Preparation of Lithium Carbonate from Manganese-Containing Desorption Solution from Salt Lakes via an Organophosphoric Acid Extraction System
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Equipment
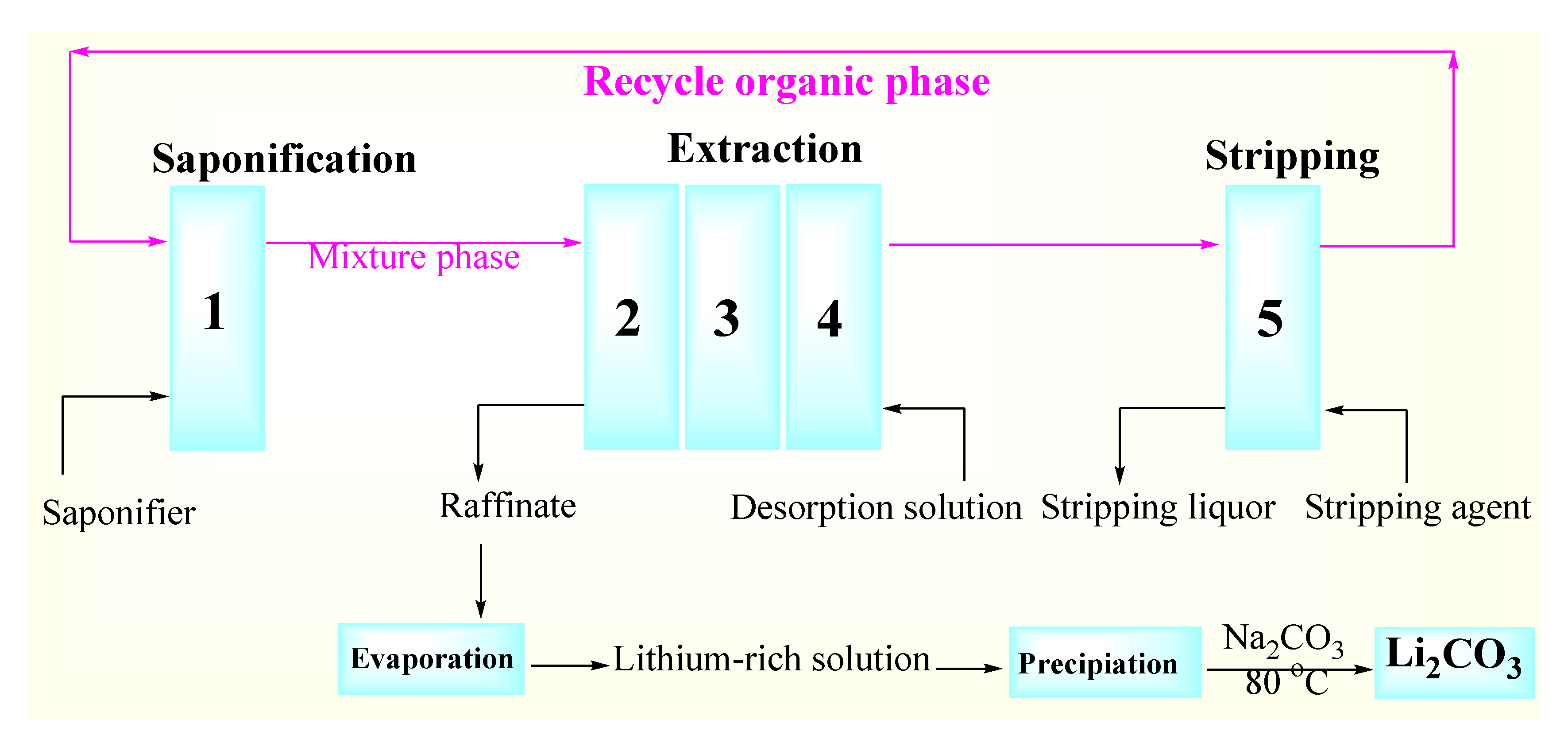
2.2. Experimental Procedure
2.3. Three-Stage Countercurrent Extraction Procedures
2.4. Analysis and Calculation
3. Results and Discussion
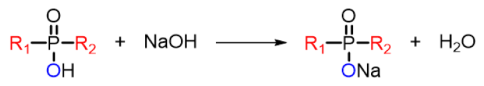
3.1. Effect of NaOH Concentration on Saponification Method
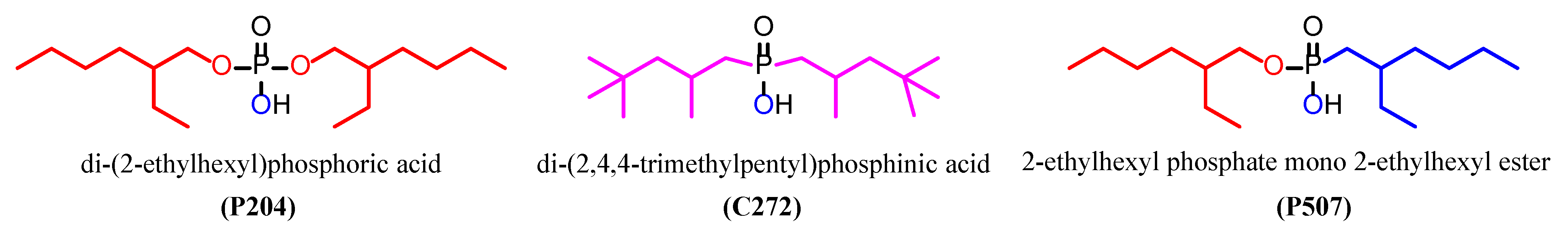
3.2. Screening of Organophosphorus Acid Extractants
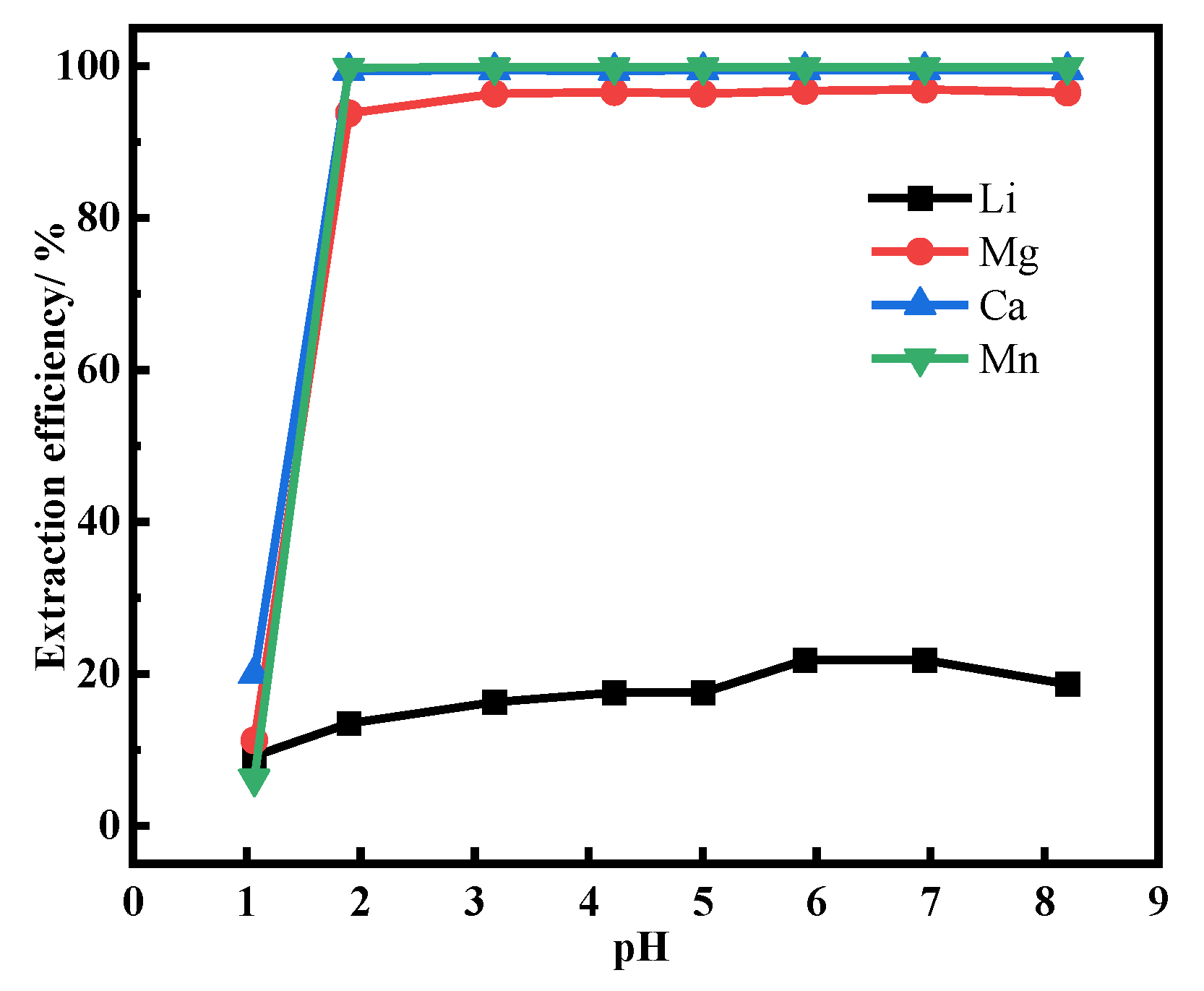
3.3. Effect of pH on Extraction Efficiency of Li+, Mg2+, Ca2+, and Mn2+ from Desorption Solution Using P204
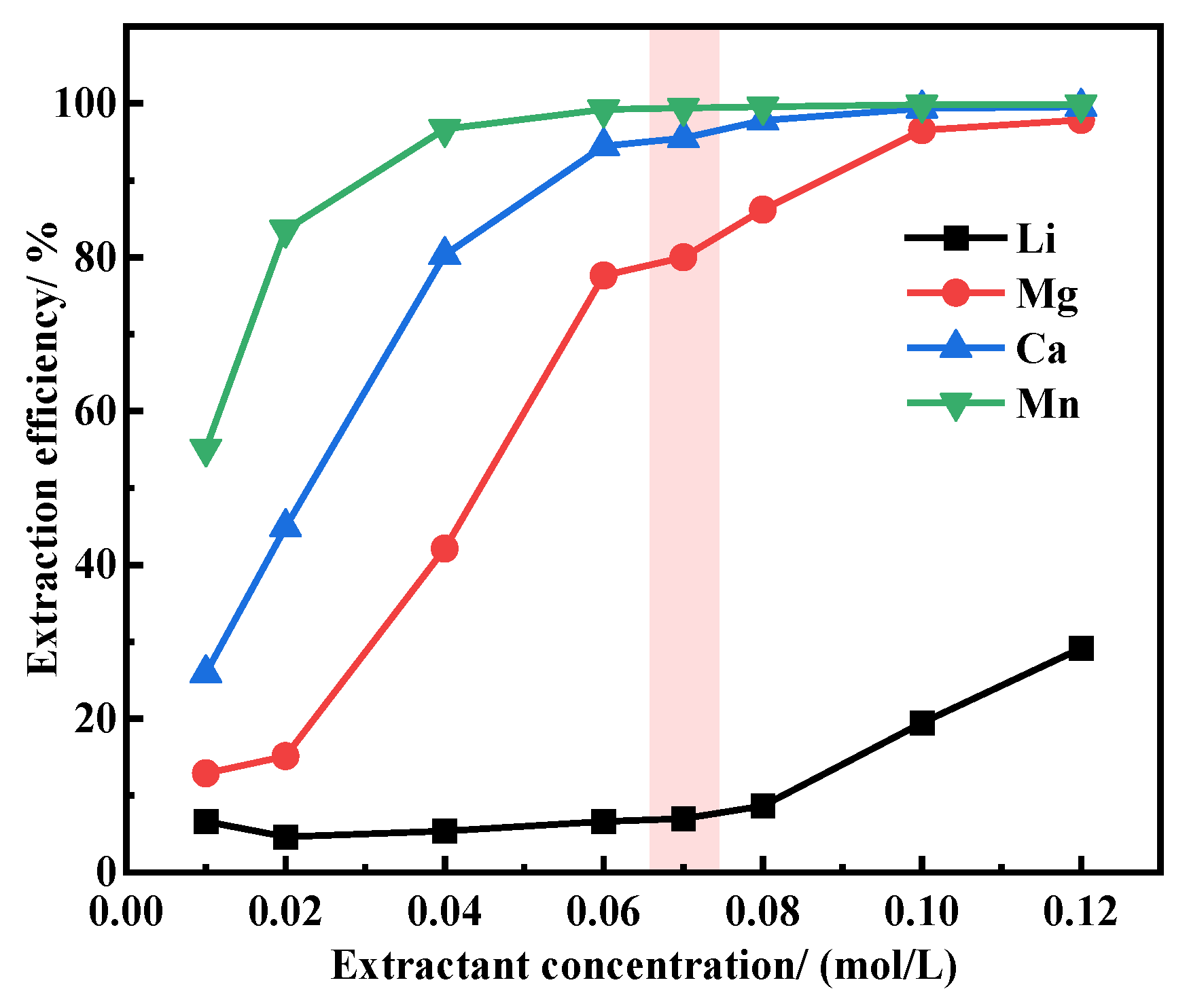
3.4. Determination of Extractant Concentration
3.5. Determination of Phase Ratio
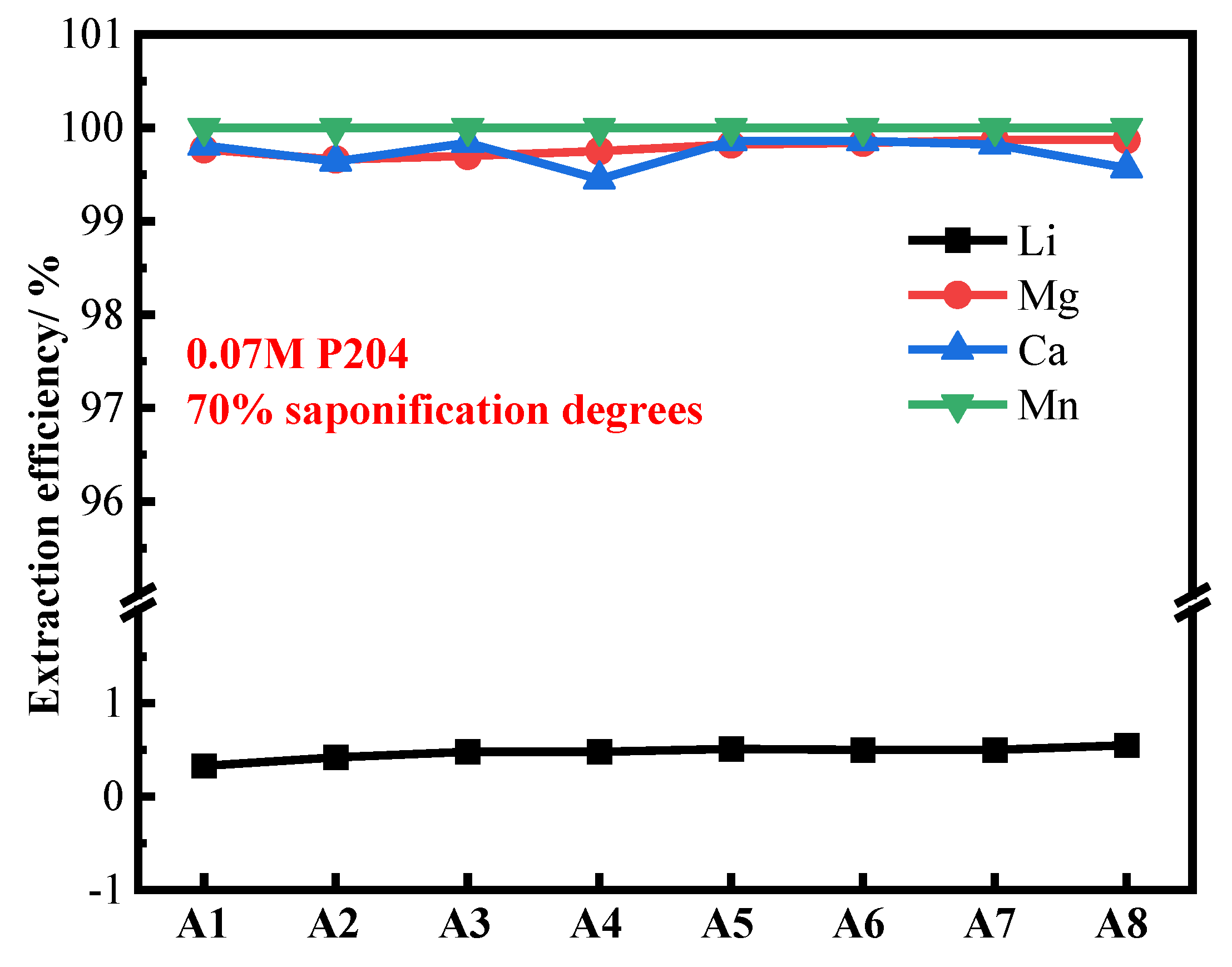
3.6. Removal of Mn2+, Ca2+, and Mg2+ by Three-Stage Continuous Countercurrent Extraction Process
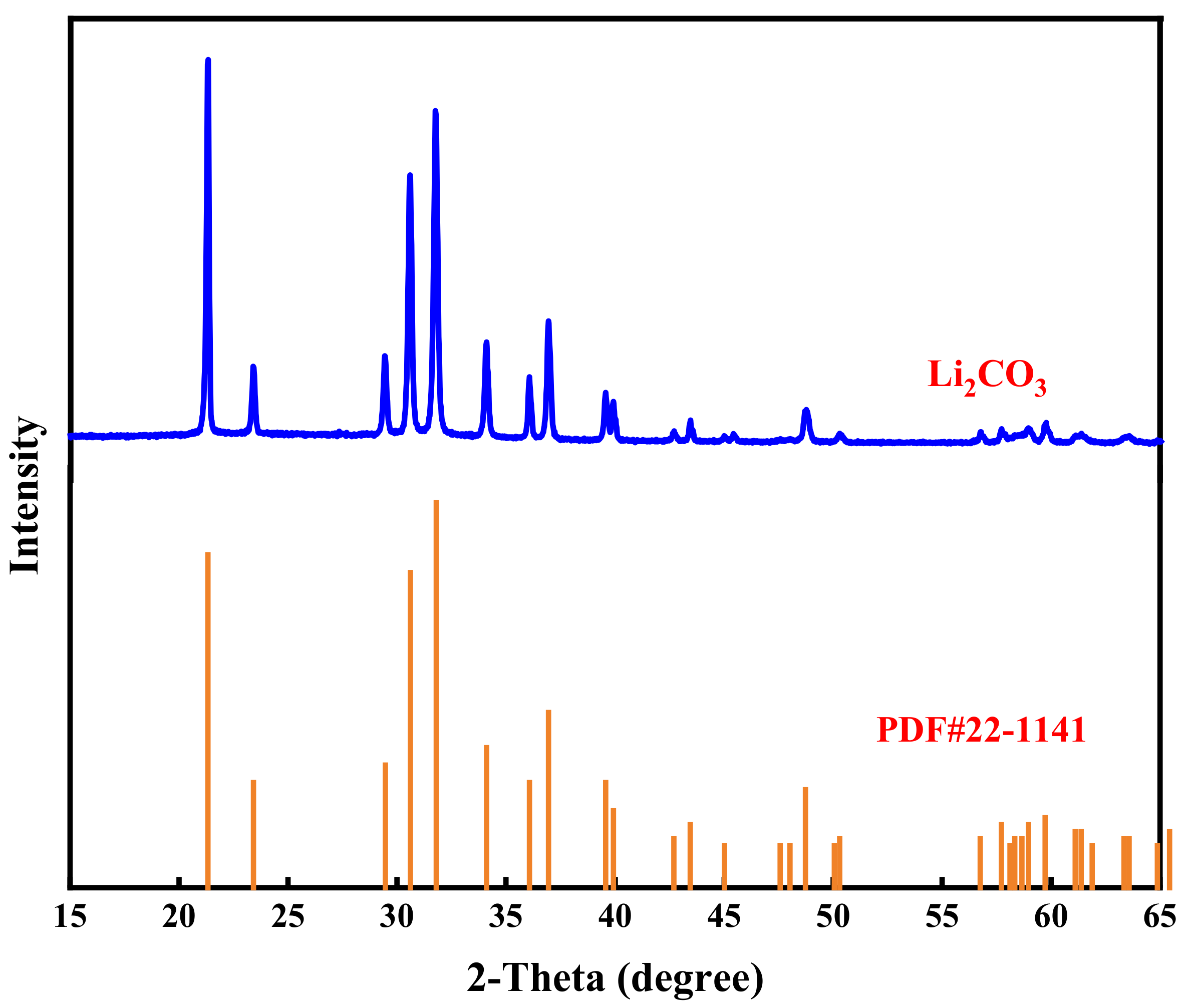
3.7. Preparation of Li2CO3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amato, A.; Becci, A.; Villen-Guzman, M.; Vereda-Alonso, C.; Beolchini, F. Challenges for sustainable lithium supply: A critical review. J. Clean. Prod. 2021, 300, 126954. [Google Scholar]
- Yang, S.; Wang, Y.; Pan, H.; He, P.; Zhou, H. Lithium extraction from low-quality brines. Nature 2024, 636, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, X.; Qing, D.; Li, J.; Wang, H. Research progress of technology of lithium extraction. Sep. Purif. Technol. 2025, 359 Pt 2, 130561. [Google Scholar] [CrossRef]
- Yu, J.; Gao, C.; Cheng, A.; Liu, Y.; Zhang, L.; He, X. Geomorphic, hydroclimatic and hydrothermal controls on the formation of lithium brine deposits in the Qaidam Basin, northern Tibetan plateau, China. Ore Geol. Rev. 2013, 50, 171–183. [Google Scholar] [CrossRef]
- Li, Z.; Mercken, J.; Li, X.; Riaño, S.A.; Binnemans, K. Efficient and sustainable removal of magnesium from brines for lithium/magnesium separation using binary extractants. ACS Sustain. Chem. Eng. 2019, 7, 19225–19234. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.; Peng, X.; Li, L.; Song, F.; Nie, F.; Ji, L.; Zhang, Y. Extraction of lithium from salt lake brine containing boron using multistage centrifuge extractors. Desalination 2018, 441, 44–51. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Ji, L.; Li, L. Separation of lithium from alkaline solutions with hydrophobic deep eutectic solvents based on β-diketone. J. Mol. Liq. 2021, 344, 117729. [Google Scholar] [CrossRef]
- Zhong, J.; Lin, S.; Yu, J. Li+ adsorption performance and mechanism using lithium/aluminum layered double hydroxides in low grade brines. Desalination 2021, 505, 114983. [Google Scholar] [CrossRef]
- Li, J.; Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z. Synthesis of granulated Li/Al-LDHs adsorbent and application for recovery of Li from synthetic and real salt lake brines. Hydrometallurgy 2022, 209, 105828. [Google Scholar] [CrossRef]
- Li, W.; Bai, B.; He, R.; Song, J.; He, T. Lithium solvent extraction by a novel multiframe flat membrane contactor module. Sep. Purif. Technol. 2024, 328, 125061. [Google Scholar] [CrossRef]
- Xu, S.; Song, J.; Bi, Q.; Chen, Q.; Zhang, W.-M.; Qian, Z.; Zhang, L.; Xu, S.; Tang, N.; He, T. Extraction of lithium from Chinese salt-lake brines by membranes: Design and practice. J. Membr. Sci. 2021, 635, 119441. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Liu, Y.; Xiang, X. Highly Efficient Lithium Extraction from Brine with a High Sodium Content by Adsorption-Coupled Electrochemical Technology. ACS Sustain. Chem. Eng. 2021, 9, 11022–11031. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A.; Oyedotun, K.O. Utilisation of adsorption as a resource recovery technique for lithium in geothermal water. J. Mol. Liq. 2022, 365, 120107. [Google Scholar] [CrossRef]
- Ji, L.; Shi, D.; Zhang, Y.; Li, J.; Peng, X.; Xie, S.; Yang, S.; Chen, N.; Lu, H.; Niu, Y.; et al. Lithium extraction from high-Mg brines using N523/esters systems. J. Water Process. Eng. 2024, 68, 106513. [Google Scholar] [CrossRef]
- Wang, S.; Rong, M.; Sun, L.-B.; Zhao, Y.; Xing, H.; Yang, L. Benzo-12-crown-4 and β-diketone bi-functionalized ionic liquids for highly selective lithium extraction from alkaline solution. Chem. Eng. J. 2024, 497, 154341. [Google Scholar] [CrossRef]
- Ding, T.; Zheng, M.; Peng, S.; Lin, Y.; Zhang, X.; Li, M. Lithium extraction from salt lakes with different hydrochemical types in the Tibet Plateau. Geosci. Front. 2023, 14, 101485. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.; Srinivasakannan, C.; Li, S.; Yin, S.; Zhang, L.; Jiang, X.; Zhou, G.; Zhang, N. Lithium extraction from salt lake brines with high magnesium/lithium ratio: A review. Environ. Chem. Lett. 2023, 21, 1611–1626. [Google Scholar] [CrossRef]
- Sun, S.; Deng, Y.; Chen, J.; Zou, D.; Han, Y.; Liu, M. Recovery of lithium from the desorption solutions of salt lakes using β-diketone synergistic extraction system. Sep. Purif. Technol. 2025, 354, 128748. [Google Scholar] [CrossRef]
- Song, J.F.; Nghiem, L.D.; Li, X.-M.; He, T. Lithium extraction from Chinese salt-lake brines: Opportunities, challenges, and future outlook. Environ. Sci. Water Res. Technol. 2017, 3, 593–597. [Google Scholar] [CrossRef]
- Chen, Z.; Du, J.; Shi, J. Mxene-based lithium-ion sieve polymer membrane for sustainable lithium adsorption. Sep. Purif. Technol. 2025, 354, 129316. [Google Scholar] [CrossRef]
- Li, E.; Zhang, F.; Cheng, F.; Cheng, H. High selective adsorption of lithium by photochromic porous SP-C/PVDF membranes with the driven force of an external electric field. J. Mol. Liq. 2023, 372, 121160. [Google Scholar] [CrossRef]
- Hu, Y.; Su, H.; Zhu, Z.; Qi, T.; Pang, Q. Environmentally benign techniques of lithium extraction from salt lakes: A review. Environ. Chem. Lett. 2024, 22, 105–120. [Google Scholar] [CrossRef]
- Gao, A.; Sun, Z.; Li, S.; Hou, X.; Li, H.; Wang, C.; Wu, W. Self-assembled layered lithium manganese oxide shows ultra-large adsorption capacity and high selectivity for lithium. Chem. Eng. J. 2023, 471, 144287. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Zhang, J.; Zhu, Z.; Wang, L.; Qi, T. Recovery of lithium from salt lake brine using a mixed ternary solvent extraction system consisting of TBP, FeCl3 and P507. Hydrometallurgy 2020, 197, 105487. [Google Scholar] [CrossRef]
- Feng, Q.; Miyai, Y.; Kanoh, H.; Ooi, K. Li+ extraction/insertion with spinel-type lithium manganese oxides Characterization of redox-type and ion-exchange-type sites. Langmuir 1992, 8, 1861–1867. [Google Scholar] [CrossRef]
- Ji, L.-M.; Shi, D.; Peng, X.-W.; Xie, S.-L.; Li, J.-F.; Zhang, Y.-Z.; Zhang, L.-C.; Niu, Y.; Chen, G.; Li, L.-J. Lithium extraction from carbonate-rich Salt Lake brine using HBTA/TBP system. Rare Met. 2024, 43, 6717–6729. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X.; Deng, H.; Jiang, Z.; Xia, B.; Yang, L.; Li, Z. Comparative Study of Four Acidic Extractants as Modifiers in the TBP/FeCl3 Solvent Extraction System for Lithium and Magnesium Separation. Ind. Eng. Chem. Res. 2024, 63, 4897–4904. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Y.; Li, R.; Ren, Z.; Zhou, Z. Selective extraction of lithium from high magnesium/lithium ratio brines with a TBP-FeCl3-P204-kerosene extraction system. Sep. Purif. Technol. 2024, 328, 125066. [Google Scholar] [CrossRef]
- Peng, X.; Shi, D.; Zhang, Y.; Zhang, L.; Ji, L.; Li, L. Recovery of boron from unacidified salt lake brine by solvent extraction with 2,2,4-trimethyl-1,3-pentanediol. J. Mol. Liq. 2021, 326, 115301. [Google Scholar] [CrossRef]
- Türkay, S.; Civelekoğlu, H. Deacidification of sulfur olive oil. II. Multi-stage liquid extraction of miscella with ethyl alcohol. J. Am. Oil Chem. Soc. 1991, 68, 818–821. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Xu, M.; Zhang, Y.; Peng, X.; Zhang, L.; Song, F.; Ji, L. Removal of calcium and magnesium from lithium concentrated solution by solvent extraction method using D2EHPA. Desalination 2020, 479, 114306. [Google Scholar] [CrossRef]
- Hano, T.; Matsumoto, M.; Ohrake, T.; Egashir, N.; Hori, F. Recovery of Lithium from geothermal water by solvent extraction technique. Solvent Extr. Ion Exch. 1992, 10, 195–206. [Google Scholar] [CrossRef]
- Virolainen, S.; Fini, M.F.; Miettinen, V.; Laitinen, A.; Haapalainen, M.; Sainio, T. Removal of calcium and magnesium from lithium brine concentrate via continuous counter current solvent extraction. Hydrometallurgy 2016, 162, 9–15. [Google Scholar] [CrossRef]
- Guimaraes, A.S.; Manur, M.B. Selection of a synergistic solvent extraction system to remove calcium and magnesium from concentrated nickel sulfate solution. Hydrometallurgy 2018, 175, 250–256. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J.; Li, H.; Han, Y.; Liu, M. High-efficiency removal of calcium and magnesium from lithium-concentrated solution via counter-current extraction using di-(2-ethylhexyl) phosphinic acid. ACS Sustain. Chem. Eng. 2022, 10, 967–974. [Google Scholar] [CrossRef]
- Pakarinen, J.; Paatero, E. Effect of temperature on Mn-Ca selectivity with organophosphorus acid extractants. Hydrometallurgy 2011, 106, 159–164. [Google Scholar] [CrossRef]
- Fei, Z.; Su, Y.; Zha, Y.; Zhao, X.; Meng, Q.; Dong, P.; Zhang, Y. Selective lithium extraction of cathode materials from spent lithium-ion batteries via low-valent salt assisted roasting. Chem. Eng. J. 2023, 464, 142534. [Google Scholar] [CrossRef]






| Ions | Li+ | Na+ | K+ | Mg2+ | Ca2+ | Mn2+ | B | Cl− | pH |
|---|---|---|---|---|---|---|---|---|---|
| Mass Conc. g·L−1 | 1.296 | 0.025 | 0.057 | 0.308 | 0.317 | 0.217 | 0.002 | 8.793 | 3.18 |
| Molar Conc. mol·L−1 | 0.187 | 1.1 × 10−3 | 1.5 × 10−3 | 0.013 | 7.9 × 10−3 | 3.9 × 10−3 | 1.8 × 10−4 | 0.248 |
| Ions (g·L−1) | Li+ | Na+ | K+ | Mg2+ | Ca2+ | Mn2+ | B | Cl− | pH |
|---|---|---|---|---|---|---|---|---|---|
| Desorption solution | 1.296 | 0.025 | 0.057 | 0.308 | 0.317 | 0.217 | 0.002 | 8.793 | 3.18 |
| Raffinate | 1.288 | 1.010 | 0.049 | 0.001 | N.D. | N.D. | 0.004 | 8.781 | 5.87 |
| Lithium-rich solution | 23.61 | 20.14 | 1.050 | 0.008 | 0.002 | N.D. | 0.034 | 130.2 | 5.11 |
| Species/% | Li2CO3 | Na | K | Mg | Mn | Cu | Ca | Cd | Ni |
|---|---|---|---|---|---|---|---|---|---|
| Sample | 99.83 | 0.0045 | <0.001 | <0.0006 | <0.0001 | 0.0003 | 0.021 | <0.0001 | <0.0001 |
| Species/% | Al | SO42− | Si | Zn | Pb | Cl− | |||
| Sample | <0.0002 | <0.005 | 0.0056 | 0.0003 | <0.0004 | 0.0022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Zhang, Y.; Peng, X.; Niu, Y.; Lu, H.; Song, F.; Shi, D.; Li, L. Preparation of Lithium Carbonate from Manganese-Containing Desorption Solution from Salt Lakes via an Organophosphoric Acid Extraction System. Separations 2025, 12, 98. https://doi.org/10.3390/separations12040098
Xie S, Zhang Y, Peng X, Niu Y, Lu H, Song F, Shi D, Li L. Preparation of Lithium Carbonate from Manganese-Containing Desorption Solution from Salt Lakes via an Organophosphoric Acid Extraction System. Separations. 2025; 12(4):98. https://doi.org/10.3390/separations12040098
Chicago/Turabian StyleXie, Shaolei, Yuze Zhang, Xiaowu Peng, Yong Niu, Hailong Lu, Fugen Song, Dong Shi, and Lijuan Li. 2025. "Preparation of Lithium Carbonate from Manganese-Containing Desorption Solution from Salt Lakes via an Organophosphoric Acid Extraction System" Separations 12, no. 4: 98. https://doi.org/10.3390/separations12040098
APA StyleXie, S., Zhang, Y., Peng, X., Niu, Y., Lu, H., Song, F., Shi, D., & Li, L. (2025). Preparation of Lithium Carbonate from Manganese-Containing Desorption Solution from Salt Lakes via an Organophosphoric Acid Extraction System. Separations, 12(4), 98. https://doi.org/10.3390/separations12040098






