Abstract
To explore anthocyanins in black bean peel, the conditions of ultrasound-assisted deep eutectic solvents (DESs) were screened and optimized using the method of response surface optimization. After that, the purification of the anthocyanins was performed before investigating their antioxidant activity and stability. The results showed that the choline chloride–citric acid system was more suitable for the extraction of anthocyanins from black bean peel, and the maximum amount of 61.00 ± 2.73 mg C3GE/100 g DW anthocyanins was obtained with the following optimized conditions: extraction time, 40 min; ultrasonic power, 60 KHz; material–liquid ratio, 1:20 g/mL; and ultrasonic temperature, 50 °C. The purity of the anthocyanins increased to 193.62 mg C3GE/100 g after purification with AB-8 resin, which also significantly improved the ability to screen DPPH and ABTS radicals. The anthocyanins from black bean peel were sensitive to light, temperature, pH, and additives.
1. Introduction
Anthocyanins are a class of flavonoid polyphenolic compound that is primarily found in the flowers, seeds, leaves, and fruits of plants. Anthocyanins have two benzene rings linked by C6-C3-C6 and are classified as 3-hydroxyanthocyanins, 3-deoxyanthocyanins, and O-methylated anthocyanins [1]. Numerous studies have demonstrated that anthocyanins exhibit many health benefits, including antioxidant, anti-inflammatory, anti-diabetic, anti-cancer, anti-obesity, and cardiovascular protective activities [2,3,4]. However, as a class of powerful active polyphenols, anthocyanins are characterized by poor stability, high hydrophilicity, and low bioavailability during digestion and absorption [5]. Extraction is an important step in the isolation, identification, and utilization of anthocyanins. Due to their special structure and physicochemical properties, anthocyanins are extremely unstable during the extraction process. Therefore, various novel methods, such as conventional solvent extraction, ultrasound-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, and supercritical fluid extraction have been applied to extract anthocyanins [6]. DESs are liquid mixtures of binary or ternary systems consisting of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs). Recently, DESs were developed as the extraction solvents of anthocyanins with some desirable properties, including physicochemical stability, good recyclability, and thermal stability; thus, they have advantages in terms of the extraction of anthocyanins, biocompatibility, and environmental friendliness [7,8].
Although legumes are typically consumed with their peel, the peel is often discarded as a byproduct during food processing, leading to significant loss of bioactive compounds and resulting in resource waste [9]. Extracting anthocyanins from bean peel not only allows for the utilization of agricultural byproducts, creating economic value, but also helps reduce resource waste in food processing, aligning with the principles of sustainable development [9]. Black bean peel is an exceptional source of biologically active components, particularly anthocyanins, which are a class of polyphenols that typically bind to sugar via a glycosidic bond [10]. Compared to other plant sources, black bean peel anthocyanins offer distinct advantages: they exhibit a unique composition dominated by delphinidin-3-glucoside, petunidin-3-glucoside, and malvidin-3-glucoside (56%, 26%, and 18%, respectively) [11], which contributes to their exceptional antioxidant capacity. They demonstrate superior stability in certain conditions compared to anthocyanins from other sources; and they are derived from an agricultural by-product, making their extraction both economically and environmentally beneficial. The anthocyanins in black bean peel not only impart a vibrant color but also exhibit strong health benefits, including antioxidant, anti-inflammatory, anti-obesity, anti-diabetic, anti-cardiovascular, and eyesight-benefiting activities [12]. Despite these advantages, the extraction and utilization of black bean peel anthocyanins present unique challenges due to their complex matrix and their sensitivity to extraction conditions. Previous studies have primarily focused on conventional extraction methods, which often result in lower yields and the potential degradation of anthocyanins [13]. The development of novel extraction techniques, particularly using deep eutectic solvents (DESs), represents a significant advancement in this field. DESs offer several advantages for the extraction of black bean peel anthocyanin, including higher selectivity, better stability preservation, and environmental friendliness. However, no comprehensive study has yet reported the optimization of DES-based extraction for black bean peel anthocyanins; nor has there been a systematic investigation of their isolation, purification, and stability. Therefore, this study aims to address these gaps by developing an efficient DES-based extraction method and comprehensively characterizing the resulting anthocyanins, with the ultimate goal of facilitating their application in functional foods and nutraceuticals.
The present study combined ultrasound-assisted technology and the novel DESs to extract anthocyanins from black bean peel. Then, the anthocyanins were purified using AB-8-type macroporous resin. After that, we investigated the antioxidant activity of anthocyanins in vitro. Finally, the effects of pH, temperature, light, storage time, sugars, metal ions, and redox agents on the stability of anthocyanins were explored. This study aims to provide more details for the further application of anthocyanins from black bean peel in functional foods.
2. Materials and Methods
2.1. Materials and Reagents
Black bean peel was provided by Ankang Biotechnology Company Limited (Ankang, Shaanxi, China). All of the material used in this study was sourced from the same lot to ensure consistency in quality and characteristics. Choline chloride, betaine, proline, lactic acid, citric acid, and malic acid were purchased from Yuanye (Shanghai, China). D101, AB-8 and HPD100 were purchased from Xi’an Lanshen Specialty Resins Company Limited (Xian, Shaanxi, China), Anhui Samsung Resins Company Limited (Bengbu, Anhui, China) and Xi’an Hanyu Resin Technology Company Limited (Xian, Shaanxi, China), respectively.
2.2. Sample Preparation and Extraction
The dried black soybean peel was ground into a powder and then passed through an 80-mesh sieve. The processed black bean peel was stored at room temperature in the dark.
2.3. Preparation of DESs and the Extraction of Anthocyanins from Black Bean Peel
The preparation of DESs was performed according to the description of Zhang et al., with some modifications [14]. In brief, different hydrogen-bonding receptors (choline chloride, betaine, proline) were mixed with different hydrogen-bonding donors (lactic acid, citric acid, malic acid) in a 1:1 molar ratio. Then, 20% distilled water was added to dissolve the mixture at 80 °C via a water bath to obtain the DESs for use.
The extraction process was carried out according to the report of Fu et al., as follows: the extraction temperature was 50 °C, the ultrasonic power was 60 KHz, the extraction time was 20 min, and the solid–liquid ratio was 1:20. After that, the extracts were centrifuged at 7000 r/min for 15 min to collect the supernatant, which was stored at 4 °C for use [15].
2.4. Determination of the Maximum Absorption Wavelength of Anthocyanins in Extracts
The absorption spectra of the diluted and clarified anthocyanin extracts were scanned in the visible wavelength ranging of 400–700 nm using a UV–visible spectrophotometer (UV-3100, Shanghai Mepda Instrument Co., Ltd., Shanghai, China). The maximum absorption wavelength of the anthocyanins was determined to be 520 nm, which is consistent with the previous report [16].
2.5. Determination of Anthocyanins Content
The content of anthocyanins in the extracts of black bean peel was measured according to the pH difference method [15]. In brief, 0.4 mL of anthocyanin extract was diluted using a KCl-HCl (pH = 1.0) and CH3COONa-HCl solution (pH = 4.5). After 30 min in the dark at 37 °C, the absorbance was determined at wavelengths of 520 nm and 700 nm. The content of anthocyanins was calculated using Formula (1):
where ΔA = (A520—A700) pH1.0—(A520—A700) pH4.5. Mw is the molar mass of cornflowerin-3-O-glucoside, 449.2 g/mol; DF is the dilution factor; V is the total volume of the extract, mL; ε is the extinction coefficient of cyanidin-3-O-glucoside (29,600 L/mol/cm); L is the light range, 1 cm; and m is the sample mass, g.
2.6. Optimization of Extraction Process for Anthocyanins
2.6.1. Screening of DESs
The anthocyanins of black bean peel were extracted using the process described in Section 2.3, and the content of anthocyanins in the extracts prepared using different DESs was comparatively analyzed to screen the best DES.
2.6.2. Single-Factor Experiments
The choline chloride–citric acid (Chcl/CA) system was chosen as the subsequent extraction solvent because it generated the highest yield of anthocyanins from black bean peel. The optimization experiments were carried out using the method of Zhang et al., with slight modifications [14]. Briefly, the Chcl/CA was added into the black bean peel powder, and the solid–liquid ratio (1:20 g/mL, 1:30 g/mL, 1:40 g/mL, 1:50 g/mL, 1:60 g/mL), extraction temperature (30 °C, 40 °C, 50 °C, 60 °C, 70 °C), extraction time (30 min, 60 min, 90 min, 120 min, 150 min), and ultrasonic power (50 KHz, 60 KHz, 70 KHz, 80 KHz, 90 KHz) were subjected to one-way tests. The effects of different processes on the yield of anthocyanins from black bean peel were investigated, and each experiment was repeated three times.
2.6.3. Response Surface Optimization Experiments
Response surface optimization was performed according to the Box–Behnken design principle. The anthocyanin content of the extracts was used as the index of investigation (Y), and the extraction temperature (A), solid–liquid ratio (B) and ultrasonication time (C) were selected as the independent variables to determine the optimal process parameters by a three-factor, three-level response surface experiment (Table S1).
The extraction process was performed according to the description in Section 2.3 and the supernatant was further concentrated under vacuum at 50 °C using a rotary evaporator (RE 52-86A, Shanghai Yarong Biochemical Equipment Co., Ltd., Shanghai, China). After that, one part of the concentrated extract was freeze-dried and used for subsequent experiments. The other part of the concentrated extract was stored at 4 °C in the dark for subsequent experiments.
2.7. Optimization of the Purification Process of Anthocyanins
2.7.1. Pretreatment of Macroporous Resins
Three macroporous resins (D101, HPD100, AB-8) were activated by soaking in 95% ethanol for 24 h. The resins were then rinsed with water until they were free of ethanol, and they were then soaked in hydrochloric acid solution (5%) and sodium hydroxide solution (5%) for 5 h. Finally, they were further rinsed into a neutral state for use.
2.7.2. Screening of Resins
D101, HPD100, and AB-8 macroporous resins (2.0 g) were mixed with black bean peel anthocyanin extract (30 mL), and the mixtures were shaken in a shaker (THZ-82, Changzhou Guohua Electric Co., Ltd., Changzhou, China) at 100 r/min for 24 h to determine the adsorption rate. Then, 50 mL of 60% (v/v) ethanol was added to the saturated macroporous resin and the mixtures were shaken for another 24 h to determine the desorption rate. The adsorption and desorption rates were calculated as follows:
where A0 is the absorbance value at 520 nm of the diluted anthocyanin extract. A1 is the absorbance value at 520 nm of the adsorbed liquid. A2 is the absorbance value at 520 nm of the desorbed liquid.
2.7.3. Static and Dynamic Optimization
The anthocyanin extract (50 mL) was mixed with AB-8 macroporous resin (2.0 g) and shaken at 100 r/min. The absorbance of the supernatant was detected at 520 nm every 60 min and the adsorption rate was calculated at different times. The static adsorption curve was plotted to determine the loading time. The adsorb-saturated macroporous resin (1.0 g) was mixed with 60% (v/v) ethanol solution (30 mL), and the absorbance was determined at 520 nm every 60 min to plot the static desorption curves.
Moreover, 2.0 g of the saturated macroporous resin was mixed with 30 mL of ethanol solution with different concentrations (40%, 50%, 60%, 70%, 80%, v/v). The mixture was shaken (100 r/min) for 10 h and the absorbance of the supernatant was measured at 520 nm to determine the optimum volume of ethanol.
2.7.4. Optimization of the Loading Concentration
The macroporous resin was poured into the column along the glass rod for wet loading. Different concentrations of anthocyanins (0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL) were added at a constant rate (1.0 mL/min). The absorbance of the effluent was measured to determine the optimal loading concentration of the sample.
2.7.5. Optimization of the Sample Flow Rate
After the wet loading of AB-8, the anthocyanin extracted from black bean peel was added with different flow rates (0.5 mL/min, 1.0 mL/min, 1.5 mL/min, 2.0 mL/min, and 2.5 mL/min), and then the absorbance of the effluent was measured to determine the optimal sample flow rate.
2.7.6. Determination of the Loading Volume
The anthocyanin extract of black bean peel was added into the column at a flow rate of 1.0 mL/min, and the effluent was collected every 1 elution volume (BV). The absorbance was measured at 520 nm and the leakage curve was plotted. In general, the absorbance of the effluent should not exceed 1/10 of the absorbance of the sample solution. Otherwise, it is regarded as anthocyanin leakage, and the sample loading should be stopped immediately.
2.7.7. Determination of the Elution Flow Rate
The anthocyanin extract flowed through the resin to saturate it at a flow rate of 1.0 mL/min, and then it was repeatedly rinsed with distilled water. After that, different flow rates (0.5 mL/min, 1.0 mL/min, 1.5 mL/min, 2.0 mL/min, and 2.5 mL/min) of the ethanol solution (60%, v/v) were used to elute the anthocyanins and the absorbance was determined to calculate the elution flow rate.
2.8. Determination of the Color Value of Anthocyanins After Purification
The purified anthocyanins from black bean peel were freeze-dried and re-dissolved with citric acid–disodium hydrogen phosphate buffer, pH 3.0. The absorbance value was detected at 520 nm and the color value was determined using Formula (4):
where A is the absorbance of the sample solution and R is the dilution.
2.9. Antioxidant Properties of Anthocyanin Extracts from Black Bean Peel
2.9.1. Determination of DPPH Scavenging Activity
The DPPH and ABTS assays were performed according to our previous study. Briefly, different concentrations (1.5 mg/mL, 2 mg/mL, 2.5 mg/mL, 3.0 mg/mL, and 3.5 mg/mL) of black bean peel anthocyanin solution (400 μL) and DPPH (600 μL) were mixed and homogenized. The samples were incubated at 25 °C for 30 min in the dark and centrifuged at 4000× g for 5 min, and the absorbance of the supernatant was measured at 700 nm with a UV spectrophotometer (UV-3100, Shanghai Mepda Instrument Co., Ltd., Shanghai, China). The DPPH radical scavenging rate was calculated using Formula (5):
where A1 is the absorbance of the blank control, and A0 is the absorbance of the experimental groups.
2.9.2. Determination of ABTS Free Radical Scavenging Activity
First, 6 mL of the ABTS working solution was mixed with 0.2 mL of the sample solution (0.05 mg/mL, 0.1 mg/mL, 0.15 mg/mL, 0.2 mg/mL, 0.25 mg/mL, or 0.3 mg/mL). After mixing, the sample was kept away from light for 30 min. The absorbance at 734 nm was measured and VC was used as a positive control.
where A1 and A0 are the absorbance of the blank control and experimental groups, respectively.
2.10. Stability of the Anthocyanins from Black Bean Peel
2.10.1. Effect of pH on the Stability of Anthocyanins from Black Bean Peel
Anthocyanins solutions with pH values of 3.0, 5.0, and 7.0 were subjected to a 70 °C. The anthocyanins concentration was measured at 0, 1, 2, 3, 4, 5, 7, 9, 11, and 12 h to determine the degradation rate. The degradation process of anthocyanins from black bean peel was analyzed using a first-order kinetic model. The kinetic equation is as follows:
where ct is the anthocyanins concentration at time t. c0 is the initial concentration. k is the rate constant (h−1). t is the heating time (hours).
2.10.2. Effect of Temperature on the Stability of Anthocyanins from Black Soybean Peel
The anthocyanin solution of black bean peel (pH 1.5) was placed at 4 °C, 25 °C, and 37 °C away from light to record the spectrum every 2 d. The effect of temperature on the stability of anthocyanins in black bean peel is represented as the retention rate.
2.10.3. Effect of Light on the Stability of Anthocyanins in Black Bean Peel
The anthocyanin solution of black bean peel (pH 1.5) was prepared and then divided into two groups, one of which was kept in natural light and the other of which was kept in darkness. The spectra were recorded every 2 days and the preservation rate of the anthocyanins was calculated.
2.10.4. Influence of Redox Agents on the Stability of Anthocyanins from Black Bean Peel
The anthocyanin solution (pH 1.5) was mixed with the same volume of H2O2 solution and the final concentrations of H2O2 were 0%, 0.1%, 0.2%, and 0.3%. Immediately, the spectra were determined at 5 min, 10 min, 20 min, 40 min, 60 min, and 120 min to investigate the influence of H2O2 on the stability of anthocyanins from black bean peel.
Similarly, the Na2SO3 solution was mixed with the anthocyanin solution (pH 1.5) to reach the final concentrations of 0 mg/mL, 0.025 mg/mL, 0.050 mg/mL, and 0.100 mg/mL. The spectra were determined at 5 min, 10 min, 20 min, 40 min, 60 min, and 120 min to investigate the influence of Na2SO3 on the stability of anthocyanins from black bean peel.
2.10.5. Influence of Additives on the Stability of Anthocyanins in Black Soybean Peel
The anthocyanin solution (pH 1.5) was mixed with the same volume of glucose and sucrose solution, and the final concentrations of glucose and sucrose were 1%, 2%, 3%, 4%, and 5%. The mixtures were placed in the dark at room temperature and the absorbance was measured every 2 days at 520 nm. The anthocyanin retention was calculated to represent the influence of glucose and sucrose on the stability of anthocyanins.
2.10.6. Effect of Metal Ions on the Stability of Anthocyanins from Black Bean Peel
Different types of metal ions, including Na+ (0.1%, 0.2%, 0.3%, 0.4%, and 0.5%), K+ (0.1%, 0.2%, 0.3%, 0.4%, and 0.5%), and Cu2+ (1%, 5%, 10%, 15%, and 20%) in the form of NaCl, KCl, and CuCl2•2H2O solution were mixed with the same volume of anthocyanin solution (pH 1.5) and then stored at room temperature in the dark. The absorbance was determined every 2 days to explore the effect of metal ions on the stability of anthocyanins.
3. Results and Discussion
3.1. Optimization of Anthocyanin Extraction from Black Bean Peel
3.1.1. Physicochemical Properties of DESs and the Effects on Anthocyanin Extraction
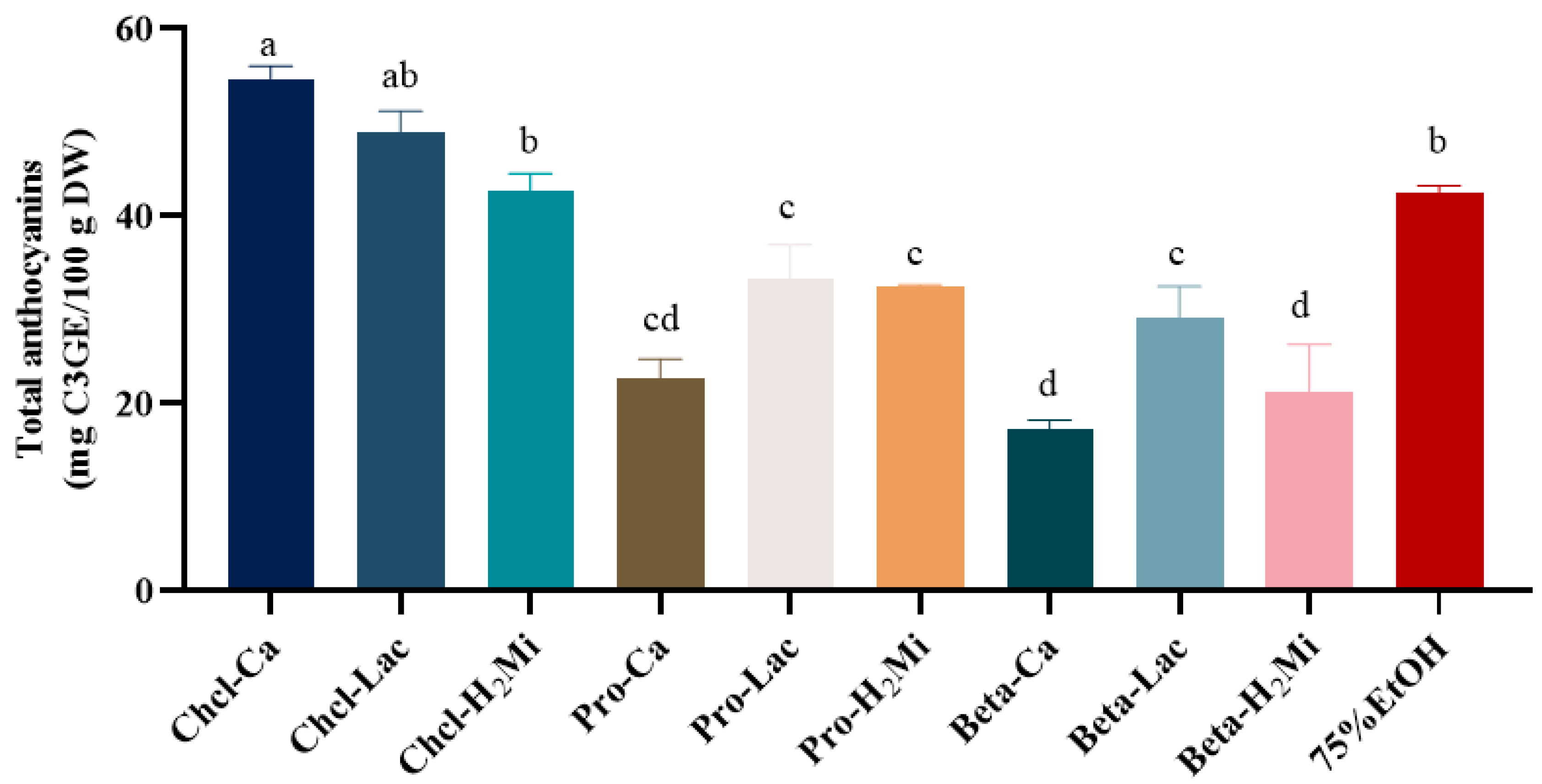
The physicochemical properties of DESs were determined and the results showed that the pH values of the DESs ranged from 0.8 (Chcl-Ca) to 3.3 (Beta-Lac) (Table 1). However, the density of these DESs showed no significant difference. After that, we measured the effects of different DESs on the extraction of anthocyanins from black bean peel. As shown in Figure 1, the Chcl/Ca system extracted the highest yield of anthocyanins, with a content of 54.18 ± 0.17 mg C3GE/100 g DW, followed by Chcl-Lac and Chcl-H2Mi. Meanwhile, the total content of anthocyanins extracted by the Chcl/Ca system was 1.26 times higher than that of 75% (v/v) ethanol under the same extraction conditions, indicating that the diffusivity of Chcl/Ca is closer to the polarity of anthocyanins in black bean peel, which promotes the dissolution and diffusion of anthocyanins. Therefore, the Chcl/Ca system was selected as the solvent for the extraction of anthocyanins from black bean peel.

Table 1.
Physicochemical properties of DESs.
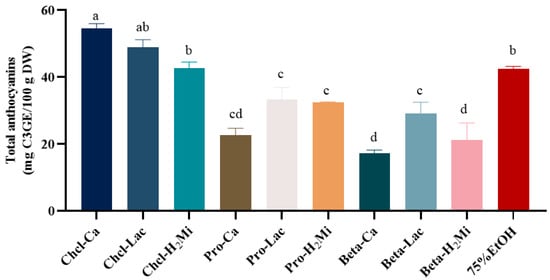
Figure 1.
Effect of different DESs on the content of anthocyanins from black bean peel. Different letters mean p < 0.05.
3.1.2. One-Way Experiments
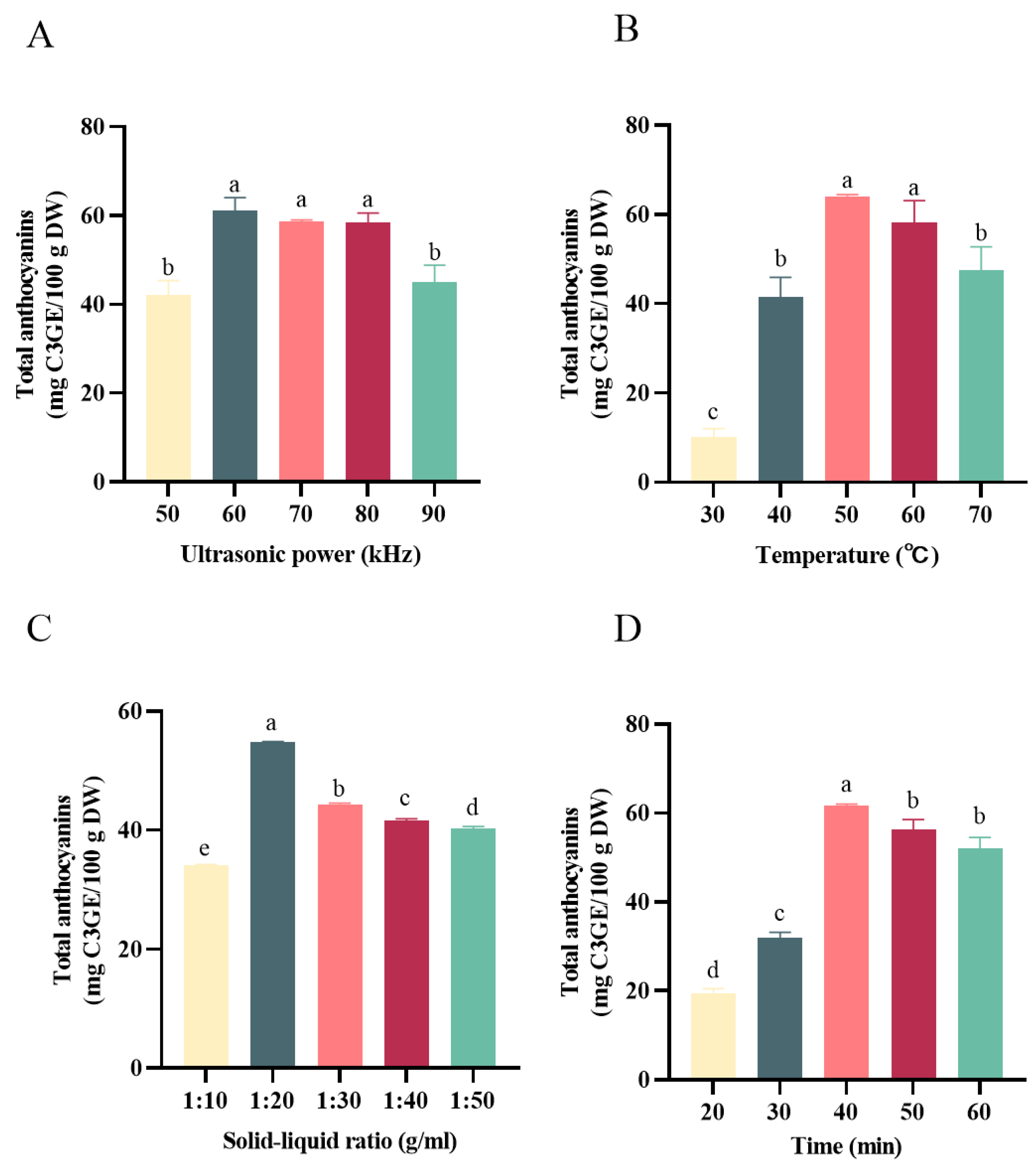
One-way experiments were employed to optimize the single factors, including the ultrasonic power, solid–liquid ratio, extraction temperature, and extraction time. The effects of ultrasonic power on the anthocyanins are shown in Figure 2A, and the results revealed that 60 KHz was the best ultrasonic power for anthocyanin extraction. Therefore, the following experiments were performed with the ultrasonic power of 60 KHz. As shown in Figure 2B, the yield of anthocyanins was increased with the elevation of the ultrasonication temperature, which reached the maximum amount at 50 °C. However, when the ultrasonication temperature was greater than 50 °C, negative effects were observed when obtaining the anthocyanins. The reasons might be related to the degradation of anthocyanins and the decrease in the polarity and hydrogen bonding stability of the DESs [17]. As can be inferred from Figure 2C, when the solid–liquid ratio was 1:20 g/mL, the anthocyanin extraction from the black bean peel reached the highest value of 54.78 ± 0.12 mg C3GE/100 g. Therefore, the optimum solid–liquid ratio selected for the DESs was 1:20 g/mL. Similarly, the extracted anthocyanins increased with the increase in the ultrasonic time from 20 min to 40 min; however, continued prolonging of the extraction time induced a decreased yield of anthocyanins. The reason for this phenomenon might be the long ultrasonic time and high power, which led to a large number of impurities in the solvent system, disturbing the dissolution or inducing the degradation of anthocyanins in the black bean peel [16]. Therefore, the solid–liquid ratio of 1:20 g/mL, an ultrasonication temperature of 50 °C, and an ultrasonication time of 40 min were selected as the center values for the response surface test.
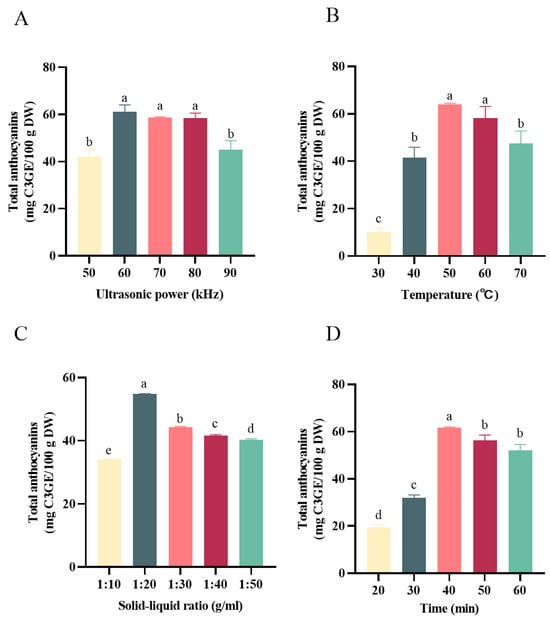
Figure 2.
Effect of different extraction factors on the content of anthocyanins. (A) Effect of the solid–liquid ratio on the content of anthocyanins; (B) effect of temperature on the content of anthocyanins; (C) effect of ultrasonic power on the content of anthocyanins; (D) effect of time on the content of anthocyanins. Different letters mean p < 0.05.
3.1.3. Response Surface Results and ANOVA
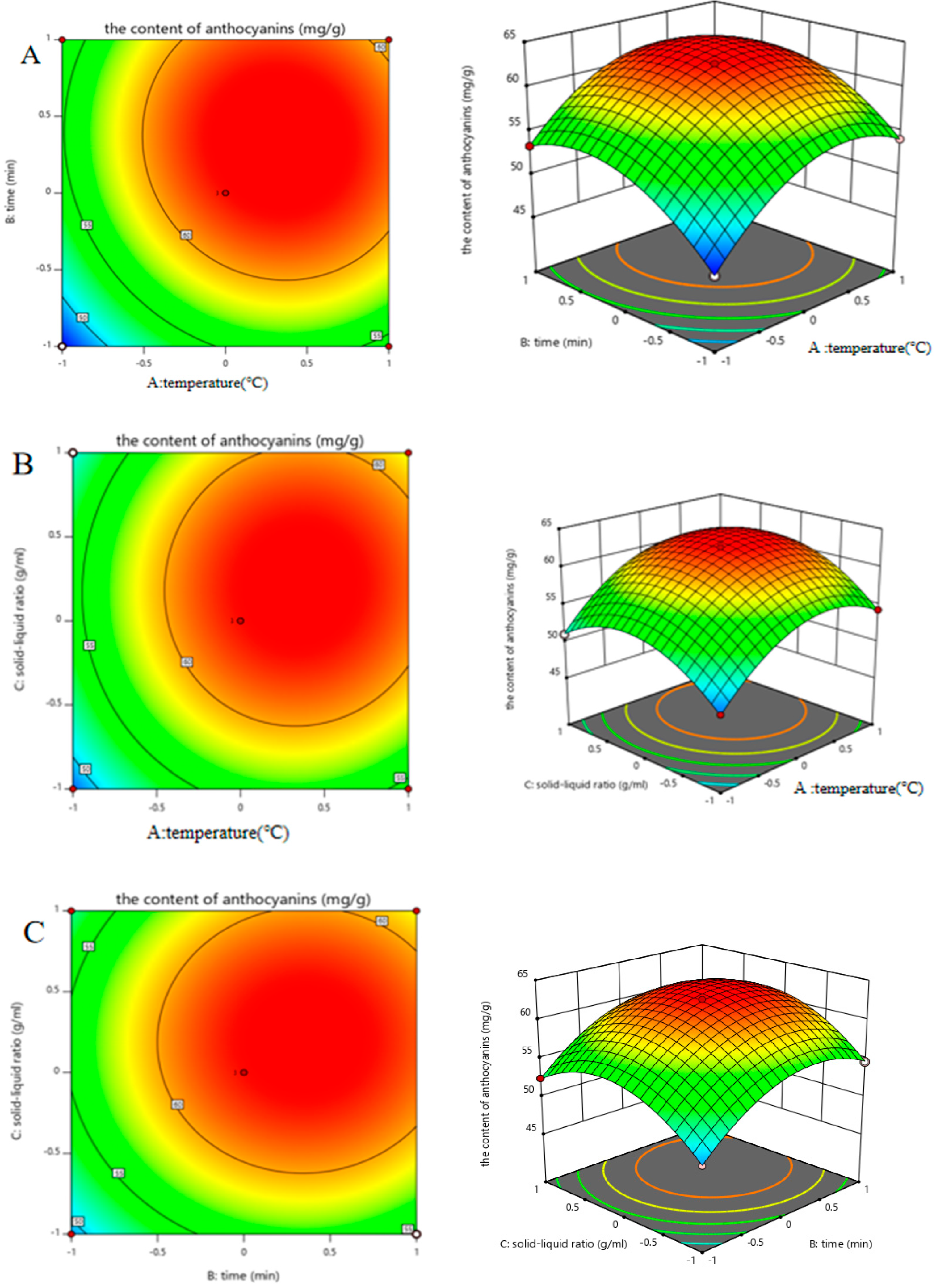
Based on the experimental protocol optimally designed using the Box–Behnken response surface method (Figure 3), the optimal experiments were performed and the regression analysis was analyzed according to the results of Table 2 to obtain a quadratic polynomial regression equation:
Y = 62.37 + 3.36∗A + 2.95∗B + 1.77∗C − 0.3900∗AB + 0.3100∗AC + 0.1250∗BC − 4.85∗A2 − 4.20∗B2 − 4.54∗C2
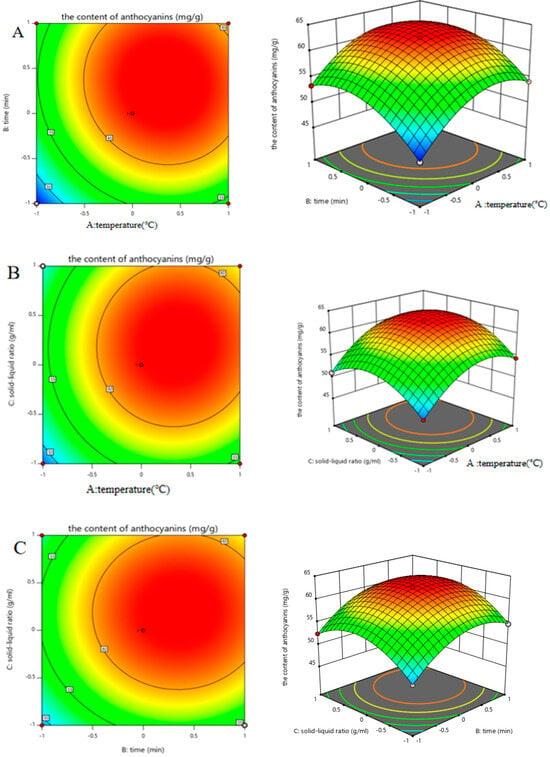
Figure 3.
The relationships between different factors. (A) Effects of the interaction between temperature and time; (B) effects of the interaction between the solid–liquid ratio and temperature; (C) effects of the interaction between time and the solid–liquid ratio.

Table 2.
Response surface design and results.
The corresponding ANOVA of variance was performed on the regression model, and the analysis results were shown in Table 3. The model p < 0.0001 indicated that the regression model was highly significant, and the misfit term p = 0.75869 > 0.05 implied that the misfit term of the model was not significant; the correlation coefficient of the model R2 = 0.9876 revealed that the model was able to explain 98.76% of the variation. In conclusion, the model analysis can be used to predict the optimal process for the extraction of anthocyanins from black bean peel via ultrasound-assisted DESs.

Table 3.
Analysis of variance of regression model.
3.2. Optimization of the Process of Purifying Anthocyanin from Black Bean Peel
3.2.1. Resin Screening
Macroporous resins mainly rely on weak interaction forces such as van der Waals forces or the hydrogen bonds formed between their surfaces and organic molecules to adsorb organic molecules, and the adsorption and desorption effects are not only affected by the polarity of the resin and the size of the particles, but also by the specific surface area, pore size, and other spatial structures [19]. As shown in Table S2, the adsorption and desorption effects of different types of macroporous resins on anthocyanins were different; specifically, the AB-8 type of macroporous resin had a better adsorption effect. The AB-8 type resin had a weaker polarity and was able to react with the weakly polar components of the anthocyanins in the black bean peel to form hydrogen bonds, thus increasing the adsorption. In terms of the desorption effect, the desorption rate of AB-8 was also higher than that of the other two macroporous resins (Table S2). Therefore, the AB-8 macroporous resin was selected for the next study.
3.2.2. Dynamic and Static Optimization Results
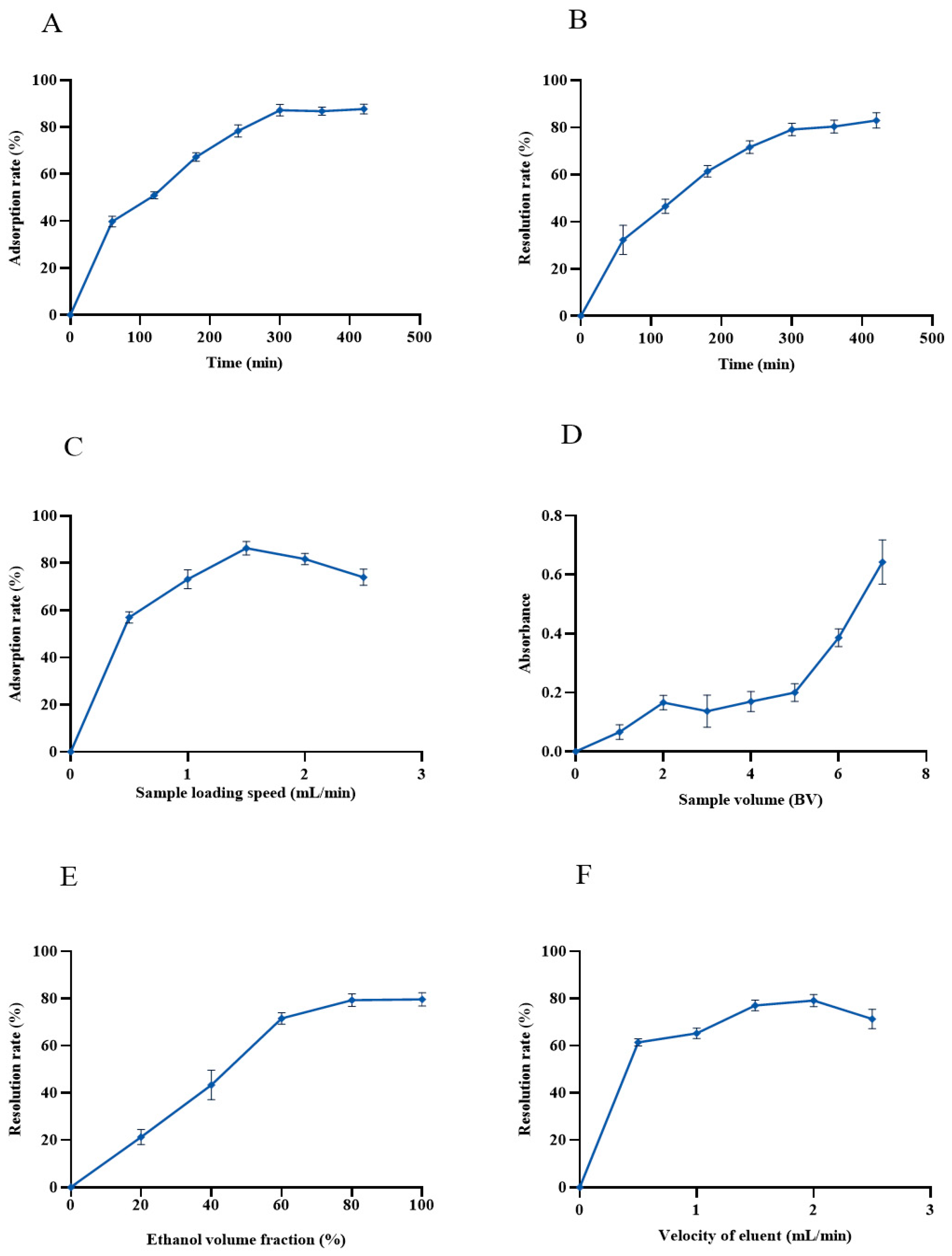
As shown in Figure 4A, the adsorption capacity of AB-8 macroporous resin on anthocyanins of black bean peel was linearly increased over the first 60 min and then leveled off. Until 300 min, the adsorption of anthocyanins by the AB-8 resin was nearly saturated, and the maximum adsorption rate reached 89.27%. Therefore, 300 min was used as the sampling time in the subsequent experiments.
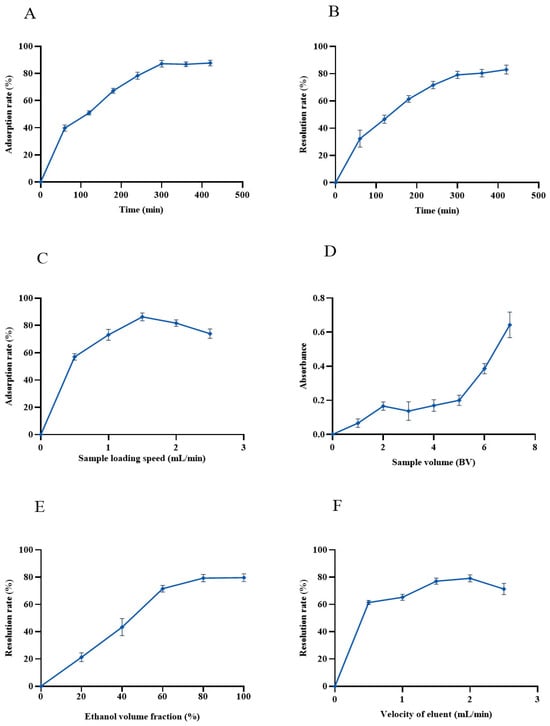
Figure 4.
Purification of anthocyanins from black bean peel by AB-8 resin. (A) Static adsorption curve of macroporous resin for anthocyanins in black bean peel; (B) dynamic elution curve of macroporous resin for anthocyanins in black bean peel; (C) the effect of the sample loading speed on the adsorption rate of the AB-8 resin; (D) the influence of the sample volume on the adsorption rate of the AB-8 resin; (E) the effect of the ethanol volume on the desorption rate of the AB-8 resin; (F) the effect of the elution flow rate on the desorption rate of the AB-8 resin.
As shown in Figure 4B, the desorption rate of the AB-8 macroporous resin gradually increased with the extension of time, reaching a steady level at 300 min. Hence, 300 min was selected as the optimum static desorption time.
The effect of sample flow rate on the adsorption of anthocyanins from black bean peel is shown in Figure 4C. With the increase in the sample flow rate, the adsorbed amount showed an obvious upward trend, and the adsorbed amount decreased obviously when the sample flow rate reached 1.5 mL/min, so the sample flow rate was set at 1.5 mL/min. The flow rate is a key factor that affects the adsorption efficiency of the macroporous resin. If the flow rate is too high, the amount of anthocyanin adsorbed will be reduced, resulting in a waste of samples. However, if the flow rate is too slow, it will take a long time to affect the efficiency, although the macroporous resin can fully adsorbs the anthocyanins [20].
The influence of the sample volume on the amount of AB-8 resin adsorbed is shown in Figure 4D. With the increase in the sample volume, the absorbance of the effluent showed an upward trend. When the sample volume was 5 BV, the absorbance reached 1/10 of the sample solution, which was considered to be the leakage point, so the sample volume of the subsequent tests was set at 5 BV.
The effect of ethanol concentration on the efficiency of desorption is shown in Figure 4E. With the increase in the ethanol concentration, the anthocyanins desorbed from the black bean peel rapidly increased, reaching its maximum when the ethanol concentration was 80% (Figure 4E). Due to the principle of similar compatibility between the eluent and the anthocyanin, this result indicated that the polarity of 80% ethanol is similar to the anthocyanins from black bean peel, promoting the dissolution and elution of anthocyanins from AB-8 resin. Therefore, the ethanol solution with a concentration of 80% was selected as the eluent for the desorption of the anthocyanins.
The effect of the elution flow rate on the desorption rate is shown in Figure 4F. When the elution flow rate was 2 mL/min, the desorption rate reached the highest value of 81%, so this was selected as the optimal elution flow rate.
3.3. Antioxidant Activity of Anthocyanins from Black Bean Peel
The scavenging capacity of black bean peel anthocyanins against DPPH and ABTS free radicals increased in a dose-dependent manner. Specifically, purified anthocyanins from black bean peel exhibited increased DPPH and ABTS scavenging abilities with the elevation of concentrations (Figure 5). Meanwhile, the antioxidant activity of the anthocyanins was obviously enhanced after purification, exhibiting maximum scavenging rates of 82% and 30%. Nevertheless, anthocyanins from black bean peel showed weaker antioxidant activity than VC, an antioxidant commonly used in the food industry.

Figure 5.
Antioxidant capacity of anthocyanins from black bean peel. (A) DPPH scavenging ability of anthocyanins from black bean peel; (B) ABTS scavenging ability of anthocyanins from black bean peel.
3.4. Stability of Anthocyanins from Black Bean Peel
3.4.1. Environmental Effects on the Stability of Anthocyanins from Black Bean Peel
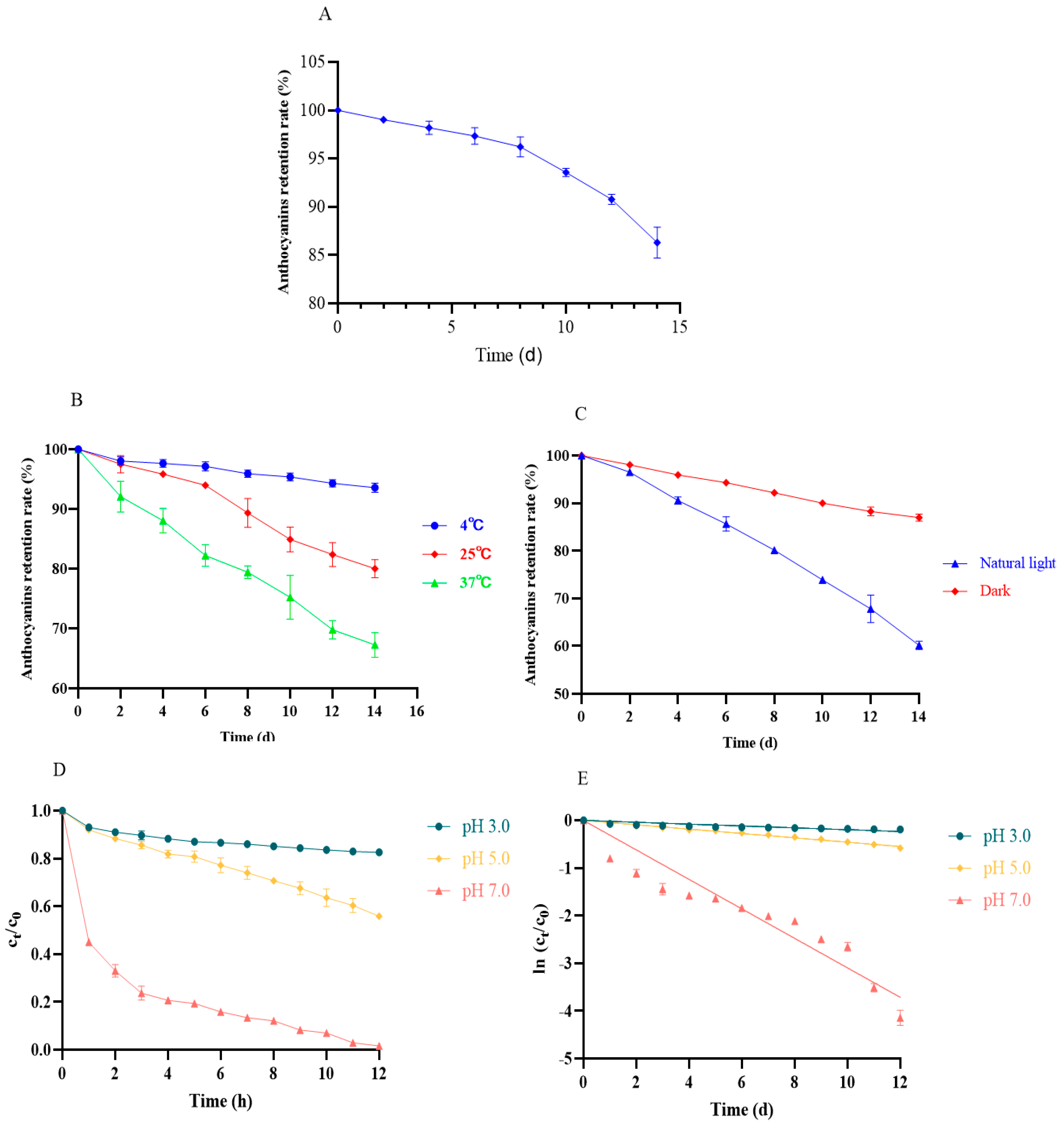
As shown in Figure 6A, prolonged exposure (14 d) caused more than 15% loss of anthocyanins in the same environment (Figure 6A). As a result, we investigated the effects of temperature and light on the stability of anthocyanins. As seen in Figure 5B, low temperatures (4 °C) showed a protective effect for anthocyanins against degradation (Figure 6B). With the increase in the storage time and temperature, the stability of the anthocyanins suffered serious challenges, rapidly decreasing within 14 days (Figure 6C). Similarly, dark conditions could maintain the relative stability of the anthocyanins, but natural light rapidly destroyed anthocyanins (Figure 6C). These results are consistent with many previous reports [4]. High temperatures and light might accelerate the hydrolysis or deglycosylation ring-opening reaction of anthocyanins, which can in turn be converted into colorless pseudobasic bases and chalcones [21].Therefore, we recommend preserving the anthocyanins of black bean peel at low temperatures in the dark.
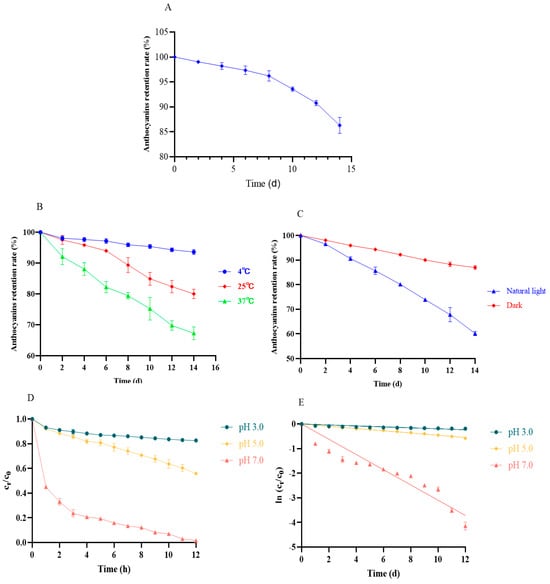
Figure 6.
Effect of environment (temperature/light/time/pH) on the stability of anthocyanins in black bean peel. (A) Effect of time on the stability of anthocyanins from black bean peel; (B) effect of temperature on the stability of anthocyanins in black bean peel; (C) effect of light on the stability of anthocyanins from black bean peel; (D) changes in the concentration ratio (Ct/C0) of the black bean anthocyanin solution under different pH conditions at 70 °C; (E) thermal degradation kinetics plot.
pH is one of the most important factors that leads to anthocyanins’ degradation. A low pH can maintain their stability, but an elevated pH gradually induces the degradation of anthocyanins, which gradually transform from a stable yellow-salt cation to a less stable chalcone and, ultimately, to an extremely unstable quinone-type base [22]. The thermal stability of the anthocyanin solution was markedly influenced by pH. When subjected to a 70 °C water bath, the anthocyanin solution at pH 3.0 exhibited the highest thermal stability, retaining 82.33% of its initial concentration after 12 h. In contrast, the solution at pH 5.0 displayed reduced stability compared to that at pH 3.0, while the pH 7.0 solution degraded the most rapidly, with only 1.60% of anthocyanins remaining after 12 h (Figure 6D,E and Table 4). A kinetic analysis demonstrated that the isothermal degradation of anthocyanins from black bean seed coats followed first-order reaction kinetics, as supported by the linear relationships and high determination coefficients (R2) of the fitted models. Notably, the degradation half-life (t1/2) decreased significantly with an increasing pH, indicating a decline in thermal stability and accelerated degradation rates under neutral to alkaline conditions. These findings suggest that an elevated pH promotes molecular instability, leading to the faster structural breakdown of anthocyanins.

Table 4.
Kinetic model fitting equation and related parameters.
3.4.2. Influence of Additives on the Stability of Anthocyanins from Black Soybean Peel
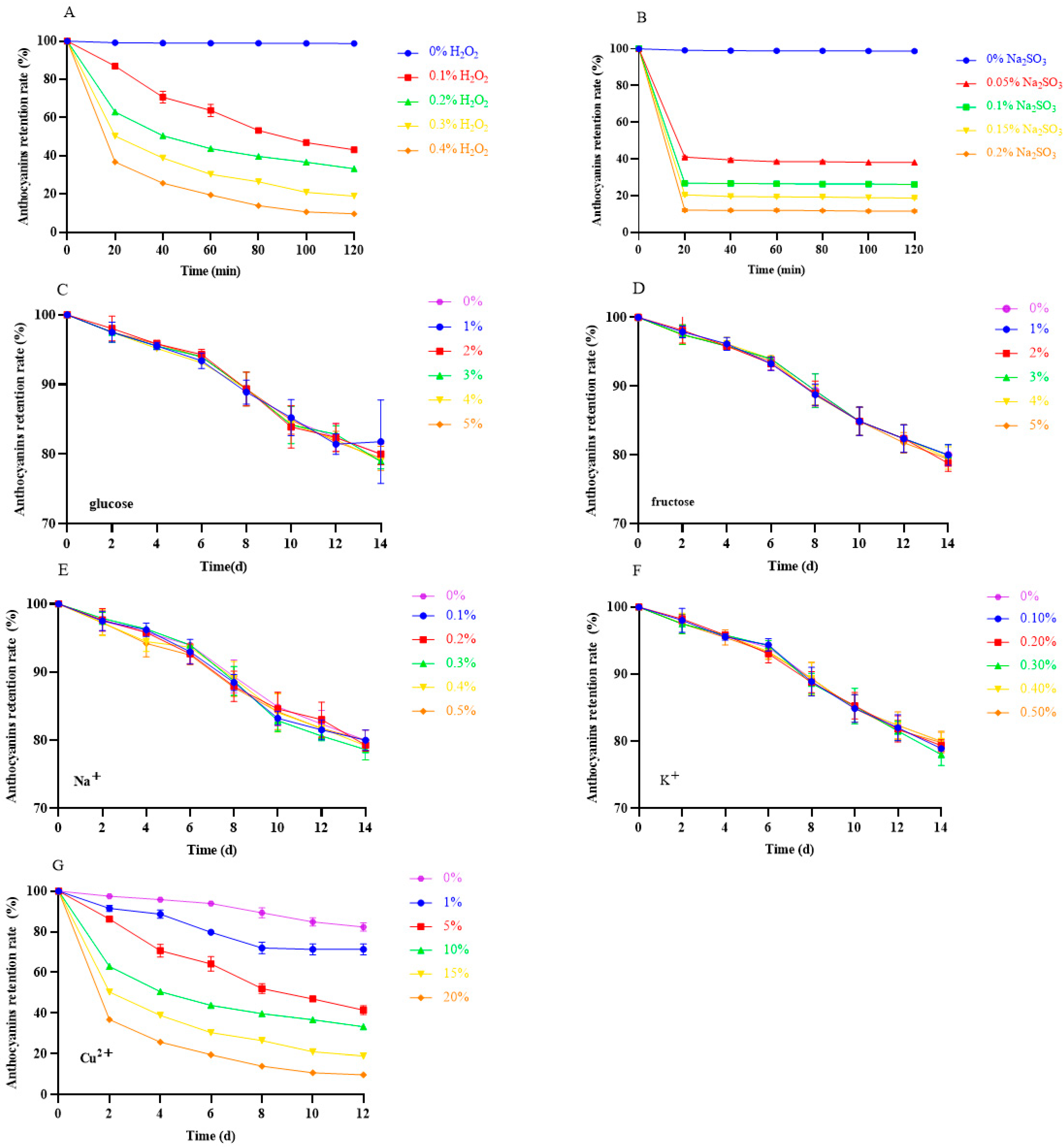
The effect of different additives on the stability of anthocyanins in black bean peel is shown in Figure 6. H2O2 and Na2SO3 are two commonly used oxidants in the food industry. When H2O2 and Na2SO3 were added, the content of anthocyanins markedly decreased with the increase in the concentration and incubation time (Figure 7A,B), and the anthocyanin retention rate was lower than 20% in the group with 0.4% H2O2 for 120 min and with 0.2% NA2SO3 for 60 min, implying that oxidants had greater negative effects on the stability of anthocyanins from black bean peel [23].
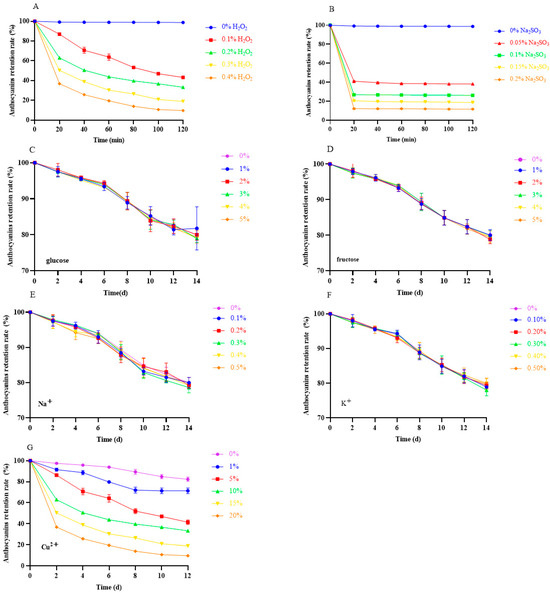
Figure 7.
Effect of additives on the stability of anthocyanins in black bean peel. (A) Effect of H2O2 on the stability of anthocyanins from black bean peel; (B) effect of Na2SO3 on the stability of anthocyanins from black bean peel; (C) effect of glucose on the stability of anthocyanins from black bean peel; (D) effect of sucrose on the stability of anthocyanins from black bean peel; (E) effect of NaCl on the stability of anthocyanins from black bean peel; (F) effect of KCl on the stability of anthocyanins from black bean peel; (G) effect of CuCl2•2H2O on the stability of anthocyanins from black bean peel.
It was found that glycosylation enabled the attachment of sugar groups to anthocyanins through O-bonding and intermolecular forces, while improving the stability and water solubility of anthocyanins by exposing hydrophobic surfaces, reducing the number of electronic domains and enhancing the chain resistance [24]. However, in this experiment, we found that, after the addition of sugar (glucose, sucrose), the content of anthocyanins gradually decreased over time, but there was no significant difference between the different concentrations (Figure 7C,D).
The addition of different metal ions (Na+, K+, Cu2+) showed different influences on the stability of anthocyanins in black bean peel. As shown in Figure 7E–G, the anthocyanin content in the groups with the addition of Na+ and K+ gradually decreased during the storage time, but there was no significant difference between the groups with different concentrations (Figure 7E,F). In the Cu2+ group, the anthocyanin retention rate decreased gradually over time, but low concentrations of Cu2+ revealed the protective capacity of anthocyanins, which showed a slower degradation rate than that of the Na+ and K+ groups (Figure 7G). Conversely, the stability of the anthocyanins rapidly decreased with the increased Cu2+ concentration, indicating that anthocyanins are relatively stable in low-concentration divalent ion solutions.
4. Conclusions
In this study, we optimized the process of extracting anthocyanins from black bean peel using ultrasound-assisted DESs, and the optimization of the extraction process showed that the maximum amount of anthocyanins reached 61.00 ± 2.73 mg C3GE/100 g DW under the optimal conditions of an ultrasound temperature of 51 °C; an ultrasound time of 40 min; and a solid–liquid ratio of 1:20. This relatively high yield, achieved under optimized yet straightforward conditions, demonstrates the method’s promising scalability potential for industrial-scale production. The optimization of the purification process showed that the AB-8 resin had the best purification effects with the conditions of 300 min for the up-sampling time, 1.5 mL/min for the up-sampling flow rate, 5 BV for the up-sampling volume, an 80% ethanol volume fraction of the eluent, and 2.0 mL/min for the elution flow rate. After purification, anthocyanins showed better activity in terms of eliminating DPPH and ABTS free radicals. Moreover, anthocyanins from black bean peel were sensitive to various environmental factors, including temperature, light, and pH. Meanwhile, the stability of the anthocyanins was reduced when they came into contact with the food additives of H2O2, Na2SO3, glucose, sucrose, as well as metal ions. These results provided more insights about the anthocyanins from black bean peel, which may assist in their further application in functional foods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12040073/s1, Table S1: Factors and levels of Box-Behnken design; Table S2: Properties and adsorption/desorption rates of different macroporous resins.
Author Contributions
Conceptualization, S.X. and R.F.; methodology, H.W.; software, S.X. and P.G.; validation, S.X., Z.G. and P.G.; formal analysis, S.X.; investigation, L.W.; resources, H.W.; data curation, S.X.; writing—original draft preparation, S.X.; writing—review and editing, S.X.; visualization, L.H.; supervision, M.W.; project administration, L.H.; funding acquisition, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China, grant number 32001701. This research was funded by the China Agriculture Research System, grant number CARS-08-E2-01. The APC was funded by grant number 32001701.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Escalante-Aburto, A.; Mendoza-Córdova, M.Y.; Mahady, G.B.; Luna-Vital, D.A.; Gutiérrez-Uribe, J.A.; Chuck-Hernández, C. Consumption of dietary anthocyanins and their association with a reduction in obesity biomarkers and the prevention of obesity. Trends Food Sci. Technol. 2023, 140, 104140. [Google Scholar] [CrossRef]
- Han, L.; Li, R.; Jin, X.; Li, Y.; Chen, Q.; He, C.; Wang, M. Metabolomic analysis, extraction, purification and stability of the anthocyanins from colored potatoes. Food Chem. X 2024, 22, 101423. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; He, C.; Guo, Z.; Li, Y.; Li, Y.; Gao, J.; Wang, M.; Han, L. The hypoglycemic activity of buckwheat and the underlying mechanisms: A mechanistic review. Food Biosci. 2024, 62, 105046. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Liu, J.; Si, D.; Zhang, X. Guideline for extraction, qualitative, quantitative, and stability analysis of anthocyanins. eFood 2023, 4, e59. [Google Scholar] [CrossRef]
- Tang, R.; He, Y.; Fan, K. Recent advances in stability improvement of anthocyanins by efficient methods and its application in food intelligent packaging: A review. Food Biosci. 2023, 56, 103164. [Google Scholar] [CrossRef]
- Foroutani, Z.; Afshar Mogaddam, M.R.; Ghasempour, Z.; Ghareaghajlou, N. Application of deep eutectic solvents in the extraction of anthocyanins: Stability, bioavailability, and antioxidant property. Trends Food Sci. Technol. 2024, 144, 104324. [Google Scholar] [CrossRef]
- Rabiei, M.R.; Hosseini, M.; Xu, G. Deep eutectic solvents: A review on their sensing applications. Microchem. J. 2024, 203, 110909. [Google Scholar] [CrossRef]
- Shoshtari-Yeganeh, B.; Giesy, J.P.; Yeganeh, M.S.; Badibostan, H. Deep eutectic solvents (DESs) in microextraction of Parabens: A review. Microchem. J. 2024, 201, 110699. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Machado-Velarde, L.X.; Dávila-Vega, J.P.; Gutiérrez-Uribe, J.; Espinosa-Ramírez, J.; Martínez-Ávila, M.; Guajardo-Flores, D.; Chuck-Hernández, C. Black bean hulls as a byproduct of an extraction process to enhance nutraceutical and glycemic-related properties of nixtamalized maize tostadas. Foods 2023, 12, 1915. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D.J. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Mali, P.S.; Kumar, P. Optimization of microwave assisted extraction of bioactive compounds from black bean waste and evaluation of its antioxidant and antidiabetic potential in vitro. Food Chem. Adv. 2023, 3, 100543. [Google Scholar] [CrossRef]
- Meenu, M.; Chen, P.; Mradula, M.; Chang, S.K.C.; Xu, B. New insights into chemical compositions and health-promoting effects of black beans (Phaseolus vulgaris L.). Food Front. 2023, 4, 1019–1038. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Liu, Z.-T.; Chen, X.-Q.; Zhang, T.-T.; Zhang, Y. Deep eutectic solvent combined with ultrasound technology: A promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food Chem. 2023, 422, 136224. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT-Food Sci. Technol. 2021, 144, 111220. [Google Scholar] [CrossRef]
- Teng, Z.; Jiang, X.; He, F.; Bai, W. Qualitative and quantitative methods to evaluate anthocyanins. eFood 2020, 1, 339–346. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Sivapragasam, N.; Ali Redha, A.; Maqsood, S. Sustainable green extraction of anthocyanins and carotenoids using deep eutectic solvents (DES): A review of recent developments. Food Chem. 2024, 448, 139061. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Anthocyanin content and antioxidant capacity of black bean (Phaseolus vulgaris L.) peel extracts. J. Agric. Food Chem. 2009, 57, 8388–8396. [Google Scholar]
- Li, K.; Xiao, Y.; Bian, J.; Han, L.; He, C.; El-Omar, E.; Gong, L.; Wang, M. Ameliorative effects of gut microbial metabolite urolithin A on pancreatic diseases. Nutrients 2022, 14, 2549. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Du, J.; Liu, Y.; Zhang, Y.; Liu, J.; Wang, M.; Han, L. Research progress of anthocyanins regulating intestinal microorganisms. Food Nutr. Chem. 2024, 2, 185. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in embedding techniques of anthocyanins: Improving stability, bioactivity and bioavailability. Food Chem. X 2023, 20, 100983. [Google Scholar] [CrossRef] [PubMed]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, J.; Cheng, T.; Liu, Y.; Han, F. Methylation, hydroxylation, glycosylation and acylation affect the transport of wine anthocyanins in Caco-2 cells. Foods 2022, 11, 3793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).