Physicochemical and Phytochemical Determinations of Greek “Kollitsida’’ (Arctium lappa L.) from Different Regions and Evaluation of Its Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Kollitsida Fine Powders
2.2. Chemicals and Reagents
2.3. Extraction of Phytochemicals
2.4. Determination of Total Phenolic Content
2.5. Determination of Antioxidant Activity
2.6. Determination of Caffeic Acid Content
2.7. Determination of Quercetin Content
2.8. Determination of pH
2.9. Determination of Color Parameters
2.10. Determination of Oxalic Acid
2.11. Determination of Antibacterial Activity
2.12. Statistical Analysis
3. Results
3.1. Physicochemical Parameters and Phytochemical Content of Greek “Kollitsida’’ Extracted with Deionized Water
3.2. Physicochemical Parameters and Phytochemical Content of Greeκ “Kollitsida’’ Extracted with Ethanol of Grape Origin
3.3. Antimicrobial Activity of Greek “Kollitsida’’ Samples According to Geographical Origin
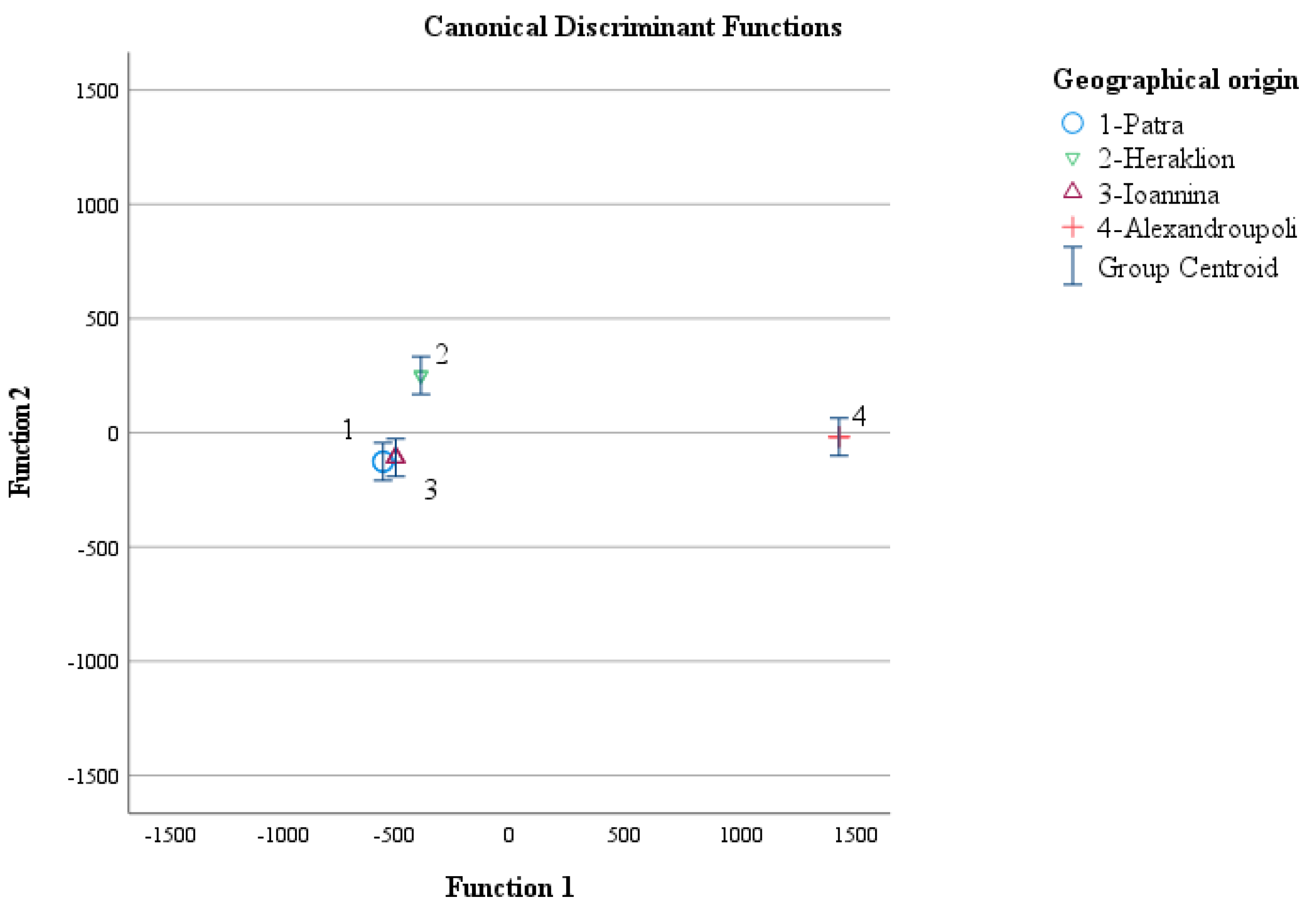
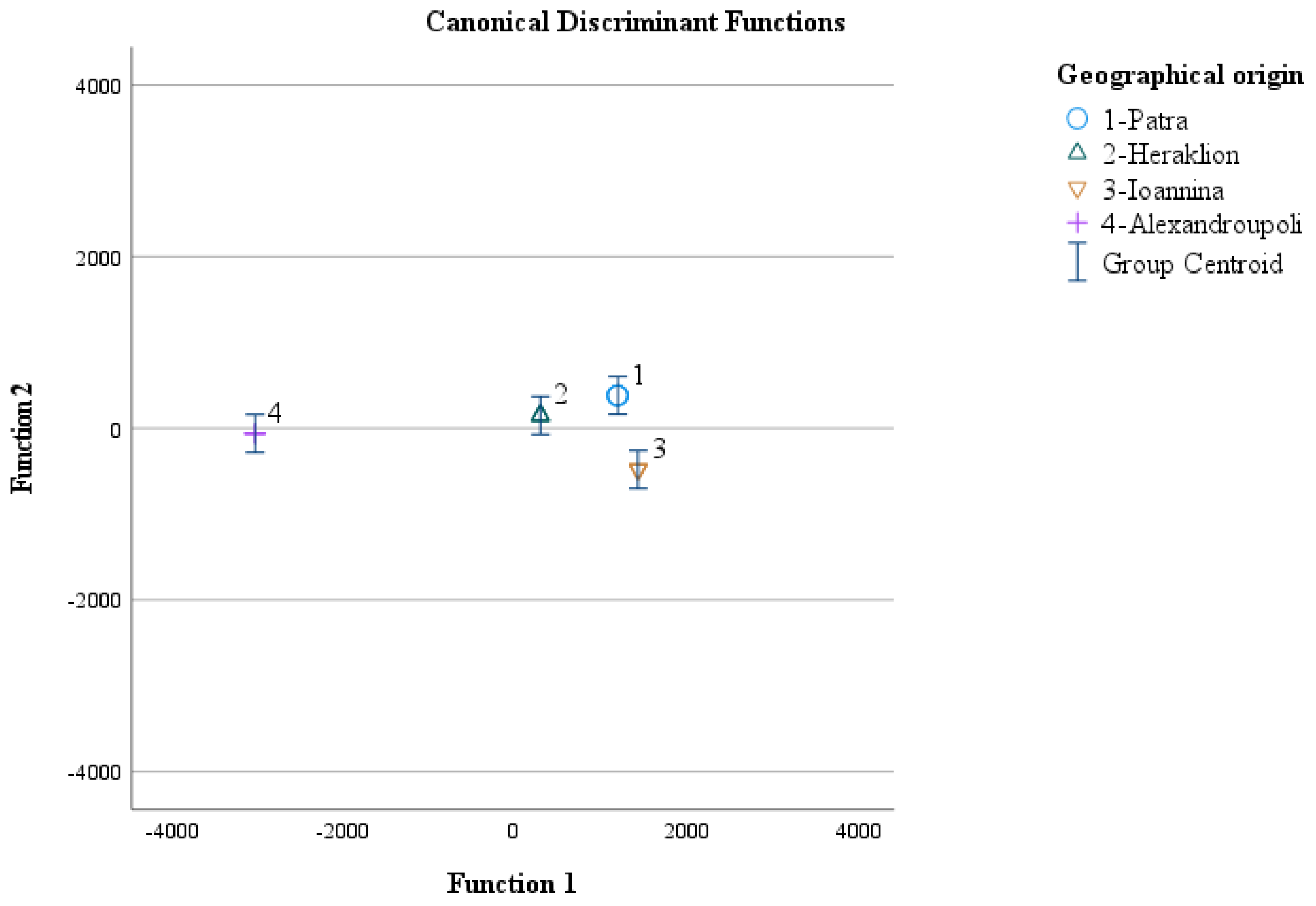
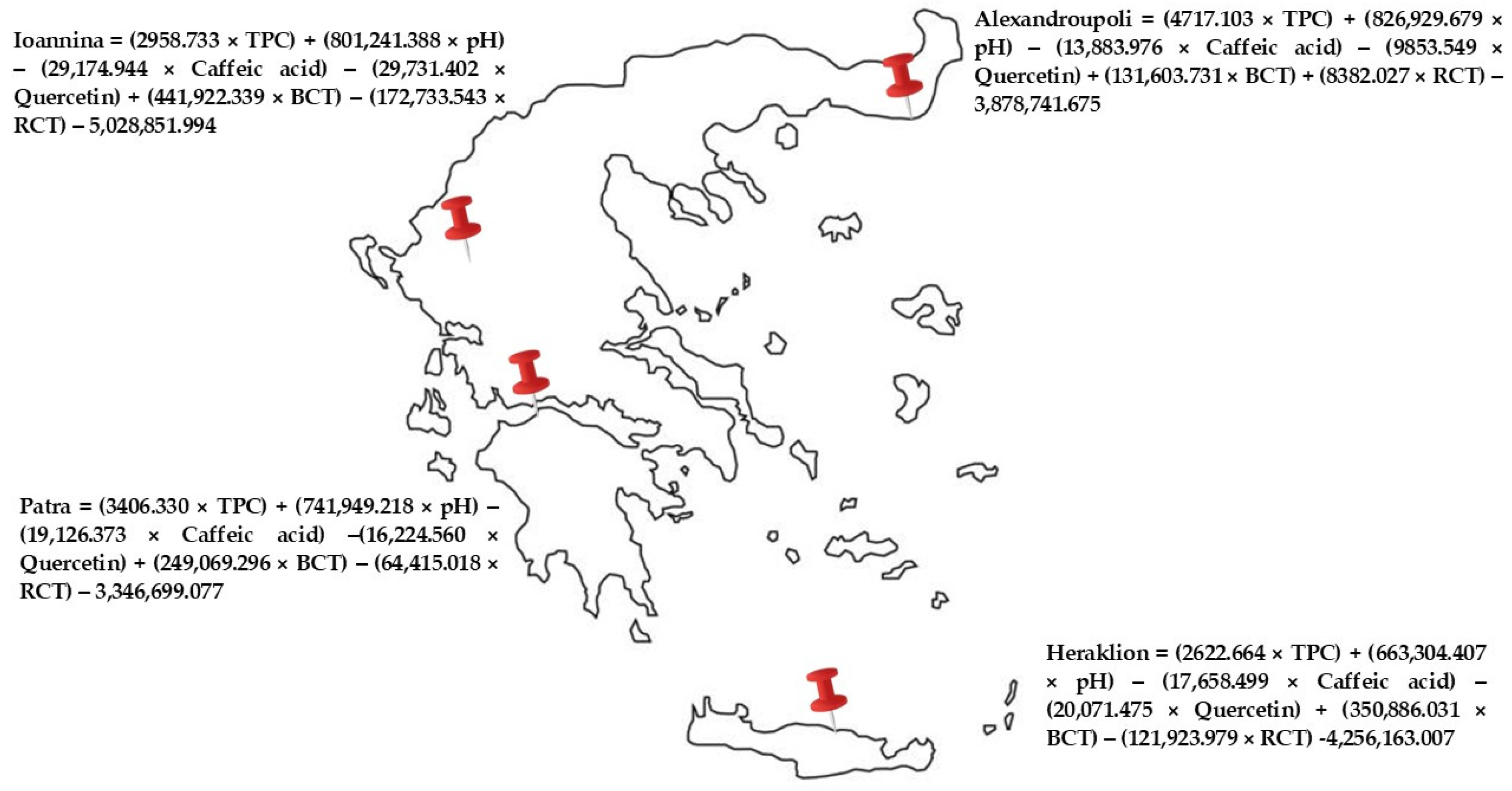
3.4. Geographical Origin Recognition of Greek “Kollitsida’’ According to Geographical Origin
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arctium lappa. Arctium lappa. Germplasm Resources Information Network. Agricultural Research Service, United States Department of Agriculture. Available online: https://www.ars-grin.gov/ (accessed on 8 February 2025).
- Chan, Y.-S.; Cheng, L.-N.; Wu, J.-H.; Chan, E.; Kwan, Y.-W.; Lee, S.M.-Y.; Leung, G.P.-H.; Yu, P.H.-F.; Chan, S.-W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2010, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ding, J.; Li, Y.; Cao, G.; Zhu, L.; Bian, Y.; Liu, Y. Extraction, Structure and Bioactivities of Polysaccharide from Root of Arctium lappa L.: A Review. Int. J. Biol. Macromol. 2024, 265, 131035. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Sun, Y.; Wang, X.; Zhai, Y.; Sun, Y.; Sun, S.; Yu, A.; Zhang, H.; Wang, Y. Determination of the Major Constituents in Fruit of Arctium lappa L. By Matrix Solid-Phase Dispersion Extraction Coupled with HPLC Separation and Fluorescence Detection. J. Chromatogr. B 2010, 878, 2707–2711. [Google Scholar] [CrossRef]
- da Silva, L.M.; Allemand, A.; Mendes, D.A.G.B.; dos Santos, A.C.; André, E.; de Souza, L.M.; Cipriani, T.R.; Dartora, N.; Marques, M.C.A.; Baggio, C.H.; et al. Ethanolic Extract of Roots from Arctium lappa L. Accelerates the Healing of Acetic Acid-Induced Gastric Ulcer in Rats: Involvement of the Antioxidant System. Food Chem. Toxicol. 2013, 51, 179–187. [Google Scholar] [CrossRef]
- Petknova, N.; Hambarlyiska, I.; Tumbarski, Y.; Vrancheva, R.; Raeva, M.; Ivanov, I. Phytochemical Composition and Antimicrobial Properties of Burdock (Arctium lappa L.) Roots Extracts. Biointerface Res. Appl. Chem. 2022, 12, 2826–2842. [Google Scholar] [CrossRef]
- Carlotto, J.; Souza, L.M.d.; Baggio, C.H.; Fernanda, M.; Maria-Ferreira, D.; Sassaki, G.L.; Iacomini, M.; Cipriani, T.R. Polysaccharides from Arctium lappa L.: Chemical Structure and Biological Activity. Int. J. Biol. Macromol. 2016, 91, 954–960. [Google Scholar] [CrossRef]
- Hayashi, K.; Narutaki, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza. Biol. Pharm. Bull. 2010, 33, 1199–1205. [Google Scholar] [CrossRef]
- Mekki, I.; Camin, F.; Perini, M.; Smeti, S.; Hajji, H.; Mahouachi, M.; Piasentier, E.; Atti, N. Differentiating the geographical origin of Tunisian indigenous lamb using stable isotope ratio and fatty acid content. J. Food Compos. Anal. 2016, 53, 40–48. [Google Scholar] [CrossRef]
- Gaiad, J.E.; Hidalgo, M.J.; Villafañe, R.N.; Marchevsky, E.J.; Pellerano, R.G. Tracing the Geographical Origin of Argentinean Lemon Juices Based on Trace Element Profiles Using Advanced Chemometric Techniques. Microchem. J. 2016, 129, 243–248. [Google Scholar] [CrossRef]
- Aprea, E.; Carlin, S.; Versini, G.; Märk, T.D.; Gasperi, F. Assessing truffle origin by PTR-MS fingerprinting and comparison with GC-MS for the headspace analysis. In Proceedings of the 3rd International Conference on Proton Transfer Reaction Mass Spectrometry, Obergurgl, Austria, 27 January–1 February 2007; Hansel, A., Märk, T.D., Eds.; University Press: Innsbruck, Austria, 2007; pp. 132–135, ISBN 3902571039. Available online: http://hdl.handle.net/10449/16072 (accessed on 10 April 2025).
- Povolo, M.; Contarini, G.; Mele, M.; Secchiari, P.L. Study on the Influence of Pasture on Volatile Fraction of Ewes’ Dairy Products by Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. J. Dairy Sci. 2007, 90, 556–569. [Google Scholar] [CrossRef]
- Camin, F.; Pavone, A.; Bontempo, L.; Wehrens, R.; Paolini, M.; Faberi, A.; Marianella, R.M.; Capitani, D.; Vista, S.; Mannina, L. The Use of IRMS, 1H NMR and Chemical Analysis to Characterise Italian and Imported Tunisian Olive Oils. Food Chem. 2016, 196, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Morillo, A.; Jurado, J.M.; Alcázar, Á.; de Pablos, F. Geographical Characterization of Spanish PDO Paprika by Multivariate Analysis of Multielemental Content. Talanta 2014, 128, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Marquetti, I.; Link, J.V.; Makimori, G.Y.F.; Arca, V.d.C.; Lemes, A.G.L.; Ferreira, J.M.G.; Scholz, M.B.d.S.; Valderrama, P.; Poppi, R.J. Support Vector Machines in Tandem with Infrared Spectroscopy for Geographical Classification of Green Arabica Coffee. LWT-Food Sci. Technol. 2017, 76, 330–336. [Google Scholar] [CrossRef]
- Diniz, P.H.G.D.; Gomes, A.A.; Pistonesi, M.F.; Band, B.S.F.; de Araújo, M.C.U. Simultaneous Classification of Teas according to Their Varieties and Geographical Origins by Using NIR Spectroscopy and SPA-LDA. Food Anal. Methods 2014, 7, 1712–1718. [Google Scholar] [CrossRef]
- Mandrile, L.; Zeppa, G.; Giovannozzi, A.M.; Rossi, A.M. Controlling Protected Designation of Origin of Wine by Raman Spectroscopy. Food Chem. 2016, 211, 260–267. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Giannitto, A.; Maggi, M.A.; Ruggieri, F. Geographical Classification of Italian Saffron (Crocus sativus L.) Based on Chemical Constituents Determined by High-Performance Liquid-Chromatography and by Using Linear Discriminant Analysis. Food Chem. 2016, 212, 110–116. [Google Scholar] [CrossRef]
- Etièvant, P.; Schlich, P.; Cantagrel, R.; Bertrand, M.; Bouvier, J. Varietal and Geographic Classification of French Red Wines in Terms of Major Acids. J. Sci. Food Agric. 1989, 46, 421–438. [Google Scholar] [CrossRef]
- Rizelio, V.M.; Gonzaga, L.V.; Campelo, S.; Maltez, H.F.; Carolina, A.; Fett, R. Fast Determination of Cations in Honey by Capillary Electrophoresis: A Possible Method for Geographic Origin Discrimination. Talanta 2012, 99, 450–456. [Google Scholar] [CrossRef]
- Lazaridis, D.G.; Karabagias, V.K.; Karabagias, I.K.; Andritsos, N.D.; Giannakas, A.E. Physicochemical and Phytochemical Characterization of Green Coffee, Cinnamon Clove, and Nutmeg EEGO, and Aroma Evaluation of the Raw Powders. Eur. Food Res. Technol. 2023, 250, 83–96. [Google Scholar] [CrossRef]
- Kitsios, A.-P.M.; Lazaridis, D.G.; Koutoulis, A.S.; Karabagias, V.K.; Andritsos, N.D.; Karabagias, I.K. Geographical Origin Estimation of Miniaturized Samples of “Nova” Mandarin Juice Based on Multiple Physicochemical and Biochemical Parameters Conjointly with Bootstrapping. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Kokkosi, E.K.; Mylonaki, E.N.; Karabagias, V.K.; Andritsos, N.D.; Giannakas, A.E.; Karabagias, I.K. Shelf Life Extension of “Gyros’’ Pork Meat Using Plant Extracts with Antioxidant and Antimicrobial Activities. Food Biosci. 2024, 62, 105542. [Google Scholar] [CrossRef]
- Lazaridis, D.G.; Kokkosi, E.K.; Mylonaki, E.N.; Karabagias, V.K.; Andritsos, N.D.; Karabagias, I.K. Rapid Classification of Unroasted Green Coffee Beans and Spices Based on the Tentative Determination of Volatile Compounds by Solid-Phase Dynamic Extraction (SPDE) and Gas Chromatography–Mass Spectrometry (GC–MS) with Supervised Learning. Separations 2024, 11, 351. [Google Scholar] [CrossRef]
- Vanaki, E.; Kamkar, A.; Noori, N.; Azizian, A.; Mohammadkhan, F. The Effect of Aqueous Extract of Arctium lappa Root on the Survival of Lactobacillus Acidophilus La-5 and Bifidobacterium bifidum Bb-12 and Sensorial and Physicochemical Properties of Synbiotic Yogurt. Food Sci. Nutr. 2023, 12, 2182–2191. [Google Scholar] [CrossRef]
- Predes, F.S.; Ruiz, A.L.; Carvalho, J.E.; Foglio, M.A.; Dolder, H. Antioxidative and in Vitro Antiproliferative Activity of Arctium lappa Root Extracts. BMC Complement. Altern. Med. 2011, 11, 25. [Google Scholar] [CrossRef]
- Funaki, N.; Hattori, S.; Kimura, Y.; Sato, K.; Tsukada, M.; Tsumura, A.; Sano, M.; Toyoda, M.; Kozuka, K.; Kadokura, M.; et al. Determination of the Geographic Origin of Edible Burdocks by Inorganic Analysis. Nippon Shokuhin Kagaku Kogaku Kaishi 2010, 57, 70–77. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Ma, S.; Liu, Z. Tracing the Geographical Origin of Burdock Root Based on Fluorescent Components Using Multi-Way Chemometrics Techniques. Microchem. J. 2018, 137, 456–463. [Google Scholar] [CrossRef]

| Region | pH | Oxalic Acid (mg/100 mL) | YCT (%) | BCT (%) | RCT (%) | Caffeic Acid (mg/L) | Quercetin (mg/L) | TPC (mg GAE/L) | AA (%) | Ash (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Patra | 5.20± 0.01 a | 11.70 ± 1.80 a | 65.42 ± 0.02 a | 25.37 ± 0.02 a | 9.21 ± 0.01 a | 109.47 ± 0.20 a | 102.81 ± 0.48 a | 423.00 ± 0.00 a | 54.85 ± 0.82 a | 90.83 ± 2.17 a |
| Heraklion | 5.15 ± 0.01 b | 9.00± 4.50 a | 52.24 ± 0.03 b | 27.25 ± 0.03 b | 20.51 ± 0.12 b | 91.79 ± 0.21 b | 68.14 ± 0.48 b | 673.00 ± 1.43 b | 48.42 ± 1.80 b | 93.63 ± 0.29 a |
| Ioannina | 5.09 ± 0.01 c | 16.20 ± 0.90 ab | 69.80 ± 0.03 c | 22.50 ± 0.02 c | 7.70 ± 0.03 c | 90.56 ± 0.20 c | 58.51 ± 0.48 c | 495.90 ± 1.43 c | 55.01 ± 1.74 a | 92.00 ± 2.83 a |
| Alexandroupoli | 4.78 ± 0.01 d | 20.26 ± 2.26 b | 49.44 ± 0.32 d | 28.77 ± 0.15 d | 21.79 ± 0.17 d | 206.20 ± 0.41 d | - | 867.29 ± 1.42 d | 56.54 ± 3.48 a | 92.63 ± 1.01 a |
| Region | pH | Oxalic Acid (mg/100 mL) | YCT (%) | BCT (%) | RCT (%) | Caffeic Acid (mg/L) | Quercetin (mg/L) | TPC (mg GAE/L) | AA (%) |
|---|---|---|---|---|---|---|---|---|---|
| Patra | 5.13 ± 0.01 a | 14.86 ± 3.14 a | 76.90 ± 0.04 a | 17.68 ± 0.03 a | 5.42 ± 0.02 a | 123.35 ± 0.10 a | 29.37 ± 0.24 a | 490.14 ± 1.43 a | 51.58 ± 2.67 a |
| Heraklion | 4.77 ± 0.01 b | 19.90 ± 2.70 a | 70.52 ± 0.04 a | 23.38 ± 0.03 b | 6.10 ± 0.02 b | 150.19 ± 0.20 b | 67.42 ± 0.25 b | 721.57 ± 1.43 b | 55.99 ± 0.76 a |
| Ioannina | 4.81 ± 0.01 b | 16.66 ± 2.24 a | 84.00 ± 13.83 a | 18.69 ± 0.00 c | 5.28 ± 0.02 c | 86.34 ± 0.10 c | 8.19 ± 0.24 c | 547.29 ± 1.42 c | 53.98 ± 3.21 a |
| Alexandroupoli | 4.91 ± 0.01 c | 21.16 ± 0.46 a | 78.64 ± 0.03 a | 17.64 ± 0.01 a | 3.72 ± 0.02 d | 202.7 ± 0.21 d | - | 881.57 ± 1.43 d | 55.72 ± 1.14 a |
| Salmonella typhimurium | Staphylococcus aureus | |||
|---|---|---|---|---|
| Inhibition Zone (mm) | Inhibition Zone (mm) | |||
| Region/Solvent | Deionized Water | Ethanol of Grape Origin | Deionized Water | Ethanol of Grape Origin |
| Patra | 4.61 ± 0.52 a | 5.65 ± 0.21 a | 5.28 ± 0.43 a | 7.50 ± 0.48 a |
| Heraklion | 0.75 ± 0.09 b | 4.32 ± 0.24 b | 7.08 ± 0.09 b | 8.94 ± 0.16 b |
| Ioannina | 6.46 ± 0.13 c | 0.62 ±0.1 c | 5.77 ± 0.27 a | 7.08 ± 0.46 a |
| Alexandroupoli | 5.24 ± 0.48 a | 6.16 ± 0.45 a | 6.61 ± 0.09 c | 9.47 ± 0.53 c |
| MANOVA Indices * | Interval Values | F-Value | df | p |
|---|---|---|---|---|
| Pillai’s Trace | 3.000 | 40713.817 | 24 | <0.001 |
| Wilks’ Lambda | 0.000 | 41210.933 | 24 | <0.001 |
| Physicochemical and Phytochemical Parameters | Wilks’ Lambda | F | df1 | df2 | Significance (p-Value) |
|---|---|---|---|---|---|
| TPC | 0.000 | 46,264.254 | 3 | 8 | <0.001 |
| AA | 0.510 | 2.559 | 3 | 8 | 0.128 * |
| pH | 0.003 | 779.000 | 3 | 8 | <0.001 |
| Caffeic acid | 0.000 | 270,980.520 | 3 | 8 | <0.001 |
| Quercetin | 0.000 | 62,080.764 | 3 | 8 | <0.001 |
| YCT | 0.579 | 1.938 | 3 | 8 | 0.202 * |
| BCT | 0.000 | 68,972.684 | 3 | 8 | <0.001 |
| RCT | 0.000 | 6416.766 | 3 | 8 | <0.001 |
| Oxalic acid | 0.401 | 3.990 | 3 | 8 | 0.052 * |
| Discriminant Function (s) | Eigenvalue | Variance (%) | Cumulative Variance (%) | Canonical Correlation | Test of Function(s) | Wilks’ Lambda | Chi-Square (X 2) | Degrees of Freedom (df) | Significance (p-Value) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 422974.384 a | 80.0 | 80.0 | 1.000 | 1 through 3 | 0.000 | 207.532 | 18 | <0.001 |
| 2 | 70733.792 a | 13.4 | 93.4 | 1.000 | 2 through 3 | 0.000 | 129.802 | 10 | <0.001 |
| 3 | 35133.860 a | 6.6 | 100.0 | 1.000 | 3 | 0.000 | 62.802 | 4 | <0.001 |
| Physicochemical and Phytochemical Parameters | |||
|---|---|---|---|
| Discriminant Function (s) | |||
| 1 | 2 | 3 | |
| Caffeic acid | 0.468 * | −0.100 | 0.483 * |
| BCT | −0.041 | −0.575 * | 0.221 |
| Quercetin | −0.021 | −0.557 * | −0.182 |
| TPC | 0.148 | −0.055 | 0.474 * |
| RCT | −0.046 | −0.132 * | −0.090 |
| pH | 0.008 | 0.026 | −0.079 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridis, D.G.; Giannoulis, S.D.; Simoni, M.; Karabagias, V.K.; Andritsos, N.D.; Triantafyllidis, V.; Karabagias, I.K. Physicochemical and Phytochemical Determinations of Greek “Kollitsida’’ (Arctium lappa L.) from Different Regions and Evaluation of Its Antimicrobial Activity. Separations 2025, 12, 151. https://doi.org/10.3390/separations12060151
Lazaridis DG, Giannoulis SD, Simoni M, Karabagias VK, Andritsos ND, Triantafyllidis V, Karabagias IK. Physicochemical and Phytochemical Determinations of Greek “Kollitsida’’ (Arctium lappa L.) from Different Regions and Evaluation of Its Antimicrobial Activity. Separations. 2025; 12(6):151. https://doi.org/10.3390/separations12060151
Chicago/Turabian StyleLazaridis, Dimitrios G., Sokratis D. Giannoulis, Maria Simoni, Vassilios K. Karabagias, Nikolaos D. Andritsos, Vasileios Triantafyllidis, and Ioannis K. Karabagias. 2025. "Physicochemical and Phytochemical Determinations of Greek “Kollitsida’’ (Arctium lappa L.) from Different Regions and Evaluation of Its Antimicrobial Activity" Separations 12, no. 6: 151. https://doi.org/10.3390/separations12060151
APA StyleLazaridis, D. G., Giannoulis, S. D., Simoni, M., Karabagias, V. K., Andritsos, N. D., Triantafyllidis, V., & Karabagias, I. K. (2025). Physicochemical and Phytochemical Determinations of Greek “Kollitsida’’ (Arctium lappa L.) from Different Regions and Evaluation of Its Antimicrobial Activity. Separations, 12(6), 151. https://doi.org/10.3390/separations12060151











