Abstract
The rapid isolation of target constituents from natural products poses a significant challenge and is a key focus in current research. The Hosta plantaginea flower (HPF), a traditional Chinese medicinal herb, is primarily used to treat inflammatory diseases, with kaempferol as one of its major bioactive constituents. In this study, macroporous adsorption resin was used to purify total flavonoids (TF) from the HPFs. The 50% ethanol–water elution fraction of the TF was then recrystallized to yield kaempferol with a purity of 99.44%. Network pharmacology analysis identified 61 potential kaempferol-inflammation targets, which were linked to the PI3K-Akt and TNF signaling pathways. Molecular docking and molecular dynamics simulations revealed the stability and binding of kaempferol to PI3K, Akt, and TNF-α proteins. The analysis metrics included binding ability, the root mean square deviation (RMSD), radius of gyration, free energy landscape, solvent-accessible surface area, hydrogen bond count, RMS fluctuation, free binding energy, amino acid residue free energy decomposition, and principal component analysis. The anti-inflammatory mechanism of kaempferol was further validated in an LPS-induced RAW264.7 cell model, where it was shown to inhibit the PI3K-Akt and TNF-α signaling pathways. This study provides new insights into the anti-inflammatory mechanism of kaempferol and presents novel strategies for the rapid isolation of target constituents from natural products.
1. Introduction
The Hosta flower, the dried flower of Hosta plantaginea (Lam.) Aschers (HPF) in the Hostaceae family, is a traditional Chinese medicinal herb [1]. These flowers are primarily used to treat various inflammatory diseases [2,3]. Previous studies have identified kaempferol and its derivates as the major chemical constituents of Hosta flowers, demonstrating their pharmacological activities, including anti-inflammatory and antioxidant effects [3,4].
Traditional separation and purification methods have significantly advanced chemical research on natural products. However, these methods are time-consuming, labor-intensive, and prone to overlooking low-concentration bioactive constituents [5,6]. Therefore, the rapid isolation of target compounds has become a central focus in natural product research. Kaempferol, a flavonoid constituent, is primarily enriched using macroporous adsorption resin, which has been shown to have effective separation and purification results [7,8].
Network pharmacology is a widely used approach for elucidating the potential mechanisms of action for phytochemicals [9,10]. It has been extensively applied to screen targets for natural products, traditional Chinese medicines, and their active components. Additionally, molecular docking and molecular dynamics simulations are commonly used to investigate the interactions between small molecules and target proteins, offering insights into their binding affinities [11].
This study utilized a two-step method, involving macroporous adsorption resin and recrystallization, to rapidly obtain high-purity kaempferol from the HPF. Network pharmacology was subsequently used to predict the key targets and potential mechanisms underlying the anti-inflammatory effects of kaempferol. Finally, molecular docking, molecular dynamics simulations, and an LPS-induced RAW264.7 cell model were used to validate the anti-inflammatory effects and mechanisms of kaempferol. These findings provide scientific evidence for the anti-inflammatory mechanism of kaempferol and offer new insights and strategies for the rapid isolation of target compounds from natural products.
2. Materials and Methods
2.1. Chemicals and Materials
Analytical grade ethanol and chromatographic grade acetonitrile were purchased from TEDIA Company (Fairfield, OH, USA). Water was prepared in the laboratory. The culture medium for RAW264.7 cells was purchased from Haixing Biotechnology Co., Ltd (Guangzhou, China). Nitric oxide (NO) and CCK-8 assay kits were obtained from Beyotime Biotechnology Co., Ltd. (Shanghai, China) and Dalian Boglin Biotechnology Co., Ltd. (Dalian, China), respectively.
The p85 antibody, along with its phosphorylated forms, p-p85 and p-Akt, were both purchased from Huaan Biotechnology Co., Ltd. (Hangzhou, China). The Akt antibody was obtained from Cell Signaling Technology, while the TNF-α and GAPDH antibodies were sourced from Proteintech Group. The dilution ratios for the p85, p-p85, Akt, p-Akt, and TNF-α antibodies were 1:1000, whereas the dilution ratio for the GAPDH antibody was 1:10,000.
2.2. The Separation of Kaempferol from HPF
The HPFs were collected from Anguo City, Hebei Province (China), which has been described in our previous work [1].
A multi-factorial experimental design and response surface methodology were employed to optimize the extraction process of total flavonoids (TF) from the HPFs, and the extraction rate was 65.98% [2]. In this study, macroporous adsorption resin (D101) was used to purify the TF, which was then eluted in a gradient of water, 40%, 50%, and 60% ethanol–water, and ethanol. The 50% ethanol–water elution fraction was recrystallized with methanol to obtain kaempferol.
2.3. Comparative Analysis of TF, Its Fractions, and Kaempferol
The TF and its fractions were analyzed via high-performance liquid chromatography (HPLC). The chromatographic conditions were as follows: mobile phase A, 0.1% formic acid in water; mobile phase B, 0.1% formic acid in acetonitrile. The gradient program was: 10–26% B (0–30 min), 26–30% B (30–35 min), 30–40% B (35–40 min), 40–65% B (40–48 min), 65–100% B (48–52 min), and 100–100% B (52–60 min). The flow rate was set to 1 mL/min, the column temperature to 30 °C, the injection volume to 10 μL, and the detection wavelength to 254 nm.
The purity of the kaempferol was also determined using HPLC under the following conditions: 38% acetonitrile–0.1% formic acid in water as the mobile phase, a flow rate of 1 mL/min, column temperature of 30 °C, an injection volume of 10 μL, and detection at 367 nm.
HPLC analyses were conducted using a Shimadzu LC-20ADXR system equipped with a Shimadzu InertSustain C18 column (5 μm, 4.6 × 250 mm).
2.4. Network Pharmacology
Kaempferol target identification was performed using the SwissTarget, STITCH, PharmMapper, and SuperPred databases, while inflammation-related targets were sourced from the GeneCard, DisGenet, OMIM, and DrugBank databases. A protein–protein interaction (PPI) network was constructed based on the common targets shared by kaempferol and inflammation. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were then performed.
2.5. Molecular Docking
The PDB IDs for PI3K, Akt, and TNF-α were 7OI4, 3OW3, and 7KP7, respectively, and all were derived from murine sources. In the molecular docking experiment, which was performed using MOE2019, a total of 30 conformations were obtained. The binding pocket was predicted using MOE, and conformational sampling and scoring were conducted using the induced fit method. The first round of scoring employed the London dG function, while the second round used the GBVI/WSA dG function. Conformations were ranked according to their docking scores, and the top 10 conformations were selected. These conformations were further selected based on their binding sites. Interaction diagrams were generated using the Discovery Studio software (version 4.5, BIOVIA, Paris, France), and schematic diagrams were created using PyMOL (https://www.pymol.org/).
2.6. Molecular Dynamics (MD) Simulation
The Gromacs2022 was selected as the MD simulation software. The Amber99sb-ildn force field was applied to the proteins, and the GAFF force field to the small molecules. The TIP3P model was employed to add water molecules to the system, and a water box of size 10 × 10 × 10 nm3 was constructed, ensuring a minimum distance of 1.2 nm between the protein and the box edge. Ions were added to automatically balance the system, including 115,715, 32,758, and 32,031 water molecules, along with 7, 0, and 7 sodium ions for PI3K, Akt, and TNF-α, respectively. Electrostatic interactions were handled using the Particle–Mesh Ewald, and energy minimization was carried out using the steepest descent method with a maximum of 50,000 steps. In addition, the binding free energy simulated by molecular dynamics was calculated using GMX_MMPBSA. The Coulombic and van der Waals cutoff distances were both set to 1 nm. The system was equilibrated under the canonical ensemble and isothermal–isobaric ensemble conditions. Finally, a 100 ns MD simulation was performed at a constant temperature and pressure (300 K and 1 bar, respectively). The nonbonded interaction cutoff was set to 10 Å, and the temperature was controlled using the Langevin thermostat and the pressure was controlled using the Berendsen method.
2.7. In Vitro Anti-Inflammatory Mechanism
2.7.1. Cell Viability
RAW264.7 cells (Suzhou Starfish Biotechnology Co., Ltd., Suzhou, China) were seeded at a density of 5 × 105 cells per milliliter of culture medium. Lipopolysaccharides (LPSs) were applied at a concentration of 1 μg/mL, while the kaempferol was tested at concentrations of 80, 40, 20, 10, 5, and 2.5 μM. Eight experimental groups were included: a control group, a model group, and six treatment groups (model + kaempferol). Each group was tested in triplicate.
2.7.2. Nitric Oxide (NO) Determination
NO levels were determined using an NO assay kit, as previously described by our team [12]. The kaempferol concentrations were 80, 40, 20, 10, 5, and 2.5 μM.
2.7.3. Western Blotting Assay
Western blotting assay was performed following the protocols previously established by our group [13]. The kaempferol concentrations were 20 and 10 μM. Protein expression levels were analyzed using the Image Lab 6.1 software. The relative protein expression was calculated as the ratio of the target protein’s grayscale value to that of the reference protein.
2.8. Statistical Analysis
Experimental data are presented as mean ± SD (n = 3). All data were analyzed and plotted using the GraphPad Prism 8.0.1 software. Differences between the groups were assessed using one-way analysis of variance (ANOVA). A p-value of <0.05 was considered statistically significant.
3. Results and Discussion
3.1. The Purity and Identification of Kaempferol
Figure 1A shows the separation of TF (200.0 g, from 303.1 g HPFs) using macroporous adsorption resin D101, followed by elution with water, 40% ethanol–water, 50% ethanol–water, 60% ethanol–water, and ethanol. The kaempferol was enriched in the 50% and 60% ethanol–water fractions. The 50% ethanol–water fraction (59.3 mg) was then recrystallized with methanol as the solvent, yielding kaempferol (7.4 mg) with a purity of 99.44%, as shown in Figure 1B. The structure of the kaempferol was identified via nuclear magnetic resonance, which was consistent with our previously data [4]. Furthermore, the isolated kaempferol was also validated by comparison with reference substance using the HPLC method, including retention time and UV absorption characteristics.

Figure 1.
HPLC comparison of TF and its five eluted fractions with kaempferol. (A) HPLC comparison of TF and its fractions, (B) The purity of kaempferol.
3.2. The Results of Network Pharmacology
3.2.1. Screening of the Kaempferol and Inflammation-Related Targets
SwissTarget, STITCH, PharmMapper, and SuperPred are commonly used public databases for querying small molecule target genes [14,15,16]. In this work, a search of these databases identified 100, 10, 158, and 6 kaempferol-related genes, respectively (Figure 2A). In addition, there are 9 common genes among the four databases. Therefore, the total number of genes is 265 (Figure 2C).
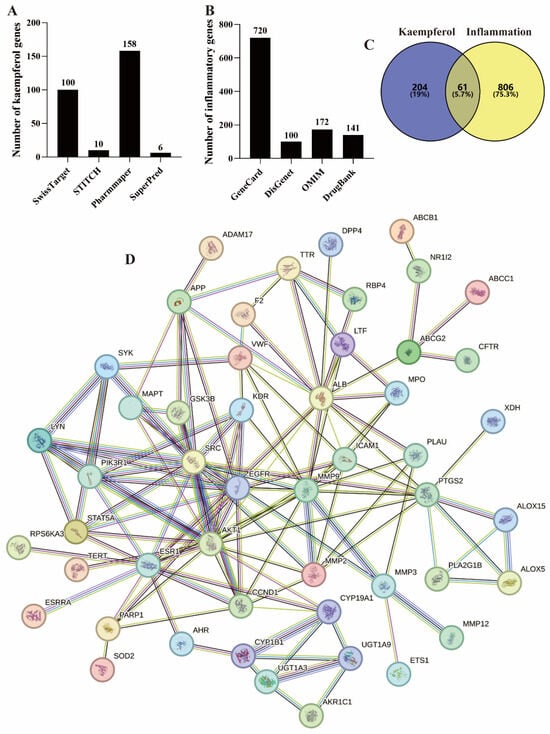
Figure 2.
Targets screening of kaempferol–inflammation. (A) Kaempferol’s targets, (B) Inflammation–related targets, (C) The intersecting targets of kaempferol-inflammation, (D) The PPI network of these intersecting targets.
Meanwhile, GeneCard, DisGenet, OMIM, and DrugBank are frequently used for screening disease-related genes [14,15,16,17,18]. From these databases, a total of 16,182, 134, 172 (Figure 2B), and 141 (Figure 2B) inflammation-related genes were identified, respectively. Specifically, GeneCard and DisGenet applied thresholds of Relevance score > 5 [16] and a score greater than the average [17], identifying 720 and 100 genes, respectively (Figure 2B).
The 265 kaempferol-related genes and 867 inflammation-related genes were imported into the Venn diagram tool, resulting in 61 intersecting genes, as shown in Figure 2C and Table 1. Among these, 50 genes exhibited a node degree > 0.

Table 1.
Sixty-one overlapping genes between kaempferol and inflammation.
3.2.2. Construction of the Protein–Protein Interaction (PPI) Network
A confidence threshold greater than 0.7 is considered high confidence for constructing PPI networks and is commonly used in network pharmacology studies [18]. The 50 intersecting kaempferol–inflammation genes were imported into STRING with a confidence threshold of >0.7 to construct the PPI network, as shown in Figure 2D.
3.2.3. GO and KEGG Enrichment Analysis
GO functional analysis and KEGG pathway enrichment analysis are widely used bioinformatics methods to explore gene functions [14,15]. GO analysis includes three functional categories: cellular component (CC), molecular function (MF), and biological process (BP), while KEGG analysis focuses on identifying related signaling pathways. In the present study, the 50 intersecting kaempferol–inflammation genes underwent GO and KEGG analysis using the DAVID database. A p-value threshold of <0.05 was applied, resulting in the identification of 173 BP terms, 30 CC terms, and 66 MF terms, as well as 86 KEGG signaling pathways. The top 10 BP, CC, and MF terms are presented in Figure 3A.
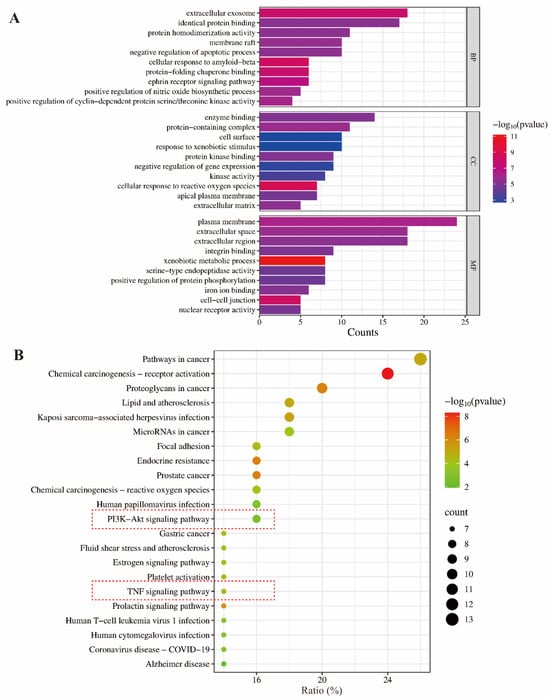
Figure 3.
GO (A) and KEGG (B) enrichment analysis of the intersection targets of kaempferol–inflammation.
As shown in Figure 3B, the PI3K-Akt and TNF signaling pathways are the primary pathways involved in the anti-inflammatory effects of kaempferol, with counts of 8 (p = 0.001855) and 7 (p = 2.40 × 10⁻⁵), respectively. Meanwhile, the other entries in Figure 3B were not selected because they do not pertain to the inflammatory pathways.
3.3. The Results of Molecular Docking
The binding affinity of small molecules to proteins is characterized by their binding ability, with values below −5 kcal/mol indicating a strong binding ability [19,20,21]. As shown in Figure 4, kaempferol exhibits strong binding affinities for PI3K, Akt, and TNF-α, with binding free energies of −6.04, −6.44, and −5.65 kcal/mol, respectively. This suggests that the anti-inflammatory effect of kaempferol is related to the modulation of PI3K, Akt, and TNF-α proteins. In fact, molecular docking is mainly used to provide reasonable initial conformations for complex MD simulations, and the free binding energy given by MD simulations is more accurate than the affinity energy in molecular docking. Further analysis reveals that the interaction between kaempferol and PI3K is primarily mediated by hydrogen bonds with the amino acid residues LEU A:211, LYS A:682, and GLN A:300. Additionally, kaempferol forms hydrogen bonds with the VAK A:123 residue of Akt and with GLY C:148 and the ARG C:33 residues of TNF-α.
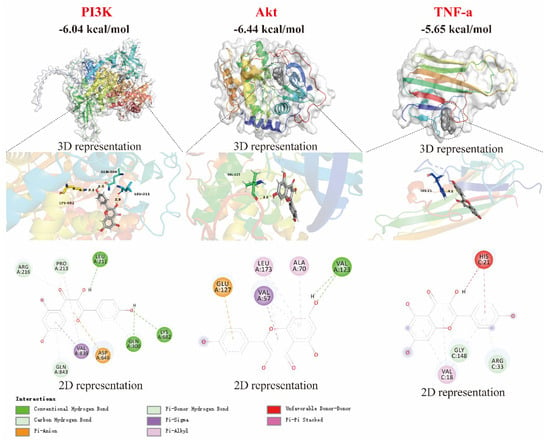
Figure 4.
Molecular docking of kaempferol with PI3K, Akt, and TNF-α proteins.
3.4. MD Simulation
MD simulation is a computational method used to study the motion of atoms and molecules within small molecule–protein ligand complexes [22,23,24]. It provides insights into the structural and macroscopic properties of these complexes. MD simulations are a key technique for assessing binding affinity and interaction sites between small molecules and protein ligands. Typical simulation times are usually 100 ns [22,23]. Root mean square deviation (RMSD) measures the deviation of atomic positions of a particular conformation from the target conformation. It is an important parameter for evaluating the stability of small molecule–protein ligand complexes. A ∆RMSD value less than 0.2 nm generally indicates a stable complex. As shown in Figure 5A, Figure 6A and Figure 7A, the ∆RMSD of kaempferol with PI3K, Akt, and TNF-α stabilizes after 20 ns, remaining below 0.2 nm. This suggests that the complex of kaempferol with PI3K, Akt, and TNF-α maintains stable conformation.

Figure 5.
MD simulation of kaempferol with PI3K protein. (A) RMSD, (B) Rg, (C) FEL, (D) SASA, (E) The number of hydrogen bond, (F) RMSF, (G) Binding free energy, (H) Binding free energy of amino acid residues, (I) PCA, (J) Snapshots from MD simulations.
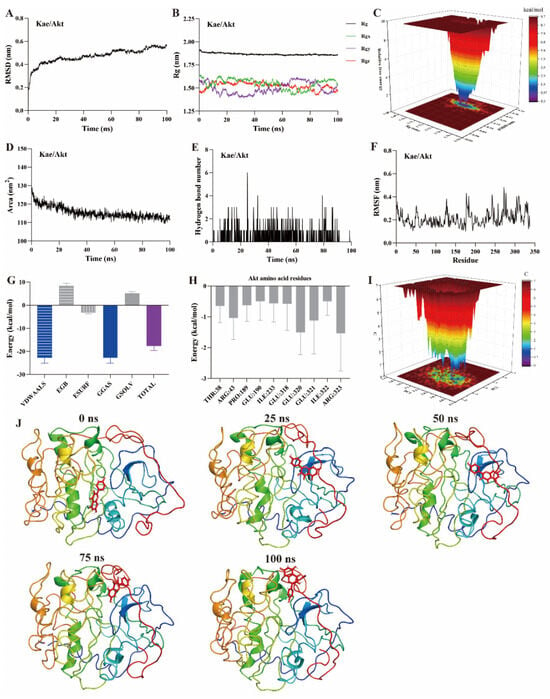
Figure 6.
MD simulation of kaempferol with Akt protein. (A) RMSD, (B) Rg, (C) FEL, (D) SASA, (E) The number of hydrogen bond, (F) RMSF, (G) Binding free energy, (H) Binding free energy of amino acid residues, (I) PCA, (J) Snapshots from MD simulations.
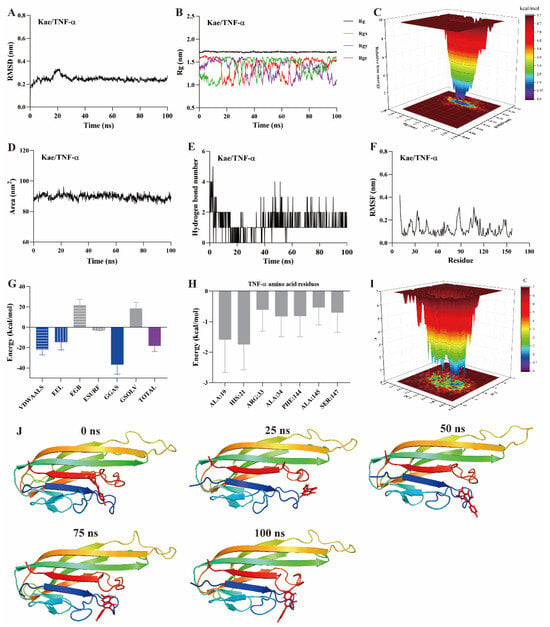
Figure 7.
MD simulation of kaempferol with TNF-α protein. (A) RMSD, (B) Rg, (C) FEL, (D) SASA, (E) The number of hydrogen bond, (F) RMSF, (G) Binding free energy, (H) Binding free energy of amino acid residues, (I) PCA, (J) Snapshots from MD simulations.
The radius of gyration (Rg) represents the average squared distance between the mass center of all atoms in a protein and its geometric center. It is a key parameter for assessing the compactness of protein structures. In this study, the Rg values for the kaempferol complexes with PI3K (Figure 5B), Akt (Figure 6B), and TNF-α (Figure 7B) remained stable, fluctuating at approximately 3.3, 1.9, and 1.7, respectively. Free energy landscapes (FEL) are graphical representations that describe changes in free energy between different conformations or states of the small molecule–protein ligand complex. The FEL plots are constructed using RMSD and Rg values as coordinates, where the blue regions correspond to the stable conformations with the lowest free energy. As shown in Figure 5C, Figure 6C, and Figure 7C, the plots reveal a region with a local free energy minimum, indicating that the kaempferol complexes with PI3K, Akt, and TNF-α maintain high stability during the 100 ns simulation.
The solvent-accessible surface area (SASA) measures the proportion of a protein accessible to a solvent, correlating with the molecular solvation free energy. SASA is used to predict the extent of conformational changes occurring during small molecule–protein interactions. In this study, the SASA values for kaempferol in complex with PI3K (Figure 5D), Akt (Figure 6D), and TNF-α (Figure 7D) ranged from 500–550, 110–120, and 80–90 nm2, respectively. These values suggest that the conformational fluctuations of kaempferol in complex with these proteins are relatively small, particularly for Akt and TNF-α.
Hydrogen bonds represent the interactions between small molecules and protein ligands. The number and nature of these bonds directly influence the stability of the complex. An increase in the number of hydrogen bonds typically indicates stronger interactions between the small molecule and the protein, thereby enhancing the stability of the complex. As shown in Figure 5E, Figure 6E, and Figure 7E, kaempferol forms multiple hydrogen bonds with PI3K, Akt, and TNF-α. The number of hydrogen bonds ranged from 2–4, 1–2, and 1–2, respectively.
Further analysis was performed on root mean square fluctuations (RMSF), free binding energy, amino acid residue free energy decomposition, and principal component analysis (PCA). RMSF takes the initial structure simulated by molecular dynamics simulation as the reference structure and measures the fluctuation degree of amino acids in the protein during the MD simulation process, reflecting local dynamic behavior. It is commonly used to assess protein flexibility, particularly for side-chain atoms. The amino acid residue free energy decomposition reflects the contribution of each amino acid residue to the binding of the small molecule to the protein. As illustrated in Figure 5F–I, Figure 6F–I, and Figure 7F–I, multiple amino acid residues contribute to the formation of the kaempferol complexes with PI3K, Akt, and TNF-α. However, the contribution of each amino acid residue to the stability of the complex differs. Additionally, the lowest energy conformation of kaempferol binding to PI3K, Akt, and TNF-α proteins can be known through the PCA diagram. Finally, molecular docking images of kaempferol with PI3K, Akt, and TNF-α at 0, 25, 50, 75, and 100 ns time points are shown in Figure 5J, Figure 6J, and Figure 7J, suggesting that kaempferol is always located within the active site of PI3K, Akt, and TNF-α proteins within 100 ns.
Based on the above evidence, kaempferol forms stable complexes with PI3K, Akt, and TNF-α, suggesting strong interactions occur between kaempferol and these proteins. These interactions suggest that kaempferol may target these proteins, which are potential mediators of its anti-inflammatory effects.
3.5. The Anti-Inflammatory Mechanism of Kaempferol In Vitro
To further investigate the anti-inflammatory mechanism of kaempferol, this study employed the classic lipopolysaccharide (LPS) (1 μg/mL)-induced RAW264.7 cell model [25,26,27].
The effect of kaempferol on the viability of LPS-induced RAW264.7 cells was first evaluated at concentrations ranging from 80 μM to 2.5 μM. The results showed that kaempferol did not induce cytotoxicity within this concentration range (Figure 8A). The NO assay kit was then used to evaluate the inhibitory effect of kaempferol on excessive NO release at these concentrations. The kaempferol significantly suppressed excessive NO release at all tested concentrations (p < 0.001, Figure 8B). A dose-response curve for NO inhibition was plotted using the Prism software, yielding an IC50 value of 11.04 μM (Figure 8C).
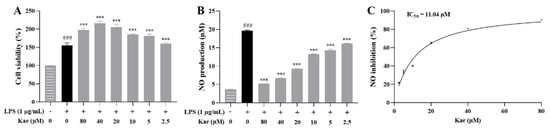
Figure 8.
Anti-inflammatory effect of kaempferol on LPS-induced RAW264.7 cell model. (A) Cell viability, (B) NO production, (C) NO inhibition curve. ### p < 0.001 vs. Control; *** p < 0.001 vs. Model.
Western blotting was performed to examine the effects of the kaempferol at 10 μM and 20 μM on the expression of p85, p-p85, Akt, p-Akt, and TNF-α in the LPS-induced RAW264.7 cell model. Compared to the control group, the LPS treatment significantly increased the expression of p-p85, p-Akt, and TNF-α (p < 0.001, Figure 9), indicating that LPS stimulation induced an exaggerated inflammatory response in the RAW264.7 cells, consistent with previous reports [12]. Treatment with the kaempferol (10 and 20 μM) remarkedly suppressed the elevated expression of these proteins (p < 0.05 or p < 0.001, Figure 9), further suggesting that kaempferol exhibits an anti-inflammatory effect, likely through the inhibition of the PI3K-Akt and TNF-α signaling pathways.
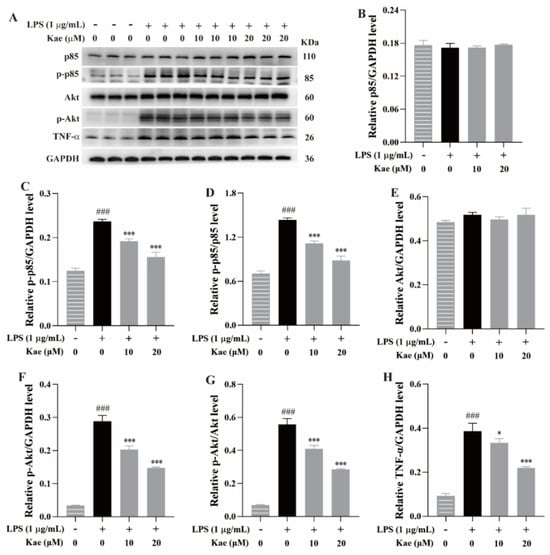
Figure 9.
Anti-inflammatory mechanism of kaempferol on PI3K, Akt, and TNF-α signaling pathways in LPS-induced RAW264.7 cell model. (A) Protein bands, (B) p85/GAPDH level, (C) p-p85/GAPDH level, (D) p-p85/p85 level, (E) Akt/GAPDH level, (F) p-Akt/GAPDH level, (G) p-Akt/Akt level, (H) TNF-α/GAPDH level. ### p < 0.001 vs. Control; *** p < 0.001 and * p < 0.05 vs. Model.
The findings of this study provide scientific evidence supporting the potential of kaempferol in treating inflammatory diseases and offer valuable insights into its mechanism of action [28]. However, three limitations remain: (1) MD simulations require multiple repetitions; (2) further anti-inflammatory studies using additional models are required, such as mice and zebrafish; and (3) more in-depth investigations into its molecular mechanisms are necessary.
4. Conclusions
This study is the first to use a two-step method to rapidly isolate kaempferol from HPF, achieving a purity of 99.44%. By integrating network pharmacology, molecular docking, molecular dynamics simulations, and the LPS-induced RAW264.7 cell model, we elucidated the anti-inflammatory mechanism of kaempferol through the inhibition of the PI3K-Akt and TNF-α signaling pathways. These findings provide new insights into the anti-inflammatory mechanisms of kaempferol and present a novel approach for the rapid isolation of active natural product components.
Author Contributions
Conceptualization, L.Y. and J.H.; methodology, Y.Y., B.X., and H.O.; software, J.H., H.O., and J.G.; validation, Y.Y., B.X., J.G., and Q.H.; formal analysis, Y.Y., J.G., and Q.H.; investigation, J.H.; resources, J.H.; data curation, Y.Y., B.X., H.O., J.G., and Q.H.; writing—original draft preparation, L.Y.; writing—review and editing, J.H.; visualization, L.Y.; supervision, J.H.; project administration, L.Y.; funding acquisition, L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangxi Chinese Medicine Young and Middle-aged Talents Project (for Li Yang) and 2024 Annual Project for Practical Research and Innovation Training in Traditional Chinese Medicine at Jiangxi University of Chinese Medicine (No. 24KYCX-YB048 and 24KYCX-YB061).
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, H.L.; Mu, Z.Q.; Liang, J.; Li, X.M.; Yang, L.; He, J.W. Hosta plantaginea (Lam.) Aschers flower modulates inflammation and amino acid metabolism by inhibiting NF-κB/MAPK/JAK-STAT/PI3K-Akt and AMPK pathways to alleviate benign prostatic hyperplasia in rats. J. Ethnopharmacol. 2025, 337, 118970. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, F.X.; He, W.W.; Zhao, B.Y.; Zhang, T.; Wang, S.; Zhou, L.F.; He, J.W. Extraction optimization and constituent analysis of total flavonoid from Hosta plantaginea (Lam.) Aschers flowers and its ameliorative effect on chronic prostatitis via inhibition of multiple inflammatory pathways in rats. J. Ethnopharmacol. 2024, 318, 116922. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.X.; Chang, A.K.; Wang, Z.N.; Su, W.P.; Li, Y.N.; Liu, W.B.; Ai, J.; Tao, X.; Zheng, P.Y.; et al. Potential active constituents responsible for treating acute pharyngitis in the flowers of Hosta plantaginea (Lam.) Aschers and their pharmacokinetics. Food Funct. 2022, 13, 3308–3317. [Google Scholar] [CrossRef]
- He, J.W.; Yang, L.; Mu, Z.Q.; Zhu, Y.Y.; Zhong, G.Y.; Liu, Z.Y.; Zhou, Q.G.; Cheng, F. Anti-inflammatory and antioxidant activities of flavonoids from the flowers of Hosta plantaginea. RSC Adv. 2018, 8, 18175–18179. [Google Scholar] [CrossRef]
- Shuai, E.; Xiao, S.L.; Huang, J.; Zeng, Z.H.; Liu, S.Q.; Tan, J.J.; Zhang, H.; Cai, W. Screening of anti-inflammatory active components in Sabia schumanniana Diels by affinity ultrafiltration and UHPLC-Q-Exactive Orbitrap mass spectrometry. J. Ethnopharmacol. 2025, 337, 118845. [Google Scholar] [CrossRef]
- Hu, L.H.; Luo, J.M.; Wen, G.Q.; Sun, L.L.; Liu, W.; Hu, H.; Li, J.; Wang, L.S.; Su, W.W.; Lin, L.Z. Identification of the active compounds in the Yi-Fei-San-Jie formula using a comprehensive strategy based on cell extraction/UPLC-MS/MS, network pharmacology, and molecular biology techniques. Phytomedicine 2023, 115, 154843. [Google Scholar] [CrossRef]
- Li, S.R.; Wang, S.W.; Zhang, L.; Ka, Y.X.; Zhou, M.J.; Wang, Y.W.; Tang, Z.; Zhang, J.M.; Wang, W.; Liu, W. Research progress on pharmacokinetics, anti-inflammatory and immunomodulatory effects of kaempferol. Int. Immunopharmacol. 2025, 152, 114387. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, S.Q.; Li, L.Y.; Gai, Q.Y. Deep eutectic solvent-based microwave-assisted extraction for the extraction of seven main flavonoids from Ribes mandshuricum (Maxim.) Kom. leaves. Separations 2023, 10, 191. [Google Scholar] [CrossRef]
- Zhao, W.B.; Wang, B.Y.; Li, S. Network pharmacology for traditional Chinese medicine in era of artificial intelligence. Chin. Herb Med. 2024, 16, 558–560. [Google Scholar] [CrossRef]
- Xu, Y.L.; Bao, L.; Zhao, R.H.; Geng, Z.H.; Li, S.R.; Pang, B.; Sun, Q.Y.; Guo, S.S.; Cui, X.L.; Sun, J. Mechanisms of Shufeng Jiedu capsule in treating bacterial pneumonia based on network pharmacology and experimental verification. Chin. Herb Med. 2024, 16, 656–666. [Google Scholar] [CrossRef]
- Liang, J.; Dai, W.G.; Liu, C.H.; Wen, Y.F.; Chen, C.; Xu, Y.F.; Huang, S.; Hou, S.Z.; Li, C.; Chen, Y.M.; et al. Gingerenone A attenuates ulcerative colitis via targeting IL-17RA to inhibit inflammation and restore intestinal barrier function. Adv. Sci. 2024, 11, 2400206. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.W.; Yang, L.; He, J.W. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-κB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 143, 112104. [Google Scholar] [CrossRef]
- He, J.W.; Zhang, Q.C.; Xia, X.Y.; Yang, L. Lagopsis supina ameliorates myocardial ischemia injury by regulating angiogenesis, thrombosis, inflammation, and energy metabolism through VEGF, ROS and HMGB1 signaling pathways in rats. Phytomedicine 2023, 120, 155050. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Shi, R.J.; Yan, S.H.; Zhang, S.B.; Lu, B.; Huang, Z.L.; Ji, L.L. Integrating network pharmacology, experimental validation and molecular docking to reveal the alleviation of Yinhuang granule on idiopathic pulmonary fibrosis. Phytomedicine 2024, 128, 155368. [Google Scholar] [CrossRef]
- Wang, C.; Fu, R.J.; Xu, D.Q.; Zuo, Q.; Liu, J.P.; Tang, Y.P. A study integrated metabolomics and network pharmacology to investigate the effects of Shicao in alleviating acute liver injury. J. Ethnopharmacol. 2024, 319, 117369. [Google Scholar] [CrossRef]
- Tu, Y.B.; Wang, K.; Tan, L.H.; Han, B.; Hu, Y.J.; Ding, H.; He, C.W. Dolichosin A, a coumestan isolated from Glycine tabacina, inhibits IL-1β-induced inflammation in SW982 human synovial cells and suppresses RANKL-induced osteoclastogenesis: From network pharmacology to experimental pharmacology. J. Ethnopharmacol. 2020, 258, 112855. [Google Scholar] [CrossRef]
- Huang, X.J.; Wang, J.; Muhammad, A.; Tong, H.Y.; Wang, D.G.; Li, J.; Ihsan, A.; Yang, G.Z. Systems pharmacology-based dissection of mechanisms of Tibetan medicinal compound Ruteng as an effective treatment for collagen-induced arthritis rats. J. Ethnopharmacol. 2021, 272, 113953. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Bian, Y.; Gu, J.F.; Yin, G.; Sun, R.L.; Liang, Y.; Wan, L.L.; Yin, Q.H.; Wang, X.; Gao, J.; et al. Exploring the anti-metastatic effects of Astragalus mongholicus Bunge-Curcuma aromatica Salisb. on colorectal cancer: A network-based metabolomics and pharmacology approach. Phytomedicine 2023, 114, 154772. [Google Scholar] [CrossRef]
- Zhang, J.W.; Han, M.T.; Wang, S.; Wu, R.X.; Zhao, Q.P.; Chen, M.H.; Yang, Y.M.; Zhang, J.; Meng, X.L.; Zhang, Y.; et al. Study on the anti-mitochondrial apoptosis mechanism of Erigeron breviscapus injection based on UPLC-Q-TOF-MS metabolomics and molecular docking in rats with cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2024, 319, 117310. [Google Scholar] [CrossRef]
- Zhai, M.Y.; Chen, T.X.; Shao, M.Q.; Yang, X.S.; Qi, Y.; Kong, S.; Jiang, L.J.; Yang, Q.P. Unveiling the molecular mechanisms of Haitang-Xiaoyin Mixture in psoriasis treatment based on bioinformatics, network pharmacology, machine learning, and molecular docking verification. Comput. Biol. Chem. 2025, 115, 108352. [Google Scholar] [CrossRef]
- Wu, D.J.; Hong, L.; Xu, S.Y.; Zhong, Z.; Gong, Q.Y.; Wang, Q.; Yan, L.J. Integrating network pharmacology and experimental validation via PPAR signaling to ameliorate rheumatoid arthritis: Insights from Corydalis Decumbentis Rhizoma (Xiatianwu). Fitoterapia 2025, 183, 106541. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Shen, Y.Q.; Qiu, F.B. Lycopene alleviates age-related cognitive deficit via activating liver-brain fibroblast growth factor-21 signaling. Redox Biol. 2024, 77, 103363. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, W.; Shi, Y.; Wang, L.; Khan, G.J.; Luom, M.M.; Zhou, J.; Yang, J.H.; He, C.H.; Li, F.; et al. Anti-colorectal cancer actions of Glycyrrhiza uralensis Fisch. and its underlying mechanism via HPLC integration and network pharmacological approaches. Phytomedicine 2025, 138, 156370. [Google Scholar] [CrossRef]
- Li, F.F.; Zhang, Z.X.; Shi, Q.Y.; Wang, R.B.; Ji, M.; Chen, X.G.; Li, Y.; Liu, Y.B.; Yu, S.S. Thermal proteome profiling (TPP) reveals NAMPT as the anti-glioma target of phenanthroindolizidine alkaloid PF403. Acta Pharm. Sin. B 2025, 15, 2008–2023. [Google Scholar] [CrossRef]
- Li, J.H.; Guo, X.W.; Luo, Z.L.; Wu, D.; Shi, X.; Xu, L.X.; Zhang, Q.; Xie, C.F.; Yang, C. Chemical constituents from the flowers of Inula japonica and their anti-inflammatory activity. J. Ethnopharmacol. 2024, 318, 117052. [Google Scholar] [CrossRef]
- Meng, W.S.; Sun, J.; Lu, Y.; Cao, T.T.; Chi, M.Y.; Gong, Z.P.; Li, Y.T.; Zheng, L.; Liu, T.; Huang, Y. Biancaea decapetala (Roth) O.Deg. extract exerts an anti-inflammatory effect by regulating the TNF/Akt/NF-κB pathway. Phytomedicine 2023, 119, 154983. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, j.h.; Shin, J.Y.; Kang, E.S.; Cho, B.O. Anti-inflammatory effects of Peucedanum japonicum Thunberg leaves extract in Lipopolysaccharide-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 309, 116362. [Google Scholar] [CrossRef]
- Herrera, T.E.S.; Tello, I.P.S.; Mustafa, M.A.; Jamil, N.Y.; Alaraj, M.; Altameem, K.K.A.; Alasheqi, M.Q.; Hamoody, A.M.; Alkhafaji, A.T.; Shakir, M.N.; et al. Kaempferol: Unveiling its anti-inflammatory properties for therapeutic innovation. Cytokines 2025, 186, 156846. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).