Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative condition and one of the most prevalent types of dementia in older adults. Currently, the primary drugs used to treat AD are acetylcholinesterase (AChE) inhibitors. The development of natural substances has become a research hotspot due to the high number of adverse effects of synthetic drugs. In this study, a new assay based on ultrafiltration–liquid chromatography–high-speed counter-current chromatography (UF-HPLC-HSCCC) was developed for the rapid screening and identification of AChE inhibitors from Olea europaea L. fruit. In this research, we screened and isolated two AChE inhibitors from O. europaea fruit extracts, identified by EI-MS and NMR as secologanoside and oleuroside-11-methyl ester. These compounds were identified for the first time from O. europaea and found to possess AChE inhibitory activity using an in vitro AChE inhibition assay and molecular docking. The IC50 values of the two compounds were 0.76 ± 0.04 mM and 1.08 ± 0.05 mM. The results demonstrated that secologanoside showed better AChE inhibition activity than oleuroside-11-methyl ester, suggesting that this compound is a promising AChE inhibitor. At the same time, the results showed that the combination of UF-HPLC- HSCCC provides a powerful tool for screening and isolating AChE inhibitors in complex samples.
1. Introduction
Among elderly individuals, Alzheimer’s disease (AD) stands out as a leading neurodegenerative disorder and a primary format of dementia [1]. Its pathogenic mechanisms include the Aβ hypothesis, the hyperphosphorylation of Tau, and the cholinergic hypothesis [2]. Cholinergic neurons are broadly dispersed in the human brain and play an essential role in cognition. Currently, the primary drugs used to treat AD are AChE inhibitors [3,4]. These are mainly huperzine A, tacrine, and donepezil [5]. Among them, huperzine A is extracted from the natural product huperziaceae to obtain alkaloids. It has been widely used because it exhibits better enzyme inhibitory activity due to its unique C15N2 central skeleton structure [6]. Since synthetic products have adverse effects in long-term use, with donepezil inducing psychosis and tacrine being hepatotoxic [7,8], screening AChE inhibitors with good activity and low side effects from natural products has become a hot spot of research in medicine. For example, the fruit of Platycodon grandiflorus (Jacq.) A.DC [9] and the components of Tripterygium wilfordii Hook. F. [10] and O. europaea [11] have been proven to have significant anti-AD effects.
Conventional methodologies for bioactive compound discovery and isolation in natural product research have been constrained by multistep protocols, significant solvent expenditure, and labor-intensive operational demands. With the continuous development of modern analytical techniques, researchers have also developed several simple, efficient, and high-throughput methods to screen bioactive compounds from natural products, such as bioaffinity chromatography [12], cell membrane affinity chromatography [13], and UF [14]. However, these methods have limitations of application and are only capable of efficiently screening active compounds. They need to be combined with other methods to separate large-scale target components. HSCCC is characterized by large sample loading volumes, no irreversible adsorption, and a wide range of solvent systems. UF-HPLC-HSCCC represents a cutting-edge, enzyme-based approach to identify and isolate target compounds from complex matrices, optimizing sample usage and accelerating drug discovery processes [15]. Thus, this method can be successfully applied to screen and prepare potential enzyme inhibitors from complex systems.
O. europaea holds significant commercial value in the Mediterranean, primarily for olive oil production, and both its leaves and fruits have demonstrated diverse biological properties [16]. Related studies have shown that ethanol extracts of O. europaea can exhibit better anti-AD activity [17]. O. europaea fruit is a promising nutritious food. Studies have shown that its extract contains several polyphenolic compounds with potential anti-AD activity [18].
In this study, we used O. europaea fruit as raw material and separated the extracts by HSCCC after 90% ethanol extraction. Then, using AChE as the target enzyme, we rapidly screened, isolated, and prepared potential AChE inhibitors from O. europaea fruit using the UF-HPLC-HSCCC method. Finally, in vitro enzyme assays and molecular docking studies were employed to validate the AChE inhibitory activity of the inhibitors and to analyze their interactions with the enzyme.
2. Materials and Methods
2.1. Reagents and Materials
AChE (C16363435, 200 u/g) was extracted from Electrophorus electricus, while huperzine A (≥99%), Acetylthiocholine iodide (ATChI, 98%), and 5,5-Dithiobis (2-nitrobenzoic acid) (DTNB) were supplied by Macklin Biochemical Technology (Shanghai, China). Formic acid, purified water, methanol, and acetonitrile of HPLC grade were procured from Mreda Technology Company (St. Louis, MO, USA) and Wahaha Group (Hangzhou, China). Tianjin Kaixin Chemical Industry Co., Ltd. (Tianjin, China) supplied DMSO. The HSCCC analytical solvents came from Tianjin Damao Chemical Reagent Factory (Tianjin, China) and O. europaea fruit from Yunnan, China.
2.2. Apparatus
The separation of active compounds was carried out using a TBE-300B preparative-HSCCC system (Taut Biotech, Shanghai, China), featuring a 300 mL total column volume, a 20 mL injection loop, and an adjustable helical tube speed range of 0–1000 rpm. The setup included a DC-0506 cryostat (Shanghai, China), a TBP5002 pump (Shanghai, China), a detector (Sanotac, Shanghai, China), and a BSZ-100 automatic fraction collector (Shanghai Husi Analytical Instrument Co., Ltd., Shanghai, China). HPLC analyses were executed using an Agilent 1260 Infinity II system, equipped with a G7111 pump and a G7115A detector, and supported by the USA Agilent Technologies workstation (Santa Clara, CA, USA). The Microplate Reader was Mulitiskan FC from Thermo Fisher Scientific.
2.3. Preparation of Sample Extracts
First, we accurately weighed 100 g of O. europaea fruit, crushed, and extracted with 90% ethanol (1:10, g/mL) by ultrasonic extraction for one hour; the extraction was performed three times. The filtrates were combined, spun dry, and kept in the refrigerator (4 °C) for storage.
2.4. Compound Analysis with High-Performance Liquid Chromatography
The chromatographic analysis was conducted using a Hedera ODS-2 column. The mobile phase was composed of 0.1% formic acid in water (phase A), methanol (phase B), and acetonitrile (phase C). Gradient elution was performed as follows: 0–20 min, 80–70% (A), 10–15% (B); 20–30 min, 70–75% (A), 15–12.5% (B); 30–60 min, 75–70% (A), 12.5–15% (B). Other conditions were a flow rate of 1 mL/min and an injection volume of 20 µL, and the detection was set at a wavelength of 240 nm.
2.5. Selection of Two-Phase Solvent Systems for High-Speed Counter-Current Chromatography
Compounds 1 and 2 had their partition coefficients (K) determined in two-phase solvents through HPLC analysis. Initially, a series of immiscible solvent systems were systematically designed according to the polarity gradient of target compounds; we mixed them thoroughly and waited for the upper and lower phases to equilibrate after some time. The precise measurement of 2 mL of solvent from the upper and lower phases was placed into 5 mL disposable test tubes. Then, the samples were precisely weighed and added to the test tubes. The tubes were tightly sealed and shaken vigorously for 1 min to allow the sample in the two-phase solvents to reach equilibrium in distribution. From each phase, 500 µL was pipetted, evaporated, and dissolved in 300 µL of methanol. The redissolved samples were analyzed via the HPLC method outlined in Section 2.4. The partition coefficient K was computed using the formula K = A1/A2, with A1 and A2 representing the peak areas in the upper and lower phases, respectively.
2.6. Separation of Crude Extracts and Active Components by High-Speed Counter-Current Chromatography
In this study, two steps were used to separate the active compounds. In the first step, the extracts were fractionated in elution–extrusion mode. To obtain a two-phase solvent system, water, methanol, n-hexane, and ethyl acetate (1:1:1:1, v/v/v) were thoroughly mixed in a separatory funnel and equilibrated for a sufficient time. The upper phase functioned as the stationary phase, while the lower phase acted as the mobile phase. Before being used, both phases underwent ultrasonic degassing for 30 min. A solution containing 400 mg of the extracted sample, as described in Section 2.3, was dissolved in 20 mL of the lower-phase solvent. The upper phase was then pumped into the PTFE column of the HSCCC apparatus at 18 mL/min. Once filled, the system was configured to operate at a temperature of 25 °C, with 900 rpm, and a flow rate of 2 mL/min. Then, the lower phase started to be pumped in. The mobile phase flowed out from the tail-end outlet of the column, which indicated that the two-phase solvents had already reached the kinetic equilibrium. At this point, the sample solution was loaded into the HSCCC to isolate the extracts under 254 nm detection. The resulting fractions were collected, rid of organic solvents by rotary evaporation s, and assessed for in vitro enzyme activities.
In the second stage, the target compounds screened by UF were separated. A suitable solvent system of water/acetic acid/n-butanol (2:0.02:2, v/v/v) was obtained through the screening of the K value with the 2.5 method for the separation of the active fractions under the following HSCCC conditions: a temperature of 25 °C, 900 rpm, a flow rate of 2 mL/min, and a detection wavelength of 240 nm; the rest of the operating procedures were the same as above.
2.7. In Vitro Acetylcholinesterase Inhibition Assay to Determine the Rate of Inhibition
A slightly modified version of the method in the literature was used for the in vitro AChE inhibition assay [19]. Firstly, a solution of 0.1 M phosphate buffer (PBS) at pH 7.34 was used to dissolve AtCHI, DTNB, and AChE. Then, 80 μL of PBS, 20 μL of 1.2 U/mL AChE solution, and 20 μL of different sample concentrations (dissolved in 5% DMSO) were sequentially added to the wells of a 96-well plate, followed by incubation at 37 °C for 10 min. Following the addition of 40 μL of 15 mM AtCHI and 40 μL of 2.5 mmol/L DTNB, the mixture was incubated at 37 °C for 20 min to initiate the reaction. Afterward, the 96-well plate was placed at −20 °C for 10 min to stop the reaction. Absorbance at 405 nm was measured using a Microplate Reader, with three parallel measurements taken for averaging. The inhibition rate was calculated according to the following formula. The positive control during the experiment was huperzine A, and the blank control was 5% DMSO solution.
In the formula, Abc, Ac, Abs, and As refer to the absorbance of the blank control group (no enzyme and no inhibitor), control group (with enzyme and no inhibitor), experimental control group (with inhibitor and no enzyme), and experimental group (with enzyme and inhibitor), respectively.
2.8. Screening of Acetylcholinesterase Inhibitors
To quickly identify potential AChE inhibitors, a UF screening technique was developed using a slightly adjusted approach derived from the literature, focusing on the active fractions of O. europaea [20]. A UF tube containing 300 µL of 12 U/mL AChE solution and 300 µL of a 10.0 mg/mL active fraction was incubated for 30 min at 37 °C. After centrifugation at 10,000 rpm for 10 min, 300 µL of 0.1 M PBS was added, allowed to sit for 15 min, and centrifuged again, repeating this cycle three times. Afterward, 300 µL of 50% methanol solution was added to deactivate the enzyme and dissociate the inhibitors. This methanol step was also repeated three times after centrifugation at 10,000 rpm for 10 min each. The methanol eluate was collected, dried under nitrogen, and reconstituted in 50% methanol for HPLC analysis.
2.9. Molecular Docking Analysis of the Binding Interactions Between Acetylcholinesterase and Compounds
To examine the binding relationship between AChE and potential inhibitors, molecular docking was performed. Compound structures in 3D format were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/, (accssed on 15 May 2024)), converted to the required format using OpenBabel, and saved as ligand. pdbqt files with torsional and rotational properties assigned by AutoDockTools (ADT). The AChE crystal structure (PDB ID: 1QT1; resolution: 2.50 Å) was retrieved from the Protein Data Bank (https://www.rcsb.org/ (accessed on 15 May 2024)). ADT software (4.2) was then used to strip the original ligand, water, and unnecessary atoms, while adding hydrogen atoms, and save the edited structure as AChE.pdbqt. AutoDock Vina was used for the semi-flexible docking of active small molecules and enzymes [21]. Based on the molecular docking results, the best conformation of the active compound was selected for the analysis of the docking results with the enzyme, and PyMOL analyzed the docking results for visualization and analysis.
2.10. Statistical Analysis
Mean values and standard deviations from triplicate measurements were used to present the data. It is worth noting that the drug concentrations in the text are all final concentrations. GraphPad Prism software (version 9.5) was used to calculate IC50 values. Plotting was performed using Origin 2021.
3. Results and Discussion
3.1. Acetylcholinesterase Inhibitory Activity of Different Fractions
The catalytic activity of AChE was determined by measuring its absorbance, and the inhibitory activity of the five fractions of O. europaea fruit extract HSCCC against AChE was evaluated. In the present study, we conducted the in vitro enzyme inhibition screening of compounds with reference to protocols established in the literature, employing identical positive control drugs to ensure experimental comparability. After verifying the consistency of the experimental conditions with published methods, we determined an IC50 value of 1.31 ± 0.04 µM for positive control, which aligns with reported values (1.6 µM) in the literature, and this verified the feasibility of the experimental protocol [19].
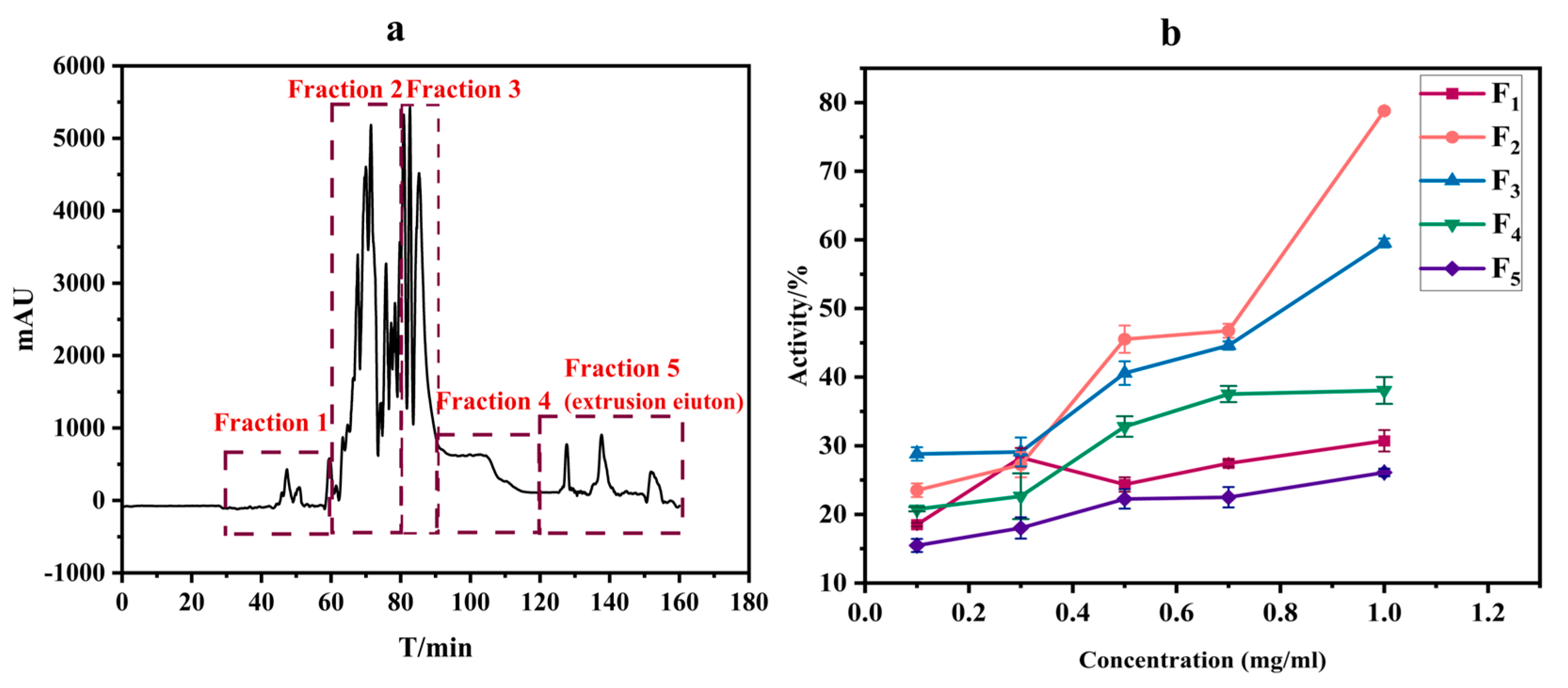
In this study, the crude extract was subjected to separation using an equal-volume solvent system comprising water, methanol, n-hexane, and ethyl acetate (1:1:1:1, v/v/v/v). The strategic advantage of this approach lies in its harmonization of solvent polarity gradients and density disparities, aiming to achieve multi-component fractionation. Under isovolumetric conditions, water (high polarity) and methanol (moderate polarity) synergistically formed a miscible layer capable of dissolving polar constituents such as glycosides and alkaloids. Concurrently, n-hexane (non-polar) and ethyl acetate (moderate-to-low polarity) selectively partitioned lipophilic compounds (e.g., terpenoids and phytosterols) through stratified extraction. This solvent ratio effectively mitigated cross-contamination risks arising from polarity overlaps, which often occurs when a single solvent predominates in conventional systems. According to the HSCCC separation results, the extract fractions were divided into five parts. The separation results are shown in Figure 1a. By measuring the activity of the five fractions at the same concentration, the activity increased gradually with the increase in the final concentration of the fractions (0.1–1 mg/mL), among which the inhibition rate of compound 2 was higher; the result is shown in Figure 1b. The IC50 value was measured as 0.56 ± 0.03 mg/mL. Therefore, fraction 2 may contain potential inhibitors, which will be used as the object of further study.
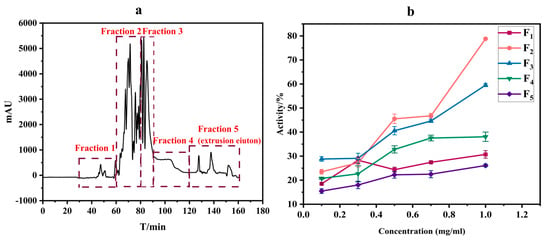
Figure 1.
Separation of crude extract of O. europaea fruit by high-speed counter-current chromatography (a). Acetylcholinesterase inhibitory activity of different fractions of an extract isolated by high-speed counter-current chromatography (b).
3.2. Screening of Acetylcholinesterase Inhibitors by Ultrafiltration
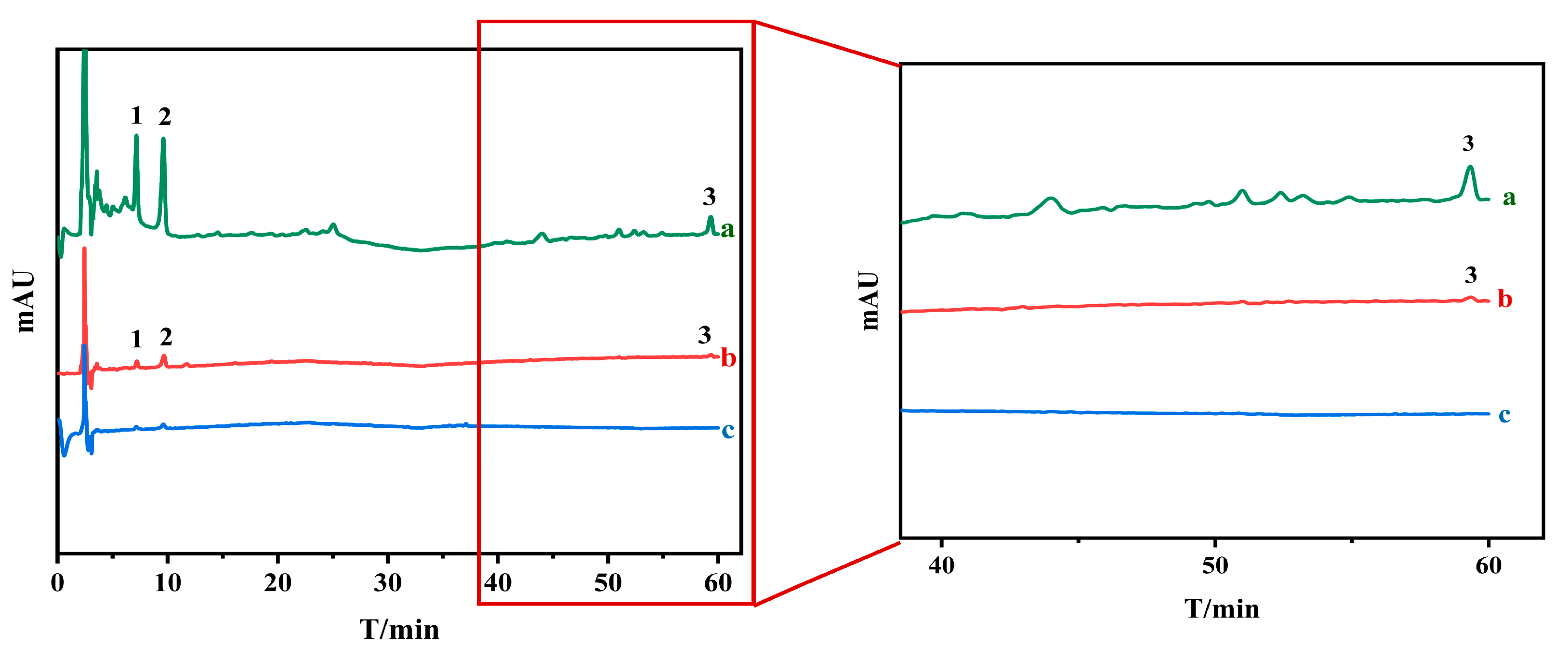
To swiftly screen potential AChE inhibitors, a UF-HPLC method was designed and applied to fraction 2 obtained through the initial HSCCC isolation of O. europaea After the incubation of fraction 2 with AChE and screening by UF, inactive compounds or weakly bound compounds were removed by centrifugation through the UF membrane. The more active components formed macromolecular complexes with AChE that was trapped in the UF tubes, which were washed with buffer solution and dissociated by 50% methanol solution, after which the dissociated solution was determined by HPLC as shown in Figure 2. As shown in the figure, three compounds were considered as potentially active compounds, which were taken as the next step in the isolation study.
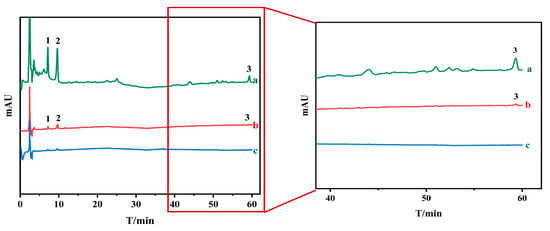
Figure 2.
Screening for acetylcholinesterase inhibitors by ultrafiltration (a: fraction 2; b: experiments with extracts and acetylcholinesterase; and c: experiments with denatured acetylcholinesterase, 1,2,3: compounds 1, 2 and 3).
3.3. Separation of Compounds 1 and 2 by High-Speed Counter-Current Chromatography
3.3.1. Selection of Two-Phase Solvent Systems and Calculation of Partition Coefficients
HSCCC separations are mainly based on determining the partition coefficients (K) of the target compounds in the solvent systems to determine the suitable solvent system. Suitable solvent systems generally require a K in the range of 0.5 to 2 for the target compound [22]. In this study, the solvent systems were screened for compounds 1 and 2 against the results under 3.2 (as shown in Table 1), and the solvent system for compound 3 was not screened because of the lower content of compound 3 and its lesser polarity relative to other compounds, which span a wider range of polarities. During the study, the traditional HEMW system was first selected. Still, compounds 1 and 2 did not have suitable K values, so it was guessed that the compounds were more polar, and the ethyl acetate/n-butanol/water system was subsequently selected. After adjusting the proportion of ethyl acetate, the K value of compound 1 changed significantly, so the n-butanol/water system was chosen, but the results showed that the compounds were be distributed in the upper and lower phases, but the range of K failed to reach the standard. It was shown that the addition of acetic acid to the system modulated the K value [23]. The mechanism by which acetic acid modulates the partition coefficient involves its role as a polarity regulator. For acidic compounds, acetic acid facilitates their partitioning into the lower phase through hydrogen bonding and protonation effects, thereby altering the distribution coefficient. Therefore, acetic acid was added to the system to adjust K, and the K value was distributed in the range of 0.5–2 after adding acetic acid. The n-butanol/acetic acid/water (2:0.02:2, v/v) solvent system was finally selected to isolate compounds 1 and 2 based on the amount of acetic acid and the size of K.

Table 1.
The K of compounds in different solvent systems.
3.3.2. Optimization Results of Rotation Speed and Flow Rate
After selecting a solvent system with suitable K values, the stationary phase retention (Sf) is also an essential factor determining whether the HSCCC separation is successful. The stationary phase retention is related to the HSCCC operating parameters, generally including speed, flow rate, and mode of operation. The change in flow rate is negatively correlated with the separation of compounds; a low flow rate can improve the separation but will increase the analysis time. Rotational speed changes are positively related to the separation of compounds. At a higher rotational speed, separation can be improved. Studies have shown that the higher the rotational speed, the greater the stationary phase retention. In general, when the retention rate is greater than 50%, satisfactory separation results can be obtained.
In order to determine the optimal separation conditions, three flow rates and three rotational speeds were selected for one-way investigation under the head-to-tail elution mode. The results are shown in Table 2, from which it can be seen that the retention rate of 950 rpm was higher than that of 900 rpm. Still, the two were not very different, so 2 mL/min and 900 rpm were selected for the separation of HSCCC in consideration of the wear and tear of the instrument.

Table 2.
Optimization results of rotation speed and flow rate.
3.3.3. Optimization Results of Injection Concentration
One of the advantages of HSCCC over solid–liquid chromatography is that it can achieve large preparation volumes. Increasing the injection volume can increase the preparation volume of compounds, reduce the number of separations, and reduce the consumption of solvents. Still, the injection volume is too large to facilitate the separation of compounds and disrupt the hydrodynamic equilibrium, so to obtain the optimal injection volume, we investigated the effect of injection volumes of 400 mg, 600 mg, and 800 mg on the separation effect. The results showed that an injection volume that was too large resulted in a serious loss of stationary phase, the reduced solubility of the sample, and peak broadening, which affected the separation efficiency. In contrast, a good separation was achieved at an injection volume of 400 mg.
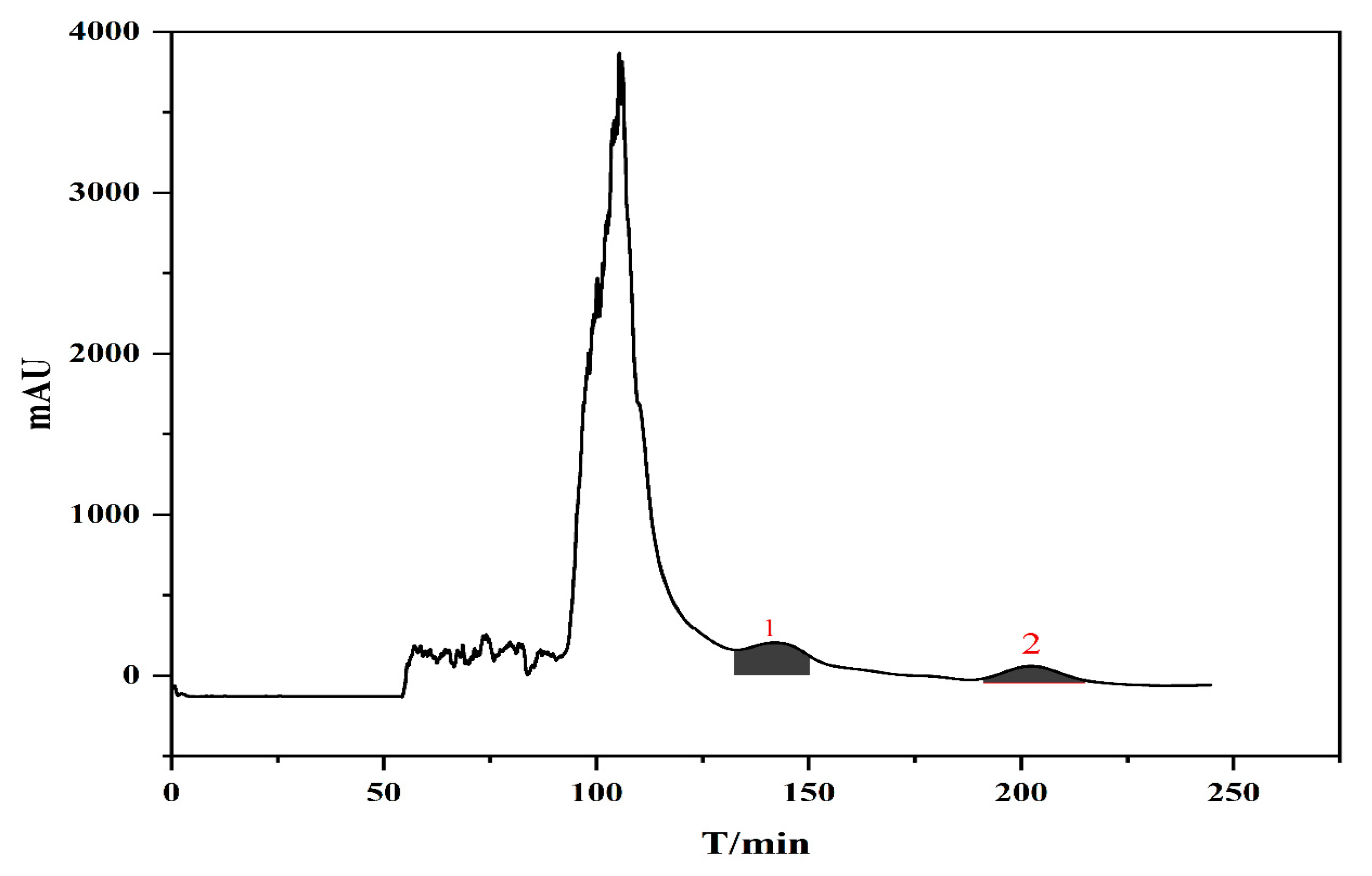
In summary, the optimal HSCCC separation conditions were as follows: two-phase solvent system: n-butanol/acetic acid/water (2:0.02:2, v/v/v); mobile phase flow rate: 2 mL/min; rotational speed: 900 rpm; working temperature: 25 °C; injection volume: 20 mg/mL; detection wavelength: 240 nm. Under these conditions, compounds 1 and 2 were well separated within 240 min, and the chromatograms are shown in Figure 3.
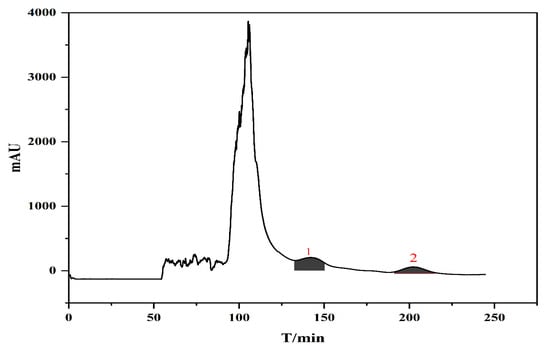
Figure 3.
Separation of compounds 1 and 2 by high-speed counter-current chromatography.
3.4. Structural Identification of the Isolated Compounds
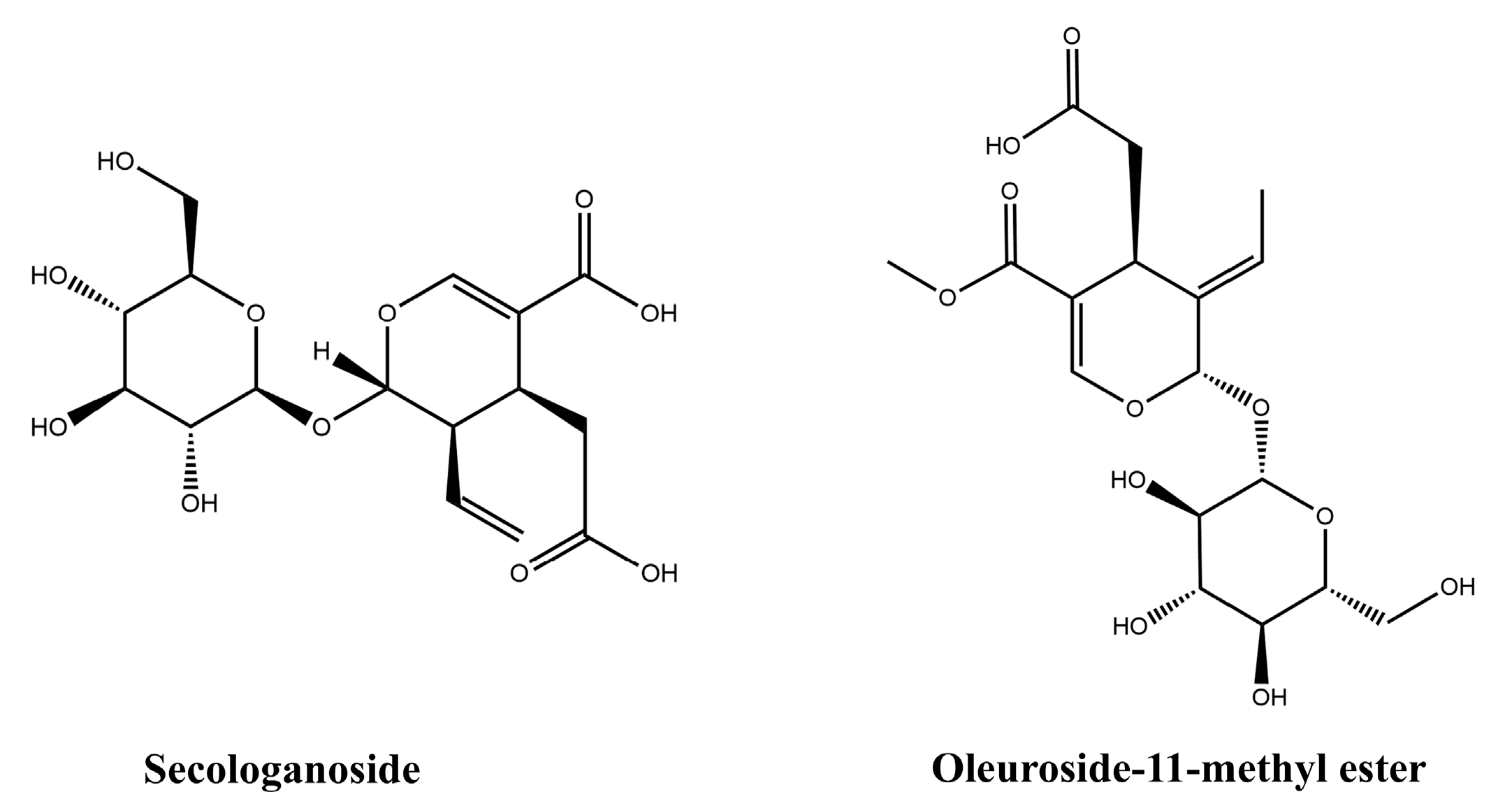
The chemical structures of the active compounds obtained by HSCCC isolation, secologanoside (compound 1, CAS: 59472-23-0) and oleuroside-11-methyl ester (compound 2, CAS: 60539-23-3), were identified by MS and NMR spectroscopy. The compound data are given below, and the structural formulas are shown in Figure 4.
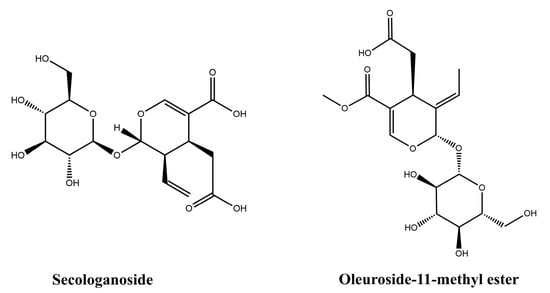
Figure 4.
Chemical structures of compound 1 and compound 2.
Compound 1: Pale yellow powder. ESI-MS m/z: 413.3 [M + Na]+. 1H-NMR (400 MHz, CD4O) δ: 5.47 (1H, d, J = 3.7 Hz, H-1), 7.49 (1H, S, H-3), 3.35 (1H, dd, J = 11.8, 6.2 Hz, H-5), 2.82 (1H, m, H-6a), 2.24 (1H, m, H-6b), 5.64 (1H, m, H-8), 3.06 (1H, m, H-9), 5.47 (1H, s, H-10a), 5.22 (1H, m, H-10b), 4.65 (1H, d, J = 7.9 Hz, H-1′). 13C-NMR (100 MHz, DMSO-d6) δ: 95.26 (C-1), 151.19 (C-3), 108.96 (C-4), 26.91 (C-5), 33.74 (C-6), 173.30 (C-7), 133.51 (C-8), 43.08 (C-9), 119.65 (C-10), 167.70 (C-11), 98.44 (C-1′), 72.93 (C-2′), 77.22 (C-3′), 69.89 (C-4′), 76.68 (C-5′), 60.97 (C-6′). Comparing these data with the literature [24], the target compound was identified as secologanoside.
Compound 2: Light yellow oil. ESI-MS m/z: 403.3 [M − H]−. 1H-NMR (400 MHz, CD4O) δ: 5.98 (1H, s, H-1), 7.45 (1H, s, H-3), 4.77 (1H, d, J = 7.74 Hz, H-5), 2.63 (1H, dd, H-6a), 2.19 (1H, dd, J = 12.99, 10.04 Hz, H-6b), 6.04 (1H, s, H-8), 1.76 (3H, dd, J = 7.1, 1.5 Hz, H-10), 3.70 (3H, s, O-CH3). 13C-NMR (100 MHz, DMSO-d6) δ: C-1 (99.05), C-3 (153.29), C-4 (108.20), C-5 (30.29), C-6 (34.73), C-7 (172.67), C-8 (122.88), C-9 (129.50), C-10 (13.24), C-11 (166.31), OCH3 (51.28), 1′ (99.05), 2′ (73.35), 3′ (77.41), 4′ (69.98), 5′ (76.58), 6′ (61.09). Comparing these data with the literature [25], the target compound was identified as oleuroside-11-methyl ester.
3.5. Inhibition of Acetylcholinesterase by Active Compounds and Molecular Docking Results
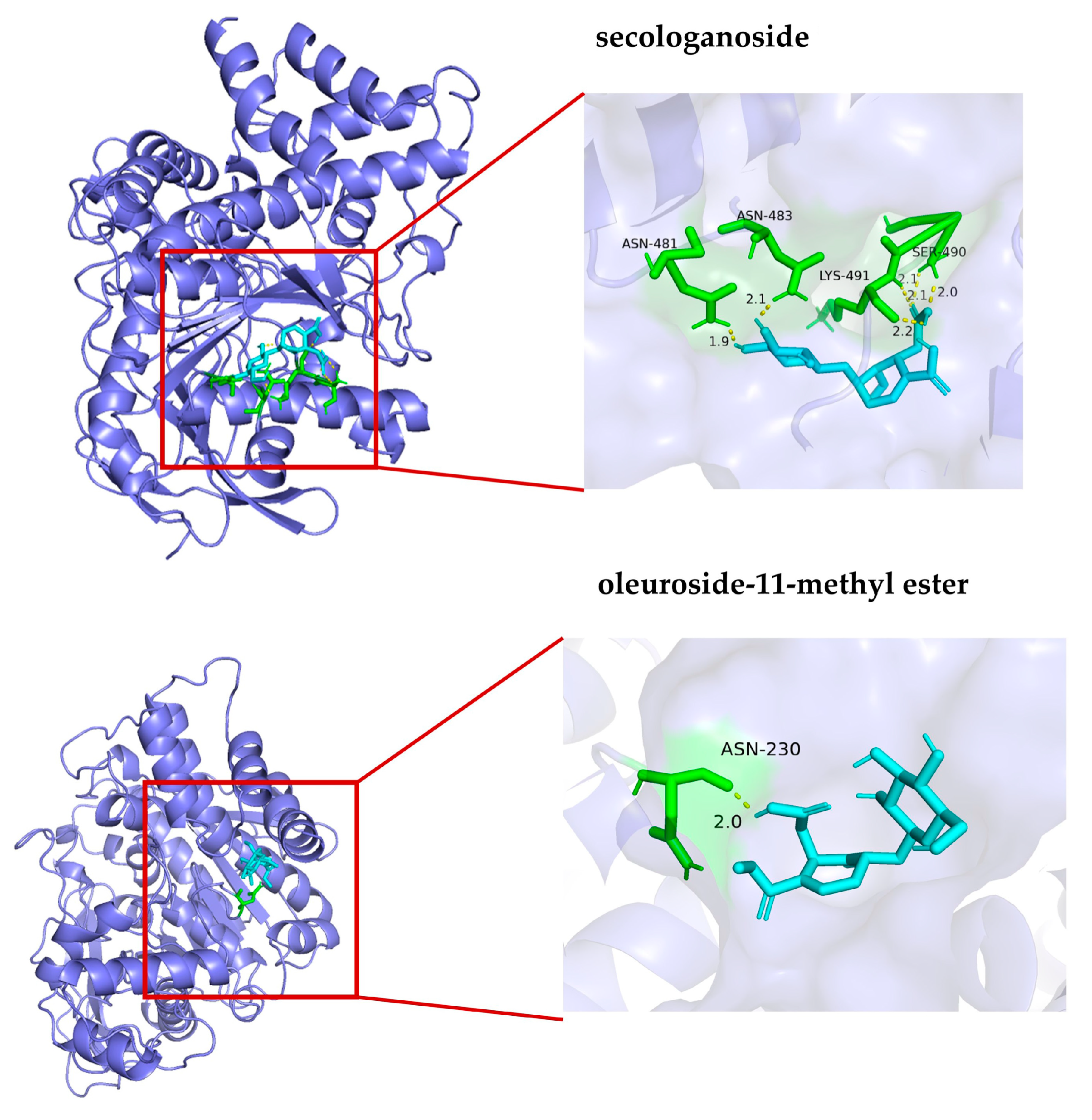
To verify the reliability of the established UF-UPLC-HSCCC method, an in vitro enzyme activity inhibition assay and a molecular docking assay (see Section 2.7) were used to evaluate the inhibitory activity of the screened active compounds against AChE. According to the literature, for samples that are difficult to dissolve in water, using 5% DMSO solution not only ensures the complete dissolution of the sample but also ensures the enzyme activity [21]. This phenomenon can be attributed to the fact that high concentrations of DMSO competitively disrupt the hydrogen bond network within the enzyme’s active site, inducing conformational changes. Furthermore, its strong hygroscopicity compromises the essential hydration shell surrounding the enzyme molecule, adversely affecting protein flexibility and catalytic efficiency. In contrast, 5% DMSO optimally modulates compound solubility to facilitate access to the active site while maintaining the stability of the enzyme–substrate complex, thereby accelerating choline release. Therefore, in this study, 5% DMSO was used to prepare the sample solution, and the experimental results showed that the IC50 values of compounds 1 and 2 were 0.76 ± 0.04 mM and 1.08 ± 0.05 mM, respectively. The interactions of potential inhibitors with AChE were simulated by molecular docking. As shown in Table 3 and Figure 5, the affinity binding energies of secologanoside and oleuroside-11-methyl ester were −6.21 and −4.52 kcal/mol, respectively. They formed six and one hydrogen bonds, respectively, with the major amino acid residues of AChE, such as SER490, LYS491, ASN483, and ASN230. The hydrogen bonds may play a key role in the enzyme catalysis mechanism. The lower binding energy of molecular docking indicates that the compounds have a strong binding effect with AChE. Meanwhile, the experimental results showed that compound 1 has a promising application in anti-AD compared to compound 2. Relevant studies have shown that the positive control compound huperzine A demonstrated a binding affinity of −8.7 kcal·mol−1 toward AChE, with specific interactions mediated through dual hydrogen bonding (His440-Ser200) and hydrophobic contacts (Phe330-Phe331) within the catalytic pocket 1011 [26]. Notably, the oleanolic acid ligand exhibited stabilizing CH-π interactions between its core scaffold and a conserved aromatic cluster comprising Phe288/290/330/331, Tyr121/334, and Trp84/279 side chains, which collectively form the peripheral anionic site adjacent to the catalytic triad [27].

Table 3.
IC50 values of active compounds, affinity binding, and interaction with acetylcholinesterase.
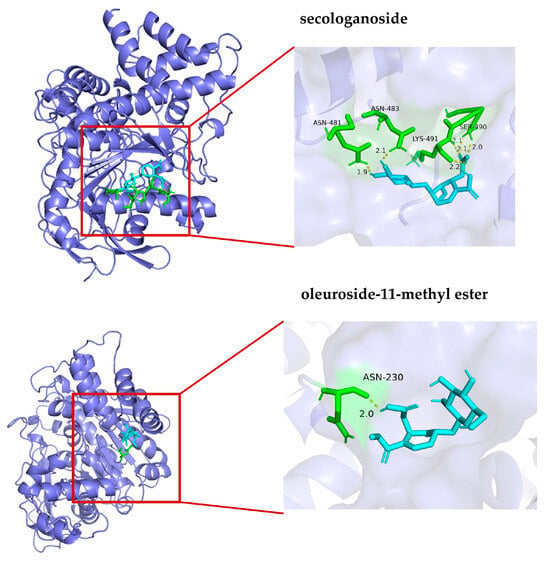
Figure 5.
Molecular docking of compounds 1 and 2 with acetylcholinesterase.
4. Conclusions
In this study, the UF-HPLC-HSCCC method was established to screen and isolate the potential AChE inhibitors secologanoside and oleuroside-11-methyl ester from O. europaea fruit. A search of the SciFinder database revealed that the two compounds are the first to be isolated from O. europaea fruit. The inhibition of AChE by these compounds was further assessed and confirmed via an in vitro AChE inhibition assay. Molecular docking was utilized to study both the binding sites and the binding affinity of the active compounds to AChE. The results demonstrate that the established screening method can be used to identify and isolate small molecules with specific molecular targets from the complex system of traditional Chinese medicines simply and rapidly. The findings also suggest that O. europaea may be an important source of potential natural AChE inhibitors.
This study provides an efficient screening and isolation method for the preparation of active compounds, which can be obtained in large quantities, and the elution–extrusion method of HSCCC avoids the loss of active fractions. In addition, the combination of molecular docking and UF screening methods was also able to maximize the exclusion of false positive results. Thus, this method is a good approach for systematically screening and purifying active compounds from crude extracts.
Author Contributions
X.W.: writing—original draft, data curation, validation, methodology, and conceptualization; Y.Z.: resources and writing—review and editing; J.M.: investigation and software. D.D.: project administration and writing—review and editing; X.H.: writing—review and editing; D.P.: writing—review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation (No. ZR2024MB018); the Key R&D Program of Yunnan Province (No. 202203AD150003); the Longnan Science and Technology Plan Project (2023-S·QKJ-02); the Yunnan Province Major Scientific and Technological Project (202402AA310034); the Qingdao Municipal Bureau of Science and Technology (No. 24-1-4-xxgg-17-nsh); and the Longnan Science and Technology Plan Project (No. 2024ZD01).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors are sincerely grateful for the financial support from the Shandong Provincial Natural Science Foundation (No. ZR2024MB018); the Key R&D Program of Yunnan Province (No. 202203AD150003); the Longnan Science and Technology Plan Project (2023-S·QKJ-02); the Yunnan Province Major Scientific and Technological Project (202402AA310034); the Qingdao Municipal Bureau of Science and Technology (No. 24-1-4-xxgg-17-nsh); and the Longnan Science and Technology Plan Project (No. 2024ZD01).
Conflicts of Interest
Author Dong Pei was employed by the company Yunnan Olive Health Industry Innovation Research and Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| HSCCC | high-speed counter-current chromatography |
| UF | ultrafiltration |
References
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s disease and vascular aging: JACC focus seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Almadodi, R.; Sultana, S.; Sebastian, M.G.; Kavani, K.S.; Lyenouq, V.E.; Shankar, A. Alzheimer’s disease: A review on the current trends of the effective diagnosis and therapeutics. Front. Aging Neurosci. 2024, 16, 1429211–1429241. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Zhou, Y.; Seven, E.; Lakshmana, M.K.; Kaushik, A.K.; Chand, H.S.; Leblanc, R.M. Nanoparticle-mediated approAChEs for Alzheimer’s disease pathogenesis, diagnosis, and therapeutics. J. Control. Release 2019, 314, 125–140. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of cholinergic signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Ozsahin, I.; Onakpojeruo, E.P.; Uzun, B.; Ozsahin, D.U.; Butler, T.A. Comparative evaluation of FDA-approved drugs for managing the symptoms of AD. Alzheimer’s Dement. 2023, 19, e075669–e075679. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Tian, P.; Tan, T. Delineating biosynthesis of Huperzine A, A plant-derived medicine for the treatment of Alzheimer’s disease. Biotechnol. Adv. 2022, 60, 108026–108037. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, K.R.; Hutchings, B.K.; Ratnakaran, B.; Kablinger, A.S. Donepezil-induced psychosis: A cautionary report of a rare adverse reaction. Eur. Psychiatry 2023, 66, S357–S358. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Qiao, O.; Ji, H.; Zhang, Y.; Han, X.; Wang, W.; Li, X.; Wang, J.; Guo, L.; et al. Rosmarinic acid potentiates and detoxifies tacrine in combination for Alzheimer’s high-performance liquid chromatography disease. Phytomedicine 2023, 109, 154600–154612. [Google Scholar] [CrossRef]
- Nam, Y.; Shin, S.J.; Park, Y.H.; Kim, M.J.; Jeon, S.G.; Lee, H.; Choi, Y.; Kim, T.J.; Shin, S.M.; Kim, J.J.; et al. Platycodon grandiflorum root protects against Aβ-Induced cognitive dysfunction and pathology in female models of Alzheimer’s Disease. Antioxidants 2021, 10, 207. [Google Scholar] [CrossRef]
- Refaey, M.S.; Abdelhamid, R.A.; Elimam, H.; Elshaier, Y.A.M.M.; Ali, A.A.; Orabi, M.A. A bioactive constituent from thunbergia erecta as potential anticholinesterase and anti-ageing agents: Experimental and in silico studies. Bioorg. Chem. 2021, 108, 104643–104653. [Google Scholar] [CrossRef]
- Li, J.G.; Mutreja, Y.; Servili, M.; Leone, A.; Praticò, D. The anti-neuroinflammatory effect of extra-virgin olive oil in the triple transgenic mouse model of Alzheimer’s Disease. JAD 2024, 100, 119–126. [Google Scholar] [CrossRef]
- Guo, J.; Lin, H.; Wang, J.; Lin, Y.; Zhang, T.; Jiang, Z. Recent advances in bio-affinity chromatography for screening bioactive compounds from natural products. JPBA 2019, 165, 182–197. [Google Scholar] [CrossRef]
- Cieśla, Ł.; Moaddel, R. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat. Prod. Rep. 2016, 33, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Hou, X. Recent advances in screening active components from natural products based on bioaffinity techniques. Acta Pharm. Sin. B 2020, 10, 1800–1813. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, J.; Qin, J.; Yang, M. Screening techniques for the identification of bioactive compounds in natural products. J. Pharm. Biomed. Anal. 2019, 168, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, I.Y.; Habibie, H.; Bahar Muh, A.; Budán, F.; Csupor, D. Anticancer effects of secoiridoids—A scoping review of the molecular mechanisms behind the chemopreventive effects of the olive tree components oleocanthal, oleacein, and oleuropein. Nutrients 2024, 16, 2755. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Senol, F.S.; Ankli, A.; Reich, E.; Orhan, I.E. HPTLC fingerprinting and cholinesterase inhibitory and metal-chelating capacity of various citrus cultivars and Olea europaea. Food Technol. Biotechnol. 2016, 54, 275–281. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Li, Y.J.; He, F.Q.; Zhao, H.H.; Li, Y.; Chen, J. Screening and identification of acetylcholinesterase inhibitors from Terminalia chebula fruits by immobilized enzyme on cellulose filter paper coupled with ultra-performance liquid chromatography-quadrupole time-of-flight spectrometry and molecular docking. J. Chromatogr. A 2022, 1663, 462784–462798. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Liu, C.; Li, S.; Li, R.; Zhang, Y.; Chen, M.; Sun, R. High-speed countercurrent chromatography isolation of active components from Evodia rutaecarpa and affinity ultrafiltration Screening for their acetylcholinesterase inhibitor activity. J. Sep. Sci. 2024, 47, e70002–e70012. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, P.; Wang, N.; Sun, C.; Yang, X.; Li, H.; Zhou, G.; Li, Y. Separation of three polar compounds from rheum tanguticum by high-speed countercurrent chromatography with an ethyl acetate/glacial acetic acid/water system. J. Sep. Sci. 2018, 41, 1775–1780. [Google Scholar] [CrossRef]
- Bailleul, F.; Leveau, A.M.; Durand, M. Nouvel iridoide des fruits de lonicera alpigena. J. Nat. Prod. 1981, 44, 573–575. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, S.L.; Chein, C.C. Jaspolyside, a secoiridoid glycoside from Jasminum polyanthum. Phytochemistry 1996, 42, 1629–1631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).