Abstract
Herein, a simple and effective analytical method was developed to monitor etoricoxib concentrations in human urine samples. Etoricoxib is a nonsteroidal anti-inflammatory drug for pain and inflammation relief in conditions such as osteoarthritis and rheumatoid arthritis. To determine its concentration, fabric phase sorptive extraction (FPSE) was combined with high-performance liquid chromatography and diode array detection (HPLC-DAD). FPSE is a green sample preparation technique that utilizes sol–gel-coated fabric substrates as extraction devices, offering numerous benefits in bioanalysis. Initially, different materials were tested for their affinity towards etoricoxib. The most critical FPSE parameters (i.e., sample amount, stirring rate, and adsorption time) were optimized using a face-centered central composite design (FC-CCD), while the remaining ones were explored by means of the one-variable-at-a-time approach. Afterwards, the analytical method was validated in terms of its selectivity, linearity, sensitivity, accuracy, and precision, while the environmental sustainability and the practicality of the method were also examined. The limit of detection was 0.03 μg mL−1, and the lower limit of quantification was 0.10 μg mL−1. The relative standard deviation was less than 7.2% in all cases, showing good precision. The proposed approach was successfully used to monitor urinary etoricoxib concentrations in real samples obtained from a volunteer after oral drug administration.
1. Introduction
Etoricoxib is a selective inhibitor of cyclooxygenase-2 (COX-2), which reduces the production of the prostaglandins responsible for pain, inflammation, and fever. It belongs to the oxicam class of nonsteroidal anti-inflammatory drugs (NSAIDs) and is primarily used for rheumatoid arthritis, osteoarthritis, acute gouty arthritis, and dental surgery, as well as gout and chronic pelvic pain. Clinical trials have proven its analgesic and anti-inflammatory performance to be similar or even better compared to nonselective NSAIDs in several treatment settings [1,2]. Etoricoxib shows better gastrointestinal safety in comparison with nonselective NSAIDs and has a favourable overall tolerability and safety profile [1,3]. More than 90% of etoricoxib is metabolized and excreted through feces and urine, while only about 1% of the oral dose is recovered intact in urine [1]. Given this low concentration, analytical methods for its determination must be highly sensitive and specific to accurately quantify the drug and its metabolites. Several analytical techniques have been developed and validated for this purpose, including UV spectrophotometry [4] and high-performance liquid chromatography (HPLC) [5,6].
Sample preparation is an integral part of any bioanalytical method that aims at the selective isolation of the analyte of interest from the matrix, the minimization/elimination of other matrix components, and, if required, preconcentration. For this purpose, several sample preparation techniques such as protein precipitation, liquid–liquid extraction (LLE), and solid-phase extraction (SPE) have been reported [7]. However, these techniques are characterized by various fundamental drawbacks. For example, LLE is considered laborious and time-consuming, and it utilizes toxic or flammable chemicals [8]. Today, there is an ongoing need for advancements in sample preparation and microfluidics-based techniques to promote research in bioanalysis [9] while reducing sample volumes and reagent consumption based on the principles of Green Sample Preparation (GSP) [10] and Green Analytical Chemistry (GAC) [11]. Specifically, rapid, miniaturized, and low-energy-consumption methods are preferred over the conventional techniques to eliminate the environmental impact of sample preparation, which is profound in many analytical protocols.
The typical paradigms of greener microextraction techniques that have been used in bioanalysis to minimize harm to the environment include solid-phase microextraction (SPME), liquid-phase microextraction (LPME), stir bar sorptive extraction (SBSE), dispersive solid-phase extraction (d-SPE), dispersive liquid–liquid microextraction (DLLME), fabric phase sorptive extraction (FPSE), and magnet integrated fabric phase sorptive extraction (MI-FPSE) [12,13,14,15].
Fabric phase sorptive extraction (FPSE) is an advanced sample preparation approach that was developed by Kabir and Furton in 2014 [16]. This technique combines the inherent benefits of solid-phase extraction (SPE), an exhaustive extraction technique, and SPME, an equilibrium extraction technique. FPSE uses fabric substrates coated with sol–gel organic–inorganic hybrid sorbents for the extraction of compounds of interest from complex samples. Due to the strong covalent bonding between the sol–gel sorbent coating and the fabric substrate, the coated FPSE membranes possess high chemical, solvent, and thermal stability. In a typical FPSE protocol, the membrane is added in the aqueous sample and external stimuli (e.g., magnetic stirring) are used to assist the procedure. Accordingly, the membrane is immersed in an appropriate solvent or mixture of solvents for the desorption of the target analyte(s). The sponge-like porous structure of the sol–gel-derived hybrid sorbents enables the rapid achievement of extraction equilibrium. The simplified workflow eliminates the need for sample pre-treatment steps such as filtration, protein precipitation, or centrifugation, allowing direct extraction from complex samples. FPSE has found to be a valuable tool in modern analytical chemistry for analysing environmental, biological, and food samples, offering an attractive alternative to traditional extraction techniques [17].
In this work, an efficient FPSE protocol was proposed to extract urinary etoricoxib levels combined with high-performance liquid chromatography–diode array detection (HPLC-DAD) analysis. Method optimization was conducted by means of statistical design of experiments (DoE), particularly through face-centered central composite design and the one-factor-at-a-time (OFAT) approach. The method was validated according to the FDA guidelines for bioanalytical methods considering its linearity, sensitivity, selectivity, accuracy, and precision. It was then used to measure the etoricoxib levels in the urine samples from a patient treated with etoricoxib.
2. Materials and Methods
2.1. Reagents, and Materials
All reagents were of analytical grade or higher. KH2PO4, concentrated H3PO4 (85% w/w), and HPLC grade water were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol (MeOH), acetone (ACE), and acetonitrile (ACN) were obtained from Honeywell (Morristown, NJ, USA). These organic solvents were of LC-MS grade.
The etoricoxib administered to the volunteer was in the form of a commercial pharmaceutical formulation (90 mg/tab, Iasis Pharma, Kamatero, Greece). Etoricoxib, or 5-chloro-3-[4-(methylsulfonyl)phenyl]-2-(2-methyl-5-pyridinyl)pyridine), with a purity higher than 95% was purchased from Merck (Darmstadt, Germany). The chemical structure of the compound is shown in Figure S1. The stock solution (1000 μg mL−1) was made in ACN and it was stored at +4 °C. From this solution, working standards were made on a daily basis using water as solvent for dilution.
The synthesis of the FPSE membranes has been previously described [18]. The 100% cellulose fabric substrates for the FPSE membranes were obtained from JoAnn Fabric (Miami, FL, USA). The carbowax 20 M (CW 20 M) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The solvents and chemicals, including ACE, dichloromethane, NaOH, HCl, MeOH, ammonium hydroxide, and trifluoroacetic acid, used in the FPSE synthesis were purchased from Fisher Scientific (Milwaukee, WI, USA). The methyl trimethoxysilane was obtained from Gelest Inc. (Morrisville, PA, USA).
2.2. HPLC Instrumentation
A Shimadzu 2010 HPLC-DAD system (Kyoto, Japan) was utilized for the monitoring of etoricoxib in the human urine following the FPSE procedure. The instrument had two pumps, a column compartment, a communication bus module, an autosampler, and a diode array detector. The target analyte was monitored at 284 nm. A Kinetex C18 100 A (30 × 4.6 mm, 2.6 μm) column by Phenomenex (Torrance, CA, USA) was used and it was kept at 35 °C. The mobile phase was a mixture of potassium dihydrogen phosphate buffer 0.05 M (pH adjusted to 4.2 with H3PO4) (A) and acetonitrile (B). The separation of etoricoxib was performed under the following conditions A:B v/v: 70:30, (kept for 1 min), 40:60 (changed at 1.5 min), 50:50 (at 2 min), and 30:70 (at 2.5 min). The composition was constant for 1.5 min for stationary phase equilibration. The flow rate was 1.0 mL min−1 and an aliquot of 5 μL was injected for analysis.
2.3. Sample Collection and Handling
The urine samples from healthy volunteers (n = 6) who did not undergo any treatment with etoricoxib or other medication were collected in sterilized cups. Before this procedure, all individuals were informed regarding the scope and the procedures of the proposed study, and their written informed ethical consent was obtained. The samples were stored at −18 °C until the analysis. An aliquot of 2000 μL of undiluted urine was used for the analysis. This method was also used for the analysis of the urine samples obtained from one adult volunteer following etoricoxib administration (90 mg/tab). For this purpose, the samples were collected within 1, 2, 8, 12, and 24 h after oral drug administration.
2.4. Method Validation
The validation of the FPSE-HPLC-DAD method was performed in terms of linearity, accuracy, precision, selectivity, limit of detection (LOD), limit of quantitation (LOQ), matrix effect (ME%), and carryover, taking into consideration the FDA guidelines for bioanalytical methods [19]. The selectivity of the FPSE-HPLC-DAD method was evaluated through the analysis of the etoricoxib-free and spiked urine samples (n = 6). The accuracy and precision of the FPSE-HPLC-DAD method were studied by analyzing the spiked samples at 0.10 μg mL−1 (lower limit of quantification, LLOQ), 0.50 μg mL−1 (low quality control level, LQC), 1.0 μg mL−1 (medium quality control level, MQC), and 10.0 μg mL−1 (high quality control level, HQC). Three replicate analyses were performed within the same day (intra-day accuracy and precision, n = 3) and on three consecutive days (inter-day precision and accuracy, n = 3 × 3), and the relative recovery (RR%) was calculated. A matrix-matched calibration curve was constructed by plotting the peak area of etoricoxib and its concentration within the working range of 0.10–10.0 μg mL−1 to assess method linearity. To evaluate the ME%, a calibration curve for aqueous standards was constructed within the same concentration range. All concentration levels of the calibration curve were analyzed in triplicate. The LLOQ of the method was the lowest point of the calibration curve that had a signal-to-noise ratio ≥ 10, while the LOD corresponded to a signal-to-noise ratio higher than 3. Finally, the carryover was assessed by analyzing blank samples after the calibration standard at the upper limit of quantification (i.e., 10.0 μg mL−1).
2.5. FPSE Procedure
The procedure for extracting the etoricoxib involved four consecutive steps, as follows:
- (a)
- Activation: The membranes were activated by immersion in 1 mL of a MeOH:ACN mixture (50:50, v/v) for 5 min, followed by thorough rinsing with high-purity water.
- (b)
- Extraction: The FPSE membrane was added to the glass vial containing the sample. A magnetic stirrer was also added to the vial and the sample was stirred at 100 rpm. Under these conditions, the etoricoxib was extracted within 35 min.
- (c)
- Desorption: After adsorption, high-purity water was employed to rinse the membrane, which was further transferred to an Eppendorf tube. For the elution of etoricoxib, an aliquot of 500 μL of methanol was added and the membrane was left for 5 min to ensure complete desorption. Accordingly, the eluate was placed into an HPLC vial.
- (d)
- Cleaning: The membrane was cleaned by immersing it in 1 mL of the solvent mixture of step (a) for 5 min. Finally, it was dried and kept for future use.
3. Results and Discussion
3.1. Optimization of FPSE Procedure
To obtain the highest extraction performance, the primary parameters that were expected to affect the performance of the FPSE method were studied and optimized. These parameters were the type of sol–gel coating of the FPSE membranes and its dimensions, the acidity and the salt content, the eluent and its amount, and the elution time. The one-factor-at-a-time (OFAT) approach, known for its simplicity and ease of implementation, was employed for the optimization process to individually assess the effect of each variable while keeping the others constant. The initial experimental parameters were the following: (i) 1 × 1 cm2 FPSE size, (ii) 2000 μL of sample (pH 7, no salt), (iii) extraction time: 30 min under stirring at 400 rpm, and (vi) desorption: 1 mL of MeOH for 10 min. All experiments were conducted in triplicate. Afterward, the sample amount, the stirring rate, and the extraction time were studied using an experimental design. These factors were expected to exhibit a profound effect on the extraction procedure.
3.1.1. Selection of Sol–Gel Coating and Membrane Dimensions
Initially, three sol–gel-coated membranes, i.e., sol–gel Carbowax 20 M, PTHF, and C18, were investigated. Cellulose was used as a substrate in all cases. These materials are polar, semi-polar, and non-polar sorbents, respectively. The extraction recovery (%ER) for all cases was calculated through the comparison of the theoretical and experimentally calculated concentration of etoricoxib after the FPSE procedure. The optimum performance was achieved utilizing the sol–gel-coated CW 20 M membranes (Figure 1a). This material is a highly versatile sorbent that can interact with both non-polar and polar compounds by dipole–dipole interactions, hydrogen bonding, and some hydrophobic interactions [17]. This can be attributed to its high loading capacity, which results in a high number of active sites for interaction with analytes [20].
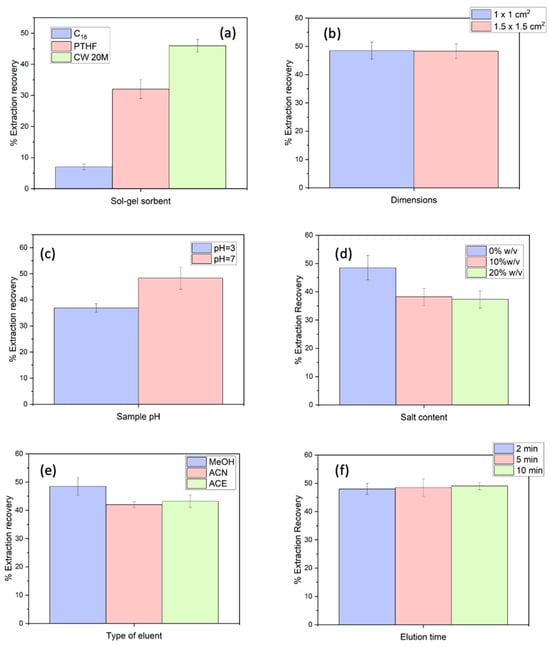
Figure 1.
Optimization of sample preparation: (a) type of sol–gel coating for the FPSE membranes, (b) membranes’ dimensions, (c) pH, (d) NaCl content, (e) eluent, and (f) elution time. Sample volume (Vs): 2.0 mL. Experiments were conducted in triplicate.
Subsequently, two dimensions (i.e., 1.0 cm × 1.0 cm and 1.5 cm × 1.5 cm) were evaluated. In principle, by enhancing the amount of the sol–gel sorbent, an enhancement of the %ER can be achieved. As derived from Figure 1b, the increase in the dimensions of the FPSE medium did not have any profound effect on the ER% of the etoricoxib. This can be attributed to the incomplete immersion of the 1.5 cm × 1.5 cm membranes in the sample solution due to their size. Thus, further experiments were conducted using 1.0 × 1.0 cm sol–gel coated FPSE membranes.
3.1.2. Study of Sample pH and Salt Content
The acidity and salinity of a sample are critical chemical parameters that can impact the %ER of etoricoxib. The effect of the pH of the sample was evaluated at pH values of 7 and 3. It was observed that at a pH value of 3, the efficiency of the extraction for etoricoxib decreased, as shown in Figure 1c. This small difference can be attributed to the higher affinity of the Carbowax sorbent towards the neutral form of etoricoxib due to more favorable hydrophobic and dipole-induced interactions. Moreover, urine samples contain various ionic species that can result to the potential “suppression” of the extraction recovery of the analyte [21]. Thus, pH 7 was selected for the rest of the study. This also ensured handling simplicity and reduced the number of sample preparation steps, while it also eliminated the sources of possible contamination.
Afterwards, the salt addition was investigated by adding variable NaCl quantities (model electrolyte) into the sample to achieve the following salt contents: 0, 10, and 20%, m/v. The solubility of medium-polar drugs can decrease at higher salt contents, enhancing the transference of the compound to the sorptive phase (salting-out). In contrast, the viscosity of the sample solution may also increase and potentially hinder the mass transfer of etoricoxib. The results of the effect of the ionic strength at the extraction performance are illustrated in Figure 1d. The %ER of etoricoxib decreased at elevated salt contents. Consequently, no NaCl was finally added to the sample solution.
3.1.3. Optimization of Sample Volume, Stirring Rate, and Extraction Time by Means of Response Surface Methodology
The extraction time (text) (Factor A), sample volume (Vs) (Factor B), and stirring rate (Factor C) were optimized by means of FC-CCD. This enabled the reliable estimation of quadratic models while preserving rotatability and uniform precision. To achieve this, 20 experiments were generated with the aid of Design Expert® 13 software (vs 22.08.0, Stat-Ease® Inc., Minneapolis, MN, USA). These runs consisted of 14 factorial and axial experiments along with 6 center points. The experimental domain is presented in Table 1. To minimize systematic errors, the generated experiments were conducted in a random order. A second-order polynomial quadratic model was developed and further refined by removing non-significant interactions and quadratic terms through multivariate regression analysis.

Table 1.
FC-CCD experimental design and the obtained responses for the experimental sequence.
After performing backward elimination and removing non-significant factors (p > 0.05), statistical validation was performed for the models. ANOVA (Table S1) indicated a non-significant lack-of-fit (LOF) (p = 0.3795). The model’s R2 and adjusted R2 values exceeded 0.7629, confirming its reliability and predictive capability. The adequate precision value (12.71) was higher than 4, indicating an adequate signal compared to noise. The response surface plots for etoricoxib’s peak area are presented in Figure 2, while the diagnostic plots (i.e., the normal probability plot of the residuals and the predicted values vs. the residuals) are summarized in Figure S2. The actual and predicted responses were strongly correlated, as revealed by the consistent aligment of all data points along the line. The predicted regression model obtained from ANOVA is the following:
Peak area (etoricoxib) = −3742.2 + 692.9 × A + 10080.1 × B + 4.7 × C − 0.34 × A × C − 9.2 × A2
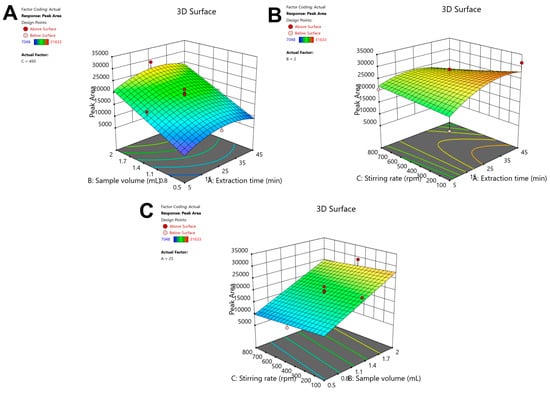
Figure 2.
Response surface plots of the effect of (A) Vs and extraction time, (B) extraction time and stirring rate, and (C) stirring rate and sample volume on the peak area of the drug.
To determine the optimal FPSE parameters, Derringer’s desirability function (D), scaled from zero to one, was applied. The desirability contour plot is shown in Figure S3. The optimal conditions were texp = 35.9 min, Vs = 2000 μL, and a stirring rate of 100 rpm. For simplicity reasons, the texp value was rounded to 35 min.
3.1.4. Optimization of Elution Parameters
After the selection of the adsorption conditions, the eluent type and quantity, as well as the desorption time, were optimized. Firstly, three elution solvents (i.e, MeOH, ACN, and ACE) were examined (Figure 1e). The optimum elution efficiency was achieved when MeOH was used. It should be noted that no etoricoxib existed in a second and consecutive desorption step with a fresh aliquot of methanol, demonstrating that the analyte was entirely eluted from the FPSE membrane and there were no unwanted carryover phenomena. Thus, MeOH was selected. The amount of MeOH was evaluated among 500–1500 μL. Ideally, the elution solvent amount should be minimized to reduce chemical usage, aligning with the guidelines of GSP [10] and GAC [11]. Similar %ER values were obtained throughout the studied range, demonstrating that a volume of 500 μL was sufficient to elute the adsorbed analyte. Thus, this value was selected for further studies. Finally, the study evaluated elution times ranging from 2 to 10 min. Generally, shorter elution times are preferable for developing rapid analytical protocols and improving sample throughput. However, they must be sufficient for the efficient desorption of etoricoxib. Among the tested intervals, 5 min demonstrated higher %ER values with good reproducibility in comparison with 2 min, while extending the time beyond 5 min showed no additional benefit (Figure 1f). Considering these factors, for the subsequent experiments 5 min was selected for desorption.
3.2. Method Validation Results
The developed method was performed in terms of linearity, precision and accuracy, selectivity, limit of detection (LOD), lower limit of quantification (LLOQ), matrix effect (ME%), and carryover as described in the Supplementary Material. As shown in Figure S4, there were no interferences eluting at the retention time of etoricoxib. Therefore, good selectivity was obtained for the determination of the drug in urine samples.
The method exhibited linearity of 0.10–10.0 μg mL−1. The regression coefficient, r, was 0.999, indicating good correlation. The calibration curve for etoricoxib was y = 7328.8x + 283. The LLOQ for etoricoxib was found to be 0.10 μg mL−1, and its LOD was 0.03 μg mL−1. Comparing the parallelism of the matrix-matched curve and the curve corresponding to the aqueous samples subjected to FPSE, a ME% of 40% was found. This can be attributed to the presence of various endogenous urine compounds that might interfere with the FPSE membrane, suppressing the extraction kinetics of the analytes. Thus, the matrix-matched calibration should be used for the quantitation of etoricoxib in real urine samples. In case the method needs to be adapted to be used for the analysis of different biological samples, an appropriate matrix-matched calibration should be prepared, while the experimental conditions should be optimized since they might differ from the optimum values used for urine analysis.
The results of the intra-day and inter-day accuracy and precision of the method for the different concentrations, i.e., LLOQ, LQC, MQC, and HQC, are presented in Table 2. As can be observed, the RSD% was below 7.2% and 6.7% for intra-day and inter-day, respectively. These values are below 15% and 20% (LLOQ) specifications. The method accuracy was between 97.3 and 109.1% (intra-day) and 94.5 and 104.1% (inter-day), which also falls in the interval of ±15% and ±20% (for the LLOQ level). As a result, satisfactory precision and accuracy were demonstrated for the proposed FPSE-HPLC-DAD method. Finally, the concentration of etoricoxib in blank urine samples analyzed after the analysis of a 10.0 μg mL−1 urine sample was below the LOD of the method, indicating that there is no carryover effect that could affect the quality of the determination.

Table 2.
Accuracy and precision data of the developed protocol.
3.3. Reusability of FPSE Media
The reusability of the sol–gel-coated FPSE media was monitored through repeated adsorption/elution cycles, utilizing a single FPSE medium for the extraction of etoricoxib from a urine sample (c = 1.0 μg mL−1). The set acceptance criterion for the loss of performance was set at a deviation of 15% among the original of the FPSE media and their performance at the end of each extraction/elution cycle, and it was measured as %RR. From the results, it was observed that the initial performance and the performance after 15 cycles were similar, indicating that the herein used FPSE media can be reused for >15 cycles. This is also in accordance with the principles of GSP [10] and GAC [11], which favor the use of reusable materials instead of single-use ones.
3.4. Stability Study
To assess the stability of etoricoxib in urine samples, different time intervals and different storing temperatures were evaluated, such as +25 °C for up to 8 h, and +4 °C and 18 °C for up to 48 h. As a threshold to evaluate the stability of etoricoxib, a ±15% deviation between the theoretical value and the experimentally found one was set. Among the examined conditions, no deviation higher than the acceptable limits was observed, showing that etoricoxib was stable in the real samples in the examined time periods.
3.5. Analysis of Urine Samples
The developed protocol was used for the determination of etoricoxib in authentic urine collected at various time intervals from an adult volunteer who had received an etoricoxib-containing tablet (90 mg/tab) orally. Following the administration, urine sampling was performed at different time points (1, 2, 8, 12, and 24 h). Accordingly, sample preparation was conducted as described in Section 2.3 and Section 2.4. The results of these analyses are shown in Figure 3. As can be observed, in all samples taken at the pre-defined intervals, etoricoxib was detected at a concentration higher than the LLOQ. The highest concentration (i.e., 0.41 μg mL−1) was determined in the time frame between 2 and 8 h.
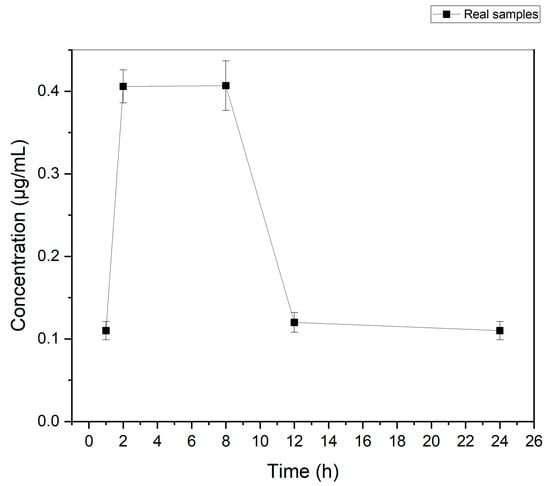
Figure 3.
Concentration of etoricoxib in the urine of one volunteer after administration of an etoricoxib tablet (90 mg of etoricoxib) at 1, 2, 8, 12, and 24 h.
3.6. Greenness and Practicality of the Method
The green potential of the FPSE-based analytical method was assessed using the ComplexGAPI index [22], which evaluated the environmental impact of the analytical method across all its procedural steps. Moreover, ComplexGAPI can assess the impact of the synthetic process of the coated membranes. A pictogram is generated with a colour scale of green, yellow, and red to show high, medium, and low environmental friendliness. Figure 4a shows the ComplexGAPI evaluation results for the herein proposed method. Most components of the method demonstrated high compliance with GAC principles [11], as indicated by the green colour in the index. The FPSE membranes were found to align with sustainability standards due to their low E-factor and minimal use of organic solvents. Moreover, miniaturized extraction was employed, eliminating the need for chemical use, and thus making the analytical method more environmentally sustainable. Furthermore, the protocol resulted in minimal waste generation, the majority of which can be attributed to the HPLC mobile phase and not to the FPSE procedure. Finally, the method’s practicality was studied using the Blue Applicability Grade Index (BAGI) [23]. BAGI considers the different characteristics of sample preparation and instrumental analysis that are required in an analytical method. It generates a pictogram with an asteroid shape that is divided into different subsections, each of them corresponding to the assessment criteria. Each section has an individual colour ranging from dark blue to white for high to no practicality. Simultaneously, a score is produced and depicted on the pictogram. This score is recommended to be beyond 60.0 to consider the method practical. As shown in Figure 4b, the BAGI score of the analytical method was 67.5, showing good practicality. This is because of the increased sample throughput, the good obtained sensitivity, and the selection of microextraction. Other benefits of these techniques that are not included in the BAGI evaluation include the membrane stability in a wide range of sample pH, the good figures of merit, the low mobile phase consumption (4 mL), and the absence of column clogging after FPSE.
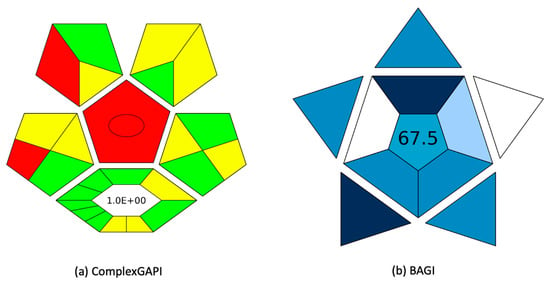
Figure 4.
(a) ComplexGAPI and (b) BAGI evaluation of the proposed method.
To further improve the practicality of the proposed method, the automation of the sample preparation step could be considered with the simultaneous reduction of the sample amount. However, in this case, the analytical performance of the method and its overall practicality should also be taken into consideration. For example, in pharmacokinetic studies where sample availability is limited, the sample amount can be reduced. Since this can negatively affect method sensitivity, the use of a more sensitive detection system (e.g., mass spectrometer or fluorescent detector) and/or sample evaporation and reconstitution may be necessary. Moreover, the dimensions of the FPSE membrane can also be reduced in this case to ensure complete immersion into the sample solution and waste reduction.
It should be noted that SPE is traditionally used for the determination of etoricoxib in urine samples. In this case, higher volumes of chemicals are required, including more environmentally hazardous ones (i.e., methylene chloride). Moreover, eluent evaporation and reconstitution are typically performed, limiting the practicality of the method by introducing time-consuming and error-prone steps [24,25]. Thus, although SPE can be performed in a semi-automated 96-well format, its practicality can be reduced in the case of advanced additional treatments. The approach used in this study exhibited similar accuracy and precision but better sensitivity due to the utilization of more sensitive detection systems and/or additional preconcentration steps. Moreover, it did not require more expensive and energy-demanding instrumentation, such as mass spectrometers, and it overcame the need for derivatization, maintaining high practicality and green character. Thus, FPSE can replace these conventional approaches with greener and more practical ones in cases where the sensitivity criteria are met.
4. Conclusions
In this work, an FPSE-HPLC-DAD protocol was developed for monitoring etoricoxib concentration levels using sol–gel-coated CW 20 M membranes. The validated analytical procedure offers significant benefits, such as simplicity of operation, fast sample preparation with the ability to perform parallel handling of numerous samples, and cost-effectiveness. The FPSE media can be reused at least 15 times, drastically reducing the need for materials during sample preparation as supported by the principles of GSP. Moreover, the method demonstrated a low environmental impact, as highlighted by the ComplexGAPI index, with low solvent consumption and waste production. Under optimized conditions, it delivers good linearity, precision, and accuracy, serving as a good option for determining etoricoxib in urine samples. Future perspectives include the expansion of the proposed FPSE protocol combined with chromatographic determination to include the main metabolites of etoricoxib for a more comprehensive assessment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12060141/s1, Figure S1. Chemical structure of etoricoxib; Figure S2. (A) Residuals vs. predicted and (B) Normal probability plots the for the %ER of etoricoxib; Figure S3. Desirability contour plots for defining the optimal conditions of the peak area of the drug, Figure S4. Representative FPSE-HPLC chromatograms at 284 nm of the analysis of the urine sample (black line), spiked with etoricoxib (pink line); Table S1. ANOVA table for the peak area of etoricoxib.
Author Contributions
Conceptualization, N.M., A.K. (Abuzar Kabir), and E.R.; methodology, A.K. (Anastasia Korpeti), N.M., A.K. (Abuzar Kabir), C.K.Z., and E.R.; software, N.M. and C.K.Z.; validation, A.K. (Anastasia Korpeti) and N.M.; formal analysis, A.K. (Anastasi Korpeti), N.M., A.K. (Abuzar Kabir), C.K.Z., and E.R.; investigation, A.K. (Anastasia Korpeti), N.M., A.K. (Abuzar Kabir), C.K.Z., and E.R.; resources, A.K. (Abuzar Kabir) and E.R.; data curation, A.K. (Anastasia Korpeti) and N.M.; writing—original draft preparation, A.K. (Anastasia Korpeti) and N.M.; writing—review and editing, A.K. (Abuzar Kabir), C.K.Z., and E.R.; visualization, A.K. (Anastasia Korpeti), N.M., C.K.Z., and E.R.; supervision, E.R.; project administration, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Acetone |
| ACN | Acetonitrile |
| BAGI | Blue Applicability Grade Index |
| ComplexGAPI | Complementary Green Analytical Procedure Index |
| COX-2 | Cyclooxygenase-2 |
| CW 20 M | Carbowax 20 M |
| d-SPE | Dispersive solid-phase extraction |
| DLLME | Dispersive liquid–liquid microextraction |
| DoE | Design of experiments |
| FC-CCD | Face-centered central composite design |
| FPSE | Fabric phase sorptive extraction |
| GAC | Green Analytical Chemistry |
| GSP | Green Sample Preparation |
| HPLC-DAD | High-performance liquid chromatography and diode array detection |
| HQC | High quality control |
| LLE | Liquid–liquid extraction |
| LLOQ | Lower limit of quantification |
| LOD | Limit of detection |
| LOF | Lack-of-fit |
| LPME | Liquid-phase microextraction |
| LQC | Low quality control |
| MeOH | Methanol |
| MI-FPSE | Magnet integrated fabric phase sorptive extraction |
| MQC | Medium quality control |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OFAT | One-factor-at-a-time |
| RR | Relative recovery |
| RSD | Relative standard deviation |
| SBSE | Stir bar sorptive extraction |
| SPE | Solid-phase extraction |
| SPME | Solid-phase microextraction |
| text | Extraction time |
| VS | Sample volume |
| %ER | Extraction recovery (%) |
References
- Onn, G.; Loh, K.; Yii, E.; Wong, L.; Tze, Y.; Tan, F.; Heng, S.C.; Saaid, M.; Cheah, K.Y.; Diyana, N.; et al. Fast and Sensitive HPLC-ESI-MS/MS Method for Etoricoxib Quantification in Human Plasma and Application to Bioequivalence Study. Molecules 2022, 27, 5706. [Google Scholar]
- Agrawal, N.G.P.; Matthews, C.Z.; Mazenko, R.S.; Kline, W.F.; Woolf, E.J.; Porras, A.G.; Geer, L.A.; Wong, P.H.; Cho, M.; Cote, J.; et al. Pharmacokinetics of Etoricoxib in Patients with Renal Impairment. J. Clin. Pharmacol. 2004, 44, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Contreras, A.; Cervantes, J.V.M.; Collantes-Estevez, E. Update on the clinical pharmacology of etoricoxib, a potent cyclooxygenase-2 inhibitor. Fut. Rheumatol. 2007, 2, 545–565. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Verma, A.; Ghosh, A.K.; Mishra, A.K. A simple ultraviolet spectrophotometric method for the determination of etoricoxib in dosage formulations. J. Adv. Pharm. Technol. Res. 2012, 3, 237–240. [Google Scholar]
- Chandrasekhar, T.G.; Mathrusri, M. Validated RP-HPLC Method for the Assay of Etoricoxib (A Non-Steroidal Anti-Inflammatory Drug) in Pharmaceutical Dosage Forms. J. Chem. 2012, 9, 832–838. [Google Scholar]
- Prajapati, M.S.; Yamgar, D.B.; Desale, M.N.; Fegade, B. A Review on Various Analytical Methodologies for Etoricoxib. Adv. J. Grad. Res. 2022, 11, 61–70. [Google Scholar] [CrossRef]
- Kole, P.L.; Venkatesh, G.; Kotecha, J.; Sheshala, R. Recent advances in sample preparation techniques for effective bioanalytical methods. Biomed. Chromatogr. 2011, 25, 199–217. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Ingle, R.G.; Zeng, S.; Jiang, H.; Fang, W.J. Current developments of bioanalytical sample preparation techniques in pharmaceuticals. J. Pharm. Anal. 2022, 12, 517–529. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Vaghela, A.; Patel, A.; Patel, A.; Vyas, A.; Patel, N. Sample Preparation In Bioanalysis: A Review. Int. J. Sci. Technol. Res. 2016, 5, 6–10. [Google Scholar]
- Lee, J.; Lee, H.K.; Rasmussen, K.E.; Pedersen-Bjergaard, S. Environmental and bioanalytical applications of hollow fiber membrane liquid-phase microextraction: A review. Anal. Chim. Acta 2008, 624, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Nazyropoulou, C.; Samanidou, V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 2015, 7, 2241–2250. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; A Zachariadis, G. Green bioanalytical sample preparation: Fabric phase sorptive extraction. Bioanalysis 2021, 13, 693–710. [Google Scholar] [CrossRef]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric phase sorptive extraction explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Kabir, A.; Samanidou, V. Fabric Phase Sorptive Extraction: A Paradigm Shift Approach in Analytical and Bioanalytical Sample Preparation. Molecules 2021, 26, 865. [Google Scholar] [CrossRef]
- Manousi, N.; Alampanos, V.; Ferracane, A.; Efstratiadis, G.; Kabir, A.; Furton, K.G.; Tranchida, P.Q.; Zachariadis, G.A.; Mondello, L.; Rosenberg, E.; et al. Magnet integrated fabric phase sorptive extraction as a stand-alone extraction device for the monitoring of benzoyl urea insecticides in water samples by HPLC-DAD. J. Chromatogr. A 2022, 1672, 463026. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA) M10. Bioanalytical Method Validation and Study Sample Analysis; ICH Harmonised Guideline: Geneva, Switzerland, 2022. [Google Scholar]
- Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. [Google Scholar] [CrossRef]
- Korpeti, A.; Manousi, N.; Kabir, A.; Furton, K.G.; Tzanavaras, P.D.; Zacharis, C.K. Investigating the applicability of polar fabric phase sorptive extraction for the HPLC quantitation of salivary vitamin B12 following administration of sublingual tablets and oral sprays. Talanta 2023, 258, 124482. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V.F. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Matthews, C.Z.; Woolf, E.J.; Lin, L.; Fang, W.; Hsieh, J.; Ha, S.; Simpson, R.; Matuszewski, B.K. High-throughput, semi-automated determination of a cyclooxygenase II inhibitor in human plasma and urine using solid-phase extraction in the 96-well format and high-performance liquid chromatography with post-column photochemical derivatization-fluorescen. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Subhahar, M.B.; Singh, J.; Albert, P.H.; Kadry, A.M. Detection and Pharmacokinetics of Etoricoxib in Thoroughbred Horses. J. Equine Vet. Sci. 2020, 88, 102942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).