Methods of Analysis of Phytoestrogenic Compounds: An Up-to-Date of the Present State
Abstract
1. Introduction
2. Methods
3. Characterization and Classification of Phytoestrogens
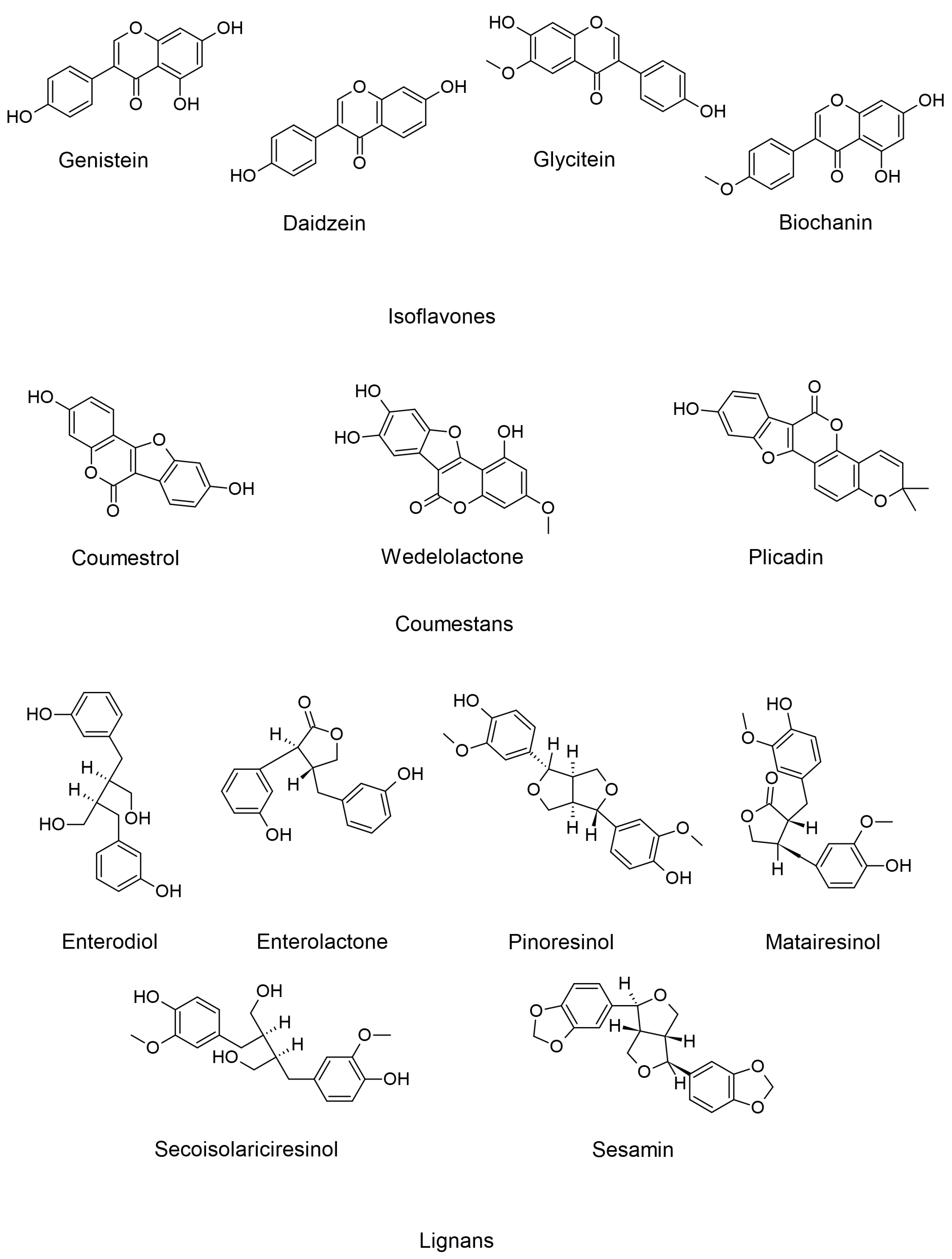
3.1. Isoflavones
3.2. Coumestans
3.3. Lignans
3.4. Possible Mechanisms of Action
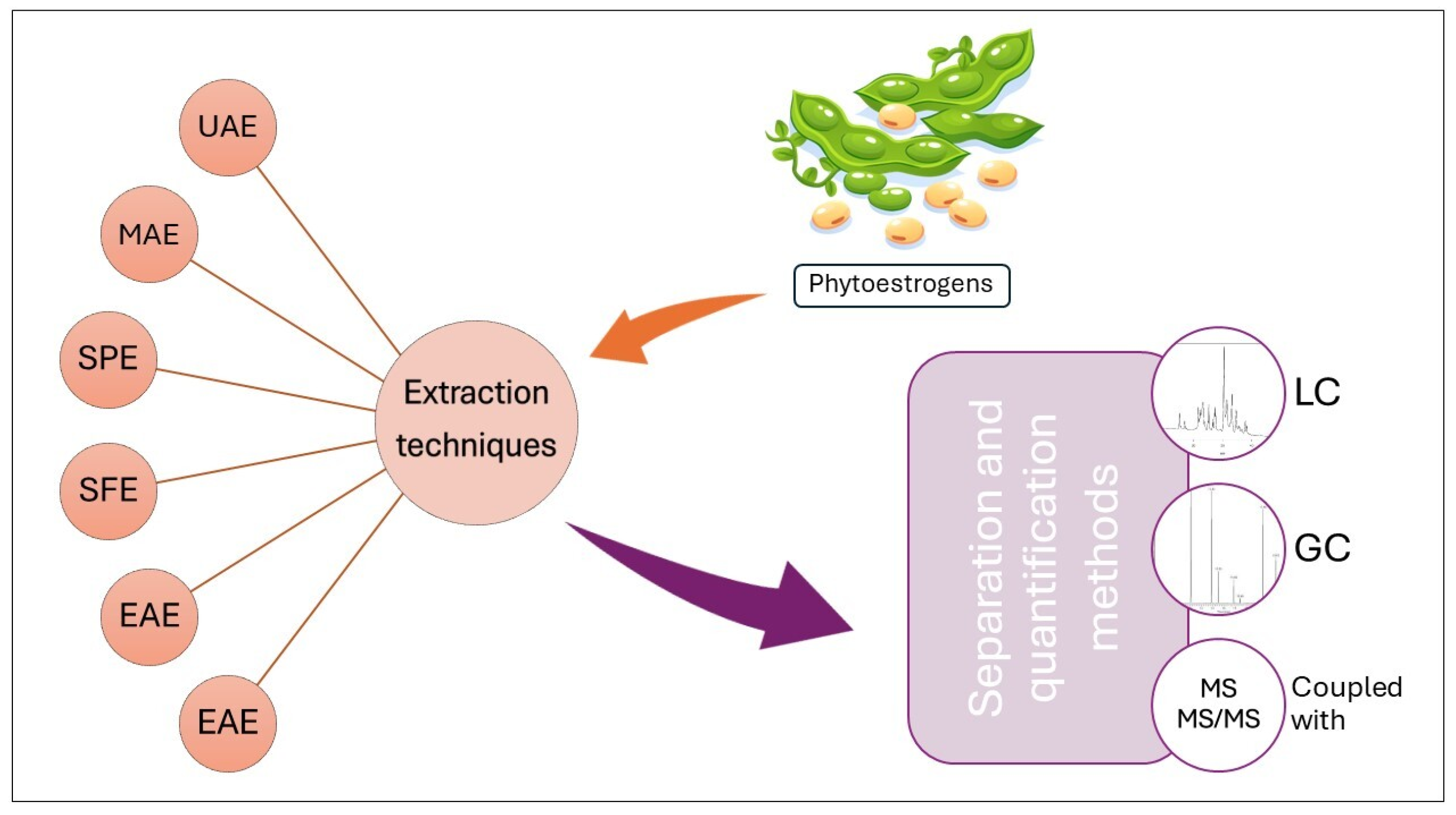
4. Phytoestrogens Analysis from Plant Material
5. Phytoestrogens Analysis in Food
5.1. Extraction of Phytoestrogens from Food Matrix
5.2. Analytical Techniques for Phytoestrogens in Food Matrix
5.2.1. Gas Chromatography
5.2.2. Liquid Chromatography
5.2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
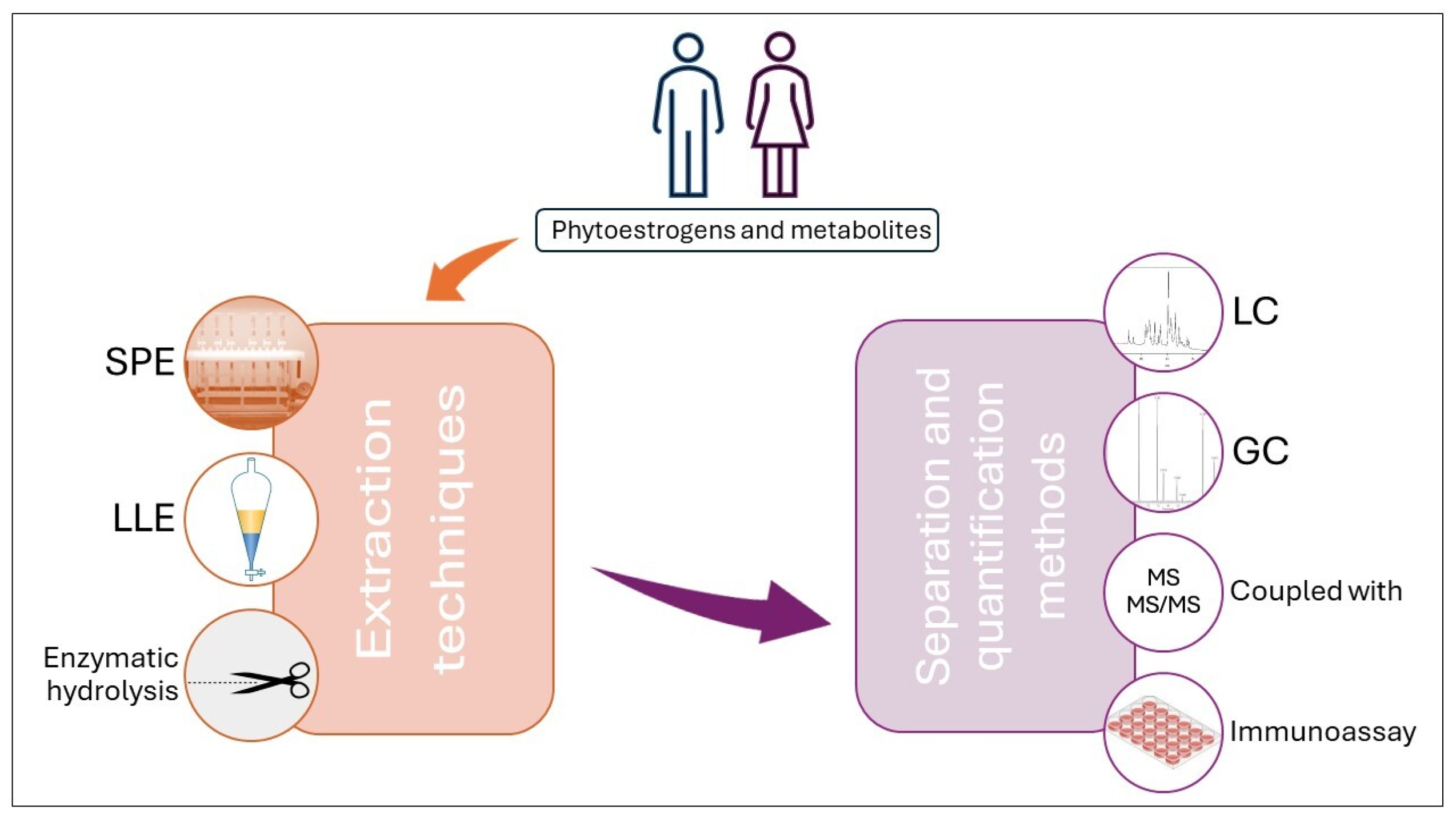
6. Phytoestrogens Analysis in Biological Samples
6.1. Liquid Chromatography
6.2. Liquid Chromatography Coupled with Mass Spectrometry Methods
6.3. Gas Chromatography
6.4. Immunoassay
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chavda, V.P.; Chaudhari, A.Z.; Balar, P.C.; Gholap, A.; Vora, L.K. Phytoestrogens: Chemistry, potential health benefits, and their medicinal importance. Phytother. Res. 2024, 38, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Cvejić, J.; Bursać, M.; Atanacković, M. Phytoestrogens: “estrogene-like” phytochemicals. Stud. Nat. Prod. Chem. 2012, 38, 1–35. [Google Scholar]
- Wang, C.-C.; Prasain, J.K.; Barnes, S. Review of the methods used in the determination of phytoestrogens. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.P.; Wähälä, K.; Williamson, G. Identification and quantification of polyphenol phytoestrogens in foods and human biological fluids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 93–109. [Google Scholar] [CrossRef]
- Hoikkala, A.A.; Schiavoni, E.; Wähälä, K. Analysis of phyto-oestrogens in biological matrices. Br. J. Nutr. 2003, 89 (Suppl. 1), S5–S18. [Google Scholar] [CrossRef] [PubMed]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Barnes, S.L.; Lee, M.M.; Coward, L.H.; Mandel, E.M.; Koo, J.; John, E.M.; Smith, M.S. Phytoestrogen content of foods consumed in the United States. J. Natl. Cancer Inst. 2000, 92, 134–146. [Google Scholar]
- Rostagno, M.A.; Villares, A.; Guillamón, E.; García-Lafuente, A.; Martínez, J.A. Sample preparation for the analysis of isoflavones from soybeans and soy foods. J. Chromatogr. A 2009, 1216, 2–29. [Google Scholar] [CrossRef]
- Moors, S.; Blaszkewicz, M.; Bolt, H.M.; Degen, G.H. Simultaneous determination of daidzein, equol, genistein and bisphenol A in human urine by a fast and simple method using SPE and GC-MS. Mol. Nutr. Food Res. 2007, 51, 787–798. [Google Scholar] [CrossRef]
- Nørskov, N.P.; Kyrø, C.; Olsen, A.; Tjønneland, A.; Knudsen, K.E. High-throughput LC-MS/MS method for direct quantification of glucuronidated, sulfated, and free enterolactone in human plasma. J. Proteome Res. 2016, 15, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Shi, H.; Hewett, J.E.; Ruhlen, R.L.; MacDonald, R.S.; Rottinghaus, G.E.; Chen, Y.C.; Sauter, E.R. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef]
- Lóránd, T.; Vigh, E.; Garai, J. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: Phytoestrogens and xenoestrogens. Curr. Med. Chem. 2010, 17, 3542–3574. [Google Scholar] [CrossRef]
- Reed, K.F.M. Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species. Agriculture 2016, 6, 35. [Google Scholar] [CrossRef]
- Daly, D.C.; Cameron, K.M.; Stevenson, D.W. Plant systematics in the age of genomics. Plant Physiol 2001, 127, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Ibarreta, D.; Daxenberger, A.; Meyer, H.H. Possible health impact of phytoestrogens and xenoestrogens in food. APMIS 2001, 109, 161–184. [Google Scholar] [CrossRef]
- Jung, Y.S.; Rha, C.S.; Baik, M.Y.; Baek, N.I.; Kim, D.O. A brief history and spectroscopic analysis of soy isoflavones. Food Sci. Biotechnol. 2020, 29, 1605–1617. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones derived from plant raw materials: Bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 2023, 63, 261–287. [Google Scholar] [CrossRef]
- Popa, D.-S.; Rusu, M.E.; Popa, D.-S.; Rusu, M.E. Isoflavones: Vegetable sources, biological activity, and analytical methods for their assessment. In Superfood and Functional Food-The Development of Superfoods and Their Roles as Medicine; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Alipour, M.R.; Karimi-Sales, E. Molecular mechanisms of protective roles of isoflavones against chemicals-induced liver injuries. Chem. Biol. Interact. 2020, 329, 109213. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.J. Food sources of phyto-oestrogens and their precursors in Europe. Br. J. Nutr. 2003, 89 (Suppl. 1), S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Delmonte, P.; Rader, J.I. Analysis of isoflavones in foods and dietary supplements. J. AOAC Int. 2006, 89, 1138–1146. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, Y.; Li, Y.; He, C. Naturally occurring coumestans from plants, their biological activities and therapeutic effects on human diseases. Pharmacol. Res. 2021, 169, 105615. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kumar, A.; Raghuvanshi, A. Synthesis, stereochemistry, structural classification, and chemical reactivity of natural pterocarpans. Chem. Rev. 2013, 113, 1614–1640. [Google Scholar] [CrossRef] [PubMed]
- Murkies, A.L.; Wilcox, G.; Davis, S.R. Clinical review 92: Phytoestrogens. J. Clin. Endocrinol. Metab. 1998, 83, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, S.; Chiodelli, G.; Rocchetti, G.; Kane, D.; Lucini, L. UHPLC-ESI-QTOF-MS screening of lignans and other phenolics in dry seeds for human consumption. J. Funct. Foods 2017, 34, 229–236. [Google Scholar] [CrossRef]
- Liggins, J.; Grimwood, R.; Bingham, S.A. Extraction and quantification of lignan phytoestrogens in food and human samples. Anal. Biochem. 2000, 287, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Kreft, S. Gut Microbiota and the Metabolism of Phytoestrogens. Rev. Bras. De Farmacogn. 2020, 30, 145–154. [Google Scholar] [CrossRef]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Muñoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- Wang, L.Q. Mammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Singh, S.; Naseem, I. Cytotoxic activity of soy phytoestrogen coumestrol against human breast cancer MCF-7 cells: Insights into the molecular mechanism. Food Chem. Toxicol. 2017, 99, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. the potential effects of phytoestrogens: The role in neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef] [PubMed]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Ji, Z.N.; Zhao, W.Y.; Liao, G.R.; Choi, R.C.; Lo, C.K.; Dong, T.T.; Tsim, K.W. In vitro estrogenic activity of formononetin by two bioassay systems. Gynecol. Endocrinol. 2006, 22, 578–584. [Google Scholar] [CrossRef]
- Sicilia, T.; Niemeyer, H.B.; Honig, D.M.; Metzler, M. Identification and stereochemical characterization of lignans in flaxseed and pumpkin seeds. J. Agric. Food Chem. 2003, 51, 1181–1188. [Google Scholar] [CrossRef]
- Mazur, W.M.; Wähälä, K.; Rasku, S.; Salakka, A.; Hase, T.; Adlercreutz, H. Lignan and isoflavonoid concentrations in tea and coffee. Br. J. Nutr. 1998, 79, 37–45. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Murphy, P.A.; Barua, K.; Hauck, C.C. Solvent extraction selection in the determination of isoflavones in soy foods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, X.; Zhang, Y.; Sheng, X. Molecular interaction between MeOH and genistein during soy extraction. RSC Adv. 2019, 9, 39170–39179. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.P.; Brighenti, V.; Rodolfi, M.; Mongelli, A.; dall’Asta, C.; Ganino, T.; Bruni, R.; Pellati, F. Development of a new high-performance liquid chromatography method with diode array and electrospray ionization-mass spectrometry detection for the metabolite fingerprinting of bioactive compounds in Humulus lupulus L. J. Chromatogr. A 2014, 1349, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ceslová, L.; Holcapek, M.; Fidler, M.; Drsticková, J.; Lísa, M. Characterization of prenylflavonoids and hop bitter acids in various classes of Czech beers and hop extracts using high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Buckett, L.; Schinko, S.; Urmann, C.; Riepl, H.; Rychlik, M. Stable isotope dilution analysis of the major prenylated flavonoids found in beer, hop tea, and hops. Front. Nutr. 2020, 7, 619921. [Google Scholar] [CrossRef]
- Kac, J.; Zakrajsek, J.; Mlinaric, A.; Kreft, S.; Filipic, M. Determination of xanthohumol in hops (Humulus lupulus L.) by nonaqueous CE. Electrophoresis 2007, 28, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.E.; Hall, C.; Kubátová, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, J.P.; Xu, S.P.; Wang, J.H.; Liu, X. Separation and determination of secoisolariciresinol diglucoside oligomers and their hydrolysates in the flaxseed extract by high-performance liquid chromatography. J. Chromatogr. A 2008, 1185, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of beverages, nuts, seeds, and oils. J. Agric. Food Chem. 2008, 56, 7311–7315. [Google Scholar] [CrossRef]
- Angeloni, S.; Navarini, L.; Khamitova, G.; Sagratini, G.; Vittori, S.; Caprioli, G. Quantification of lignans in 30 ground coffee samples and evaluation of theirs extraction yield in espresso coffee by HPLC-MS/MS triple quadrupole. Int. J. Food Sci. Nutr. 2020, 71, 193–200. [Google Scholar] [CrossRef]
- Hloucalová, P.; Skládanka, J.; Horký, P.; Klejdus, B.; Pelikán, J.; Knotová, D. Determination of phytoestrogen content in fresh-cut legume forage. Animals 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, M.; Simon, J.E. Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J. Chromatogr. A 2003, 1016, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Unterrieder, I.; Ruprechter, R. Quantification of isoflavones in red clover by high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 123–128. [Google Scholar] [CrossRef] [PubMed]
- De Rijke, E.; Zafra-Gómez, A.; Ariese, F.; Brinkman, U.A.; Gooije, C. Determination of isoflavone glucoside malonates in Trifolium pratense L. (red clover) extracts: Quantification and stability studies. J. Chromatogr. A 2001, 932, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, J.; Kleinová, T.; Loučka, R. The determination of coumestrol in alfalfa (Medicago sativa) by capillary electrophoresis. Rostl. Vyrob. 2002, 48, 224–229. [Google Scholar] [CrossRef]
- Soto-Zarazúa, M.G.; Rodrigues, F.; Pimentel, F.B.; Bah, M.M.; Oliveira, M.B. The isoflavone content of two new alfalfa-derived products for instant beverage preparation. Food Funct. 2016, 7, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Uchiyama, H.; Yusakul, G.; Kyokong, N.; Pongkitwitoon, B.; Putalun, W.; Tanaka, H.; Morimoto, S. Open sandwich fluorescence-linked immunosorbent assay for detection of soy isoflavone glycosides. Food Chem. 2021, 361, 129829. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Yusakul, G.; Pongkitwitoon, B.; Paudel, M.K.; Tanaka, H.; Morimoto, S. Simultaneous determination of soy isoflavone glycosides, daidzin and genistin by monoclonal antibody-based highly sensitive indirect competitive enzyme-linked immunosorbent assay. Food Chem. 2015, 169, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Yusakul, G.; Pongkitwitoon, B.; Tanaka, H.; Morimoto, S. Colloidal gold-based indirect competitive immunochromatographic assay for rapid detection of bioactive isoflavone glycosides daidzin and genistin in soy products. Food Chem. 2016, 194, 191–195. [Google Scholar] [CrossRef]
- Fiechter, G.; Opacak, I.; Raba, B.; Mayer, H.K. A new ultra-high pressure liquid chromatography method for the determination of total isoflavone aglycones after enzymatic hydrolysis: Application to analyze isoflavone levels in soybean cultivars. Food Res. Int. 2013, 50, 586–592. [Google Scholar] [CrossRef]
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745. [Google Scholar] [CrossRef]
- Arráez-Román, D.; Cortacero-Ramírez, S.; Segura-Carretero, A.; Martín-Lagos Contreras, J.A.; Fernández-Gutiérrez, A. Characterization of the methanolic extract of hops using capillary electrophoresis-electrospray ionization-mass spectrometry. Electrophoresis 2006, 27, 2197–2207. [Google Scholar] [CrossRef]
- Fritsche, J.; Angoelal, R.; Dachtler, M. On-line liquid-chromatography–nuclear magnetic resonance spectroscopy–mass spectrometry coupling for the separation and characterization of secoisolariciresinol diglucoside isomers in flaxseed. J. Chromatogr. A 2002, 972, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Beck, V.; Rohr, U.; Jungbauer, A. Phytoestrogens derived from red clover: An alternative to estrogen replacement therapy? J. Steroid Biochem. Mol. Biol. 2005, 94, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Wyse, J.M.; Latif, S.; Gurusinghe, S.; Berntsen, E.D.; Weston, L.A.; Stephen, C.P. Characterization of phytoestrogens. Metabolites 2021, 11, 550. [Google Scholar] [CrossRef]
- Tucak, M.; Horvat, D.; Cupic, T.; Krizmanic, G.; Tomas, V.; Ravlic, M.; Popovic, S. Forage legumes as sources of bioactive phytoestrogens for use in pharmaceutics: A review. Curr. Pharm. Biotechnol. 2018, 19, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tucak, M.; Čupić, T.; Horvat, D.; Popović, S.; Krizmanić, G.; Ravlić, M. Variation of phytoestrogen content and major agronomic traits in alfalfa (Medicago sativa l.) populations. Agronomy 2020, 10, 87. [Google Scholar] [CrossRef]
- Bettaiah, A.; Prabhushankar, H.B. Screening of novel source for genistein by rapid and sensitive UPLC-APCI-TOF mass spectrometry. Int. J. Food Sci. 2021, 2021, 5537917. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Vincken, J.P.; Mol, L.A.; The, S.A.; Bovee, T.F.; Luijendijk, T.J.; Verbruggen, M.A.; Gruppen, H. Agonistic and antagonistic estrogens in licorice root (Glycyrrhiza glabra). Anal. Bioanal. Chem. 2011, 401, 305–313. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Simons, R.; Vincken, J.P.; Bakx, E.J.; Verbruggen, M.A.; Gruppen, H. A rapid screening method for prenylated flavonoids with ultra-high-performance liquid chromatography/electrospray ionisation mass spectrometry in licorice root extracts. Rapid Commun. Mass Spectrom. 2009, 23, 3083–3093. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Rizzo, S.; Campone, L.; Di Sanzo, R.; Carabetta, S.; Rastrelli, L.; Russo, M. Specialized metabolite profiling of different Glycyrrhiza glabra organs by untargeted UHPLC-HRMS. Ind. Crops Prod. 2021, 170, 113688. [Google Scholar] [CrossRef]
- Mahrous, R.S.; Fathy, H.; Ibrahim, R.S. Metabolic bioprofiling of different Glycyrrhiza glabra solvent fractions for the identification of anti-adenoviral compounds using LC-HRMS/MS and in-vitro cytopathic assay coupled with chemometry. BMC Complement. Med. Ther. 2023, 23, 259. [Google Scholar] [CrossRef] [PubMed]
- Docimo, T.; Celano, R.; Lambiase, A.; Di Sanzo, R.; Serio, S.; Santoro, V.; Coccetti, P.; Russo, M.; Rastrelli, L.; Piccinelli, A.L. Exploring influence of production area and harvest time on specialized metabolite content of glycyrrhiza glabra leaves and evaluation of antioxidant and anti-aging properties. Antioxidants 2024, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Bursać, M.; Krstonošić, M.A.; Miladinović, J.; Malenčić, Đ.; Gvozdenović, L.; Cvejić, J.H. Isoflavone composition, total phenolic content and antioxidant capacity of soybeans with colored seed coat. Nat. Prod. Commun. 2017, 12, 1934578X1701200417. [Google Scholar] [CrossRef]
- Hsu, C.; Wang, S.-T.; Wu, B.-Y.; Hung, Y.-T.; Su, N.-W. Isolation of individual isoflavone species from soybean by solvent extraction followed by the combination of macroporous resin and aluminium oxide separation. Food Chem. 2020, 331, 127312. [Google Scholar] [CrossRef] [PubMed]
- Lante, A.; Barion, G.; Zannoni, S.; Pastore, M.R.; Tinello, F.; Dal Cortivo, C.; Vamerali, T.; Mosca, G. An ecofriendly procedure to extract isoflavones from soybean seeds. J. Clean. Prod. 2018, 170, 1102–1110. [Google Scholar] [CrossRef]
- Magiera, S.; Sobik, A. Ionic liquid-based ultrasound-assisted extraction coupled with liquid chromatography to determine isoflavones in soy foods. J. Food Compos. Anal. 2017, 57, 94–101. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Carabias-Martínez, R. QuEChERS method for the extraction of isoflavones from soy-based foods before determination by capillary electrophoresis-electrospray ionization-mass spectrometry. Microchem. J. 2013, 108, 203–209. [Google Scholar] [CrossRef]
- Bacciottini, L.; Falchetti, A.; Pampaloni, B.; Bartolini, E.; Carossino, A.M.; Brandi, M.L. Phytoestrogens: Food or drug? Clin. Cases Miner. Bone Metab. 2007, 4, 123–130. [Google Scholar]
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary intake, bioavailability, and protective mechanisms against colorectal neoproliferative lesions. Nutrients 2019, 11, 1709. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Hallund, J.; Talbot, D.; Schroot, J.; Williams, C.M.; Bugel, S.; Cassidy, A. Absorption of isoflavones in humans: Effects of food matrix and processing. J. Nutr. Biochem. 2006, 17, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary phytoestrogens and their metabolites as epigenetic modulators with impact on human health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L.; Natarajan, S.S. Influence of sample preparation on the assay of isoflavones. Planta Med. 2009, 75, 704–710. [Google Scholar] [CrossRef]
- Achouri, A.; Boye, J.I.; Belanger, D. Soybean isoflavones: Efficacy of extraction conditions and effect of food type on extractability. Food Res. Int. 2005, 38, 1199–1204. [Google Scholar] [CrossRef]
- Lin, X.; Duan, N.; Wu, J.; Lv, Z.; Wang, Z.; Wu, S. Potential food safety risk factors in plant-based foods: Source, occurrence, and detection methods. Trends Food Sci. Technol. 2023, 138, 511–522. [Google Scholar] [CrossRef]
- Fahmi, R.; Khodaiyan, F.; Pourahmad, R.; Emam-Djomeh, Z. Effect of ultrasound assisted extraction upon the Genistin and Daidzin contents of resultant soymilk. J. Food Sci. Technol. 2014, 51, 2857–2861. [Google Scholar] [CrossRef]
- Benedetti, B.; Di Carro, M.D.; Magi, E. Phytoestrogens in soy-based meat substitutes: Comparison of different extraction methods for the subsequent analysis by liquid chromatography-tandem mass spectrometry. J. Mass Spectrom. 2018, 53, 862–870. [Google Scholar] [CrossRef]
- da Silva, B.; Kupski, L.; Badiale-Furlong, E. Central composite design-desirability function approach for optimum ultrasound-assisted extraction of daidzein and genistein from soybean and their antimycotoxigenic potential. Food Anal. Methods 2019, 12, 258–270. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Microwave assisted extraction of soy isoflavones. Anal. Chim. Acta 2007, 588, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Nemes, S.M.; Orsat, V. Microwave-assisted extraction of secoisolariciresinol diglucoside-method development. Food Bioprocess Technol. 2011, 4, 1219–1227. [Google Scholar] [CrossRef]
- Nemes, S.M.; Orsat, V. Evaluation of a microwave-assisted extraction method for lignan quantification in flaxseed cultivars and selected oil seeds. Food Anal. Methods 2012, 5, 551–563. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Arau´jo, J.M.A.; Sandi, D. Supercritical fluid extraction of isoflavones from soybean flour. Food Chem. 2002, 78, 111–117. [Google Scholar] [CrossRef]
- Pyo, D.; Yoo, J.; Surh, J. Comparison of supercritical fluid extraction and solvent extraction of isoflavones from soybeans. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 923–932. [Google Scholar] [CrossRef]
- Palma-Duran, S.A.; Caire-Juvera, G.; Campa-Siqueiros, M.M.; Chávez-Suárez, K.M.; Robles-Burgueño, M.d.R.; Gutiérrez-Coronado, M.L.; Bermúdez-Almada, M.d.C.; Saucedo-Tamayo, M.d.S.; Grajeda-Cota, P.; Valenzuela-Quintanar, A.I. A comprehensive HPLC-DAD-ESI-MS validated method for the quantification of 16 phytoestrogens in food, serum and urine. Appl. Sci. 2020, 10, 8147. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Caprioli, G.; Maggi, F.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Effective clean-up and ultra high-performance liquid chromatography-tandem mass spectrometry for isoflavone determination in legumes. Food Chem. 2015, 174, 487–494. [Google Scholar] [CrossRef]
- Nørskov, N.P.; Knudsen, K.E.B. Validated LC-MS/MS method for the quantification of free and bound lignans in cereal-based diets and feces. J. Agric. Food Chem. 2016, 64, 8343–8351. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of isoflavones from soybeans. Anal. Chim. Acta 2004, 522, 169–177. [Google Scholar] [CrossRef]
- Angeloni, S.; Navarini, L.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. Development of an extraction method for the quantification of lignans in espresso coffee by using HPLC-MS/MS triple quadrupole. J. Mass Spectrom. 2018, 53, 842–848. [Google Scholar] [CrossRef]
- de Queirós, L.D.; Dias, F.F.G.; de Ávila, A.R.A.; Macedo, J.A.; Macedo, G.A.; Leite Nobrega de Moura Bell, J.M. Effects of enzyme-assisted extraction on the profile and bioaccessibility of isoflavones from soybean flour. Food Res. Int. 2021, 147, 110474. [Google Scholar] [CrossRef]
- Arya, P.; Munshi, M.; Kumar, P. Diosgenin: Chemistry, extraction, quantification and health benefits. Food Chem. Adv. 2023, 2, 100170. [Google Scholar] [CrossRef]
- Kim, Y.R.; Pyo, H.S.; Chung, B.C.; Moon, M.H.; Lee, J. GC-MS Analysis of various phytoestrogens in health functional foods. Bull. Korean Chem. Soc. 2017, 38, 448–458. [Google Scholar] [CrossRef]
- Benedetti, B.; Di Carro, M.; Mirasole, C.; Magi, E. Fast derivatization procedure for the analysis of phytoestrogens in soy milk by gas chromatography tandem mass spectrometry. Microchem. J. 2018, 137, 62–70. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, R.H.; Kim, J.H.; Na, H.; Lee, S.J.; Choi, Y.M.; Yoon, H.; Kim, S.Y.; Kim, Y.S.; Lee, S.H.; et al. Changes in isoflavone profile from soybean seeds during cheonggukjang fermentation based on high-resolution UPLC-DAD-QToF/MS: New succinylated and phosphorylated conjugates. Molecules 2022, 27, 4120. [Google Scholar] [CrossRef] [PubMed]
- Myrtsi, E.D.; Koulocheri, S.D.; Haroutounian, S.A. A novel method for the efficient simultaneous quantification of 67 phytoestrogens in plants and foodstuffs. Food Biosci. 2023, 56, 103357. [Google Scholar] [CrossRef]
- Gu, W.; Pang, Y.H.; Li, Y.L.; Shen, X.F.; Liu, J. Alkaline-enzymatic hydrolysis for improved quantitation of total isoflavones in soy protein-based infant food by UPLC-MS/MS. ACS Food Sci. Technol. 2024, 4, 263–271. [Google Scholar] [CrossRef]
- Bensaada, S.; Chabrier, F.; Ginisty, P.; Ferrand, C.; Peruzzi, G.; Valat, M.; Bennetau-Pelissero, C. Improved food-processing techniques to reduce isoflavones in soy-based foodstuffs. Foods 2023, 12, 1540. [Google Scholar] [CrossRef]
- Fujii, S.; Ohta, T.; Ehama, R.; Irikida, M.; Nomura, S.; Shoyama, Y.; Uto, T. Development of an indirect competitive enzyme-linked immunosorbent assay for formononetin and its application in a cell-based assay using MC3T3-E1 cells. Food Chem. 2023, 403, 134339. [Google Scholar] [CrossRef]
- Lampe, J.W. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J. Nutr. 2003, 133 (Suppl. 3), 956S–964S. [Google Scholar] [CrossRef]
- Franke, A.A.; Custer, L.J. High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J. Chromatogr. B Biomed. Appl. 1994, 662, 47–60. [Google Scholar] [CrossRef]
- Hosoda, K.; Furuta, T.; Ishii, K. Simultaneous determination of glucuronic acid and sulfuric acid conjugated metabolites of daidzein and genistein in human plasma by high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Furuta, T.; Yokokawa, A.; Ishii, K. Identification and quantification of daidzein-7-glucuronide-4’-sulfate, genistein-7-glucuronide-4’-sulfate and genistein-4’,7-diglucuronide as major metabolites in human plasma after administration of kinako. Anal. Bioanal. Chem. 2010, 397, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Redruello, B.; Guadamuro, L.; Cuesta, I.; Álvarez-Buylla, J.R.; Mayo, B.; Delgado, S. A novel UHPLC method for the rapid and simultaneous determination of daidzein, genistein and equol in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1005, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Bolca, S.; De Keukeleire, D.; Heyerick, A. Development of a high-throughput LC/APCI-MS method for the determination of thirteen phytoestrogens including gut microbial metabolites in human urine and serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 949–956. [Google Scholar] [CrossRef]
- Valentín-Blasini, L.; Blount, B.C.; Rogers, H.S.; Needham, L.L. HPLC-MS/MS method for the measurement of seven phytoestrogens in human serum and urine. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 799–807. [Google Scholar] [CrossRef][Green Version]
- Rybak, M.E.; Parker, D.L.; Pfeiffer, C.M. Determination of urinary phytoestrogens by HPLC-MS/MS: A comparison of atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 861, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kuijsten, A.; Buijsman, M.N.; Arts, I.C.; Mulder, P.P.; Hollman, P.C. A validated method for the quantification of enterodiol and enterolactone in plasma using isotope dilution liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 822, 178–184. [Google Scholar] [CrossRef]
- Prasain, J.K.; Arabshahi, A.; Moore, D.R.; Greendale, G.A.; Wyss, J.M.; Barnes, S. Simultaneous determination of 11 phytoestrogens in human serum using a 2 min liquid chromatography/tandem mass spectrometry method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 994–1002. [Google Scholar] [CrossRef]
- Jung, H.R.; Kim, S.J.; Ham, S.H.; Cho, J.H.; Lee, Y.B.; Cho, H.Y. Simultaneous determination of puerarin and its active metabolite in human plasma by UPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 971, 64–71. [Google Scholar] [CrossRef]
- Grace, P.B.; Mistry, N.S.; Carter, M.H.; Leathem, A.J.; Teale, P. High throughput quantification of phytoestrogens in human urine and serum using liquid chromatography/tandem mass spectrometry (LC-MS/MS). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 853, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.I.; Grace, P.B.; Bingham, S.A. Optimization of conditions for the enzymatic hydrolysis of phytoestrogen conjugates in urine and plasma. Anal. Biochem. 2005, 341, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kunisue, T.; Tanabe, S.; Isobe, T.; Aldous, K.M.; Kannan, K. Profiles of phytoestrogens in human urine from several Asian countries. J. Agric. Food Chem. 2010, 58, 9838–9846. [Google Scholar] [CrossRef] [PubMed]
- Fotsis, T.; Heikkinen, R.; Adlercreutz, H.; Axelson, M.; Setchell, K.D. Capillary gas chromatographic method for the analysis of lignans in human urine. Clin. Chim. Acta 1982, 121, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Fotsis, T.; Bannwart, C.; Wähälä, K.; Brunow, G.; Hase, T. Isotope dilution gas chromatographic-mass spectrometric method for the determination of lignans and isoflavonoids in human urine, including identification of genistein. Clin. Chim. Acta 1991, 199, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.B.; Taylor, J.I.; Botting, N.P.; Fryatt, T.; Oldfield, M.F.; Bingham, S.A. Quantification of isoflavones and lignans in urine using gas chromatography/mass spectrometry. Anal. Biochem. 2003, 315, 114–121. [Google Scholar] [CrossRef]
- Degen, G.H.; Blaszkewicz, M.; Shi, L.; Buyken, A.E.; Remer, T. Urinary isoflavone phytoestrogens in German children and adolescents—A longitudinal examination in the DONALD cohort. Mol. Nutr. Food Res. 2011, 55, 359–367. [Google Scholar] [CrossRef]
- Uehara, M.; Lapcík, O.; Hampl, R.; Al-Maharik, N.; Mäkelä, T.; Wähälä, K.; Mikola, H.; Adlercreutz, H. Rapid analysis of phytoestrogens in human urine by time-resolved fluoroimmunoassay. J. Steroid Biochem. Mol. Biol. 2000, 72, 273–282. [Google Scholar] [CrossRef]
| Botanical Name | Phytoestrogens | Method | LoD/LoQ | Reference |
|---|---|---|---|---|
| Humulus lupulus L. | Prenylated flavonoids (8-PN; 6-PN; Xanthohumol) | HPLC/DAD (assay) HPLC–ESI–MS (fingerprinting) | LoD: 0.3 to 1.0 µg/mL LoQ: 1.3 to 3.8 µg/mL (HPLC/DAD) | [44] |
| Prenylflavonoids and bitter acids | HPLC (APCI–MS and UV detection) | For 8-PN with MS detection: LoD: 0.006 mg/L; LoQ: 0.02 mg/L; For 8-PN with UV detection: LoD: 0.03 mg/L; LoQ: 0.1 mg/mL | [45] | |
| Prenylated flavonoids | SIDA–LC–MS/MS | LoD: 0.04 µg/L; LoQ: 3.2 µg/L | [46] | |
| Xanthohumol | Nonaqueous reversed polarity CE |
For xanthohumol
LoD: 0.05 mg/L; LoQ: 0.15 μg/mL, | [47] | |
| Linum usitatissimum L. | Lignans (SECO, SDG-oligomers) | TOF–MS | LoDs for SECO and SDG-containing oligomers: 0.008 pg | [48] |
| SECO, SDG, SDG-oligomers | reversed-phase HPLC | LoDs for SDG oligomers, SDG and SECO: 0.065 µg/mL, 0.087 µg/mL and 0.039 µg/mL, LoQs: 0.217 µg/mL, 0.288 µg/mL and 0.130 µg/ml | [49] | |
| Coffea arabica L. | Lignans (SECO, lariciresinol, matairesinol) | LC–MS/MS | LoD: 1.5 µg/100 g of dry weight | [50] |
| SECO, lariciresinol, matairesinol | HPLC–MS/MS | SECO: LoD: 2 µg/L; LoQ: 5 µg/L Lariciresinol: LoD: 2 µg/L; LoQ: 5 µg/L Matairesinol: LoD: 3 µg/L; LoQ: 10 µg/L | [51] | |
| Trifolium pratense L. | Isoflavones (genistein, daidzein, biochanin A, and formononetin); coumestans | LC–MS |
LoDs: 0.06 to 1.81 ng/mL LoQs: 0.19 to 6.02 ng/mL | [52] |
| Daidzein, genistein, biochanin A, formononetin | HPLC/UV/ESI–MS |
LoQ(UV detection): 24 ng/mL LoQ(MS detection): 6 ng/ml | [53] | |
| Daidzein, genistein, biochanin A, formononetin |
HPLC/UV with internal standard (TFA hydrolysis) | LoQ daidzein: 2.0 ng LoQ formononetin 10.0 ng | [54] | |
| Biochanin A, formononetin, genistein, ononin, sissotrin, daidzein | LC–MS |
LoD: 0.06 to 1.81 ng/mL LoQs: 0.19 to 6.02 ng/mL | [52] | |
| Isoflavones and their glucoside malonates | Reversed-phase LC (APCI–MS), DAD, and fluorescence detectors | LC-DAD: LoD (biochanin A): 20 µg/mL, LoD (daidzin): 35 µg/mL. | [55] | |
| Medicago sativa L. | Coumestrol | CE/diode-array detection | LoD: 0.39 mg.dm−3 | [56] |
| Isoflavones | HPLC/DAD method | LoD: 0.03 to 3.72 µg/ mL LoQ: 0.10 to 11.27 µg/mL | [57] | |
| Glycine max L. Merr. | Daidzin and genistin | os-FLISA | LoQ: 0.1 μg/mL daidzin | [58] |
| Daidzin and genistin | icELISA | LoD: 1.95 ng/ml | [59] | |
| daidzin and genistin | ICA | LoD: 125 ng/mL | [60] | |
| Daidzein, glycitein, genistein |
UHPLC (with enzymatic hydrolysis) | LoD: 67 pg; LoQ: 223 pg (for daidzein) LoD: 55 pg; LoQ: 184 pg (for glycitein) LoD: 94 pg; LoQ: 314 pg (for genistein) | [61] |
| Extraction Technique | Phytoestrogen | Source | Parameters | Reference |
|---|---|---|---|---|
| UAE | Daidzin, genistin, daidzein, genistein | soymilk | Ultrasound frequency 35 kHz, 135 kHz Time: 20 to 60 min Temperature: 20 to 40 °C | [90] |
| UAE | Daidzin, genistin, formononetin, biochaninA, coumestrol | soy burgers | Time:15 min Temperature: 30 °C | [91] |
| UAE | Daidzin, genistin | soybeans | Ultrasound frequency 20 to 90 kHz Time 10 to 50 min Temperature: 32 to 168 °C (hydrolysis temperature) | [92] |
| MAE | Daidzein, genistein, glycitein, daidzin, genistin, glycitin | soybeans | Microwave power: 500 W Solvents: EtOH, MeOH (50–70%) Temperature: 50 to 150 °C Extraction time: 10–30 min | [93] |
| MAE | Secoisolariciresinol diglucoside | flaxseed | Microwave power: 30 to 360 W; Time: 1 to 25 min; Solvent: NaOH 0.25 to 1 M; Pmode: power on 30 and 60 s/min. | [94] |
| MAE | Secoisolariciresinol diglucoside | flaxseed cultivars, flaxseed hulls, sesame seeds, chia seeds, almonds, sunflower seeds | Microwave power: 135 W; Time: 3 min; Solvent: NaOH 0.5 M; Pmode: power on 30 s/min. | [95] |
| SFE | Genistin, genistein daidzein | soybean flour | Temperature: 40–70 °C, Pressure: 200–360 bar, Modifier: 0, 5, and 10 mol % of MeOH 70% in water Time: 30 min | [96] |
| SFE | Daidzein genistein | soybean | Temperature: 45–65 °C, Pressure: 80–120 bar, Modifier: 6.5–8.5% EtOH Time: 120 min | [97] |
| SPE | Biochanin A, secoisolariciresinol, matairesinol, enterodiol, enterolactone, equol, quercetin, genistein, glycitein, luteolin, naringenin, kaempferol, formononetin, daidzein, resveratrol and coumestrol | boiled rice potatoes | C18 SPE 120 μL, 65% water, 35% MeOH | [98] |
| SPE | Daidzein, Genistein, Biochanin A | lentils | C18 SPE 4 mL, MeOH | [99] |
| SPE | Secoisolariciresinol, Enterodiol, Matairesinol, Enterolactone | bread | C18 SPE 1.6 mL, 25% ACN | [100] |
| PLE | Daidzein, Genistein, Biochanin A | soybeans | Solvent: MeOH, EtOh (30–80%) Temperature: 60–200 °C Pressure: 100–200 atm | [101] |
| EAE | Secoisolariciresinol | coffee | 4 Different enzymes: Taka-Diastase (Aspergillus oryzae), Clara-Diastase (papaya latex), Papain (papaya latex), Protease(Rhizopus sp) | [102] |
| EAE | Daidzin, genistin, daidzein, genistein | soybean flour | Protease derived from Bacillus subtilis | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam-Dima, I.; Olteanu, A.A.; Olaru, O.T.; Popa, D.E.; Purdel, C. Methods of Analysis of Phytoestrogenic Compounds: An Up-to-Date of the Present State. Separations 2024, 11, 205. https://doi.org/10.3390/separations11070205
Adam-Dima I, Olteanu AA, Olaru OT, Popa DE, Purdel C. Methods of Analysis of Phytoestrogenic Compounds: An Up-to-Date of the Present State. Separations. 2024; 11(7):205. https://doi.org/10.3390/separations11070205
Chicago/Turabian StyleAdam-Dima, Ines, Andreea Alexandra Olteanu, Octavian Tudorel Olaru, Daniela Elena Popa, and Carmen Purdel. 2024. "Methods of Analysis of Phytoestrogenic Compounds: An Up-to-Date of the Present State" Separations 11, no. 7: 205. https://doi.org/10.3390/separations11070205
APA StyleAdam-Dima, I., Olteanu, A. A., Olaru, O. T., Popa, D. E., & Purdel, C. (2024). Methods of Analysis of Phytoestrogenic Compounds: An Up-to-Date of the Present State. Separations, 11(7), 205. https://doi.org/10.3390/separations11070205








