for adsorption of micropollutants.
| Feed Water Characteristics | Micropollutants | Cellulose-Based Hydrogels | Qe (mg/g) | Conditions | Cycles | Reference | ||
|---|---|---|---|---|---|---|---|---|
| pH | T (°C) | Dose (g) | ||||||
| Antibiotics wastewater | LEV | βCCH | 1376.9 | 6 | 25 | 5 | [303] | |
| TC | FMIH | 544.4 | 3 | 45 | 3.5 | 6 | [304] | |
| DOXY | SA-DCNC | 594.6 | 7 | 45 | 5 | [312] | ||
| DOXY | LCCH | 1686 | 6 | 60 | 5 | [313] | ||
| TC | CH | 541.3 | 8 | 45 | 4 | [314] | ||
| Pharmaceutical and personal care products wastewater | DCF | P/TCNF | 498 | 3.4 | 25 | [305] | ||
| DCF | FG-CMC3% | 666.7 | 4.2 | 25 | 4 | [315] | ||
| Ibuprofen | Alg/AC/CMC | 28.3 | 10 | [308] | ||||
| Fluorides wastewater | F- | CMC-g-AMPS/Fe/Al/AC | 67.114 | 6 | [306] | |||
| Endocrine disruptors wastewater | BPA | SCZC | 1696 | 7 | 15 | 5 | [310] | |
| BPA | FeN@CP | 309.17 | 2 | 25 | 5 | [316] | ||
| Microplastic wastewater | MFs | BCH | 93.6% flocculation | 5.37 | 25 | [311] | ||
5. Challenges and Future Perspectives
5.1. Mechanical Performance Challenges
Despite the encouraging potential demonstrated by CBHs, challenges continue to hinder their advancement. For instance, the mechanical properties and stability of hydrogels frequently fall short of the requirements for specific applications, significantly restricting their use. In this regard, the mechanical properties and stability of CBHs can be enhanced through the optimization of cross-linking strategies, the integration of nanocomposite technology, and the implementation of molecular-level design. For instance, the incorporation of nanocellulose or nanoparticles has been shown to enhance the hydrogel structure, while the employment of advanced crosslinking methods has been demonstrated to increase crosslink density and homogeneity. Achieving the optimal equilibrium between porosity and stability in CBHs represents a significant challenge. An increase in porosity can result in an enhancement of adsorption sites; nevertheless, excessive porosity may also precipitate a decline in mechanical properties. Exploration of the scope for improving the mechanical durability of CBHs is also important, as is the improvement in their self-healing ability after the swollen state.
5.2. Solvent Use and Biodegradability Challenges
The development of these materials is frequently contingent upon the utilization of solvents, creating environmental burdens and scalability challenges. Addressing this requires adopting green solvents—particularly deep eutectic solvents (DESs), supercritical fluids (SCFs), and ionic liquids (ILs)—to circumvent hazardous solvent dependency while maintaining process efficiency. Furthermore, the biodegradability of CBHs signifies added value for their environmental applications, as it ensures complete and safe degradation without the release of toxic by-products. However, it is important to note that the rates and patterns of degradation are often challenging to accurately control, which may result in reduced performance or unexpected biological interactions in specific applications. Concurrently, their stability in real water, which is naturally home to diverse microorganisms, must be contemplated and optimized to identify an acceptable compromise between the kinetics of the adsorption process and the biodegradation time scale. Resolving this persistence–performance paradox requires mechanistic studies of hydrogel–environment interplay under extended field conditions to establish predictive behavior models.
5.3. Fouling and Long-Term Stability Challenges
In real wastewater treatment scenarios, the engineering of hydrogel materials also needs to focus on two key issues: fouling and long-term stability. The former includes biological fouling, inorganic fouling, and organic fouling. Biological fouling is due to microorganisms (e.g., algae) on the surface of the hydrogel to form a biofilm, resulting in pore blockage; at the same time, its metabolic waste will also corrode the hydrogel, resulting in a substantial reduction in mechanical properties. Inorganic fouling is due to the deposition of inorganic crystals (e.g., CaCO3) within the pores, shielding the adsorption active sites on the hydrogel. In addition, hydrophobic substances such as oils and fats can also preferentially occupy the active sites, resulting in organic fouling, competing with the target contaminants for adsorption while preventing water molecules from penetrating due to their hydrophobicity. A potential strategy to address the fouling problem is the use of bionic structures or amphoteric Ion Coatings, thus enabling the design of superhydrophobic/super-hydrophilic surfaces. The compatibility of hydrogels can also be utilized to alleviate this limitation by carrying different functional components for self-cleaning purposes. For instance, the incorporation of inorganic particles endowed with catalytic properties (e.g., Ag NPs) or enzymes (e.g., laccase) into the hydrogel network can facilitate the regeneration of its adsorption capabilities through the catalysis of surface fouling degradation, concomitantly impeding biological adhesion. Furthermore, the integration of Fe3O4 nanoparticles within a hydrogel network, inducing a thermal effect through its magnetic responsiveness, facilitates the automated removal of fouling. The long-term stability of these materials is compromised by various factors, including mechanical fatigue due to repetitive swelling and shrinkage, acidic hydrolysis, oxidative corrosion, and interference resulting from high ionic concentrations. Recent studies have demonstrated that the addition of nanocomposites can enhance the stability of the material, particularly through the formation of specialized structures resulting from the oriented alignment of cellulose nanocrystals. Additionally, the construction of double crosslinked networks and hydrophobic microregions has been shown to improve stability while increasing resistance to swelling.
5.4. Scalability and Cost-Effectiveness
A further key issue is the enhancement of the scalability and cost-effectiveness of the processes employed in the production of CBHs. It is imperative to develop scalable production processes that can reduce costs while maintaining the quality and performance of these hydrogels, given that processes involving advanced modifications, such as TEMPO oxidation or the incorporation of functional additives, can be very complex and costly. This objective encompasses the optimization of raw material usage, the refinement of synthesis methodologies, and reductions in the necessity for costly reagents or extensive purification steps. In the context of contemporary technological capabilities, it remains challenging to achieve uniformity in the properties of hydrogels. The prevailing synthesis methodologies employed for industrial applications are susceptible to an imbalanced distribution of cross-linking densities. A promising approach to address this challenge is the implementation of continuous synthesis via microfluidic chips, a strategy that enables precise control over the pore size distribution of hydrogels. The synthesis of high-performance CBHs necessitates high cellulose purity, which implies a higher raw material cost; further, the employment of conventional solvents with toxicity remains prevalent. Moreover, the prevailing technological capabilities remain inadequate in addressing the substantial energy demands inherent in freeze–drying processes, which are indispensable for ensuring the integrity of hydrogel pores. Conversely, the utilization of hot-air drying is susceptible to pore collapse, a conundrum that necessitates technological breakthroughs for resolution. In the context of adsorption application processes, the economic viability of the process is contingent on the regeneration capacity of the material. Despite the fact that the regeneration capacity of hydrogels has been the subject of numerous studies, the majority of these focus primarily on the adsorption process, with little attention paid to the effective reuse process of hydrogels. In this context, there is a necessity for more in-depth studies to be conducted in order to understand the regeneration capacity of hydrogels in various cycles.
5.5. Variability of Natural Water Bodies
A multitude of physical and chemical properties inherent in natural water bodies, including pH, temperature, salinity, oxygen content, and light, can exert an influence on the process of water treatment. This is attributable to the inherent variability of these properties, which constitutes an additional factor demanding consideration. Consequently, priority must be given to the effectiveness of hydrogels under these challenging conditions in real water samples or simulated environments. Real wastewater constitutes multicomponent systems containing heavy metals, dyes, microplastics, pharmaceuticals, and persistent organics, frequently compounded by additives like surfactants or preservatives. The presence of multiple ions in water has been demonstrated to affect the adsorption of particular ions. Consequently, future studies of wastewater treatment with CBHs should be extended to solutions containing different contaminants to assess possible cross-interference, rather than just focusing on a single contaminant, systematically evaluating competitive sorption mechanisms.
5.6. Strategies for Improving Practicality
In light of the enduring prevalence of traditional water treatment processes, the potential of innovative technologies, such as cellulose-based hydrogels, to enhance their market competitiveness by integrating with fluidized beds, adsorption towers, and other well-established treatment equipment is a subject of significant interest. The integration of these novel technologies with existing wastewater treatment infrastructure has the potential to enhance their practical applicability, paving the way for large-scale applications, particularly in the case of bead-based hydrogels. This form of hydrogel utilizes a high surface area, resulting in better contact with water and a larger adsorption area that can be exposed to a uniform water flow, thus speeding up the process. Moreover, the integration of CBHs with advanced water treatment technologies, including membrane filtration, photocatalysis, and electrochemical methods, has the potential to enhance the efficiency and sustainability of water treatment processes.
The necessity to functionalize and tailor the physical and chemical properties of CBHs to meet specific applications is also a challenge. Particularly, the development of smart hydrogels that exhibit responsiveness to temperature, pH, and light, thereby becoming sensitive to environmental changes, necessitates sophisticated design and engineering in conjunction with innovative preparation methodologies.
It is imperative to address the challenges and limitations currently faced by the field, which will require the development of innovative production methods, the optimization of processing conditions, and a deeper understanding of the parameters that affect their properties and performance, to unlock the full potential of CBHs.
6. Conclusions
This paper provides a comprehensive review of recent studies exploring the use of cellulose-based hydrogels (CBHs) for wastewater treatment, with a focus on the removal of heavy metals, dyes, and micropollutants. The review encompasses the synthesis of CBHs, the adsorption mechanisms, and measures to optimize adsorption capacity. The properties of cellulose and its derivative-based hydrogels that render them a compelling class of materials for sustainable and efficient remediation of contaminated water are reviewed, including their high removal efficiency, good mechanical properties, biocompatibility and degradability, low cost, process simplicity, and versatility. Furthermore, given that the removal of pollutants typically necessitates non-covalent interactions (e.g., hydrogen bonding and electrostatic forces), cellulose systems can be reused numerous times, a factor that is of significant importance in today’s society, which places considerable emphasis on sustainable and environmentally friendly development.
In recent years, there has been a notable advancement in the development of preparation strategies and functional properties of CBHs, leading to an increased focus on their application in wastewater treatment. However, challenges remain to be overcome, including their relatively low mechanical strength and durability, before they can be recognized as promising and pioneering materials for a wide range of applications in the field. It has been demonstrated by a considerable number of studies that the integration of cellulose-based hydrogels with other materials, including nanomaterials (e.g., GO, ZnO, TiO2, CCD, CNT, MMTNS, and Fe2O3), results in a substantial enhancement of the hydrogels’ adsorption properties. This is attributable to the significant increase in surface area and porosity that the nanoparticles provide, as well as an improvement in the mechanical strength and durability of the hydrogels. Furthermore, the employment of novel evaluation methodologies for complex scenarios in actual water bodies has the potential to further propel the field, facilitating the analysis of their performance and efficacy. It is imperative that adsorption studies of CBHs do not concentrate exclusively on single pollutant studies but, rather, extend to multi-pollutant specific evaluations. Furthermore, the entire process of CBHs, from raw material to degradation, is quantified through the evaluation of scientific models. This establishes a standardized evaluation system for researching the development of the same type of materials.
In addition, research on their life cycle will be strengthened to promote their environmental adaptability. Of paramount importance is the emphasis placed on the utilization of emerging techniques, such as simulation analysis, to explore the potential mechanisms of hydrogel adsorption in greater depth and for the design of novel hydrogels. It is anticipated that through the continued refinement of formulations and the optimization of synthesis conditions, more efficient and sustainable wastewater treatment solutions will be developed that exhibit greater adaptability to complex and changing water environments. In future research, the development of cellulose-based hydrogels will focus more on combining them with other functional materials, leading to the development of new multifunctional and integrated materials. This multifunctional integration will break through the traditional application boundaries of CBHs and promote their deep development into multidisciplinary, cross-cutting fields. These include the fixation of heavy metals in soil for environmental remediation; use as a wound dressing for antibacterial and healing promotion; use as a scaffolding material for tissue engineering, promoting cellular differentiation; and utilization of its pH responsiveness to achieve the controlled release of medicines. Moreover, it has been demonstrated that the CNT/CBHs composites have extremely high specific capacitance and are good energy materials. Consequently, CBHs are anticipated to have considerable potential in environmental remediation and pollution control, biomedicine, energy, smart materials, and other fields. Technological breakthroughs in this area are expected to lead to a new generation of green materials.
Author Contributions
Conceptualization, Z.Z. and Y.L.; methodology, Z.Z.; validation, S.G. and S.W.; formal analysis, Z.Z.; investigation, Y.L.; resources, Z.Z.; writing—original draft preparation, Z.Z.; writing—review and editing, S.G. and S.W.; supervision, S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [the National Natural Science Foundation of China] grant number [51808263], and [the Practice Innovation Training Program Projects of Jiangsu University] grant number [202410299298X, 202410299112Y].
Data Availability Statement
No new data were created.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51808263), and the Practice Innovation Training Program Projects of Jiangsu University (202410299298X, 202410299112Y).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CBHs | Cellulose-based hydrogels | CNC | Cellulose nanocrystals | CNF | Cellulose nanofibrils |
| BNC | Bacterial nanocellulose | -OH | Hydroxyl group | CD | Cellulose derivatives |
| PVA | Polyvinyl alcohol | CMC | Carboxymethyl cellulose | BC | Bacterial cellulose |
| GO | Graphene oxide | ATP | Attapulgite | CMCF | Carboxymethylcellulose nanofiber |
| CA | Citric acid | IG | Ionotropic gelation | SA | Sodium alginate |
| HPC | Hydroxypropyl cellulose | CGG | Cationic guar gum | TOCN | TEMPO (2,2,6,6-tetramethylpiperidin-1-yloxy)-oxidized cellulose nanofibers |
| MLB | Methyl blue | MB | Methylene blue | KPS | Potassium persulfate |
| TEMED | Tetramethylene diamine | APS | Ammonium persulfate | MBA | N, N′-methylene-bis(acrylamide) |
| AA | Acrylic acid | AM | Acrylamide | HEC | Hydroxyethyl cellulose |
| TA | Tannic acid | ZnO | Zinc oxide | MCC | Microcrystalline cellulose |
| MV | Methyl violet | RhB | Rhodamine B | DCMC | Dialdehyde carboxymethyl cellulose |
| CH | Cysteamine hydrochloride | CM | Cellulose methacrylate | BHNC | Bifunctional hairy nanofibrillar cellulose |
| MA | Maleic anhydride | ECH | Epichlorohydrin | MA | 3-mercaptopropionic acid |
| PEG | Polyethylene glycol | CP | Chlorpyrifos | PAH | Poly-(allylamine hydrochloride) |
| MO | Methyl orange | CA | Cellulose acetate | CMC | Carboxymethyl cellulose |
| CDs | Carbon dots | CS | Chitosan | CNs | Cellulose nanofibrils |
| ECH | Epichlorohydrin | HA | Hydroxyapatite | LDH | Laminar double hydroxide |
| PDA | Polydopamine | PEI | Polyethyleneimine | MoS2 | Molybdenum disulfide |
| SA | Sodium alginate | PAA | Polyacrylic acid | G-C3N4 | Graphitic carbon nitride |
| TAPB | 1,3,5-tris(4-aminophenyl)-benzene | BP | 2,2′-bipyridine-5,5′-formaldehyde | TEMPO | (2,2,6,6-Tetramethylpiperidin-1-yl) oxyl |
| PEI | Polyethyleneimine | DMSO | Dimethyl-sulfoxide | MOFs | Metal-organic frameworks |
| PAM | Polyacrylamide | NFC | Nano-fibrillated cellulose | COFs | Covalent organic frameworks |
| FGL | Fish gelatin | MFC | Microfibrillar cellulose | NMMO | N-methyl morpholine-N-oxide |
| PSCA | Pore structure control agent | BSP | Bamboo shoot particles | DMAEMA | 2-dimethylaminoethyl methacrylate monomer |
| CR | Congo red | κ-CG | Kappa-carrageenan | HEMA | Tert-butyl acrylate-co-2-hydroxyethyl methacrylate |
| MG | Malachite green | BF | Basic fuchsin | DES | Deep eutectic solvent |
| BR46 | Basic red 46 | AgZ | Ag-zeolite | MCCP | Cellulose phosphonate |
| CV | Crystalline violet | LEV | Levofloxacin | SNC | Spherical nanocellulose |
| TC | Tetracycline | WCNs | Wood-derived cellulose nanocrystals | PDChNF | Partially deacetylated chitin nanofibers |
| DOXY | Doxycycline | DCF | Diclofenac | AMPSA | 2-acrylamido-2-methylpropanesulfonic acid |
| BPA | Bisphenol A | AC | Activated carbon | DS | Sodium dextran Sulfate |
| Alg | Alginate | MFs | Microfibers |
References
- Radoor, S.; Karayil, J.; Jayakumar, A.; Kandel, D.R.; Kim, J.T.; Siengchin, S.; Lee, J. Recent advances in cellulose- and alginate-based hydrogels for water and wastewater treatment: A review. Carbohydr. Polym. 2024, 323, 121339. [Google Scholar] [CrossRef]
- Ali, A.B.; Li, H.; Elshaikh, N.A.; Yan, H.F. Assessing impacts of water harvesting techniques on the water footprint of sorghum in Western Sudan. Outlook Agric. 2016, 45, 185–191. [Google Scholar] [CrossRef]
- Mao, F.; Miller, J.D.; Young, S.L.; Krause, S.; Hannah, D.M.; Network, H.R.C. Inequality of household water security follows a Development Kuznets Curve. Nat. Commun. 2022, 13, 4525. [Google Scholar] [CrossRef]
- Marcal, J.; Antizar-Ladislao, B.; Hofman, J. Addressing Water Security: An Overview. Sustainability 2021, 13, 13702. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Zhao, X.; Zhao, D.; Ding, X.; Yin, Y.; Liu, Y. Study on Evaluation and Dynamic Early Warning of Urban Water Resources Security. Water 2025, 17, 242. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.Q.; Cui, M.M.; Yu, H.C.; Fan, X.; Zhu, Z.Q.; Zhang, H.Y.; Dai, Z.C.; Sun, J.F.; Yang, B.; Du, D.L. Global Environmental Change Shifts Ecological Stoichiometry Coupling Between Plant and Soil in Early-Stage Invasions. J. Soil Sci. Plant Nutr. 2024, 24, 2402–2412. [Google Scholar] [CrossRef]
- Antunes, L.N.; Ghisi, E.; Souza, J.C. Stormwater harvested from a permeable pavement for use in the fire extinguishing system and non-potable uses of a building: A case study. Urban Water J. 2022, 19, 433–440. [Google Scholar] [CrossRef]
- Foster, S.; Andreo, B. Public groundwater supplies: Minimising operational costs and carbon footprints. Hydrogeol. J. 2024, 32, 1253–1257. [Google Scholar] [CrossRef]
- Tow, E.W.; Hartman, A.L.; Jaworowski, A.; Zucker, I.; Kum, S.; AzadiAghdam, M.; Blatchley, E.R.; Achilli, A.; Gu, H.; Urper, G.M.; et al. Modeling the energy consumption of potable water reuse schemes. Water Res. X 2021, 13, 100126. [Google Scholar] [CrossRef]
- Mohamed, T.M.K.; Gao, J.M.; Abuarab, M.E.; Kassem, M.; Wasef, E.; El-Ssawy, W. Applying Different Magnetic Water Densities as Irrigation for Aeroponically and Hydroponically Grown Strawberries. Agriculture 2022, 12, 819. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.F.; Zhang, C.; Wang, G.Q.; He, B.; Hao, B.B.; Han, Y.J.; Wang, B.Y.; Bao, R.X.; Syed, T.N.; et al. A Review of Precision Irrigation Water-Saving Technology under Changing Climate for Enhancing Water Use Efficiency, Crop Yield, and Environmental Footprints. Agriculture 2024, 14, 1141. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, Y.; Yin, L.; Chen, Y.; Xue, Y.; Wang, X.; Mao, D.; Luo, Y. Unraveling the influence of human fecal pollution on antibiotic resistance gene levels in different receiving water bodies using crAssphage indicator gene. J. Hazard. Mater. 2023, 442, 130005. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Klink, J.; Perello, L.A.; Abily, M.; Salo, J.; Rodriguez-Roda, I.; Marce, R.; Gernjak, W.; Corominas, L. Coupling hydrological and sanitation datasets to simulate wastewater-derived contamination in European rivers: Model development and calibration. Environ. Model. Softw. 2024, 178, 106049. [Google Scholar] [CrossRef]
- Shi, W.J.; Xu, C.; Cai, J.W.; Wu, S.P. Advancements in material selection and application research for mixed matrix membranes in water treatment. J. Environ. Chem. Eng. 2023, 11, 106049. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ramadan, H.; Elsamahy, T.; Eltawab, R.; Mostafa, S.; Zhou, X.; Cheng, L. Multifaced features and sustainability of using pure oxygen in biological wastewater treatment: A review. J. Water Process Eng. 2023, 53, 103883. [Google Scholar] [CrossRef]
- Feng, C.; Wu, F.; Zhang, L.; Yang, X.; Zhuang, Y. Assessing provincial integrated wastewater treatment efficiency and influencing factors considering triple bottom line. J. Clean. Prod. 2025, 491, 144724. [Google Scholar] [CrossRef]
- Feng, H.; Jin, L.; Chen, Y.; Ji, J.; Gong, Z.; Hu, W.; Ying, C.; Liang, Y.; Li, J. Tofu wastewater as a carbon source flowing into municipal wastewater treatment plants for reductions of costs and greenhouse gas emissions. J. Environ. Manag. 2024, 370, 122550. [Google Scholar] [CrossRef]
- Zhang, C.; Quan, B.; Tang, J.; Cheng, K.; Tang, Y.; Shen, W.; Su, P.; Zhang, C. China’s wastewater treatment: Status quo and sustainability perspectives. J. Water Process Eng. 2023, 53, 103708. [Google Scholar] [CrossRef]
- Cai, R.; Chen, Y.; Hu, J.; Xiong, J.; Lu, J.; Liu, J.; Tan, X.; Liu, W.; Zhou, Y.; Chen, Y. A self-supported sodium alginate composite hydrogel membrane and its performance in filtering heavy metal ions. Carbohydr. Polym. 2023, 300, 120278. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Q.; Lu, J.; Wu, Y.L.; Meng, M.J.; Yu, C.; Sun, C.; Chen, M.N.; Da, Z.L.; Yan, Y.S. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Shi, W.J.; Li, K.H.; Cai, J.W.; Xu, C.; Gao, L.; Lu, J.W.; Ding, F.Y. Chitosan-based hollow nanofiber membranes with polyvinylpyrrolidone and polyvinyl alcohol for efficient removal and filtration of organic dyes and heavy metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, Y.; Liu, L.; Li, Z.; Chen, X.; Xia, M.; Fan, G.; Ding, A. Membrane Distillation for Wastewater Treatment: A Mini Review. Water 2021, 13, 3480. [Google Scholar] [CrossRef]
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Abdul Manaf, S.F.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2022, 12, 26. [Google Scholar] [CrossRef]
- Hu, R.-Z.; Zhang, Z.-F.; Yu, B.-Q.; Wang, J.; Yao, X.-H.; Chen, T.; Zhao, W.-G.; Zhang, D.-Y. Natural phenolics and flavonoids modified the hierarchical cellular cellulose sponges for efficient water disinfection. Carbohydr. Polym. 2022, 296, 119962. [Google Scholar] [CrossRef]
- Fotoohi, E.; Mokhtarian, N.; Farahbod, F. Operational analysis of the biological treatment unit’s ultraviolet-wave disinfection method for wastewater outflow. Appl. Water Sci. 2024, 14, 27. [Google Scholar] [CrossRef]
- Kalli, M.; Noutsopoulos, C.; Mamais, D. The Fate and Occurrence of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes during Advanced Wastewater Treatment and Disinfection: A Review. Water 2023, 15, 2084. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Li, L.; Song, B.; Zhou, C.; Zeng, G.; Zhang, Y.; Xiao, R. Microplastics act as an important protective umbrella for bacteria during water/wastewater disinfection. J. Clean. Prod. 2021, 315, 128188. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, H.; Cai, J.; Liu, J.; Wang, Y.; Deng, J. Research progress of electrochemical oxidation and self-action of electric field for medical wastewater treatment. Front. Microbiol. 2023, 13, 1083974. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Guo, X.; Cao, J.; Yang, S.; Qiu, Z.; Wang, J.; Shen, Z. The occurrence, ecological risk, and control of disinfection by-products from intensified wastewater disinfection during the COVID-19 pandemic. Sci. Total Environ. 2023, 900, 165602. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Shi, H.-J. UV/chlorinated cyanurates as an emerging advanced oxidation process for drinking water and potable reuse treatments. Water Res. 2022, 211, 118075. [Google Scholar] [CrossRef] [PubMed]
- Antinolo Bermudez, L.; Martin Pascual, J.; Munio Martinez, M.d.M.; Poyatos Capilla, J.M. Effectiveness of Advanced Oxidation Processes in Wastewater Treatment: State of the Art. Water 2021, 13, 2094. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Reyes-Vidal, Y.; Irigoyen-Campuzano, J.R.; Reynoso-Cuevas, L. Simultaneous Oxidation of Emerging Pollutants in Real Wastewater by the Advanced Fenton Oxidation Process. Catalysts 2023, 13, 748. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced oxidation process: A sustainable technology for treating refractory organic compounds present in industrial wastewater. Environ. Sci. Pollut. Res. 2023, 30, 25477–25505. [Google Scholar] [CrossRef]
- Yeneneh, A.M.; Al Balushi, K.; Jafary, T.; Al Marshudi, A.S. Hydrodynamic Cavitation and Advanced Oxidation for Enhanced Degradation of Persistent Organic Pollutants: A Review. Sustainability 2024, 16, 4601. [Google Scholar] [CrossRef]
- Soliman, A.I.A.; Díaz Baca, J.A.; Fatehi, P. One-pot synthesis of magnetic cellulose nanocrystal and its post-functionalization for doxycycline adsorption. Carbohydr. Polym. 2023, 308, 120619. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.P.; Qiu, F.X.; Zhang, T.; Xu, J.C. Controllable preparation of FeOOH/CuO@WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Alaoui, S.B.; Achak, M.; Lamy, E. Hydrodynamic Behavior of Natural Adsorbents Filters for Water Treatment Technology. Chem. Eng. Technol. 2023, 46, 1235–1240. [Google Scholar] [CrossRef]
- Eniola, J.O.O.; Sizirici, B.; Fseha, Y.; Shaheen, J.F.; Aboulella, A.M. Application of conventional and emerging low-cost adsorbents as sustainable materials for removal of contaminants from water. Environ. Sci. Pollut. Res. 2023, 30, 88245–88271. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Xie, Y.; Xie, W.; He, Z.; Zhong, H. A critical review on adsorption and recovery of fluoride from wastewater by metal-based adsorbents. Environ. Sci. Pollut. Res. 2022, 29, 82740–82761. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose hydrogel and its derivatives: A review of application in heavy metal adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.P.; Zhang, T.; Zhu, Y.L.; Qiu, F.X. Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.J.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.Y.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Fu, W.G. Sponge effect of aerated concrete on phosphorus adsorption-desorption from agricultural drainage water in rainfall. Soil Water Res. 2020, 15, 220–227. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Tenhu, H.; Khattab, S.A.; Eldeeb, A.A.; Azaam, M.M. Highly efficient adsorbent material for removal of methylene blue dye based on functionalized polyacrylonitrile. Eur. Polym. J. 2022, 169, 111138. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Hsu, W.-L.; Wang, Y.-S.; Kuo, H.-Y.; Tsai, H.-A.; Lee, K.-R. Using Tannic-Acid-Based Complex to Modify Polyacrylonitrile Hollow Fiber Membrane for Efficient Oil-In-Water Separation. Membranes 2023, 13, 351. [Google Scholar] [CrossRef]
- Chen, X.; Wan, C.; Yu, R.; Meng, L.; Wang, D.; Chen, W.; Duan, T.; Li, L. A novel carboxylated polyacrylonitrile nanofibrous membrane with high adsorption capacity for fluoride removal from water. J. Hazard. Mater. 2021, 411, 125113. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, V.; Mishra, P.K.; Ekielski, A. A Review on Polyacrylonitrile as an Effective and Economic Constituent of Adsorbents for Wastewater Treatment. Molecules 2022, 27, 8689. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wu, H.; Xu, L.; Dong, F.; Jia, Y.; Liu, X. Removal of Acidic Organic Ionic Dyes from Water by Electrospinning a Polyacrylonitrile Composite MIL101(Fe)-NH2 Nanofiber Membrane. Molecules 2022, 27, 2035. [Google Scholar] [CrossRef] [PubMed]
- Ruzmetov, U.U.; Jumayeva, E.S.; Smanova, Z.A. Adsorption-Atomic-Absorption Determination of Cu(II) Ions in Technogenic Waters. J. Anal. Chem. 2024, 79, 578–584. [Google Scholar] [CrossRef]
- Lin, H.; Jie, B.; Ye, J.; Zhai, Y.; Luo, Z.; Shao, G.; Chen, R.; Zhang, X.; Yang, Y. Recent advance of macroscopic metal-organic frameworks for water treatment: A review. Surf. Interfaces 2023, 36, 102564. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, C.; Zhao, Y.-G.; Ye, M.-L.; Yang, K. Orientation Growth of N-Doped and Iron-Based Metal-Organic Framework and Its Application for Removal of Cr(VI) in Wastewater. Molecules 2024, 29, 1007. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Xia, G.; Tang, Y.; Liu, Q.; Li, Y.; Fang, B.; Yao, J. Metal-Organic Framework Templated Z-Scheme ZnIn2S4Bi2S3 Hierarchical Heterojunction for Photocatalytic H2O2 Production from Wastewater. Small 2024, 20, 2403268. [Google Scholar] [CrossRef]
- Kaur, M.; Yusuf, M.; Tsang, Y.F.; Kim, K.-H.; Malik, A.K. Amine/hydrazone functionalized Cd(II)/Zn(II) metal-organic framework for ultrafast sensitive detection of hazardous 2,4,6-trinitrophenol in water. Sci. Total Environ. 2023, 857, 159385. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Fu, L.; Wang, H.; Wang, S.; Xiong, C.; Wang, S.; Zhang, L. Ligand design of a novel metal-organic framework for selective capturing of Pb(II) from wastewater. J. Clean. Prod. 2023, 386, 135841. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Recent Developments in Porphyrin-Based Metal-Organic Framework Materials for Water Remediation under Visible-Light Irradiation. Int. J. Mol. Sci. 2024, 25, 4183. [Google Scholar] [CrossRef]
- Xu, X.; Eguchi, M.; Asakura, Y.; Pan, L.; Yamauchi, Y. Metal-organic framework derivatives for promoted capacitive deionization of oxygenated saline water. Energy Environ. Sci. 2023, 16, 1815–1820. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Kydyrbekova, A.; Devrishov, D.; Nurbala, U.; Tuyakbayeva, A.; Zhangazin, S.; Ualiyeva, R.; Kolpakova, V.; Yeremeyeva, Y.; Mkilima, T. Comparative Analysis of Natural and Synthetic Zeolite Filter Performance in the Purification of Groundwater. Water 2023, 15, 588. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef]
- Pan, J.; Wang, B.; Liu, S.; Liu, S.; Yan, W. Synthesis and Application of LTA Zeolite for the Removal of Inorganic and Organic Hazardous Substances from Water: A Review. Molecules 2025, 30, 554. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.O.A.; El-Kamash, A.M.; Hung, Y.-T. Applications of Nano-Zeolite in Wastewater Treatment: An Overview. Water 2022, 14, 137. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; Moreside, E.; Berruti, F. High-Temperature Pyrolysis for Elimination of Per- and Polyfluoroalkyl Substances (PFAS) from Biosolids. Processes 2022, 10, 2187. [Google Scholar] [CrossRef]
- Grechanik, S.V.; Klymenko, N.A.; Bunetskyi, V.A.; Smolin, S.K.; Zabneva, O.V.; Nevynna, L.V. Production of Activated Biochar from Wood Raw Materials for Water Treatment and Water Purification Applications. J. Water Chem. Technol. 2024, 46, 512–523. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Jiao, Y.; Lv, P.; Liu, Y.; Zuo, W.; Tian, Y.; Zhang, J. Transformation and Mitigation of Tar and Related Secondary Pollutants during Sewage Sludge Pyrolysis. Water 2024, 16, 2066. [Google Scholar] [CrossRef]
- Zuhara, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G. A review of prospects and current scenarios of biomass co-pyrolysis for water treatment. Biomass Convers. Biorefinery 2024, 14, 6053–6082. [Google Scholar] [CrossRef]
- Weerasundara, L.; Gabriele, B.; Figoli, A.; Ok, Y.-S.; Bundschuh, J. Hydrogels: Novel materials for contaminant removal in water—A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1970–2014. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, S.; Zhang, S.; Na, B.; Lin, S.; Lv, R. Supramolecular alginate-polyethyleneimine composite hydrogels for enhanced uranium adsorption. J. Radioanal. Nucl. Chem. 2023, 332, 4463–4470. [Google Scholar] [CrossRef]
- Loo, S.-L.; Vasquez, L.; Athanassiou, A.; Fragouli, D. Polymeric Hydrogels-A Promising Platform in Enhancing Water Security for a Sustainable Future. Adv. Mater. Interfaces 2021, 8, 2100580. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M.; Naushad, M. Advances in the role of natural gums-based hydrogels in water purification, desalination and atmospheric-water harvesting. Int. J. Biol. Macromol. 2022, 222, 2888–2921. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.F.; Reck Paulino, I.M.; Bergamasco, R.; Vieira, M.F.; Salcedo Vieira, A.M. Hydrogels produced from natural polymers: A review on its use and employment in water treatment. Braz. J. Chem. Eng. 2023, 40, 23–38. [Google Scholar] [CrossRef]
- Ge, W.J.; Cao, S.; Yu, H.; Wang, X.H. Tough polyacrylic acid hydrogels with stable swelling and active functionalities enabled by quaternized cellulose nanofibrils and iron ions for absorbent pad interlayers. Carbohydr. Polym. 2024, 345, 122491. [Google Scholar] [CrossRef]
- Le, V.T.; Joo, S.-W.; Berkani, M.; Mashifana, T.; Kamyab, H.; Wang, C.; Vasseghian, Y. Sustainable cellulose-based hydrogels for water treatment and purification. Ind. Crops Prod. 2023, 205, 117525. [Google Scholar] [CrossRef]
- Su, C.Y.; Li, D.; Sun, W.H.; Wang, L.J.; Wang, Y. Green, tough, and heat-resistant: A GDL-induced strategy for starch-alginate hydrogels. Food Chem. 2024, 449, 139188. [Google Scholar] [CrossRef]
- Wei, B.X.; Zou, J.; Pu, Q.Q.; Shi, K.; Xu, B.G.; Ma, Y.K. One-step preparation of hydrogel based on different molecular weights of chitosan with citric acid. J. Sci. Food Agric. 2022, 102, 3826–3834. [Google Scholar] [CrossRef]
- Duman, O.; Polat, T.G.; Diker, C.Ö.; Tunç, S. Agar/κ-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water. Int. J. Biol. Macromol. 2020, 160, 823–835. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.J.; Zhao, Y.M.; Cui, L.J.; Xu, C.; Wu, S.P. Current Developments in Chitosan-Based Hydrogels for Water and Wastewater Treatment: A Comprehensive Review. Chemistryselect 2025, 10, e202404061. [Google Scholar] [CrossRef]
- Soleimani, S.; Heydari, A.; Fattahi, M.; Motamedisade, A. Calcium alginate hydrogels reinforced with cellulose nanocrystals for methylene blue adsorption: Synthesis, characterization, and modelling. Ind. Crops Prod. 2023, 192, 115999. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Li, Y.; Ren, J.; Pei, C. Effect of cellulose nanocrystals on bacterial cellulose hydrogel for oil-water separation. Sep. Purif. Technol. 2023, 304, 122349. [Google Scholar] [CrossRef]
- Malik, R.; Saxena, R.; Warkar, S.G. Organic Hybrid Hydrogels: A Sustenance Technique in Waste-Water Treatment. Chemistryselect 2023, 8, e202203670. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Environmental and Wastewater Treatment Applications of Stimulus-Responsive Hydrogels. Gels 2025, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Tanweer, M.S.; Mir, T.A.; Alam, M.; Ikram, S.; Sheikh, J.N. Antimicrobial gum based hydrogels as adsorbents for the removal of organic and inorganic pollutants. J. Water Process Eng. 2023, 51, 103377. [Google Scholar] [CrossRef]
- Dong, Y.; Ghasemzadeh, M.; Khorsandi, Z.; Sheibani, R.; Nasrollahzadeh, M. Starch-based hydrogels for environmental applications: A review. Int. J. Biol. Macromol. 2024, 269, 131956. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Malitesta, C.; Mazzotta, E. Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal. Polymers 2024, 16, 1292. [Google Scholar] [CrossRef]
- Wu, S.P.; Li, K.H.; Shi, W.J.; Cai, J.W. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [CrossRef]
- Xie, B.S.; Zhang, Z.; Lu, Y.J.; Cui, L.J.; Xu, C.; Shi, W.J.; Wu, S.P. Fabrication of Sustainable Sodium Alginate/Polyethyleneimine/Polyvinyl Alcohol Multilayer Composite Electrospun Nanofiber Membrane for Efficient Cu2+ Removal. Sustainability 2024, 16, 5993. [Google Scholar] [CrossRef]
- Javed, M.; Huang, H.; Ma, Y.R.; Ettoumi, F.E.; Wang, L.; Xu, Y.Q.; El-Seedi, H.R.; Ru, Q.M.; Luo, Z.S. Construction of self-assembled nano cellulose crystals/chitosan nanobubbles composite hydrogel with improved gallic acid release property. Food Chem. 2024, 438, 137948. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; Liang, Q.F.; Sun, Y.F.; Zhong, M.M.; Tufail, T.; Rashid, A.; Qayum, A.; Rehman, A.; Ekumah, J.N.; et al. Enhancing storage and gastroprotective viability of Lactiplantibacillus plantarum encapsulated by sodium caseinate-inulin-soy protein isolates composites carried within carboxymethyl cellulose hydrogel. Food Res. Int. 2024, 187, 114432. [Google Scholar] [CrossRef]
- Tanpichai, S.; Phoothong, F.; Boonmahitthisud, A. Superabsorbent cellulose-based hydrogels cross-liked with borax. Sci. Rep. 2022, 12, 8920. [Google Scholar] [CrossRef] [PubMed]
- Hamidon, T.S.; Adnan, R.; Haafiz, M.K.M.; Hussin, M.H. Cellulose-based beads for the adsorptive removal of wastewater effluents: A review. Environ. Chem. Lett. 2022, 20, 1965–2017. [Google Scholar] [CrossRef]
- Hammouda, S.b.; Chen, Z.; An, C.; Lee, K. Recent advances in developing cellulosic sorbent materials for oil spill cleanup: A state-of-the-art review. J. Clean. Prod. 2021, 311, 127630. [Google Scholar] [CrossRef]
- Liu, H.; Shang, J.; Wang, Y.; Wang, Y.; Lan, J.; Dou, B.; Yang, L.; Lin, S. Ag/AgCl nanoparticles reinforced cellulose-based hydrogel coated cotton fabric with self-healing and photo-induced self-cleaning properties for durable oil/water separation. Polymer 2022, 255, 125146. [Google Scholar] [CrossRef]
- Ren, J.-X.; Zhu, J.-L.; Shi, S.-C.; Yin, M.-Q.; Huang, H.-D.; Li, Z.-M. In-situ structuring a robust cellulose hydrogel with ZnO/SiO2 heterojunctions for efficient photocatalytic degradation. Carbohydr. Polym. 2022, 296, 119957. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.; Wang, R. Uranium and lithium extraction from seawater: Challenges and opportunities for a sustainable energy future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Lehtonen, J.; Hassinen, J.; Kumar, A.A.; Johansson, L.-S.; Mäenpää, R.; Pahimanolis, N.; Pradeep, T.; Ikkala, O.; Rojas, O.J. Phosphorylated cellulose nanofibers exhibit exceptional capacity for uranium capture. Cellulose 2020, 27, 10719–10732. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, S.; Li, Z.; Na, B.; Lin, S.; Zhang, S. Tough polyamidoxime-nanocellulose supramolecular composite hydrogels for effective uranium extraction from seawater. Polymer 2024, 298, 126895. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, Z.; Lee, H.-J. Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review. Gels 2024, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.J.; Hong, X.; Ni, Y.S.; Li, Y.Z.; Pang, J.; Wang, Q.; Xiao, J.B.; Zheng, Y.F. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Sugawara, A.; Asoh, T.-A.; Takashima, Y.; Harada, A.; Uyama, H. Thermoresponsive Hydrogels Reinforced with Supramolecular Cellulose Filler. Chem. Lett. 2022, 51, 145–148. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Huang, M.G.; Huang, W.Y.; Feng, J. Green preparation of holocellulose nanocrystals from burdock and their inhibitory effects against α-amylase and α-glucosidase. Food Funct. 2022, 13, 170–185. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Rice straw structure changes following green pretreatment with petha wastewater for economically viable bioethanol production. Sci. Rep. 2022, 12, 10443. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Nath, P.C.; Mohanta, Y.K.; Bhunia, B.; Mishra, B.; Sharma, M.; Suri, S.; Bhaswant, M.; Nayak, P.K.; Sridhar, K. Recent advances in cellulose-based sustainable materials for wastewater treatment: An overview. Int. J. Biol. Macromol. 2024, 256, 128517. [Google Scholar] [CrossRef]
- Sun, L.Y.; Han, J.; Wu, J.C.; Huang, W.R.; Li, Y.Y.; Mao, Y.L.; Wang, L.; Wang, Y. Cellulose pretreatment with inorganic salt hydrate: Dissolution, regeneration, structure and morphology. Ind. Crops Prod. 2022, 180, 114722. [Google Scholar] [CrossRef]
- Lu, Q.M.; Yu, X.J.; Yagoub, A.A.; Wahia, H.; Zhou, C.S. Application and challenge of nanocellulose in the food industry. Food Biosci. 2021, 43, 101285. [Google Scholar] [CrossRef]
- Gao, C.; Liu, S.; Edgar, K.J. Regioselective chlorination of cellulose esters by methanesulfonyl chloride. Carbohydr. Polym. 2018, 193, 108–118. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, Z.; Qu, J.; Wang, C.; Wang, A. Evaluation of Ultraviolet Light and Hydrogen Peroxide Enhanced Ozone Oxidation Treatment for the Production of Cellulose Nanofibrils. ACS Sustain. Chem. Eng. 2020, 8, 2688–2697. [Google Scholar] [CrossRef]
- Li, H.X.; Liang, J.K.; Chen, L.; Ren, M.N.; Zhou, C.S. Utilization of walnut shell by deep eutectic solvents: Enzymatic digestion of cellulose and preparation of lignin nanoparticles. Ind. Crops Prod. 2023, 192, 116034. [Google Scholar] [CrossRef]
- Shi, W.; Cai, J.; Yang, Y.; Xu, C.; Lu, J.; Wu, S. Electrospun Carboxymethyl Cellulose/Polyvinyl Alcohol Nanofiber Membranes for Enhanced Metal Ion Removal. Sustainability 2023, 15, 11331. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Chen, S. The self-assembly of dialdehyde-cellulose-nanofiber-based hydrogels with high compression resilience. Cellulose 2022, 29, 5645–5658. [Google Scholar] [CrossRef]
- Cafiso, D.; Septevani, A.A.; Noe, C.; Schiller, T.; Pirri, C.F.; Roppolo, I.; Chiappone, A. 3D printing of fully cellulose-based hydrogels by digital light processing. Sustain. Mater. Technol. 2022, 32, e00444. [Google Scholar] [CrossRef]
- Ghalhari, M.R.; Sanaei, D.; Nabizadeh, R.; Mahvi, A.H. Cellulose-based hydrogel beads derived from wastepapers: Application for organic dye adsorption. Cellulose 2023, 30, 9669–9691. [Google Scholar] [CrossRef]
- Li, M.; Yang, M.; Liu, B.; Guo, H.; Wang, H.; Li, X.; Wang, L.; James, T.D. Self-assembling fluorescent hydrogel for highly efficient water purification and photothermal conversion. Chem. Eng. J. 2022, 431, 134245. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, X.H.; Hou, B.X.; Hao, C.; Li, X.; Wu, J.B. Construction of a Lignosulfonate-Lysine Hydrogel for the Adsorption of Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, M.; Yu, N.; Su, S.; Zhang, X. Fabrication of AgCl@tannic acid-cellulose hydrogels for NaBH4-mediated reduction of 4-nitrophenol. Cellulose 2021, 28, 3515–3529. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.C.; Li, Y.Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of Multienzyme Co-immobilized Hybrid Nanoflowers for an Efficient Conversion of Cellulose into Glucose in a Cascade Reaction. J. Agric. Food Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.; Rehman, A.; Hussain, A.; Karim, A.; Sharif, H.R.; Siddiquy, M.; Lianfu, Z. Optimization of Extraction Process and Estimation of Flavonoids from Fenugreek Using Green Extracting Deep Eutectic Solvents Coupled with Ultrasonication. Food Bioprocess Technol. 2024, 17, 887–903. [Google Scholar] [CrossRef]
- Naseem, Z.; Iqbal, J.; Zahid, M.; Shaheen, A.; Hussain, S.; Yaseen, W. Use of hydrogen-bonded supramolecular eutectic solvents for eco-friendly extraction of bioactive molecules from Cymbopogon citratus using Box-Behnken design. J. Food Meas. Charact. 2021, 15, 1487–1498. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, B.; Zhao, H.; Liao, X.; Xu, H. Preparation and performance of poly(AM/NVCL) temperature-sensitive composite hydrogels enhanced by laponite. Iran. Polym. J. 2024, 33, 555–565. [Google Scholar] [CrossRef]
- Ortega, A.; Valencia, S.; Rivera, E.; Segura, T.; Burillo, G. Reinforcement of Acrylamide Hydrogels with Cellulose Nanocrystals Using Gamma Radiation for Antibiotic Drug Delivery. Gels 2023, 9, 602. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hu, Y.-J.; Bian, J.; Li, M.-F.; Hao, X.; Peng, F.; Sun, R.-C. Enhanced mechanical performance of xylan-based composite hydrogel via chain extension and semi-interpenetrating networks. Cellulose 2020, 27, 4407–4416. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Das, D. Cellulose: A fascinating biopolymer for hydrogel synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhang, C.; Zhang, X.; Xia, C.; Lin, H.; Lin, C.Y.; Lam, S.S. Cellulose-based thermosensitive supramolecular hydrogel for phenol removal from polluted water. Environ. Res. 2022, 214, 113863. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoo, S.; Cho, S.-M.; Kelley, S.S.; Park, S. Production of single-component cellulose-based hydrogel and its utilization as adsorbent for aqueous contaminants. Int. J. Biol. Macromol. 2023, 243, 125085. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Samadi, A.; Esmaeili, J.; Arshad, R.; Rahdar, A.; Tavangarian, F.; Pandey, S. Novel carboxymethyl cellulose based nanocomposite: A promising biomaterial for biomedical applications. Process Biochem. 2023, 130, 211–226. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, W.; Wu, Z.; Zhang, X.; Chu, Z.; Yang, Z. Preparation of cellulose-based porous adsorption materials derived from corn straw for wastewater purification. Int. J. Biol. Macromol. 2023, 233, 123595. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F.; et al. Dual physically cross-linked carboxymethyl cellulose-based hydrogel with high stretchability and toughness as sensitive strain sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Feng, Y.B.; Tan, C.P.; Zhou, C.S.; Yagoub, A.A.; Xu, B.G.; Sun, Y.H.; Ma, H.L.; Xu, X.; Yu, X.J. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020, 324, 126883. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yagoub, A.A.; Yu, X.J.; Ma, H.L.; Zhou, C.S. Effects of ultrasound, freeze-thaw pretreatments and drying methods on structure and functional properties of pectin during the processing of okra. Food Hydrocoll. 2021, 120, 106965. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Guo, Y.T.; Liu, W.; Wu, B.G.; Wu, P.; Duan, Y.Q.; Yang, Q.R.; Ma, H.L. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Wang, M.-J. Removal of Heavy Metal Ions by Poly(vinyl alcohol) and Carboxymethyl Cellulose Composite Hydrogels Prepared by a Freeze–Thaw Method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Zhang, J.; Jiao, C.; Ding, L.; Yang, S. Synthesis and Adsorption Properties of Novel Bacterial Cellulose/Graphene Oxide/Attapulgite Materials for Cu and Pb Ions in Aqueous Solutions. Materials 2020, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Nankawa, T.; Yunoki, S.; Sugita, T.; Nakagawa, H.; Yamada, T. Eco-friendly Carboxymethyl Cellulose Nanofiber Hydrogels Prepared via Freeze Cross-Linking and Their Applications. ACS Appl. Polym. Mater. 2020, 2, 5482–5491. [Google Scholar] [CrossRef]
- Li, M.Z.; Shi, T.; Wang, X.; Bao, Y.L.; Xiong, Z.Y.; Monto, A.R.; Jin, W.A.; Yuan, L.; Gao, R.C. Plasma-activated water promoted the aggregation of Aristichthys nobilis myofibrillar protein and the effects on gelation properties. Curr. Res. Food Sci. 2022, 5, 1616–1624. [Google Scholar] [CrossRef]
- Shi, H.B.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.Y.; Xu, W.M. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef]
- Monto, A.R.; Li, M.Z.; Wang, X.; Wijaya, G.Y.A.; Shi, T.; Xiong, Z.Y.; Yuan, L.; Jin, W.G.; Li, J.R.; Gao, R.C. Recent developments in maintaining gel properties of surimi products under reduced salt conditions and use of additives. Crit. Rev. Food Sci. Nutr. 2022, 62, 8518–8533. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Soleh Setiyawan, A.; Guerrero, R.; Acibar, C.; Alarde, C.M.; Maslog, J.; Pacilan, C.J.; Dwi Ariesyady, H.; Nastiti, A.; Roosmini, D.; Sonny Abfertiawan, M. Evaluation of Pb (II) Removal from Water Using Sodium Alginate/Hydroxypropyl Cellulose Beads. E3S Web Conf. 2020, 148, 2002. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, R. Fe3+ Metal Ion-Doped Ionic and Double Network Hydrogels Based on Sodium Carboxymethyl Cellulose (NaCMC): Broadband Dielectric Spectroscopy Investigations. Arab. J. Sci. Eng. 2023, 49, 1131–1139. [Google Scholar] [CrossRef]
- Jacobs, M.; Lopez, C.G.; Dobrynin, A.V. Quantifying the Effect of Multivalent Ions in Polyelectrolyte Solutions. Macromolecules 2021, 54, 9577–9586. [Google Scholar] [CrossRef]
- Barajas-Ledesma, R.M.; Hossain, L.; Wong, V.N.L.; Patti, A.F.; Garnier, G. Effect of the counter-ion on nanocellulose hydrogels and their superabsorbent structure and properties. J. Colloid Interface Sci. 2021, 599, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cheng, T.; Xi, X.; Nie, S.; Ke, H.; Liu, Y.; Tong, S.; Chen, Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose 2019, 27, 915–925. [Google Scholar] [CrossRef]
- Fan, L.L.; Xie, P.J.; Wang, Y.; Liu, X.L.; Li, Y.; Zhou, J.Z. Influences of mannosylerythritol lipid-A on the self-assembling structure formation and functional properties of heat-induced β-lactoglobulin aggregates. Food Hydrocoll. 2019, 96, 310–321. [Google Scholar] [CrossRef]
- Bai, M.; Li, C.Z.; Cui, H.Y.; Lin, L. Preparation of self-assembling Litsea cubeba essential oil/ diphenylalanine peptide micro/nanotubes with enhanced antibacterial properties against Staphylococcus aureus biofilm. LWT-Food Sci. Technol. 2021, 146, 111394. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.C.; Chen, Q.Y.; Peng, X.M.; Yang, D.Y.; Qiu, F.X. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Lv, Y.; Xi, X.; Xue, Y.; Jiang, F.; Zhu, X.; Dai, L.; Chen, Z. A sustainable filtering material for efficient removal of volatile organic compounds from their aqueous mixtures. Cellulose 2021, 28, 6353–6360. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; Li, W.; Wu, M.; Zhan, W.; Cheng, T.; He, P.; Xiong, C. A self-assembly all-polysaccharide hydrogel for the aquatic heavy metal ions management and utilization. Ind. Crops Prod. 2023, 203, 117236. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.I.B. Biohybrid Hydrogel and Aerogel from Self-Assembled Nanocellulose and Nanochitin as a High-Efficiency Adsorbent for Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, K.; Yang, Y.; Kim, M.-S.; Lee, C.-H.; Zhang, R.; Xu, T.; Choi, S.-E.; Si, C. Hemicellulose-based hydrogels for advanced applications. Front. Bioeng. Biotechnol. 2023, 10, 1110004. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Peng, Q.; Yan, Y.; Ding, Y.; Wang, Z. Biomimetic, strong, and tough hydrogels by integrating cellulose nanocrystals into polymer networks. Ind. Crops Prod. 2020, 158, 112973. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.T.; Bao, Q.H.; Zheng, J.; Deng, D.; Duan, Y.Q.; Shen, L.Q. The physicochemical characterization, equilibrium, and kinetics of heavy metal ions adsorption from aqueous solution by arrowhead plant (Sagittaria trifolia L.) stalk. J. Food Biochem. 2018, 42, 12448. [Google Scholar] [CrossRef]
- Qin, C.C.; Guo, W.L.; Liu, Y.; Liu, Z.C.; Qiu, J.; Peng, J.B. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles-Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.; Bang, J.; Jung, M.; Park, S.; Yun, H.; Kwak, H.W. pH-sensitive cellulose/chitin nanofibrillar hydrogel for dye pollutant removal. Carbohydr. Polym. 2023, 317, 121090. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.-F.; Shu, L.; Yao, J. Copper sulfide integrated functional cellulose hydrogel for efficient solar water purification. Carbohydr. Polym. 2023, 319, 121161. [Google Scholar] [CrossRef]
- Topare, N.S.; Wadgaonkar, V.S. A review on application of low-cost adsorbents for heavy metals removal from wastewater. Mater. Today Proc. 2023, 77, 8–18. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Chen, H.; Wei, P.; Xie, Y.; Huang, X.; Cheng, Z. Acrylic-grafted nanocellulose hybrid double-network hydrogel with super-high toughness for water shutoff treatments. Chem. Eng. Res. Des. 2023, 197, 136–147. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, J.; Kang, M.; Ma, C.; Li, H.; Qiu, Z. Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2020, 138, 49880. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Lamkhao, S.; Tandorn, S.; Rujijanagul, G.; Randorn, C. A practical approach using a novel porous photocatalyst/hydrogel composite for wastewater treatment. Mater. Today Sustain. 2023, 23, 100482. [Google Scholar] [CrossRef]
- Eskandani, M.; Derakhshankhah, H.; Zare, S.; Jahanban-Esfahlan, R.; Jaymand, M. Enzymatically crosslinked magnetic starch-grafted poly(tannic acid) hydrogel for “smart” cancer treatment: An in vitro chemo/hyperthermia therapy study. Int. J. Biol. Macromol. 2023, 253, 127214. [Google Scholar] [CrossRef]
- Cui, H.Y.; Cheng, Q.; Li, C.Z.; Khin, M.N.; Lin, L. Schiff base cross-linked dialdehyde β-cyclodextrin/gelatin-carrageenan active packaging film for the application of carvacrol on ready-to-eat foods. Food Hydrocoll. 2023, 141, 108744. [Google Scholar] [CrossRef]
- Heidarian, P.; Kaynak, A.; Paulino, M.; Zolfagharian, A.; Varley, R.J.; Kouzani, A.Z. Dynamic nanocellulose hydrogels: Recent advancements and future outlook. Carbohydr. Polym. 2021, 270, 118357. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Lv, X.; Huang, Y.; Hu, M.; Wang, Y.; Dai, D.; Ma, L.; Zhang, Y.; Dai, H. Recent advances in nanocellulose based hydrogels: Preparation strategy, typical properties and food application. Int. J. Biol. Macromol. 2024, 277, 134015. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Kaith, B.S.; Kaur, M.; Sharma, N.; Khullar, S. A hydrogel based on dialdehyde carboxymethyl cellulose–gelatin and its utilization as a bio adsorbent. J. Chem. Sci. 2019, 132, 15. [Google Scholar] [CrossRef]
- Nia, M.H.; Tavakolian, M.; Kiasat, A.R.; van de Ven, T.G.M. Hybrid Aerogel Nanocomposite of Dendritic Colloidal Silica and Hairy Nanocellulose: An Effective Dye Adsorbent. Langmuir 2020, 36, 11963–11974. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Q.; Liu, Z.; Liu, J.; Zheng, X.; Pei, Y.; Tang, K. Modified nano microfibrillated cellulose/carboxymethyl chitosan composite hydrogel with giant network structure and quick gelation formability. Int. J. Biol. Macromol. 2019, 135, 561–568. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wang, W.; Wang, W.; Hao, Y.; Xue, C.; Mao, X. A Double-Layer Polysaccharide Hydrogel (DPH) for the Enhanced Intestine-Targeted Oral Delivery of Probiotics. Engineering 2024, 34, 187–194. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Li, Z.; Zeng, W.; Meng, D.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Click chemistry-induced selective adsorption of cationic and anionic dyes using functionalized cellulose methacrylate hydrogels. Cellulose 2022, 29, 8843–8861. [Google Scholar] [CrossRef]
- Wei, H.; Li, S.; Liu, Z.; Chen, H.; Liu, Y.; Li, W.; Wang, G. Preparation and characterization of starch-cellulose interpenetrating network hydrogels based on sequential Diels-Alder click reaction and photopolymerization. Int. J. Biol. Macromol. 2022, 194, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Wang, B.; Zeng, X.; Wang, J.; Ren, B.; Yang, X.; Bai, X. Preparation of non-swelling hydrogels and investigation on the adsorption performance of iron ions. J. Appl. Polym. Sci. 2022, 139, e52411. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Li, S.; Wang, G.; Guo, T.; Han, H. Facile fabrication of semi-IPN hydrogel adsorbent based on quaternary cellulose via amino-anhydride click reaction in water. Int. J. Biol. Macromol. 2022, 207, 622–634. [Google Scholar] [CrossRef]
- Morozova, S.M. Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef]
- Kramer, R.K.; Belgacem, M.N.; Carvalho, A.J.F.; Gandini, A. Thermally reversible nanocellulose hydrogels synthesized via the furan/maleimide Diels-Alder click reaction in water. Int. J. Biol. Macromol. 2019, 141, 493–498. [Google Scholar] [CrossRef]
- Wang, C.P.; He, G.; Meng, J.; Wang, S.M.; Kong, Y.Z.; Jiang, J.X.; Hu, R.B.; Zhou, G.K. Improved lignocellulose saccharification of a<i>Miscanthus</i>reddish stem mutant induced by heavy-ion irradiation. Glob. Change Biol. Bioenergy 2020, 12, 1066–1077. [Google Scholar] [CrossRef]
- Huang, L.R.; Zhang, W.X.; Ding, X.N.; Wu, Z.F.; Li, Y.L. Effects of dual-frequency ultrasound with different energy irradiation modes on the structural and emulsifying properties of soy protein isolate. Food Bioprod. Process. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Sayed, A.; Hany, F.; Abdel-Raouf, M.E.-S.; Mahmoud, G.A. Gamma irradiation synthesis of pectin-based biohydrogels for removal of lead cations from simulated solutions. J. Polym. Res. 2022, 29, 372. [Google Scholar] [CrossRef]
- Betraoui, A.; Seddiki, N.; Souag, R.; Guerfi, N.; Semlali, A.; Aouak, T.; Aliouche, D. Synthesis of New Hydrogels Involving Acrylic Acid and Acrylamide Grafted Agar-Agar and Their Application in the Removal of Cationic Dyes from Wastewater. Gels 2023, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, S.C.; Banik, N.; Islam, M.; Rahman Khan, M.M.; Jeong, J.-H. Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal. Gels 2024, 10, 785. [Google Scholar] [CrossRef]
- Masry, B.A.; Gayed, H.M.; Daoud, J.A. Gamma radiation synthesis of hydroxyethyl cellulose/acrylic acid/CYANEX 471X hydrogel for silver ions capture from acidic nitrate medium. Cellulose 2024, 31, 4329–4346. [Google Scholar] [CrossRef]
- Khiewsawai, N.; Rattanawongwiboon, T.; Ummartyotin, S. Cellulose fiber derived from sugarcane bagasse and polyethylene glycol/acrylic acid/ branched polyethylenimine-based hydrogel composite prepared by gamma irradiation: A platform for mercury (II) ions adsorption. Environ. Adv. 2024, 17, 100561. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Bundjaja, V.; Kurniawan, A.; Yuliana, M.; Hsieh, C.-W.; Go, A.W.; Cheng, K.-C.; Soetaredjo, F.E.; Ismadji, S. Investigation of the influence of crosslinking activation methods on the physicochemical and Cu(II) adsorption characteristics of cellulose hydrogels. J. Environ. Chem. Eng. 2022, 10, 106971. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.N.; Zhang, L.; Yu, X.J.; Liu, H.; Yagoub, A.A.; Zhou, C.S. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020, 149, 112361. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.H.; Zou, X.B.; Xu, Y.W.; Xu, X.C. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef]
- Baiya, C.; Nannuan, L.; Tassanapukdee, Y.; Chailapakul, O.; Songsrirote, K. The Synthesis of Carboxymethyl Cellulose-Based Hydrogel from Sugarcane Bagasse Using Microwave-Assisted Irradiation for Selective Adsorption of Copper(II) Ions. Environ. Prog. Sustain. Energy 2018, 38, S157–S165. [Google Scholar] [CrossRef]
- Nishitha, M.; Narayana, B.; Sarojini, B.K.; Kodoth, A.K. Environmentally Benign Cellulose Acetate Hydrogel Beads for Solid Phase Extraction of Chlorpyrifos Pesticide from Water. Water Air Soil Pollut. 2024, 236, 23. [Google Scholar] [CrossRef]
- Roa, K.; Tapiero, Y.; Thotiyl, M.O.; Sánchez, J. Hydrogels Based on Poly([2-(acryloxy)ethyl] Trimethylammonium Chloride) and Nanocellulose Applied to Remove Methyl Orange Dye from Water. Polymers 2021, 13, 2265. [Google Scholar] [CrossRef]
- Jiao, X.; Jia, K.; Yu, Y.; Liu, D.; Zhang, J.; Zhang, K.; Zheng, H.; Sun, X.; Tong, Y.; Wei, Q.; et al. Nanocellulose-based functional materials towards water treatment. Carbohydr. Polym. 2025, 350, 122977. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Shen, Z. Nanocellulose Based Filtration Membrane in Industrial Waste Water Treatment: A Review. Materials 2021, 14, 5398. [Google Scholar] [CrossRef]
- Saud, A.; Saleem, H.; Zaidi, S.J. Progress and Prospects of Nanocellulose-Based Membranes for Desalination and Water Treatment. Membranes 2022, 12, 462. [Google Scholar] [CrossRef]
- Yang, J.; Han, X.; Yang, W.; Hu, J.; Zhang, C.; Liu, K.; Jiang, S. Nanocellulose-based composite aerogels toward the environmental protection: Preparation, modification and applications. Environ. Res. 2023, 236, 116736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, X.; Wu, X.; Liu, L.; Guo, H.; Xu, K.; Zhang, L.; Du, G. Preparation of Nanocellulose-Based Aerogel and Its Research Progress in Wastewater Treatment. Molecules 2023, 28, 3541. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Goswami, R.; Singh, S.; Narasimhappa, P.; Ramamurthy, P.C.; Mishra, A.; Mishra, P.K.; Joshi, H.C.; Pant, G.; Singh, J.; Kumar, G.; et al. Nanocellulose: A comprehensive review investigating its potential as an innovative material for water remediation. Int. J. Biol. Macromol. 2024, 254, 127465. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, D.; Zhao, Y.; Zhao, R.; Russell, S.J.; Ning, X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers 2022, 14, 2343. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef]
- James, A.; Rahman, M.R.; Said, K.A.M.; Namakka, M.; Kuok, K.K.; Khandaker, M.U.; Al-Humaidi, J.Y.; Althomali, R.H.; Rahman, M.M. Lithium Chloride-Mediated enhancement of dye removal capacity in Borneo bamboo derived nanocellulose-based nanocomposite membranes (NCMs). J. Mol. Liq. 2024, 413, 125973. [Google Scholar] [CrossRef]
- Nitodas, S.; Skehan, M.; Liu, H.; Shah, R. Current and Potential Applications of Green Membranes with Nanocellulose. Membranes 2023, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Aoudi, B.; Boluk, Y.; Gamal El-Din, M. Recent advances and future perspective on nanocellulose-based materials in diverse water treatment applications. Sci. Total Environ. 2022, 843, 156903. [Google Scholar] [CrossRef]
- Alves, D.F.; Camani, P.H.; Souza, A.G.; Rosa, D.S. A novel sustainable composite hydrogel containing nanocellulose to remove potentially toxic metals from contaminated water. Polym. Bull. 2023, 81, 5939–5966. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, J.; Qi, X.; Huang, Y.; Qiao, J.; Guo, Y.; Guo, X.; Wu, Y. Nanocellulose/carbon dots hydrogel as superior intensifier of ZnO/AgBr nanocomposite with adsorption and photocatalysis synergy for Cr(VI) removal. Int. J. Biol. Macromol. 2023, 233, 123566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yi, X.; Ouyang, J.; Wang, S.; Xu, D.; Qi, X.; Jiang, P.; Guo, X.; Wu, Y. Chitosan/carbon dots-loaded nanocellulose/layered double hydroxides composite hydrogel for effective detection and removal of iodide ion. Chem. Eng. J. 2024, 479, 147753. [Google Scholar] [CrossRef]
- Rodrigues, F.H.A.; de C. Magalhães, C.E.; Medina, A.L.; Fajardo, A.R. Hydrogel composites containing nanocellulose as adsorbents for aqueous removal of heavy metals: Design, optimization, and application. Cellulose 2019, 26, 9119–9133. [Google Scholar] [CrossRef]
- Wang, G.; Lu, T.; Zhang, X.; Feng, M.; Wang, C.; Yao, W.; Zhou, S.; Zhu, Z.; Ding, W.; He, M. Structure and properties of cellulose/HAP nanocomposite hydrogels. Int. J. Biol. Macromol. 2021, 186, 377–384. [Google Scholar] [CrossRef]
- Wong, S.M.; Zulkifli, M.Z.A.; Nordin, D.; Teow, Y.H. Synthesis of Cellulose/Nano-hydroxyapatite Composite Hydrogel Absorbent for Removal of Heavy Metal Ions from Palm Oil Mill Effluents. J. Polym. Environ. 2021, 29, 4106–4119. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Jia, F.; Song, S. Adsorption of heavy metals on molybdenum disulfide in water: A critical review. J. Mol. Liq. 2019, 292, 111390. [Google Scholar] [CrossRef]
- Thangavelu, K.; Zou, L. Evaluating oil removal by amphiphilic MoS2/cellulose acetate fibrous sponge in a flow-through reactor and by artificial neural network. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100684. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Ungureanu, E.; Belosinschi, D.; Tofanica, B.-M.; Volf, I. Cellulose-Based Metallogels—Part 3: Multifunctional Materials. Gels 2023, 9, 878. [Google Scholar] [CrossRef]
- Liang, N.N.; Shi, B.Q.; Hu, X.T.; Li, W.T.; Huang, X.W.; Li, Z.H.; Zhang, X.N.; Zou, X.B.; Shi, J.Y. A ternary heterostructure aptasensor based on metal-organic framework and polydopamine nanoparticles for fluorescent detection of sulfamethazine. Food Chem. 2024, 460, 140570. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Hu, W.W.; Hassan, M.M.; Zhang, Z.Z.; Chen, Q.S. A facile and sensitive SERS-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles. J. Food Meas. Charact. 2019, 13, 259–268. [Google Scholar] [CrossRef]
- Yang, Z.K.; Zhai, X.D.; Zhang, C.C.; Shi, J.Y.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Gong, Y.Y.; Holmes, M.; Povey, M.; et al. Agar/TiO2/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2022, 123, 107187. [Google Scholar] [CrossRef]
- Wang, W.; Ni, J.; Chen, L.; Ai, Z.; Zhao, Y.; Song, S. Synthesis of carboxymethyl cellulose-chitosan-montmorillonite nanosheets composite hydrogel for dye effluent remediation. Int. J. Biol. Macromol. 2020, 165, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Peng, W.; Fang, Z.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2019, 356, 904–914. [Google Scholar] [CrossRef]
- Si, Z.; Pei, M.; Liu, Y.; Li, B.; Kang, F. Boosting the photocatalytic activity of β-FeOOH catalyst for toluene oxidation by constructing internal electric field at 0D/1D homojunction interfaces. J. Colloid Interface Sci. 2024, 654, 300–307. [Google Scholar] [CrossRef]
- Yang, X.; Ci, Y.; Zhu, P.; Chen, T.; Li, F.; Tang, Y. Preparation and characterization of cellulose-chitosan/β-FeOOH composite hydrogels for adsorption and photocatalytic degradation of methyl orange. Int. J. Biol. Macromol. 2024, 274, 133201. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Zhou, W.S.; Li, C.H.; Sun, C.; Yang, X.D. Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem. 2016, 192, 351–357. [Google Scholar] [CrossRef]
- Gopika, G.; Sathish, A.; Kumar, P.S.; Nithya, K.; Rangasamy, G. A review on current progress of graphene-based ternary nanocomposites in the removal of anionic and cationic inorganic pollutants. Chemosphere 2022, 309, 136617. [Google Scholar] [CrossRef]
- Xu, Y.W.; Zhang, W.; Shi, J.Y.; Zou, X.B.; Li, Y.X.; Tahir, H.E.; Huang, X.W.; Li, Z.H.; Zhai, X.D.; Hu, X.T. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem. 2017, 237, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Gomez-Maldonado, D.; Zhang, K.; Du, H.; Whitehead, D.C.; Li, M.; Zhang, X.; Peresin, M.S. Polyethylenimine functionalized graphene oxide and cellulose nanofibril composite hydrogels: Synthesis, characterization and water pollutants adsorption. Carbohydr. Polym. Technol. Appl. 2024, 8, 100585. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; McClements, D.J.; Goodarzi, V.; Chen, W.-H. Removal of methylene blue from wastewater using ternary nanocomposite aerogel systems: Carboxymethyl cellulose grafted by polyacrylic acid and decorated with graphene oxide. J. Hazard. Mater. 2022, 421, 126752. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose—A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 2021, 167, 1248–1261. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Dai, X.; Wang, C.; Lv, X.; Waterhouse, G.I.N.; Fan, H.; Ai, S. Highly flexible and stable carbon nitride/cellulose acetate porous films with enhanced photocatalytic activity for contaminants removal from wastewater. J. Hazard. Mater. 2020, 384, 121417. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wang, L.; Liu, P.F.; Zhao, K.R.; Ye, S.Y.; Liang, G.X. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pan, Y.; Cai, P. Sugarcane cellulose-based composite hydrogel enhanced by g-C3N4 nanosheet for selective removal of organic dyes from water. Int. J. Biol. Macromol. 2022, 205, 37–48. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, J.; Yang, K.; Cai, P.; Xiao, H. Novel multi-responsive and sugarcane bagasse cellulose-based nanogels for controllable release of doxorubicin hydrochloride. Mater. Sci. Eng. C 2021, 118, 111357. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Gao, J.; Yao, J.; Zhang, Q. Recent progress in metal-organic frameworks-based hydrogels and aerogels and their applications. Coord. Chem. Rev. 2019, 398, 213016. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhang, M.M.; Chen, Q.S.; Ouyang, Q. Multifunctional Metal-Organic Frameworks Driven Three-Dimensional Folded Paper-Based Microfluidic Analysis Device for Chlorpyrifos Detection. J. Agric. Food Chem. 2024, 72, 14375–14385. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Lu, L.; Zhu, C.; Xu, J.; Fang, Q.; Liu, R.; Shen, Y. Enhanced adsorption and photocatalytic removal of PFOA from water by F-functionalized MOF with in-situ-growth TiO2: Regulation of electron density and bandgap. Sep. Purif. Technol. 2022, 297, 121449. [Google Scholar] [CrossRef]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef]
- Wu, S.P.; Shi, W.J.; Cui, L.J.; Xu, C. Enhancing contaminant rejection efficiency with ZIF-8 molecular sieving in sustainable mixed matrix membranes. Chem. Eng. J. 2024, 482, 148954. [Google Scholar] [CrossRef]
- Cui, J.; Xu, X.; Yang, L.; Chen, C.; Qian, J.; Chen, X.; Sun, D. Soft foam-like UiO-66/Polydopamine/Bacterial cellulose composite for the removal of aspirin and tetracycline hydrochloride. Chem. Eng. J. 2020, 395, 125174. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Du, Y.; Zhang, M.; Yang, Z.; Su, J.; Peng, X.; Liu, X.; Sun, G.; Cui, Y. In-situ immobilization of CuMOF on sodium alginate/chitosan/cellulose nanofibril composite hydrogel for fast and highly efficient removal of Pb2+ from aqueous solutions. J. Solid State Chem. 2023, 322, 123928. [Google Scholar] [CrossRef]
- Gendy, E.A.; Ifthikar, J.; Ali, J.; Oyekunle, D.T.; Elkhlifia, Z.; Shahib, I.I.; Khodair, A.I.; Chen, Z. Removal of heavy metals by covalent organic frameworks (COFs): A review on its mechanism and adsorption properties. J. Environ. Chem. Eng. 2021, 9, 105687. [Google Scholar] [CrossRef]
- Wang, S.L.; Liang, N.N.; Hu, X.T.; Li, W.T.; Guo, Z.; Zhang, X.A.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Zhao, B.; Fu, X.; Di, Y.; Wei, L.; Shao, G.; Cui, H.; Wei, L.; Liu, N.; An, Q.; Zhai, S. Covalent organic framework@cellulose nanofibrils@carboxymethyl cellulose composite hydrogel beads for the removal of nickel ions from aqueous solutions. J. Mol. Struct. 2024, 1312, 138619. [Google Scholar] [CrossRef]
- Li, M.; Sun, L.; Gao, W.; Qing, B.; Yao, H.; Dai, W.; Zhang, H.; Shou, Q.; Liang, X.; Liu, H. In-situ bioprocessing of bacterial cellulose aerogel with covalent organic frameworks for enhanced uranium extraction. Sep. Purif. Technol. 2025, 355, 129654. [Google Scholar] [CrossRef]
- Cho, I.S.; Ooya, T. Tuned cell attachments by double-network hydrogels consisting of glycol chitosan, carboxylmethyl cellulose and agar bearing robust and self-healing properties. Int. J. Biol. Macromol. 2019, 134, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Zhao, W.Y.; Li, Z.H.; Zhang, N.; Wang, S.; Shi, J.Y.; Zhai, X.D.; Zhang, J.J.; Shen, T.T. Preparation of a Dual-Functional Active Film Based on Bilayer Hydrogel and Red Cabbage Anthocyanin for Maintaining and Monitoring Pork Freshness. Foods 2023, 12, 4520. [Google Scholar] [CrossRef]
- Lin, T.; Bai, Q.; Peng, J.; Xu, L.; Li, J.; Zhai, M. One-step radiation synthesis of agarose/polyacrylamide double-network hydrogel with extremely excellent mechanical properties. Carbohydr. Polym. 2018, 200, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, D.; Lin, J.; Wen, Z.; Zhang, K.; Xia, Z.; Wang, D. Preparation of double-network hydrogel consisting of chitosan, cellulose and polyacrylamide for enrichment of tetracyclines. Microchem. J. 2022, 182, 107931. [Google Scholar] [CrossRef]
- Liu, H.; Pan, B.; Wang, Q.; Niu, Y.; Tai, Y.; Du, X.; Zhang, K. Crucial roles of graphene oxide in preparing alginate/nanofibrillated cellulose double network composites hydrogels. Chemosphere 2021, 263, 128240. [Google Scholar] [CrossRef]
- Cai, C.; Gao, L.; Xiong, Y. Green recyclable chitosan/carboxymethyl cellulose/sodium alginate magnetic double-network composite hydrogels for adsorption of MB and Cu2+. J. Water Process Eng. 2024, 63, 105420. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Bao, C.; Zhang, J.; Tian, Y.; Shen, J.; Feng, X. Freezing-induced chemical crosslinking to fabricate nanocellulose-based cryogels for efficient bilirubin removal. Sep. Purif. Technol. 2022, 300, 121865. [Google Scholar] [CrossRef]
- Li, C.; Shen, J.; Wang, J.; Bao, C.; Li, B.; Liu, L.; Wang, H.; Zhang, X. Highly compressible and macro-porous hydrogels via the synergy of cryogelation and double-network for efficient removal of Cr(VI). Int. J. Biol. Macromol. 2023, 238, 124160. [Google Scholar] [CrossRef]
- Ahmadian, M.; Jaymand, M. Interpenetrating polymer network hydrogels for removal of synthetic dyes: A comprehensive review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, S. Bioinspired highly anisotropic, robust and environmental resistant wood aerogel composite with semi-interpenetrating polymer networks for Cu(II) ion removal. Cellulose 2022, 29, 8353–8370. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Han, J.; Yu, L.; Chen, J.; Wu, Q.; Jiang, J. Effects of nanocellulose on sodium alginate/polyacrylamide hydrogel: Mechanical properties and adsorption-desorption capacities. Carbohydr. Polym. 2019, 206, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Rinoldi, C.; Lanzi, M.; Fiorelli, R.; Nakielski, P.; Zembrzycki, K.; Kowalewski, T.; Urbanek, O.; Grippo, V.; Jezierska-Woźniak, K.; Maksymowicz, W.; et al. Three-Dimensional Printable Conductive Semi-Interpenetrating Polymer Network Hydrogel for Neural Tissue Applications. Biomacromolecules 2021, 22, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, X.; Wang, P.; Wang, X.; Yang, R.; Liu, S.; Ye, Z.; Chi, B. Covalently Adaptable Hydrogel Based on Hyaluronic Acid and Poly(γ-glutamic acid) for Potential Load-Bearing Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 4036–4043. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Shahrousvand, M.; Shojaei, S.; Khonakdar, H.A.; Asefnejad, A.; Goodarzi, V. Preparation of superabsorbent eco-friendly semi-interpenetrating network based on cross-linked poly acrylic acid/xanthan gum/graphene oxide (PAA/XG/GO): Characterization and dye removal ability. Int. J. Biol. Macromol. 2020, 152, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Ismaeilimoghadam, S.; Jonoobi, M.; Ashori, A.; Shahraki, A.; Azimi, B.; Danti, S. Interpenetrating and semi-interpenetrating network superabsorbent hydrogels based on sodium alginate and cellulose nano-crystals: A biodegradable and high-performance solution for adult incontinence pads. Int. J. Biol. Macromol. 2023, 253, 127118. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Wu, W.; Jing, Y.; Dai, H.; Fang, G. Nanocellulose/Poly(2-(dimethylamino)ethyl methacrylate)Interpenetrating polymer network hydrogels for removal of Pb(II) and Cu(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 474–480. [Google Scholar] [CrossRef]
- Bai, H.; Li, Z.; Zhang, S.; Wang, W.; Dong, W. Interpenetrating polymer networks in polyvinyl alcohol/cellulose nanocrystals hydrogels to develop absorbent materials. Carbohydr. Polym. 2018, 200, 468–476. [Google Scholar] [CrossRef]
- Han, Z.X.; Wang, N.; Zhang, H.L.; Yang, X.Y. Heavy metal contamination and risk assessment of human exposure near an e-waste processing site. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2017, 67, 119–125. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Zhang, C.; Yuan, Z.; Chi, H. Real-Time Uranyl Ion Adsorption Monitoring Based on Cellulose Hydrogels. ACS Appl. Polym. Mater. 2024, 6, 13193–13201. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Nanocellulose-Based Materials for Water Pollutant Removal: A Review. Int. J. Mol. Sci. 2024, 25, 8529. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.; Li, J.; Kamal, N.; Mahmoud, E.; Omara, A.E.D.; Du, D.L. Assessing and Predicting Soil Quality in Heavy Metal-Contaminated Soils: Statistical and ANN-Based Techniques. J. Soil Sci. Plant Nutr. 2023, 23, 6510–6526. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, R.; Zhao, X.; Liu, T.; Bai, Z. A novel chitosan/cellulose phosphonate composite hydrogel for ultrafast and efficient removal of Pb(II) and Cu(II) from wastewater. Carbohydr. Polym. 2024, 336, 122104. [Google Scholar] [CrossRef]
- Zeng, R.; Zheng, J.; Zuo, Y.; Xiao, C.; Zhu, Y. Synergistic and simultaneous removal of heavy metal ions over waste bamboo shoot particles encapsulated carboxymethyl cellulose/gelatin composite hydrogel. Int. J. Biol. Macromol. 2024, 283, 137578. [Google Scholar] [CrossRef]
- Abdul Rahman, A.S.; Fizal, A.N.S.; Khalil, N.A.; Ahmad Yahaya, A.N.; Hossain, M.S.; Zulkifli, M. Fabrication and Characterization of Magnetic Cellulose–Chitosan–Alginate Composite Hydrogel Bead Bio-Sorbent. Polymers 2023, 15, 2494. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, M.; Liu, H.; Lei, H.; Jian, B.; Liu, R.; Li, X.; Wang, Y.; Zhou, B. Preparation of green magnetic hydrogel from soybean residue cellulose for effective and rapid removal of copper ions from wastewater. J. Environ. Chem. Eng. 2022, 10, 108213. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- He, X.; Jia, H.; Sun, N.; Hou, M.; Tan, Z.; Lu, X. Fluorescent hydrogels based on oxidized carboxymethyl cellulose with excellent adsorption and sensing abilities for Ag+. Int. J. Biol. Macromol. 2022, 213, 955–966. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Zhou, R.; Qiao, J.; Liu, J.; Cai, R.; Liu, J.; Rong, J.; Chen, Y. Porous sodium alginate/cellulose nanofiber composite hydrogel microspheres for heavy metal removal in wastewater. Int. J. Biol. Macromol. 2024, 278, 135000. [Google Scholar] [CrossRef]
- Kalaiselvi, K.; Mohandoss, S.; Ahmad, N.; Khan, M.R.; Manoharan, R.K. Adsorption of Pb2+ Ions from Aqueous Solution onto Porous Kappa-Carrageenan/Cellulose Hydrogels: Isotherm and Kinetics Study. Sustainability 2023, 15, 9534. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, B.; Ma, J.; Zhu, R. Preparation and characterization of carboxymethyl cellulose/chitosan/alginic acid hydrogels with adjustable pore structure for adsorption of heavy metal ions. Eur. Polym. J. 2022, 179, 111577. [Google Scholar] [CrossRef]
- Yang, S.C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, Q.; Xu, X.; Zhang, X.; Su, Y.; Yue, Q.; Gao, B. A wheat straw cellulose-based hydrogel for Cu (II) removal and preparation copper nanocomposite for reductive degradation of chloramphenicol. Carbohydr. Polym. 2018, 190, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Song, Y.-L.; Yang, H.-R.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Carboxymethyl cellulose-based cryogels for efficient heavy metal capture: Aluminum-mediated assembly process and sorption mechanism. Int. J. Biol. Macromol. 2020, 164, 3275–3286. [Google Scholar] [CrossRef]
- Han, S.Q.; Xie, H.H.; Zhang, L.; Wang, X.H.; Zhong, Y.; Shen, Y.T.; Wang, H.L.; Hao, C. High-performance polyethylenimine-functionalized lignin/silica porous composite microsphere for the removal of hexavalent chromium, phosphate and Congo red from aqueous solutions. Ind. Crops Prod. 2023, 194, 116289. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N. Removal of organic dyes and free radical assay by encapsulating polyvinylpyrrolidone and Tinospora Cordifolia in dual (Co–Cu) doped TiO2 nanoparticles. Environ. Pollut. 2023, 335, 122229. [Google Scholar] [CrossRef]
- Hussain, S.; Salman, M.; Farooq, U.; Zahid, F.; Yasmeen, S.; Al-Ahmary, K.M.; Ahmed, M. Fabrication of carboxymethyl cellulose/graphene oxide/ZnO composite hydrogel for efficient removal of fuchsin dye from aqueous media. Int. J. Biol. Macromol. 2024, 277, 134104. [Google Scholar] [CrossRef]
- Qiao, A.; Cui, M.; Huang, R.; Ding, G.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef]
- Jamwal, P.; Chauhan, G.S.; Kumar, P.; Kumari, B.; Kumar, K.; Chauhan, S. A study in the synthesis of new Pinus wallichiana derived spherical nanocellulose hydrogel and its evaluation as malachite green adsorbent. Sustain. Chem. Pharm. 2023, 32, 100950. [Google Scholar] [CrossRef]
- Benhalima, T.; Sadi, A.; Dairi, N.; Ferfera-Harrar, H. Multifunctional carboxymethyl cellulose-dextran sulfate/AgNPs@zeolite hydrogel beads for basic red 46 and methylene blue dyes removal and water disinfection control. Sep. Purif. Technol. 2024, 342, 127001. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, D.; Xu, W.; Ding, W.-P.; Zhu, Z.-Z.; He, J.-R.; Cheng, S.-Y. Polysaccharide-Based Hydrogels Derived from Cellulose: The Architecture Change from Nanofibers to Hydrogels for a Putative Dual Function in Dye Wastewater Treatment. J. Agric. Food Chem. 2020, 68, 9725–9732. [Google Scholar] [CrossRef]
- Nguyen, B.C.; Truong, T.M.; Nguyen, N.T.; Dinh, D.N.; Hollmann, D.; Nguyen, M.N. Advanced cellulose-based hydrogel TiO2 catalyst composites for efficient photocatalytic degradation of organic dye methylene blue. Sci. Rep. 2024, 14, 10935. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, S.; Vishalakshi, B. Development of banana pseudo stem cellulose fiber based magnetic nanocomposite as an adsorbent for dye removal. Int. J. Biol. Macromol. 2024, 278, 134877. [Google Scholar] [CrossRef]
- Xu, H.Y.; Yang, X.; Yu, R.; Zuo, T.; Liu, Q.; Jia, S.; Jia, L.Y. Adsorption properties of cellulose-derived hydrogel and magnetic hydrogels from Sophora flavescens on Cu2+ and Congo red. Int. J. Biol. Macromol. 2024, 274, 133209. [Google Scholar] [CrossRef]
- Salama, A. Preparation of CMC-g-P(SPMA) super adsorbent hydrogels: Exploring their capacity for MB removal from waste water. Int. J. Biol. Macromol. 2018, 106, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Chen, L. A green composite hydrogel based on cellulose and clay as efficient absorbent of colored organic effluent. Carbohydr. Polym. 2019, 210, 314–321. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- Kasbaji, M.; Mennani, M.; Grimi, N.; Oubenali, M.; Mbarki, M.; El Zakhem, H.; Moubarik, A. Adsorption of cationic and anionic dyes onto coffee grounds cellulose/sodium alginate double-network hydrogel beads: Isotherm analysis and recyclability performance. Int. J. Biol. Macromol. 2023, 239, 124288. [Google Scholar] [CrossRef]
- Medeiros, D.C.C.d.S.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.-H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; Gamal El-Din, M. Pristine and engineered biochar for the removal of contaminants co-existing in several types of industrial wastewaters: A critical review. Sci. Total Environ. 2022, 809, 151120. [Google Scholar] [CrossRef]
- Azam, S.M.R.; Ma, H.L.; Xu, B.G.; Devi, S.; Siddique, M.A.; Stanley, S.L.; Bhandari, B.; Zhu, J.S. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Song, J.; Huang, M.; Lin, X.; Li, S.F.Y.; Jiang, N.; Liu, Y.; Guo, H.; Li, Y. Novel Fe-based metal–organic framework (MOF) modified carbon nanofiber as a highly selective and sensitive electrochemical sensor for tetracycline detection. Chem. Eng. J. 2022, 427, 130913. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, X.; Zhang, X.; Huang, Y.; Ma, Q.; Guo, X.; Wu, Y. β-Cyclodextrin/carbon dots-grafted cellulose nanofibrils hydrogel for enhanced adsorption and fluorescence detection of levofloxacin. Carbohydr. Polym. 2024, 340, 122306. [Google Scholar] [CrossRef]
- Luo, Q.; He, S.; Huang, Y.; Lei, Z.; Qiao, J.; Li, Q.; Xu, D.; Guo, X.; Wu, Y. Non-toxic fluorescent molecularly imprinted hydrogel based on wood-derived cellulose nanocrystals and carbon dots for efficient sorption and sensitive detection of tetracycline. Ind. Crops Prod. 2022, 177, 114528. [Google Scholar] [CrossRef]
- Tie, L.; Ke, Y.; Gong, Y.; Zhang, W.-x.; Deng, Z. Nanocellulose fine-tuned poly(acrylic acid) hydrogel for enhanced diclofenac removal. Int. J. Biol. Macromol. 2022, 213, 1029–1036. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Synthesis and evaluation of CMC-g-AMPS/Fe/Al/AC composite hydrogel and their use in fluoride removal from aqueous solution. Environ. Technol. Innov. 2020, 17, 100620. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, J.; Choi, Y.-K.; Park, S.; Lee, S.H. Reswellable alginate/activated carbon/carboxymethyl cellulose hydrogel beads for ibuprofen adsorption from aqueous solutions. Int. J. Biol. Macromol. 2023, 249, 126053. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.Y.; Li, L.; Cai, J.R. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhang, X.; Qi, X.; Wang, C.; Yuan, Y.; Xie, X.; Qiao, J.; Guo, X.; Wu, Y. Enhanced sorption and fluorescent detection of bisphenol A by using sodium alginate/cellulose nanofibrils/ZIF-8 composite hydrogel. Int. J. Biol. Macromol. 2024, 271, 132198. [Google Scholar] [CrossRef]
- Rodrigues, F.; Faria, M.; Mendonça, I.; Sousa, E.; Ferreira, A.; Cordeiro, N. Efficacy of bacterial cellulose hydrogel in microfiber removal from contaminated waters: A sustainable approach to wastewater treatment. Sci. Total Environ. 2024, 919, 170846. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lee, C.-S.; Zhang, K.; Alhamzani, A.G.; Hsiao, B.S. Sodium Alginate–Aldehyde Cellulose Nanocrystal Composite Hydrogel for Doxycycline and Other Tetracycline Removal. Nanomaterials 2023, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qi, X.; Ouyang, J.; Jiang, P.; Guo, X.; Wu, Y. Lignin/carbon dots-embedded cellulose nanofibrils hydrogel: An effective, inexpensive and versatile platform for doxycycline adsorption and determination. Ind. Crops Prod. 2024, 220, 119300. [Google Scholar] [CrossRef]
- Luo, Q.; Ren, T.; Lei, Z.; Huang, Y.; Huang, Y.; Xu, D.; Wan, C.; Guo, X.; Wu, Y. Non-toxic chitosan-based hydrogel with strong adsorption and sensitive detection abilities for tetracycline. Chem. Eng. J. 2022, 427, 131738. [Google Scholar] [CrossRef]
- Barragan Medina, Y.P.; Alvarez, V.A.; Mendoza Zélis, P.; Gonzalez, J.S. Ecofriendly magnetic gels beads based on carboxymethylcellulose and iron oxides for diclofenac adsorption. Discov. Chem. Eng. 2024, 4, 22. [Google Scholar] [CrossRef]
- Tie, L.; Zhang, W.-X.; Deng, Z. Composite hydrogel derived iron/nitrogen co-doped carbon for bisphenol A removal. Sep. Purif. Technol. 2024, 340, 126752. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).