Synthesis of Geopolymer-Based Fenton-like Catalytic Tubular Membrane for Dye Wastewater Treatment
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Materials and Reagents
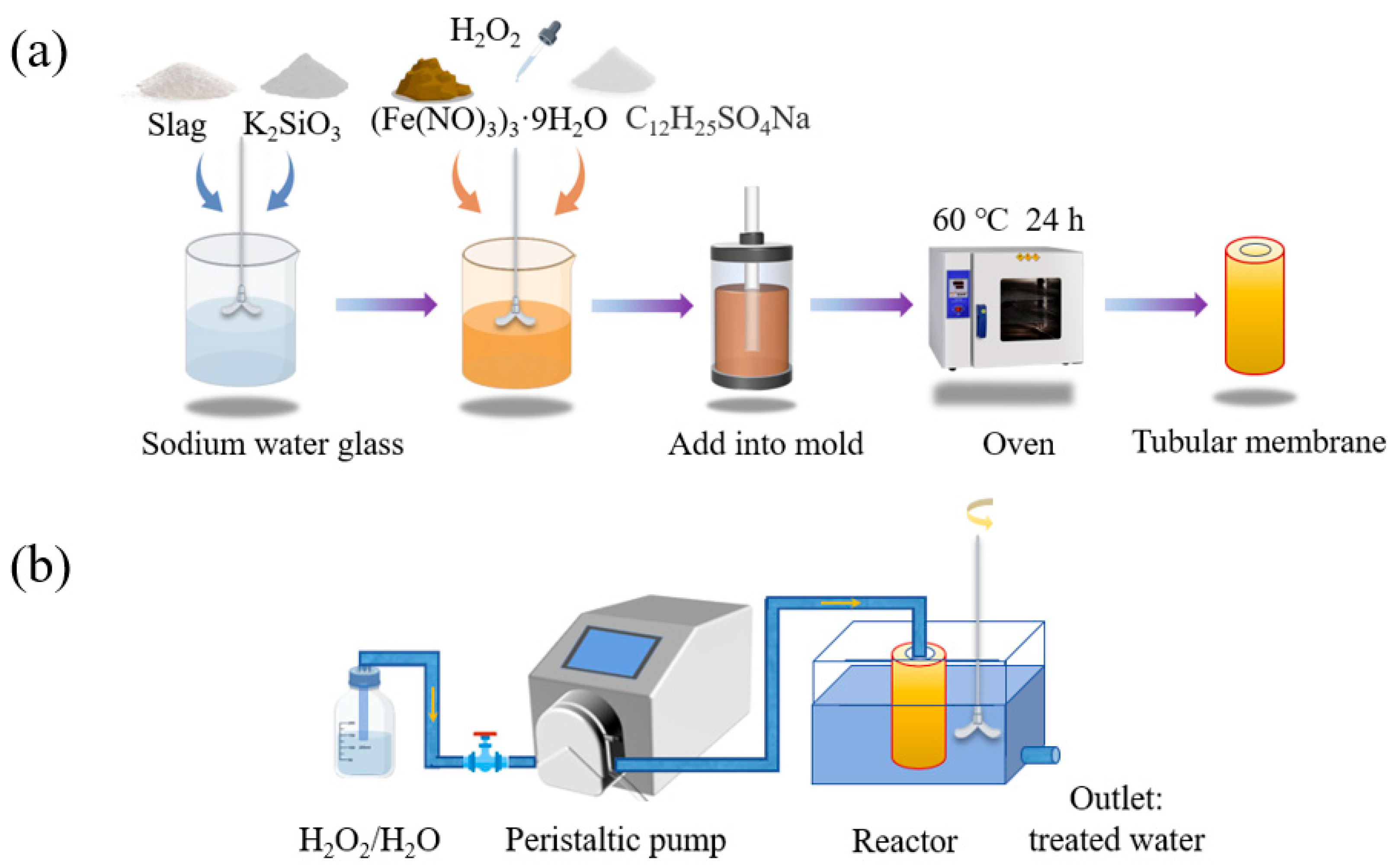
2.2. Preparation of Catalytic Tubular Membrane
2.3. Experimental Equipment and Apparatus
2.4. Study on Dye Wastewater Removal Efficiency
3. Results and Discussion
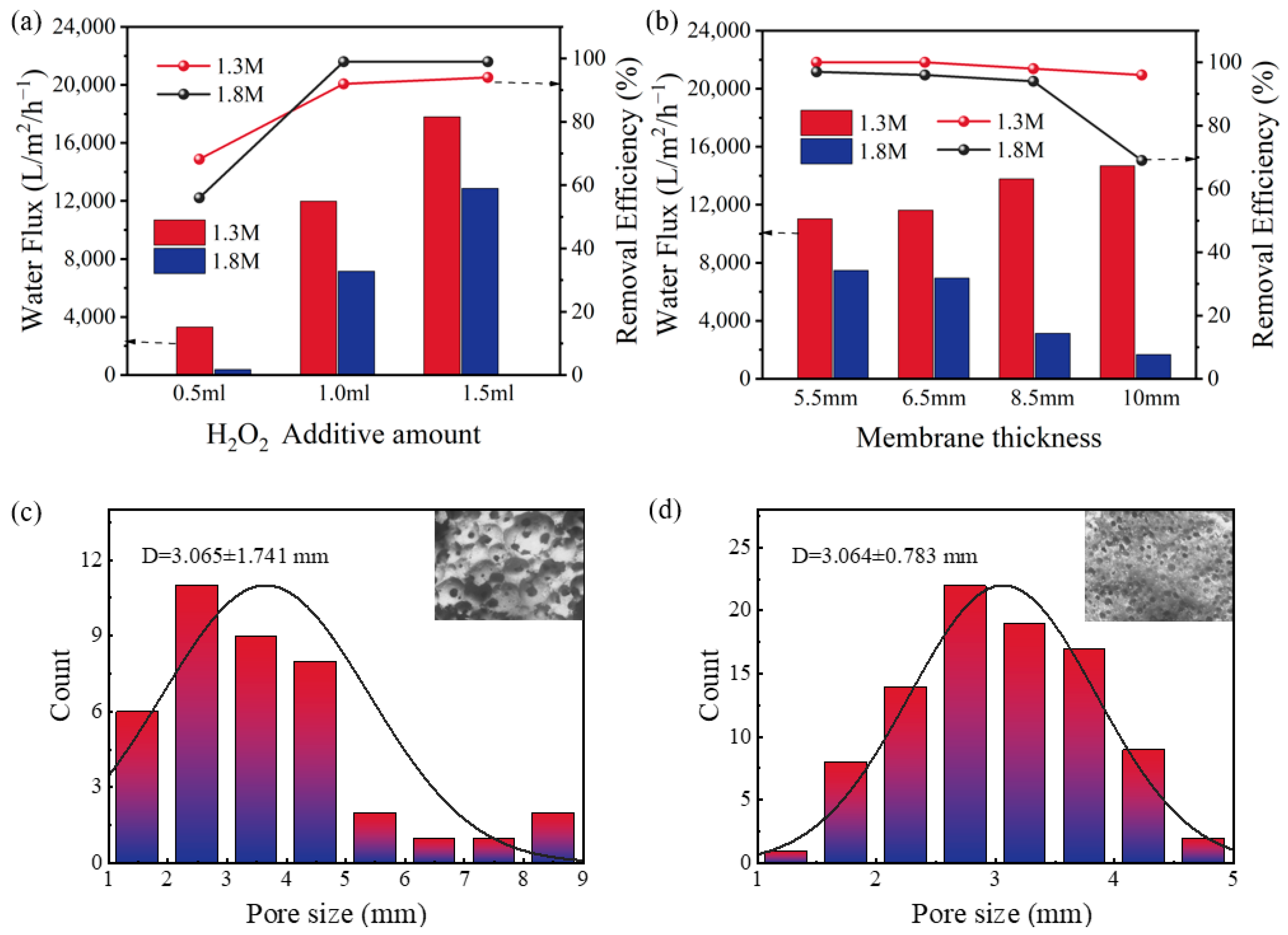
3.1. Study of the Preparation Conditions of Tubular Membranes
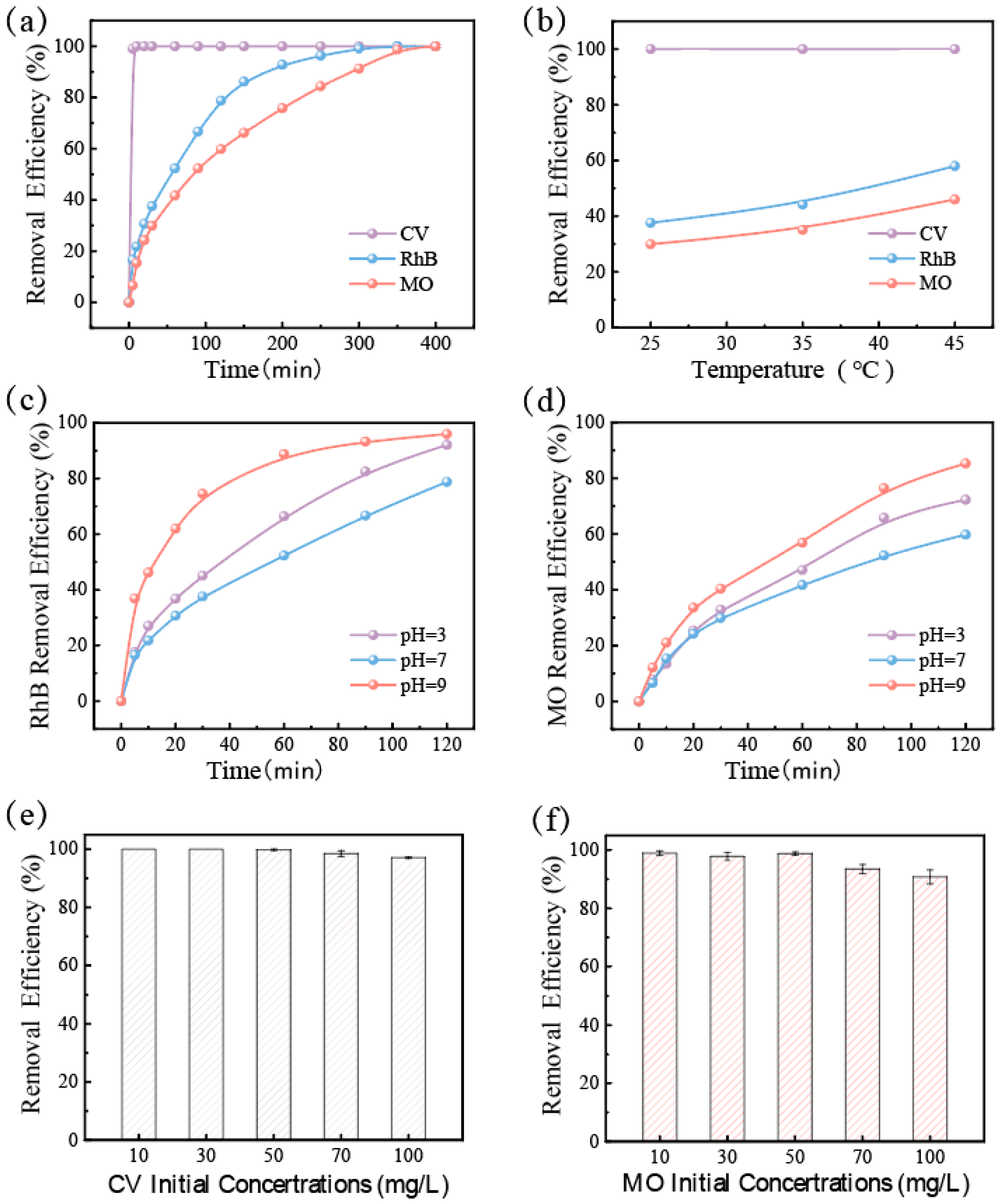
3.2. Study on Dye Wastewater Removal Efficiency of GFM
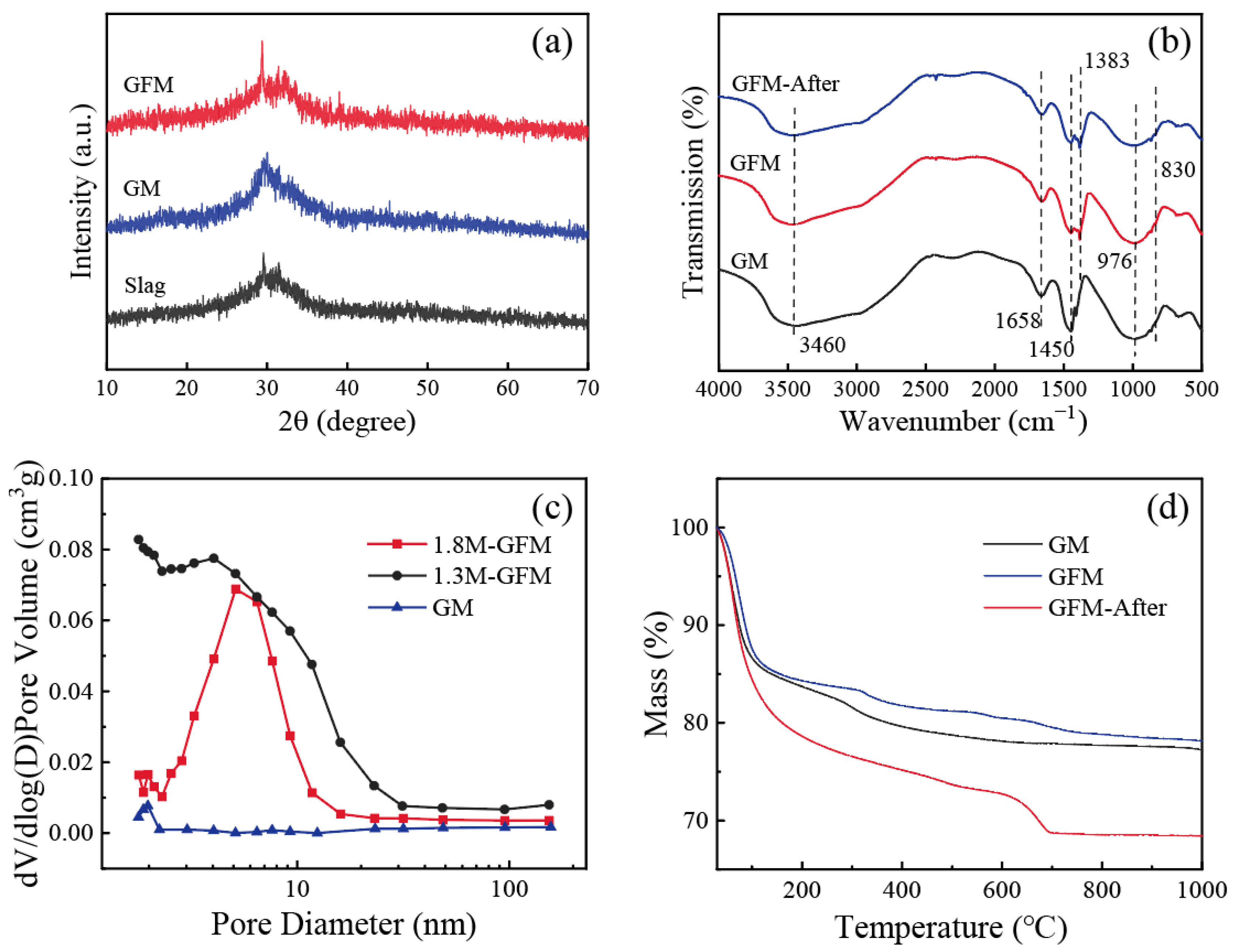
3.3. Characterization of GFM
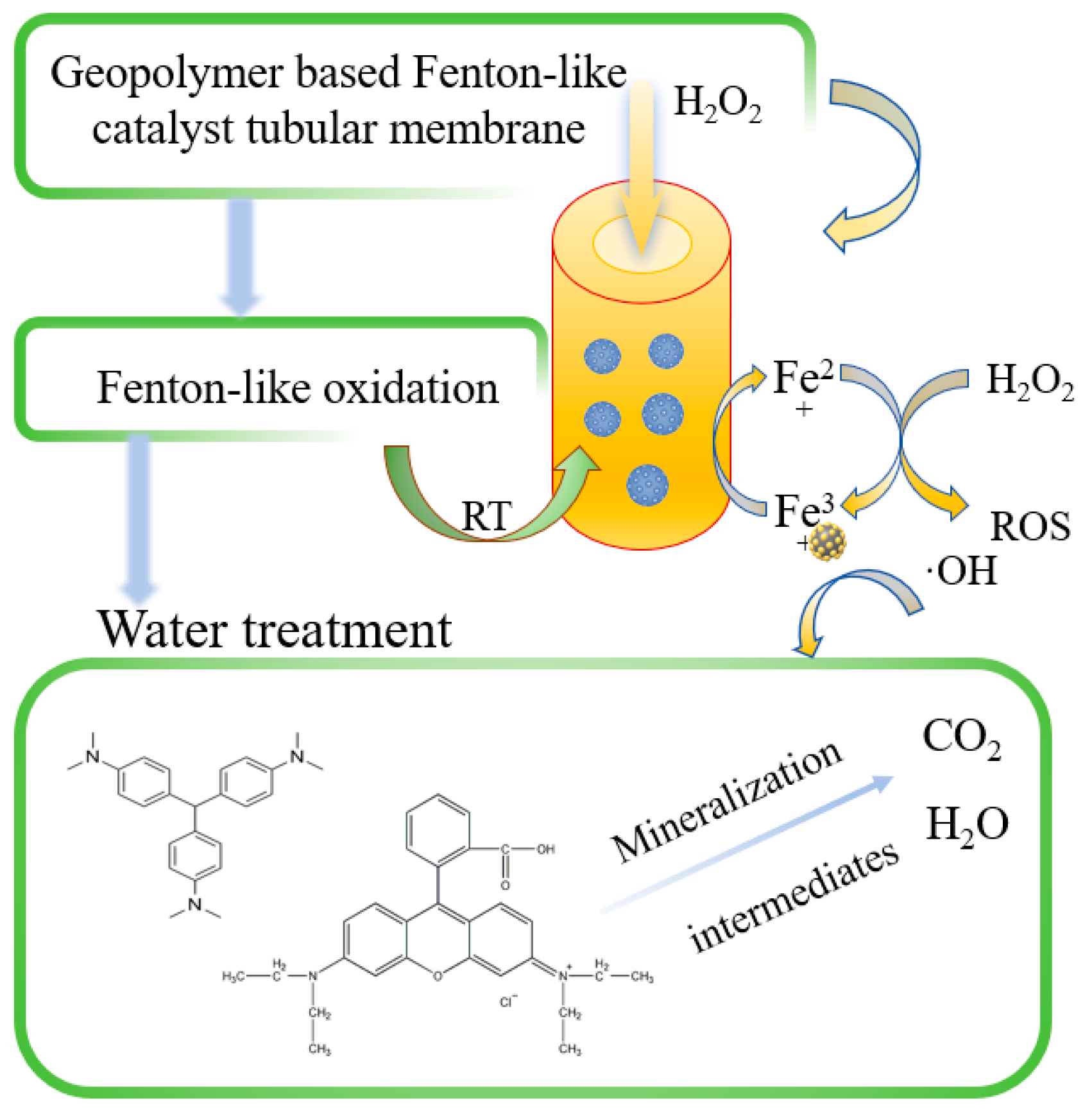
3.4. The Mechanism of Dye Removal by GFM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Rasero, C.R.; Jimenez, V.M.; Franco, M.F.A.; González, C.F.; Tercero, J.P.; Correa, E.M.C. Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review. Water 2024, 16, 1607. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, D.V.; Hatamoto, M.; Takimoto, Y.; Watari, T.; Do, K.-U.; Yamaguchi, T. Harnessing iron materials for enhanced decolorization of azo dye wastewater: A comprehensive review. Environ. Res. 2024, 258, 119418. [Google Scholar] [CrossRef] [PubMed]
- Artifon, W.; Mazur, L.P.; de Souza, A.A.U.; de Oliveira, D. Production of bioflocculants from spent brewer’s yeast and its application in the treatment of effluents with textile dyes. J. Water Process Eng. 2022, 49, 102997. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, L.; Liu, F.; Yu, Q. Adsorption experiments and mechanisms of methylene blue on activated carbon from garden waste via deep eutectic solvents coupling KOH activation. Biomass Bioenergy 2024, 182, 107074. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Ding, J.; Pang, J.-W.; Wu, C.-D.; Sun, H.-J.; Fang, R.; Ren, N.-Q.; Yang, S.-S. Promotion of anaerobic biodegradation of azo dye RR2 by different biowaste-derived biochars: Characteristics and mechanism study by machine learning. Bioresour. Technol. 2024, 396, 130383. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Nawaz, I.; Ullah, A.; Tauseef Khawar, M.; Nikiel, M.; Razzaq, H.; Siddiq, M.; Liu, X. Hybrid PVDF/PANI Membrane for Removal of Dyes from Textile Wastewater. Adv. Eng. Mater. 2022, 24, 2100719. [Google Scholar] [CrossRef]
- Patel, R.P.; Pataniya, P.M.; Siraj, S.; Sahatiya, P.; Sumesh, C.K. Simultaneous production of green hydrogen and decontamination of dye wastewater using WSe2–CuO/graphite electrochemical cell. Int. J. Hydrogen Energy 2024, 55, 815–827. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, S.; Li, X.; Zhang, S.; Thang Nguyen, T.; Guo, M.; Gao, X. Ultrasound-assisted heterogeneous Fenton-like process for methylene blue removal using magnetic MnFe2O4/biochar nanocomposite. Appl. Surf. Sci. 2021, 566, 150654. [Google Scholar] [CrossRef]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile Industry Effluent Treatment Techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- AlHadithy, Z.E.; AbdulRazak, A.A.; Al-Ghaban, A.M.H.A.; Alsalhy, Q.F.; Meskher, H.; Al-Juboori, R.A.; Katibi, K.K.; Lawal, D.U. Advancements in Water Treatment with MXene-Enhanced Membranes: A Review of Current Progress and Future Directions. Water Air Soil Pollut. 2024, 235, 809. [Google Scholar] [CrossRef]
- Grylewicz, A.; Mozia, S. Polymeric mixed-matrix membranes modified with halloysite nanotubes for water and wastewater treatment: A review. Sep. Purif. Technol. 2021, 256, 117827. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Chong, K.C.; Lai, S.O.; Lau, W.J.; Maldonado, E.A.L.; Camacho, L.M.; Shirazi, M.M.A.; Ali, A.; Mamba, B.B.; Osial, M.; et al. Progress in membrane distillation processes for dye wastewater treatment: A review. Chemosphere 2024, 360, 142347. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Tanveer, R.; Yasar, A.; Nizami, A.-S.; Tabinda, A.B. Integration of physical and advanced oxidation processes for treatment and reuse of textile dye-bath effluents with minimum area footprint. J. Clean. Prod. 2023, 383, 135366. [Google Scholar] [CrossRef]
- Huddersman, K.; Ekpruke, A.; Asuelimen, L. Application of AOPs in the treatment of OSPAR chemicals and a comparative cost analysis. Crit. Rev. Environ. Sci. Technol. 2019, 49, 277–317. [Google Scholar] [CrossRef]
- Xavier, S.; Gandhimathi, R.; Nidheesh, P.V.; Ramesh, S.T. Comparison of homogeneous and heterogeneous Fenton processes for the removal of reactive dye Magenta MB from aqueous solution. Desalination Water Treat. 2015, 53, 109–118. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Xu, N.; Han, J.; Feng, Y.; Xiao, C. Polyacrylonitrile/poly(acrylic acid) layer-by-layer superimposed composite nanofiber membrane with low iron ion leaching-out and stable methylene blue-removing performance. J. Membr. Sci. 2022, 641, 119935. [Google Scholar] [CrossRef]
- Chen, B.; Hu, X.; Wang, J.; Li, R.; Shen, L.; Xu, Y.; Zhang, M.; Hong, H.; Lin, H. Novel catalytic self-cleaning membrane with peroxymonosulfate activation for dual-function wastewater purification: Performance and mechanism. J. Clean. Prod. 2022, 355, 131858. [Google Scholar] [CrossRef]
- Nurlina, N.; Pratama, J.H.; Pambudi, A.B.; Rahmawati, Z.; Subaer, S.; Abdullah, M.M.A.B.; Gusrizal, G.; Fansuri, H. A review of geopolymer membrane for water treatment. Appl. Clay Sci. 2024, 251, 107301. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, G.; Liu, R.; Qu, J.; Liu, H. Potential applications of solid waste-based geopolymer materials: In wastewater treatment and greenhouse gas emission reduction. J. Clean. Prod. 2024, 443, 141144. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Bibliographic and visualized analysis of geopolymer research and its application in heavy metal immobilization: A review. J. Environ. Manag. 2019, 231, 256–267. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Zhang, J.; Zhang, Y.; Ge, Y.; Cui, X. In-situ synchronous carbonation and self-activation of biochar/geopolymer composite membrane: Enhanced catalyst for oxidative degradation of tetracycline in water. Chem. Eng. J. 2020, 397, 125528. [Google Scholar] [CrossRef]
- He, P.; Zhang, F.; Zhang, Y.; Chen, H. Multifunctional fly ash-based GO/geopolymer composite membrane for efficient oil-water separation and dye degradation. Ceram. Int. 2023, 49, 1855–1864. [Google Scholar] [CrossRef]
- Liu, L.; Deng, X.; He, Y.; Cui, X.-M. Synthesis of composite zeolite membrane by low-temperature water bath for efficient dynamic hardwater softening. J. Environ. Chem. Eng. 2023, 11, 109482. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Vickers, L.; Bignozzi, M.C.; van Riessen, A. A comparison between different foaming methods for the synthesis of light weight geopolymers. Ceram. Int. 2014, 40, 13891–13902. [Google Scholar] [CrossRef]
- Xu, M.-X.; He, Y.; Liu, Z.-H.; Tong, Z.-F.; Cui, X.-M. Preparation of geopolymer inorganic membrane and purification of pulp-papermaking green liquor. Appl. Clay Sci. 2019, 168, 269–275. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Landi, E. Geopolymer-hydroxyapatite composite foams for wastewater remediation. Ceram. Int. 2024, 50, 50377–50387. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Xu, M.; Cui, X. Preparation, characterization and application of geopolymer-based tubular inorganic membrane. Appl. Clay Sci. 2021, 203, 106001. [Google Scholar] [CrossRef]
- Chen, C.; Xu, M.-x.; Deng, X.; He, Y.; Cui, X. Preparation of metakaolin-based geopolymer membrane and its application in black liquor treatment. Appl. Clay Sci. 2023, 232, 106773. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, X.; He, Y.; Xiao, P.; Dhmees, A.S.; Cui, X. Carbonic anhydrase immobilized on Zn(II)-geopolymer membrane for CO2 capture. Biochem. Eng. J. 2024, 208, 109364. [Google Scholar] [CrossRef]
- Lv, X.; Qin, Y.; Liang, H.; Cui, X. Effects of modifying agent on rheology and workability of alkali-activated slag paste for 3D extrusion forming. Constr. Build. Mater. 2021, 302, 124062. [Google Scholar] [CrossRef]
- Al-Sammarraie, E.S.A.; Sabirova, T.M.; Meskher, H.; Al-Juboori, R.A.; Zyryanov, G.V.; Alsalhy, Q.F. Nanocomposite UF membrane of PVC/nano-silica modified with SDS for carwash wastewater treatment. Environ. Sci. Adv. 2025, 4, 469–488. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, L.; Wang, H.; Lin, H.; Li, J.; Yan, Y.; Cui, J.; Jiang, J. Bio-Inspired Underwater Superoleophobic Aramid Nanofiber-Based Aerogel Membranes for Highly Efficient Removal of Emulsified Oils and Organic Dyes. Langmuir 2024, 40, 13995–14006. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Wei, X.; Liu, K.; Wang, J.; Hu, J.; Lin, J. An integrated strategy for achieving oil-in-water separation, removal, and anti-oil/dye/bacteria-fouling. Chem. Eng. J. 2021, 413, 127493. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Gao, A.; Zhang, Q.; Cui, J.; Zhao, S.; Zhan, X.; Yan, Y. Bio-inspired underwater superoleophobic PVDF membranes for highly-efficient simultaneous removal of insoluble emulsified oils and soluble anionic dyes. Chem. Eng. J. 2019, 369, 576–587. [Google Scholar] [CrossRef]
- Mansor, E.S.; Ali, H.; Abdel-Karim, A. Efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloid Interface Sci. Commun. 2020, 39, 100314. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Lyu, B.; Guo, Z.; Xing, N.; Pang, X.; Wu, T.; Jiang, Y.; Zhang, R.; El-Gendi, A.; et al. Micropore engineering of polyamide loose nanofiltration membrane for efficient dye/salt separation. J. Membr. Sci. 2024, 705, 122932. [Google Scholar] [CrossRef]
- Alani, H.A.; Alsalhy, Q.F.; Al-Saadi, S.; Alani, F.H.; Meskher, H.; Al-Juboori, R.A.; Russo, F.; Chiappetta, G.; Di Luca, G.; Figoli, A. Terephthalic-co-glycerol-g-fumaric Acid: A Promising Nanopolymer for Enhancing PPSU Membrane Properties. ChemEngineering 2025, 9, 12. [Google Scholar] [CrossRef]
- Shen, Y.; Xiao, Y.; Zhang, H.; Fan, H.; Li, Y.; Yan, Z.; Zhang, W.-H. Synthesis of magnetic biochar-supported Fe-Cu bimetallic catalyst from pulp and paper mill wastes for the Fenton-like removal of rhodamine B dye. Chem. Eng. J. 2023, 477, 146823. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Zhang, Q.; Hu, H.; Zhao, Z.; Li, J.; Li, J.; Qiao, Y.; Gogotsi, Y. Self-Sensing, Ultralight, and Conductive 3D Graphene/Iron Oxide Aerogel Elastomer Deformable in a Magnetic Field. ACS Nano 2015, 9, 3969–3977. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Qin, J.; Wu, G.; Lian, C.; Zhang, J.; Wang, S. Iron encapsulated in boron and nitrogen codoped carbon nanotubes as synergistic catalysts for Fenton-like reaction. Water Res. 2016, 101, 281–291. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Yuan, Y.; Jin, Y.; Liu, Y.; Jiang, Z.; Li, X.; Dai, J.; Zhang, Y.; Siyal, A.A.; et al. Catalytic degradation of crystal violet and methyl orange in heterogeneous Fenton-like processes. Chemosphere 2023, 344, 140406. [Google Scholar] [CrossRef]
- Samir, B.; Bakhta, S.; Bouazizi, N.; Sadaoui, Z.; Allalou, O.; Le Derf, F.; Vieillard, J. TBO Degradation by Heterogeneous Fenton-like Reaction Using Fe Supported over Activated Carbon. Catalysts 2021, 11, 1456. [Google Scholar] [CrossRef]
- Mateus, L.; Moreno-Castilla, C.; López-Ramón, M.V.; Cortés, F.B.; Álvarez, M.Á.; Medina, O.E.; Franco, C.A.; Yebra-Rodríguez, Á. Physicochemical characteristics of calcined MnFe2O4 solid nanospheres and their catalytic activity to oxidize para-nitrophenol with peroxymonosulfate and n-C7 asphaltenes with air. J. Environ. Manag. 2021, 281, 111871. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.; Zhang, H.; Yang, W.; Li, J.; Zhou, K. Enhanced photocatalytic degradation of organic contaminants over CaFe2O4 under visible LED light irradiation mediated by peroxymonosulfate. J. Mater. Sci. Technol. 2021, 62, 34–43. [Google Scholar] [CrossRef]


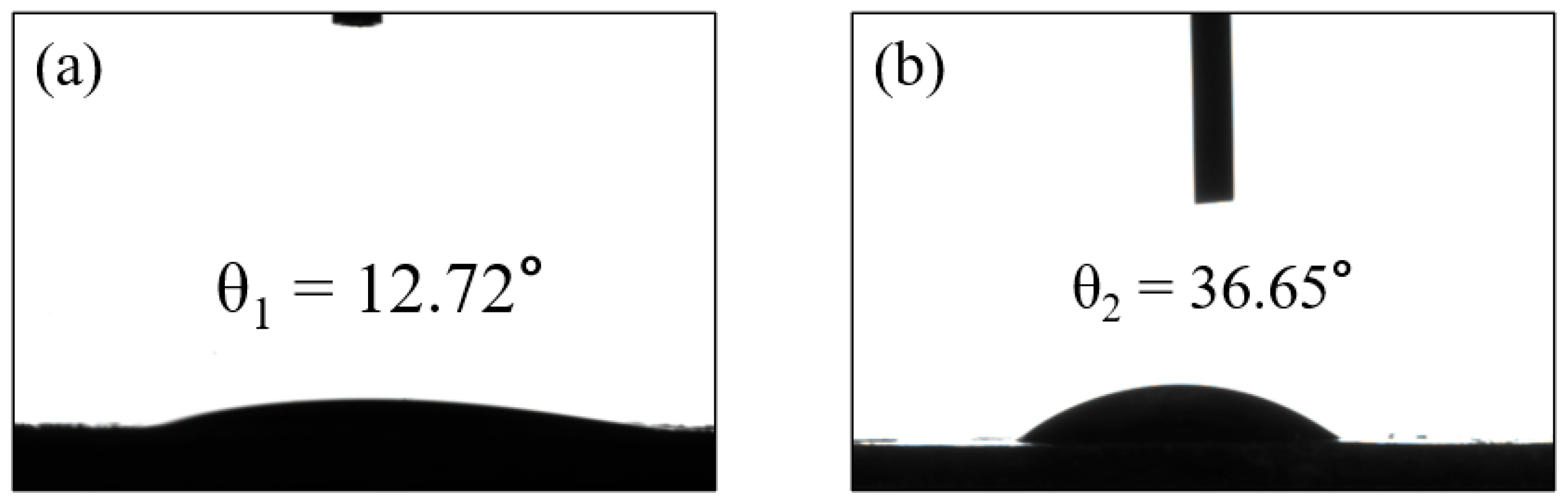

| Chemical Composition | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O |
|---|---|---|---|---|---|---|---|
| Slag (wt.%) | 27.85 | 13.22 | 43.30 | 0.29 | 0.60 | 9.95 | 0.54 |
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | |
|---|---|---|---|
| GM | 1.43 | 0.0022 | 6.11 |
| 1.3M-GFM | 20.41 | 0.0317 | 6.21 |
| 1.8M-GFM | 68.74 | 0.0732 | 4.26 |
| Membrane Types | Water Flux (L/m2/h−1) | Water Contact Angle | Removal Efficiency Performance | Ref. |
|---|---|---|---|---|
| CS&PDA@ANFs | 3729 | 5° | MG: 96.7% CV: 65% RhB: 62% MB: 48% | [36] |
| H-PVDF@ZnO/Ag | 1700 | 27° | MB: 99.2%(photocatalysis) | [37] |
| PVDF/CS&DA | 201.3 | 25.3° | MB: 96.8% CV: 19.9% MeB: 14.4% RhB: 4.2% | [38] |
| PEO/PANI | 277 | 108.4° | MO: 95.3% | [39] |
| Ad-PA-NaOH | 87.72 | 88° | EvB: 99.88% CR: 99.73% | [40] |
| TGF/PPSU | 220.7 | 65.1° | CR: 97.8% MO: 30% | [41] |
| GFM | 6942 | 36.65° | CV: 100% MO: 98.82% RhB: 99.43% | This work |
| Sample | C (%) | O (%) | Fe (%) |
|---|---|---|---|
| GFM | 41.88 | 57.3 | 0.82 |
| GFM-After | 59.22 | 40.05 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, P.; Yang, Q.; Deng, X.; Chu, K.; Cui, X. Synthesis of Geopolymer-Based Fenton-like Catalytic Tubular Membrane for Dye Wastewater Treatment. Separations 2025, 12, 99. https://doi.org/10.3390/separations12040099
Xiao P, Yang Q, Deng X, Chu K, Cui X. Synthesis of Geopolymer-Based Fenton-like Catalytic Tubular Membrane for Dye Wastewater Treatment. Separations. 2025; 12(4):99. https://doi.org/10.3390/separations12040099
Chicago/Turabian StyleXiao, Pei, Qing Yang, Xingfa Deng, Kunyu Chu, and Xuemin Cui. 2025. "Synthesis of Geopolymer-Based Fenton-like Catalytic Tubular Membrane for Dye Wastewater Treatment" Separations 12, no. 4: 99. https://doi.org/10.3390/separations12040099
APA StyleXiao, P., Yang, Q., Deng, X., Chu, K., & Cui, X. (2025). Synthesis of Geopolymer-Based Fenton-like Catalytic Tubular Membrane for Dye Wastewater Treatment. Separations, 12(4), 99. https://doi.org/10.3390/separations12040099






