LC-MS/MS-QTOF Identification of Phenolic Compounds of Sideritis Species Cultivated in Greece
Abstract
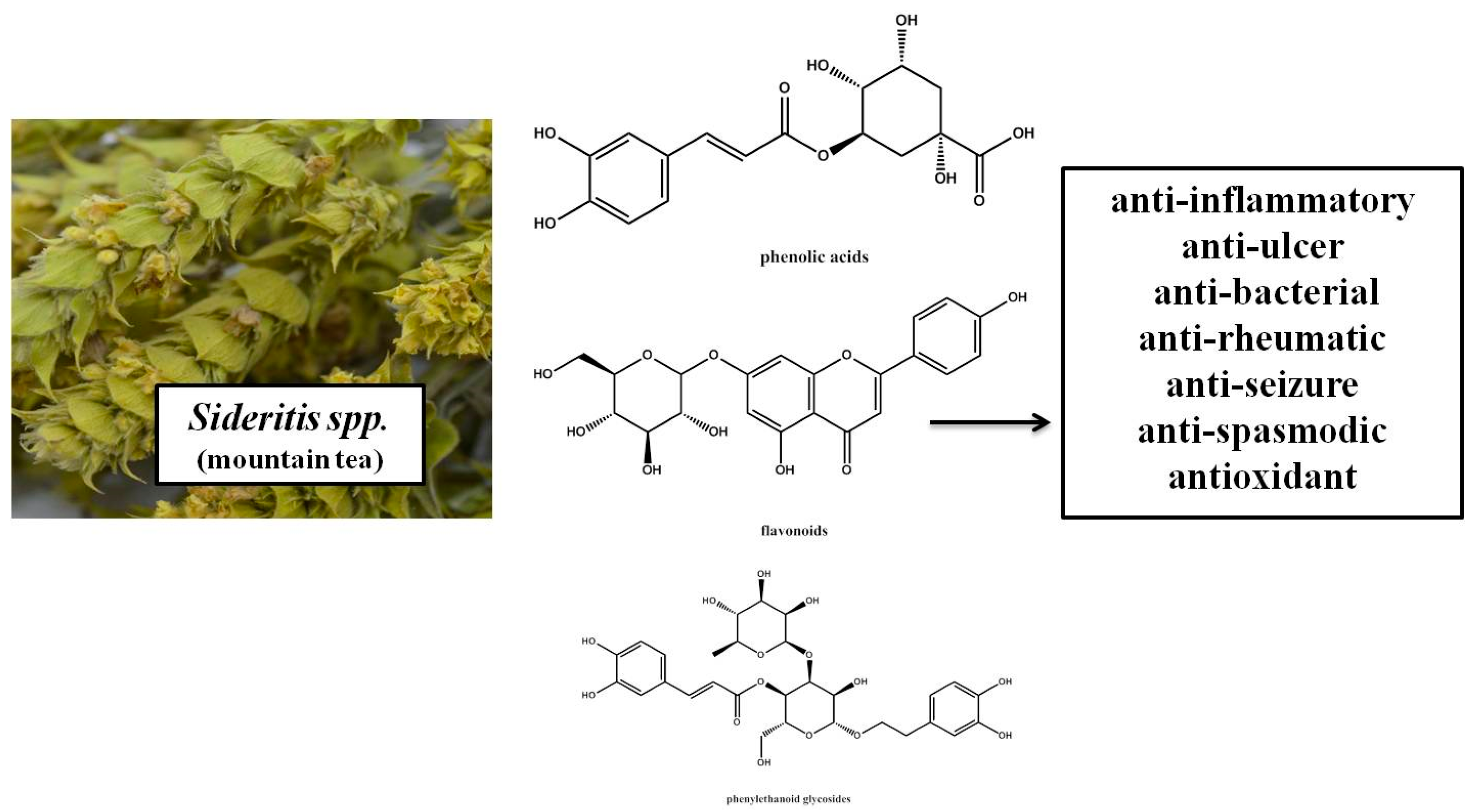
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
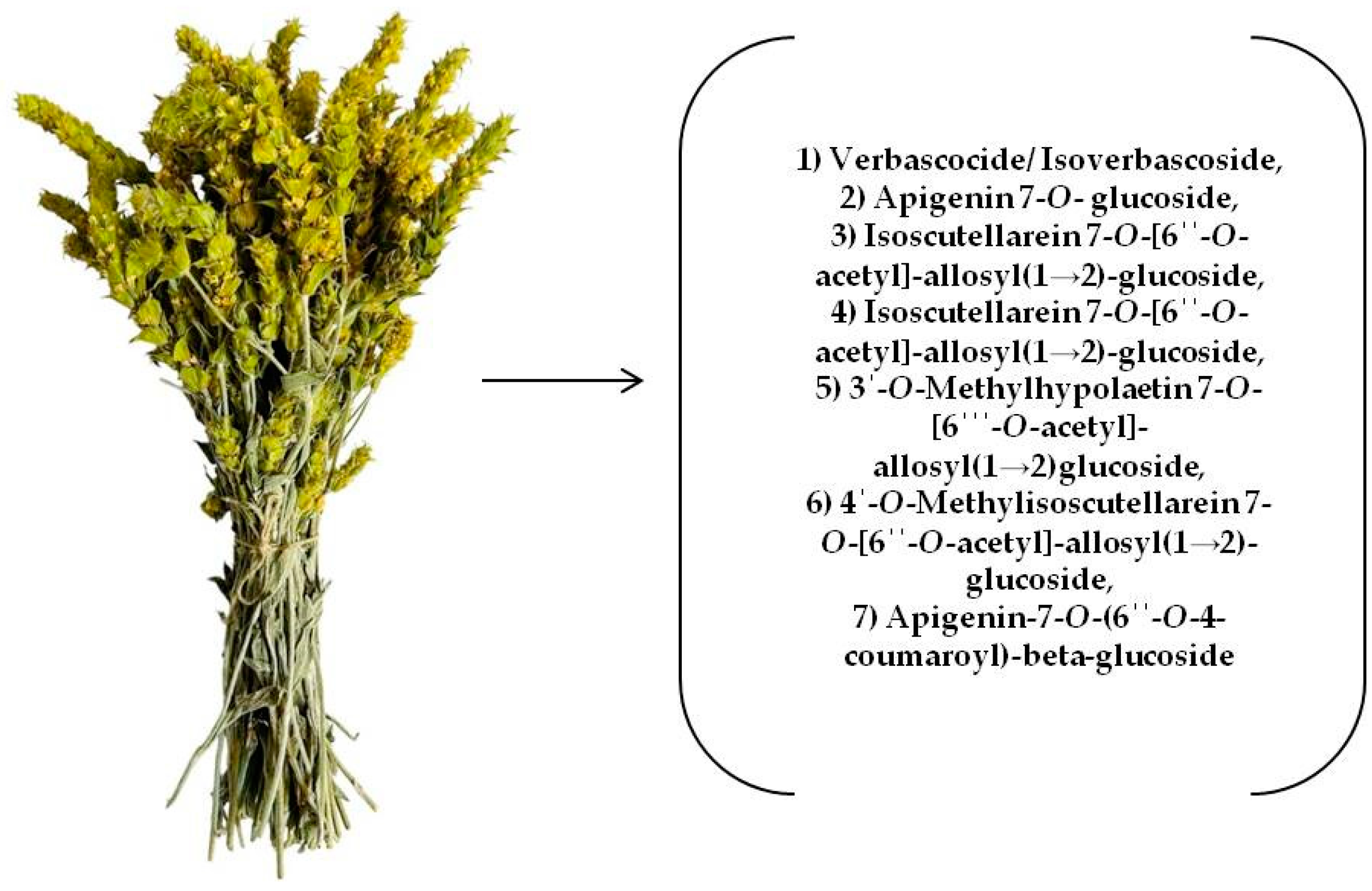
2.2. Plant Material
2.3. Preparation of Extracts
2.4. LC-MS/MS-QTOF
2.5. Identification of Phenolic Compounds and Statistical Analysis of LC-MS/MS-QTOF Data
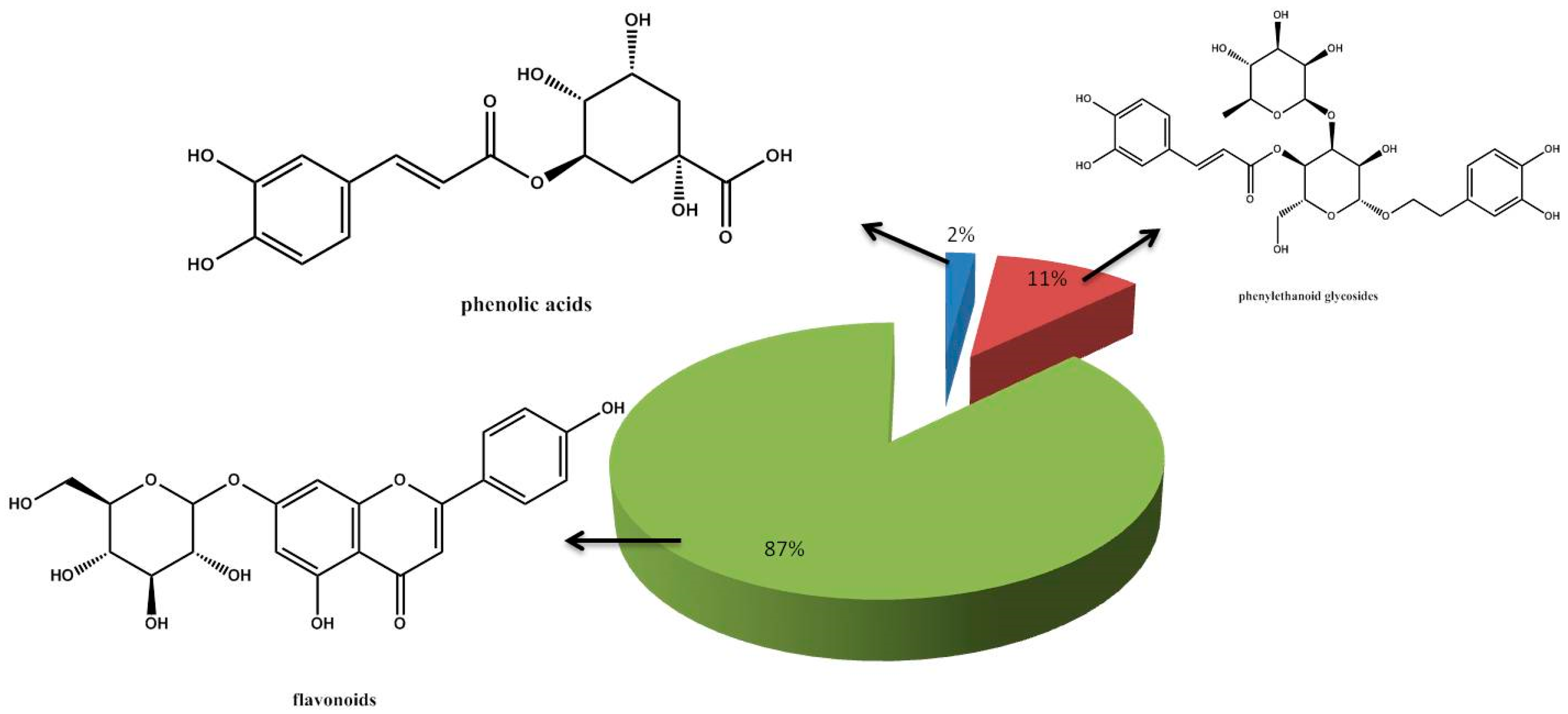
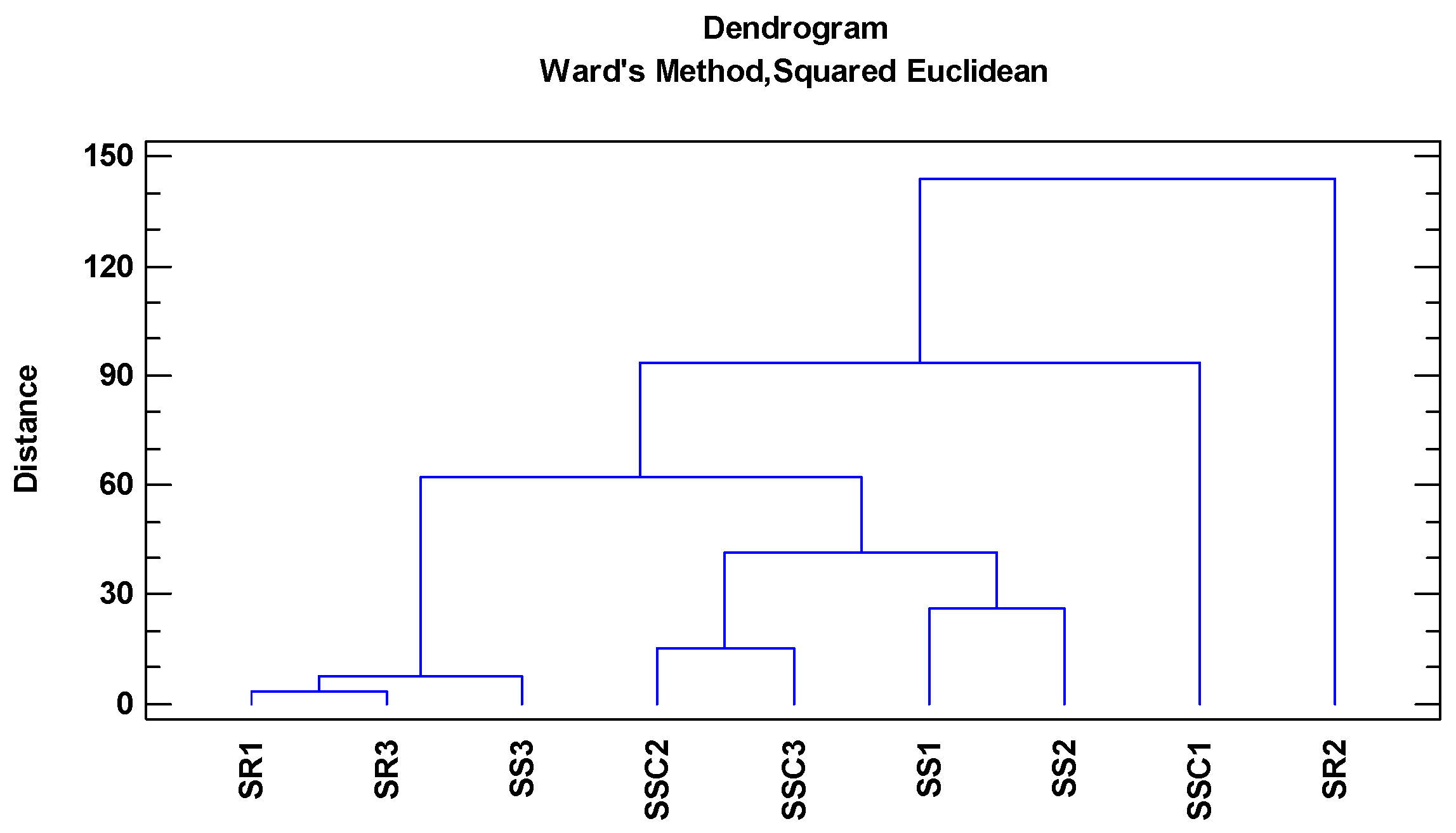
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lattanzio, V. Natural Products. Phenolic Compounds: Introduction; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. 10—Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In Engineering Tools in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 283–314. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from Medicinal and Aromatic Plants: Characterisation and Potential as Biostimulants and Bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; Volume 661, pp. 23–67. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in Plants. In Polyphenols in Plants Isolation Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 263–284. [Google Scholar]
- Costa, D.; Costa, H.; Albuquuerque, T.; Ramos, F.; Castilho, M.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicine 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifacetedcompounds: Bioactivity, industrial prospects and role of positive-stress. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Aghakhani, F.; Kharazian, N.; Gooini, Z.L. Flavonoid Constituents of Phlomis (Lamiaceae) Species Using Liquid Chromatography Mass Spectrometry. Phytochem. Anal. 2017, 29, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Milevskaya, V.V.; Temerdashev, Z.A.; Butyl’Skaya, T.S.; Kiseleva, N.V. Determination of phenolic compounds in medicinal plants from the Lamiaceae family. J. Anal. Chem. 2017, 72, 342–348. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in lamiaceae spices by lc-esi-ms/ms. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Van Puyvelde, L.; Mukazayire, M.J.; Gazim, Z.C. Editorial: Ethnopharmacology of the lamiaceae: Opportunities and challenges for developing new medicines. Front. Pharmacol. 2022, 13, 961486. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants. 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Ina Aneva, I.; Evstatieva, L.N. Chemotaxonomic contribution to the Sideritis species dilemma on the Balkans. Biochem. Syst. Ecol. 2015, 61, 477–487. [Google Scholar] [CrossRef]
- Kaparakou, E.H.; Daferera, D.; Kanakis, C.D.; Skotti, E.; Kokotou, M.G.; Tarantilis, P.A. Chemical composition of the essential oils of three popular Sideritis species cultivated in Greece using GC-MS analysis. Biomolecules 2023, 13, 1157. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. Herba Sideritis: A putative adaptogen for reducing the risk of age-related cognitive decline and neurodegenerative disorders. Phytomedicine Plus 2024, 4, 100519. [Google Scholar] [CrossRef]
- Bojovic, D.; Jankovic, S.; Potpara, Z.; Tadic, V. Summary of the phytochemical research performed to date on Sideritis species. Ser. J. Exp. Clin. Res. 2011, 12, 109–122. [Google Scholar] [CrossRef]
- Heywood, V.H. Flora Europaea: Notulae Systematicae ad Floram Europaeam spectantes: No. 12. Bot. J. Linn. Soc. 1972, 65, 223–269. [Google Scholar] [CrossRef]
- Hayek, A.V. Prodromus Florae peninsulae Balcanicae2, 192–197. Feddes Repert. Sp. Nov. Beih. 1929, 30. [Google Scholar]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Carretero, M.E.; Gòmez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; Angelis, A.; Percaccio, E.; Vitalone, A.; Gullì, M.; Macone, A.; Axiotis, E.; Skaltsounis, A.L. Phytochemical Composition and Cytoprotective Properties of the Endemic Sideritis sipylea Boiss Greek Species: A Valorization Study. Pharmaceuticals 2022, 15, 987. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and other bioactive compound s of Sideritis plants and their potential biological activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.; Oliva, A.; Božović, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [PubMed]
- Aligiannis, N.; Kalpoutzakis, E.; Chinou, I.; Mitakou, S.; Gikas, E.; Tsarbopoulos, A. Composition and antimicrobial activity of the essential oils of five taxa of Sideritis from Greece. J. Agric. Food Chem. 2001, 49, 811–815. [Google Scholar] [CrossRef] [PubMed]
- EMA/HMPC/39455/2015. Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba. (2016). Committee on Herbal Medicinal Products (HMPC). Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub-hayek-sideritis_en.pdf (accessed on 12 July 2023).
- Dimaki, V.D.; Zeliou, K.; Nakka, F.; Stavreli, M.; Bakratsas, I.; Papaioannou, L.; Iatrou, G.; Lamari, F.N. Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations. Molecules 2022, 27, 7613. [Google Scholar] [CrossRef]
- Mróz, M.; Malinowska-Pańczyk, E.; Bartoszek, A.; Kusznierewicz, B. Comparative Study on Assisted Solvent Extraction Techniques for the Extraction of Biologically Active Compounds from Sideritis raeseri and Sideritis scardica. Molecules 2023, 28, 4207. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Voynikov, Y.; Gevrenova, R.; Balabanova, V. A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules 2024, 29, 204. [Google Scholar] [CrossRef] [PubMed]
- Petreska, J.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Stefova, M. Phenolic Compounds of Mountain Tea from the Balkans: LC/DAD/ESI/MSn Profile and Content. Nat. Prod. Commun. 2011, 6, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, A.; Okada, Y.; Akkol, E.K.; Duman, H.; Okuyama, T.; Calış, İ. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010, 118, 686–692. [Google Scholar] [CrossRef]
- Tian, X.Y.; Li, M.; Lin, T.; Qiu, Y.; Zhu, Y.T.; Li, X.L.; Tao, W.D.; Wang, P.; Ren, X.X.; Chen, L.P. A Review on the Structure and Pharmacological Activity of Phenylethanoid Glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]

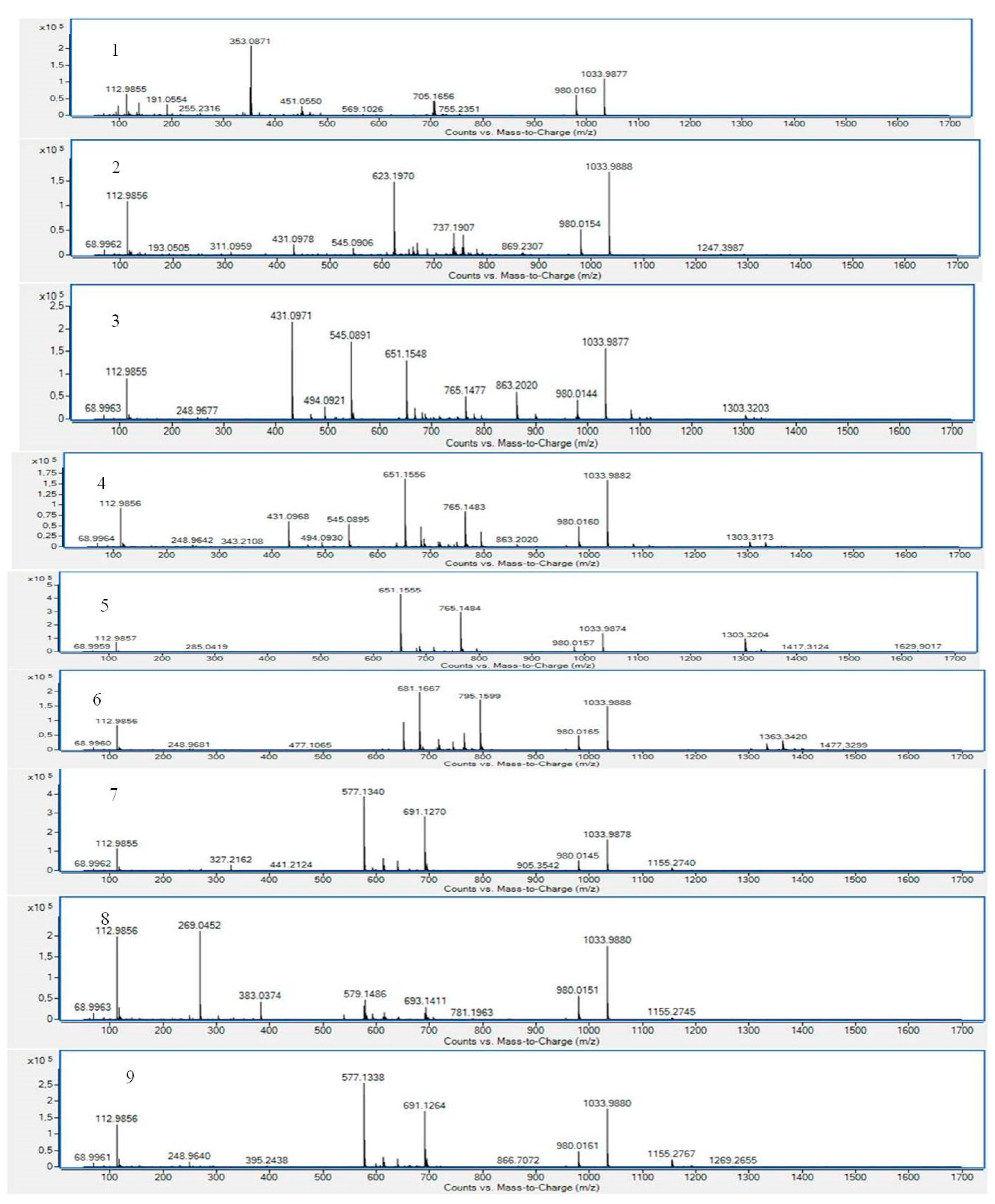
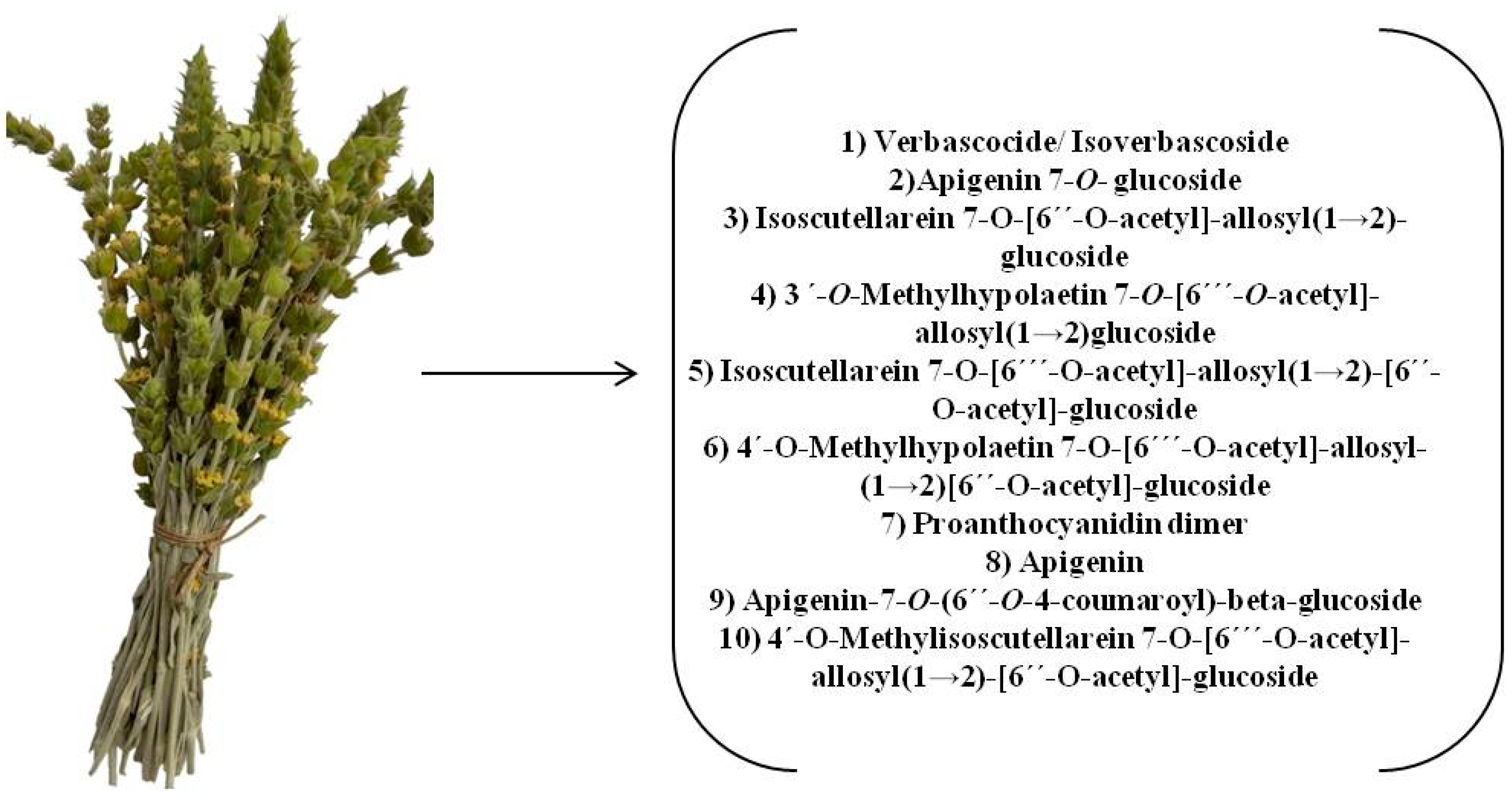
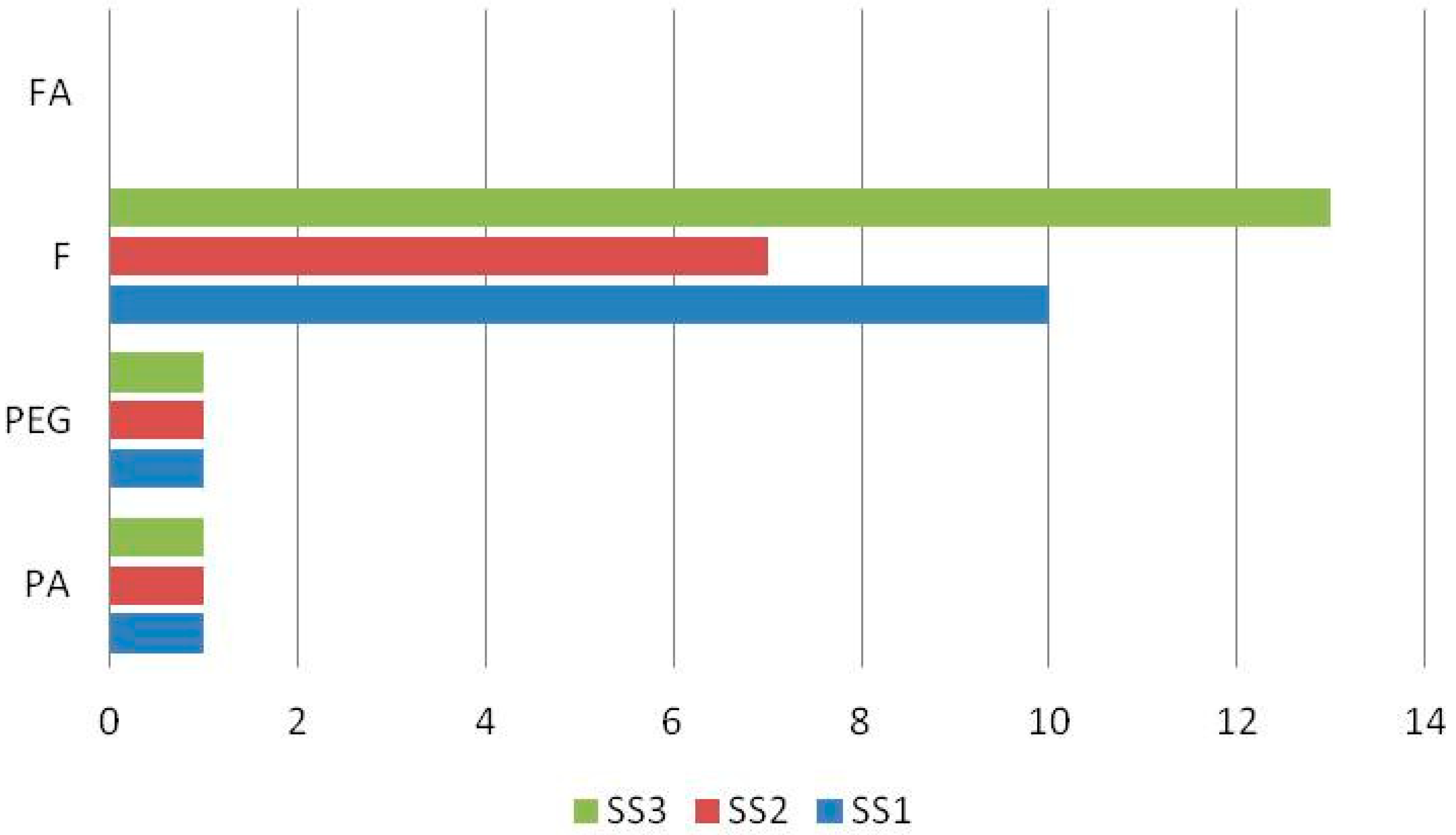
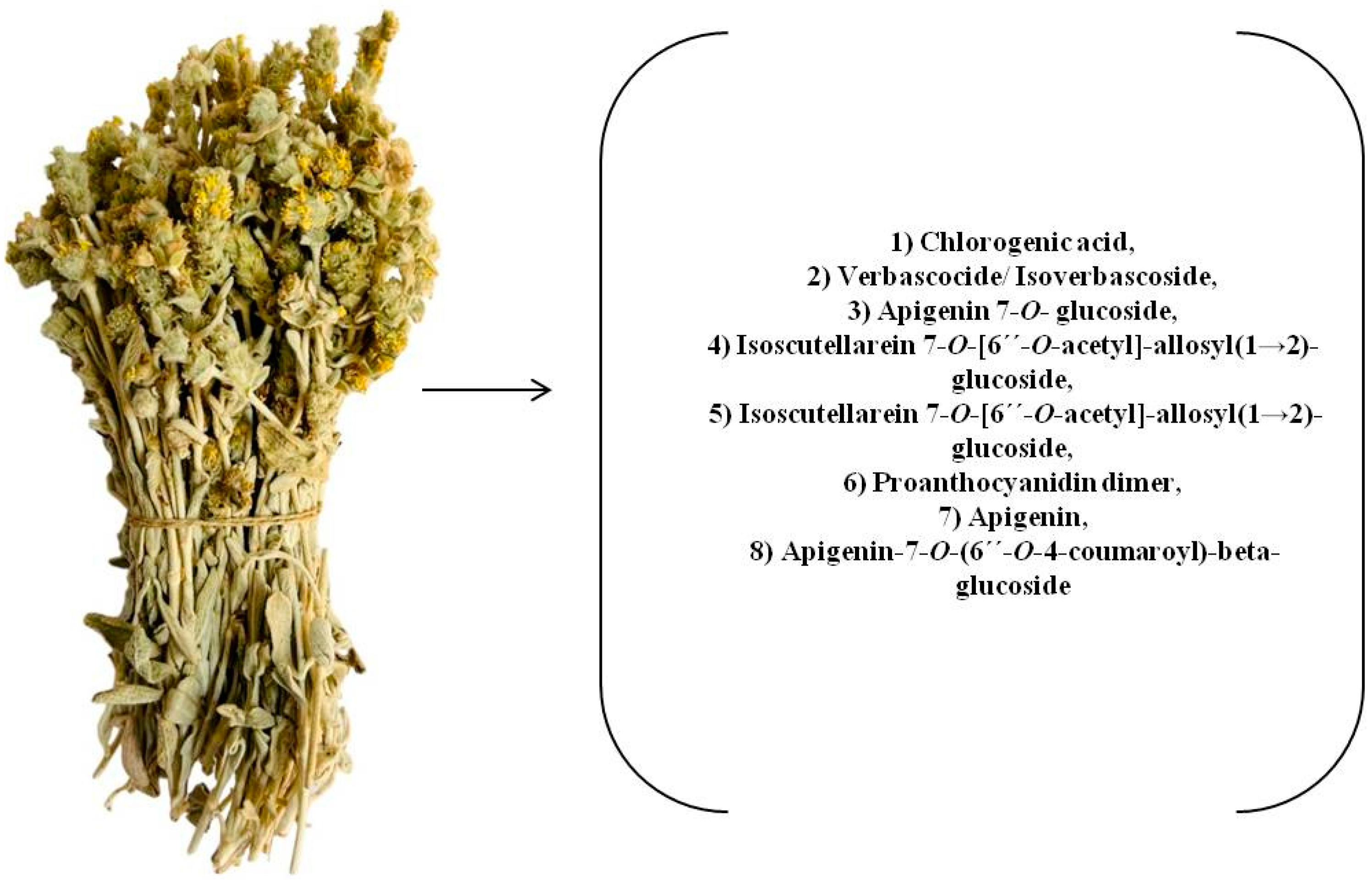
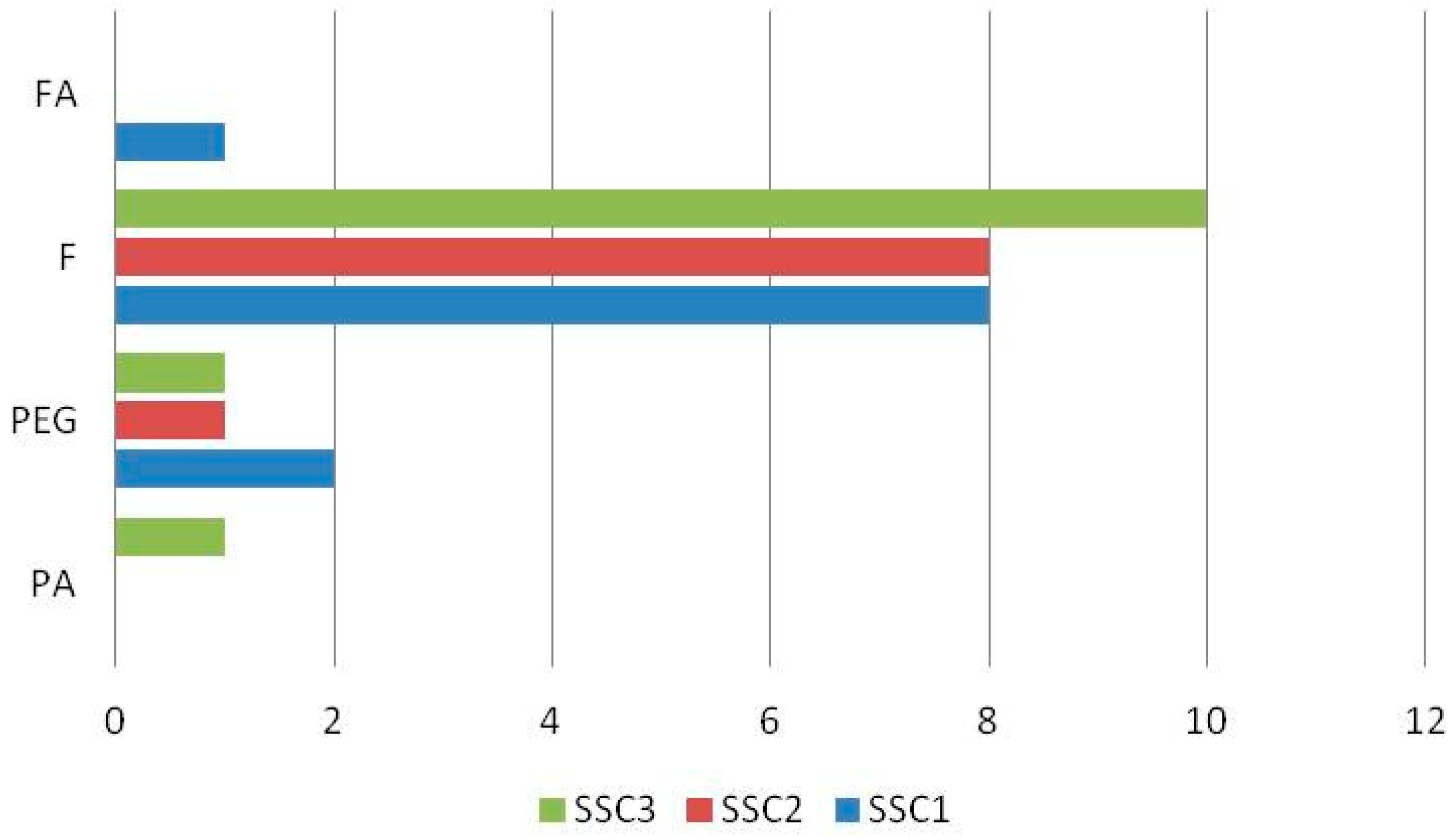


| No. | Compound | Retention Time (min) | Suggested Formula | Exact Mass [M−H]− | Fragmentation Pattern in (−) ESI-MS/MS | S. raeseri | S. scardica | S. syriaca | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR1 a | SR2 | SR3 | SSC1 | SSC2 | SSC3 | SS1 | SS2 | SS3 | |||||||
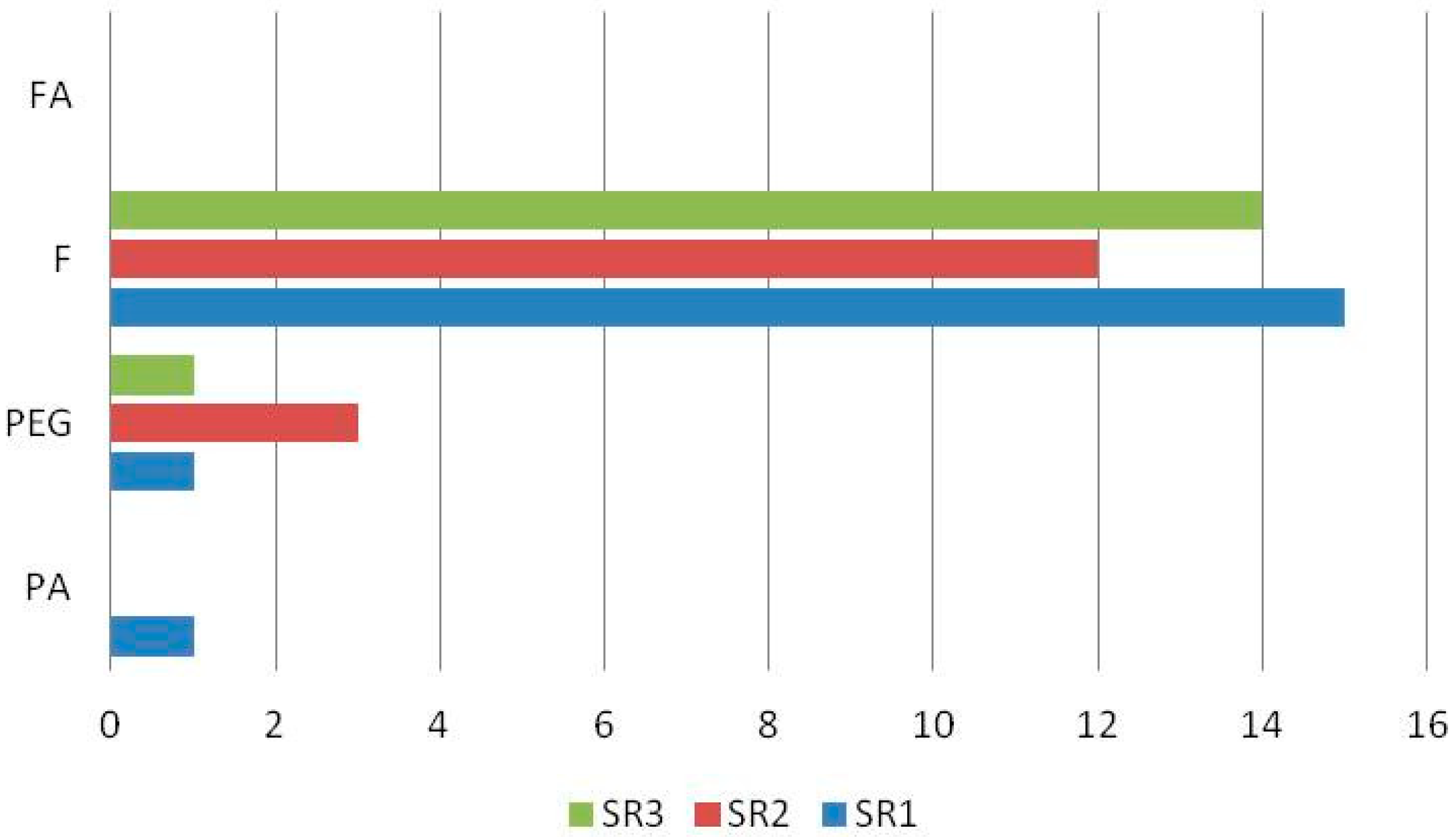
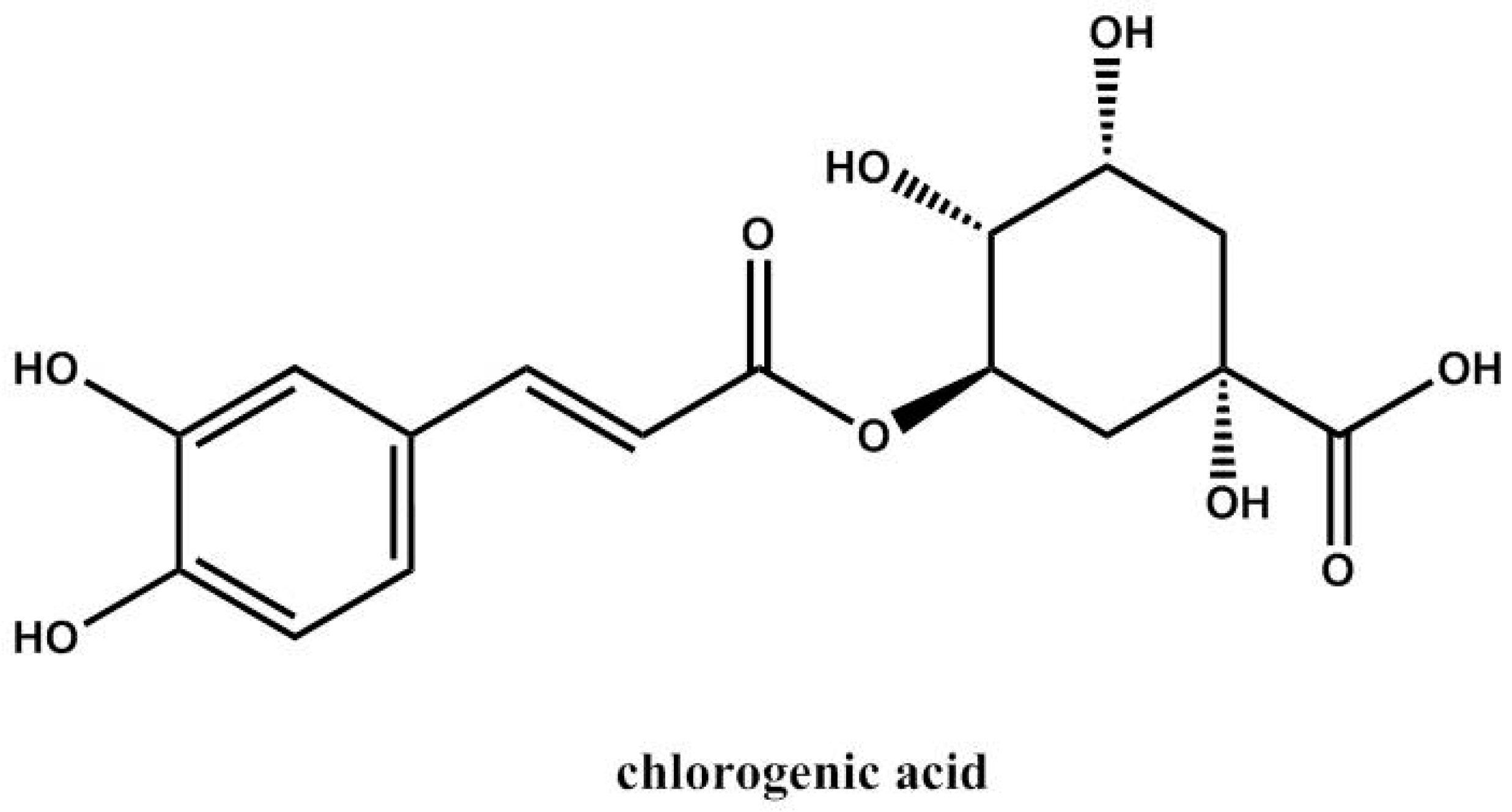
| 1 | Chlorogenic acid | 3.154 | C16H18O9 | 353.0871 | 191.0506 | + b | - c | - | - | - | + | + | + | + | [29,30,31] std |
| 2 | Forsythoside B/Lavandulifolioside | 5.755 | C34H44O19 | 755.2390 | 161.0227 | - | + | - | - | - | - | - | - | - | [15,25,29,30,31,32] |
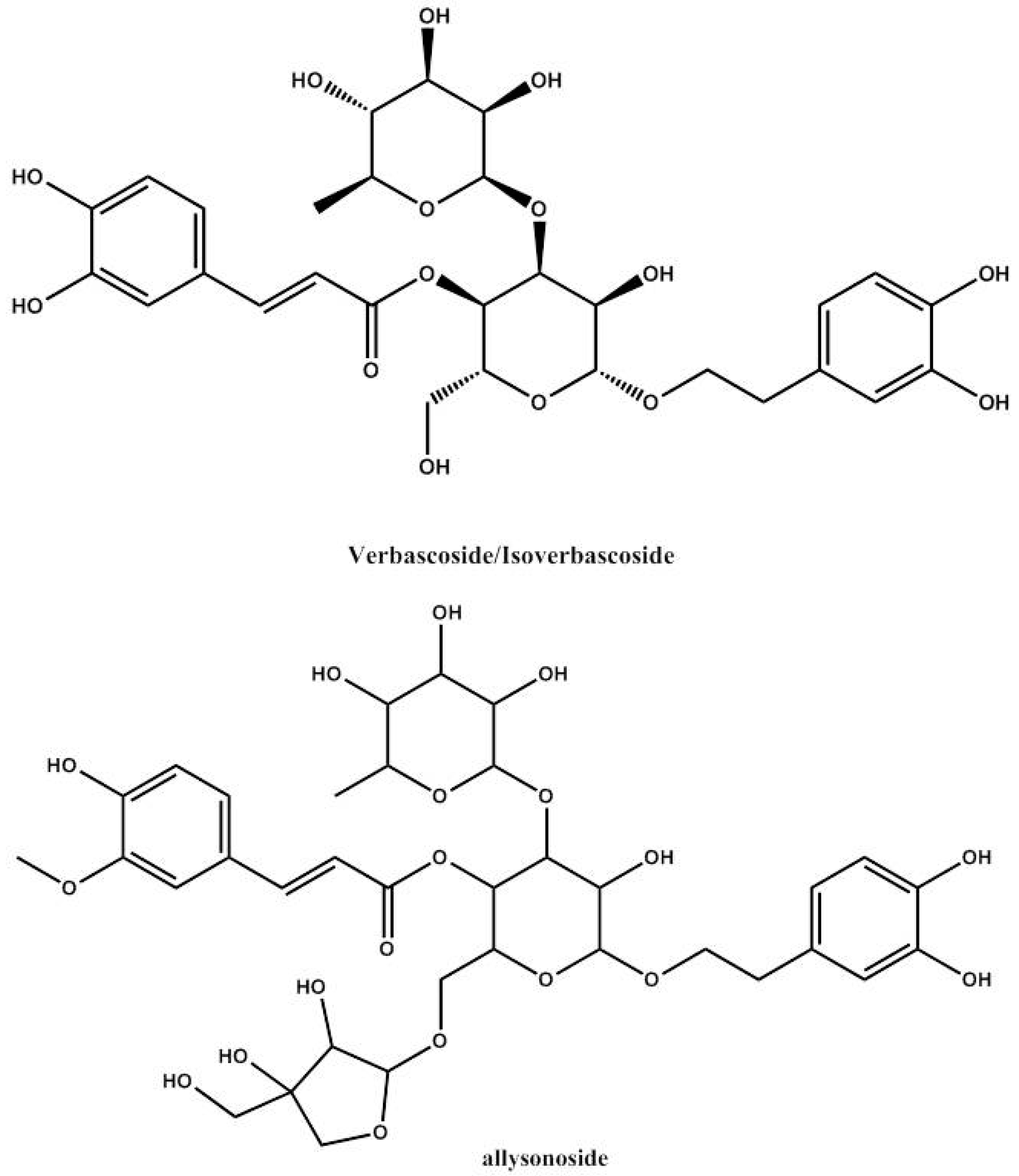
| 3 | Verbascocide/Isoverbascoside | 5.959 | C29H36O15 | 623.1970 | 161.0238 | + | + | + | + | + | + | + | + | + | [15,25,29,30,31,32] std |
| 4 | Allysonoside | 6.803 | C35H46O19 | 769.2544 | 175.0392 | - | + | - | - | - | - | - | - | - | [15,25,29,30,31,32] |
| 5 | Hypolaetin 7-O-[6‴-O-acetyl]-allosyl(1→2)-glucoside | 7.039 | C29H32O18 | 667.1507 | 301.0341 | - | + | - | - | - | - | - | - | - | [15,25,29,30,31,32] |
| 6 | Apigenin 7-O-glucoside | 7.336 | C21H20O10 | 431.0971 | 268.0393 | + | + | + | + | + | + | + | + | + | [15,25,30,31,32] |
| 7 | Isoscutellarein 7-O-[6′-O-acetyl]-allosyl(1→2)-glucoside | 7.412 | C29H32O17 | 651.1556 | 285.0396 | + | - | + | + | + | + | + | + | + | [15,25,29,30,31,32] |
| 8 | 4′-O-Methylhypolaetin7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | 7.749 | C30H34O18 | 681.1660 | 315.0510 | + | + | - | + | + | - | - | - | - | [15,29,30,31,32] |
| 9 | Isoscutellarein 7-O-[6″-O-acetyl]-allosyl(1→2)-glucoside | 7.994 | C29H32O17 | 651.1555 | 285.0394 | + | + | + | + | + | + | + | + | + | [15,25,29,30,31,32] |
| 10 | 3′-O-Methylhypolaetin 7-O-[6‴--O-acetyl]-allosyl(1→2)glucoside | 8.289 | C30H34O18 | 681.1667 | 315.0485 | + | + | + | + | + | + | - | + | + | [15,25,29,30,32] |
| 11 | Martynoside | 8.533 | C31H40O15 | 651.1971 | 175.0419 | - | - | - | + | - | - | - | - | - | [25,30,31] |
| 12 | 4′-O-Methylisoscutellarein 7-O-allosyl(1→2)glucoside | 8.662 | C28H32O16 | 623.1608 | 299.0553 | + | - | + | - | - | - | - | - | + | [15,25,30,31,32] |
| 13 | Hypolaetin 7-O-[2‴,6″-di-O-acetyl]-allosyl(1→2)glucoside | 9.405 | C31H34O19 | 709.1619 | 301.0307 | - | + | - | - | - | - | - | - | - | [15,29,30,32] |
| 14 | 4′-O-Methylisoscutellarein 7-O-[6″-O-acetyl]-allosyl(1→2)-glucoside | 10.085 | C30H34O17 | 665.1718 | 299.0554 | + | - | + | + | + | + | - | - | + | [15,25,29,30,31,32] |
| 15 | Isoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside | 10.386 | C31H34O18 | 693.1659 | 285.0398 | + | + | + | - | + | + | + | - | + | [25,29,30,31,32] |
| 16 | 4′-O-Methylhypolaetin 7-O-[6‴-O-acetyl]-allosyl-(1→2)[6″-O-acetyl]-glucoside | 10.622 | C32H36O19 | 723.1772 | 315.0518 | + | + | + | - | - | - | - | - | - | [15,25,29,30,31,32] |
| 17 | Proanthocyanidin dimer | 10.926 | C30H26O12 | 577.1340 | 269.0453 | + | + | + | - | - | - | + | + | + | [30] |
| 18 | Apigenin | 11.469 | C15H10O5 | 269.0452 | 151.0030 | + | + | + | - | - | + | + | + | + | std |
| 19 | Trihydroxy octadecenoic acid | 11.884 | C18H34O5 | 329.2327 | 211.1346 | - | - | - | + | - | - | - | - | - | [30,31] |
| 20 | Apigenin-7-O-(6″-O-4-coumaroyl)-beta-glucoside | 11.997 | C30H26O12 | 577.1338 | 269.0442 | + | + | + | + | + | + | + | + | + | [15,25,30,31,32] |
| 21 | 4′-O-Methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)-[6″-O-acetyl]-glucoside | 12.412 | C32H36O18 | 707.1821 | 299.0553 | + | + | + | - | - | - | + | - | + | [15,25,30,31,32] |
| 22 | Cirsimaritin | 13.559 | C17H14O6 | 313.0714 | 283.0251 | + | - | + | - | - | + | + | - | + | [30] |
| 23 | Usnicacid/Eupatorin | 14.509 | C18H16O7 | 343.0816 | 313.0350 | + | - | + | + | - | + | + | - | + | [30] |
| Compound | Structure | Activity | Ref. |
|---|---|---|---|
| Verbascoside/ Isoverbascoside | 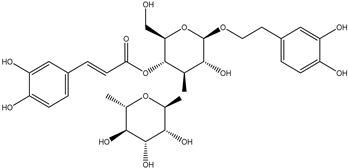 | anti-inflammatory activity prevention of red blood cell from free radical damage tyrosinase and/or melanin production inhibition activity | [22,23,25] |
| Apigenin-7-O-glucoside |  | antioxidant activity anti-inflammatory effect cytotoxicity to cancer cells promoting apoptosis of cancer cells anxiolytic effect memory improvement neuroprotective effect protective effect against amyloid-β-neurotoxicity | [25,26] |
| Isoscutellarein 7-O-[6″-O-acetyl]-allosyl(1→2)-glucoside | 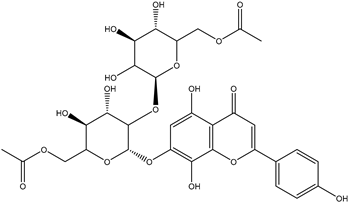 | moderate-to-weak cytotoxicity to cancer cells | [25] |
| Apigenin-7-O-(6″-O-4-cou-maroyl)-beta-glucoside | 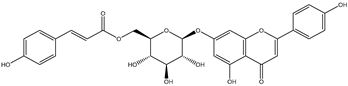 | antioxidant activity anti-inflammatory effect cytotoxicity to cancer cells promoting apoptosis of cancer cells anxiolytic effect memory improvement neuroprotective effect protective effect against amyloid-β-neurotoxicity | [25,26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaparakou, E.H.; Kanakis, C.D.; Kokotou, M.G.; Papadopoulos, G.; Tarantilis, P.A. LC-MS/MS-QTOF Identification of Phenolic Compounds of Sideritis Species Cultivated in Greece. Separations 2024, 11, 229. https://doi.org/10.3390/separations11080229
Kaparakou EH, Kanakis CD, Kokotou MG, Papadopoulos G, Tarantilis PA. LC-MS/MS-QTOF Identification of Phenolic Compounds of Sideritis Species Cultivated in Greece. Separations. 2024; 11(8):229. https://doi.org/10.3390/separations11080229
Chicago/Turabian StyleKaparakou, Eleftheria H., Charalabos D. Kanakis, Maroula G. Kokotou, Georgios Papadopoulos, and Petros A. Tarantilis. 2024. "LC-MS/MS-QTOF Identification of Phenolic Compounds of Sideritis Species Cultivated in Greece" Separations 11, no. 8: 229. https://doi.org/10.3390/separations11080229
APA StyleKaparakou, E. H., Kanakis, C. D., Kokotou, M. G., Papadopoulos, G., & Tarantilis, P. A. (2024). LC-MS/MS-QTOF Identification of Phenolic Compounds of Sideritis Species Cultivated in Greece. Separations, 11(8), 229. https://doi.org/10.3390/separations11080229









