Effects of Extraction Methods on Volatile Oil Profiles of Cinnamomi ramulus–Zingiberis rhizoma recens Couplet Medicines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials
2.3. Methods
2.3.1. Extraction of Volatile Oils by Steam Distillation (SD)
2.3.2. Extraction of Volatile Oils by Azeotropic Distillation (AD)
2.3.3. Extraction of Volatile Oils by Supercritical Fluid Extraction (SFE)
2.4. Analysis of Volatile Oils by HS-GC-MS
2.5. Data Processing and Statistical Analysis
3. Results
3.1. Extraction of Volatile Oils from CR, ZRR and CR-ZRR by SD
3.1.1. Yields of Volatile Oils from CR, ZRR and CR-ZRR by SD
3.1.2. Chemical Composition of CR, ZRR and CR-ZRR Volatile Oils
3.1.3. The Component Transfer Rate from CR and ZRR to Couplet Medicines
3.2. Effects of Different Extraction Methods on the Chemical Composition of Volatile Oil from CR-ZRR
3.2.1. Yields of Volatile Oil from CR-ZRR by Different Extraction Methods
3.2.2. Comparison of the Chemical Profiles of Volatile Oils from CR-ZRR by Different Extraction Methods
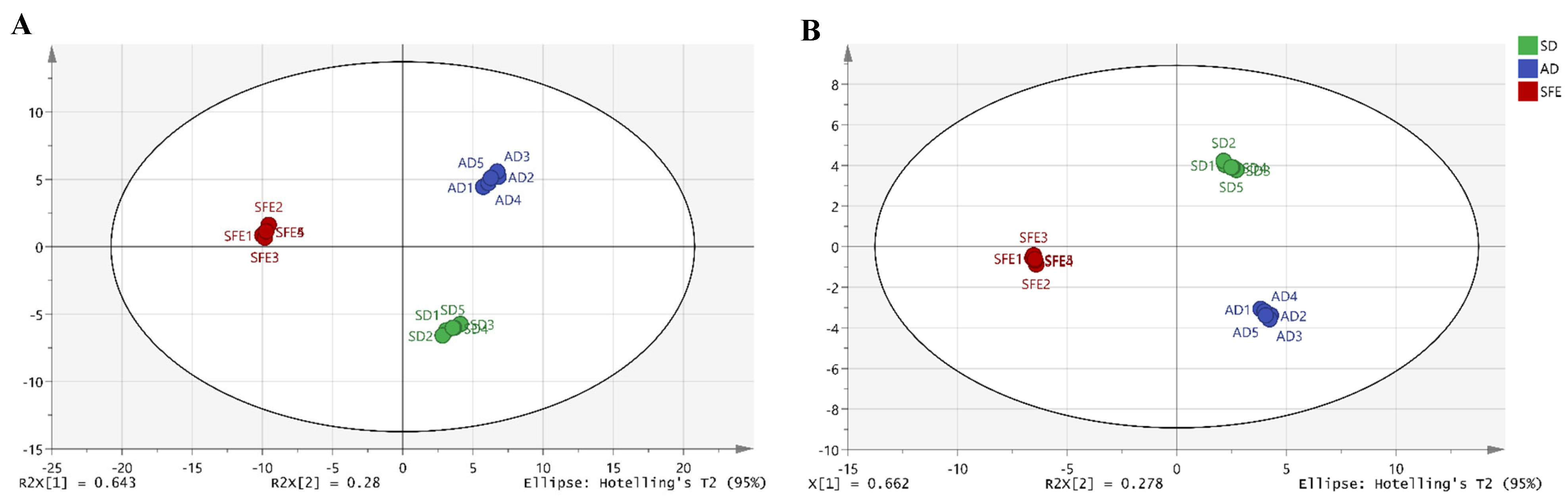
3.2.3. PCA and OPLS-DA
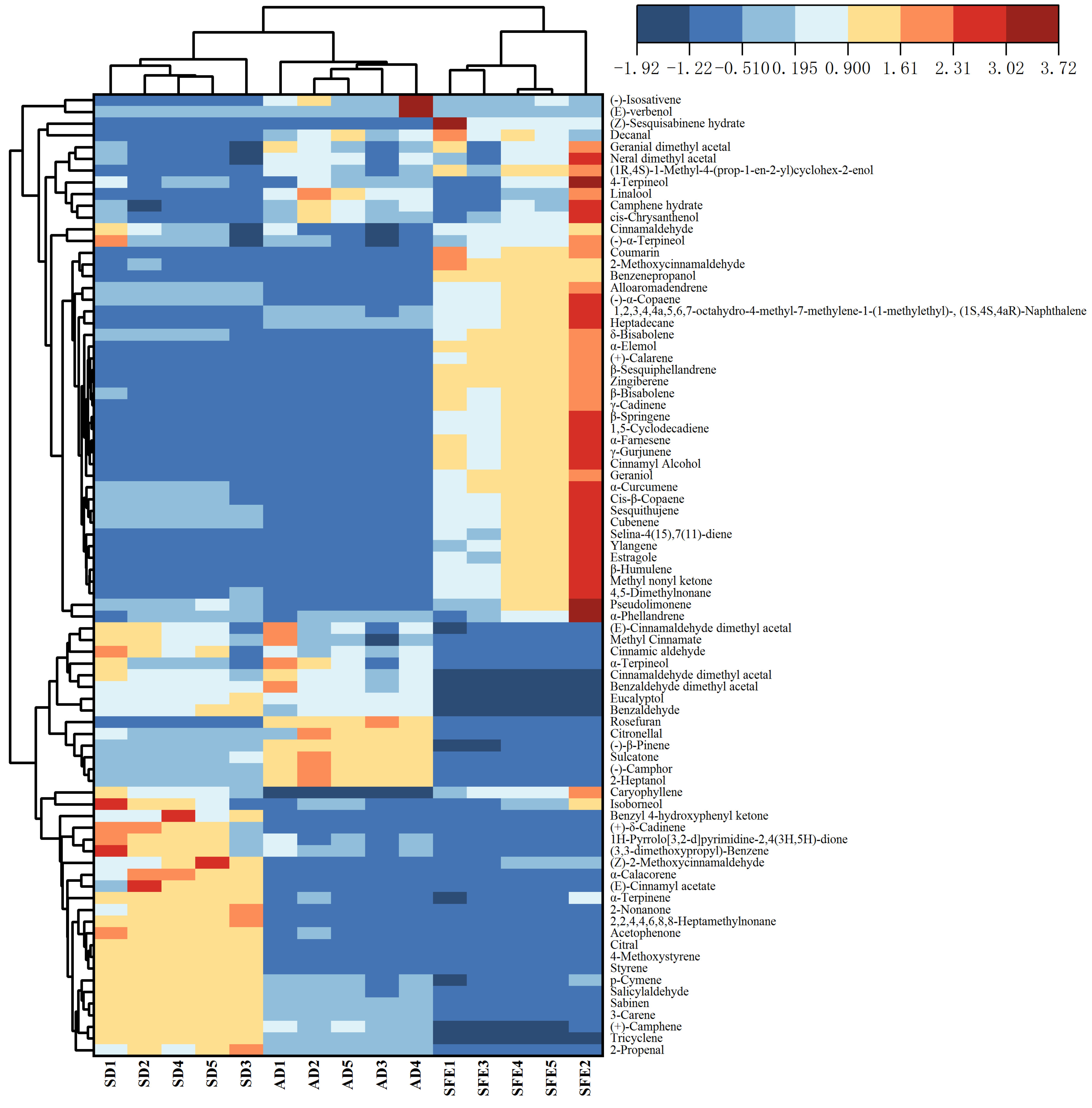
3.2.4. Clustering Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Li, J.; Luo, L. Modern research progress of traditional Chinese medicine couplet. Lishizhen Med. Mater. Medica Res. 2012, 23, 1003–1005. [Google Scholar]
- Pang, T.; Mai, L.; Chen, Y.; Xie, Z.; Tang, Y.; Li, Y.; Zhong, M. Research progress on the changes of chemical components in the compatibility of traditional Chinese medicine. J. Chin. Med. Mater. 2015, 38, 2429–2434. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, C. Discussion on the law of drug use in Treatise on Febrile Diseases and Synopsis of the Golden Chamber based on the auxiliary platform of traditional Chinese medicine inheritance. Jiangsu J. Tradit. Chin. Med. 2019, 51, 73–75. [Google Scholar]
- Shi, Y.; Zhang, Q.; Duan, J.; Zhang, L.; Xue, Y. Analysis of prescription medication rules of “Treatise on Febrile Diseases” prescriptions based on traditional Chinese medicine inheritance auxiliary platform (V2.5) software. China Pharm. 2016, 27, 2296–2298. [Google Scholar]
- Zuo, M.; Tang, T.; Wang, X.; Gu, J.; Huang, J. Analysis of Zhang Zhongjing ‘s Medication Rules for Heart Diseases Based on Data Mining. Anhui Med. Pharm. J. 2022, 26, 1254–1258. [Google Scholar]
- Liu, J.; Zhang, Q.; Li, R.; Wei, S.; Huang, C.; Gao, Y.; Pu, X. The traditional uses, phytochemistry, pharmacology and toxicology of Cinnamomi ramulus: A review. J. Pharm. Pharmacol. 2020, 72, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ao, M.; Zhang, C.; Fan, S.; Chen, Z.; Yu, L. Zingiberis rhizoma recens: A review of its traditional uses, phytochemistry, pharmacology, and toxicology. Evid.-Based Compl. Alt. 2021, 20, 6668990. [Google Scholar] [CrossRef]
- Peng, L.; Lei, Z.; Rao, Z.; Yang, R.; Zheng, L.; Fan, Y.; Luan, F.; Zeng, N. Cardioprotective activity of ethyl acetate extract of Cinnamomi ramulus against myocardial ischemia/reperfusion injury in rats via inhibiting NLRP3 inflammasome activation and pyroptosis. Phytomedicine 2021, 93, 153798. [Google Scholar] [CrossRef]
- Lee, J.S.; Lim, S. Anti-inflammatory, and anti-arthritic effects by the twigs of Cinnamomum cassia on complete Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2021, 278, 114209. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Li, R.; Wei, S.; Gao, Y.; Ai, L.; Wu, C.; Pu, X. Anti-proliferation and anti-migration effects of an aqueous extract of Cinnamomi ramulus on MH7A rheumatoid arthritis-derived fibroblast-like synoviocytes through induction of apoptosis, cell arrest and suppression of matrix metalloproteinase. Pharm. Biol. 2020, 58, 863–877. [Google Scholar] [CrossRef]
- Tian, L.; Huang, H.; Ye, X.; Li, N.; Zou, T.; Zhou, A.; Liu, Y. Anti-influenza virus activity and chemical composition of Ramulus cinnamomi–Ramulus zingiber recens, a Chinese herb pair. Chin. J. Hosp. Pharm. 2012, 32, 1100–1104. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Keshamma, E.; Sridhar, B.T.; Dakshayini, P.N.; Geethanjali, R. An overview on role of ethnomedicine in boosting human immunity to combat various viral diseases. Int. Ayurvedic Med. J. 2021, 9, 1425–1432. [Google Scholar] [CrossRef]
- da Silva Pamplona, L.; Silva, N.C. The promising activity of Zingiber officinale (ginger) against COVID-19. Health Soc. 2023, 3, 764–811. [Google Scholar] [CrossRef]
- Nagar, S.; Pigott, M.; Whyms, S.; Berlemont, A.; Sheridan, H. Effect of extraction methods on essential oil composition: A case study of Irish Bog Myrtle—Myrica gale L. Separations 2023, 10, 128. [Google Scholar] [CrossRef]
- Djapic, N. Essential Oils of Taxodium distichum Winter Leaves Obtained by Supercritical Carbon Dioxide Extraction Method and Hydrodistillation Separations. Separations 2022, 9, 436. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Zhu, X.; Xu, R.; Shen, H.; Zhang, Q.; Ge, X. The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil. Foods 2022, 11, 721. [Google Scholar] [CrossRef]
- Yi, F.; Xu, H.; Lü, C.; Wu, K.; Hao, L.; Lin, S.; Su, C. Comparison of Three Different Extraction methods on Osmanthus volatile oil: Aroma and biological activity. Chem. Biodivers. 2023, 20, e202200658. [Google Scholar] [CrossRef] [PubMed]
- Padilla-de la Rosa, J.D.; Manzano-Alfaro, M.D.; Gómez-Huerta, J.R.; Arriola-Guevara, E.; Guatemala-Morales, G.; Cardador-Martínez, A.; Estarrón-Espinosa, M. Innovation in a Continuous System of Distillation by Steam to Obtain Essential Oil from Persian Lime Juice (Citrus latifolia Tanaka). Molecules 2021, 26, 4172. [Google Scholar] [CrossRef]
- Zhen, Z.; Wang, H.; Yue, Y.; Li, D.; Song, X.; Li, J. Determination of water content of crude oil by azeotropic distillation Karl Fischer coulometric titration. Anal. Bioanal. Chem. 2020, 412, 4639–4645. [Google Scholar] [CrossRef]
- De Guido, G.; Monticelli, C.; Spatolisano, E.; Pellegrini, L. Separation of the Mixture 2-Propanol Water by Heterogeneous Azeotropic Distillation with Isooctane as an Entrainer. Energies 2021, 14, 5471. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. Supercritical Carbon Dioxide (scCO2) Extraction of Phenolic Compounds from Lavender (Lavandula angustifolia) Flowers: A Box-Behnken Experimental Optimization. Molecules 2019, 24, 3354. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ge, D.; Dai, Y.; Chen, Y.; Fu, Q.; Jin, Y. Extraction and isolation of diphenylheptanes and flavonoids from Alpinia officinarum Hance using supercritical fluid extraction followed by supercritical fluid chromatography. J. Sep. Sci. 2023, 46, 2300156. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.; Chua, M.; Wang, S.; Chang, S. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, T.; Yang, B.; Liu, X.; Duan, G. Development of gas chromatography-mass spectrometry with microwave distillation and simultaneous solid-phase microextraction for rapid determination of volatile constituents in ginger. J. Pharm. Biomed. Anal. 2007, 43, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.J.; Lin, K.W.; Li, X.L.; Ou, H.Y.; Tan, Y.F.; Wang, M.; Wei, N. Chemical Constituents and Anti-Gastric Ulcer Activity of Essential Oils of Alpinia officinarum (Zingiberaceae), Cyperus rotundus (Cyperaceae), and Their Herbal Pair. Chem. Biodivers. 2021, 18, e2100214. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.; Lu, H.; Liang, Y. Analysis of volatile components in herbal pair Semen Persicae-Flos Carthami by GC-MS and chemometric resolution. J. Sep. Sci. 2012, 35, 2940–2948. [Google Scholar] [CrossRef]
- Li, X.R.; Liang, Y.Z.; Guo, F.Q. Analysis of volatile oil in Rhizoma ligustici chuanxiong-Radix paeoniae rubra by gas chromatography-mass spectrometry and chemometric resolution. Acta Pharmacol. Sin. 2006, 27, 491–498. [Google Scholar] [CrossRef]

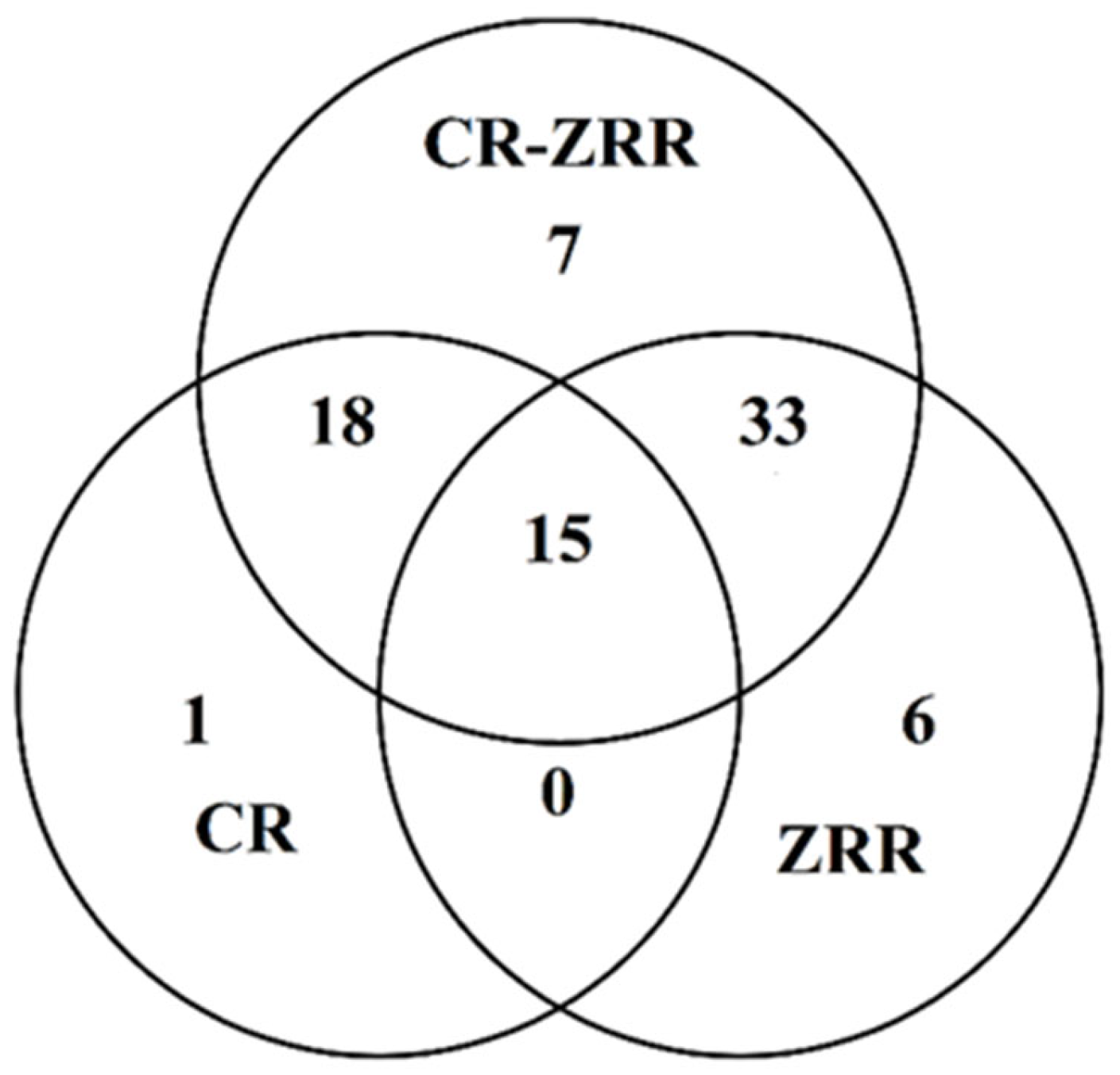

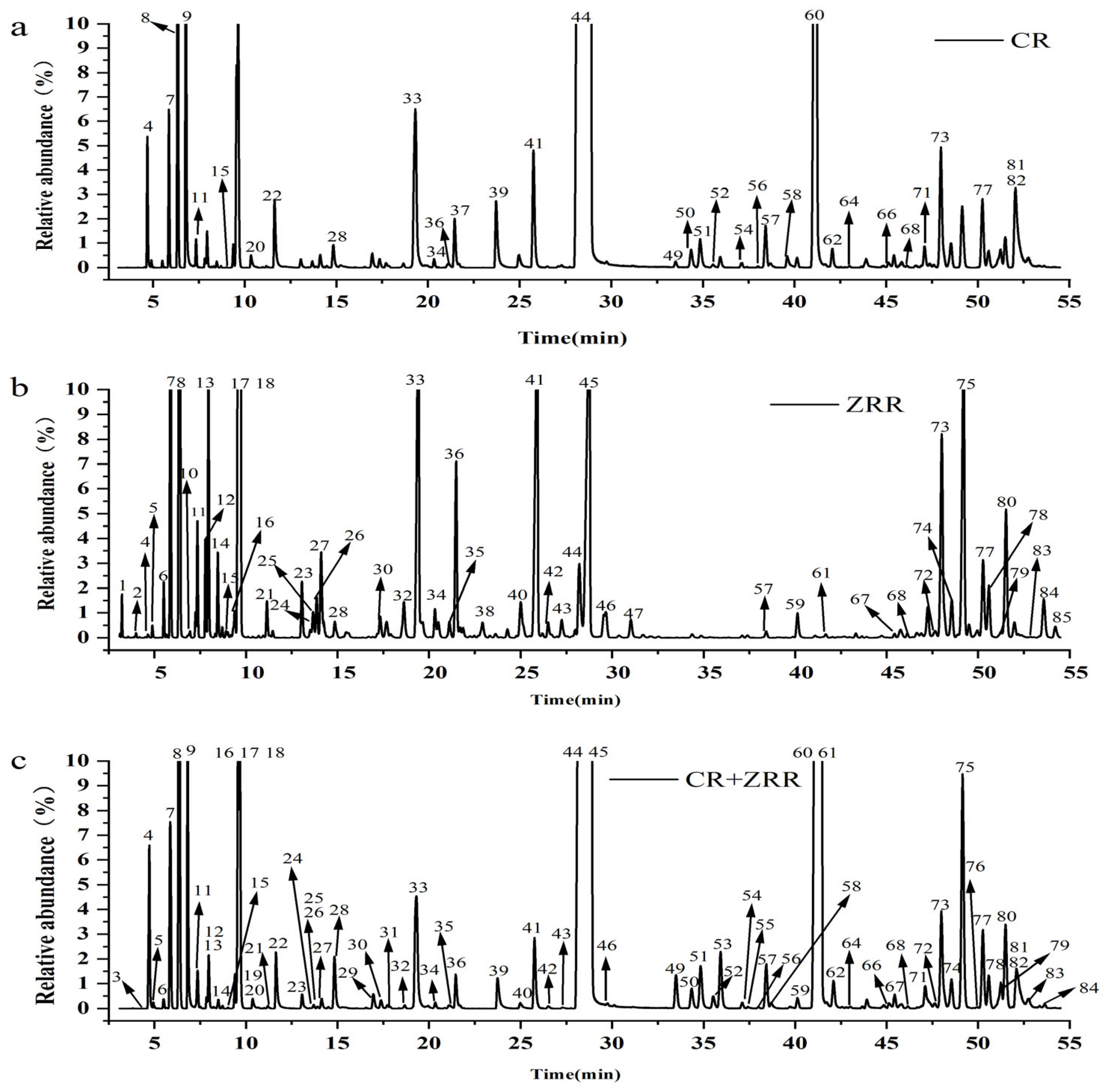
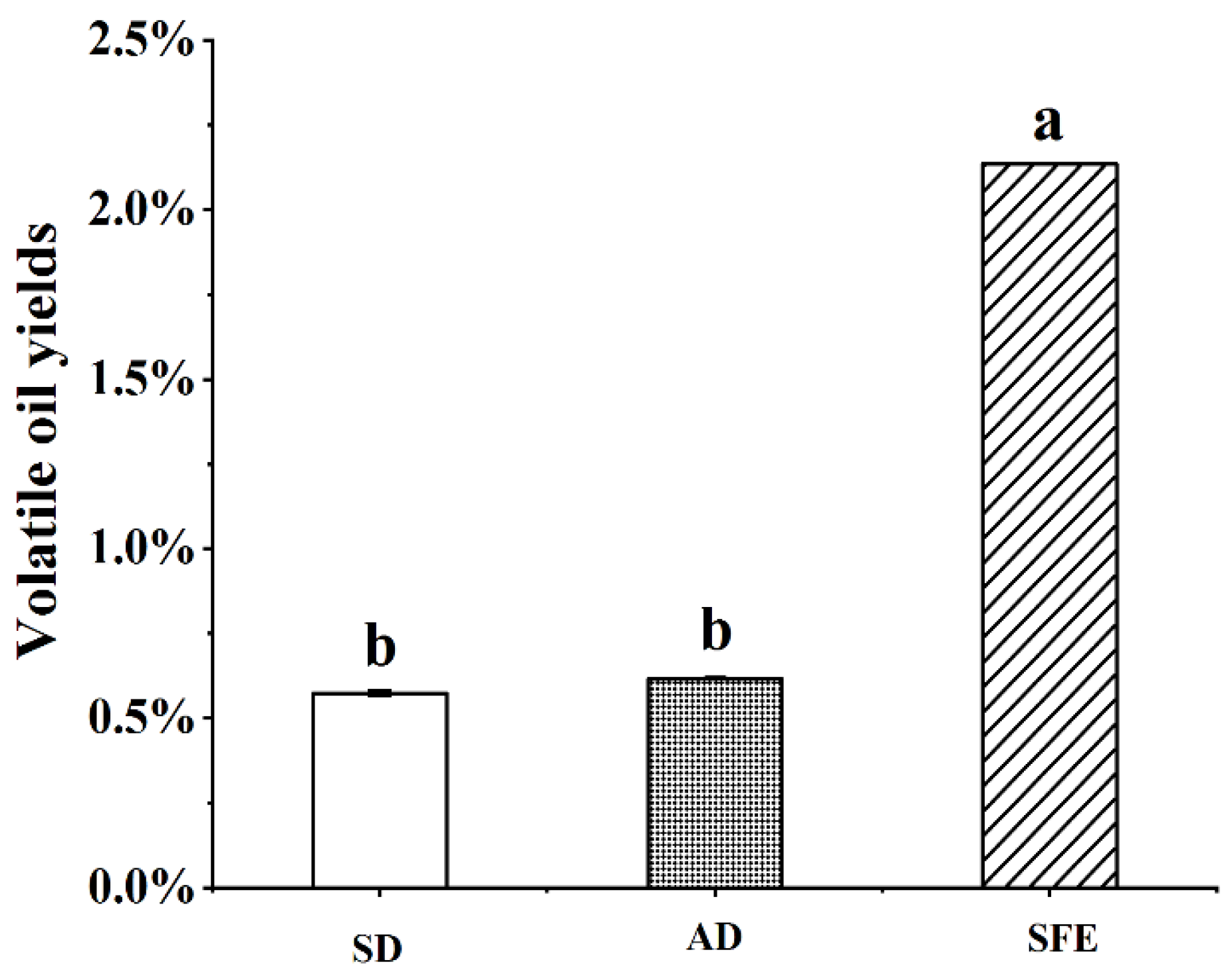
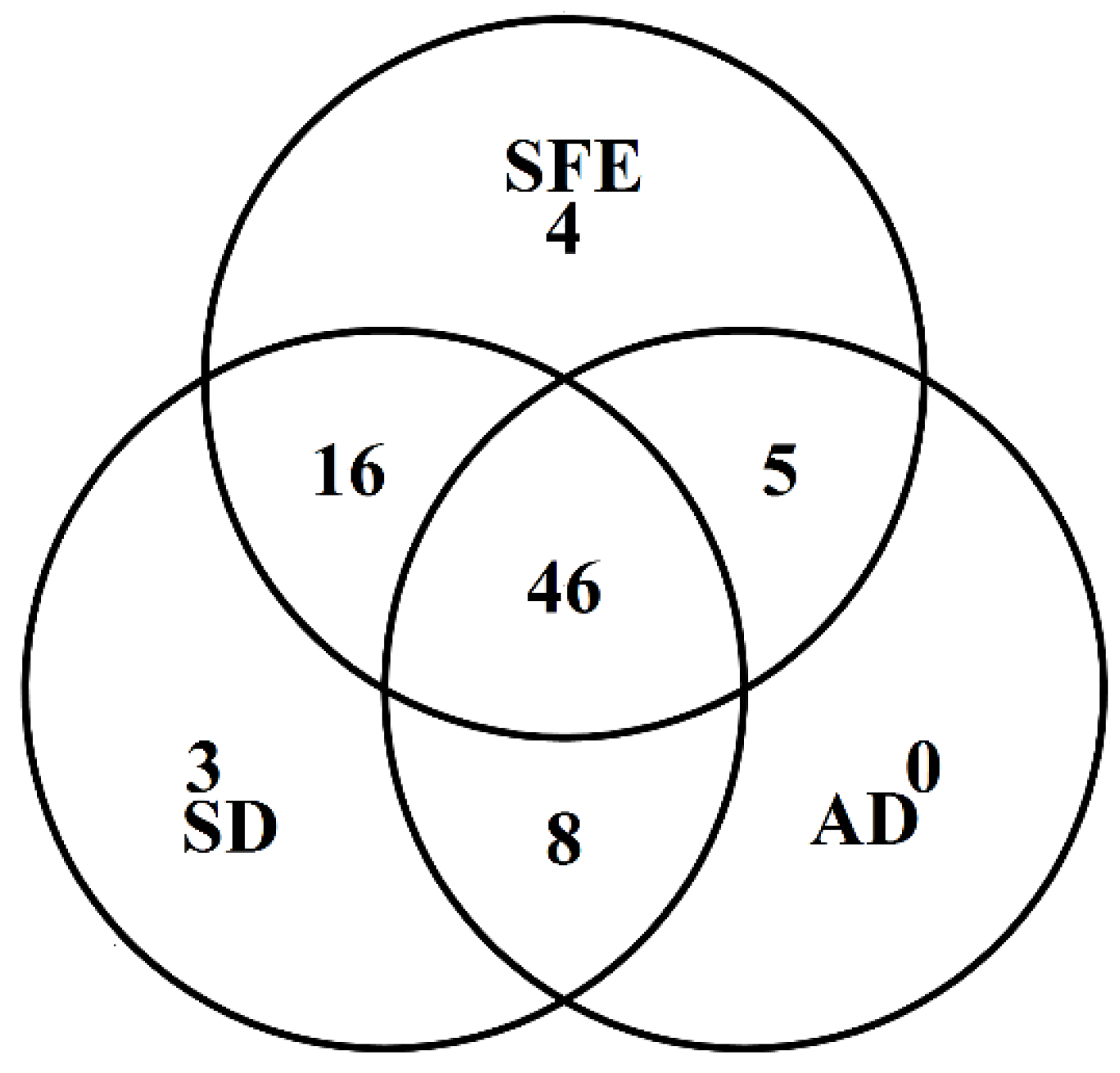
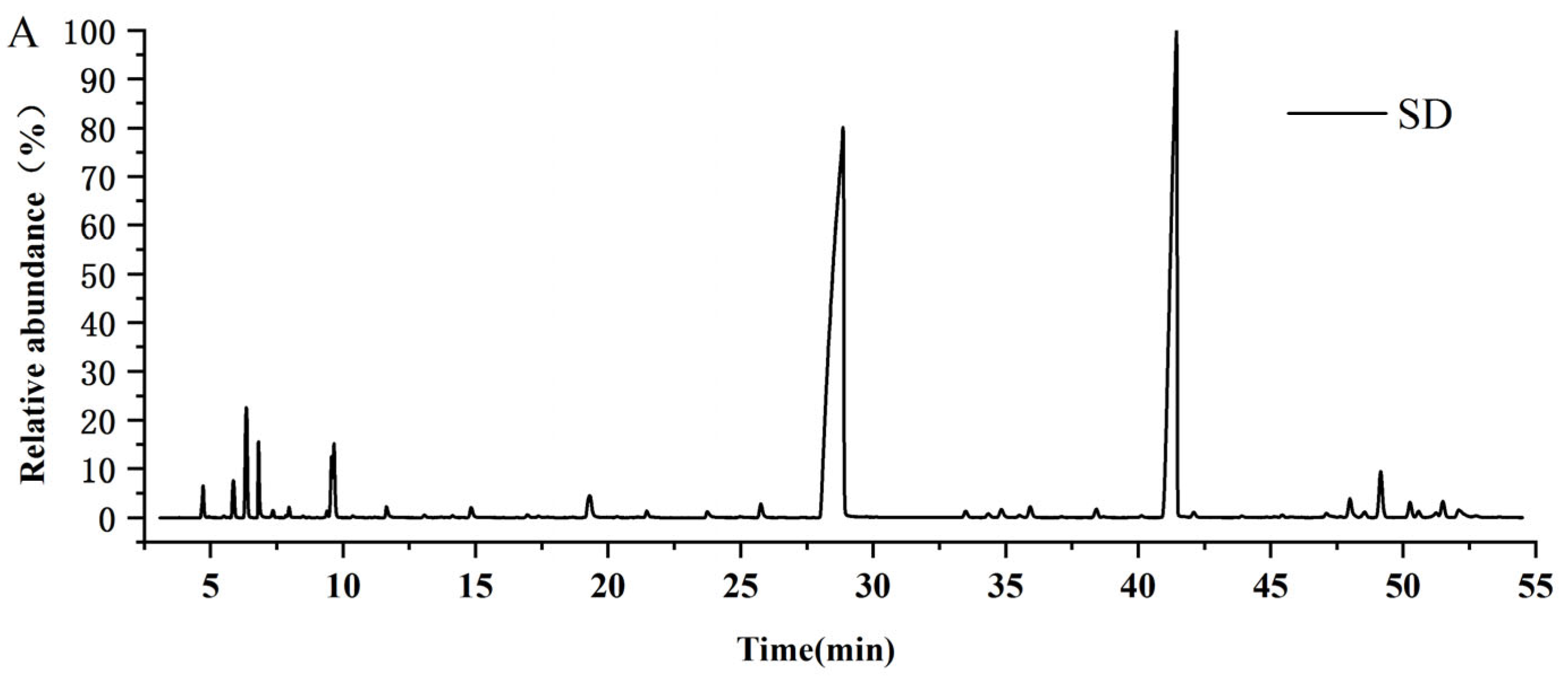
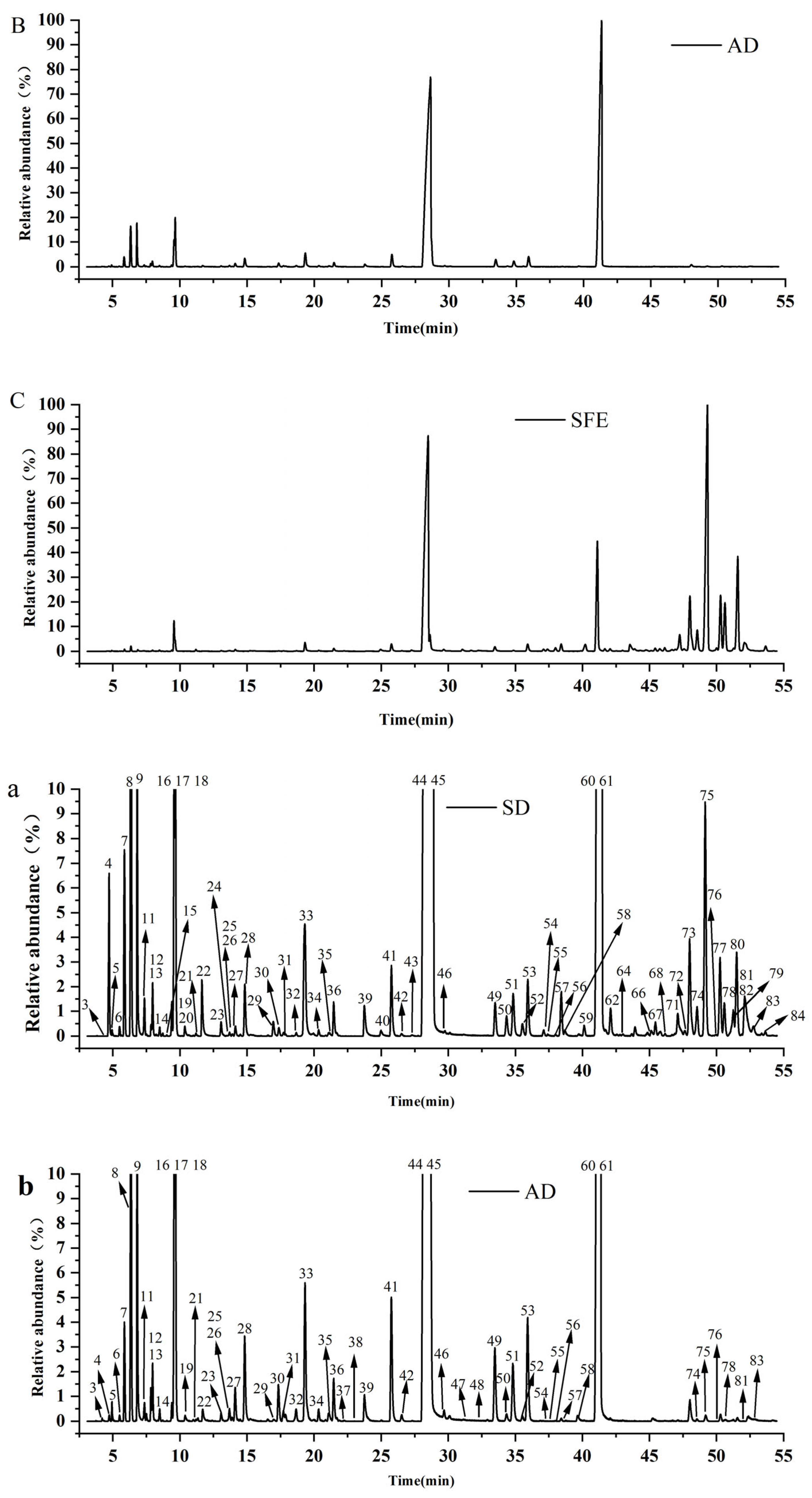
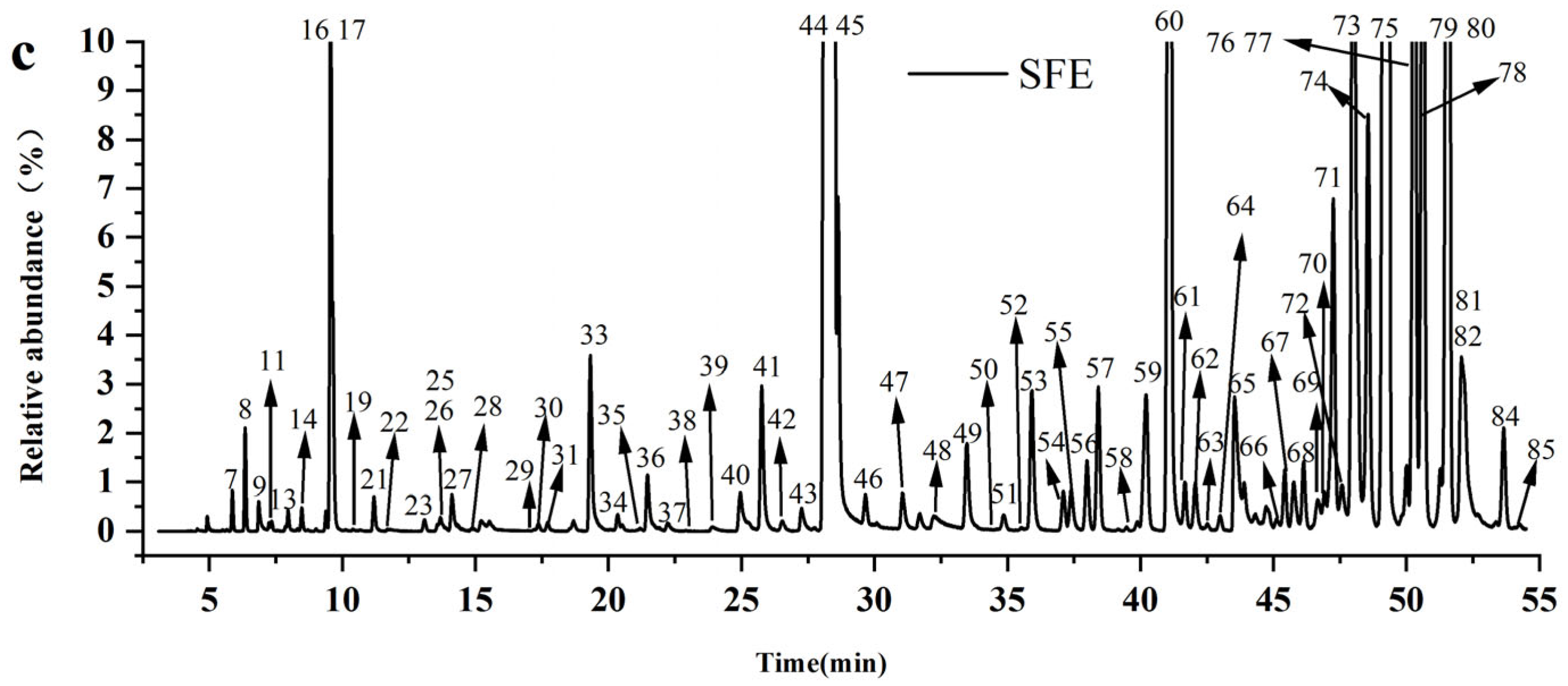

| Peak No. | RT /min | RI | Compounds | Molecular Formula | Molecular Weight | CR —SD (%) | ZRR —SD (%) | CR+ZRR — SD (%) | CR+ZRR —AD (%) | CR+ZRR —SFE (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.223 | 982 | 2-(pentyloxy)tetrahydro-2H-pyran | C10H20O2 | 172.27 | - | 0.25 | - | - | - |
| 2 | 3.990 | 991 | 4-Methyloctane | C9H20 | 128.26 | - | 0.04 | - | - | - |
| 3 | 4.197 | 993 | 2-Propenal | C3H4O | 56.00 | - | - | 1.50 × 10−5 | 1.31 × 10−5 | - |
| 4 | 4.685 | 998 | Styrene | C8H8 | 104.15 | 0.02 | 0.04 | 0.44 | 0.03 | - |
| 5 | 4.909 | 1001 | 2-Heptanol | C7H16O | 116.10 | - | 0.10 | 0.01 | 0.05 | - |
| 6 | 5.500 | 1007 | Tricyclene | C10H16 | 136.23 | - | 0.43 | 0.44 | 0.03 | - |
| 7 | 5.866 | 1011 | 3-Carene | C10H16 | 136.23 | 0.05 | 5.93 | 0.61 | 0.50 | 0.05 |
| 8 | 6.342 | 1016 | (+)-Camphene | C10H16 | 136.23 | 0.04 | 24.03 | 1.86 | 2.14 | 0.14 |
| 9 | 6.783 | 1021 | Benzaldehyde | C7H6O | 106.12 | 1.63 | - | 0.85 | 1.35 | 0.06 |
| 10 | 6.945 | 1023 | Cosmene | C10H14 | 134.23 | - | 0.10 | - | - | - |
| 11 | 7.346 | 1027 | Sabinen | C10H16 | 136.23 | 0.01 | 1.02 | 0.11 | 0.08 | 0.01 |
| 12 | 7.828 | 1032 | Sulcatone | C8H14O | 126.20 | - | 0.87 | 0.02 | 0.10 | - |
| 13 | 7.946 | 1034 | (-)-β-Pinene | C10H16 | 136.23 | - | 2.93 | 0.07 | 0.26 | 0.02 |
| 14 | 8.471 | 1039 | α-Phellandrene | C10H16 | 136.23 | - | 0.83 | 0.02 | 0.04 | 0.03 |
| 15 | 8.911 | 1044 | 2,2,4,4,6,8,8-Heptamethylnonane | C10H16 | 136.23 | 3.02 × 10−5 | 0.07 | 2.07 × 10−5 | - | - |
| 16 | 9.373 | 1049 | p-Cymene | C10H14 | 134.23 | - | 0.40 | 0.09 | 0.06 | 0.02 |
| 17 | 9.553 | 1051 | Pseudolimonene | C10H16 | 136.23 | - | 0.75 | 0.86 | 1.10 | 1.29 |
| 18 | 9.700 | 1052 | Eucalyptol | C10H18O | 154.23 | - | 7.16 | 1.09 | 2.09 | - |
| 19 | 10.343 | 1059 | Salicylaldehyde | C7H6O2 | 122.12 | - | - | 0.04 | 0.02 | 1.34 × 10−5 |
| 20 | 10.421 | 1060 | Benzyl 4-hydroxyphenyl ketone | C14H12O2 | 212.24 | 0.03 | - | 2.33 × 10−5 | - | - |
| 21 | 11.176 | 1068 | 4,5-Dimethylnonane | C11H24 | 156.31 | - | 0.48 | 0.01 | 0.01 | 0.06 |
| 22 | 11.624 | 1073 | Acetophenone | C8H8O | 120.15 | 0.06 | - | 0.26 | 0.06 | 4.72 × 10−7 |
| 23 | 13.057 | 1089 | α-Terpinene | C10H16 | 136.23 | - | 0.79 | 0.05 | 0.04 | 0.02 |
| 24 | 13.495 | 1094 | 2-Nonanone | C9H18O | 136.23 | - | 0.04 | 1.18 × 10−5 | - | - |
| 25 | 13.685 | 1096 | cis-Chrysanthenol | C10H16O | 152.22 | - | 0.24 | 0.01 | 0.04 | 0.03 |
| 26 | 13.870 | 1098 | Rosefuran | C10H14O | 150.22 | - | 0.36 | 1.58 × 10−5 | 0.01 | 3.46 × 10−5 |
| 27 | 14.129 | 1100 | Linalool | C10H18O | 154.25 | - | 0.95 | 0.04 | 0.16 | 0.06 |
| 28 | 14.841 | 1100 | Benzaldehyde dimethyl acetal | C9H12O2 | 152.15 | 0.11 | 0.31 | 0.30 | 0.58 | 1.18 × 10−5 |
| 29 | 16.968 | 1100 | 4-Methoxystyrene | C9H10O | 134.15 | - | - | 0.07 | 2.43 × 10−4 | 1.73 × 10−5 |
| 30 | 17.365 | 1100 | (-)-Camphor | C10H16O | 152.23 | - | 0.43 | 0.04 | 0.21 | 1.69 × 10−5 |
| 31 | 17.707 | 1100 | Camphene hydrate | C10H18O | 154.10 | - | - | 0.01 | 0.04 | 0.02 |
| 32 | 18.662 | 1101 | Citronellal | C10H18O | 154.25 | - | 0.75 | 0.01 | 0.05 | - |
| 33 | 19.315 | 1101 | Isoborneol | C10H18O | 154.23 | 0.75 | 6.83 | 0.76 | 0.93 | 0.58 |
| 34 | 20.334 | 1101 | 4-Terpineol | C10H18O | 154.23 | 0.01 | 0.44 | 0.03 | 0.05 | 0.03 |
| 35 | 21.133 | 1101 | α-Terpineol | C10H18O | 154.23 | - | 0.30 | 0.01 | 0.05 | 9.82 × 10−6 |
| 36 | 21.463 | 1101 | (-)-α-Terpineol | C10H18O | 154.25 | 0.04 | 2.77 | 0.15 | 0.24 | 0.19 |
| 37 | 22.162 | 1101 | Estragole | C10H12O | 148.20 | 0.29 | - | - | 2.70 × 10−4 | 0.02 |
| 38 | 22.940 | 1205 | Decanal | C10H20O | 156.27 | - | 0.35 | - | 3.17 × 10−4 | 1.87 × 10−4 |
| 39 | 23.723 | 1213 | Cinnamic aldehyde | C9H8O | 132.16 | 0.86 | - | 0.15 | 0.22 | 1.45 × 10−4 |
| 40 | 24.985 | 1227 | Benzenepropanol | C9H12O | 136.15 | - | 0.88 | 4.26 × 10−4 | - | 0.16 |
| 41 | 25.753 | 1236 | (E)-verbenol | C10H16O | 152.23 | 0.08 | 7.58 | 0.35 | 0.93 | 0.50 |
| 42 | 26.505 | 1244 | (1R,4S)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-2-enol | C10H16O | 153.23 | - | 0.30 | 1.29 × 10−4 | 0.04 | 0.03 |
| 43 | 27.242 | 1252 | Geraniol | C10H18O | 154.25 | - | 0.34 | 1.52 × 10−4 | - | 0.09 |
| 44 | 28.212 | 1262 | Cinnamaldehyde | C9H8O | 132.16 | 74.56 | 1.64 | 35.15 | 47.77 | 38.77 |
| 45 | 28.622 | 1267 | Citral | C10H16O | 152.23 | - | 9.89 | 30.86 | 1.44 × 10−4 | 0.80 |
| 46 | 29.652 | 1278 | Heptadecane | C21H44 | 296.57 | - | 0.45 | 6.59 × 10−4 | 0.04 | 0.07 |
| 47 | 31.056 | 1293 | Methyl nonyl ketone | C11H22O | 170.29 | - | 0.40 | - | 2.53 × 10−4 | 0.10 |
| 48 | 32.237 | 1306 | Cinnamyl Alcohol | C9H10O | 134.10 | - | - | - | 3.91 × 10−4 | 0.08 |
| 49 | 33.481 | 1318 | Neral dimethyl acetal | C12H22O2 | 198.20 | 4.60 × 10−5 | - | 0.17 | 0.56 | 0.32 |
| 50 | 34.364 | 1327 | (3,3-dimethoxypropyl)-Benzene | C11H16O2 | 180.21 | 2.90 × 10−5 | - | 0.12 | 0.08 | 1.24 × 10−5 |
| 51 | 34.824 | 1332 | (Z)-Cinnamaldehyde dimethyl acetal | C11H14O2 | 178.10 | 5.81 × 10−5 | - | 0.27 | 0.52 | 1.36 × 10−5 |
| 52 | 35.542 | 1339 | 1H-Pyrrolo[3,2-d]pyrimidine-2,4(3H,5H)-dione | C6H5N3O2 | 151.12 | 1.16 × 10−5 | - | 0.05 | 0.03 | 1.70 × 10−5 |
| 53 | 35.952 | 1343 | Geranial dimethyl acetal | C12H22O2 | 198.20 | - | - | 0.26 | 0.83 | 0.53 |
| 54 | 37.143 | 1355 | Cubenene | C15H24 | 204.35 | 8.22 × 10−5 | - | 0.03 | 1.60 × 10−5 | 0.11 |
| 55 | 37.398 | 1358 | β-Humulene | C15H24 | 204.20 | - | - | 4.02 × 10−3 | 3.06 × 10−5 | 0.12 |
| 56 | 37.982 | 1364 | Ylangene | C15H24 | 204.20 | 1.03 × 10−4 | - | 0.01 | 3.35 × 10−5 | 0.16 |
| 57 | 38.421 | 1368 | (-)-α-Copaene | C15H24 | 204.35 | 0.01 | 0.12 | 0.23 | 0.01 | 0.44 |
| 58 | 39.611 | 1380 | Methyl Cinnamate | C10H10O2 | 162.18 | 0.14 | - | 0.05 | 0.07 | 0.01 |
| 59 | 40.132 | 1385 | 1,5-Cyclodecadiene | C15H24 | 204.35 | - | 0.48 | 0.05 | - | 0.55 |
| 60 | 41.181 | 1396 | (E)-Cinnamaldehyde dimethyl acetal | C11H14O2 | 178.21 | 20.28 | - | 21.53 | 38.47 | 8.20 |
| 61 | 41.663 | 1401 | Sesquithujene | C15H24 | 204.35 | - | 3.49 × 10−5 | 0.02 | 5.50 × 10−5 | 0.10 |
| 62 | 42.086 | 1406 | Caryophyllene | C15H24 | 204.20 | 1.27 × 10−4 | - | 0.13 | - | 0.13 |
| 63 | 42.499 | 1411 | (-)-Isosativene | C15H24 | 204.20 | - | - | - | 3.48 × 10−5 | 7.93 × 10−6 |
| 64 | 42.981 | 1417 | Cis-β-Copaene | C15H24 | 204.20 | 3.40 × 10−4 | - | 0.01 | - | 0.04 |
| 65 | 43.590 | 1424 | Coumarin | C9H6O2 | 146.00 | - | - | - | - | 0.56 |
| 66 | 45.126 | 1442 | (E)-Cinnamyl acetate | C11H12O2 | 176.21 | 0.25 | - | 0.02 | - | 3.05 × 10−5 |
| 67 | 45.418 | 1445 | Alloaromadendrene | C15H24 | 204.35 | - | 0.06 | 0.05 | - | 0.16 |
| 68 | 46.139 | 1454 | β-Springene | C20H32 | 272.47 | 0.08 | 0.11 | 0.01 | - | 0.17 |
| 69 | 46.649 | 1460 | γ-Gurjunene | C15H24 | 204.20 | - | - | - | - | 0.06 |
| 70 | 46.913 | 1463 | Selina-4(15),7(11)-diene | C15H24 | 204.20 | - | - | - | - | 0.04 |
| 71 | 47.094 | 1465 | γ-Cadinene | C15H24 | 204.35 | 0.03 | - | 0.09 | - | 1.09 |
| 72 | 47.638 | 1472 | δ-Bisabolene | C15H24 | 204.35 | - | 0.17 | 0.01 | - | 0.08 |
| 73 | 47.992 | 1476 | α-Curcumene | C15H22 | 202.35 | 0.06 | 4.30 | 0.46 | - | 3.72 |
| 74 | 48.549 | 1483 | (+)-Calarene | C15H24 | 204.35 | - | 0.78 | 0.13 | 0.01 | 1.41 |
| 75 | 49.152 | 1490 | Zingiberene | C15H24 | 204.35 | - | 6.04 | 0.28 | 0.05 | 22.45 |
| 76 | 49.929 | 1499 | 1,2,3,4,4a,5,6,7-octahydro-4-methyl-7-methylene-1-(1-methylethyl)-, (1S,4S,4aR)-Naphthalene | C15H24 | 204.20 | - | - | 1.50 × 10−4 | 0.05 | 0.15 |
| 77 | 50.252 | 1503 | β-Bisabolene | C15H24 | 204.35 | 0.03 | 1.61 | 0.36 | - | 3.75 |
| 78 | 50.594 | 1507 | α-Farnesene | C15H24 | 204.35 | - | 1.06 | 0.14 | 3.72 × 10−5 | 3.35 |
| 79 | 51.250 | 1515 | (+)-δ-Cadinene | C15H24 | 204.35 | - | 0.13 | 0.12 | - | 1.26 × 10−4 |
| 80 | 51.505 | 1518 | β-Sesquiphellandrene | C15H24 | 204.35 | - | 2.57 | 0.37 | - | 6.93 |
| 81 | 52.065 | 1524 | 2-Methoxycinnamaldehyde | C10H10O2 | 162.18 | 0.58 | - | 0.26 | 4.35 × 10−5 | 1.71 |
| 82 | 52.385 | 1528 | (Z)-2-Methoxycinnamaldehyde | C10H10O2 | 162.10 | 1.15 × 10−4 | - | 1.00 × 10−3 | - | 1.63 × 10−4 |
| 83 | 52.764 | 1532 | α-Calacorene | C15H20 | 200.20 | - | 3.85 × 10−5 | 1.21 × 10−3 | 4.24 × 10−5 | - |
| 84 | 53.591 | 1542 | α-Elemol | C15H26O | 222.37 | - | 0.88 | 0.01 | - | 0.42 |
| 85 | 54.197 | 1549 | (Z)-Sesquisabinene hydrate | C15H26O | 222.37 | - | 0.24 | - | - | 1.42 × 10−4 |
| No. | Compounds | Peak Area | Transfer Rates (%) | ||
|---|---|---|---|---|---|
| CR | ZRR | CR-ZRR | |||
| 4 | Styrene | 14691 | 39598 | 926761 | 3414.18 |
| 5 | 2-Heptanol | - | 112327 | 20940 | 37.28 |
| 6 | Tricyclene | - | 481270 | 926761 | 385.13 |
| 7 | 3-Carene | 42254 | 6629112 | 1292984 | 38.76 |
| 8 | (+)-Camphene | 29636 | 26853528 | 3958503 | 29.45 |
| 9 | Benzaldehyde | 1350684 | - | 1810546 | 268.09 |
| 11 | Sabinen | 4930 | 1141339 | 223776 | 39.04 |
| 16 | p-Cymene | - | 451937 | 188831 | 83.57 |
| 17 | Pseudolimonene | - | 834509 | 1818212 | 435.76 |
| 18 | Eucalyptol | - | 8007077 | 2323724 | 58.04 |
| 22 | Acetophenone | 50856 | - | 544685 | 2142.07 |
| 23 | α-Terpinene | - | 878417 | 97914 | 22.29 |
| 25 | cis-Chrysanthenol | - | 269432 | 29053 | 21.57 |
| 27 | Linalool | - | 1064598 | 90822 | 17.06 |
| 28 | Benzaldehyde dimethyl acetal | 90889 | 348585 | 631424 | 287.35 |
| 30 | (-)-Camphor | - | 481313 | 77278 | 32.11 |
| 33 | Isoborneol | 624216 | 7628668 | 1611529 | 39.05 |
| 34 | 4-Terpineol | 5554 | 492279 | 53678 | 21.56 |
| 36 | (-)-α-Terpineol | 36921 | 3091945 | 313114 | 20.01 |
| 39 | Cinnamic aldehyde | 711804 | - | 318950 | 89.62 |
| 41 | (E)-verbenol | 63296 | 8466831 | 745173 | 17.47 |
| 44 | Cinnamaldehyde | 61651195 | 1837030 | 74628567 | 235.09 |
| 45 | Citral | - | 11056141 | 65508050 | 1185.01 |
| 57 | (-)-α-Copaene | 7144 | 130396 | 480610 | 698.87 |
| 58 | Methyl Cinnamate | 114634 | - | 104134 | 181.68 |
| 59 | 1,5-Cyclodecadiene | - | 538380 | 98747 | 36.68 |
| 60 | (E)-Cinnamaldehyde dimethyl acetal | 16766672 | - | 45702592 | 545.16 |
| 66 | (E)-Cinnamyl acetate | 208628 | - | 45470 | 43.59 |
| 67 | Alloaromadendrene | - | 65881 | 111250 | 337.73 |
| 68 | β-Springene | 64871 | 121349 | 17654 | 18.96 |
| 71 | γ-Cadinene | 23786 | - | 186971 | 1572.11 |
| 72 | δ-Bisabolene | - | 190706 | 27706 | 29.06 |
| 73 | α-Curcumene | 50342 | 4803278 | 984293 | 40.56 |
| 74 | (+)-Calarene | - | 869494 | 286210 | 65.83 |
| 77 | β-Bisabolene | 28633 | 1801059 | 773487 | 84.55 |
| 78 | α-Farnesene | - | 1188897 | 300444 | 50.54 |
| 79 | (+)-δ-Cadinene | - | 146588 | 244839 | 334.05 |
| 80 | β-Sesquiphellandrene | - | 2877551 | 777295 | 54.02 |
| 81 | 2-Methoxycinnamaldehyde | 478059 | - | 556297 | 232.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, X.; Li, X.; Chen, J.; Shen, C.; Shi, X.; Wang, L.; Li, C. Effects of Extraction Methods on Volatile Oil Profiles of Cinnamomi ramulus–Zingiberis rhizoma recens Couplet Medicines. Separations 2024, 11, 206. https://doi.org/10.3390/separations11070206
Xin X, Li X, Chen J, Shen C, Shi X, Wang L, Li C. Effects of Extraction Methods on Volatile Oil Profiles of Cinnamomi ramulus–Zingiberis rhizoma recens Couplet Medicines. Separations. 2024; 11(7):206. https://doi.org/10.3390/separations11070206
Chicago/Turabian StyleXin, Xiaodong, Xinnong Li, Jiabao Chen, Chuanghui Shen, Xiaohan Shi, Lei Wang, and Chunhua Li. 2024. "Effects of Extraction Methods on Volatile Oil Profiles of Cinnamomi ramulus–Zingiberis rhizoma recens Couplet Medicines" Separations 11, no. 7: 206. https://doi.org/10.3390/separations11070206
APA StyleXin, X., Li, X., Chen, J., Shen, C., Shi, X., Wang, L., & Li, C. (2024). Effects of Extraction Methods on Volatile Oil Profiles of Cinnamomi ramulus–Zingiberis rhizoma recens Couplet Medicines. Separations, 11(7), 206. https://doi.org/10.3390/separations11070206





