Vanadium, Titanium, and Iron Extraction from Titanomagnetite Ore by Salt Roasting and 21st-Century Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Sample and Preparation
2.3. Characterisation of Sample
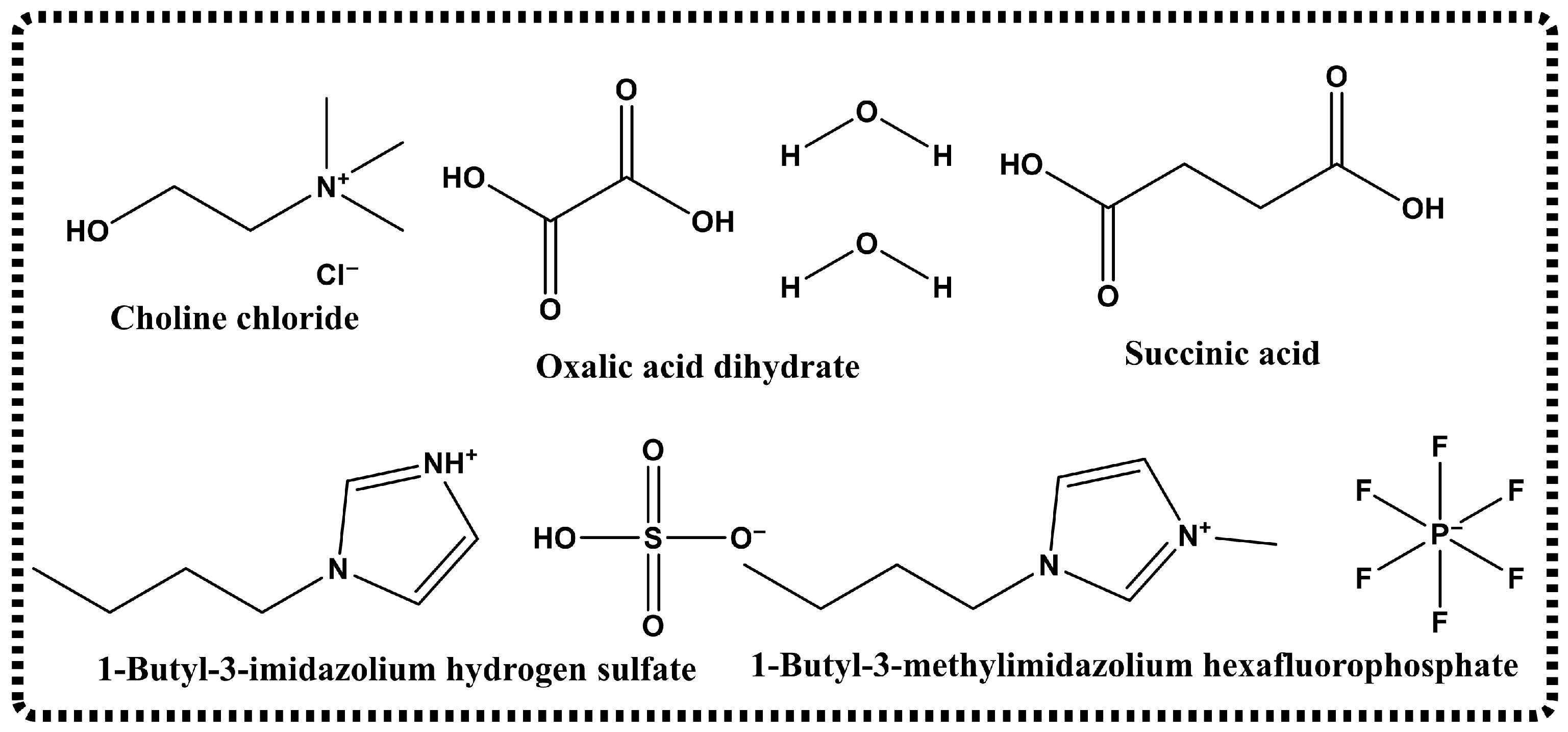
2.4. Synthesis of the DESs
2.5. Extraction Procedures
2.5.1. Roasting with Salts
2.5.2. Leaching with ILs and DESs
3. Results and Discussion
3.1. Roasting with Sodium Salts
3.2. Leaching with ILs
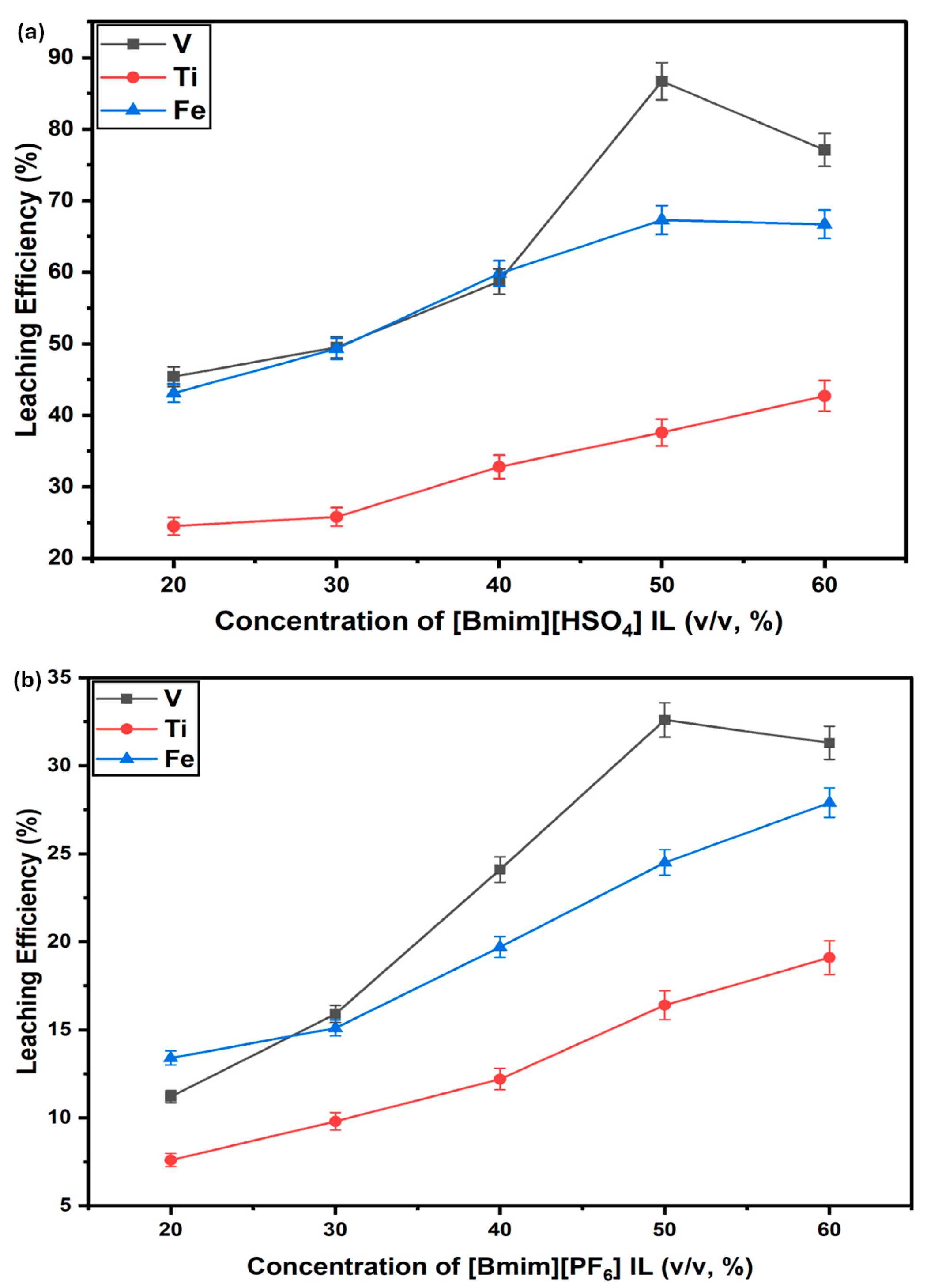
3.2.1. Effect of Varying IL Concentration
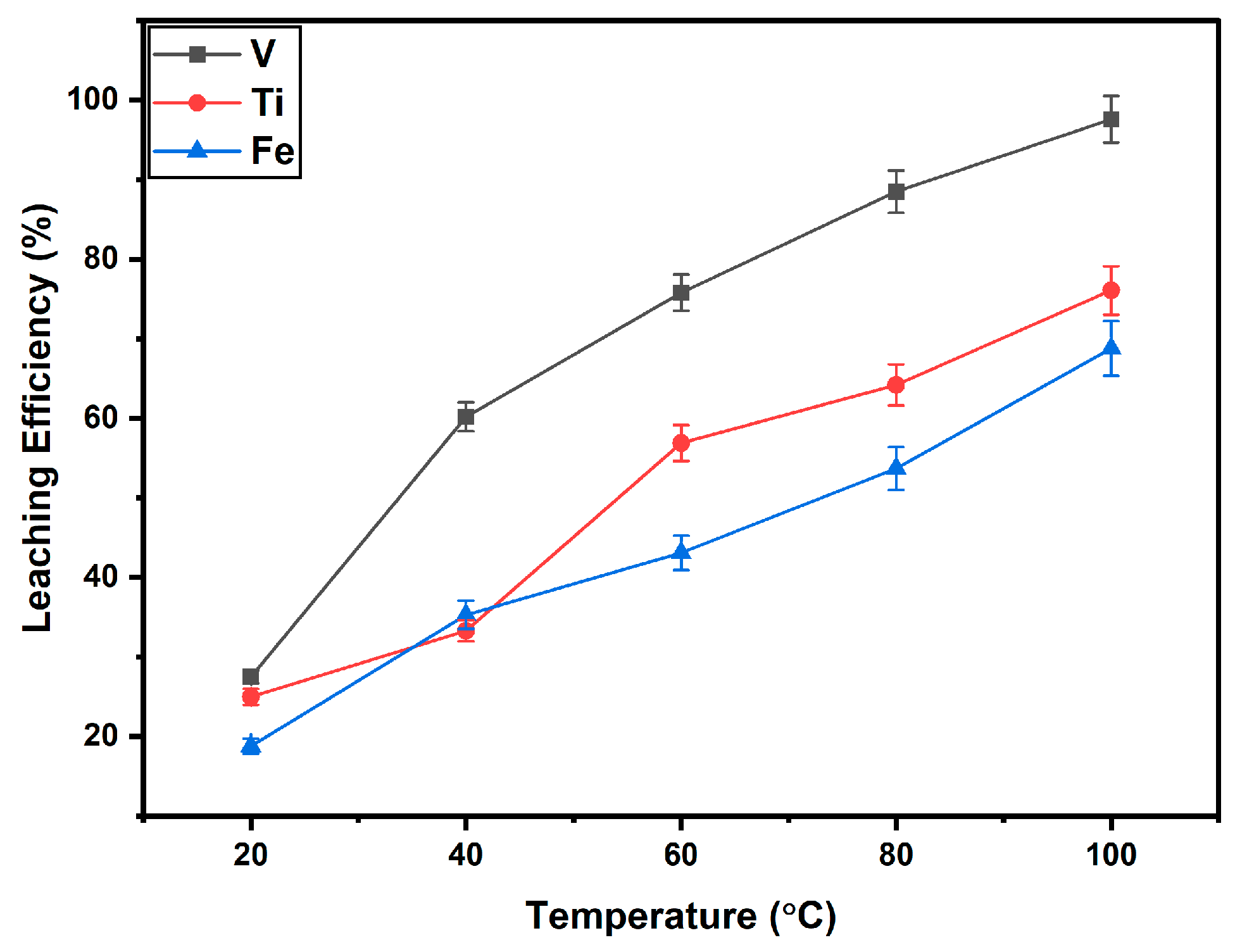
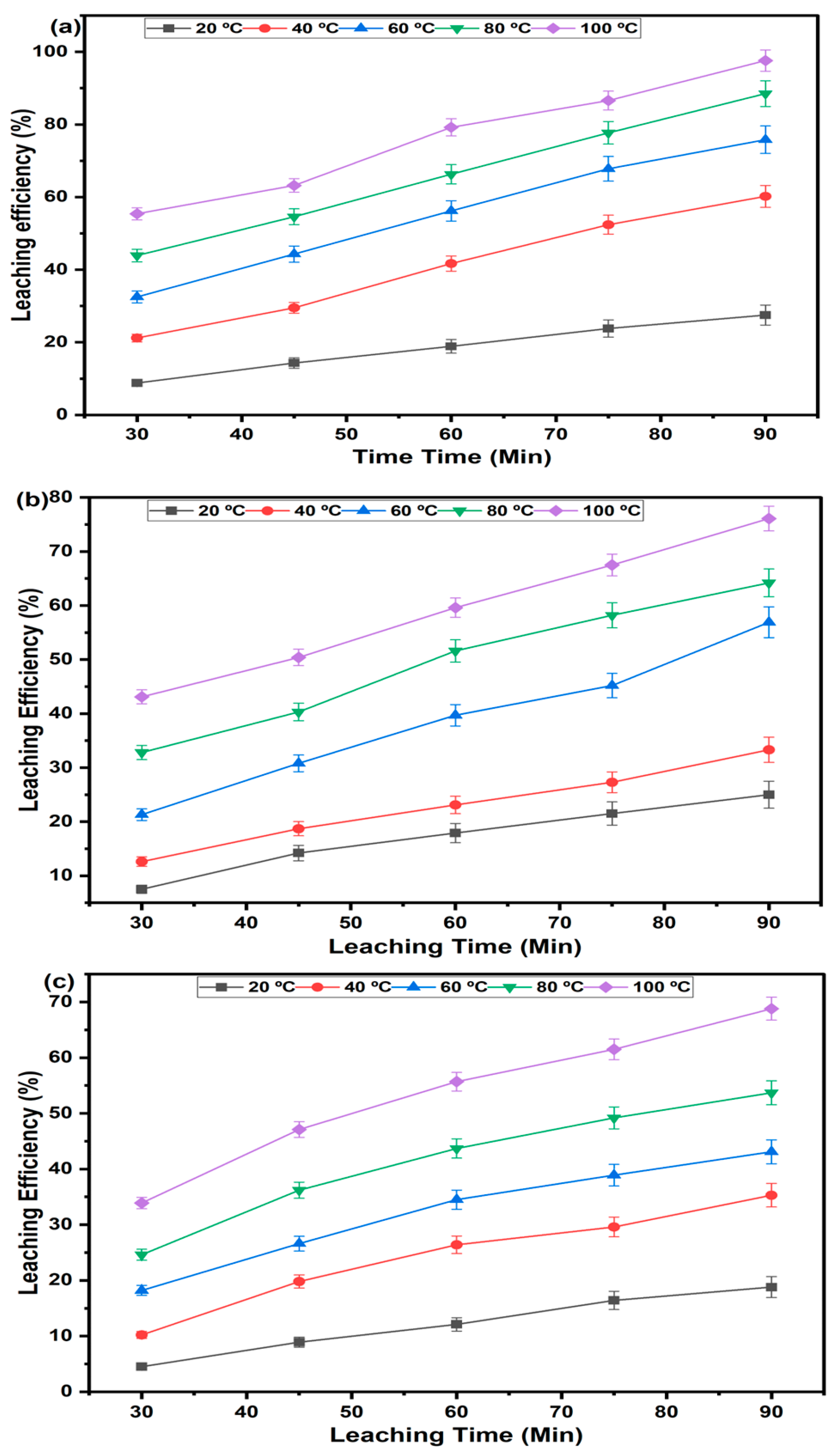
3.2.2. Effect of Varying Temperature
3.3. Leaching with DESs
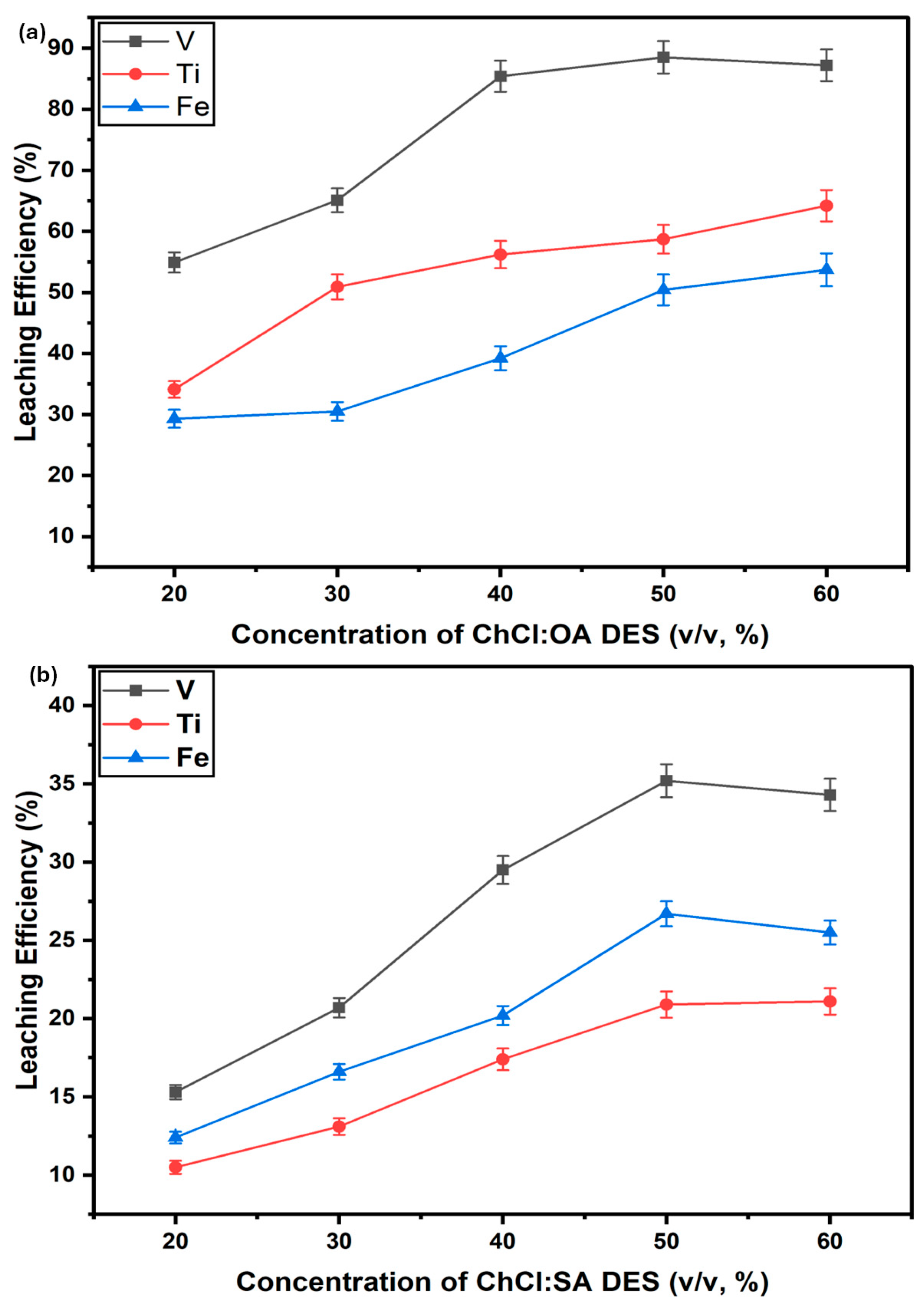
3.3.1. Effect of Varying DES Concentration
3.3.2. Effect of Varying Temperature
3.4. Comparative Performance of the Investigated Methods
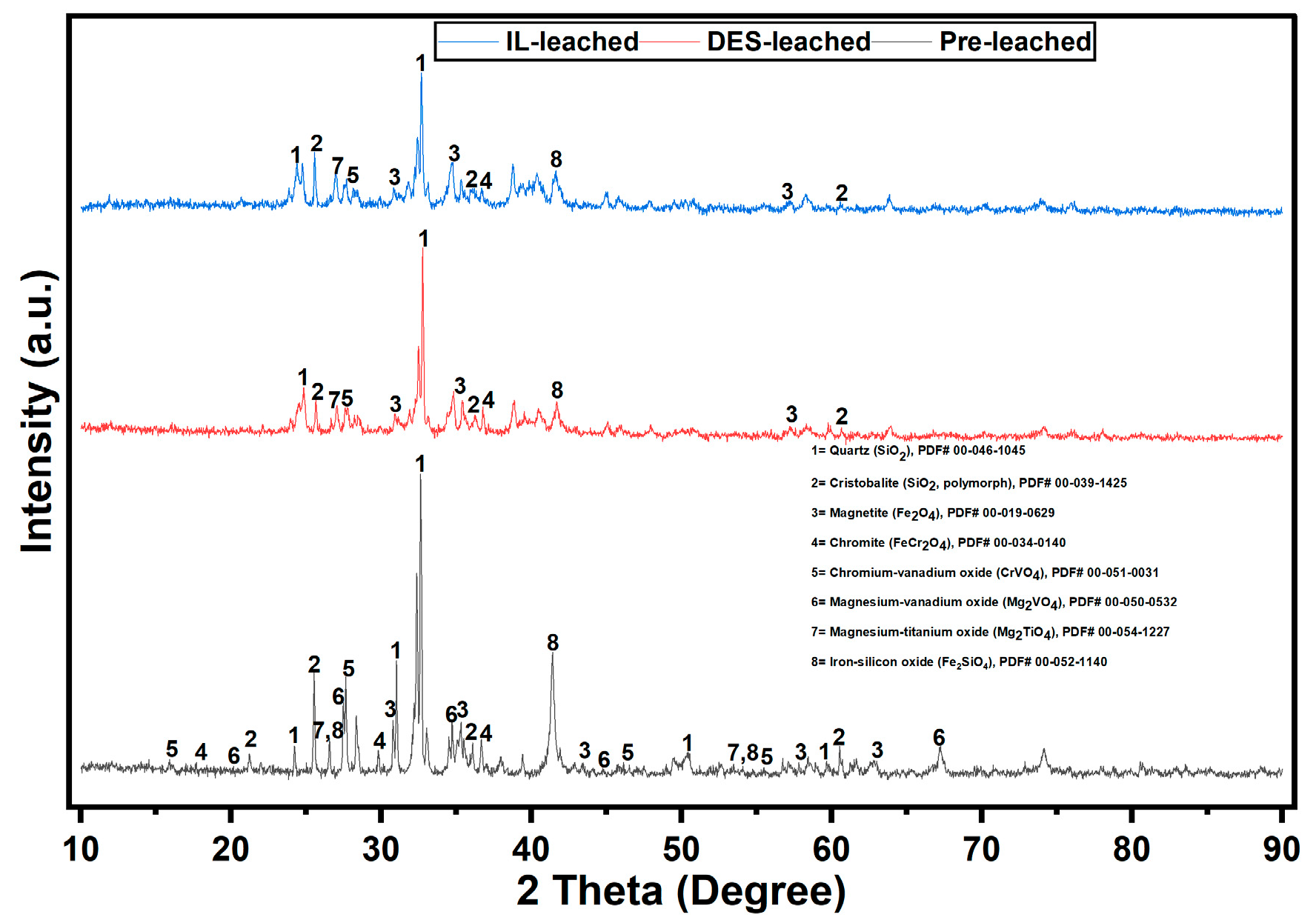
3.5. Analysis of the Pre- and Post-Leached Titanomagnetite Ore
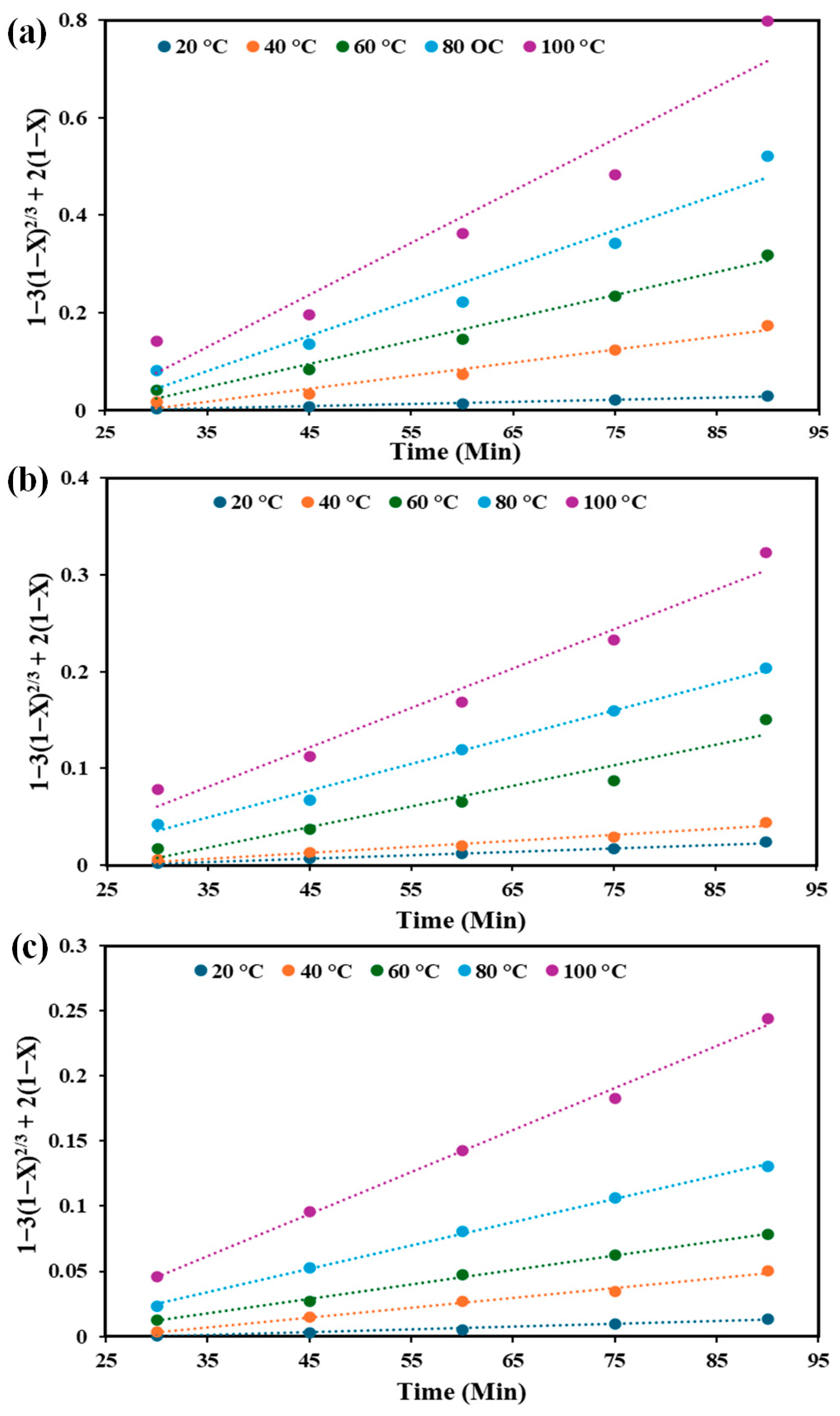
3.6. Kinetic Model of Leaching Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ILs | Ionic liquids |
| DESs | Deep eutectic solvents |
| TOMAC | Tri-n-octylmethylammonium chloride |
| [BmimCF3SO3] | 1-butyl-3-methylimidazolium trifluoromethane sulfonate |
| [BmimHSO4] | 1-butyl-3-methylimidazolium hydrogen sulphate |
| [Bmim][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| ChCl | Choline chloride |
| HBA | Hydrogen bond acceptor |
| HBDs | Hydrogen bond donors |
| SA | Succinic acid |
| OA | Oxalic acid |
| XRF | X-ray fluorescence |
| XRD | X-ray diffraction |
| LE | Leaching efficiency |
| SCM | Shrinking core model |
| Ea | Apparent activation energy |
References
- Hu, P.; Zhang, Y.; Huang, J.; Liu, T.; Yuan, Y.; Xue, N. Eco-Friendly Leaching and Separation of Vanadium over Iron Impurity from Vanadium-Bearing Shale Using Oxalic Acid as a Leachant. ACS Sustain. Chem. Eng. 2018, 6, 1900–1908. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Xue, N. Source Separation of Vanadium over Iron from Roasted Vanadium-Bearing Shale during Acid Leaching via Ferric Fluoride Surface Coating. J. Clean. Prod. 2018, 181, 399–407. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Lv, G.; Zhang, G.; Liu, Y.; Zhang, W. Synergistic Extraction of Vanadium(IV) in Sulfuric Acid Media Using a Mixture of D2ehpa and Ehehpa. Hydrometallurgy 2016, 166, 87–93. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Lyu, X.; Sharafeldin, H.E.; ElDeeb, A.B. Eco-Friendly and Complex Processing of Vanadium-Bearing Waste for Effective Extraction of Valuable Metals and Other By-Products: A Critical Review. Recycling 2025, 10, 6. [Google Scholar] [CrossRef]
- Kelley, K.D.; Scott, C.; Polyak, D.E.; Kimball, B.E. Vanadium; Schulz, K.J., DeYoung, J.H., Seal, R.R., Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2017. [Google Scholar]
- Yang, J.; Wang, Y.; Gao, X.; Zuo, R.; Song, L.; Jin, C.; Wang, J.; Teng, Y. Vanadium: A Review of Different Extraction Methods to Evaluate Bioavailability and Speciation. Minerals 2022, 12, 642. [Google Scholar] [CrossRef]
- Luo, D.; Huang, J.; Zhang, Y.; Liu, H.; Hu, P. Highly Efficient Separation and Extraction of Vanadium from a Multi-Impurity Leachate of Vanadium Shale Using Tri-n-Octylmethylammonium Chloride. Sep. Purif. Technol. 2020, 230, 115842. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wang, Y.-F.; Li, Y.-T.; Wu, C.; Han, X.-L.; Zhao, N.-N.; Zhang, Z.-K.; Dai, L.; Wang, L.; He, Z.-X. A Review on Vanadium Extraction Techniques from Major Vanadium-Containing Resources. Rare Met. 2024, 43, 4115–4131. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of Vanadium: A Review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Bao, S.-X.; Liu, T.; Chen, T.-J.; Huang, J. The Technology of Extracting Vanadium from Stone Coal in China: History, Current Status and Future Prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Li, C.; Jiang, T.; Wen, J.; Yu, T.; Li, F. Review of Leaching, Separation and Recovery of Vanadium from Roasted Products of Vanadium Slag. Hydrometallurgy 2024, 226, 106313. [Google Scholar] [CrossRef]
- Han, C.; Li, L.; Yang, H.; Xue, X.-X. Preparation of V2O5 from Converter Slag Containing Vanadium. Rare Met. 2018, 37, 904–912. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhao, T.S.; An, L.; Zhou, X.L.; Wei, L. A Comparative Study of All-Vanadium and Iron-Chromium Redox Flow Batteries for Large-Scale Energy Storage. J. Power Sources 2015, 300, 438–443. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, Z.; Chen, L.; Liang, B.; Zhu, Y.; Wu, K.; Luo, D. Recovery of Low Valence Vanadium from Vanadium Slag for the Preparation of VOSO4 Electrolyte. Process Saf. Environ. Prot. 2023, 174, 298–309. [Google Scholar] [CrossRef]
- Nagata, M.; Matsuda, M.; Himeno, Y.; Shida, K.; Matsuda, M. Magnéli Phase Vanadium Oxide Thin Films Produced by Stepwise Oxidation of Vanadium Metal Foil. Scr. Mater. 2022, 219, 114884. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Ho, H.-C.; Lai, Y.-C.; Nagao, T.; Hsueh, C.-H. Thermochromic Vanadium Dioxide Film on Textured Silica Substrate for Smart Window with Enhanced Visible Transmittance and Tunable Infrared Radiation. Infrared Phys. Technol. 2019, 102, 103019. [Google Scholar] [CrossRef]
- An, Y.; Ma, B.; Li, X.; Chen, Y.; Wang, C.; Wang, B.; Gao, M.; Feng, G. A Review on the Roasting-Assisted Leaching and Recovery of V from Vanadium Slag. Process Saf. Environ. Prot. 2023, 173, 263–276. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, Y.; Zhang, M.; Wei, W.; Xie, B. A Novel Anion Exchange Method Based on in Situ Selectively Reductive Desorption of Cr(VI) for Its Separation from V(V): Toward the Comprehensive Use of Hazardous Wastewater. J. Hazard. Mater. 2019, 368, 670–679. [Google Scholar] [CrossRef]
- Ju, J.; Fu, H.-G.; Fu, D.-M.; Wei, S.-Z.; Sang, P.; Wu, Z.-W.; Tang, K.-Z.; Lei, Y.-P. Effects of Cr and V Additions on the Microstructure and Properties of High-Vanadium Wear-Resistant Alloy Steel. Ironmak. Steelmak. 2018, 45, 176–186. [Google Scholar] [CrossRef]
- Dong, F.; Venezuela, J.; Li, H.; Shi, Z.; Zhou, Q.; Chen, L.; Chen, J.; Du, L.; Atrens, A. Effect of Vanadium and Rare Earth Microalloying on the Hydrogen Embrittlement Susceptibility of a Fe-18Mn-0.6C TWIP Steel Studied Using the Linearly Increasing Stress Test. Corros. Sci. 2021, 185, 109440. [Google Scholar] [CrossRef]
- Gao, F.; Du, H.; Wang, S.; Chen, B.; Li, J.; Zhang, Y.; Li, M.; Liu, B.; Olayiwola, A.U. A Comparative Study of Extracting Vanadium from Vanadium Titano-Magnetite Ores: Calcium Salt Roasting Vs Sodium Salt Roasting. Miner. Process. Extr. Metall. Rev. 2023, 44, 352–364. [Google Scholar] [CrossRef]
- Trinh, H.B.; Kim, S.; Lee, J.; Oh, S. Efficient Recovery of Vanadium and Titanium from Domestic Titanomagnetite Concentrate Using Molten Salt Roasting and Water Leaching. Materials 2023, 16, 6918. [Google Scholar] [CrossRef]
- Bai, L.; Xiang, J.; Lu, X.; Xu, Y.; Zhu, K.; Yu, J.; Huang, Q.; Pei, G.; Lv, X. Extraction of Vanadium from Vanadium–Titanium Magnetite: Enhanced by Sodium–Calcium Synergistic Roasting–Water Leaching Process. Metall. Mater. Trans. B 2025, 56, 552–563. [Google Scholar] [CrossRef]
- Petranikova, M.; Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Tunsu, C. Vanadium Sustainability in the Context of Innovative Recycling and Sourcing Development. Waste Manag. 2020, 113, 521–544. [Google Scholar] [CrossRef]
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of Vanadium Production Part I: Primary Resources. Miner. Process. Extr. Metall. Rev. 2022, 43, 466–488. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, T.; Wen, J.; Yang, G.; Yu, T.; Zhu, F.; Hu, P. High-Chromium Vanadium–Titanium Magnetite All-Pellet Integrated Burden Optimization and Softening–Melting Behavior Based on Flux Pellets. Int. J. Miner. Metall. Mater. 2024, 31, 498–507. [Google Scholar] [CrossRef]
- Sun, H.; Adetoro, A.A.; Wang, Z.; Zhu, Q. Behavior and Mechanism of Pre-Oxidation Improvement on Fluidization in the Fluidized Reduction of Titanomagnetite. Int. J. Miner. Metall. Mater. 2024, 31, 2458–2465. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yuan, Y.; Hu, P. An Insight on the Mechanism of Efficient Leaching of Vanadium from Vanadium Shale Induced by Microwave-Generated Hot Spots. Int. J. Miner. Metall. Mater. 2023, 30, 293–302. [Google Scholar] [CrossRef]
- Dong, Y.; Chong, S.; Lin, H. Bioleaching and Biosorption Behavior of Vanadium-Bearing Stone Coal by Bacillus Mucilaginosus. Int. J. Miner. Metall. Mater. 2023, 30, 283–292. [Google Scholar] [CrossRef]
- Dong, Y.; Zan, J.; Lin, H. Bioleaching of Vanadium from Stone Coal Vanadium Ore by Bacillus Mucilaginosus: Influencing Factors and Mechanism. Int. J. Miner. Metall. Mater. 2024, 31, 1828–1838. [Google Scholar] [CrossRef]
- Tian, G.; Liu, H. Review on the Mineral Processing in Ionic Liquids and Deep Eutectic Solvents. Miner. Process. Extr. Metall. Rev. 2024, 45, 130–153. [Google Scholar] [CrossRef]
- Al-Bodour, A.; Alomari, N.; Perez-Duran, G.; Rozas, S.; Iglesias-Silva, G.; Aparicio, S.; Gutierrez-Vega, A.; Atilhan, M. Ionic Liquids as Multidimensional Materials: A Review from Fundamentals to Applications. Energy Fuels 2025, 39, 12791–12829. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Hallett, J.; Shiflett, M. Introduction: Ionic Liquids for Diverse Applications. Chem. Rev. 2024, 124, 7533–7535. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, T.; Zhang, Y.; Hu, P.; Liu, H.; He, Y. Mechanism of Selectivity of Tri-n-Octylmethylammonium Chloride for Vanadium. Miner. Eng. 2024, 218, 109052. [Google Scholar] [CrossRef]
- Potgieter, H.; Teimouri, S. Investigation into the Possibility of Using a Novel Ionic Liquid Leaching Method to Obtain Vanadium from Vanadium Slag. Environ. Res. Technol. 2023, 6, 1–7. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, T.; Zhang, Y.; Hu, P. Optimizing Vanadium Extraction from Shale Leachate Using Salicylhydroxamic Acid-Tri-n-Octylmethylammonium. J. Clean. Prod. 2025, 496, 145144. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, H. Recent Chemical Methods for Metals Recovery from Printed Circuit Boards: A Review. J. Mater. Cycles Waste Manag. 2024, 26, 1349–1368. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, J.H. Sustainable Leaching of Metals from Waste Printed Circuit Boards Using Efficient Carboxylic Acid-Based Deep Eutectic Solvents. Sep. Purif. Technol. 2025, 374, 133712. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Surauzhanov, K.; Ultarakova, A. Extraction of Vanadium from the Dust of Ore-Thermal Melting of Ilmenite Concentrate Using Deep Eutectic Solvents. Chem. Eng. Trans. 2021, 88, 1015–1020. [Google Scholar] [CrossRef]
- Chen, S.-C.; Chou, F.-C.; Shirke, S.; Lin, S.-Y.; Lin, Y.-C. Efficiency Comparison of Succinic Acid and Oxalic Acid with Choline Chloride Deep-Eutectic Solvent through Response Surface Methodology. J. Taiwan Inst. Chem. Eng. 2025, 174, 106230. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Y.; Yan, X.; Hu, T.; Jing, S.; Wang, Z.; Ge, X. Closed-Loop Cobalt Recycling from Spent Lithium-Ion Batteries Based on a Deep Eutectic Solvent (DES) with Easy Solvent Recovery. J. Energy Chem. 2022, 72, 532–538. [Google Scholar] [CrossRef]
- Oke, E.A.; Fedai, Y.; Potgieter, J.H. Hydrometallurgical Leaching of Copper and Cobalt from a Copper–Cobalt Ore by Aqueous Choline Chloride-Based Deep Eutectic Solvent Solutions. Minerals 2025, 15, 815. [Google Scholar] [CrossRef]
- Tan, J.; Huang, R.; Li, K.; Yan, X.; Guo, L.; Guo, Z.; Zhang, W.; Chai, L. Achieving High Solid–Liquid Ratio through Competitive Coordination towards Efficient Recovery of Metals from Spent Batteries. Angew. Chem. 2025, 137, e202422313. [Google Scholar] [CrossRef]
- Septioga, K.; Fajar, A.T.N.; Wakabayashi, R.; Goto, M. Deep Eutectic Solvent-Aqueous Two-Phase Leaching System for Direct Separation of Lithium and Critical Metals. ACS Sustain. Resour. Manag. 2024, 1, 2482–2491. [Google Scholar] [CrossRef]
- Nkosi, S.B.; Goso, X.C.; Mokone, T.; Petersen, J. Preliminary investigations into the extraction of vanadium from titaniferous slags using a modified vanadium primary production process. In Proceedings of the Hydrometallurgy for the Future, Stellenbosch, South Africa, 3–9 September 2024. [Google Scholar]
- Xu, S.; Long, M.; Li, Y.; Chen, D.; Fan, H.; Zhao, Y.; Ma, Y. Study on Roasting Parameters for Extraction of Vanadium from a High Ca/V Ratio Vanadium Slag. Metal. Int. 2013, 18, 38. [Google Scholar]
- Gilligan, R.; Nikoloski, A.N. The Extraction of Vanadium from Titanomagnetites and Other Sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q. Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes 2021, 9, 1923. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, J.; Zhu, Y.; Han, Y.; Bai, Z.; Tang, Z. Effect of Roasting Temperature on Vanadium Extraction, Kinetics, Phase Transformation, and Microstructure Evolution of Vanadium-Bearing Shale during Suspension Oxidation Roasting Process. Adv. Powder Technol. 2023, 34, 104233. [Google Scholar] [CrossRef]
- Kuzmina, O.; Symianakis, E.; Godfrey, D.; Albrecht, T.; Welton, T. Ionic Liquids for Metal Extraction from Chalcopyrite: Solid, Liquid and Gas Phase Studies. Phys. Chem. Chem. Phys. 2017, 19, 21556–21564. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Hua, Y.; Zhang, Q.; Zhou, D. Leaching of Chalcopyrite with Brønsted Acidic Ionic Liquid. Hydrometallurgy 2009, 99, 33–38. [Google Scholar] [CrossRef]
- Rodríguez, M.; Ayala, L.; Robles, P.; Sepúlveda, R.; Torres, D.; Carrillo-Pedroza, F.R.; Jeldres, R.I.; Toro, N. Leaching Chalcopyrite with an Imidazolium-Based Ionic Liquid and Bromide. Metals 2020, 10, 183. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, H.; Mondlane, F.; Skosana, N.P.; Teimouri, S.; Nyembwe, J.K. Concurrent Leaching of Copper and Cobalt from a Copper–Cobalt Ore Using Sulfuric and Organic Acids. Miner. Eng. 2024, 216, 108853. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Z.; Li, Y.; Zhu, Y.; Xie, K. Selective Sulfuric Acid Cyclic Leaching of Vanadium from the Calcification Roasting Pellets of Vanadium Titanomagnetite. J. Mater. Res. Technol. 2023, 23, 778–790. [Google Scholar] [CrossRef]
- Pateli, I.M.; Thompson, D.; Alabdullah, S.S.M.; Abbott, A.P.; Jenkin, G.R.T.; Hartley, J.M. The Effect of PH and Hydrogen Bond Donor on the Dissolution of Metal Oxides in Deep Eutectic Solvents. Green Chem. 2020, 22, 5476–5486. [Google Scholar] [CrossRef]
- Olaoluwa, D.T.; Baba, A.A.; Oyewole, A.L. Beneficiation of a Nigerian Lepidolite Ore by Sulfuric Acid Leaching. Miner. Process. Extr. Metall. 2023, 132, 134–140. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, J.H. Effectiveness of Acidic Deep Eutectic Solvents in Recovery of Hazardous Base Metals from Waste Printed Circuit Boards. Environ. Sci. Pollut. Res. 2025, 32, 16361–16379. [Google Scholar] [CrossRef]
- Cheng, W.; Li, J.; Deng, J.; Li, Y.; Cheng, F. Leaching Vanadium from the Spent Denitration Catalyst in the Sulfuric Acid/Oxalic Acid Combined Solvent. ACS Omega 2024, 9, 9286–9294. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The PH Behavior of Seventeen Deep Eutectic Solvents. Bioresources 2018, 13, 5042–5051. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Chen, L.; Zhu, Y.; Wu, K.; Luo, D. Optimization and Kinetic Analysis of Co-Extraction of Vanadium(IV) and Chromium(III) from High Chromium Vanadium Slag with Titanium Dioxide Waste Acid. Miner. Eng. 2025, 233, 109604. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Hai, D.; Chen, X.; Diao, J.; Xie, B. Acidic Leaching Kinetics Study on Vanadium from Magnesiation-Roasted Vanadium Slag. Trans. Nonferrous Met. Soc. China 2024, 34, 669–680. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, H.; Peng, T.; Zhao, D.; Zhang, X. The Leaching Kinetics of Iron from Titanium Gypsum in a Citric Acid Medium and Obtain Materials by Leaching Liquid. Molecules 2023, 28, 952. [Google Scholar] [CrossRef]
- Gao, L.; Rao, B.; Dai, H.; Xie, H.; Wang, P.; Ma, F. Kinetics of Sulphuric Acid Leaching of Titanium from Refractory Anatase under Atmospheric Pressure. Physicochem. Probl. Miner. Process. 2019, 55, 467–478. [Google Scholar] [CrossRef]
- Peng, H.; Shang, Q.; Chen, R.; Leng, Y.; Guo, J.; Liu, Z.; Tao, C. Oxidative Leaching Kinetics of Vanadium from the Vanadium-Chromium-Reducing Residue with K2Cr2O7. ACS Omega 2020, 5, 8777–8783. [Google Scholar] [CrossRef]
- Salem, A.R. Efficient Leaching Process of Valuable Elements from Gibbsite Ore Materials in Talet Seleim, Southwestern, Sinai, Egypt Using Green and Ecofriendly Lixiviant Agent: Optimization, Kinetic and Thermodynamic Study. Anal. Chem. Lett. 2023, 13, 141–158. [Google Scholar] [CrossRef]



| Composition | Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | TiO2 | V2O5 | MnO | Fe2O3 | CuO |
| Mass % | 1.20 | 2.10 | 17.10 | 39.8 | 0.23 | 0.20 | 11.80 | 3.00 | 0.56 | 0.21 | 23.50 | 0.13 |
| Method | V LE (%) | Ti LE (%) | Fe LE (%) | Sustainability/Environmental Impact | Remarks |
|---|---|---|---|---|---|
| Roasting with Na2CO3 | 86.5 | 50.6 | 66.6 | High energy demand; CO2 emissions; sintering risk | Good V recovery; moderate Ti and Fe. |
| Roasting with Na2SO4 | 82.3 | 36.5 | 80.1 | High temperature; sulphate residues | Best Fe recovery; weak for V and Ti. |
| [Bmim][HSO4] | 95.6 | 53.2 | 74.8 | Low-temp operation; toxicity/recycling concerns | Excellent V; high Fe; Ti moderate. |
| [Bmim][PF6] | 31.3 | 19.1 | 27.9 | Persistent; poor efficiency | Not viable. |
| ChCl:OA | 97.6 | 76.1 | 68.8 | Biodegradable, low-cost, low toxicity | Best overall recovery. |
| ChCl:SA | 34.3 | 21.1 | 25.5 | Greener than ILs, but poor yields | Inefficient. |
| Temperature (°C) | ChCl:OA (50% v/v Aqueous) pH | [Bmim][HSO4] (50% v/v Aqueous) pH |
|---|---|---|
| 20 | 1.40 | 2.90 |
| 40 | 1.20 | 2.70 |
| 60 | 1.10 | 2.60 |
| 80 | 0.90 | 2.40 |
| 100 | 0.80 | 2.10 |
| V | (Chemical control) | (Diffusion control) | ||
| T (°C) | R2 | kc | R2 | kd |
| 20 | 0.9681 | 0.0012 | 0.9872 | 0.0004 |
| 40 | 0.9444 | 0.0032 | 0.9714 | 0.0027 |
| 60 | 0.9676 | 0.0043 | 0.9809 | 0.0047 |
| 80 | 0.9389 | 0.0056 | 0.9533 | 0.0072 |
| 100 | 0.9299 | 0.0077 | 0.9307 | 0.0107 |
| Ti | (Chemical control) | (Diffusion control) | ||
| T (°C) | R2 | kc | R2 | kd |
| 20 | 0.9738 | 0.0011 | 0.9958 | 0.0004 |
| 40 | 0.9345 | 0.0013 | 0.9688 | 0.0006 |
| 60 | 0.9434 | 0.0027 | 0.9417 | 0.0021 |
| 80 | 0.9836 | 0.0028 | 0.9913 | 0.0028 |
| 100 | 0.9503 | 0.0035 | 0.9725 | 0.0041 |
| Fe | (Chemical control) | (Diffusion control) | ||
| T (°C) | R2 | kc | R2 | kd |
| 20 | 0.9637 | 0.0009 | 0.9801 | 0.0002 |
| 40 | 0.9758 | 0.0016 | 0.9900 | 0.0007 |
| 60 | 0.9825 | 0.0018 | 0.9973 | 0.0011 |
| 80 | 0.9786 | 0.0022 | 0.9982 | 0.0018 |
| 100 | 0.9809 | 0.0031 | 0.9959 | 0.0032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oke, E.A.; Potgieter, J.H.; Nkhoesa, D.; Dyk, L.D.v. Vanadium, Titanium, and Iron Extraction from Titanomagnetite Ore by Salt Roasting and 21st-Century Solvents. Separations 2025, 12, 285. https://doi.org/10.3390/separations12100285
Oke EA, Potgieter JH, Nkhoesa D, Dyk LDv. Vanadium, Titanium, and Iron Extraction from Titanomagnetite Ore by Salt Roasting and 21st-Century Solvents. Separations. 2025; 12(10):285. https://doi.org/10.3390/separations12100285
Chicago/Turabian StyleOke, Emmanuel Anuoluwapo, Johannes Hermanus Potgieter, David Nkhoesa, and Lizelle Doreen van Dyk. 2025. "Vanadium, Titanium, and Iron Extraction from Titanomagnetite Ore by Salt Roasting and 21st-Century Solvents" Separations 12, no. 10: 285. https://doi.org/10.3390/separations12100285
APA StyleOke, E. A., Potgieter, J. H., Nkhoesa, D., & Dyk, L. D. v. (2025). Vanadium, Titanium, and Iron Extraction from Titanomagnetite Ore by Salt Roasting and 21st-Century Solvents. Separations, 12(10), 285. https://doi.org/10.3390/separations12100285








