Abstract
This review systematically summarizes the novel preparation methods of cyclodextrin-based chromatographic stationary phases and their applications for chiral recognition in separation techniques such as capillary gas chromatography and high-performance liquid chromatography. Aiming at the current situation that enantiomers of chiral compounds present significant differences at the pharmacological, pharmacodynamic, and toxicological levels, the core value of chromatographic chiral separation technology in the field of drug discovery and development is emphasized. By analyzing the unique cavity structure and excellent stereoselective properties of cyclodextrins, the mechanism of their action as a chromatographic stationary phase was elaborated. Combined with the typical applications of different derivatized cyclodextrin stationary phases in drug analysis, environmental testing, and biological samples, the value and potential of cyclodextrin stationary phases in stereoisomer separation are systematically demonstrated.
1. Introduction
With the advancement of related disciplines, chiral compounds have found increasingly extensive applications in pharmaceuticals [1], fragrances [2], and pesticides [3]. Particularly, chiral drug research has emerged as the central focus in chiral science [4,5]. Currently, the majority of chiral drugs are still administered as racemic mixtures. However, in non-chiral environments, although enantiomers possess identical physicochemical properties, they ultimately exhibit distinctly different pharmacological activities, metabolic behaviors, or toxicological characteristics within biological systems due to selective differences in their interactions with chiral biomacromolecules. This characteristic not only highlights the crucial role of chiral substances in biological systems but also drives the demand for developing high-efficiency and low-toxicity pharmaceuticals. Chiral separation analysis constitutes not only a key technology but also an imperative requirement for ensuring drug safety and environmental security. Existing separation techniques include crystallization [6], chromatography [7], liquid membrane separation [8], and chiral extraction [9], among which chromatographic methods employing chiral stationary phases have been demonstrated as the most effective separation approach [10,11].
In modern chromatographic analysis, chiral stationary phases have established sophisticated enantioseparation systems through diversified molecular recognition mechanisms. Polysaccharide-derived systems, represented by cellulose tribenzoate (CTB) and cellulose tris(3,5-dimethylphenylcarbamate) (CDMPC), dominate chiral drug separation with their rigid helical cavity structures, serving as core technologies for enantiomeric resolution in pharmaceutical applications [12]. Macrocyclic antibiotic-based phases leverage three-dimensional interaction networks constructed by molecules like vancomycin and teicoplanin, demonstrating unique advantages in separating amino acids and β-blockers through synergistic hydrogen bonding, π-π stacking, and steric hindrance effects [13,14]. Protein affinity systems employing biomacromolecules such as bovine serum albumin (BSA) achieve precise isolation of complex chiral compounds via specific molecular interactions [15]. For aromatic compound resolution, Pirkle-type phases establish well-defined chiral recognition models through meticulously designed π-acceptor/donor groups coordinated with hydrogen bonding sites. Crown ether systems exemplified by 18-crown-6 derivatives excel in analyzing amino acid-derived pharmaceuticals through host-guest chemistry targeting primary amine-containing chiral molecules [16]. Meanwhile, cyclodextrin-modified materials maintain their significance in resolving volatile and semi-volatile chiral compounds by synergizing conical hydrophobic cavities with surface-functionalized groups.
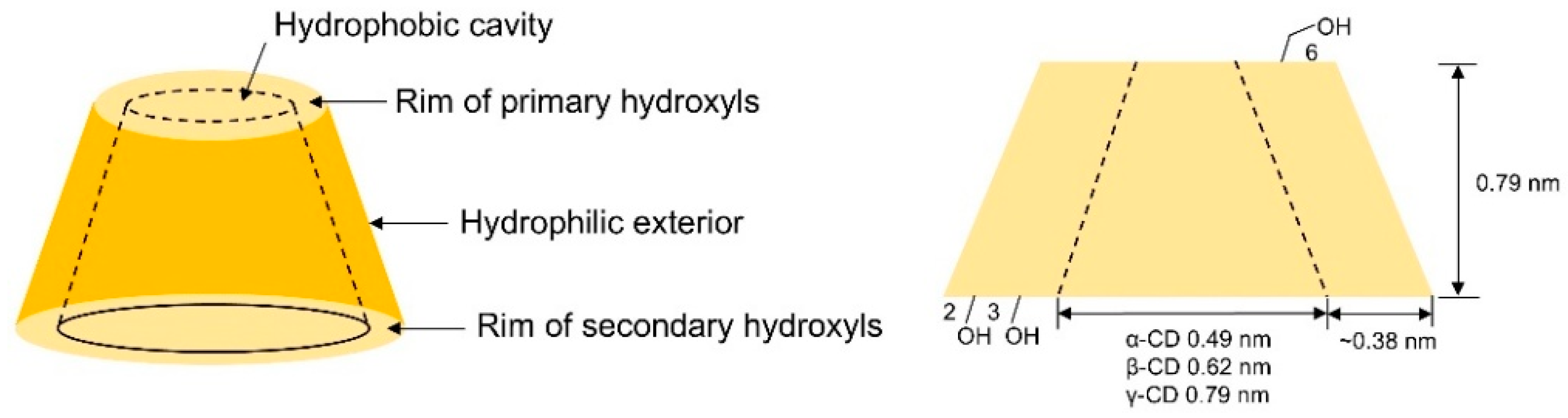
Cyclodextrins (CDs) are cage-like oligosaccharides formed by D-glucopyranose units linked through α-1,4-glycosidic bonds, with common α-, β-, and γ-CDs containing 6–8 glucose units, respectively. Their structural characteristics include the inner cavity is composed of C-H bonds and the oxygen atom of the glycosidic group, exhibiting distinct hydrophobicity, and a hydrophilic exterior decorated with hydroxyl groups (see Figure 1). Each glucose unit contains five chiral centers, resulting in 5n chiral sites for an n-unit cyclodextrin, providing advantageous conditions for chiral resolution. In chromatographic separation, enantiomers form inclusion complexes by entering the hydrophobic cavity, enabling isomer separation through differential complex stability [17], thus earning recognition as “second-generation supramolecular compounds”. Physical parameters of native cyclodextrins are detailed in Table 1.
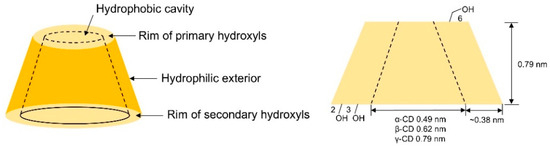
Figure 1.
Schematic shape and size of CDs.
Natural cyclodextrins often require chemical modification due to limitations such as high melting points and poor film-forming properties. Their hydroxyl groups at 2-, 3-, and 6-positions demonstrate differential reactivity: the 6-position hydroxyl group exhibits the strongest nucleophilicity, the 2-position shows the highest acidity, while the 3-position suffers from significant steric hindrance. This reactivity hierarchy enables selective modification, typically prioritizing the 6-position hydroxyl with potential extension to 2- or 3-position hydroxyls under specific conditions, thereby facilitating the preparation of diverse derivatives [18,19]. The cyclodextrin framework contains multiple stereoisomeric centers, with twisted glucose unit arrangements forming unique chiral cavities in three-dimensional structures [20]. This stereochemical differentiation allows derivatives to establish inclusion complexes with chiral molecules through intermolecular interactions, including hydrophobic effects, hydrogen bonding, and van der Waals forces [21,22], enabling enantioselective recognition. Cyclodextrin-derived chiral stationary phases developed based on these characteristics can construct asymmetric separation environments with specific selectivity, currently finding extensive applications in chromatographic chiral separation [23,24,25,26,27,28,29].
According to the Web of Science literature search results based on the keywords of “cyclodextrin” and “chiral stationary phase”, there are about 580 related research papers in the past ten years (2015–2025), which involve a variety of separation techniques, such as gas chromatography (GC), high-performance liquid chromatography (HPLC), capillary electrochromatography (CEC), and supercritical fluid chromatography (SFC), etc. In terms of basic research, a relatively complete theoretical system has been formed for cyclodextrin chiral stationary phases, and Professor Schurig’s group systematically reviewed the use of cyclodextrin derivatives as chiral selective agents in gas chromatography in 2010. In terms of basic research, the chiral stationary phase of cyclodextrins has formed a relatively complete theoretical system, and Prof. Schurig’s team [30] systematically reviewed the application of cyclodextrin derivatives as chiral selective agents in the separation of enantiomers for gas chromatography in 2010. On this basis, Wang et al. [31] published a review paper in 2012, which systematically summarized the construction strategies and application progress of cyclodextrin chiral stationary phases in different separation systems, such as liquid chromatography, capillary electrochromatography, gas chromatography, and supercritical fluid chromatography, during the period from January 2007 to March 2012. It is noteworthy that the development of new chiral stationary phases for cyclodextrin derivatives has shown a significant growth trend, although no new systematic review has appeared in the last decade. Although the application of cyclodextrin chiral stationary phases in capillary electrophoresis has been reported in the last two years [32,33], gas chromatography and liquid chromatography are still the main research vehicles. In this paper, we focus on the latest research on cyclodextrin-based chiral stationary phases (CD-CSPs) in GC and HPLC and provide theoretical references and practical guidance for chiral separation research by systematically reviewing their development status and key technological breakthroughs.

Table 1.
Cavity dimensions and physicochemical properties of native cyclodextrins.
Table 1.
Cavity dimensions and physicochemical properties of native cyclodextrins.
| Cyclodextrin | Glucose Unit | Molecular Weight | Cavity Height (Å) | Cavity Diameter (Å) | Cavity Volume (Å3) | Specific Optical Rotation ([α]25D) | Water Solubility (g/100 mL) |
|---|---|---|---|---|---|---|---|
| α-CD | 6 | 973 | 7.9 ± 0.1 | 4.70~5.30 | 174.0 | +150.5° | 14.5 |
| β-CD | 7 | 1135 | 7.9 ± 0.1 | 6.00~6.50 | 262.0 | +162.5° | 1.85 |
| γ-CD | 8 | 1297 | 7.9 ± 0.1 | 7.50~8.30 | 427.0 | +177.4° | 23.2 |
2. Chiral Resolution Mechanisms of Cyclodextrin Derivatives
The position and type of substituents on cyclodextrin molecules directly influence their chiral selectivity; however, no definitive substituent-selectivity correlation has been established. To achieve efficient separations, it is imperative to thoroughly elucidate the underlying chiral resolution mechanisms [34]. Lipkowitz et al. [35] employed molecular dynamics simulations to investigate the spatial localization of chiral recognition in cyclodextrin derivatives, yet the chiral resolution mechanisms of these derivatives remain incompletely understood. Current research proposes the following primary mechanisms:
2.1. Mechanism of Inclusion Complexation
Armstrong et al. [36] experimentally confirmed that cyclodextrin molecules and solute molecules can form size-matched inclusion complexes. The stability of these complexes is predominantly governed by van der Waals forces and hydrogen bonding, with differences in solute molecular volume, shape, and thermodynamic parameters (ΔH and ΔS) serving as critical factors for separation.
2.2. Conformation-Induced Recognition Mechanism
Venema et al. [37,38] discovered that when chiral molecules interact with 2,3,6-position modified cyclodextrin stationary phases, the latter undergo conformational adjustments to achieve stereochemical matching with solute molecules, thereby enhancing intermolecular interactions.
2.3. Association Mechanism
Bradshaw proposed two distinct association modes [39,40]: nonpolar molecules are separated via cavity-inclusion binding, while polar molecules form external associations with cyclodextrins through dipole-dipole interactions and hydrogen bonding. In practice, chiral resolution often results from synergistic interactions of multiple mechanisms.
2.4. Host-Guest Synergy Mechanism
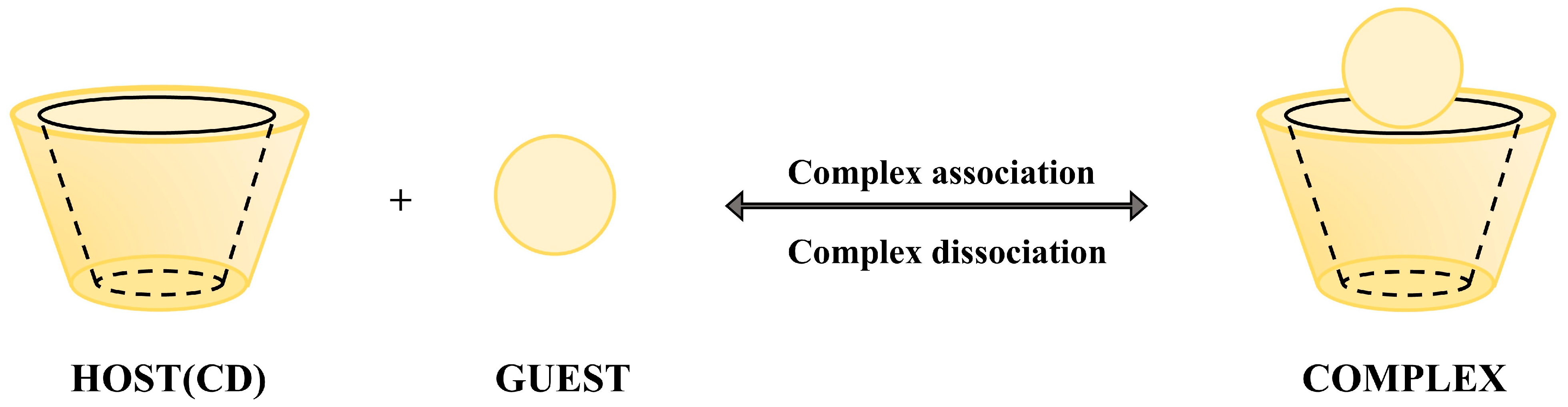
König et al. emphasize that hydrogen bonding, dipole interactions, and van der Waals forces collectively influence the chiral recognition process, with multiple intermolecular interactions between the host and guest forming the stereoselective basis [41,42], and the schematic diagram of the host-guest mechanism is shown in Figure 2. The chiral chromatographic separation mechanism based on the three-point interaction model arises from stereochemical matching differences between enantiomers and the chiral selector [43,44]. When three functional groups of one enantiomer precisely align with the binding sites of the selector, it forms a more stable complex compared to its counterpart that only achieves two-point binding. This stability difference governs retention time variations. Key interactions include van der Waals forces, dipole interactions, and hydrogen bonding. Moreover, bulky substituents on the selector create steric hindrance, selectively blocking access for specific enantiomers to critical binding sites, thereby enhancing separation efficiency.
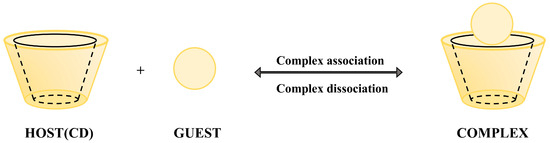
Figure 2.
Host-guest synergy mechanism schematic.
2.5. Multimodal Interaction Mechanisms
Temperature significantly impacts separation performance, with selectivity of derivatives converging at elevated temperatures (>90 °C), while impurities may improve separation efficiency at lower temperatures [45]. Studies demonstrate that randomly substituted tert-butyldimethylsilyl (TBS) cyclodextrins exhibit significantly superior separation capability (16/24 pairs) compared to site-specifically substituted counterparts (6/24 pairs). However, as the purity-performance relationship has not been fully elucidated, practical applications prioritize functional performance over purity control.
3. Cyclodextrin-Derived Stationary Phases in Gas Chromatography
The selection of chiral stationary phases constitutes the core of chiral separation in gas chromatography. Since the advent of the first gas chromatography chiral column, cyclodextrin (CD)-based stationary phases have progressively become the mainstream choice. Their advantage lies in the ability to prepare diverse derivatives through the introduction of acyl, alkyl, hydroxyalkyl, and other functional groups, forming versatile stationary phase systems with multiple molecular recognition sites that have been successfully applied to chiral separation of pesticides and various other compounds. Research on cyclodextrins as gas chromatography stationary phases originated in the 1960s, but underivatized cyclodextrins exhibited limited practical application due to their high chemical reactivity [46,47]. Compared to other chiral stationary phases, the uniqueness of cyclodextrin systems resides in their broad-spectrum separation mechanisms based on inclusion effects, hydrogen bonding, and diverse intermolecular interactions. Modified cyclodextrin derivatives combine high thermal stability, large specific surface area, and optimal pore characteristics, perfectly meeting the requirements of gas chromatography stationary phases. Recent research focuses include the development of novel cyclodextrin derivatives, construction of composite stationary phases based on metal-organic framework (MOF)/covalent organic framework (COF) materials, and innovative applications of column coupling techniques, which are summarized in Table 2. These advancements further demonstrate the broad application prospects of cyclodextrin systems as new-generation high-selectivity chromatographic stationary phases.
3.1. Cyclodextrin-Derived Stationary Phases
Functional group substitution of cyclodextrin hydroxyl groups can significantly enhance molecular recognition capability. Due to variations in substitution degree and positions, most derivatives exhibit random substitution characteristics, forming mixtures containing multiple isomers [15]. Among these, multi-substituted derivatives introduce additional interaction sites, enabling diversified chiral recognition modes.
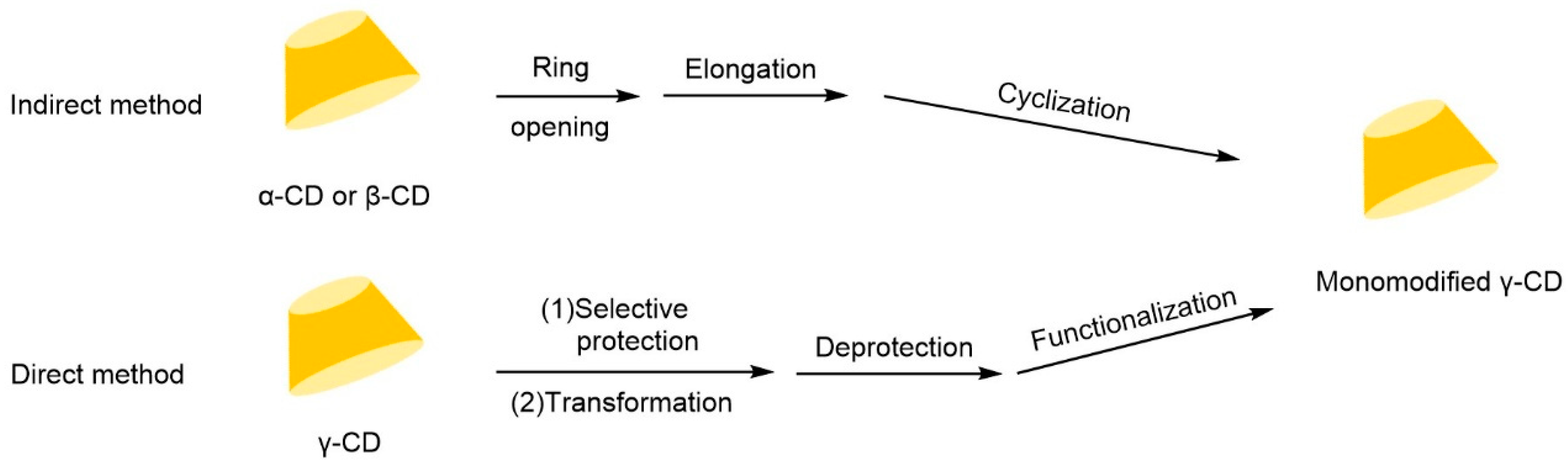
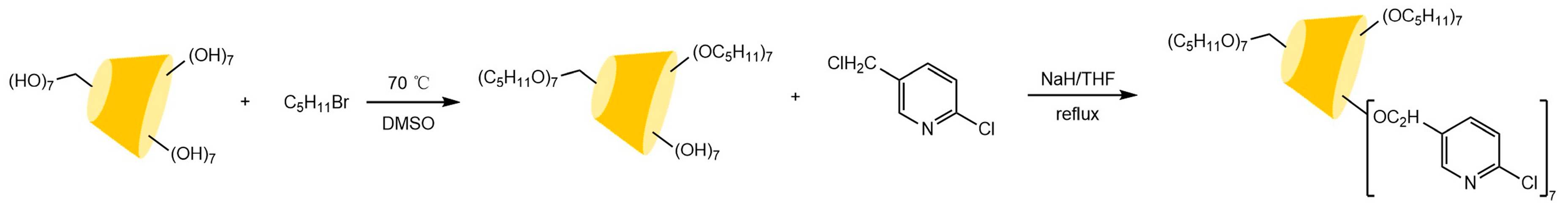
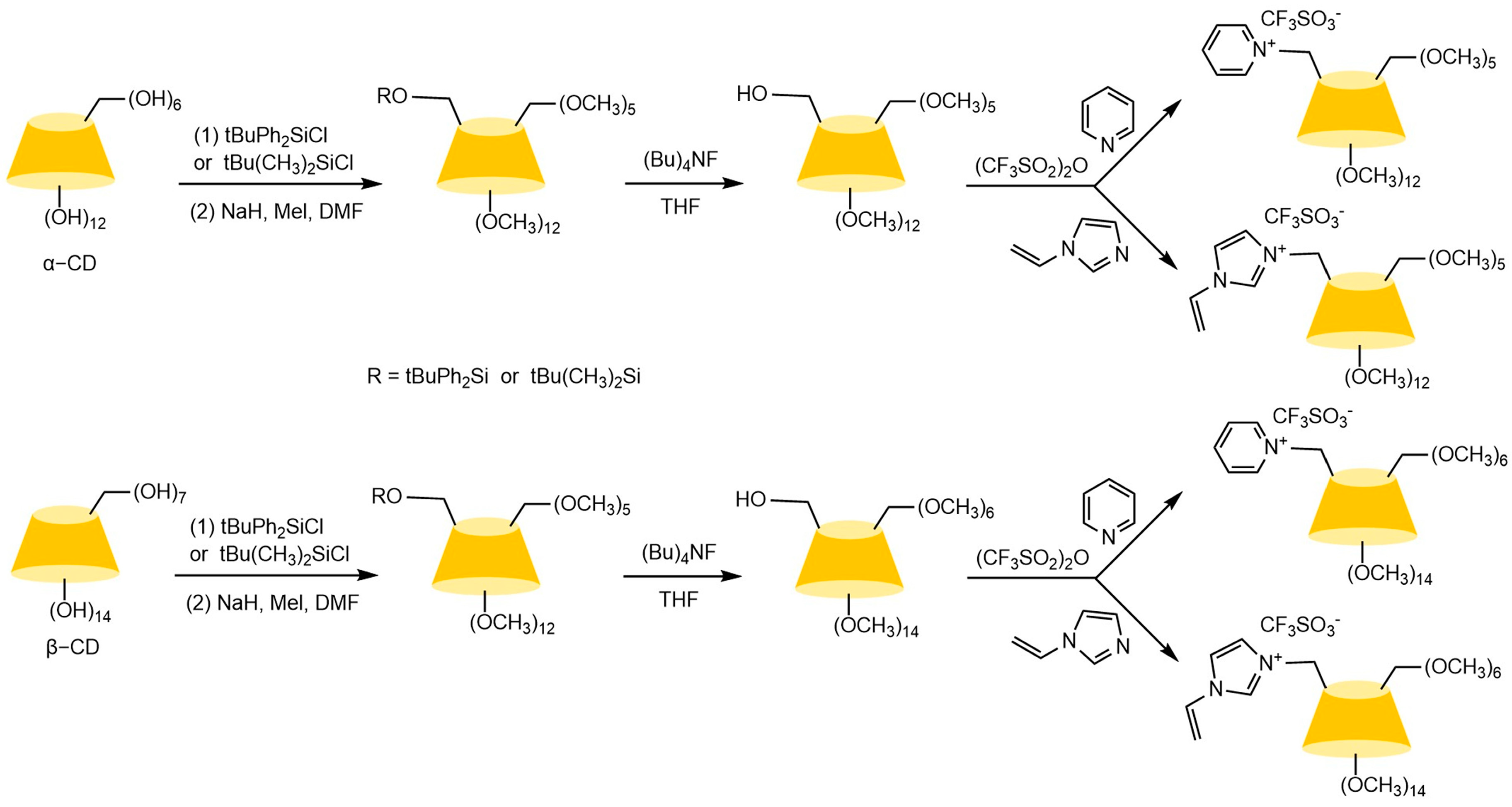
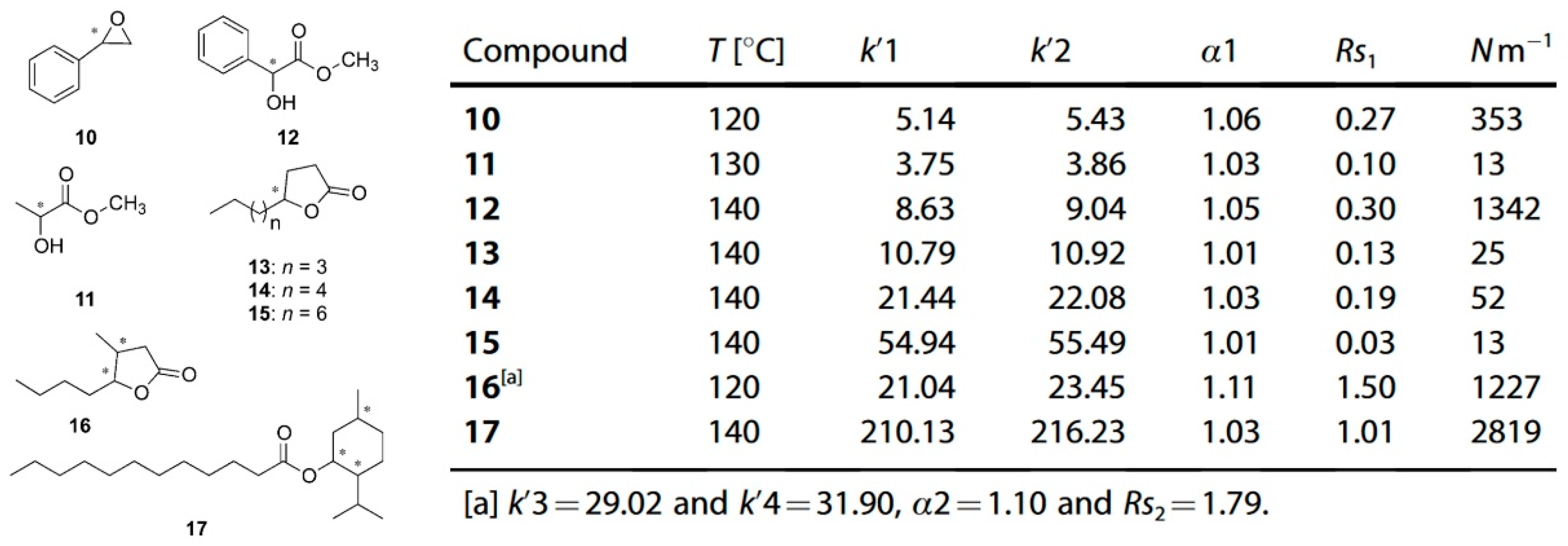
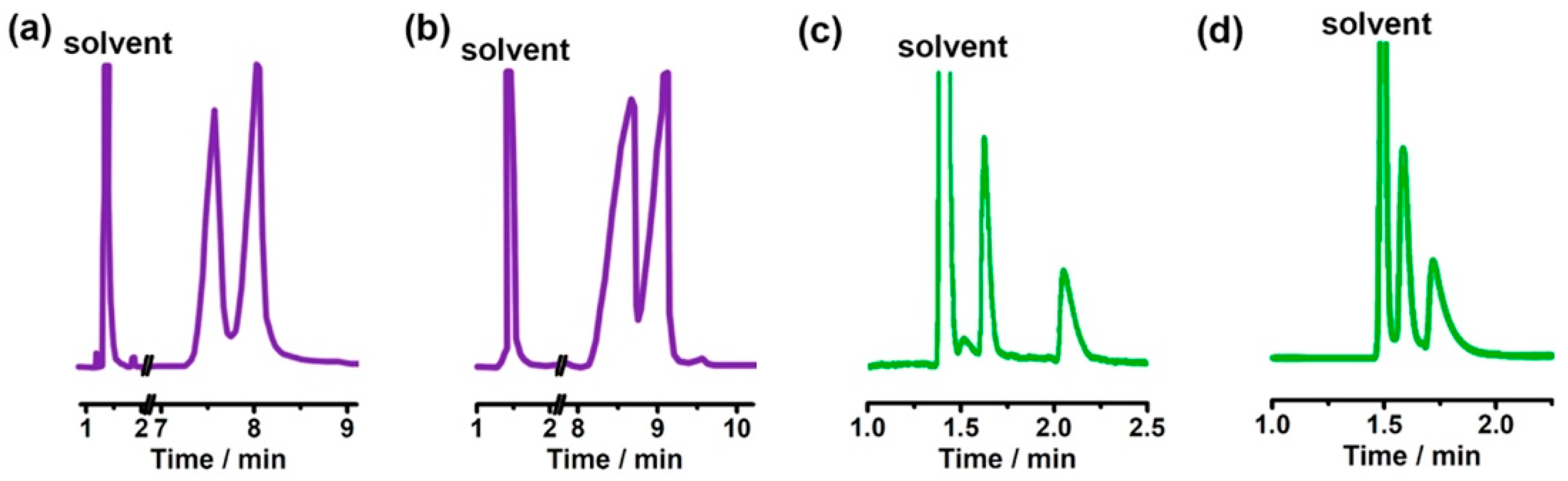
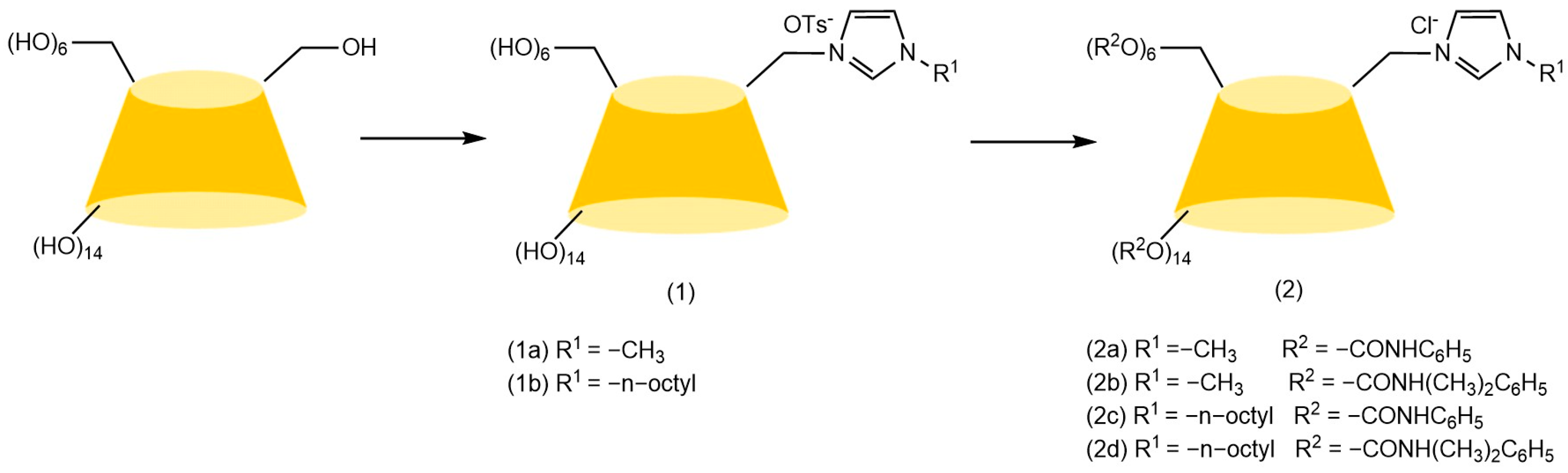
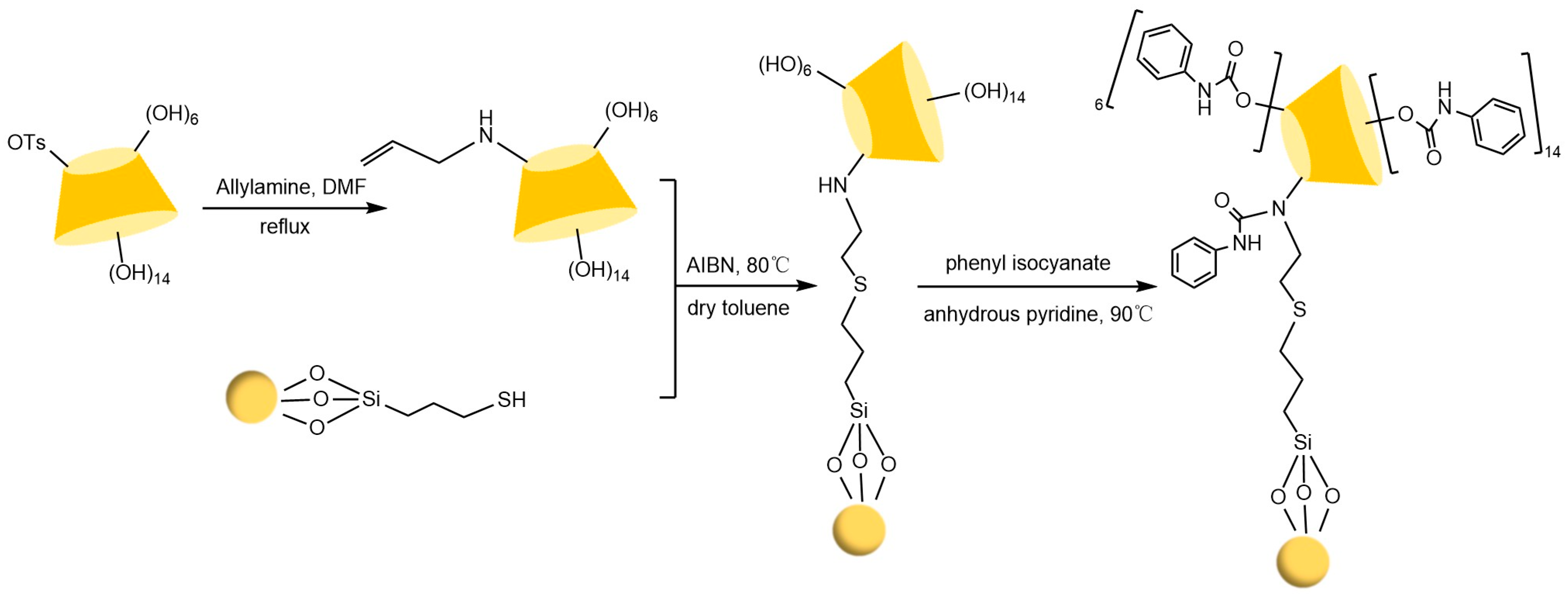
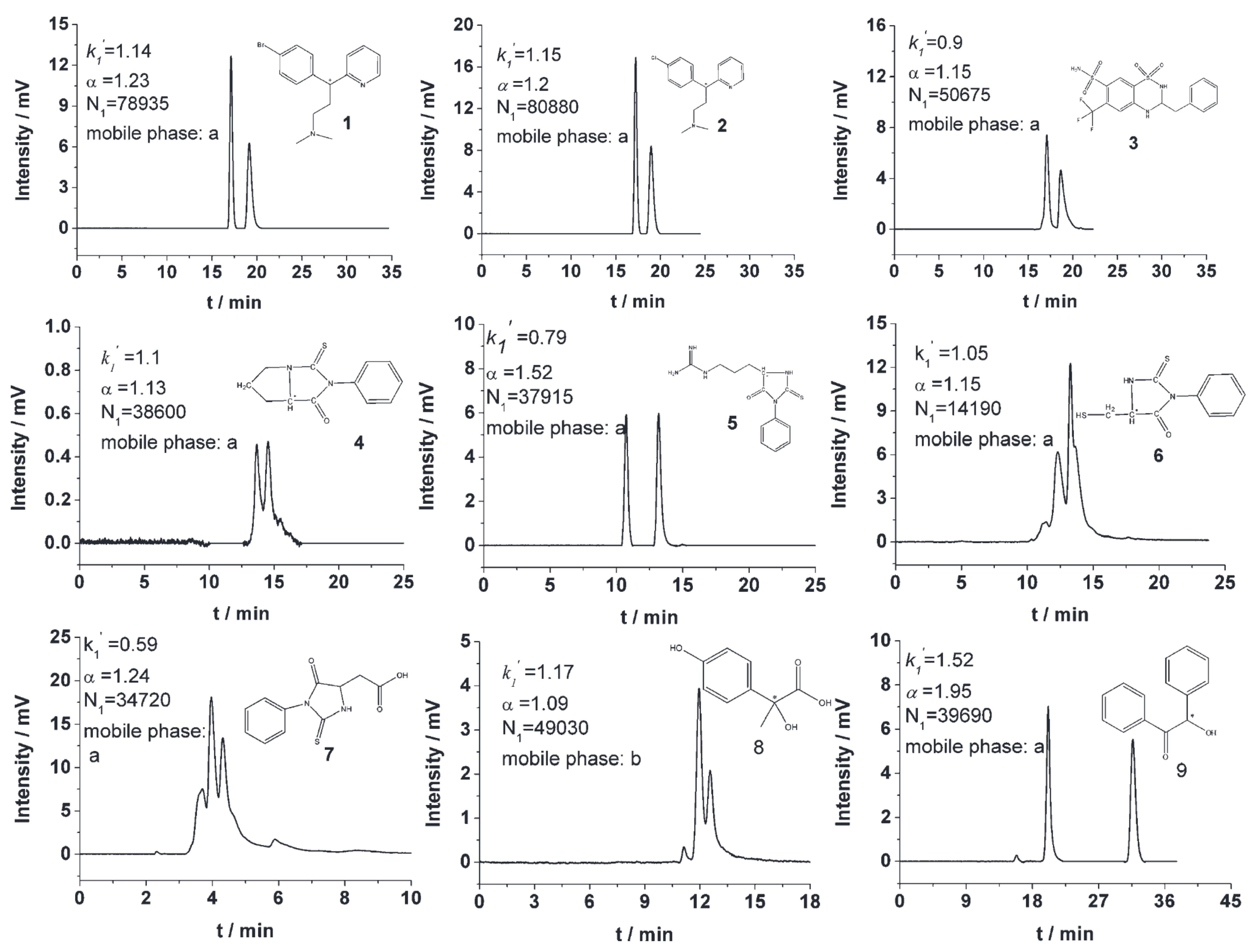
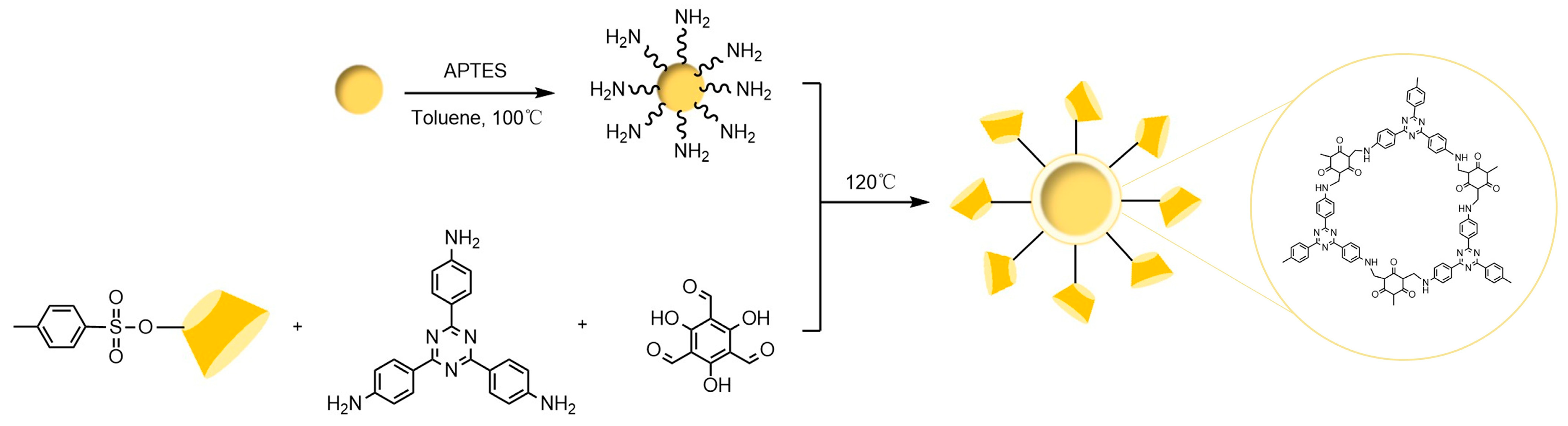
The chiral selectivity of β-CD primarily originates from the synergistic effects of 2- and 3-hydroxyl groups. The 3-position substituents, oriented toward the cavity interior, play a critical role in stereoselectivity [48]. The Kartsova group [49] constructed PEG-3000/β-CD composite stationary phases, employing solvation parameter models to quantitatively evaluate interaction mechanisms with organic compounds, revealing regulatory effects of polar groups on separation performance. Chaise et al. [50] developed dual-path synthesis strategies (see Figure 3): The indirect method achieves precise substitution via α-CD ring-opening reconstruction, while the direct method employs γ-CD selective protection-deprotection sequences for directional modification. The Shen team [51] designed novel β-CD derivatives shown in Figure 4, introducing pyridyl groups at the 3-position to enhance van der Waals interactions and pentylation at 2/6-positions to reduce melting points. This stationary phase combines strong inclusion capacity with hydrogen bond acceptor properties, significantly enhancing chiral separation selectivity. Costa et al. [52] developed four types of ionic liquid stationary phases (synthetic procedure shown in Figure 5), achieving baseline separation of racemic esters, lactones, and epoxides (see Figure 6). Experimental results demonstrate that the unique charge distribution and multiple interaction sites in ionic liquids synergistically enhance enantiomer recognition capability.
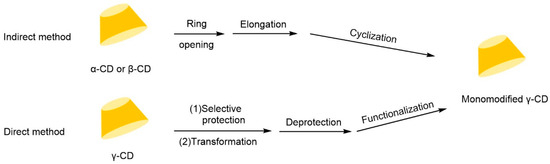
Figure 3.
Strategies for synthesizing single-modified γ-CD by direct and indirect methods [50].
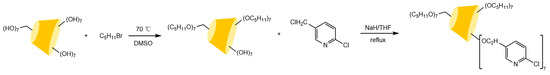
Figure 4.
Scheme for the synthesis of pyridinyl β-CD derivatives [51].
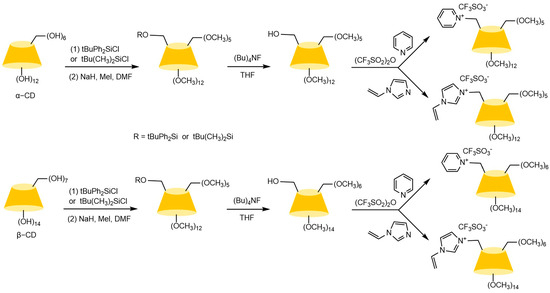
Figure 5.
Synthesis routes of four new ionic liquid cyclodextrin derivative stationary phases [52].
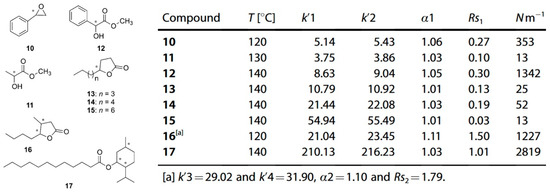
Figure 6.
Chromatographic parameters of enantiomeric separation of eight compounds (10–17) in an ionic liquid cyclodextrin stationary phase column. Figures adapted and reprinted with permission from Ref. [52].
3.2. MOF/COF-Cyclodextrin Stationary Phases
The application of cyclodextrin-based chiral stationary phases (CD-CSPs) in gas chromatography (GC) is more constrained compared to high-performance liquid chromatography (HPLC), primarily due to GCs stringent thermal stability requirements for stationary phases and the fabrication challenges of capillary column coatings. Recent advancements in novel porous materials, including metal-organic frameworks (MOFs) [53,54], covalent organic frameworks (COFs) [55], porous organic cages [56,57], and metal-organic cages (MOCs) [58], have brought breakthroughs to chiral separation technologies.
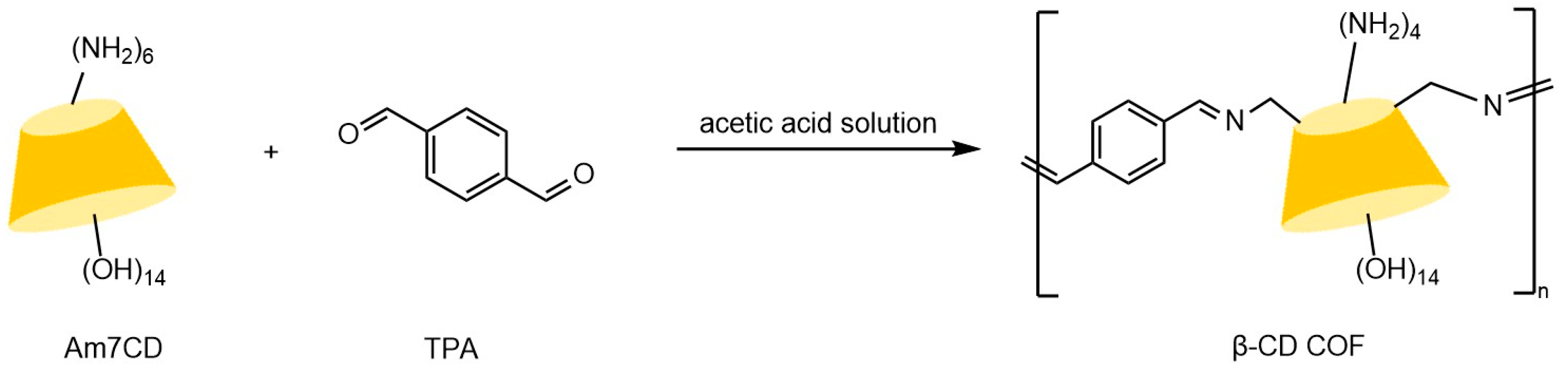
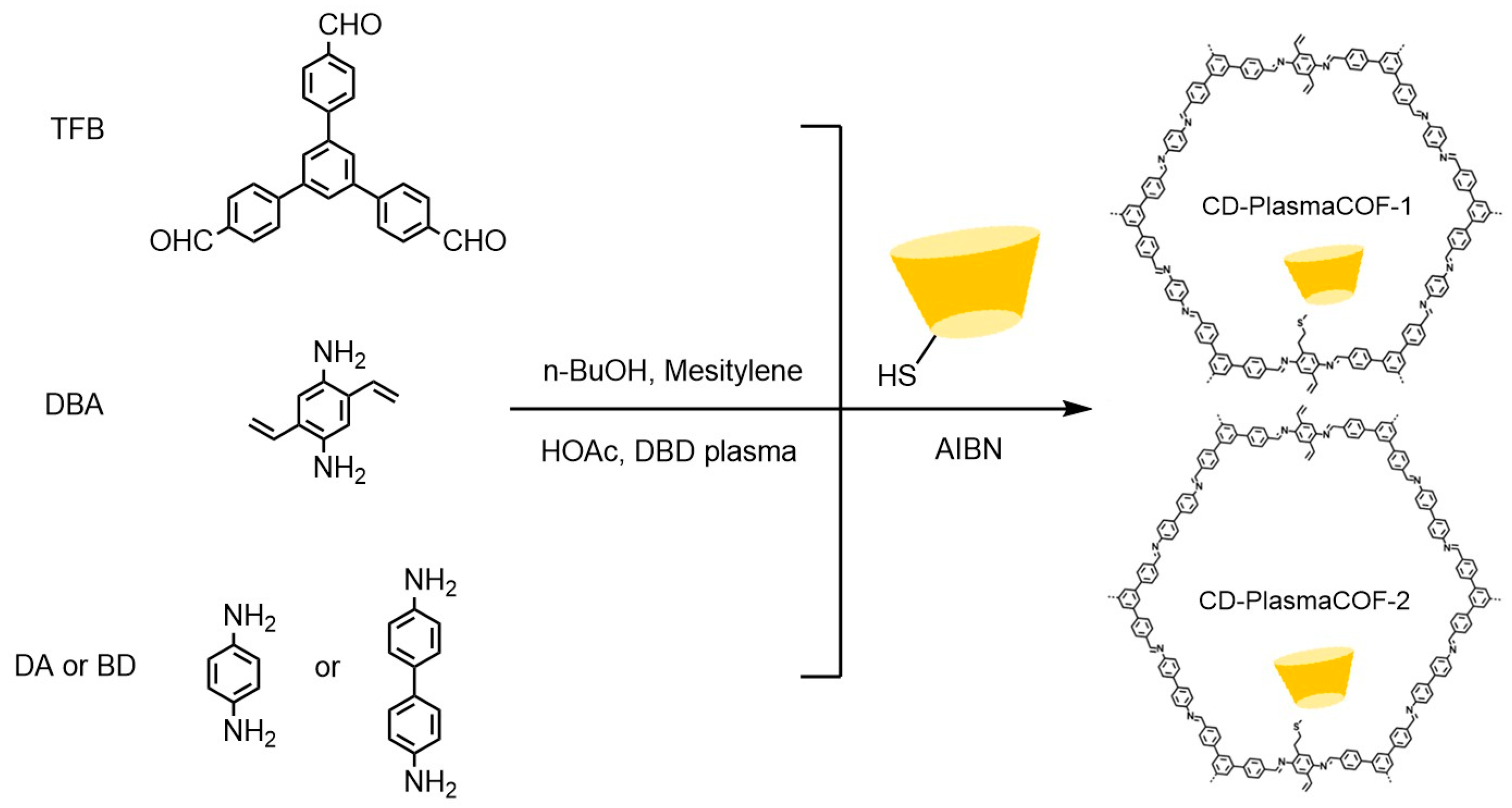
Yang et al. [59] demonstrated that hybrid CSPs prepared by integrating β-CD derivatives with chiral MOFs exhibit significantly enhanced enantioselectivity through synergistic effects, outperforming single-component materials. The development of covalent organic framework (COF) materials provides innovative solutions to address GC column preparation difficulties. These crystalline materials, constructed from organic units via reversible covalent bonds, feature highly ordered architectures with precisely tunable pore dimensions, shapes, and arrangements, coupled with exceptional specific surface areas and chemical stability. Tang et al. [60] developed a β-CD-based COF chiral stationary phase, synthesized through acid-catalyzed condensation of heptyl (6-amino-6-deoxy)-β-CD with terephthalaldehyde in Figure 7. Its permanent chiral cavities and high thermal stability demonstrated superior selectivity in GC separation of racemates. Yuan et al. [61] innovatively employed liquid DBD plasma technology to synthesize β-CD-COF composites within 4 h in Figure 8. This material combines the high-surface-area interface of COFs with the chiral recognition advantages of β-CD, with chromatograms from fabricated columns (see Figure 9) confirming remarkable separation efficiency for multiple enantiomers.
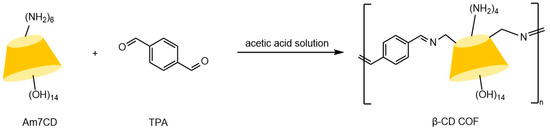
Figure 7.
Synthetic route to β-cyclodextrin covalent organic framework (β-CD COF) [60].
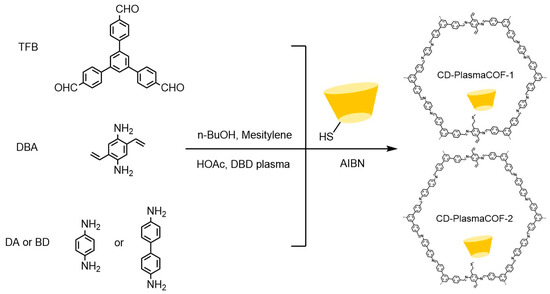
Figure 8.
Synthesis of CD-PlasmaCOF-1 and CD-PlasmaCOF-2 using plasma-induced polymerization combined with a post-synthesis modification strategy [61].
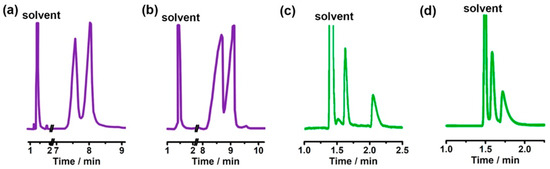
Figure 9.
(a,b) are enantiomeric separations on a CD-PlasmaCOF-1 coated GC column; (c,d) are enantiomeric separations on a CD-PlasmaCOF-2 coated GC column. (a) alanine: column temperature 100 °C under an N2 flow rate of 16.91 cm/s, and (b) valine: column temperature 120 °C under an N2 flow rate of 17.25 cm/s; (c) glutamine: column temperature 150 °C under an N2 flow rate of 16.7 cm/s, and (d) serine: column temperature 155 °C under an N2 flow rate of 15.9 cm/s on CD-PlasmaCOF-1 and CD-PlasmaCOF-2 coated GC columns. Figures adapted and reprinted with permission from ref. [61].
3.3. Column Coupling Technology
Chromatographic column coupling technology significantly enhances gas chromatographic separation efficiency by serially connecting complementary selectivity columns, effectively overcoming the limitations of single-column separation efficiency [62,63]. This technique has recently achieved critical breakthroughs in chiral separation through synergistic enhancement of separation performance by combining different stationary phases. The Tian team [64] employed a tandem configuration of Cyclosil-B chiral columns (stationary phase: 2,3-di-O-methyl-6-O-tert-butyldimethylsilyl-β-cyclodextrin) with BGB-175 chiral columns (stationary phase: 2,3-diacetyl-6-tert-butyldimethylsilyl-γ-cyclodextrin) in gas chromatography-mass spectrometry (GC-MS), successfully achieving high-efficiency separation of eight chiral menthol isomers in natural mint plants.

Table 2.
Cyclodextrin-based chiral stationary phase in GC.
Table 2.
Cyclodextrin-based chiral stationary phase in GC.
| Chiral Stationary Phase | Types of Cyclodextrin | Synthesis Protocol | Chiral Compounds | Ref. |
|---|---|---|---|---|
| mono-6-O-benzyl methylated γ-cyclodextrin | α, β, γ-CD | indirect and direct strategies | Menthol/Linalool/α-Terpineol p-Fluorophenylethan-1-ol p-Chlorophenylethan-1-ol Phenylethan-1-ol Pentan-2,4-diol Methyl mandelate 5-Methyl-5-propylhydantoin | [50] |
| heptakis [2,6-di-O-pentyl-3-O-(49-chloro-59-pyridylmethyl)]- β-CD | β-CD | two-step procedure | 1-Phenylethylamine 5-Hydroxy-4,4-dimethyl-dihydro-furan-2-one 1-(4-Chlorophenyl) ethanol 1-(2,4-Dichlorophenyl)-ethanol Methyl a-chloropropionate 2-Bromooctane Methyl 2,2-dimethyl-3-form-acylcyclopropanecarboxylate Methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcycl opropane-carboxylate | [51] |
| mono-6-deoxy-6-pyridin-1-ium/ mono-6-deoxy-6-(1-vinyl-1H-imidazol-3-ium)-α/ β-cyclodextrin trifluoromethanesulfonate ionic liquids | α, β, γ-CD | one-pot reaction solvent-free procedure | lactone epoxides hydroxy esters heavy ester | [52] |
| InH(D-C10H14O4)2-Peramylated β-Cyclodextrin | permethylated β-Cyclodextrin | coated | (±)-Citronellal (±)-Methyl l-β-hydroxyisobutyrate (±)-Limonene/(±)-Dihydrocarvyl (±)-Menthol/(±)-citronellal (±)-1-Phenylethanol-Leucine | [59] |
| β-CD-COF | heptakis(6-amino6-deoxy)-β-CD | condensation reaction | (±)-1-(3-methylphenyl)ethanol (±)-butyl glycidyl ether (±)-rose oxide dl-methionine/dl-histidine dl-glutamine/dl-serine dl-tyrosine/dl-glutamic acid | [60] |
| CD-PlasmaCOF-1/ CD-PlasmaCOF-2 | 6-deoxy-6-mercapto-β-cyclodextrin | thiol-ene click chemistry reaction | 3-benzyloxy-1,2-propanediol trans-stilbene oxide/furoin 2-methoxy-2-phenylethanol 1-phenylethylalcohol N-(3,5-dinitrobenzoyl)- leucine 2-methyl butyric acid 2-chloropropionic acid butyl glycidyl ether styrene oxide/α-pinene ethyl 3-hydroxybutyrate 1-(1-naphthyl)ethylamine | [61] |
| hepatkis(2,3-di-O-methyl-6O-t-butyl dimethylsilyl)-β-cyclodextrin coupled with 2,3diacetyl-6-tert-butyldimethylsilyl-γ-cyclodextrin | β, γ-CD | column coupling technology | eight menthol enantiomers | [64] |
4. Cyclodextrin-Derived Stationary Phases in High-Performance Liquid Chromatography
High-Performance Liquid Chromatography (HPLC), which evolved from gas chromatography (GC), is a rapid separation technique distinguished by its ability to analyze thermally labile and non-volatile compounds without requiring sample vaporization. Compared to GC, HPLC not only enables multidimensional separation through adjustments of stationary and mobile phases but also offers advantages in analysis speed, sensitivity, and broad applicability. Currently, HPLC has matured into the preferred method for chiral compound separation, identification, and quantitative analysis. The most common approach for preparing cyclodextrin-based chiral stationary phases (CD-CSPs) involves immobilizing CDs onto silica gel via physical coating or covalent bonding.
The physical coating method [65] involves adsorbing cyclodextrin derivatives directly onto silica gel surfaces. While straightforward (e.g., Wang et al. [66] successfully prepared a novel stationary phase by coating silica gel with cationic cyclodextrins, as show in Figure 10), this method suffers from limitations such as low mechanical strength and restricted applicability in normal-phase chromatography, resulting in limited research and application.
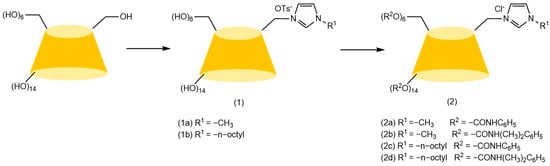
Figure 10.
Synthesis route of novel cationic functionalized cyclodextrins by physical coating method [66].
The covalent bonding method chemically anchors cyclodextrins to silica gel, offering superior stability (compatible with diverse mobile phases) and extended lifespan. Typically, spacer arms are used to covalently link the reactive hydroxyl groups of cyclodextrins or their derivatives to silica gel surfaces, with the most reactive 6-hydroxyl group preferentially reacting with the spacer. Since Fujimura pioneered the amino-bonding strategy in 1983 [67], research has focused on developing novel bonding arms and optimizing separation performance, driving innovative applications of CD-CSPs in chiral resolution of amino acids, aromatic alcohols, and other chiral compounds. Reported bonding arms include carbamate [68,69,70], urea [71,72], ether [73,74,75], and thioether [76,77,78] linkages, which are summarized in Table 3.
4.1. Ether-Linked Cyclodextrin Derivative Stationary Phases
In 1984, Armstrong’s research group [79] pioneered the development of structurally stable ether-bonded cyclodextrin stationary phases. By substituting the hydroxyl hydrogen of cyclodextrin with sodium hydride and linking it to a coupling agent, they immobilized the cyclodextrin onto a silica matrix. This stationary phase successfully achieved chiral resolution of dansyl amino acids and barbiturates under reversed-phase chromatographic conditions. Li et al. [80] reported mono-6-deoxy-(2,4-dihydroxybenzylimino)-cyclodextrin (MDHB-CD) as a novel chiral selector, demonstrating excellent performance in the separation of various chiral compounds (see Figure 11). Chen’s research team [81] utilized a long-chain spacer arm to covalently bond mono(6A-N-1-(2-hydroxyl)phenylethylimino-6A-deoxy)-β-cyclodextrin (L-PGCD-CSP) onto silica surfaces in Figure 12. The resulting novel chiral stationary phase exhibited superior enantioselectivity for multiple enantiomers in reversed-phase chromatographic systems. Despite the favorable stability of ether-bonding strategies [82], uncertainties in the hydroxyl bonding sites (C-6, C-2, C-3) of cyclodextrin molecules and the number of bonded arms have limited the preparation reproducibility of such stationary phases, necessitating further improvements.
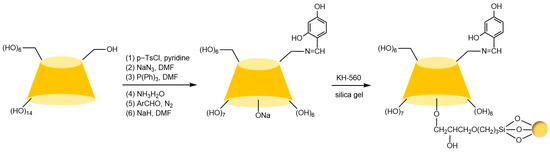
Figure 11.
Synthesis route of ether-bonded MDHB-CD chiral stationary phase [80].
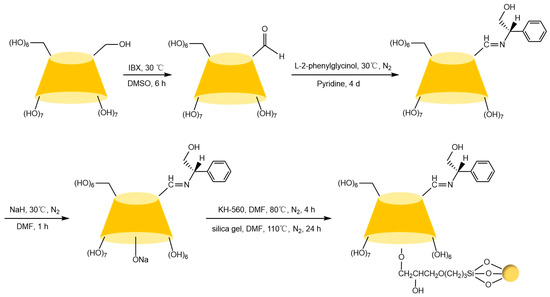
Figure 12.
Synthesis principle of ether-bonded L-PGCD-CSP columns [81].
4.2. Aminocarbamate-Bonded Cyclodextrin-Derived Chiral Stationary Phases
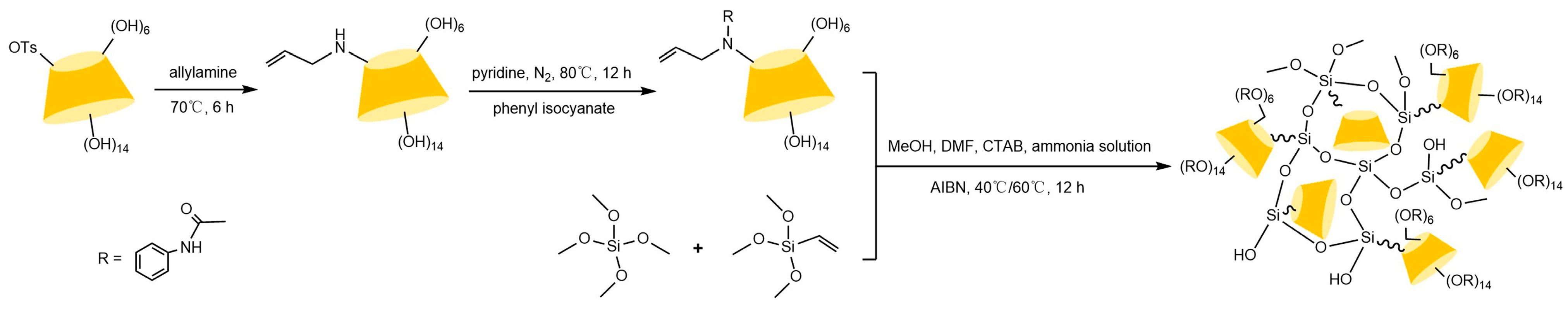
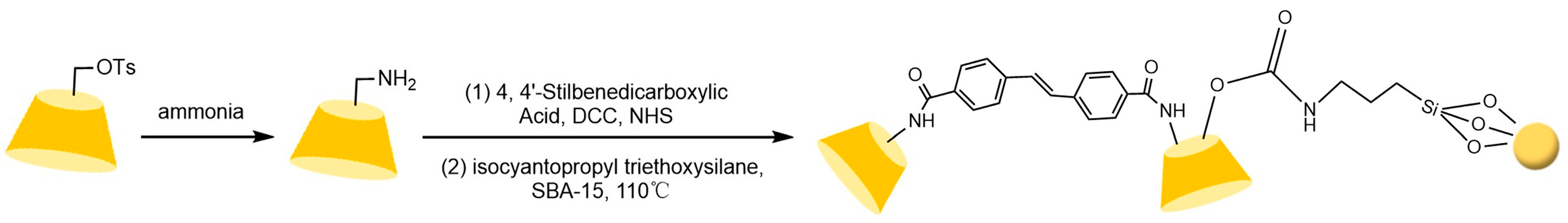
Zhong et al. [83] and subsequent research teams [84,85,86,87,88] developed multimodal CD-CSPs systems through portal functionalization of cyclodextrins (e.g., methylation, hydroxypropylation, and aryl carbamoylation). For instance, hydroxypropylated CD-CSPs successfully resolved rigid molecules such as dihydropyridines, while naphthylethyl carbamate derivatives (π-basic) achieved effective chiral resolution of pesticides (e.g., dichlorvos) and pharmaceuticals (e.g., tropicamide) under normal/reversed-phase conditions. Studies confirmed that these stationary phases operate via a three-point recognition mechanism, involving cavity expansion, enhanced inclusion complexation, and synergistic interactions such as hydrogen bonding, dipole-dipole, and π-π interactions. Recent advancements focus on novel carrier design: Ai et al. [89] synthesized submicron mesoporous silica carriers loaded with phenylcarbamoylated cyclodextrins through optimized protocols, shown in Figure 13, while Zhang et al. [90] innovatively prepared β-cyclodextrin-silica hybrid monolithic columns (see Figure 14) via an alkoxysilane co-condensation “one-pot” strategy, achieving high-efficiency separation of 13 racemates in capillary chromatography in Figure 15. Both approaches enhanced stationary phase performance by refining carrier morphology and bonding strategies.
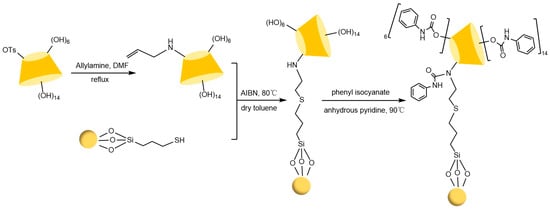
Figure 13.
Synthesis route of phenylcarbamoyl-β-CD modified mesoporous silica particles CSP [89].
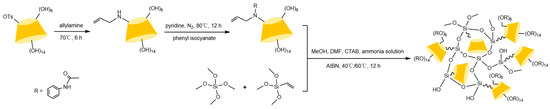
Figure 14.
Scheme for the preparation of a capillary column of mono(6A-N-allylamino-6A-deoxy)-Ph-β-CD [90].
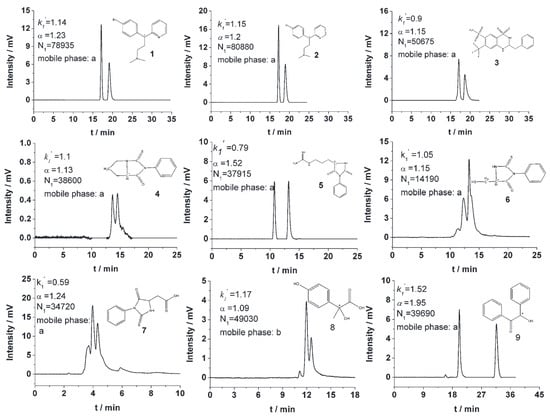
Figure 15.
Enantiomeric separation of chiral compounds on a mono(6A-N-allylamino-6A-deoxy)-Ph-β-CD silica hybrid monolithic column. Experimental conditions: effective length, 30 cm × 75 μm i.d.; mobile phase, MeOH/TEAA (pH = 4.2) = 60:40, MeOH/TEAA (pH = 4.2) = 80:20, Hexane/IPA = 90:10; flow rate, 120 nL/min; detection wavelength, 214 nm. Figures adapted and reprinted with permission from ref. [90].
4.3. Urea Bond Linkages Cyclodextrin Derivative Stationary Phases
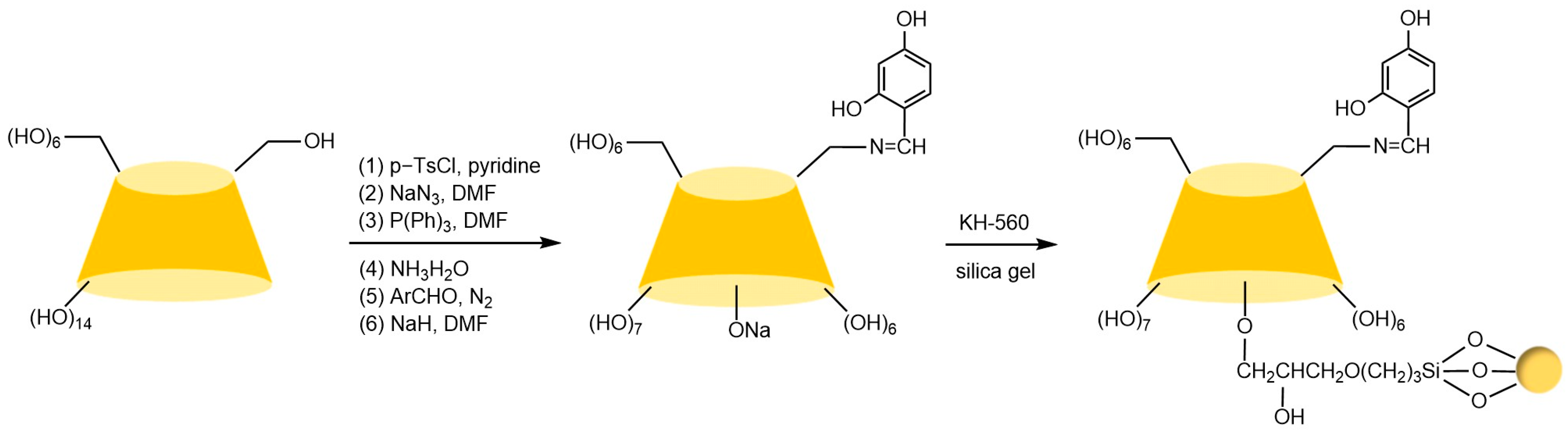
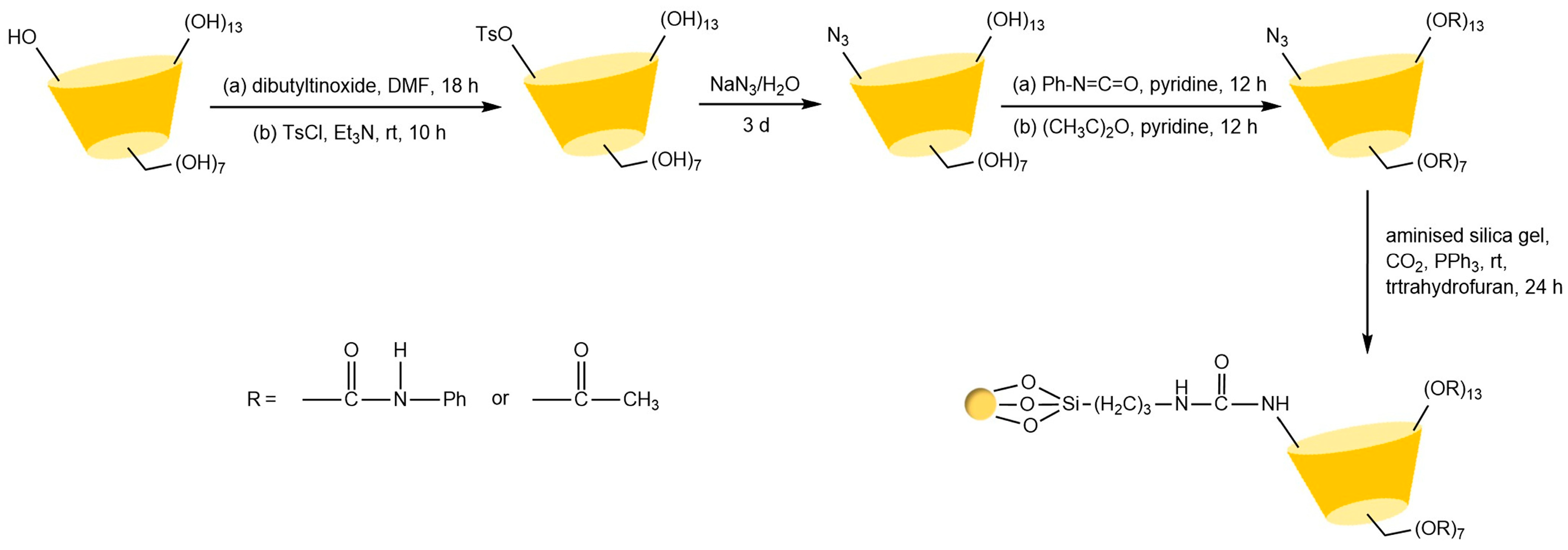
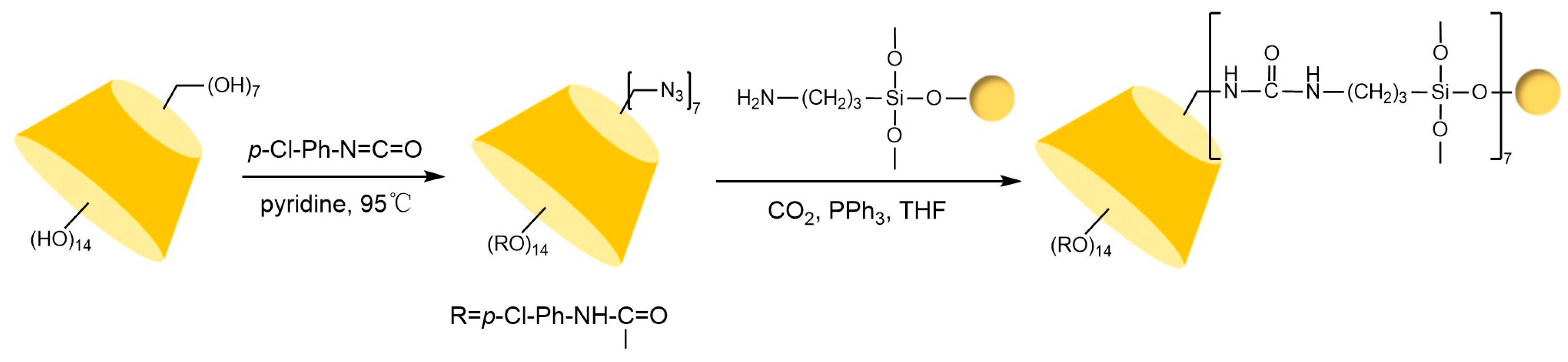
The key to preparing urea bond-linked stationary phases via the Staudinger reaction lies in the selective reaction between azido-functionalized β-cyclodextrin and aminated silica under CO2 conditions. Ng’s team [91] successfully constructed a cyclodextrin stationary phase connected by a single urea bond (see Figure 16) through precise control of reaction conditions under triphenylphosphine catalysis. This phase not only exhibited excellent chemical stability but also significantly enhanced chiral recognition capabilities due to its hydrogen bond donor properties. Notably, the hydroxyl-sensitive nature of this reaction system limits its application in the modification of certain derivatized cyclodextrins. Building on this, Lin et al. [71] innovatively immobilized hepta(6-azido-6-deoxy-2,3-di-O-(p-chlorobenzyl carbamate))-β-cyclodextrin onto silica via multiple urea bonds in Figure 17, achieving successful enantioseparation of metal-based chiral benzene complexes. However, residual amino groups on the silica surface from incomplete reactions may interfere with the separation process through hydrogen bonding interactions.
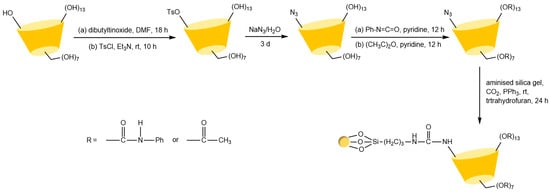
Figure 16.
Synthesis routes of two CSP stationary phases prepared by the Staudinger reaction [91].
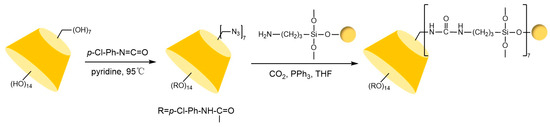
Figure 17.
Synthesis route for urea-bonded MCDP chiral stationary phases [71].
4.4. Thioether-Bonded Cyclodextrin Derivative Stationary Phases
Click chemistry, first proposed by the Sharpless team in 2001, encompasses two core reactions: “azide-alkyne” and “thiol-ene” [92]. The former generates a 1,2,3-triazole heterocyclic structure via the reaction of azide compounds with alkynes, while the latter forms thioether bonds through the interaction of thiols with alkenes. Both reactions are characterized by mild conditions, high efficiency, and minimal byproducts. Among these, the “thiol-ene” reaction [93,94] has seen increased application in recent years due to its metal catalyst-free nature and the exceptional stability of thioether bonds [95,96].
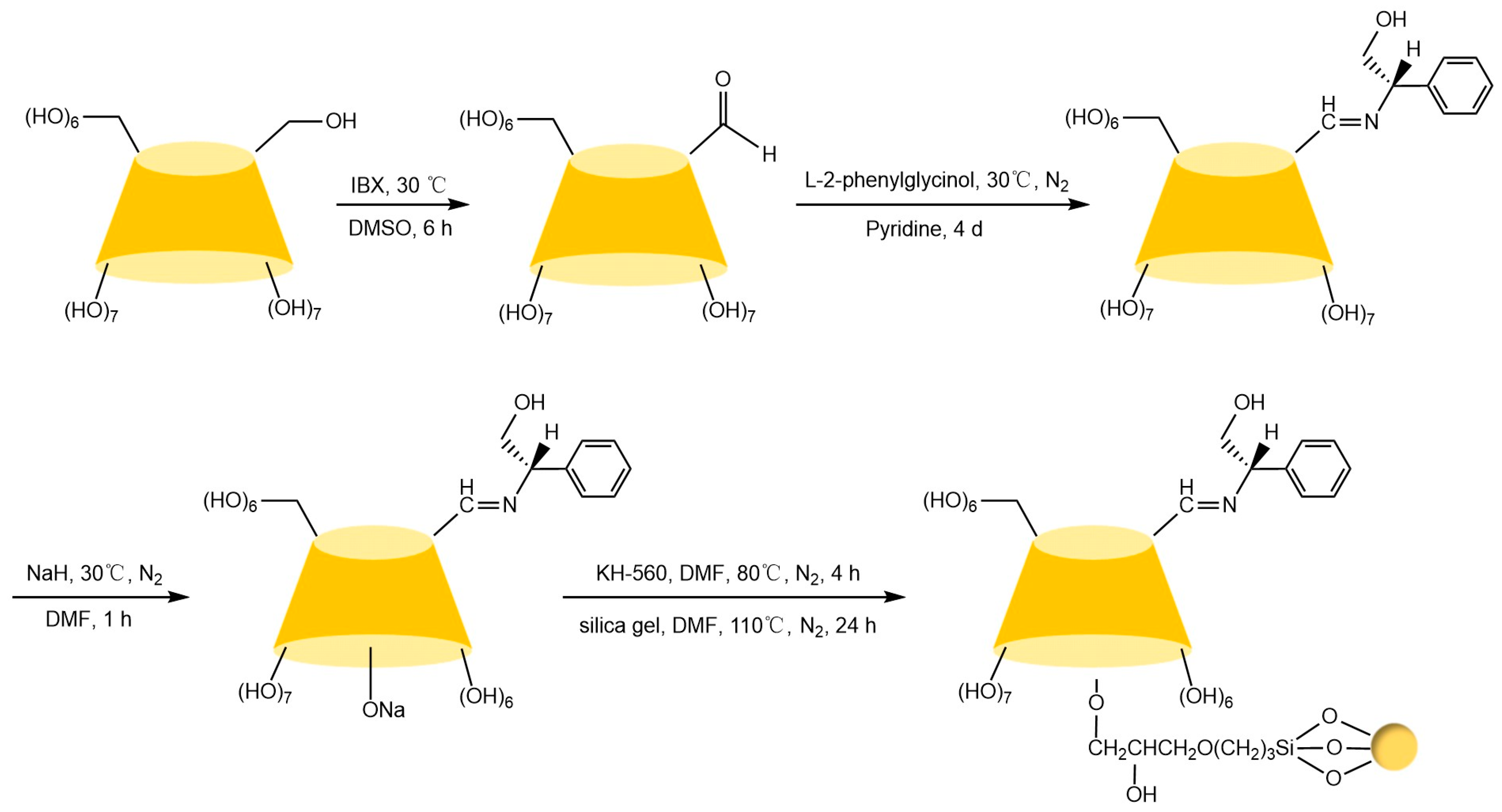
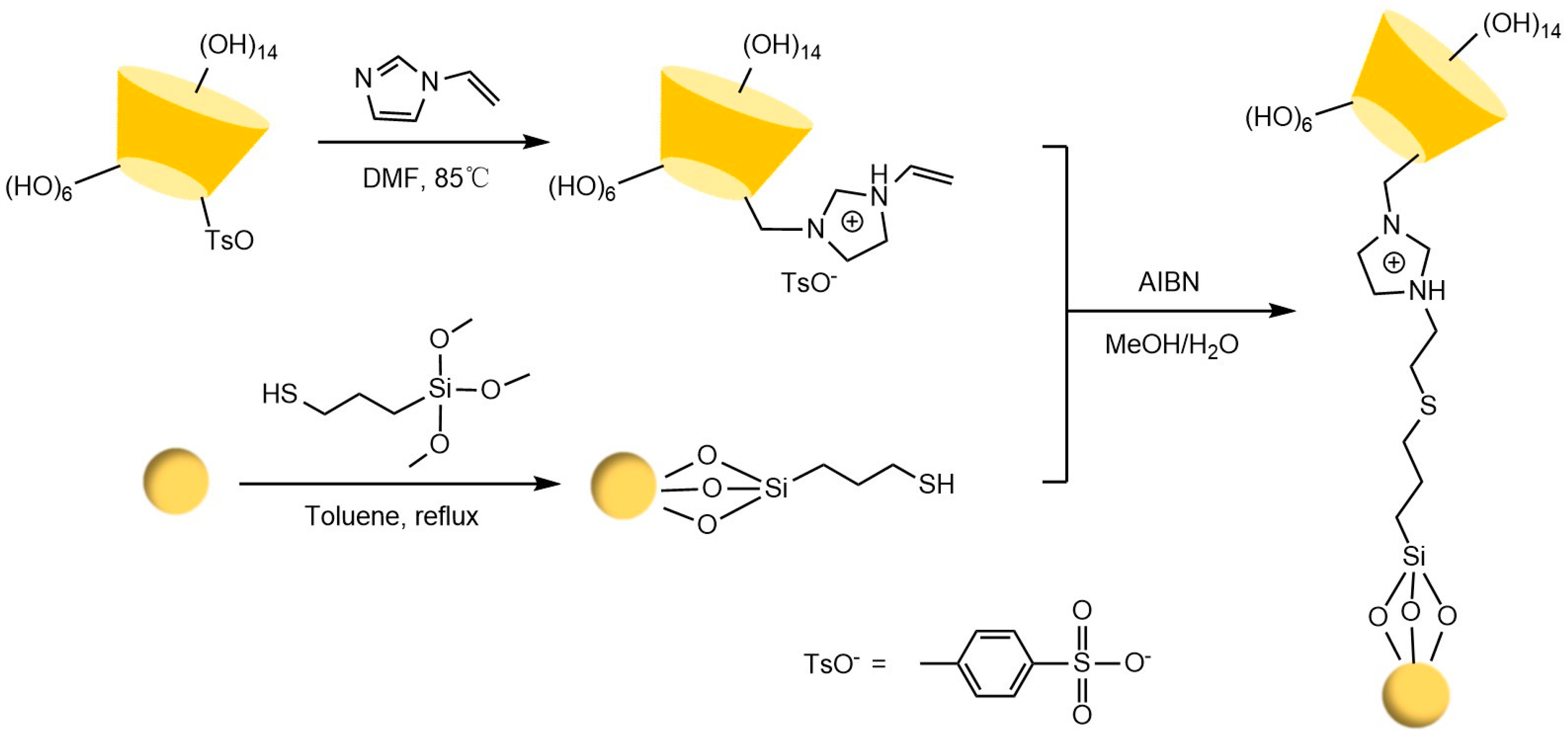
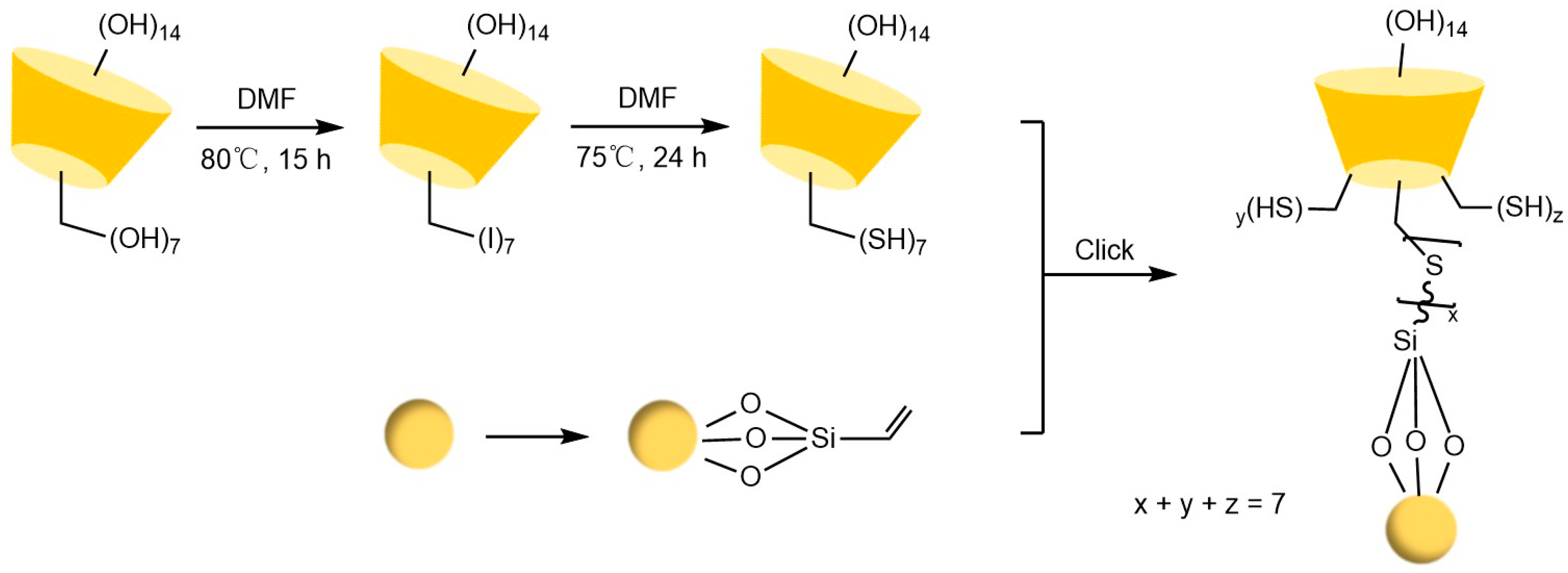
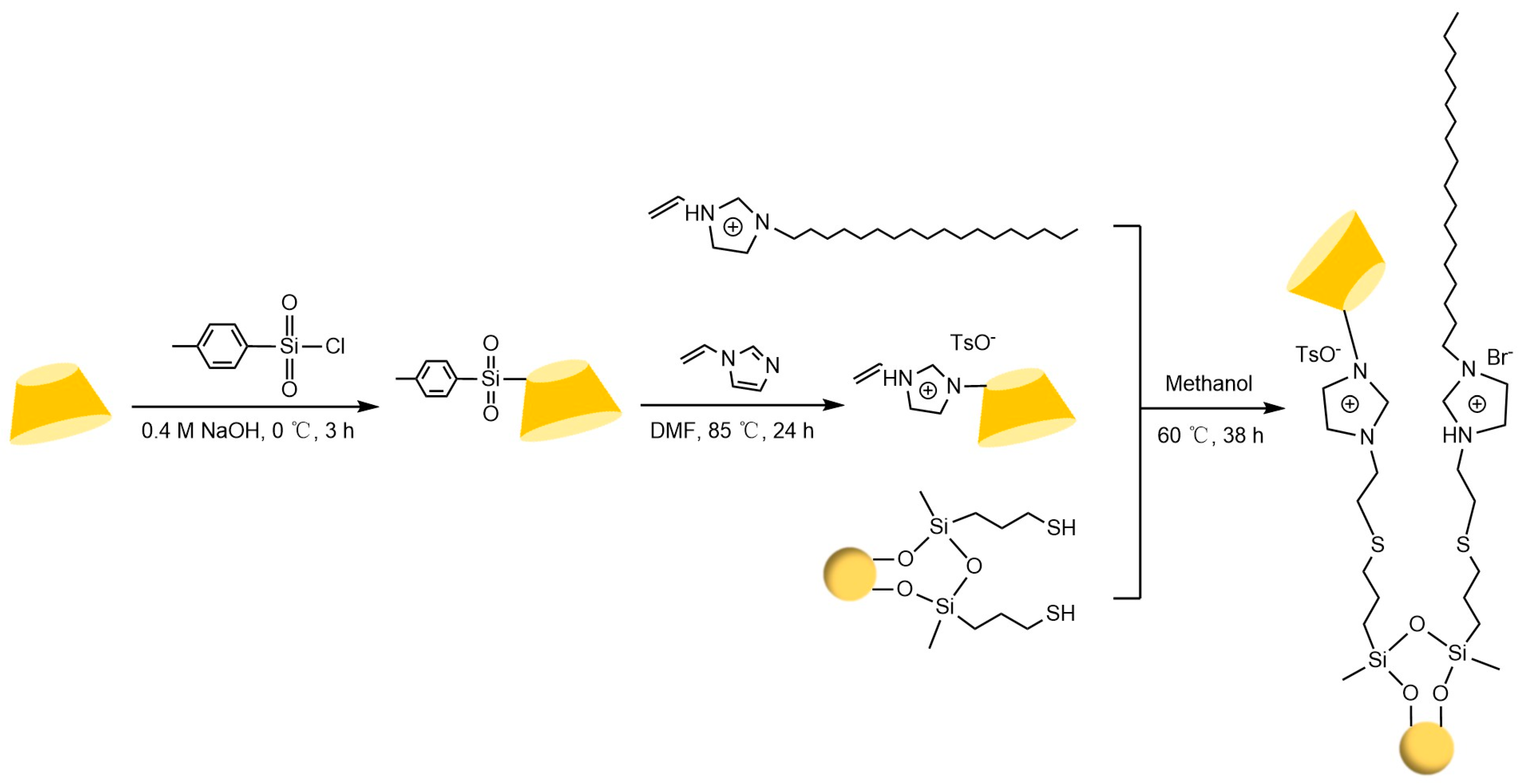
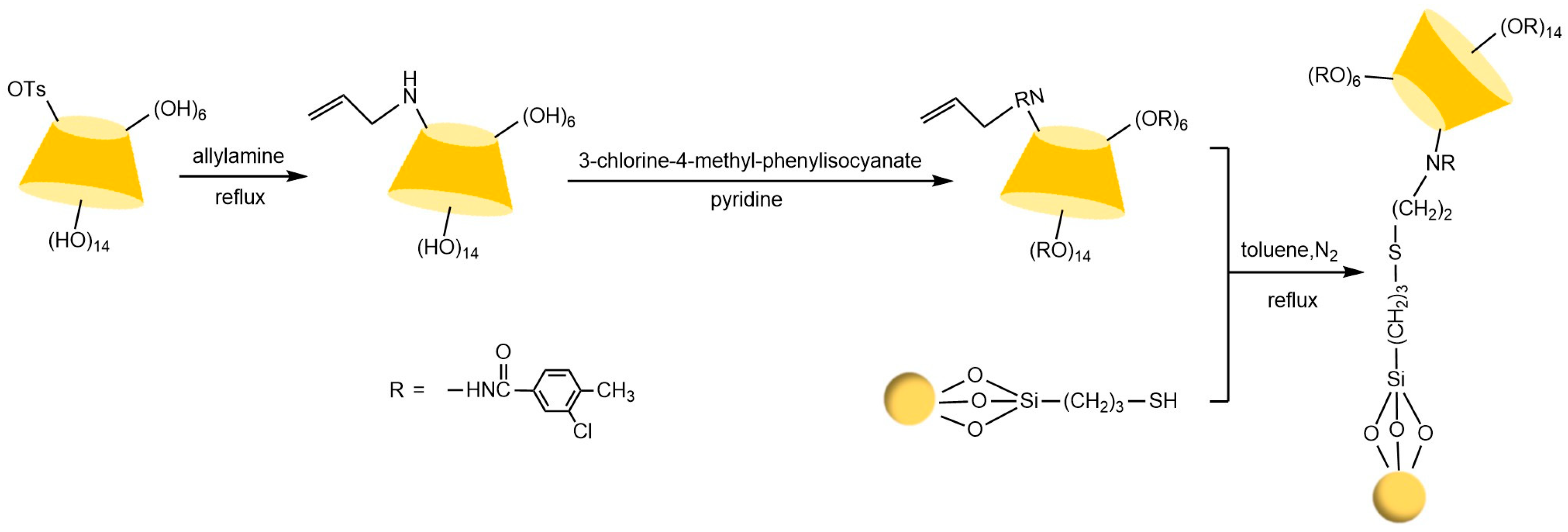
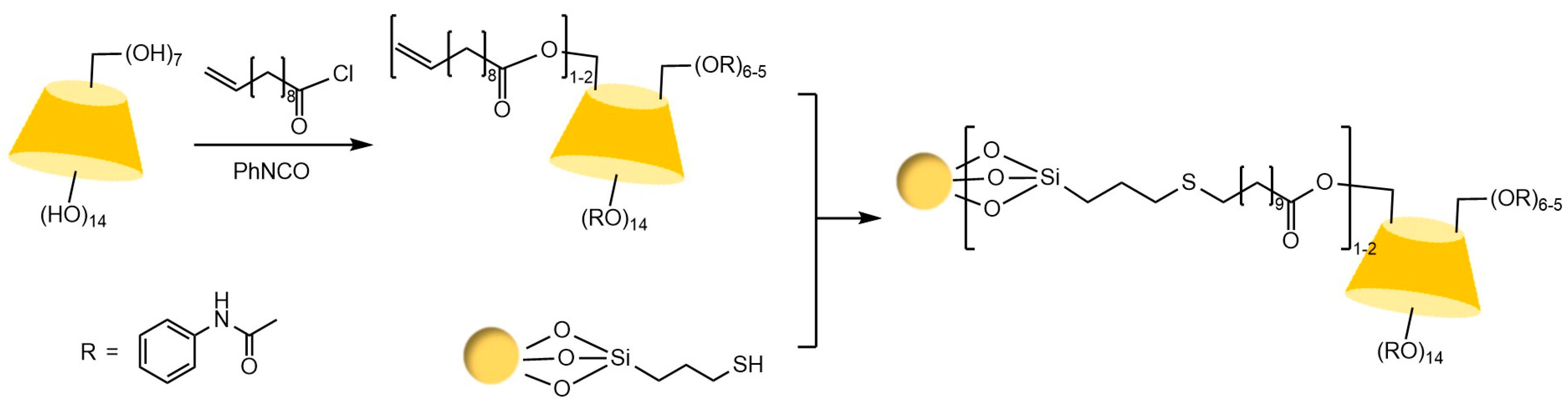
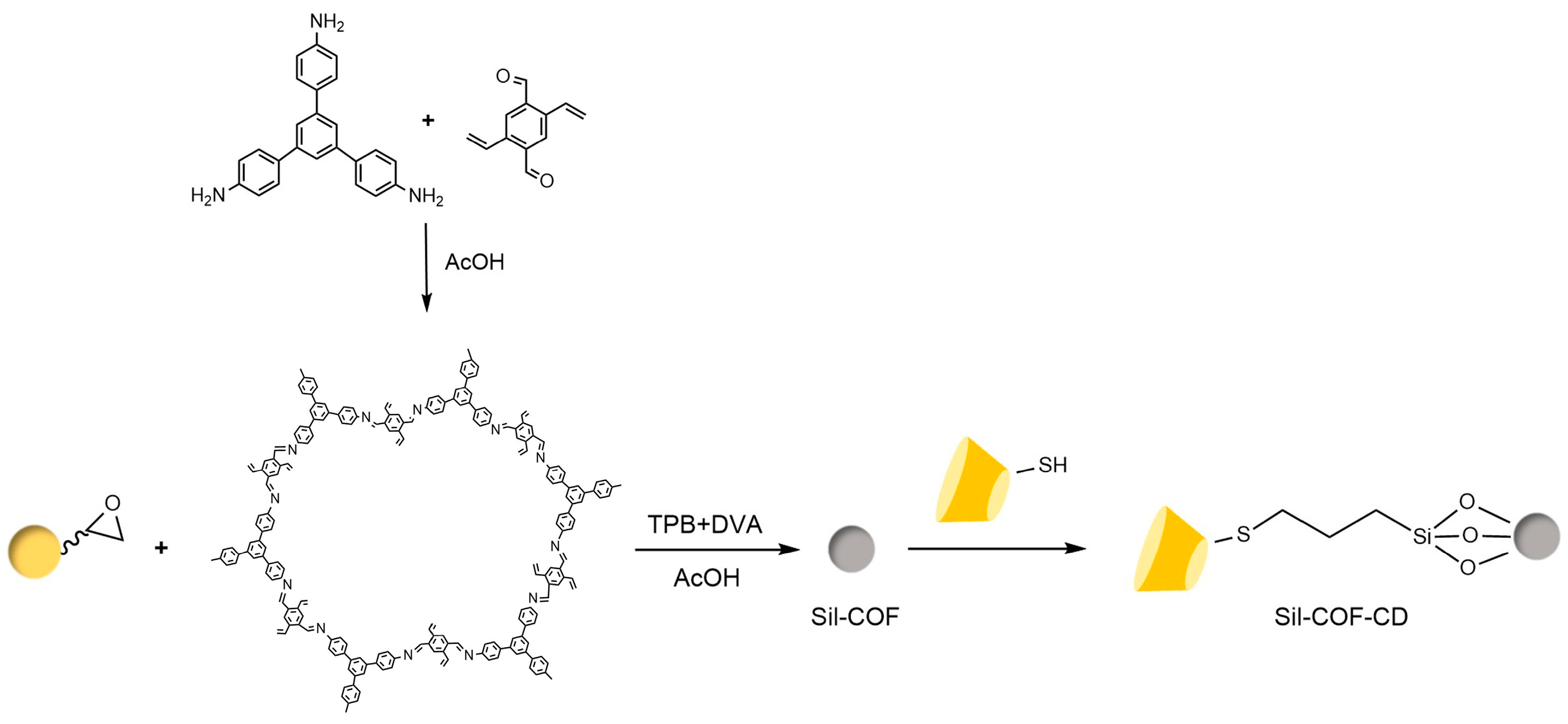
Liang et al. [77,78] pioneered the use of the “azide-alkyne” click reaction to prepare CD-CSPs with triazole-bonded arms, successfully resolving chiral molecules such as benzoin and β-blockers. Their work demonstrated that polar triazole arms enhance chiral recognition. Yao’s team [97] developed a monocationic β-CD-CSP via the “thiol-ene” reaction in Figure 18, which exhibited excellent resolution for conventional molecules (e.g., dansyl amino acids) and achieved the first efficient separation of isoxazoline derivatives. Wang’s group employed thioether bond modulation strategies to create a mono(6-thiol-6-deoxy)-β-CD-CSP [98] and multi-thioether-bridged CD-CSPs [99], shown in Figure 19, revealing complementary chiral recognition capabilities between the two. Zhou et al. [100] combined β-CD with C18 to fabricate a mixed-mode CSP (see Figure 20), demonstrating multidimensional separation performance in reversed-phase, hydrophilic, and ion-exchange chromatographic systems. Zhang et al. [101] synthesized a novel p-3-chloro-4-methylphenylcarbamoylated-β-CD-CSP via thioether bonding in Figure 21, which showed strong resolution for timolol. Huang’s team [102] introduced a benzoylated CD-CSP modified with long hydrophobic chains in Figure 22, significantly improving the separation of drugs like propranolol. Li et al. [103] designed an allylimidazolium-bridged bis-β-CD stationary phase, with molecular docking revealing that synergistic interactions underpin its high-efficiency resolution of 35 racemates. Wen’s group [104] utilized COF-modified silica technology to construct a Sil-COF-β-CD composite via thiol-click chemistry (synthesis process shown in Figure 23), where the spherical structure markedly enhanced separation efficiency in Figure 24. Zeng et al. [105] developed a bis-triazole-bridged β-CD stationary phase (BCDP, see Figure 25), which achieved the separation of 35 chiral pesticides and pharmaceuticals through multi-cavity interactions (hydrogen bonding, π-π stacking, etc.), highlighting a unique synergistic inclusion mechanism.
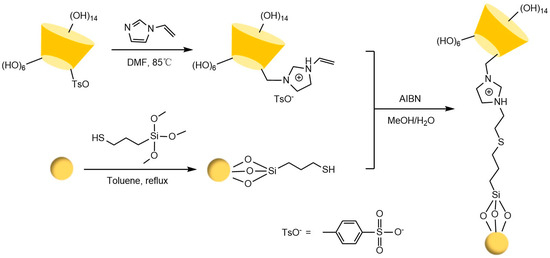
Figure 18.
Synthesis route of novel cationic CSP [97].
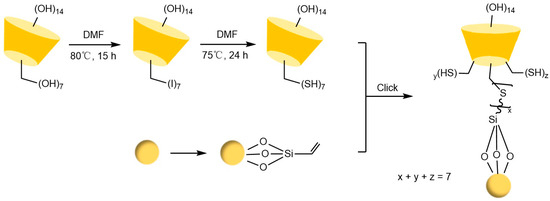
Figure 19.
Novel synthetic routes for polysulfide-bridged CD-CSP [99].
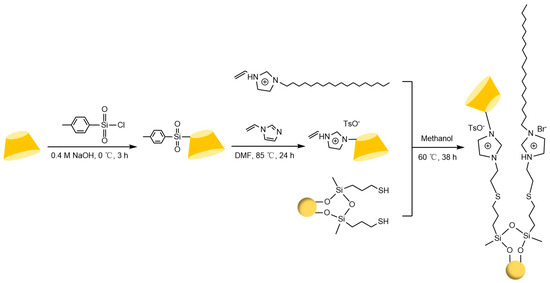
Figure 20.
Preparation route for Sil-VMBD stationary phase material [100].
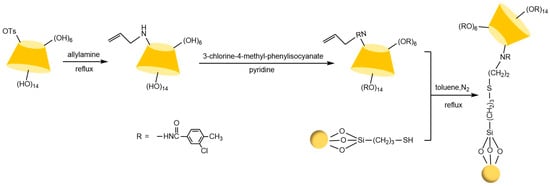
Figure 21.
Synthetic route to p-3-chloro-4-methylphenylcarbamoylated-βCD-CSP [101].
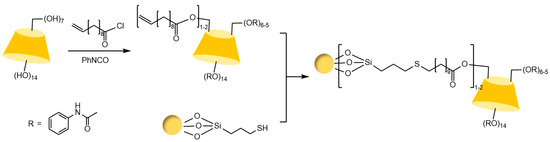
Figure 22.
Synthetic routes for novel benzoylated CD-CSPs [102].
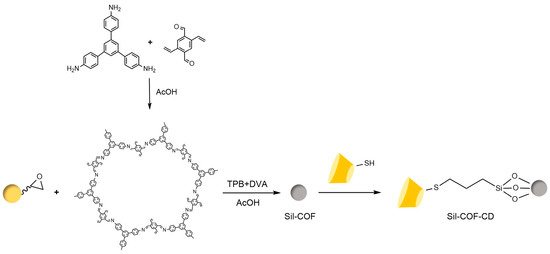
Figure 23.
Chiral stationary phase Sil-COF-CD synthesized using thio click chemistry [104].
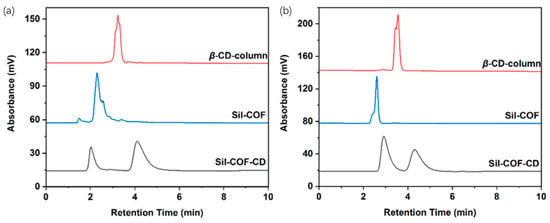
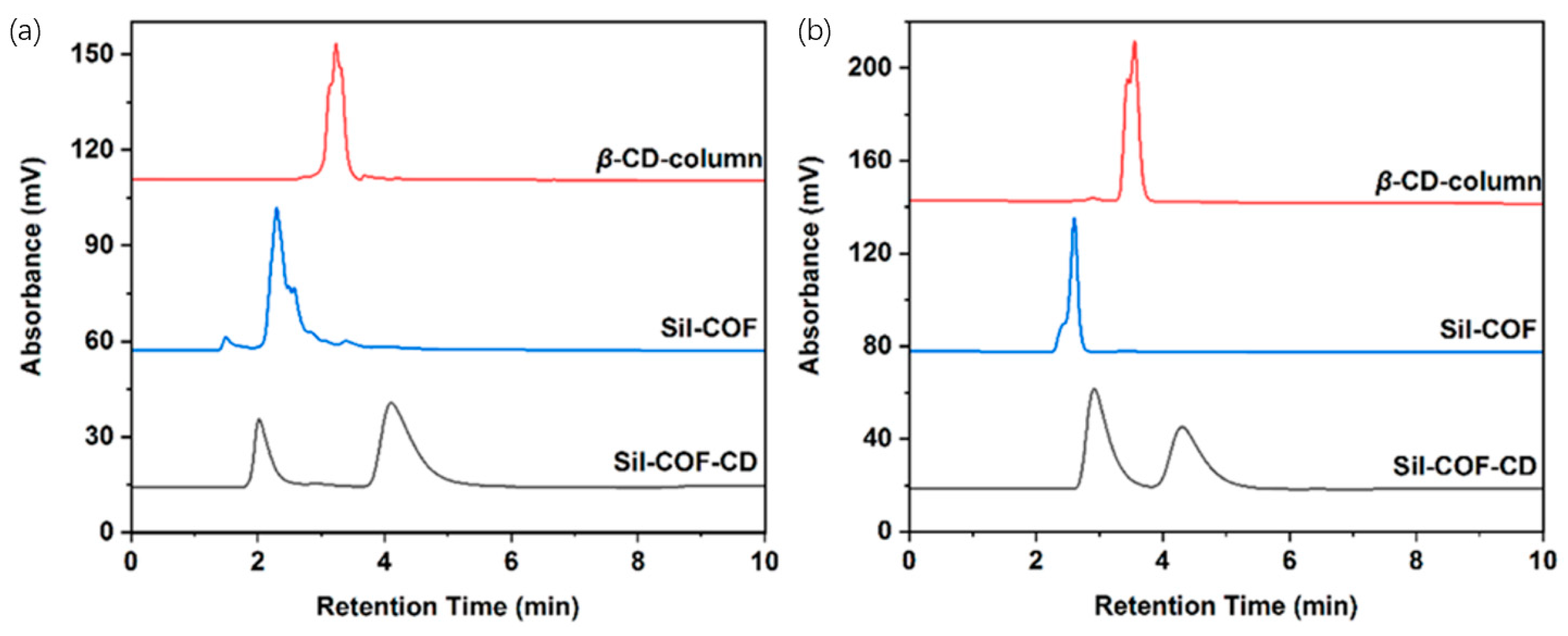
Figure 24.
Chiral enantiomers, (a) 2-phenylpropionic acid and (b) 1-phenyl-1-propanol, were chromatographically separated on a Sil-COF column, a Sil-COF-CD stationary phase, and a commercial β-CD column, respectively. Experimental conditions: (a) ACN/methanol/0.1% AcOH (80:10:10, v/v/v) and (b) ACN/0.1% AcOH (45:55, v/v); injection volume: 10 μL; column temperature: 30 °C; flow rate: 1.0 mL/min; UV detection wavelength: 254 nm. Figures adapted and reprinted with permission from ref. [104].
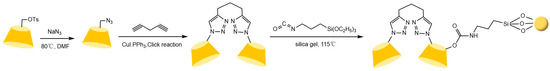
Figure 25.
Synthesis route of bis-triazole bridged β-cyclodextrin chiral stationary phase (BCDP) [105].
4.5. Bridged Cyclodextrin Derivative Stationary Phases
Bridged cyclodextrins (BCDs) are novel supramolecular systems constructed by connecting multiple cyclodextrins via bridging groups. Studies have demonstrated that their multi-cavity cooperative inclusion effects can enhance binding constants (Ks) from <105 L/mol for monomers to >1011 L/mol, reaching enzyme-like levels [106]. These systems exhibit significant potential in artificial enzymes, drug carriers, and molecular recognition [107,108,109]. Although hydroxyl derivatization can introduce chiral recognition sites, current β-CD derivatives generally suffer from two critical limitations: (1) depletion of edge hydroxyl groups leading to the loss of hydrogen-bonding recognition sites and (2) overcrowded substituents blocking the cavity and weakening inclusion capabilities. Furthermore, existing bridging strategies show limited improvements in separation performance, likely constrained by synthetic complexity and insufficient stability of the stationary phases.
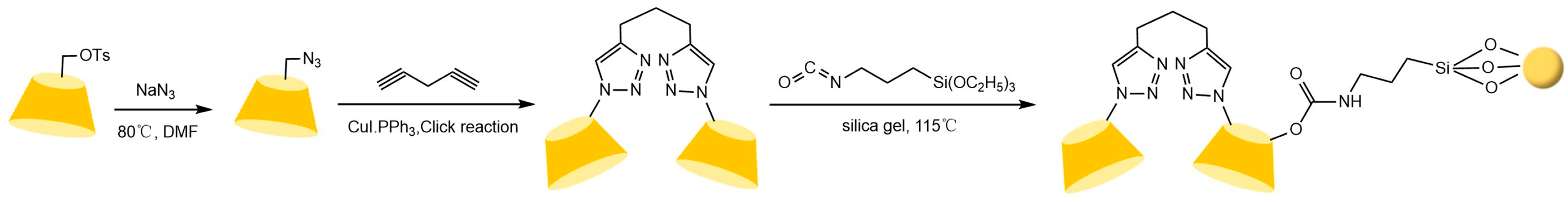
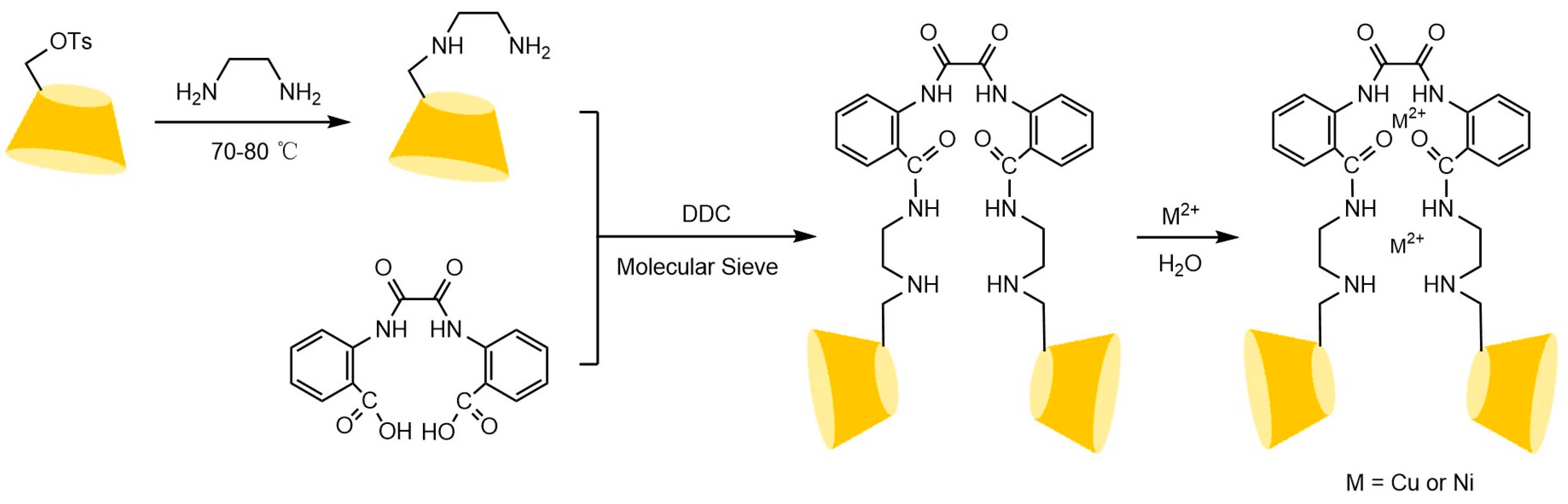
To address these issues, the research team led by Liu Yu at Nankai University [110,111,112] systematically investigated the multi-recognition mechanisms of bridged cyclodextrins. They revealed that the “pseudo-cavity” formed by synergistic interactions between the bridge and the original cavity significantly enhances spatial recognition capabilities, effectively overcoming the limitations of single cyclodextrin cavities (see Figure 26). Building on this, Shuang Yazhou et al. [113] innovatively designed a distyryl diamide-bridged bis-β-CD stationary phase with conjugated π-aryl groups in Figure 27, achieving efficient resolution of multiple classes of chiral drugs and expanding the scope of pharmaceutical analysis. In recent advancements, Liu et al. [114] developed a 4-chlorophenyl isocyanate-modified ethylenediamine-bridged β-CD dimer stationary phase (CPI-EBCD, see Figure 28), which successfully separated 12 chiral compounds under reversed-phase chromatographic conditions. Its enhanced stability and reproducibility provide new insights for designing chiral separation materials.
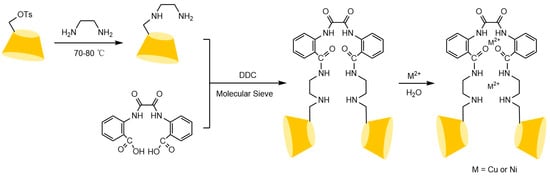
Figure 26.
Synthesis routes of bridged oxalamide bis(2-benzoic acid) carboxylate linking bis(β-CD) and its metal Cu2+ or Ni2+ [110].
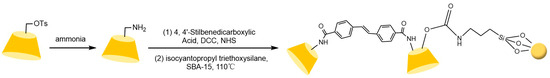
Figure 27.
Synthesis route of stilbenediamide-based bridged bis-β-cyclodextrin stationary phase (SBCDP) [113].
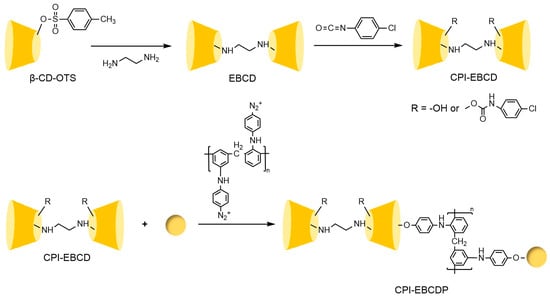
Figure 28.
Synthesis routes of bridged β-CD (CPI-EBCD)-bonded chiral stationary phases [114].
4.6. Cyclodextrin-Based Chiral Stationary Phases Utilizing Chiral Porous Materials
The evolution of porous materials has progressed through inorganic materials, inorganic-organic hybrid materials, and purely organic materials. While traditional inorganic porous materials (e.g., molecular sieves, zeolites) exhibit well-defined structures and high stability, they suffer from challenges in functional modification. The latter two categories, however, overcome this limitation through flexible organic ligand design, emerging as research hotspots due to their structural tunability and functional diversity. Recent breakthroughs have been achieved in cyclodextrin-based chiral porous materials for stationary phases. In 2017, Yan et al. [115] pioneered the use of γ-CD MOF as an HPLC chiral stationary phase, successfully separating aromatic alcohols and validating the feasibility of CD MOFs in chiral separation. In 2021, Li et al. [116] developed a diphenyl carbonate cross-linked γ-CD MOF (CL-CD-MOF) that outperformed traditional C18 columns in separating xylene isomers under both normal- and reversed-phase modes, demonstrating unique three-dimensional shape recognition capabilities. In 2022, Zheng et al. [117] constructed a β-CD-COF-functionalized silica stationary phase (COF@CD@SiO2) via a one-pot method in Figure 29, achieving efficient and rapid separation of six enantiomers. Although these chiral porous materials remain in their early developmental stages, their hierarchical pore structures and host-guest synergistic mechanisms reveal significant application potential.
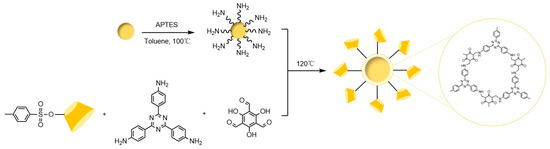
Figure 29.
Approximately 4–10 Synthesis roadmap for COF@CD@SiO2 [117].

Table 3.
Cyclodextrin-based chiral stationary phase in HPLC.
Table 3.
Cyclodextrin-based chiral stationary phase in HPLC.
| Chiral Stationary Phase | Types of Cyclodextrin | Synthesis Protocol | Chiral Compounds | Elution Mode | Ref. |
|---|---|---|---|---|---|
| mono-6-(3-methylimidazolium)-6-deoxyperphenylcarbamoyl-β-cyclodextrin chloride | β-CD | chemical reaction | racemic aromatic alcohols | NP-HPLC | [66] |
| mono-6-deoxy-(2,4-dihydroxybenzimide)-β-CD | β-CD | condensation reaction | chiral 1-phenyl-2-nitroethanol derivatives | RP-HPLC | [80] |
| mono(6A-N-1-(2-hydroxyl)-phenylethyl-imino-6Adeoxy)-β-cyclodextrin | β-CD | chemical reaction | 1-ferrocenylethanol 1-ferrocenylethyl acetate N,N-dimethyl-N-(1-ferrocenyleth-yl)amine 1-ferrocenyl-1-methoxyethane 1-ferrocenyl-1-(i-propoxy)ethane | RP-HPLC | [81] |
| 6A-(3-vinylimidazolium)-6-deoxyperphenylcarbamate-β-cyclodextrin chloride/ 6A-(N,N-allylmethylammonium)-6-deoxyperphenylcarbamoyl-β-cyclodextrin chloride | β-CD | chemically bonded | 6-Methoxyflavanone 7-Methoxyflavanone 4′-Hydroxyflavanone Hesperetin/Althiazide Bendroflumethiazide Trichlormethiazide Indapamide/Chlorthalidone | NP-HPLC | [84] |
| 3,5-dimethylphenylcarbamoylated β-cyclodextrin | mono (6A-azido-6A-deoxy)-per(3, 5-dimethylphenylcarbamoylated) β-cyclodextrin | staudinger reaction | lansoprazole/alprenolol phenylephrine hydrochloride omeprazole/ilaprazole metoprolol tartrate | NP-HPLC | [86] |
| phenylcarbamoylated-β-CD modified mesoporous silica particles CSP | mono-(6A-N-allyamino-6A-deoxy)-β-CD | chemical reaction | N-(4-aminophenyl)-3-ethyl-2,6-dioxopiperidine-4-carboxamide 3-(diethylamino)-2-methoxy-2-(1- naphthylthio)-3-oxopropionate 1-(4-iodophenyl)propan-2-ol 1-(4-methylphenyl)-3-(piperidin-1-yl)butan-2-one | NP-HPLC | [89] |
| Perphenylcarbamoylated β-cyclodextrin-silica (Ph-β-CD-silica) | mono (6A-N-allylamino-6A-deoxy)-Ph-β-CD | one-pot reaction | 4-Hydroxymandelic acid Diphenylethanone 4-(Dimethylamino)-1-(4-bromophenyl)-1-pyridin-2-ylbutan-1-ium 5-(2-Phenylethyl)-2-(trifluoromethyl) benzene-1,3-disulfonamide thiocarbamoyl chloride | NP-HPLC | [90] |
| mono-2A-azido-2A-deoxyperphenylcarbamoylated β-cyclodextrin/ mono-2A-azido-2A-deoxyperacetylated β-cyclodextrin | tosylated-CD | chemical reaction | bendroflumethiazide tolperisone/ancymidol indapamide/brompheniramine chlorpheniramine/etilefrine propranolol/flavanone | NP/RP-HPLC | [91] |
| Click β-CD | β-CD | thiol-ene click chemistry reaction | ibuprofen 5-methyluridine/uridine N4-acetylcytidine/adenosine Inosine/cytidine/guanosine Xanthosine/1-methyladenosine | HILIC/RP-HPLC | [78] |
| heptakis(6-mercapto-6-deoxy)-β-CD-CSP onto alkene functional silica | β-CD | thiol-ene click chemistry reaction | Isoxazolines/chiral lactides chiral ketones/flavanones dansyl amino acids | RP-HPLC | [99] |
| 3-n-octadecyl-1-vinylimidazolium bromide and 6-(1-allylimidazolium)-cyclodextrin | β-CD | chemical reaction | 1-phenyl-1-propanol warfarin ketoprofen | RP-HPLC/HILIC/IEC | [100] |
| mono/di(10-undecenoyl)perphenylaminocarbonyl β-CD | β-CD | thiol-ene click chemistry reaction | pindolol/propranolol alprenolol N-isopropyl-DL-noradrenaline 1-Phenylethylamine Bendroflumethiazide Ranolazine Zipiclone/Praziquantel | NP/RP-HPLC | [102] |
| phenethylamine synergistic tricarboxylic acid modified β-CD CSPs | β-CD | one-pot reaction | 1-phenylethanol 1-Phenyl-1-propanol 1-phenyl-2-propanol 2-phenyl-1-propanol 1-(4-methylphenyl)-ethanol Benzoin/mandelonitrile Flavanone/6-methoxyflavanone 6-hydroxyflavanone triadimenol/propiconazole. | NP/RP-HPLC | [118] |
| Sil-COF-CD | mono-(6-mercapto-6-deoxy)-β-cyclodextrin | thiol-ene click chemistry reaction | 2-phenylpropionic acid 1-phenyl-1-propanol | NP-HPLC | [104] |
| bis-triazolyl bridged β-cyclodextrin | azido-β-cyclodextrin | click reaction | imazalil/flutriafol Hexaconazole/tebuconazole diniconazole/uniconazole paclobutrazol/triticonazole/ metconazole | RP-HPLC | [105] |
| bridged bis(β-cyclodextrin)-bonded chiral stationary phase (SBCDP) | 6-deoxy-6-amino-β-cyclodextrin | chemical reaction | trimeprazine/praziquantel hesperidin/flavanone 2′-Hydroxy flavanone hydroxy/flavanone catechin/naringin | RP-HPLC | [113] |
| CPI-EBCD | β-CD | chemical reaction | DL-Cysteine/DL-Cysteine Cobaltic acetylacetonate DL-Tyrosine Metoprolol tartrate Benzoin/Promethazine 2-phenylcyclohexanone | RP-HPLC | [114] |
| CL-CD-MOF | γ-CD-MOF | chemical reaction | p,m,o-Xylene p,m,o-Dichlorobenzene Fluorobenzene Chlorobenzene Bromobenzene/Toluene | NP/RP-HPLC | [116] |
| COF@CD@SiO2 | β-CD | one-pot reaction | 2-phenylpropionic acid N-acetyl-L-phenylalanine Dopa/Methyldopa Menthol/Styrene oxide | NP/RP-HPLC | [117] |
5. Conclusions
Cyclodextrin (CD) derivative-based stationary phases demonstrate significant advantages in chromatographic separation, offering innovative platforms for gas chromatography (GC) and high-performance liquid chromatography (HPLC) due to their unique cavity structures and chiral recognition capabilities. In GC, CD stationary phases efficiently separate chiral compounds and volatile stereoisomers through host-guest inclusion interactions, particularly exhibiting exceptional resolution for terpenes, alcohols, and related substances. In HPLC systems, these materials achieve baseline separation of chiral drug enantiomers under reversed-phase, normal-phase, and polar organic modes while also enabling high-efficiency analysis of complex systems involving polar/nonpolar compounds and biomacromolecules. Their structural modifiability further extends application boundaries, demonstrating critical value in chiral drug separation, environmental pollutant analysis, and biomacromolecule detection. This provides innovative solutions for complex sample analysis.
Author Contributions
Conceptualization, W.D. and J.J.; methodology, J.J.; software, Y.Z.; validation, J.J., and R.H.; formal analysis, W.D.; investigation, Y.Z.; resources, A.N.; data curation, A.N.; writing—original draft preparation, W.D.; writing—review and editing, J.J.; visualization, W.D.; supervision, J.J.; project administration, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the science and technology department of Sichuan province (2025ZNSFSC0105).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Green, D.W.; Lee, J.M.; Kim, E.J.; Lee, D.J.; Jung, H.S. Chiral Biomaterials: From Molecular Design to Regenerative Medicine. Adv. Mater. Interfaces 2016, 3, 1500411. [Google Scholar] [CrossRef]
- Brandt, K.; Dötterl, S.; Fuchs, R.; Navarro, D.; Machado, I.C.S.; Dobler, D.; Reiser, O.; Ayasse, M.; Milet-Pinheiro, P. Subtle Chemical Variations with Strong Ecological Significance: Stereoselective Responses of Male Orchid Bees to Stereoisomers of Carvone Epoxide. J. Chem. Ecol. 2019, 45, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Lucci, E.; Dal Bosco, C.; Antonelli, L.; Fanali, C.; Fanali, S.; Gentili, A.; Chankvetadze, B. Enantioselective high-performance liquid chromatographic separations to study occurrence and fate of chiral pesticides in soil, water, and agricultural products. J. Chromatogr. A 2022, 1685, 463595. [Google Scholar] [CrossRef] [PubMed]
- Rayala, V.; Kandula, J.S.; Radhakrishnanand, P. Advances and challenges in the pharmacokinetics and bioanalysis of chiral drugs. Chirality 2022, 34, 1298–1310. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhou, J.; Li, H.B. Chiral Covalent Organic Framework Packed Nanochannel Membrane for Enantioseparation. Angew. Chem.-Int. Edit. 2022, 61, e202204012. [Google Scholar] [CrossRef]
- Gourlay, M.D.; Kendrick, J.; Leusen, F.J.J. Predicting the spontaneous chiral resolution by crystallization of a pair of flexible nitroxide radicals. Cryst. Growth Des. 2008, 8, 2899–2905. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Asnin, L. Chiral separation of quinolones by liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2017, 40, 2863–2882. [Google Scholar] [CrossRef]
- Shinde, S.D.; Yadav, G.D. Insight into microwave assisted immobilized Candida antarctica lipase B catalyzed kinetic resolution of RS-(±)-ketorolac. Process Biochem. 2015, 50, 230–236. [Google Scholar] [CrossRef]
- Tang, K.W.; Zhang, P.L.; Pan, C.Y.; Li, H.J. Equilibrium Studies on Enantioselective Extraction of Oxybutynin Enantiomers by Hydrophilic β-Cyclodextrin Derivatives. AIChE J. 2011, 57, 3027–3036. [Google Scholar] [CrossRef]
- Zhou, M.; Long, Y.D.; Zhi, Y.G.; Xu, X.Y. Preparation and chromatographic evaluation of a chiral stationary phase based on carboxymethyl-β-cyclodextrin for high-performance liquid chromatography. Chin. Chem. Lett. 2018, 29, 1399–1403. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, H.; Quan, K.J.; Zhao, L.; Qiu, H.D.; Li, Z.G. Preparation and applications of cellulose-functionalized chiral stationary phases: A review. Talanta 2021, 225, 121987. [Google Scholar] [CrossRef] [PubMed]
- Ikai, T.; Okamoto, Y. Structure Control of Polysaccharide Derivatives for Efficient Separation of Enantiomers by Chromatography. Chem. Rev. 2009, 109, 6077–6101. [Google Scholar] [CrossRef]
- Ilisz, I.; Berkecz, R.; Péter, A. Retention mechanism of high-performance liquid chromatographic enantioseparation on macrocyclic glycopeptide-based chiral stationary phases. J. Chromatogr. A 2009, 1216, 1845–1860. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.; El-Sayed, D.H.; Salman, B.E.; Bakr, H.H.; Adel, S.E.; Alzarak, T.M.; Mahmoud, A. Macrocyclic Antibiotics as Effective Chiral Selectors in Liquid Chromatography for Enantiomeric Separation of Pharmaceutical Compounds: A Review. Crit. Rev. Anal. Chem. 2024, 54, 3095–3113. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Chen, J.; Wang, J.; Qiu, H. Recent advances in chiral liquid chromatography stationary phases for pharmaceutical analysis. J. Chromatogr. A 2023, 1708, 464367. [Google Scholar] [CrossRef]
- Cavazzini, A.; Pasti, L.; Massi, A.; Marchetti, N.; Dondi, F. Recent applications in chiral high performance liquid chromatography: A review. Anal. Chim. Acta 2011, 706, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Y.; Guo, L.Z.; Xie, F.Y.; Yao, B.X.; Zeng, Q.; Weng, W. Enantioseparation of Hydrobenzoin and Structurally Related Compounds on β-Cyclodextrin and Hydroxypropyl-β-cyclodextrin Bonded Chiral Stationary Phases. Chromatographia 2011, 73, 1049–1055. [Google Scholar] [CrossRef]
- Liu, P.; He, W.; Qin, X.Y.; Sun, X.L.; Chen, H.; Zhang, S.Y. Synthesis and Application of a Novel Single-Isomer Mono-6-Deoxy-6-((2S,3S)-(+)-2,3-O-Isopropylidene-1, 4Tetramethylenediamine)-β-Cyclodextrin as Chiral Selector in Capillary Electrophoresis. Chirality 2010, 22, 914–921. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Ong, T.T.; Ge, L.Y.; Tan, S.N.; Young, D.J.; Tan, T.T.Y.; Ng, S.C. Chiral capillary electrophoresis with cationic pyrrolidinium-β-cyclodextrin derivatives as chiral selectors. J. Sep. Sci. 2010, 33, 1797–1805. [Google Scholar] [CrossRef]
- Juvancz, Z.; Bodáné-Kendrovics, R.; Szente, L.; Maklári, D. Cyclodextrins as Dominant Chiral Selective Agents in the Capillary Separation Techniques. Period. Polytech.-Chem. Eng. 2021, 65, 580–594. [Google Scholar] [CrossRef]
- Sapte, S.; Pore, Y. Inclusion complexes of cefuroxime axetil with β-cyclodextrin: Physicochemical characterization, molecular modeling and effect of L-arginine on complexation. J. Pharm. Anal. 2016, 6, 300–306. [Google Scholar] [CrossRef]
- Haiyee, Z.A.; Saim, N.; Said, M.; Illias, R.M.; Mustapha, W.A.W.; Hassan, O. Characterization of cyclodextrin complexes with turmeric oleoresin. Food Chem. 2009, 114, 459–465. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Zhou, J.; Tang, J.; Tang, W.H. Cyclodextrin clicked chiral stationary phases with functionalities-tuned enantioseparations in high performance liquid chromatography. J. Chromatogr. A 2015, 1406, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Kharaishvili, Q.; Jibuti, G.; Farkas, T.; Chankvetadze, B. Further proof to the utility of polysaccharide-based chiral selectors in combination with superficially porous silica particles as effective chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2016, 1467, 163–168. [Google Scholar] [CrossRef]
- Kucerová, G.; Procházková, H.; Kalíková, K.; Tesarová, E. Sulfobutylether-β-cyclodextrin as a chiral selector for separation of amino acids and dipeptides in chromatography. J. Chromatogr. A 2016, 1467, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Liu, F.P.; Mao, J.Y. The CGC enantiomer separation of 2-arylcarboxylic acid esters by using β-cyclodextrin derivatives as chiral stationary phases. Anal. Chim. Acta 2016, 912, 156–162. [Google Scholar] [CrossRef]
- Fang, L.L.; Wang, P.; Wen, X.L.; Guo, X.; Luo, L.D.; Yu, J.; Guo, X.J. Layer-by-layer self-assembly of gold nanoparticles/thiols β-cyclodextrin coating as the stationary phase for enhanced chiral differentiation in open tubular capillary electrochromatography. Talanta 2017, 167, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Yin, Q.H.; Cai, P.F.; Zhao, X.Y.; Pan, Y.J. Enhancement of visual chiral sensing via an anion-binding approach: Novel ionic liquids as the chiral selectors. Anal. Chim. Acta 2017, 962, 97–103. [Google Scholar] [CrossRef]
- Wang, S.S.; Han, C.; Wang, S.S.; Bai, L.J.; Li, S.S.; Luo, J.G.; Kong, L.Y. Development of a high speed counter-current chromatography system with Cu(II)-chiral ionic liquid complexes and hydroxypropyl-β-cyclodextrin as dual chiral selectors for enantioseparation of naringenin. J. Chromatogr. A 2016, 1471, 155–163. [Google Scholar] [CrossRef]
- Schurig, V. Use of derivatized cyclodextrins as chiral selectors for the separation of enantiomers by gas chromatography. Ann. Pharm. Françaises 2010, 68, 82–98. [Google Scholar] [CrossRef]
- Xiao, Y.; Ng, S.-C.; Tan, T.T.Y.; Wang, Y. Recent development of cyclodextrin chiral stationary phases and their applications in chromatography. J. Chromatogr. A 2012, 1269, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B.; Scriba, G.K.E. Cyclodextrins as chiral selectors in capillary electrophoresis: Recent trends in mechanistic studies. TrAC Trends Anal. Chem. 2023, 160, 116987. [Google Scholar] [CrossRef]
- Hancu, G.; Modroiu, A.; Stroia, D.G.; Uilăcan, A. Analyzing the Chiral Purity of Pharmaceuticals: The Application of Cyclodextrin-Based Chiral Selectors in Capillary Electrophoresis. Symmetry 2024, 16, 1354. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and Their Inclusion Complexes. Akad. Kiado 1982, 25. Available online: https://cir.nii.ac.jp/crid/1571698599489826304 (accessed on 10 April 2025).
- Lipkowitz, K.B.; Pearl, G.; Coner, B.; Peterson, M.A. Explanation of where and how enantioselective binding takes place on permethylated beta-cyclodextrin, a chiral stationary phase used in gas chromatography. J. Am. Chem. Soc. 1997, 119, 600–610. [Google Scholar] [CrossRef]
- Zhang, Y.; Armstrong, D.W. 4,6-Di-O-pentyl-3-O-trifluoroacetyl/propionyl cyclofructan stationary phases for gas chromatographic enantiomeric separations. Analyst 2011, 136, 2931–2940. [Google Scholar] [CrossRef]
- Easton, C.J.; Lincoln, S.F. Chiral discrimination by modified cyclodextrins. Chem. Soc. Rev. 1996, 25, 163–170. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.-M.; Xu, D.; Zhang, J. Enantiomeric separation in high-performance liquid chromatography using novel β-cyclodextrin derivatives modified by R-configuration groups as chiral stationary phases. Talanta 2011, 84, 1080–1092. [Google Scholar] [CrossRef]
- Bicchi, C.; D’Amato, A.; Rubiolo, P. Cyclodextrin derivatives as chiral selectors for direct gas chromatographic separation of enantiomers in the essential oil, aroma and flavour fields. J. Chromatogr. A 1999, 843, 99–121. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Pawlak, K.; Bruening, R.L.; Tarbet, B.J. Thermodynamic and kinetic data for macrocycle interaction with neutral molecules. Chem. Rev. 1992, 92, 1261–1354. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral recognition in separation sciences. Part I: Polysaccharide and cyclodextrin selectors. TrAC Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Kano, K. MECHANISMS FOR CHIRAL RECOGNITION BY CYCLODEXTRINS. J. Phys. Org. Chem. 1997, 10, 286–291. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral recognition in separation science—An update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef]
- Dalgliesh, C. 756. The optical resolution of aromatic amino-acids on paper chromatograms. J. Chem. Soc. 1952, 3940–3942. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Li, W.; Pitha, J. Reversing enantioselectivity in capillary gas chromatography with polar and nonpolar cyclodextrin derivative phases. Anal. Chem. 1990, 62, 214–217. [Google Scholar] [CrossRef]
- Peng, L.; Liu, H.; Hu, C.; Sun, Y.; Long, W. Terephthaloyl Chloride Bridged Bis (β-cyclodextrin) and Their Synergetic Bonding Behaviors with Dyes. Chin. J. Org. Chem. 2015, 35, 1330–1334. [Google Scholar] [CrossRef]
- Sand, D.M.; Schlenk, H. Acylated Cyclodextrins as Polar Stationary Phases for Gas-Liquid Chromatography. Anal. Chem. 1961, 33, 1624–1625. [Google Scholar] [CrossRef]
- Chen, G.D.; Shi, X.Y. Capillary gas chromatographic properties of three new cyclodextrin derivatives with acyl groups in the 6-position of β-cyclodextrin. Anal. Chim. Acta 2003, 498, 39–46. [Google Scholar] [CrossRef]
- Kartsova, L.A.; Makarov, A.A.; Popova, A.M. Quantitative evaluation of interactions of organic compounds with 18-crown ethers and β-cyclodextrin as components of stationary phases for gas chromatography. J. Anal. Chem. 2007, 62, 245–250. [Google Scholar] [CrossRef]
- Chaise, T.; Cardinael, P.; Tisse, S.; Combret, J.C.; Bouillon, J.P. Indirect and direct approaches in the synthesis of a new mono-6-O-benzyl methylated γ-cyclodextrin as chiral selector for enantioselective gas chromatography. Tetrahedron-Asymmetry 2008, 19, 348–357. [Google Scholar] [CrossRef]
- Shen, G.Y.; Cui, J.; Yang, X.L.; Ling, Y. Capillary GC using pyridyl β-cyclodextrin stationary phase. J. Sep. Sci. 2009, 32, 79–87. [Google Scholar] [CrossRef]
- Costa, N.; Matos, S.; da Silva, M.; Pereira, M.M.A. Cyclodextrin-Based Ionic Liquids as Enantioselective Stationary Phases in Gas Chromatography. ChemPlusChem 2013, 78, 1466–1474. [Google Scholar] [CrossRef]
- Lin, Y.S. Metal organic framework membranes for separation applications. Curr. Opin. Chem. Eng. 2015, 8, 21–28. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Yang, C.X.; Chang, N.; Yan, X.P. Metal-Organic Frameworks for Analytical Chemistry: From Sample Collection to Chromatographic Separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yuan, C.; Hou, B.; Liu, L.J.; Li, H.Y.; Liu, Y.; Cui, Y. Chiral covalent organic frameworks: Design, synthesis and property. Chem. Soc. Rev. 2020, 49, 6248–6272. [Google Scholar] [CrossRef]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Xie, S.M.; Zi, M.; Yuan, L.M. Recent advances of application of porous molecular cages for enantioselective recognition and separation. J. Sep. Sci. 2020, 43, 134–149. [Google Scholar] [CrossRef]
- Zhang, D.W.; Ronson, T.K.; Zou, Y.Q.; Nitschke, J.R. Metal-organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Xie, S.M.; Zhang, J.H.; Chen, L.; Nong, R.Y.; Yuan, L.M. Metal-Organic Framework [Cd(LTP)2]n for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in GC. J. Chromatogr. Sci. 2016, 54, 1467–1474. [Google Scholar] [CrossRef]
- Tang, B.; Wang, W.; Hou, H.P.; Liu, Y.Q.; Liu, Z.K.; Geng, L.N.; Sun, L.Q.; Luo, A.Q. A β-cyclodextrin covalent organic framework used as a chiral stationary phase for chiral separation in gas chromatography. Chin. Chem. Lett. 2022, 33, 898–902. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Z.; Xiong, W.Q.; Huang, Z.F.; Lai, Y.L.; Fu, S.G.; Dong, J.Q.; Duan, A.H.; Hou, X.D.; Yuan, L.M.; et al. Cyclodextrin Incorporation into Covalent Organic Frameworks Enables Extensive Liquid and Gas Chromatographic Enantioseparations. J. Am. Chem. Soc. 2023, 145, 18956–18967. [Google Scholar] [CrossRef]
- Welch, C.J.; Biba, M.; Gouker, J.R.; Kath, G.; Augustine, P.; Hosek, P. Solving multicomponent chiral separation challenges using a new SFC tandem column screening tool. Chirality 2007, 19, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Tymiak, A.A.; Zhang, Y.R. Optimization and Simulation of Tandem Column Supercritical Fluid Chromatography Separations Using Column Back Pressure as a Unique Parameter. Anal. Chem. 2014, 86, 4033–4040. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Z.G.; Liu, Z.M.; Zhu, R.Z.; Zhang, F.M.; Liu, Z.H.; Si, X.X. Botanical discrimination and classification of Mentha plants applying two-chiral column tandem GC-MS analysis of eight menthol enantiomers. Food Res. Int. 2022, 162, 112035. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Q.; Ong, T.T.; Ng, S.C. Synthesis of cationic β-cyclodextrin derivatives and their applications as chiral stationary phases for high-performance liquid chromatography and supercritical fluid chromatography. J. Chromatogr. A 2008, 1203, 185–192. [Google Scholar] [CrossRef]
- Ong, T.T.; Wang, R.Q.; Muderawan, I.W.; Ng, S.C. Synthesis and application of mono-6-(3-methylimidazolium)-6-deoxyperphenylcarbamoyl-β-cyclodextrin chloride as chiral stationary phases for high-performance liquid chromatography and supercritical fluid chromatography. J. Chromatogr. A 2008, 1182, 136–140. [Google Scholar] [CrossRef]
- Fujimura, K.; Ueda, T.; Ando, T. Retention behavior of some aromatic compounds on chemically bonded cyclodextrin silica stationary phase in liquid chromatography. Anal. Chem. 1983, 55, 446–450. [Google Scholar] [CrossRef]
- Li, L.; Cheng, B.P.; Zhou, R.D.; Cao, Z.G.; Zeng, C.; Li, L.S. Preparation and evaluation of a novel N-benzyl-phenethylamino-β-cyclodextrin-bonded chiral stationary phase for HPLC. Talanta 2017, 174, 179–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.S.; Chen, B.P.; Zhou, R.D.; Nie, G.Z. Preparation and Characterization of a Novel Isatin Derivative of β-Cyclodextrin-bonded SBA-15 Stationary Phase for HPLC. Chin. J. Anal. Chem. 2014, 42, 375–383. [Google Scholar] [CrossRef]
- Shuang, Y.Z.; Cao, Z.G.; Zhang, T.C.; Li, L.S. Enantiomeric Separation of Chiral Triazole Pesticides by a mono -6-(4-Nitrophenyl)-ureido-β-cyclodextrin-Bonded Stationary Phase Using High-Performance Liquid Chromatography. Anal. Lett. 2020, 53, 2481–2500. [Google Scholar] [CrossRef]
- Lin, C.; Liu, W.N.; Fan, J.; Wang, Y.K.; Zheng, S.R.; Lin, R.; Zhang, H.; Zhang, W.G. Synthesis of a novel cyclodextrin-derived chiral stationary phase with multiple urea linkages and enantioseparation toward chiral osmabenzene complex. J. Chromatogr. A 2013, 1283, 68–74. [Google Scholar] [CrossRef]
- Lin, C.; Fan, J.; Liu, W.N.; Tan, Y.; Zhang, W.G. Comparative HPLC enantioseparation on substituted phenylcarbamoylated cyclodextrin chiral stationary phases and mobile phase effects. J. Pharm. Biomed. Anal. 2014, 98, 221–227. [Google Scholar] [CrossRef]
- Yao, B.X.; Yang, X.M.; Guo, L.Z.; Kang, S.S.; Weng, W. Development of a Composite Chiral Stationary Phase from BSA and β-Cyclodextrin-Bonded Silica. J. Chromatogr. Sci. 2014, 52, 1233–1238. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.M. Enantioseparation performance of novel benzimido-β-cyclodextrins derivatized by ionic liquids as chiral stationary phases. Anal. Chim. Acta 2014, 819, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Wang, Y.T.; Zhou, W.H.; Zhou, Z.M. Preparation of chiral oxazolinyl-functionalized β-cyclodextrin-bonded stationary phases and their enantioseparation performance in high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 4136–4146. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem.-Int. Edit. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Guo, Z.M.; Ye, J.X.; Xu, Q.; Liang, X.M.; Lei, A.W. Preparation of novel β-cyclodextrin chiral stationary phase based on click chemistry. J. Chromatogr. A 2008, 1191, 188–192. [Google Scholar] [CrossRef]
- Guo, Z.M.; Jin, Y.; Liang, T.; Liu, Y.F.; Xu, Q.; Liang, X.M.; Lei, A.W. Synthesis, chromatographic evaluation and hydrophilic interaction/reversed-phase mixed-mode behavior of a “Click β-cyclodextrin” stationary phase. J. Chromatogr. A 2009, 1216, 257–263. [Google Scholar] [CrossRef]
- Allen Chang, C.; Abdel-Aziz, H.; Melchor, N.; Wu, Q.; Pannell, K.H.; Armstrong, D.W. Liquid chromatographic retention behavior of organometallic compounds and ligands with amine-, octadecylsilica- and β-cyclodextrin-bonded phase columns. J. Chromatogr. A 1985, 347, 51–60. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.M.; Dai, L.; Zhou, W.H.; Wang, J.L. Synthesis and enantioseparation characteristics of a novel mono-6-deoxy-(2,4-dihydroxybenzimide)-β-cyclodextrin as a chiral stationary phase in high-performance liquid chromatography. Talanta 2011, 86, 452–456. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhou, Z.M.; Yuan, H.; Meng, Z.H. Preparation and chiral recognition of a novel chiral stationary phase for HPLC, based on mono(6A-N-1-(2-hydroxyl)-phenylethylimino-6A-deoxy)-β-cyclodextrin and covalently bonded silica gel. Chin. Chem. Lett. 2008, 19, 797–800. [Google Scholar] [CrossRef]
- Remsburg, J.W.; Armstrong, D.W.; Péter, A.; Tóth, G. LC enantiomeric separation of unusual amino acids using cyclodextrin-based stationary phases. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 219–230. [Google Scholar] [CrossRef]
- Zhong, Q.Q.; He, L.F.; Beesley, T.E.; Trahanovsky, W.S.; Sun, P.; Wang, C.L.; Armstrong, D.W. Development of dinitrophenylated cyclodextrin derivatives for enhanced enantiomeric separations by high-performance liquid chromatography. J. Chromatogr. A 2006, 1115, 19–45. [Google Scholar] [CrossRef]
- Wang, R.Q.; Ong, T.T.; Tang, W.H.; Ng, S.C. Cationic cyclodextrins chemically-bonded chiral stationary phases for high-performance liquid chromatography. Anal. Chim. Acta 2012, 718, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.H.; Tang, W.H.; Ng, S.C. Novel β-cyclodextrin chiral stationary phases with different length spacers for normal-phase high performance liquid chromatography enantioseparation. J. Chromatogr. A 2011, 1218, 3496–3501. [Google Scholar] [CrossRef]
- Lin, C.; Fan, J.; Liu, W.N.; Chen, X.D.; Ruan, L.J.; Zhang, W.G. A new single-urea-bound 3,5-dimethylphenylcarbamoylated β-cyclodextrin chiral stationary phase and its enhanced separation performance in normal-phase liquid chromatography. Electrophoresis 2018, 39, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Zhang, W.G.; Luo, W.J.; Fan, J. Preparation and enantioseparation characteristics of a novel chiral stationary phase based on mono (6A-azido-6A-deoxy)-per(p-chlorophenylcarba moylated) β-cyclodextrin. J. Chromatogr. A 2008, 1213, 162–168. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, D.; Chelvi, S.K.T.; Yong, E.L.; Lee, H.K.; Gong, Y.H. Preparation and application of rifamycin-capped (3-(2-O-β-cyclodextrin)-2-hydroxypropoxy)-propylsilyl-appended silica particles as chiral stationary phase for high-performance liquid chromatography. Talanta 2010, 83, 286–290. [Google Scholar] [CrossRef]
- Ai, F.; Li, L.S.; Ng, S.C.; Tan, T.T.Y. Sub-1-micron mesoporous silica particles functionalized with cyclodextrin derivative for rapid enantioseparations on ultra-high pressure liquid chromatography. J. Chromatogr. A 2010, 1217, 7502–7506. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Wu, M.H.; Wu, R.A.; Done, J.; Ou, J.J.; Zou, H.F. Preparation of Perphenylcarbamoylated β-Cyclodextrin-silica Hybrid Monolithic Column with “One-Pot” Approach for Enantioseparation by Capillary Liquid Chromatography. Anal. Chem. 2011, 83, 3616–3622. [Google Scholar] [CrossRef]
- Poon, Y.F.; Muderawan, I.W.; Ng, S.C. Synthesis and application of mono-2A-azido-2A-deoxyperphenylcarba moylated β-cyclodextrin and mono-2Aazido-2A-deoxyperacetylated β-cyclodextrin as chiral stationary phases for high-performance liquid chromatography. J. Chromatogr. A 2006, 1101, 185–197. [Google Scholar] [CrossRef]
- Faugeras, P.A.; Boëns, B.; Elchinger, P.H.; Brouillette, F.; Montplaisir, D.; Zerrouki, R.; Lucas, R. When Cyclodextrins Meet Click Chemistry. Eur. J. Org. Chem. 2012, 2012, 4087–4105. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Bowman, C.N.; Kloxin, C.J. Toward an Enhanced Understanding and Implementation of Photopolymerization Reactions. AIChE J. 2008, 54, 2775–2795. [Google Scholar] [CrossRef]
- Yin, C.X.; Huo, F.J.; Zhang, J.J.; Martínez-Máñez, R.; Yang, Y.T.; Lv, H.G.; Li, S.D. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef]
- Qiao, L.Z.; Dou, A.; Shi, X.Z.; Li, H.; Shan, Y.H.; Lu, X.; Xu, G.W. Development and evaluation of new imidazolium-based zwitterionic stationary phases for hydrophilic interaction chromatography. J. Chromatogr. A 2013, 1286, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.B.; Tan, T.T.Y.; Wang, Y. Thiol-ene click chemistry derived cationic cyclodextrin chiral stationary phase and its enhanced separation performance in liquid chromatography. J. Chromatogr. A 2014, 1326, 80–88. [Google Scholar] [CrossRef]
- Chen, M.; Jin, X.; Ma, X.; Wang, Y. Click preparation and application of chiral stationary phase based on intrinsic recognition ability of cyclodextrin. Se pu Chin. J. Chromatogr. 2020, 38, 1270–1280. [Google Scholar] [CrossRef]
- Chen, M.; Lu, X.L.; Ma, X.F.; Xiao, Y.; Wang, Y. Click preparation of multiple-thioether bridged cyclodextrin chiral materials for efficient enantioseparation in high-performance liquid chromatography†. Analyst 2021, 146, 3025–3033. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Ren, X.J.; Luo, Q.R.; Gao, D.; Fu, Q.F.; Zhou, D.; Zu, F.J.; Xia, Z.N.; Wang, L.J. Ionic liquid functionalized β-cyclodextrin and C18 mixed-mode stationary phase with achiral and chiral separation functions. J. Chromatogr. A 2020, 1634, 461674. [Google Scholar] [CrossRef]
- Zhou, J.; Pei, W.J.; Zheng, X.X.; Zhao, S.Z.; Zhang, Z.Z. Preparation and Enantioseparation Characteristics of a Novel β-Cyclodextrin Derivative Chiral Stationary Phase in High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2015, 53, 676–679. [Google Scholar] [CrossRef]
- Huang, G.; Ou, J.J.; Zhang, X.D.; Ji, Y.S.; Peng, X.J.; Zou, H.F. Synthesis of novel perphenylcarbamated β-cyclodextrin based chiral stationary phases via thiol-ene click chemistry. Electrophoresis 2014, 35, 2752–2758. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lu, X.L.; Sun, S.; Xiao, Y.; Wang, Y.; Jin, X.N.; Ma, X.F. Surface-up click access to allylimidazolium bridged cyclodextrin dimer phase for efficient enantioseparation. J. Sep. Sci. 2023, 46, 2300075. [Google Scholar] [CrossRef]
- Wan, M.J.; Zheng, Y.C.; Dai, X.M.; Yang, H.L.; Zhou, J.Q.; Ou, J.; Yang, Y.X.; Liao, M.F.; Xia, Z.N.; Wang, L.J. Click Chemistry for the Preparation of β-Cyclodextrin Grafting Uniform Spherical Covalent Organic Framework Materials for Chiral Separation. Chem. Mat. 2023, 35, 609–616. [Google Scholar] [CrossRef]
- Zeng, Q.L.; Huang, Z.Q.; Li, D.; Li, L.S. Preparation of a bis-triazolyl bridged β-cyclodextrin stationary phase and its application for enantioseparation of chiral compounds by HPLC. Chirality 2024, 36, e23644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.C.; Shi, H.; Zhu, H.Y.; Huang, R.; Chi, C.M.; Zhao, Y. Synthesis of Novel Bis(β-cyclodextrin)s Linked with Glycol and Their Inclusion Complexation with Organic Dyes. Helv. Chim. Acta 2010, 93, 1136–1148. [Google Scholar] [CrossRef]
- Baugh, S.D.P.; Yang, Z.W.; Leung, D.K.; Wilson, D.M.; Breslow, R. Cyclodextrin dimers as cleavable carriers of photodynamic sensitizers. J. Am. Chem. Soc. 2001, 123, 12488–12494. [Google Scholar] [CrossRef]
- Sun, H.L.; Chen, Y.; Zhao, J.; Liu, Y. Photocontrolled Reversible Conversion of Nanotube and Nanoparticle Mediated by -Cyclodextrin Dimers. Angew. Chem.-Int. Edit. 2015, 54, 9376–9380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y. Cooperative binding and multiple recognition by bridged bis(β-cyclodextrin)s with functional linkers. Acc. Chem. Res. 2006, 39, 681–691. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.L.; Chen, Y.; Ding, F.; Chen, G.S. Binding behavior of aliphatic oligopeptides by bridged and metallobridged bis(β-cyclodextrin)s bearing an oxamido bis(2-benzoic) carboxyl linker. Bioconjug. Chem. 2004, 15, 1236–1245. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, S.; Chen, Y.; Yang, Y.W.; Huskens, J. Photo-induced switchable binding behavior of bridged bis(β-cyclodextrin) with an azobenzene dicarboxylate linker. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 197–201. [Google Scholar] [CrossRef]
- Chen, L.X.; Zhang, Y.M.; Cao, Y.; Zhang, H.Y.; Liu, Y. Bridged bis(β-cyclodextrin)s-based polysaccharide nanoparticles for controlled paclitaxel delivery. RSC Adv. 2016, 6, 28593–28598. [Google Scholar] [CrossRef]
- Shuang, Y.Z.; Zhang, T.C.; Li, L.S. Preparation of a stilbene diamido-bridged bis(β-cyclodextrin)-bonded chiral stationary phase for enantioseparations of drugs and pesticides by high performance liquid chromatography. J. Chromatogr. A 2020, 1614, 460702. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Liu, C.; Zhou, J.H.; Zhao, X.R.; Shen, Y.Q.; Cong, H.L.; Yu, B. Short bridging and partial derivatization synergistically modified ll-cyclodextrin bonded chiral stationary phases for improved enantioseparation. Talanta 2024, 273, 125830. [Google Scholar] [CrossRef]
- Yang, C.X.; Zheng, Y.Z.; Yan, X.P. γ-Cyclodextrin metal-organic framework for efficient separation of chiral aromatic alcohols. RSC Adv. 2017, 7, 36297–36301. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, C.C.; Wu, Y.H.; Wang, C.F.; Guo, T.; Zhang, J.W.; Sun, L.X. Cross-linked γ-cyclodextrin metal-organic framework-a new stationary phase for the separations of benzene series and polycyclic aromatic hydrocarbons. Microchim. Acta 2021, 188, 245. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Wan, M.J.; Zhou, J.Q.; Dai, X.M.; Yang, H.L.; Xia, Z.N.; Wang, L.J. One-pot method for the synthesis of β-cyclodextrin and covalent organic framework functionalized chiral stationary phase with mixed-mode retention mechanism. J. Chromatogr. A 2022, 1662, 462731. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Shi, C.; Zhao, L.; Li, Z.; Qiu, H. Chiral phenethylamine synergistic tricarboxylic acid modified β-cyclodextrin immobilized on porous silica for enantioseparation. Chin. Chem. Lett. 2023, 34, 107606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).