Green Technology for Fungal Protein Extraction—A Review
Abstract
1. Introduction
2. Fungal Proteins and Their Bioactivities
| Fungal Protein and Enzyme | Fungal Strains | Bioactivities | Experimental Model | Key Findings | Refs. |
|---|---|---|---|---|---|
| E5PcF3, E3AbF6 | Pleurotus cystidiosus, Agaricus bisporus | Anti-hypertensive | In silico | The antihypertensive activity in the two mushroom species could be due to proteins with molecular masses ranging from 3 to 10 kDa. | [15] |
| L-amino acid oxidases (LAOs) | Amanita phalloides, Infundibulicybe geotropa | Antibacterial | In vitro and In vivo | The in vitro and in vivo antibacterial efficacy of LAOs against various bacterial species (R. solanacearum and other plant pathogenic Bacteria) highlights their potential as new biological phytoprotective agents. | [16] |
| D-amino acid oxidase (DAAO) | Rhodotorula gracilis | Antibacterial | In vitro | DAAO reduced bacterial growth on various foodstuffs, with 10-fold fewer colonies on grated cheese after 16 h at 37 °C when 0.01 mg (1.2 units) of DAAO was added. | [17] |
| PeAfpA | Penicillium expansum | Antifungal | In vitro | PeAfpA was demonstrated to efficiently protect against fungal infections caused by Botrytis cinerea in tomato leaves and Penicillium digitatum in oranges. | [18] |
| Trichogin | Tricholoma giganteum | Antifungal | In vitro | Trichogin exhibited antifungal activity against Fusarium oxysporum, Mycosphaerella arachidicola and Physalospora piricola. It also inhibited HIV-1 reverse transcriptase with an IC50 of 83 nM. | [19] |
| Tsa1 | Saccharomyces cerevisiae | Antioxidant | In vitro | Deregulated Tsa1 promotes translation defects, including hypersensitivity to inhibitors, increased error rates, and protein aggregation, suggesting its broader implications in stress and growth control. | [20] |
| Lectins | Paxillus involutus | Antiphytovirus activity | In vitro | The Paxillus involutus lectin possesses antiphytovirus activity against tobacco mosaic virus with 70.6% inhibition at a concentration of 200 μg/mL. | [24] |
| Ski2 | Saccharomyces cerevisiae | Antiphytovirus activity | In vitro | Ski2, a cytoplasmic RNA helicase with a broad RNA-binding specificity and distinct structural features, functions with the exosome in mRNA turnover and quality control, suggesting its potential antiviral activity through the degradation of viral RNA in the cytoplasm. | [22,23] |
| Lectins | Agaricus bisporus | Anticancer | In vitro | Mannose impeded lectin-like protein orf239342’s ability to inhibit the proliferation of the MCF-7 breast cancer cells, providing further evidence for the mannose-binding onto the protein. | [24] |
| FIP-bbo | Botryobasidium botryosum | Anticancer | In vitro | Anti-proliferation, pro-apoptosis, and inhibiting migration experiments on Hela, Spca-1 and A549 showed that rFIP-bbo has anticancer activity. The anticancer activity of the rFIP-bbo lies between that of rLZ-8 and rFIP-fve. | [25] |
3. Challenges in Fungal Protein Extraction and Maintenance Quality
3.1. Cell Wall Structure
3.2. Extraction Methods
3.3. Protein Solubility
3.4. Contaminant Removal
3.5. Protein Stability
3.6. Quantification and Quality Assessment
4. Cell Wall Disruption by Green Extraction Technologies
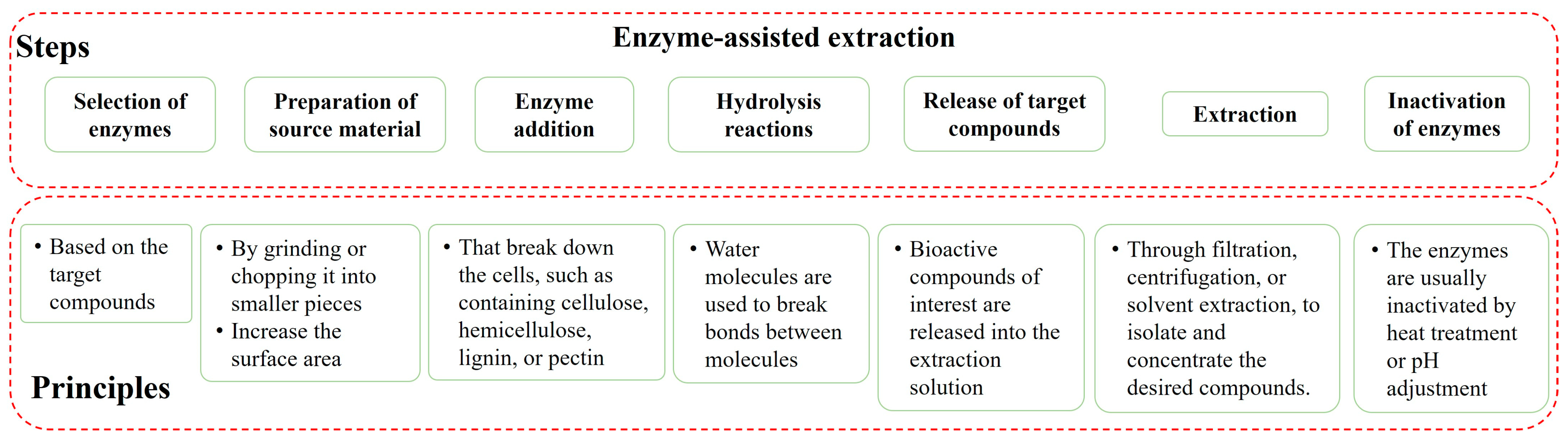
4.1. Enzyme-Assisted Extraction
4.2. Mild Mechanical Methods
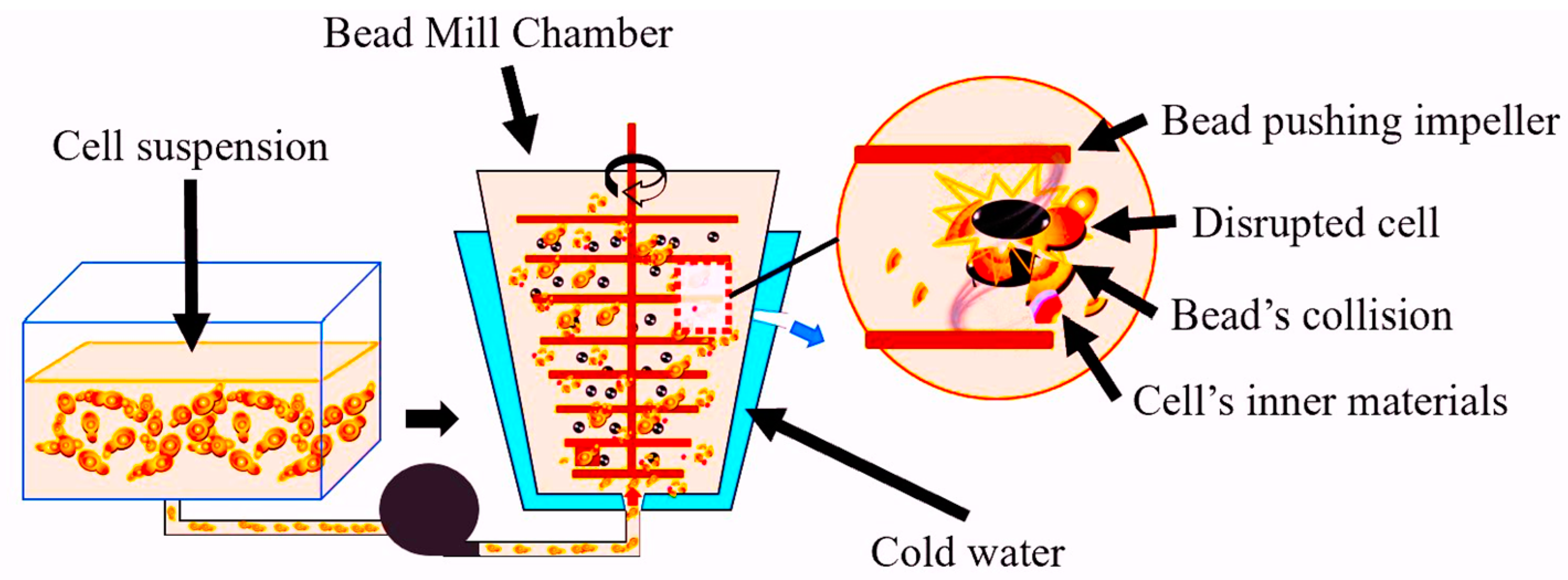
4.2.1. Bead Milling
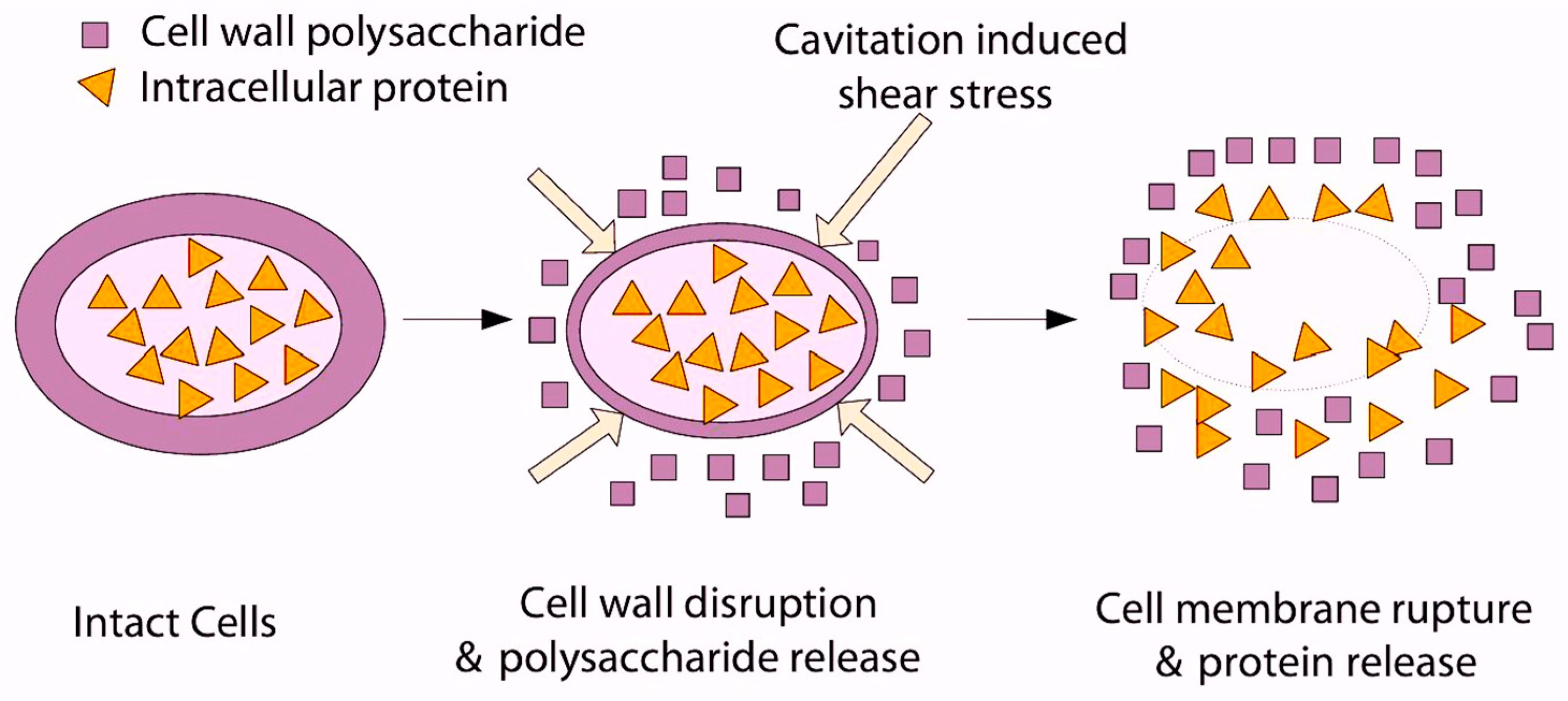
4.2.2. Ultrasonication
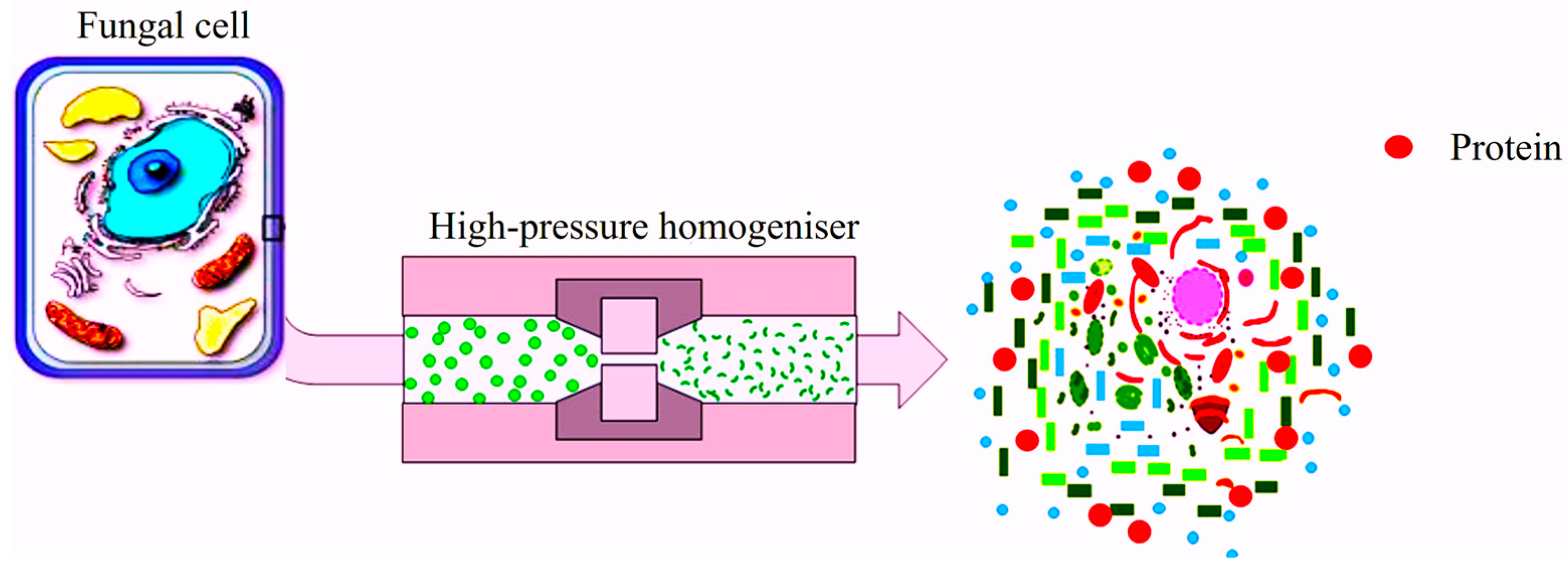
4.2.3. High-Pressure Homogenisation
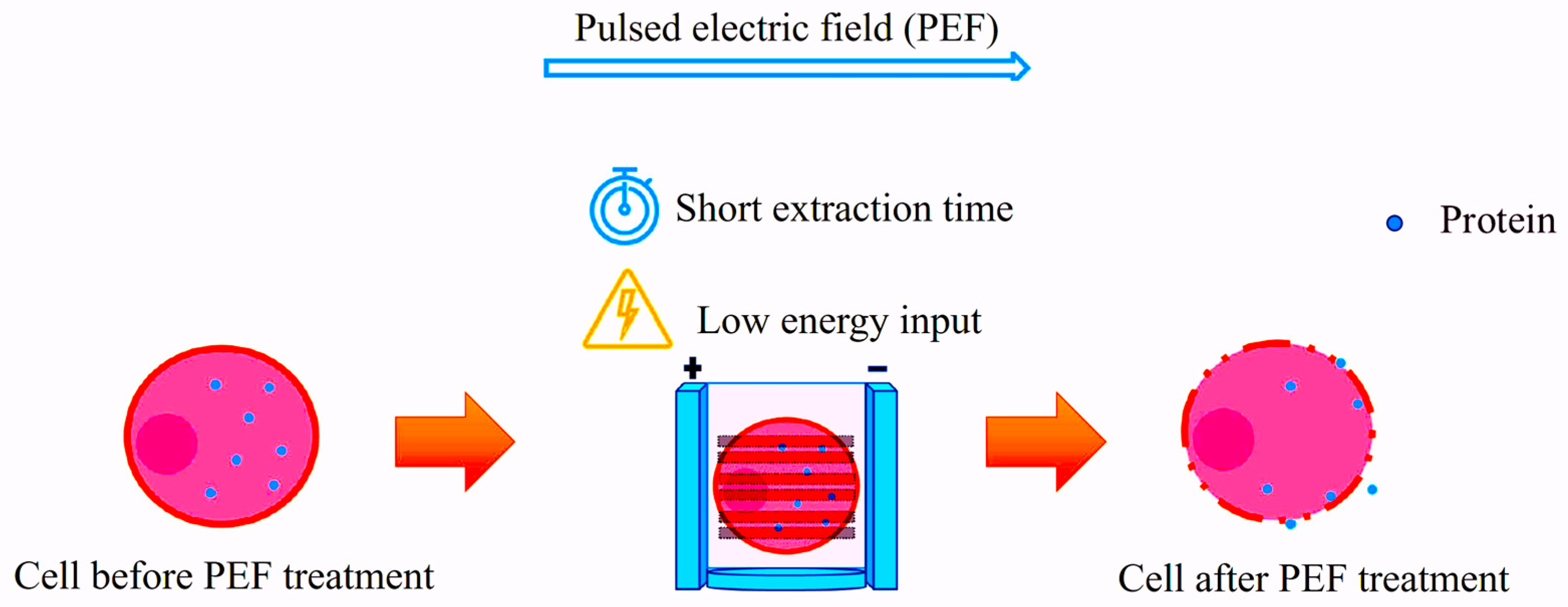
4.3. Pulsed Electric Fields
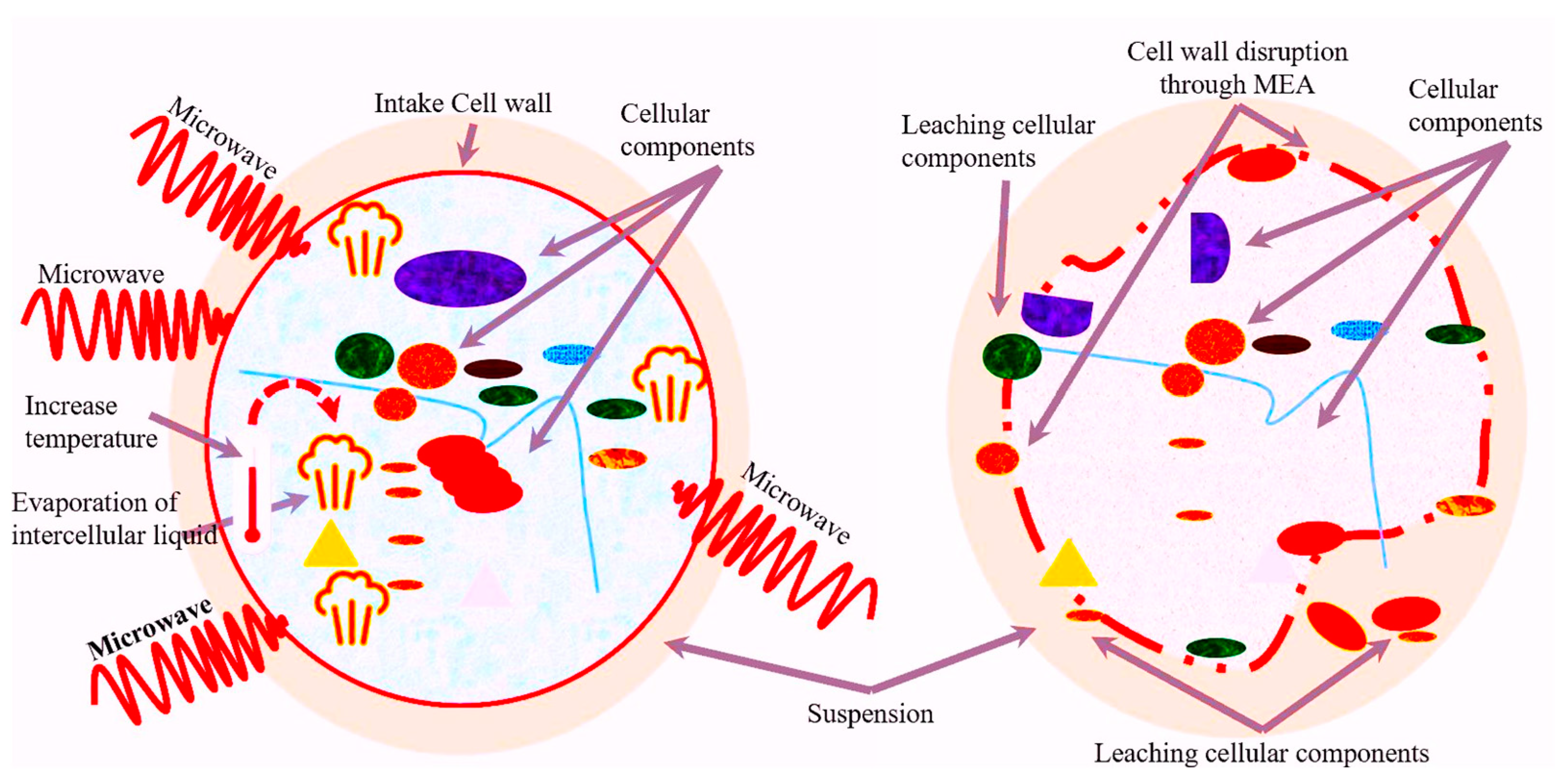
4.4. Microwave-Assisted Extraction
4.5. Supercritical Fluid Extraction
4.6. Innovative Solvent Use
5. Application of Green Extraction Technologies for Fungal Protein Extraction
| Green Extraction Technology | Fungi Species | Experimental Conditions | Protein Yield | Protein Type | Key Findings | Refs. |
|---|---|---|---|---|---|---|
| Ultrasound-assisted extraction | Saccharomyces cerevisiae | Ultrasonic conditions: power-250, 300, 350, 400, and 450 W; pH-5.5, 6.5, 7.5, 8.5, and 9.5; Solid–liquid ratio: 6%, 8%, 10%, 12%, and 14% Enzyme used: trypsin | 73.94% | Antioxidant | The polypeptide’s scavenging activity against hydroxyl radical, DPPH radical, and ABTS radical reached 95.10%, 98.37%, and 69.41%, respectively. | [79] |
| Cordyceps militaris | Ultrasonic conditions: temperature: 25 °C; power: 100 W; pH: 8.0, 8.5, and 9.0; Solid–liquid ratio: 1:25, 1:28, and 1:30; time: 3.0, 3.2, and 3.5 h Enzymes used: alkaline protease, neutral protease, papain, trypsin, and pepsin | 45.06% | Antimicrobial and anticancer polypeptides | Polypeptides (<3000 Da) showed good antibacterial activity against Escherichia coli, Bacillus subtilis, and Staphylococcus aureus, with inhibitory zones of (12.08 ± 0.22), (6.67 ± 0.12), and (10.32 ± 0.23) mm, respectively. | [82] | |
| Enzymatic-assisted extraction | Agricus bisporus | Alkaline protease, pH: 8.43, enzymolysis temperature: 44.32 °C, and enzymolysis time: 3.52 h | 6.678%. | ACE inhibitor | The average activity of the three novel ACE inhibitory peptides was 80.68%, and the IC50 value was 0.9 mg/mL. | [78] |
| Se-rich brewer’s yeast | Alkaline protease, pH: 11, temperature: 60 °C, enzyme to substrate ratio: 6000 U/g. | 100-fold | Se-rich peptide fraction | In vitro free radical scavenging and lipid peroxidation inhibition assays showed that Se-rich peptide fractions with lower MW of <1 kDa had the highest antioxidant activity compared with Se-rich peptide fractions with higher MW of <3 kDa or normal peptide fractions. | [77] | |
| Pulsed electric fields-assisted extraction | Saccharomyces cerevisiae | 10, 15, and 20 kV/cm, 39.8 and 159.3 Hz, 50–200 µs | 187.82 ± 3.75 mg/g dry weight (protein) | Antioxidant | 84–89% of the total antioxidant activity | [87] |
| Pressure extraction (PE) assisted by pulsed electric field (PEF) | Agaricus bisporus | 100–1000 V/cm, 5 bar, 0.4 s | The maximum protein yield using PE alone was about 0.26, but with PEF extraction, it increased to around 0.42. | Polyphenol-enriched protein | The PE + PEF method gave a higher ratio of nucleic acid/proteins in comparison with the PE method. | [85] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Moura, M.A.F.e.; Martins, B.d.A.; Oliveira, G.P.d.; Takahashi, J.A. Alternative Protein Sources of Plant, Algal, Fungal and Insect Origins for Dietary Diversification in Search of Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2023, 63, 10691–10708. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a Circular Economy with Fungal Biotechnology: A White Paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Niego, A.G.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Walker, A.; Hyde, K.D.; et al. The Contribution of Fungi to the Global Economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Farkaš, V. The Fungal Cell Wall. In Fungal Protoplasts; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–29. [Google Scholar]
- Goldberg, S. Mechanical/Physical Methods of Cell Disruption and Tissue Homogenization. In Proteomic Profiling: Methods and Protocols; Humana Press: New York, NY, USA, 2021; pp. 563–585. [Google Scholar]
- Krishnarjuna, B.; Ramamoorthy, A. Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a near-Native Membrane Environment. Biomolecules 2022, 12, 1076. [Google Scholar] [CrossRef]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. Industrial Use of Cell Wall Degrading Enzymes: The Fine Line between Production Strategy and Economic Feasibility. Front. Bioeng. Biotechnol. 2020, 8, 356. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent Trends in Extraction of Plant Bioactives Using Green Technologies: A Review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Safwa, S.M.; Ahmed, T.; Talukder, S.; Sarker, A.; Rana, M.R. Applications of Non-Thermal Technologies in Food Processing Industries—A Review. J. Agric. Food Res. 2023, 100917. [Google Scholar] [CrossRef]
- Geng, P.; Siu, K.-C.; Wang, Z.; Wu, J.-Y. Antifatigue Functions and Mechanisms of Edible and Medicinal Mushrooms. BioMed Res. Int. 2017, 2017, 9648496. [Google Scholar] [CrossRef]
- Liu, Y.; Bastiaan-Net, S.; Wichers, H.J. Current Understanding of the Structure and Function of Fungal Immunomodulatory Proteins. Front. Nutr. 2020, 7, 132. [Google Scholar] [CrossRef]
- Tanaka, S.; Kahmann, R. Cell Wall–Associated Effectors of Plant-Colonizing Fungi. Mycologia 2021, 113, 247–260. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 492056. [Google Scholar] [CrossRef]
- Lau, C.-C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Proteomic Analysis of Antihypertensive Proteins in Edible Mushrooms. J. Agric. Food Chem. 2012, 60, 12341–12348. [Google Scholar] [CrossRef]
- Sabotič, J.; Brzin, J.; Erjavec, J.; Dreo, T.; Tušek Žnidarič, M.; Ravnikar, M.; Kos, J. L-Amino Acid Oxidases from Mushrooms Show Antibacterial Activity against the Phytopathogen Ralstonia Solanacearum. Front. Microbiol. 2020, 11, 977. [Google Scholar] [CrossRef]
- Marcone, G.L.; Binda, E.; Rosini, E.; Abbondi, M.; Pollegioni, L. Antibacterial Properties of D-Amino Acid Oxidase: Impact on the Food Industry. Front. Microbiol. 2019, 10, 488234. [Google Scholar] [CrossRef]
- Garrigues, S.; Gandía, M.; Castillo, L.; Coca, M.; Marx, F.; Marcos, J.F.; Manzanares, P. Three Antifungal Proteins from Penicillium Expansum: Different Patterns of Production and Antifungal Activity. Front. Microbiol. 2018, 9, 2370. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Ng, T.B. Isolation of Trichogin, an Antifungal Protein from Fresh Fruiting Bodies of the Edible Mushroom Tricholoma Giganteum. Peptides 2005, 26, 575–580. [Google Scholar] [CrossRef]
- Trotter, E.W.; Rand, J.D.; Vickerstaff, J.; Grant, C.M. The Yeast Tsa1 Peroxiredoxin Is a Ribosome-Associated Antioxidant. Biochem. J. 2008, 412, 73–80. [Google Scholar] [CrossRef]
- Weids, A.J.; Grant, C.M. The Yeast Peroxiredoxin Tsa1 Protects against Protein-Aggregate-Induced Oxidative Stress. J. Cell Sci. 2014, 127, 1327–1335. [Google Scholar] [CrossRef]
- Widner, W.R.; Wickner, R.B. Evidence That the SKI Antiviral System of Saccharomyces Cerevisiae Acts by Blocking Expression of Viral MRNA. Mol. Cell. Biol. 1993, 13, 4331–4341. [Google Scholar]
- Brown, J.T.; Bai, X.; Johnson, A.W. The Yeast Antiviral Proteins Ski2p, Ski3p, and Ski8p Exist as a Complex in Vivo. RNA 2000, 6, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Zhang, G.Q.; Zhao, S.; Xu, F.; Zhou, Y.; Li Geng, X.; Liu, Y.; Wang, H.X. Purification and Characterization of a Novel Lectin with Antiphytovirus Activities from the Wild Mushroom Paxillus Involutus. Protein Pept. Lett. 2013, 20, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.N.; Bai, R.; Chen, H.Y.; Wu, Y.Y.; Shang, J.J.; Bao, D.P. Identification of a Novel Anti-Cancer Protein, FIP-Bbo, from Botryobasidium Botryosum and Protein Structure Analysis Using Molecular Dynamic Simulation. Sci. Rep. 2019, 9, 5818. [Google Scholar] [CrossRef]
- Carvalho, V.S.D.; Gómez-Delgado, L.; Curto, M.Á.; Moreno, M.B.; Pérez, P.; Ribas, J.C.; Cortés, J.C.G. Analysis and Application of a Suite of Recombinant Endo-β(1, 3)-D-Glucanases for Studying Fungal Cell Walls. Microb. Cell Fact. 2021, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Akhi, A.; Ahmed, T.; Ara, R.; Rana, M.R. Response Surface Optimization of Thermo-Sonication Conditions and Taro Mucilage Concentrations for the Preparation of Soy Yogurt. J. Agric. Food Res. 2024, 15, 100918. [Google Scholar] [CrossRef]
- Safwa, S.M.; Rana, M.R.; Ahmed, T.; Rahman, S.; Kabir, M.A. Bin Maximization and Characterization of Ultrasonic-Assisted Extraction of Taro Corms Mucilage Using Response Surface Optimization and Comparison with Conventional Methods. Food Anal. Methods 2023, 16, 1724–1737. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Zhu, G.; Luo, S.; Zhang, D.; Liu, F.; Shen, Y. Modification of the Structural and Functional Properties of Wheat Gluten Protein Using a Planetary Ball Mill. Food Chem. 2021, 363, 130251. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-Assisted Extraction and Modification of Plant-Based Proteins: Impact on Physicochemical, Functional, and Nutritional Properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Mesa, J.; Hinestroza-Córdoba, L.I.; Barrera, C.; Seguí, L.; Betoret, E.; Betoret, N. High Homogenization Pressures to Improve Food Quality, Functionality and Sustainability. Molecules 2020, 25, 3305. [Google Scholar] [CrossRef]
- Kopac, T. Emerging Applications of Process Intensification for Enhanced Separation and Energy Efficiency, Environmentally Friendly Sustainable Adsorptive Separations: A Review. Int. J. Energy Res. 2021, 45, 15839–15856. [Google Scholar] [CrossRef]
- Gouseti, O.; Larsen, M.E.; Amin, A.; Bakalis, S.; Petersen, I.L.; Lametsch, R.; Jensen, P.E. Applications of Enzyme Technology to Enhance Transition to Plant Proteins: A Review. Foods 2023, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, M.F.; Fai, C.K.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Current Pretreatment/Cell Disruption and Extraction Methods Used to Improve Intracellular Lipid Recovery from Oleaginous Yeasts. Microorganisms 2021, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.A.; Zanette, C.M.; Spier, M.R. An Overview of Cell Disruption Methods for Intracellular Biomolecules Recovery. Prep. Biochem. Biotechnol. 2020, 50, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Suzauddula, M.; Safwa, S.M.; Talukder, S.; Sharma, M.; Inbaraj, B.S.; Sridhar, K. Biotechnological Tools in Microbial Vitamins and Carotenoids Production. In Microbial Vitamins and Carotenoids in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 63–104. [Google Scholar]
- Salve, A.L.; Apotikar, S.B.; Muddebihalkar, S.V.; Joshi, K.S.; Kulkarni, S.S. Applications of Hydrophobins in Medical Biotechnology. J. Sci. Res. 2022, 66, 86–94. [Google Scholar]
- Krishnaswamy, A.; Barnes, N.; Lotlikar, N.P.; Damare, S.R. An Improved Method for Protein Extraction from Minuscule Quantities of Fungal Biomass. Indian J. Microbiol. 2019, 59, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Zhao, S.-Q.; Zhang, S.-L.; Luo, L.-H.; Liu, D.-C.; Ding, W.-H.; Fu, D.-J.; Deng, X.-D.; Yin, D.-C. Database Study on the Expression and Purification of Membrane Proteins. Protein Pept. Lett. 2021, 28, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, A.; Kilikian, B.V. Purification Process of Biomolecules. In Pharmaceutical Biotechnology; CRC Press: Boca Raton, FL, USA, 2021; pp. 225–237. [Google Scholar]
- Himel, M.A.R.; Ahmed, T.; Hossain, M.A.; Moazzem, M.S. Response Surface Optimization to Extract Antioxidants from Freeze-Dried Seeds and Peel of Pomegranate (Punica granatum L.). Biomass Convers. Biorefinery 2024, 14, 9707–9722. [Google Scholar] [CrossRef]
- Akbarian, M.; Chen, S.-H. Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 2022, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Bharmoria, P.; Tietze, A.A.; Mondal, D.; Kang, T.S.; Kumar, A.; Freire, M.G. Do Ionic Liquids Exhibit the Required Characteristics to Dissolve, Extract, Stabilize, and Purify Proteins? Past-Present-Future Assessment. Chem. Rev. 2024, 124, 3037–3084. [Google Scholar] [CrossRef]
- Ahmed, T.; Rana, M.R.; Zzaman, W.; Ara, R.; Aziz, M.G. Optimization of Substrate Composition for Pectinase Production from Satkara (Citrus macroptera) Peel Using Aspergillus Niger-ATCC 1640 in Solid-State Fermentation. Heliyon 2021, 7, e08133. [Google Scholar] [CrossRef]
- Kumar, A. Protein Purification, Estimation, Storage, and Effect on Structure—Function—Dynamics. In Frontiers in Protein Structure, Function, and Dynamics; Springer: Singapore, 2020; pp. 1–22. [Google Scholar]
- Yüce, M.; Sert, F.; Torabfam, M.; Parlar, A.; Gürel, B.; Çakır, N.; Dağlıkoca, D.E.; Khan, M.A.; Çapan, Y. Fractionated Charge Variants of Biosimilars: A Review of Separation Methods, Structural and Functional Analysis. Anal. Chim. Acta 2021, 1152, 238189. [Google Scholar] [CrossRef] [PubMed]
- Gautério, G.V.; da Silva, R.M.; Karraz, F.C.; Coelho, M.A.Z.; Ribeiro, B.D.; Lemes, A.C. Cell Disruption and Permeabilization Methods for Obtaining Yeast Bioproducts. Clean. Chem. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- Islam, M.N.; Mishra, V.K.; Munalisa, R.; Parveen, F.; Ali, S.F.; Akter, K.; Ahmed, T.; Ho, T.-J.; Huang, C.-Y. Mechanistic Insight of Mitochondrial Dysfunctions in Cardiovascular Diseases with Potential Biomarkers. Mol. Cell. Toxicol. 2024, 1–23. [Google Scholar] [CrossRef]
- Van Hung, P.; Yen Nhi, N.H.; Ting, L.Y.; Lan Phi, N.T. Chemical Composition and Biological Activities of Extracts from Pomelo Peel By-Products under Enzyme and Ultrasound-Assisted Extractions. J. Chem. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Chirinos, R.; Aquino, M.; Pedreschi, R.; Campos, D. Optimized Methodology for Alkaline and Enzyme-Assisted Extraction of Protein from Sacha Inchi (Plukenetia volubilis) Kernel Cake. J. Food Process Eng. 2017, 40, e12412. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F.; Hussain, A.I.; Shahid, M. Aqueous Enzymatic Process for Oil and Protein Extraction from Moringa Oleifera Seed. Eur. J. Lipid Sci. Technol. 2011, 113, 1012–1018. [Google Scholar] [CrossRef]
- Liu, R.; Chu, X.; Su, J.; Fu, X.; Kan, Q.; Wang, X.; Zhang, X. Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax Senticosus and Their Enrichment and Antioxidant Properties. Processes 2021, 9, 1708. [Google Scholar] [CrossRef]
- Sabuz, A.A.; Rana, M.R.; Ahmed, T.; Molla, M.M.; Islam, N.; Khan, H.H.; Chowdhury, G.F.; Zhao, Q.; Shen, Q. Health-Promoting Potential of Millet: A Review. Separations 2023, 10, 80. [Google Scholar] [CrossRef]
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. [Google Scholar] [CrossRef]
- Ahmed, T.; Rana, M.R.; Maisha, M.R.; Sayem, A.S.M.; Rahman, M.; Ara, R. Optimization of Ultrasound-Assisted Extraction of Phenolic Content & Antioxidant Activity of Hog Plum (Spondias pinnata L. f. Kurz) Pulp by Response Surface Methodology. Heliyon 2022, 8, e11109. [Google Scholar]
- Ahmed, T.; Rana, M.R.; Hossain, M.A.; Ullah, S.; Suzauddula, M. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Total Anthocyanin Content, Total Phenolic Content, and Antioxidant Activities of Roselle (Hibiscus sabdariffa L.) Calyces and Comparison with Conventional Soxhlet Extraction. Biomass Convers. Biorefinery 2023, 1–15. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, X.; Hu, A.; Zhang, L.; Jin, Y.; Abid, M. Ultrasonic Disruption of Yeast Cells: Underlying Mechanism and Effects of Processing Parameters. Innov. Food Sci. Emerg. Technol. 2015, 28, 59–65. [Google Scholar] [CrossRef]
- Li, Y.; Kiani, H.; Tiwari, B.K.; Halim, R. Unit Operations Applied to Cell Disruption of Microalgae. In 3rd Generation Biofuels; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 225–248. [Google Scholar]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of Pulsed Electric Fields and High Pressure Homogenization on the Aqueous Extraction of Intracellular Compounds from the Microalgae Chlorella Vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Guionet, A.; Hosseini, B.; Teissié, J.; Akiyama, H.; Hosseini, H. A New Mechanism for Efficient Hydrocarbon Electro-Extraction from Botryococcus Braunii. Biotechnol. Biofuels 2017, 10, 39. [Google Scholar] [CrossRef]
- Shiina, S.; Ohshima, T.; Sato, M. Extracellular Release of Recombinant α-Amylase from Escherichia Coli Using Pulsed Electric Field. Biotechnol. Prog. 2004, 20, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wen, L.; Liu, J.; Li, Y.; Zheng, F.; Min, W.; Yue, H.; Pan, P. Optimisation of Pulsed Electric Fields Extraction of Anthocyanin from Beibinghong Vitis Amurensis Rupr. Nat. Prod. Res. 2018, 32, 23–29. [Google Scholar] [CrossRef]
- Lu, C.-W.; Yin, Y.-G.; Yu, Q.-Y. Optimized Extraction of Ginsenosides from Ginseng Root (Panax ginseng CA Meyer) by Pulsed Electric Field Combined with Commercial Enzyme. J. Food Process. Preserv. 2017, 41, e12766. [Google Scholar] [CrossRef]
- Guo, J.; Dang, J.; Wang, K.; Zhang, J.; Fang, J. Effects of Nanosecond Pulsed Electric Fields (NsPEFs) on the Human Fungal Pathogen Candida Albicans: An in Vitro Study. J. Phys. D Appl. Phys. 2018, 51, 185402. [Google Scholar] [CrossRef]
- Okamoto, S.; Murakami, Y.; Urabe, G.; Katsuki, S. Nondistractive Extraction of Intracellular Molecules from Yeast Using PEF-Assisted Autolysis. Electr. Eng. Jpn. 2022, 215, e23372. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed Electric Field Applications for the Extraction of Bioactive Compounds from Food Waste and By-Products: A Critical Review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Gartshore, A.; Kidd, M.; Joshi, L.T. Applications of Microwave Energy in Medicine. Biosensors 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Tiwari, B.; Garcia-Vaquero, M. Emerging Extraction Techniques: Microwave-Assisted Extraction. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–224. [Google Scholar]
- Li, L.; Guo, H.-J.; Zhu, L.-Y.; Zheng, L.; Liu, X. A Supercritical-CO2 Extract of Ganoderma Lucidum Spores Inhibits Cholangiocarcinoma Cell Migration by Reversing the Epithelial—Mesenchymal Transition. Phytomedicine 2016, 23, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vega, S.; Salmeron, I.; Perez-Reyes, I.; Kwofie, E.; Ngadi, M. Influence of the Supercritical Fluid Extraction (SFE) on Food Bioactives. In Retention of Bioactives in Food Processing; Springer: Berlin/Heidelberg, Germany, 2022; pp. 309–340. [Google Scholar]
- El-Deen, A.K.; Shimizu, K. Deep Eutectic Solvents as Promising Green Solvents in Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplet: Recent Applications, Challenges and Future Perspectives. Molecules 2021, 26, 7406. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.Q.; Abbasi, N.M.; Smith, E.A.; Petrich, J.W.; Anderson, J.L. Characterizing the Solvation Characteristics of Deep Eutectic Solvents Composed of Active Pharmaceutical Ingredients as a Hydrogen Bond Donor and/or Acceptor. ACS Sustain. Chem. Eng. 2022, 10, 3066–3078. [Google Scholar] [CrossRef]
- Yadav, N.; Venkatesu, P. Current Understanding and Insights towards Protein Stabilization and Activation in Deep Eutectic Solvents as Sustainable Solvent Media. Phys. Chem. Chem. Phys. 2022, 24, 13474–13509. [Google Scholar] [CrossRef]
- Cao, J.; Wu, R.; Zhu, F.; Dong, Q.; Su, E. Enzymes in Nearly Anhydrous Deep Eutectic Solvents: Insight into the Biocompatibility and Thermal Stability. Enzyme Microb. Technol. 2022, 157, 110022. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, S.; Liu, H. Antioxidant Activity and Inhibition of Ultraviolet Radiation-Induced Skin Damage of Selenium-Rich Peptide Fraction from Selenium-Rich Yeast Protein Hydrolysate. Bioorg. Chem. 2020, 105, 104431. [Google Scholar] [CrossRef]
- Wang, R.; Yun, J.; Wu, S.; Bi, Y.; Zhao, F. Optimisation and Characterisation of Novel Angiotensin-Converting Enzyme Inhibitory Peptides Prepared by Double Enzymatic Hydrolysis from Agaricus bisporus Scraps. Foods 2022, 11, 394. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Feng, Y.; Yin, H.; Lai, H.; Xiao, R.; He, S.; Yang, Z.; He, Y. Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast. Molecules 2022, 27, 6825. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Khalid, W.; Al-Farga, A.; Alansari, W.S.; Shamlan, G.; Eskandrani, A.A. Ultrasound-Assisted Extraction of Protein from Moringa Oleifera Seeds and Its Impact on Techno-Functional Properties. Molecules 2023, 28, 2554. [Google Scholar] [CrossRef] [PubMed]
- Pan-Utai, W.; Pantoa, T.; Roytrakul, S.; Praiboon, J.; Kosawatpat, P.; Tamtin, M.; Thongdang, B. Ultrasonic-Assisted Extraction and Antioxidant Potential of Valuable Protein from Ulva Rigida Macroalgae. Life 2022, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, S.; Li, Y.; Guo, W.; Guo, X.; Hong, S. Ultrasound-Assisted Enzymatic Extraction and Bioactivity Analysis of Polypeptides from Cordyceps Militaris. J. Chem. 2023, 2023, 1233867. [Google Scholar] [CrossRef]
- Jacob, F.F.; Hutzler, M.; Methner, F.-J. Comparison of Various Industrially Applicable Disruption Methods to Produce Yeast Extract Using Spent Yeast from Top-Fermenting Beer Production: Influence on Amino Acid and Protein Content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Martinez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Pulsed Electric Field Assisted Pressure Extraction and Solvent Extraction from Mushroom (Agaricus bisporus). Food Bioprocess Technol. 2014, 7, 174–183. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Fang, Z. Efficient Physical Extraction of Active Constituents from Edible Fungi and Their Potential Bioactivities: A Review. Trends Food Sci. Technol. 2020, 105, 468–482. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential Extraction of Compounds of Interest from Yeast Biomass Assisted by Pulsed Electric Fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for Biofuels: A Review of Thermochemical Conversion Processes and Associated Opportunities and Challenges. Bioresour. Technol. Rep. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Nian, B.; Cao, C.; Liu, Y. How Candida Antarctica Lipase B Can Be Activated in Natural Deep Eutectic Solvents: Experimental and Molecular Dynamics Studies. J. Chem. Technol. Biotechnol. 2020, 95, 86–93. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; D’Arrigo, P.; Griffini, G.; Marzorati, S.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective. Fermentation 2022, 8, 151. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.; Suzauddula, M.; Akter, K.; Hossen, M.; Islam, M.N. Green Technology for Fungal Protein Extraction—A Review. Separations 2024, 11, 186. https://doi.org/10.3390/separations11060186
Ahmed T, Suzauddula M, Akter K, Hossen M, Islam MN. Green Technology for Fungal Protein Extraction—A Review. Separations. 2024; 11(6):186. https://doi.org/10.3390/separations11060186
Chicago/Turabian StyleAhmed, Tanvir, Md Suzauddula, Khadiza Akter, Monir Hossen, and Md Nazmul Islam. 2024. "Green Technology for Fungal Protein Extraction—A Review" Separations 11, no. 6: 186. https://doi.org/10.3390/separations11060186
APA StyleAhmed, T., Suzauddula, M., Akter, K., Hossen, M., & Islam, M. N. (2024). Green Technology for Fungal Protein Extraction—A Review. Separations, 11(6), 186. https://doi.org/10.3390/separations11060186








