Adsorption of Methylene Blue (MB) Using Novel Synthesized Phosphogypsum Flotation Tailings-Derived Zeolite (PGTZ): Experimental and Modeling Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of PGTZ
2.3. Experimental Design and Adsorption Studies
2.4. Adsorption Isotherms and Kinetic Studies
2.5. Characterization
3. Results and Discussion
3.1. Material Properties of PGTZ
3.2. Model Fitting
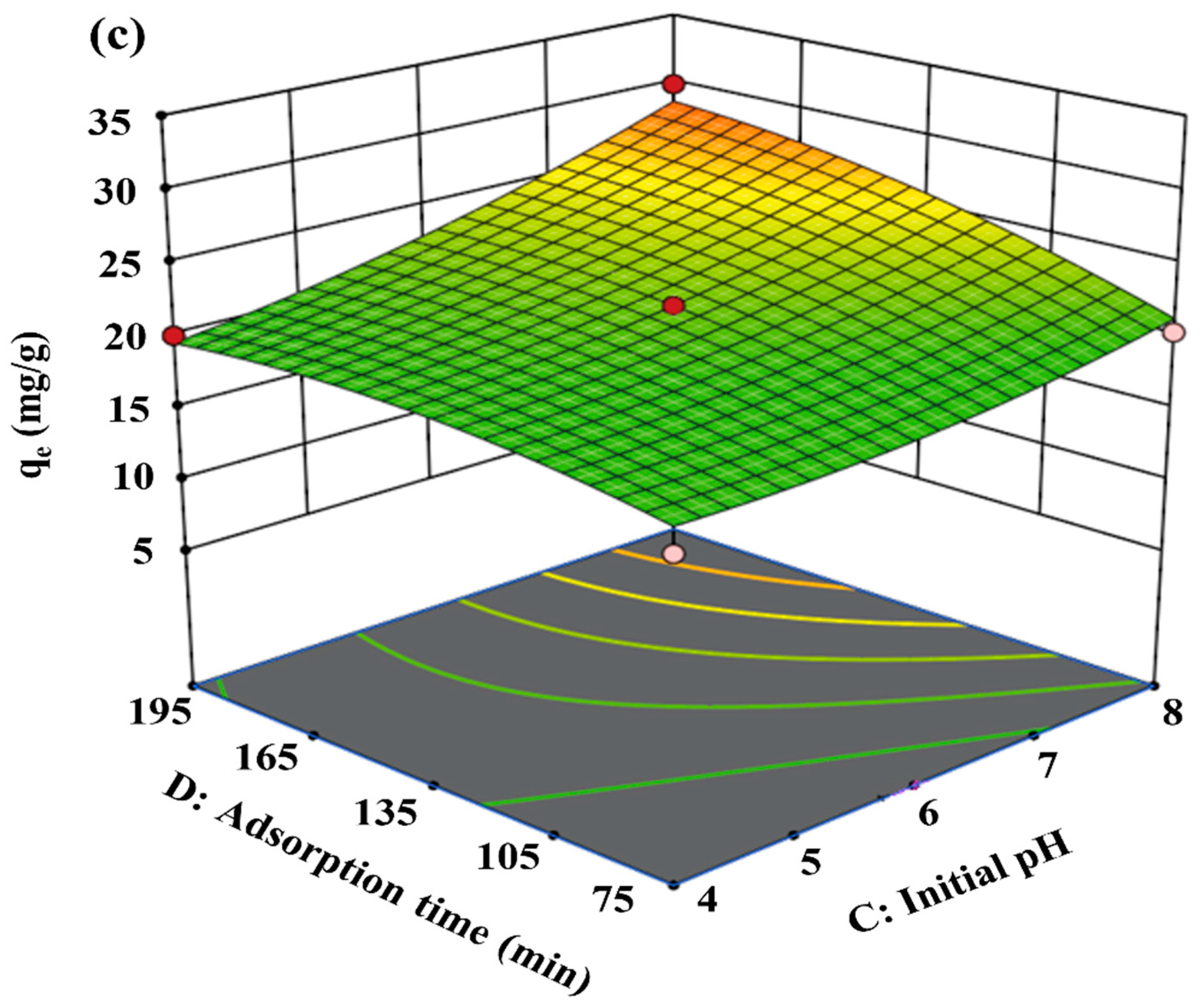
3.3. Effects of Process Variables
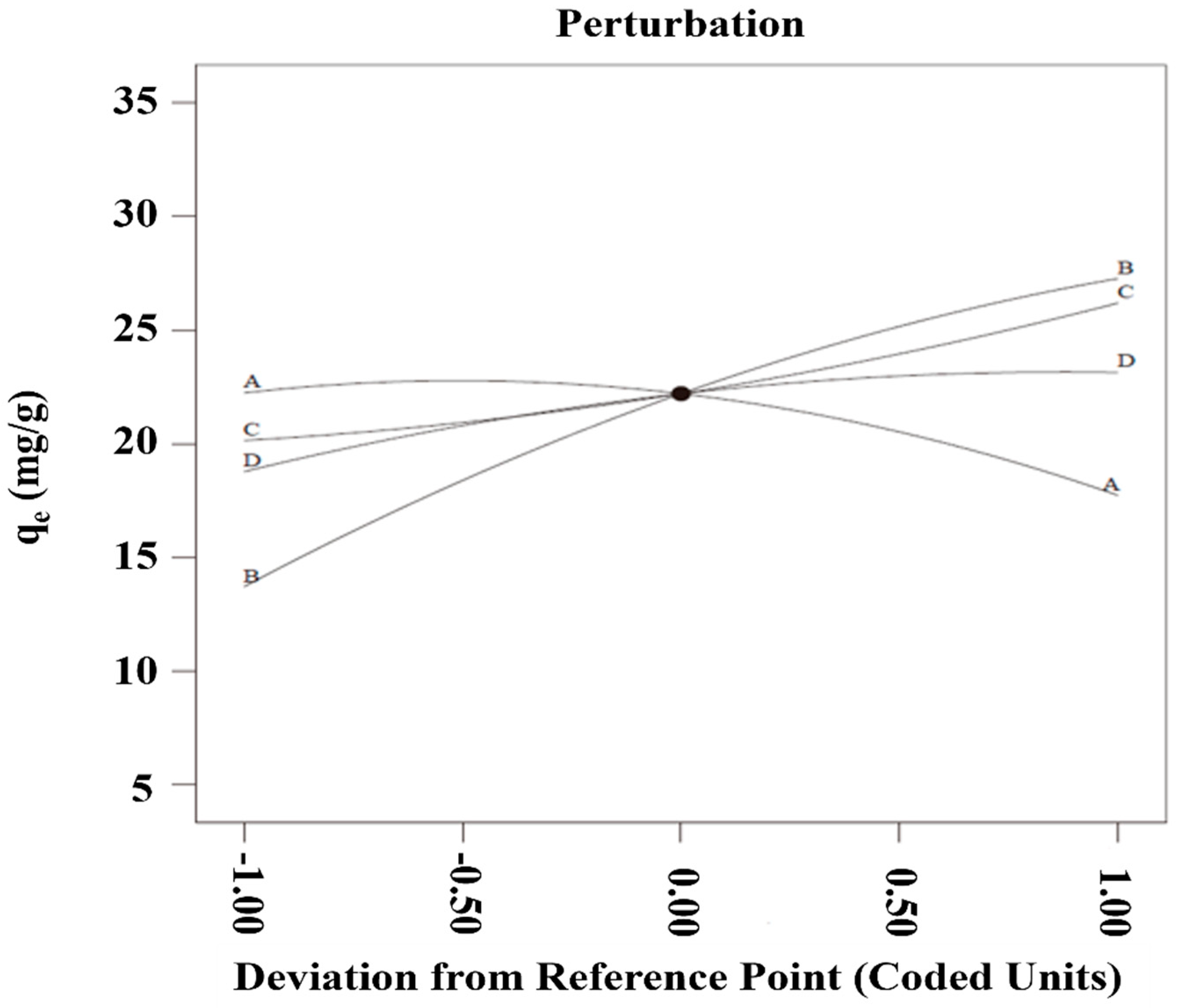
3.4. Independent Variables
3.5. Desirability Function Optimization
3.6. Adsorption Isotherms
3.7. Kinetic Studies
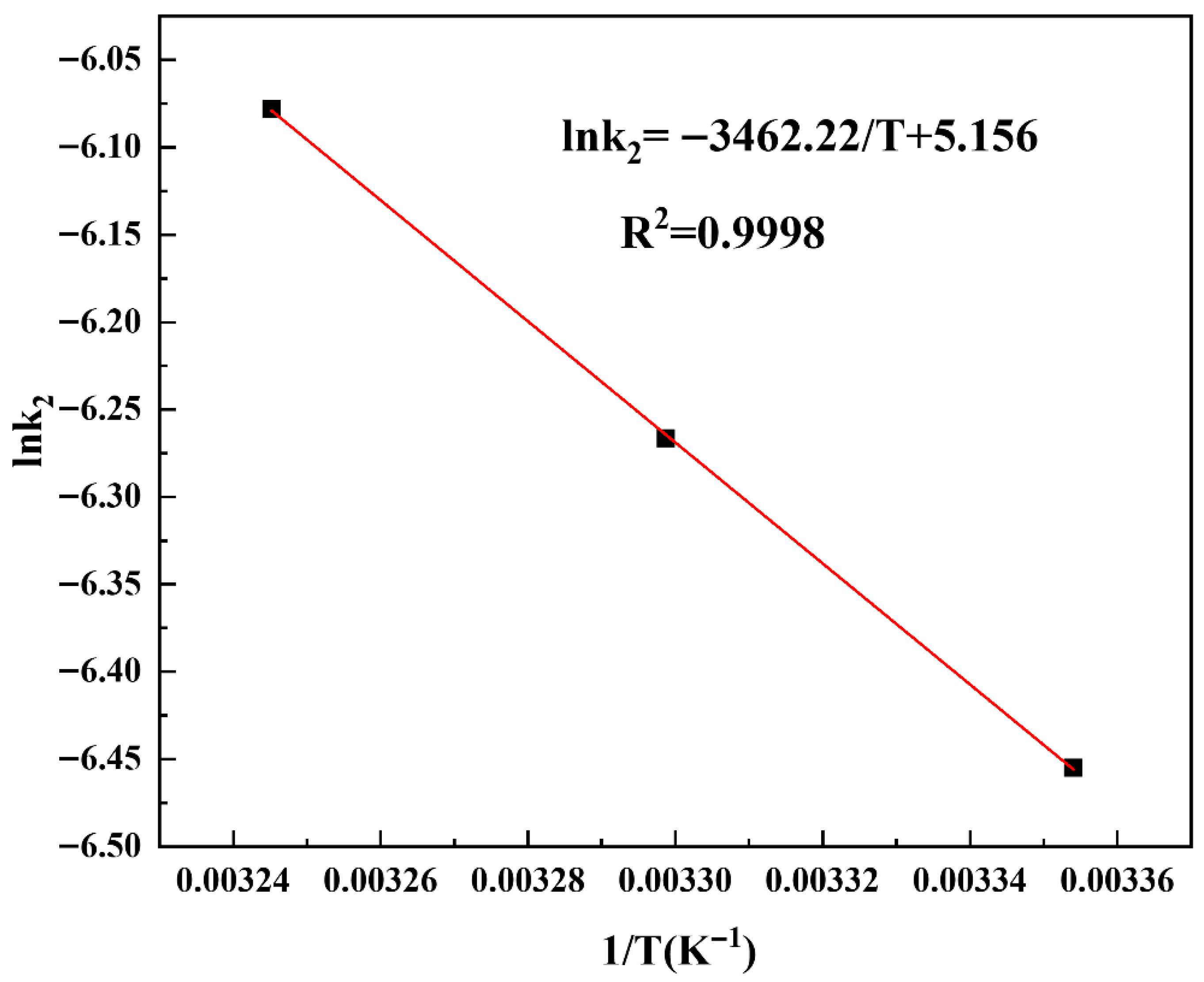
3.8. Thermodynamic Investigation and Estimation of Activation Energy
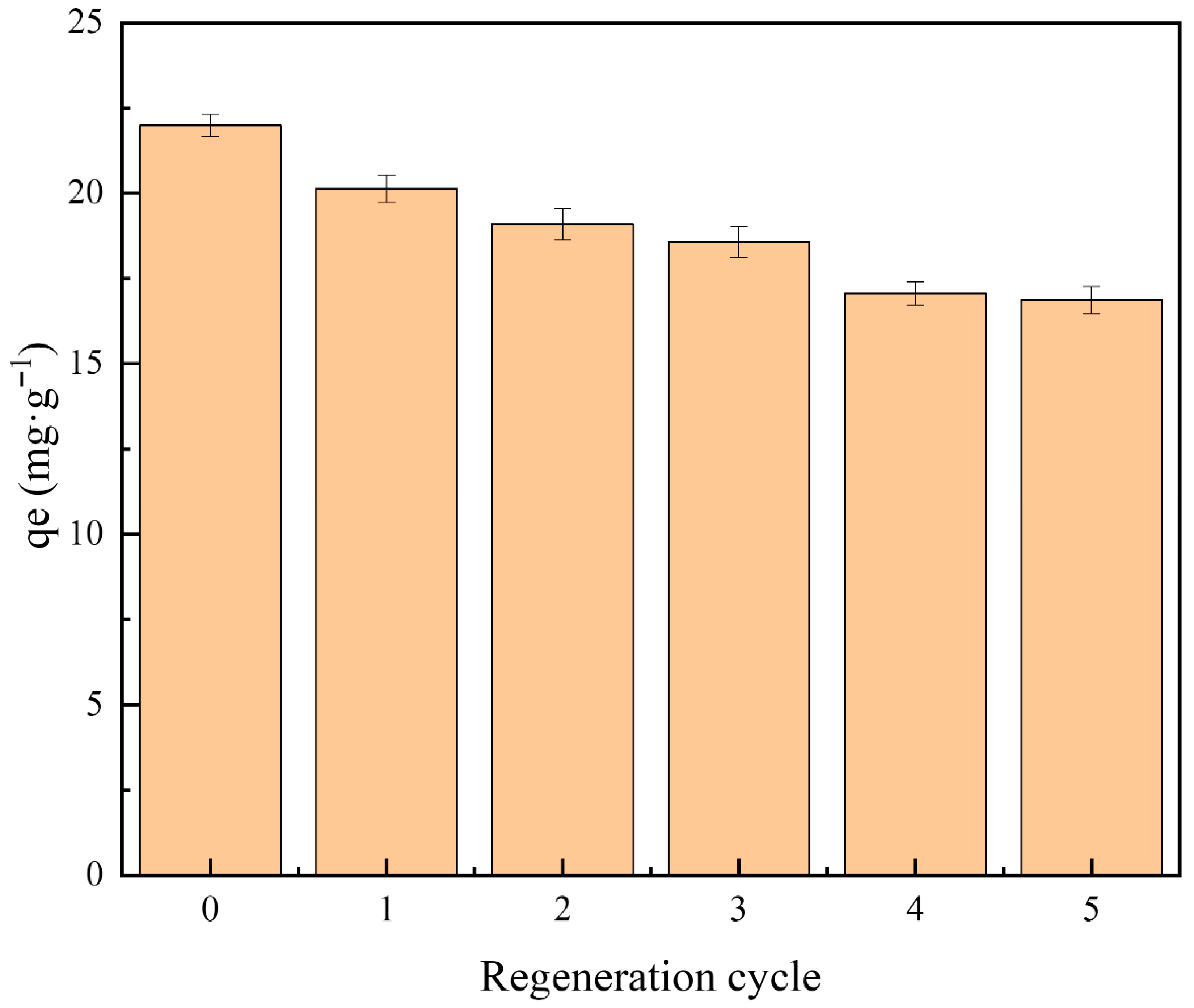
3.9. Reusability of PGTZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahimi, Z.; Zinatizadeh, A.A.; Zinadini, S.; van Loosdrecht, M. A Hydrophilic and Antifouling Nanofiltration Membrane Modified by Citric Acid Functionalized Tannic Acid (CA-f-TA) Nanocomposite for Dye Removal from Biologically Treated Baker’s Yeast Wastewater. J. Environ. Chem. Eng. 2021, 9, 104963. [Google Scholar] [CrossRef]
- Pai, S.; Kini, M.S.; Selvaraj, R. A Review on Adsorptive Removal of Dyes from Wastewater by Hydroxyapatite Nanocomposites. Environ. Sci. Pollut. Res. 2021, 28, 11835–11849. [Google Scholar] [CrossRef] [PubMed]
- Bagotia, N.; Sharma, A.K.; Kumar, S. A Review on Modified Sugarcane Bagasse Biosorbent for Removal of Dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Ren, J.; Li, R.; Wang, X.; Li, M.; Yang, W. A Superabsorbent Hydrogel for Removal of Dyes from Aqueous Solution. J. Polym. Environ. 2022, 30, 3327–3339. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-Factionalized Biomass Material for Methylene Blue Dye Removal: A Comprehensive Adsorption and Mechanism Study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef]
- Turan-Ertas, T. Biological and Physical-Chemical Treatment of Textile Dyeing Wastewater for Color and COD Removal. Ozone-Sci. Eng. 2001, 23, 199–206. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced Oxidation Processes for the Removal of Food Dyes in Wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Thillainayagam, B.P.; Nagalingam, R.; Saravanan, P. Batch and Column Studies on Removal of Methylene Blue Dye by Microalgae Biochar. Biomass Convers. Biorefinery 2022, 13, 10327–10342. [Google Scholar] [CrossRef]
- Rakass, S.; Oudghiri Hassani, H.; Mohmoud, A.; Kooli, F.; Abboudi, M.; Assirey, E.; Al Wadaani, F. Highly Efficient Methylene Blue Dye Removal by Nickel Molybdate Nanosorbent. Molecules 2021, 26, 1378. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Tenhu, H.; Khattab, S.A.; Eldeeb, A.A.; Azaam, M.M. Highly Efficient Adsorbent Material for Removal of Methylene Blue Dye Based on Functionalized Polyacrylonitrile. Eur. Polym. J. 2022, 169, 111138. [Google Scholar] [CrossRef]
- Gong, X.; Tian, W.; Wang, L.; Bai, J.; Qiao, K.; Zhao, J. Biological Regeneration of Brewery Spent Diatomite and Its Reuse in Basic Dye and Chromium (III) Ions Removal. Process. Saf. Environ. Prot. 2019, 128, 353–361. [Google Scholar] [CrossRef]
- Przystaś, W.; Zabłocka-Godlewska, E.; Grabińska-Sota, E. Biological Removal of Azo and Triphenylmethane Dyes and Toxicity of Process By-Products. Water Air Soil Pollut. 2012, 223, 1581–1592. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water. Process. Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A.; Hermosillo-Nevárez, J.J.; Ramírez-Pereda, B.; Vaca, M.; Rangel-Peraza, J.G.; Bustos-Terrones, V.; Rojas-Valencia, M.N. Removal of BB9 Textile Dye by Biological, Physical, Chemical, and Electrochemical Treatments. J. Taiwan Inst. Chem. Eng. 2021, 121, 29–37. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC. Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Alharbi, L.M.; Malik, M.A. Chitosan-Templated Synthesis of Fe2O3, NiO, and NiFe2O4 Nanoparticles for Efficient Methylene Blue Dye Removal. Polymers 2025, 17, 2750. [Google Scholar] [CrossRef]
- Yasin, S.A.; Sharaf Zeebaree, S.Y.; Sharaf Zeebaree, A.Y.; Haji Zebari, O.I.; Saeed, I.A. The Efficient Removal of Methylene Blue Dye Using CuO/PET Nanocomposite in Aqueous Solutions. Catalysts 2021, 11, 241. [Google Scholar] [CrossRef]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of Dyes by Agricultural Waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Sriram, G.; Bendre, A.; Mariappan, E.; Altalhi, T.; Kigga, M.; Ching, Y.C.; Jung, H.-Y.; Bhaduri, B.; Kurkuri, M. Recent Trends in the Application of Metal-Organic Frameworks (MOFs) for the Removal of Toxic Dyes and Their Removal Mechanism-a Review. Sustain. Mater. Technol. 2022, 31, e00378. [Google Scholar] [CrossRef]
- Salama, A.; Shoueir, K.R.; Aljohani, H.A. Preparation of Sustainable Nanocomposite as New Adsorbent for Dyes Removal. Fibers Polym. 2017, 18, 1825–1830. [Google Scholar] [CrossRef]
- Li, Z.; Yang, P.; Gao, Z.; Song, M.; Fang, Q.; Xue, M.; Qiu, S. A New ZIF Molecular-Sieving Membrane for High-Efficiency Dye Removal. Chem. Commun. 2019, 55, 3505–3508. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Yan, B.; Liu, B.; Gu, Y.; Lin, Y.; Shang, J.; Liu, W.; Chen, S.; Lan, J. Aminated Polyacrylonitrile Nanofiber Membranes for the Removal of Organic Dyes. ACS Appl. Nano Mater. 2022, 5, 1131–1140. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, U.; Kim, H.-W.; Cho, M.; Lee, J. Zero Discharge of Dyes and Regeneration of a Washing Solution in Membrane-Based Dye Removal by Cold Plasma Treatment. Membranes 2022, 12, 546. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes From Wastewater: A Review. Front. Environ. Sci. 2021, 9, 764958. [Google Scholar] [CrossRef]
- Okoniewska, E. Removal of Selected Dyes on Activated Carbons. Sustainability 2021, 13, 4300. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Inamuddin; Asiri, A.M. Nano-Engineered Adsorbent for the Removal of Dyes from Water: A Review. Curr. Anal. Chem. 2020, 16, 14–40. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated Carbon for Dyes Removal: Modeling and Understanding the Adsorption Process. J. Chem. 2020, 2020, 2096834. [Google Scholar] [CrossRef]
- Momtazan, F.; Vafaei, A.; Ghaedi, M.; Ghaedi, A.M.; Emadzadeh, D.; Lau, W.-J.; Baneshi, M.M. Application of Copper Sulfide Nanoparticles Loaded Activated Carbon for Simultaneous Adsorption of Ternary Dyes: Response Surface Methodology. Korean J. Chem. Eng. 2018, 35, 1108–1118. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Q.; Li, H. Preparation, Characterization of Chitin-Based Activated Carbon for Orange II Removal. BioResources 2023, 18, 5041–5056. [Google Scholar] [CrossRef]
- Yousef, T.A.; Sahu, U.K.; Jawad, A.H.; Abd Malek, N.N.; Al Duaij, O.K.; ALOthman, Z.A. Fruit Peel-Based Mesoporous Activated Carbon via Microwave Assisted K2 CO3 Activation: Box Behnken Design and Desirability Function for Methylene Blue Dye Adsorption. Int. J. Phytoremediat. 2023, 25, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Chumee, J.; Javadi, B.; Peungsamran, N.; Kumpun, S.; Seekakee, J.; Hoonsuwan, T.; Ohama, P. Synthesis of Zeolite P-Metal Organic Composite Beads for Superior Cationic Dye Removal. Inorg. Chem. Commun. 2025, 177, 114344. [Google Scholar] [CrossRef]
- Jia, X.; Kanbaiguli, M.; Zhang, B.; Huang, Y.; Peydayesh, M.; Huang, Q. Anisotropic Chitosan-Nanocellulose/Zeolite Imidazolate Frameworks-8 Aerogel for Sustainable Dye Removal. J. Colloid Interface Sci. 2024, 676, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Majid, Z.; AbdulRazak, A.A.; Noori, W.A.H. Modification of Zeolite by Magnetic Nanoparticles for Organic Dye Removal. Arab. J. Sci. Eng. 2019, 44, 5457–5474. [Google Scholar] [CrossRef]
- Rakanović, M.; Vukojević, A.; Savanović, M.M.; Armaković, S.; Pelemiš, S.; Živić, F.; Sladojević, S.; Armaković, S.J. Zeolites as Adsorbents and Photocatalysts for Removal of Dyes from the Aqueous Environment. Molecules 2022, 27, 6582. [Google Scholar] [CrossRef]
- Hassoune, H.; Lahhit, M.; Khalid, A.; Lachehab, A. Application of Leaching Tests on Phosphogypsum by Infiltration-Percolation. Water Sci. Technol. 2017, 76, 1844–1851. [Google Scholar] [CrossRef]
- Msila, X.; Billing, D.G.; Barnard, W. Capture and Storage of CO2 into Waste Phosphogypsum: The Modified Merseburg Process. Clean Technol. Environ. Policy 2016, 18, 2709–2715. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Z.; Yang, Z.; Peng, J. Effects of pH and Fineness of Phosphogypsum on Mechanical Performance of Cement–Phosphogypsum-Stabilized Soil and Classification for Road-Used Phosphogypsum. Coatings 2020, 10, 1021. [Google Scholar] [CrossRef]
- Du, M.; Wang, J.; Dong, F.; Wang, Z.; Yang, F.; Tan, H.; Fu, K.; Wang, W. The Study on the Effect of Flotation Purification on the Performance of α-Hemihydrate Gypsum Prepared from Phosphogypsum. Sci. Rep. 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Filippov, L.O.; Duverger, A.; Filippova, I.V.; Kasaini, H.; Thiry, J. Selective Flotation of Silicates and Ca-Bearing Minerals: The Role of Non-Ionic Reagent on Cationic Flotation. Miner. Eng. 2012, 36–38, 314–323. [Google Scholar] [CrossRef]
- Qi, M.; Peng, W.; Wang, W.; Cao, Y.; Fan, G.; Huang, Y. Simple and Efficient Method for Purification and Recovery of Gypsum from Phosphogypsum: Reverse-Direct Flotation and Mechanism. J. Mol. Liq. 2023, 371, 121111. [Google Scholar] [CrossRef]
- Zhang, H.; Chai, W.; Cao, Y. Flotation Separation of Quartz from Gypsum Using Benzyl Quaternary Ammonium Salt as Collector. Appl. Surf. Sci. 2022, 576, 151834. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of Basic Dye (Methylene Blue) from Aqueous Solution Using Zeolite Synthesized from Electrolytic Manganese Residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- GB36600—2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Development Land. China Environmental Science Press: Beijing, China, 2019.
- Murakami, T.; Sugano, Y.; Kinami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Alkali Hydrothermal Synthesis of Zeolite A Using Oxide By-Products. ISIJ Int. 2011, 51, 158–165. [Google Scholar] [CrossRef]
- Sugano, Y.; Sahara, R.; Murakami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Hydrothermal Synthesis of Zeolite A Using Blast Furnace Slag. ISIJ Int. 2005, 45, 937–945. [Google Scholar] [CrossRef]
- Zhang, K.; Van Dyk, L.; He, D.; Deng, J.; Liu, S.; Zhao, H. Synthesis of Zeolite from Fly Ash and Its Adsorption of Phosphorus in Wastewater. Green Process. Synth. 2021, 10, 349–360. [Google Scholar] [CrossRef]
- Liu, W.; Aldahri, T.; Xu, C.; Li, C.; Rohani, S. Synthesis of Sole Gismondine-Type Zeolite from Blast Furnace Slag during CO2 Mineralization Process. J. Environ. Chem. Eng. 2021, 9, 104652. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of Geopolymer-Supported Zeolites via Robust One-Step Method and Their Adsorption Potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef]
- Ren, X.; Xiao, L.; Qu, R.; Liu, S.; Ye, D.; Song, H.; Wu, W.; Zheng, C.; Wu, X.; Gao, X. Synthesis and Characterization of a Single Phase Zeolite A Using Coal Fly Ash. RSC Adv. 2018, 8, 42200–42209. [Google Scholar] [CrossRef] [PubMed]
- Mlonka-Mędrala, A.; Hasan, T.; Kalawa, W.; Sowa, M.; Sztekler, K.; Pinto, M.L.; Mika, Ł. Possibilities of Using Zeolites Synthesized from Fly Ash in Adsorption Chillers. Energies 2022, 15, 7444. [Google Scholar] [CrossRef]
- GB 6566-2010; Specification for Limit of Radionuclide Inbuilding Stone. China Standard Press: Beijing, China, 2011.
- Avramović, J.M.; Veličković, A.V.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of Sunflower Oil Ethanolysis Catalyzed by Calcium Oxide: RSM versus ANN-GA. Energy Convers. Manag. 2015, 105, 1149–1156. [Google Scholar] [CrossRef]
- Pavlović, M.D.; Buntić, A.V.; Mihajlovski, K.R.; Šiler-Marinković, S.S.; Antonović, D.G.; Radovanović, Ž.; Dimitrijević-Branković, S.I. Rapid Cationic Dye Adsorption on Polyphenol-Extracted Coffee Grounds—A Response Surface Methodology Approach. J. Taiwan Inst. Chem. Eng. 2014, 45, 1691–1699. [Google Scholar] [CrossRef]
- Krupa, A.N.D.; Abigail, M.E.A.; Santhosh, C.; Grace, A.N.; Vimala, R. Optimization of Process Parameters for the Microbial Synthesis of Silver Nanoparticles Using 3-Level Box–Behnken Design. Ecol. Eng. 2016, 87, 168–174. [Google Scholar] [CrossRef]
- Parvizi Ghaleh, S.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Comprehensive Monolayer Two-Parameter Isotherm and Kinetic Studies of Thiamine Adsorption on Clay Minerals: Experimental and Modeling Approaches. J. Mol. Liq. 2020, 306, 112942. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U.; Attarde, S.; Ingle, S. Facile Synthesis of Lead Doped Zinc-Aluminum Oxide Nanoparticles (LD-ZAO-NPs) for Efficient Adsorption of Anionic Dye: Kinetic, Isotherm and Thermodynamic Behaviors. J. Ind. Eng. Chem. 2017, 53, 294–306. [Google Scholar] [CrossRef]
- Njuguna, D.G.; Schönherr, H. Xanthan Gum Hydrogels as High-Capacity Adsorbents for Dye Removal. ACS Appl. Polym. Mater. 2021, 3, 3142–3152. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Guo, R.; Wang, Y.; Zhan, H.; Yuan, Y. Synthesis of Rectorite/Fe3O4/ZnO Composites and Their Application for the Removal of Methylene Blue Dye. Catalysts 2018, 8, 107. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, A.; Umar, K.; Nabi, S.A. Synthesis, Characterization, and Biological Applications of Nanocomposites for the Removal of Heavy Metals and Dyes. Ind. Eng. Chem. Res. 2015, 54, 76–82. [Google Scholar] [CrossRef]
- Sharma, K.; Dalai, A.K.; Vyas, R.K. Removal of Synthetic Dyes from Multicomponent Industrial Wastewaters. Rev. Chem. Eng. 2017, 34, 107–134. [Google Scholar] [CrossRef]
- He, H.; Luo, Z.; Yu, C. Diatomite-Anchored g-C3N4 Nanosheets for Selective Removal of Organic Dyes. J. Alloys Compd. 2020, 816, 152652. [Google Scholar] [CrossRef]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.Z.; Wilson, L.D.; Morin-Crini, N. Dye Removal by Biosorption Using Cross-Linked Chitosan-Based Hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666. [Google Scholar] [CrossRef]
- Coura, J.C.; Profeti, D.; Profeti, L.P.R. Eco-Friendly Chitosan/Quartzite Composite as Adsorbent for Dye Removal. Mater. Chem. Phys. 2020, 256, 123711. [Google Scholar] [CrossRef]
- Abbasi, N.; Khan, S.A.; Khan, T.A. Response Surface Methodology Mediated Process Optimization of Celestine Blue B Uptake by Novel Custard Apple Seeds Activated Carbon/FeMoO4 Nanocomposite. J. Water Process. Eng. 2021, 43, 102267. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and Multilayer Adsorption Isotherm Models for Sorption from Aqueous Media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Nithya, R. Adsorption of Acid Orange 7 Using Green Synthesized CaO/CeO2 Composite: An Insight into Kinetics, Equilibrium, Thermodynamics, Mass Transfer and Statistical Models. J. Taiwan Inst. Chem. Eng. 2020, 111, 44–62. [Google Scholar] [CrossRef]
- Sverjensky, D.A.; Fukushi, K. Anion Adsorption on Oxide Surfaces: Inclusion of the Water Dipole in Modeling the Electrostatics of Ligand Exchange. Environ. Sci. Technol. 2006, 40, 263–271. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, L.; Rao, F.; Zheng, Y.; Song, Z. Adsorption Behavior and Mechanism of MB, Pb(II) and Cu(II) on Porous Geopolymers. Ceram. Int. 2025, 51, 11455–11466. [Google Scholar] [CrossRef]
- Patel, P.; Gupta, S.; Mondal, P. Modeling and Optimization of Process Parameters of MB Dye Adsorption Using Waste-Derived Chemically Activated Biosorbents. Biomass Convers. Biorefinery 2022, 13, 13461–13480. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Song, R.; Li, S.; Yang, S.; Zhang, Q. Synthesis of Core-Double-Shell Structured Fe3O4@PDA/HKUST-1: Characterization Analysis and Adsorption Performance on Cationic MB Dyes. J. Phys. Chem. Solids 2023, 172, 111094. [Google Scholar] [CrossRef]
- Jaberi, H.; Emami, E.; Mousazadeh, M.H. CDs/HMS for Removal of MB Dye with Highly Adsorption Capacity: Isotherm, Kinetic, and Thermodynamic Study. Int. J. Environ. Sci. Technol. 2021, 19, 5167–5180. [Google Scholar] [CrossRef]
- Wang, R.; Shi, K.; Huang, D.; Zhang, J.; An, S. Synthesis and Degradation Kinetics of TiO2/GO Composites with Highly Efficient Activity for Adsorption and Photocatalytic Degradation of MB. Sci. Rep. 2019, 9, 18744. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-Promoted Synthesis of UiO-66 for Highly Selective Adsorption of Anionic Dyes: Adsorption Performance and Mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The Adsorption Kinetics and Removal of Cationic Dye, Toluidine Blue O, from Aqueous Solution with Turkish Zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef]
- Han, R.; Zhang, J.; Han, P.; Wang, Y.; Zhao, Z.; Tang, M. Study of Equilibrium, Kinetic and Thermodynamic Parameters about Methylene Blue Adsorption onto Natural Zeolite. Chem. Eng. J. 2009, 145, 496–504. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Hu, X.; Fan, Z.; Wang, Y.; Ma, P.; Niu, J.; Wang, J. Synthesis and Structure of a Copper-Based Functional Network for Efficient Organic Dye Adsorption. Inorg. Chem. 2022, 61, 19764–19772. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, Z.; Zhong, J.; Lv, Y.; Yan, X.; Wu, Y.; Zhang, H. Adsorption of Dye through Hydrochar Derived from Co-Hydrothermal Carbonization of Garden Waste and Sewage Sludge: The Adsorption Enhancement Mechanism of Lignin Component. J. Water Process. Eng. 2024, 67, 106233. [Google Scholar] [CrossRef]
- Taweekarn, T.; Wongniramaikul, W.; Boonkanon, C.; Phanrit, C.; Sriprom, W.; Limsakul, W.; Towanlong, W.; Phawachalotorn, C.; Choodum, A. Starch Biocryogel for Removal of Methylene Blue by Batch Adsorption. Polymers 2022, 14, 5543. [Google Scholar] [CrossRef]
- Deori, N.; Paul, S.; Lahkar, S.; Brahma, S. Ultrasonic-Assisted Nitrate Anion Incorporation in Triaminoguanidium Chloride Based Covalent Organic Polymer for Methylene Blue Dye Adsorption. Chem.-Asian J. 2024, 19, e202400046. [Google Scholar] [CrossRef]
- Taşdelen, B.; Çifçi, D.İ.; Meriç, S. Preparation and Characterization of Chitosan/AMPS/Kaolinite Composite Hydrogels for Adsorption of Methylene Blue. Polym. Bull. 2021, 79, 9643–9662. [Google Scholar] [CrossRef]
- Shukor, H.; Yaser, A.Z.; Shoparwe, N.F.; Makhtar, M.M.Z.; Mokhtar, N. Biosorption Study of Methylene Blue (MB) and Brilliant Red Remazol (BRR) by Coconut Dregs. Int. J. Chem. Eng. 2022, 2022, 8153617. [Google Scholar] [CrossRef]

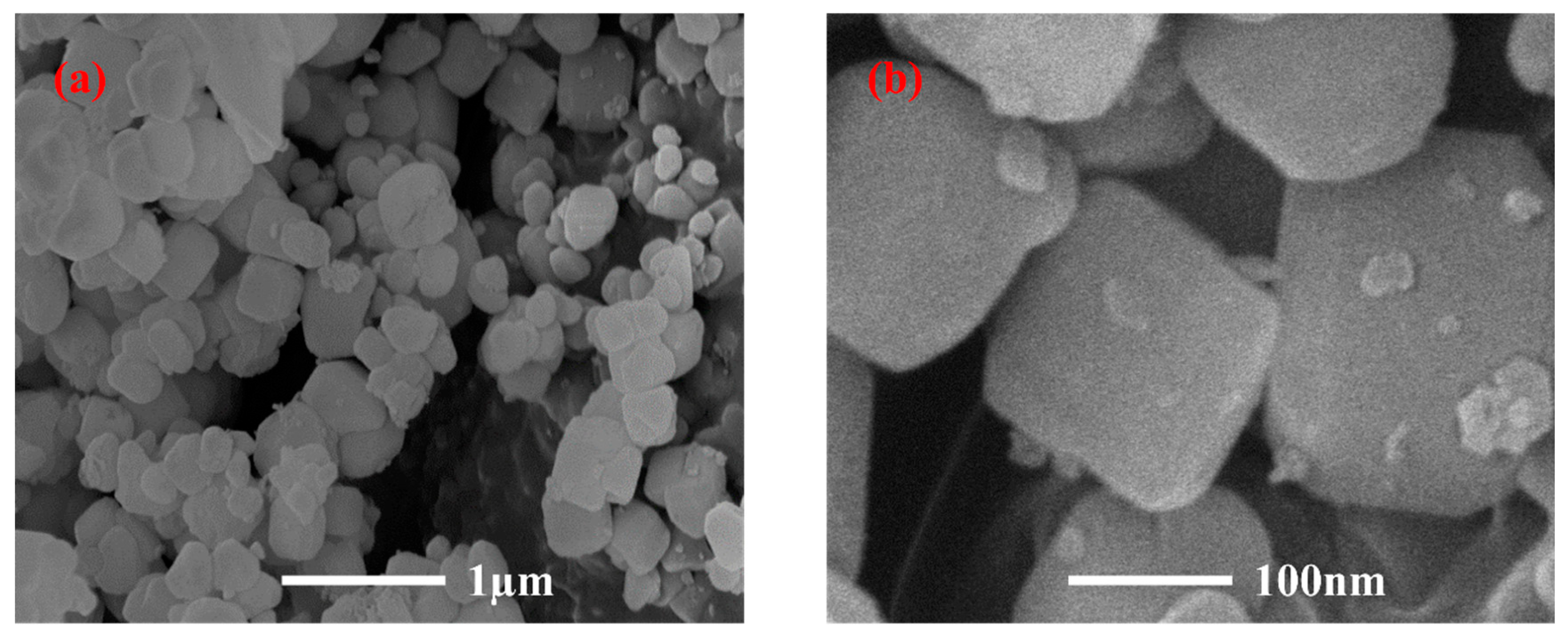
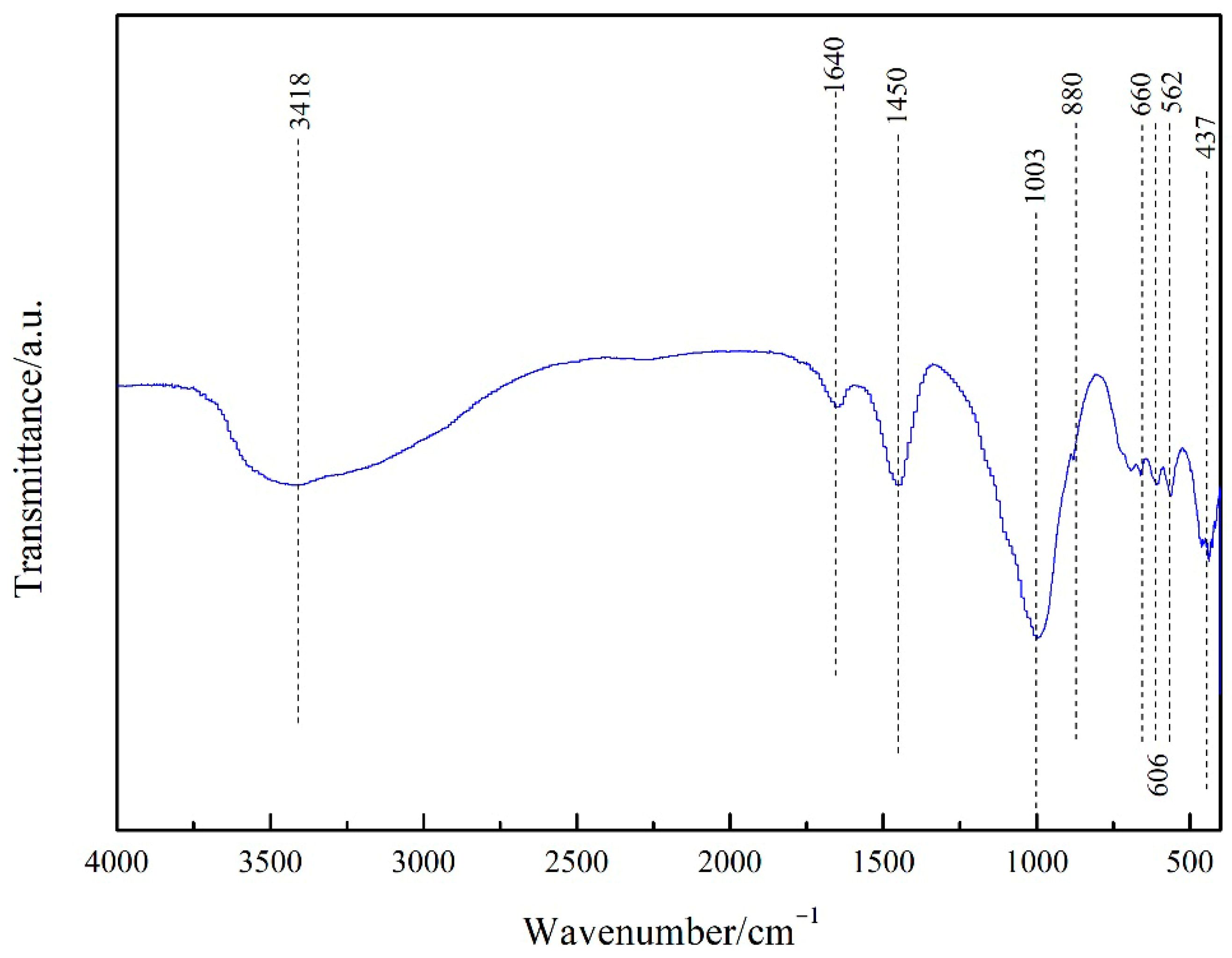
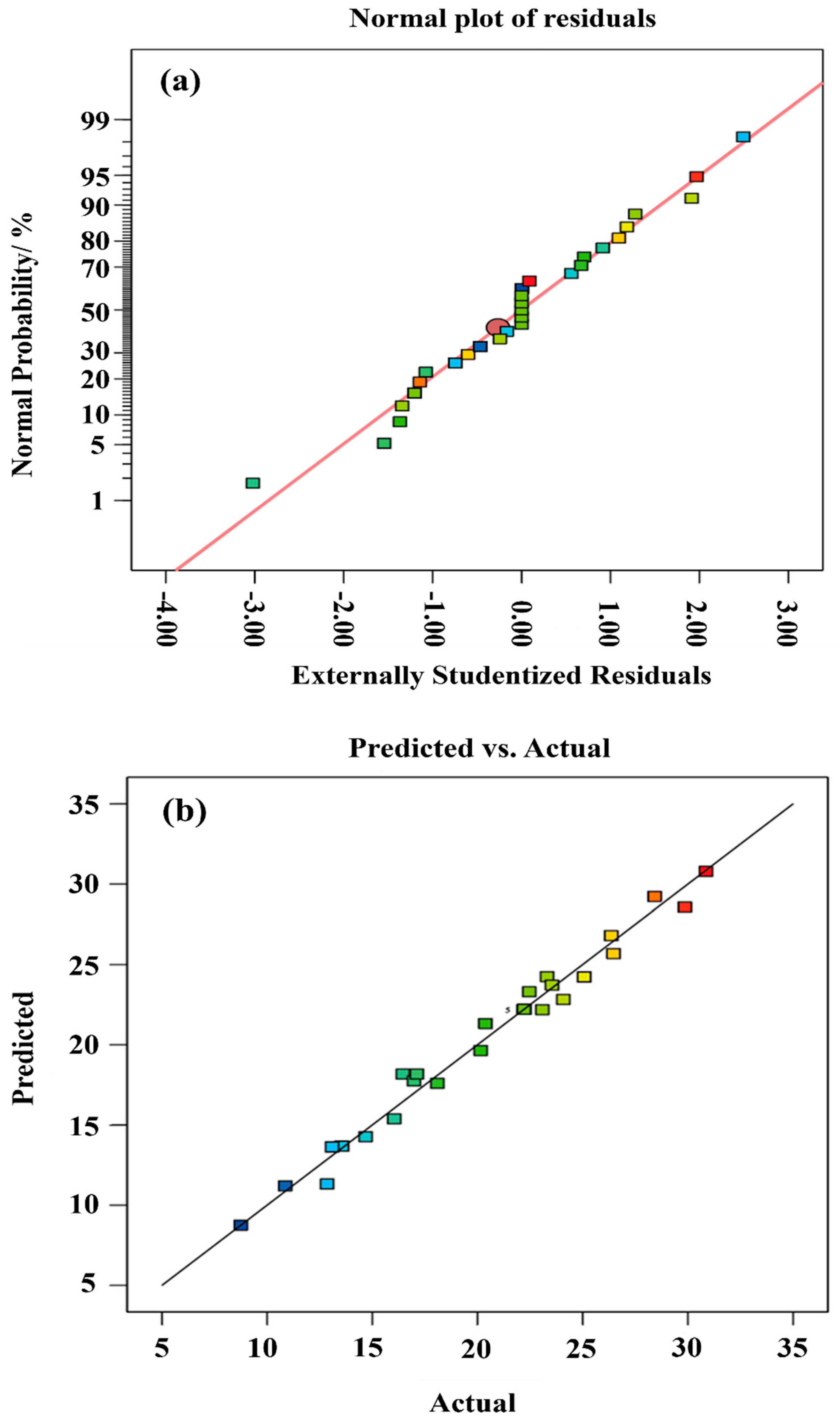
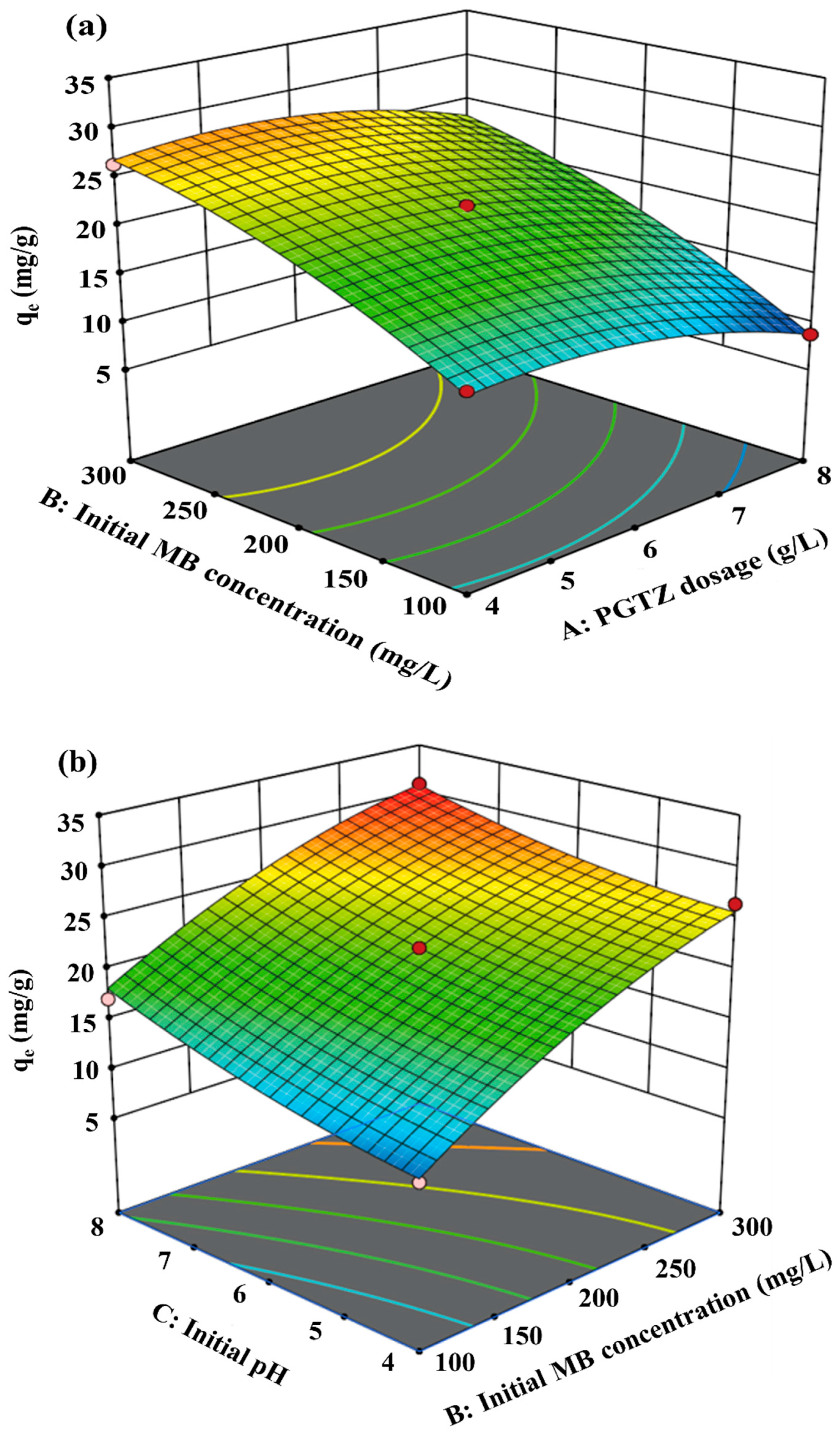
| Component | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Total |
|---|---|---|---|---|---|---|
| Mass (wt% a) | 4.82 | 50.36 | 6.68 | 3.46 | 0.26 | 65.58 b |
| Variables | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| A: PGTZ dosage (g·L−1) | 4 | 6 | 8 |
| B: Initial MB concentration (mg·L−1) | 100 | 200 | 300 |
| C: Initial pH | 4.0 | 6.0 | 8.0 |
| D: Adsorption time (min) | 75 | 135 | 195 |
| Source | Sum of Squares | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 878.69 | 62.76 | 50.87 | <0.0001 | Significant |
| A-PGTZ dosage | 60.93 | 60.93 | 49.38 | <0.0001 | |
| B-Initial MB concentration | 550.67 | 550.67 | 446.32 | <0.0001 | |
| C-Initial pH | 109.57 | 109.57 | 88.80 | <0.0001 | |
| D-Adsorption time | 56.99 | 56.99 | 46.19 | <0.0001 | |
| AB | 1.03 | 1.03 | 0.8350 | 0.3763 | |
| AC | 15.96 | 15.96 | 12.94 | 0.0029 | |
| AD | 4.58 | 4.58 | 3.71 | 0.0746 | |
| BC | 0.8649 | 0.8649 | 0.7010 | 0.4165 | |
| BD | 4.20 | 4.20 | 3.41 | 0.0862 | |
| CD | 8.38 | 8.38 | 6.79 | 0.0207 | |
| A2 | 31.93 | 31.93 | 25.88 | 0.0002 | |
| B2 | 18.97 | 18.97 | 15.37 | 0.0015 | |
| C2 | 6.02 | 6.02 | 4.88 | 0.0443 | |
| D2 | 10.09 | 10.09 | 8.18 | 0.0126 | |
| Lack of fit | 17.27 | 1.73 | 5.55 | 0.0565 | Not significant |
| Item | Value |
|---|---|
| Standard deviation (SD) | 1.11 |
| Mean | 20.47 |
| C.V.% | 5.43 |
| R2 | 0.9807 |
| Adjusted R2 | 0.9614 |
| Predicted R2 | 0.8890 |
| Adequate precision | 27.5997 |
| Response | Solution No. | PGTZ Dosage (g·L−1) | Initial MB Concentration (mg·L−1) | Initial pH | Adsorption Time (min) | qe (mg·g−1) | Desirability |
|---|---|---|---|---|---|---|---|
| Adsorbent capacity (mg·g−1) | 1 | 6.10 | 281.40 | 7.64 | 162.97 | 31.05 | 1.000 |
| 5 | 5.31 | 294.59 | 7.42 | 187.89 | 32.31 | 1.000 | |
| 14 | 5.03 | 299.08 | 6.65 | 190.40 | 31.01 | 1.000 | |
| 20 | 5.93 | 283.62 | 7.69 | 179.41 | 32.11 | 1.000 | |
| 28 | 6.75 | 277.55 | 7.90 | 158.01 | 30.87 | 1.000 |
| Adsorption Isotherm | Temperature (°C) | ||
|---|---|---|---|
| 25 | 30 | 35 | |
| Langmuir isotherm | KL = 0.0212 | KL = 0.0346 | KL = 0.0789 |
| q0 = 31.65 | q0 = 32.78 | q0 = 32.89 | |
| R2 = 0.9834 | R2 = 0.9866 | R2 = 0.9869 | |
| R2adj = 0.9773 | R2adj = 0.9821 | R2adj = 0.9825 | |
| RMSE = 0.3209 | RMSE = 0.2791 | RMSE = 0.2923 | |
| Freundlich isotherm | KF = 3.4449 | KF = 6.0225 | KF = 12.4497 |
| 1/n = 0.3965 | 1/n = 0.3025 | 1/n = 0.1651 | |
| R2 = 0.9058 | R2 = 0.9508 | R2 = 0.8561 | |
| R2adj = 0.8743 | R2adj = 0.9344 | R2adj = 0.8082 | |
| RMSE = 0.1187 | RMSE = 0.0767 | RMSE = 0.1165 | |
| Tempkin isotherm | AT = 0.1972 | AT = 0.5359 | AT = 0.6488 |
| BT = 7.3864 | BT = 6.2052 | BT = 3.6411 | |
| R2 = 0.9252 | R2 = 0.9437 | R2 = 0.9129 | |
| R2adj = 0.9002 | R2adj = 0.9249 | R2adj = 0.7879 | |
| RMSE = 1.9131 | RMSE = 1.6901 | RMSE = 2.8559 | |
| Dubinin–Radushkevich (D-R) isotherm | q0 = 24.2108 | q0 = 24.5320 | q0 = 25.3987 |
| K = 9.4708 × 10−5 | K = 2.4990 × 10−5 | K = 1.1939 × 10−5 | |
| R2 = 0.8447 | R2 = 0.7065 | R2 = 0.5738 | |
| R2adj = 0.7928 | R2adj = 0.6087 | R2adj = 0.4318 | |
| RMSE = 0.1496 | RMSE = 0.1874 | RMSE = 0.2043 | |
| Adsorption Kinetic | Temperature (°C) | ||
|---|---|---|---|
| 25 | 30 | 35 | |
| Pseudo-first-order Model | qe(exp) = 22.98 | qe(exp) = 25.93 | qe(exp) = 33.57 |
| qe = 13.56 | q0 = 14.82 | q0 = 15.30 | |
| k1 = 0.0140 | k1 = 0.0167 | k1 = 0.0092 | |
| R2 = 0.9649 | R2 = 0.9787 | R2 = 0.9572 | |
| R2adj = 0.9614 | R2adj = 0.9744 | R2adj = 0.9486 | |
| RMSE = 0.1749 | RMSE = 0.1513 | RMSE = 0.1193 | |
| Pseudo-second-order Model | qe(exp) = 22.98 | qe(exp) = 25.93 | qe(exp) = 33.57 |
| qe = 24.27 | qe = 25.06 | qe = 26.18 | |
| k2 = 0.001573 | k2 = 0.001899 | k2 = 0.002293 | |
| R2 = 0.9944 | R2 = 0.9962 | R2 = 0.9904 | |
| R2adj = 0.9937 | R2adj = 0.9958 | R2adj = 0.9901 | |
| RMSE = 0.2019 | RMSE = 0.1622 | RMSE = 0.2685 | |
| Intra-particle diffusion model (first line) | qe(exp) = 22.98 | qe(exp) = 25.93 | qe(exp) = 33.57 |
| kid1 = 2.0263 | kid1 = 1.6802 | kid1 = 1.6876 | |
| R2 = 0.9788 | R2 = 0.9601 | R2 = 0.9998 | |
| R2adj = 0.9565 | R2adj = 0.9213 | R2adj = 0.9996 | |
| RMSE = 0.6074 | RMSE = 0.4388 | RMSE = 0.04505 | |
| Intra-particle diffusion model (second line) | qe(exp) = 22.98 | qe(exp) = 25.93 | qe(exp) = 33.57 |
| kid2 = 0.9573 | kid2 = 0.8646 | kid2 = 0.8825 | |
| R2 = 0.9881 | R2 = 0.9144 | R2 = 0.9058 | |
| R2adj = 0.9842 | R2adj = 0.8858 | R2adj = 0.8743 | |
| RMSE = 0.2533 | RMSE = 0.6393 | RMSE = 0.6879 | |
| Dye | ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J·mol−1·K−1) | ||
|---|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | |||
| MB | −4.53 | −5.29 | −6.05 | 40.75 | 151.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yang, J.; Liu, S.; Liu, N.; Zhang, L.; Ren, L. Adsorption of Methylene Blue (MB) Using Novel Synthesized Phosphogypsum Flotation Tailings-Derived Zeolite (PGTZ): Experimental and Modeling Approaches. Separations 2025, 12, 286. https://doi.org/10.3390/separations12100286
Li C, Yang J, Liu S, Liu N, Zhang L, Ren L. Adsorption of Methylene Blue (MB) Using Novel Synthesized Phosphogypsum Flotation Tailings-Derived Zeolite (PGTZ): Experimental and Modeling Approaches. Separations. 2025; 12(10):286. https://doi.org/10.3390/separations12100286
Chicago/Turabian StyleLi, Changxin, Jinyu Yang, Shanpei Liu, Nan Liu, Lili Zhang, and Lu Ren. 2025. "Adsorption of Methylene Blue (MB) Using Novel Synthesized Phosphogypsum Flotation Tailings-Derived Zeolite (PGTZ): Experimental and Modeling Approaches" Separations 12, no. 10: 286. https://doi.org/10.3390/separations12100286
APA StyleLi, C., Yang, J., Liu, S., Liu, N., Zhang, L., & Ren, L. (2025). Adsorption of Methylene Blue (MB) Using Novel Synthesized Phosphogypsum Flotation Tailings-Derived Zeolite (PGTZ): Experimental and Modeling Approaches. Separations, 12(10), 286. https://doi.org/10.3390/separations12100286





