Synthesis of Quaternary-Ammonium-Lignin-Based Ionic Liquids and Comparison of Extraction Behavior of Co(II) and Ni(II) with 2-Ethylhexyl Phosphoric Acid Mono-2-Ethylhexyl Ester
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
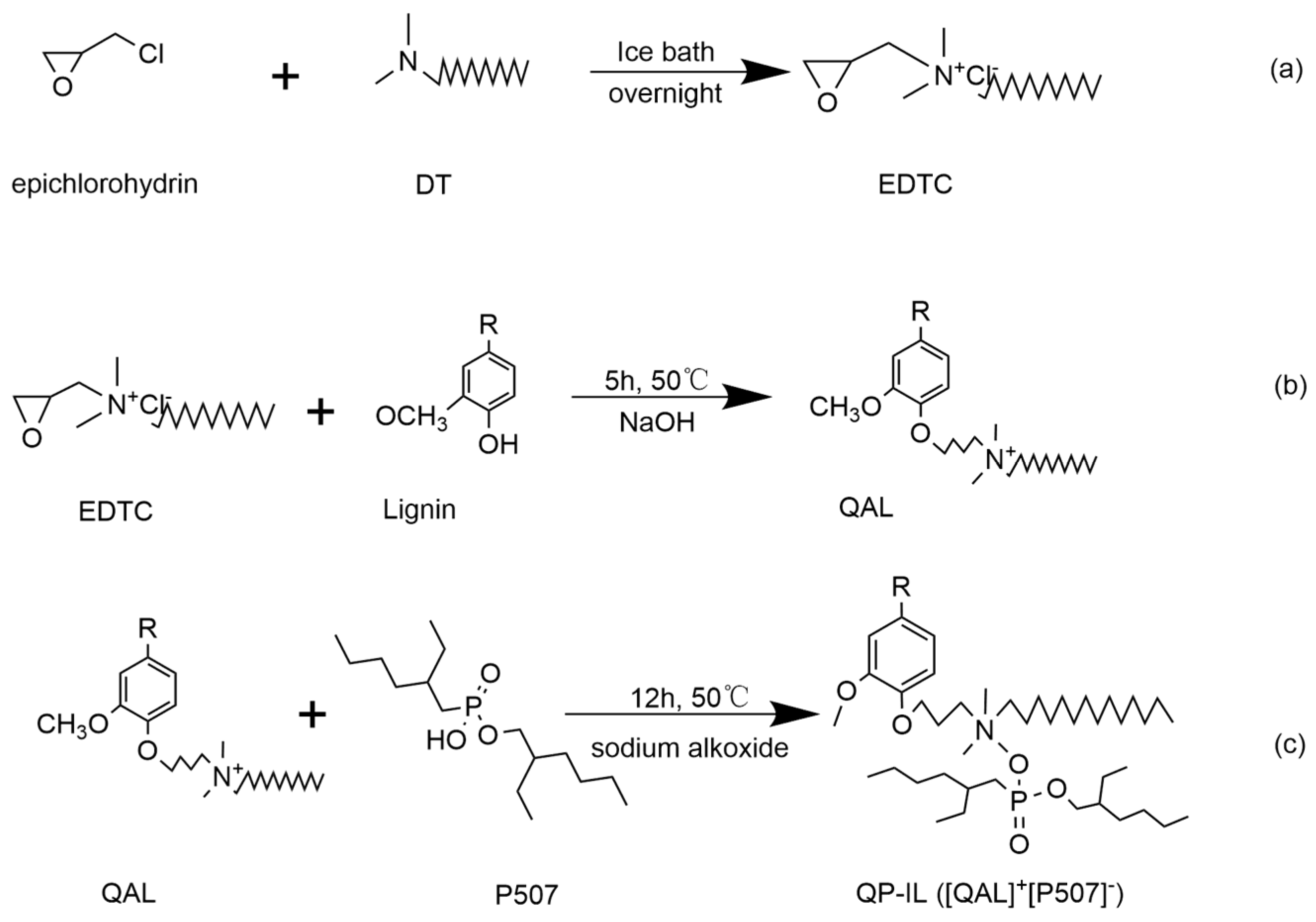
2.2. Synthesis of QP–IL
2.2.1. Synthesis of Quaternary Ammonium Lignin (QAL)
2.2.2. Synthesis of Quaternary–Ammonium–Lignin–Based IL (QP–IL)
2.3. General Procedure for Co and Ni Extraction
2.4. Characterization
3. Results
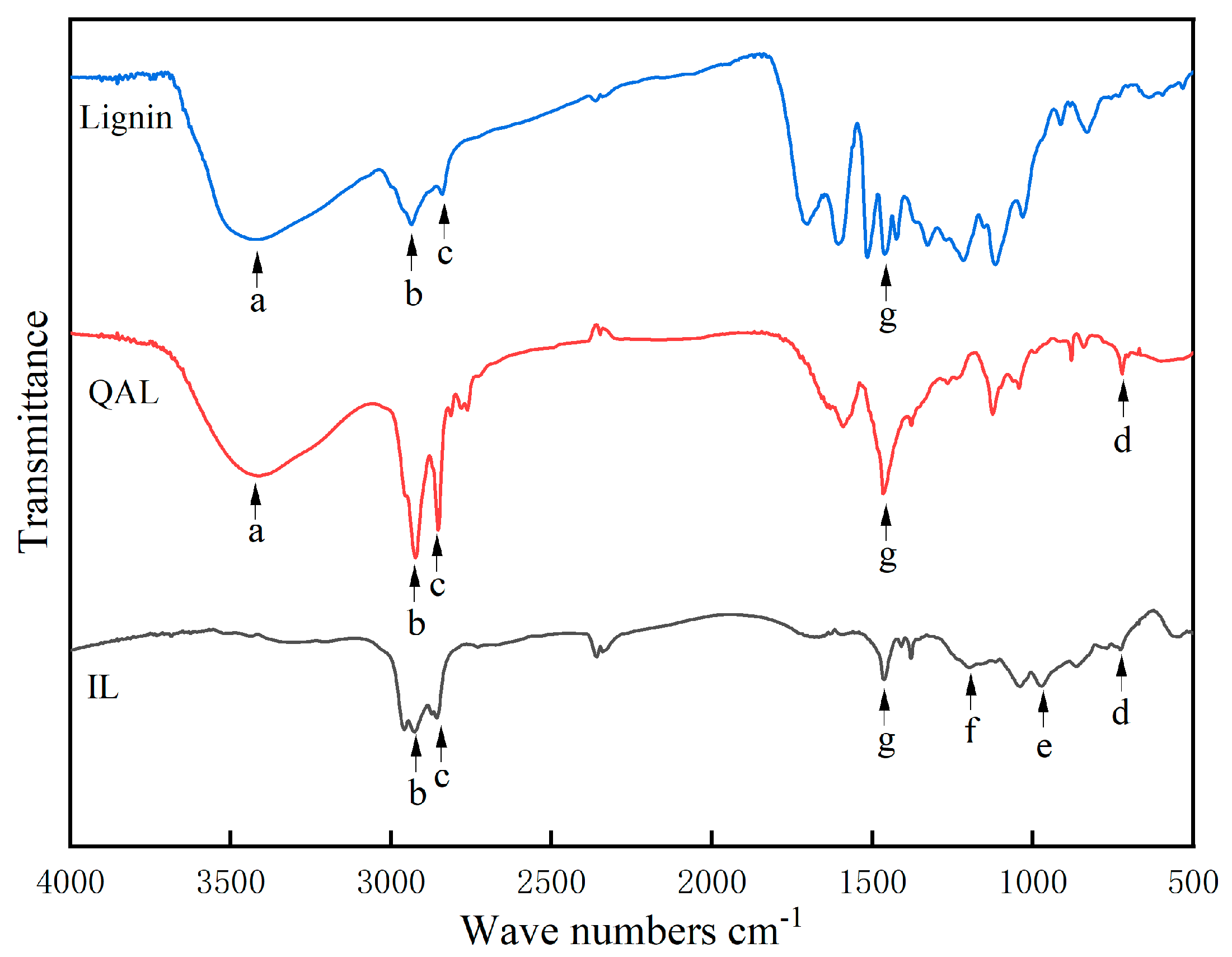
3.1. FTIR Spectra of QP–IL
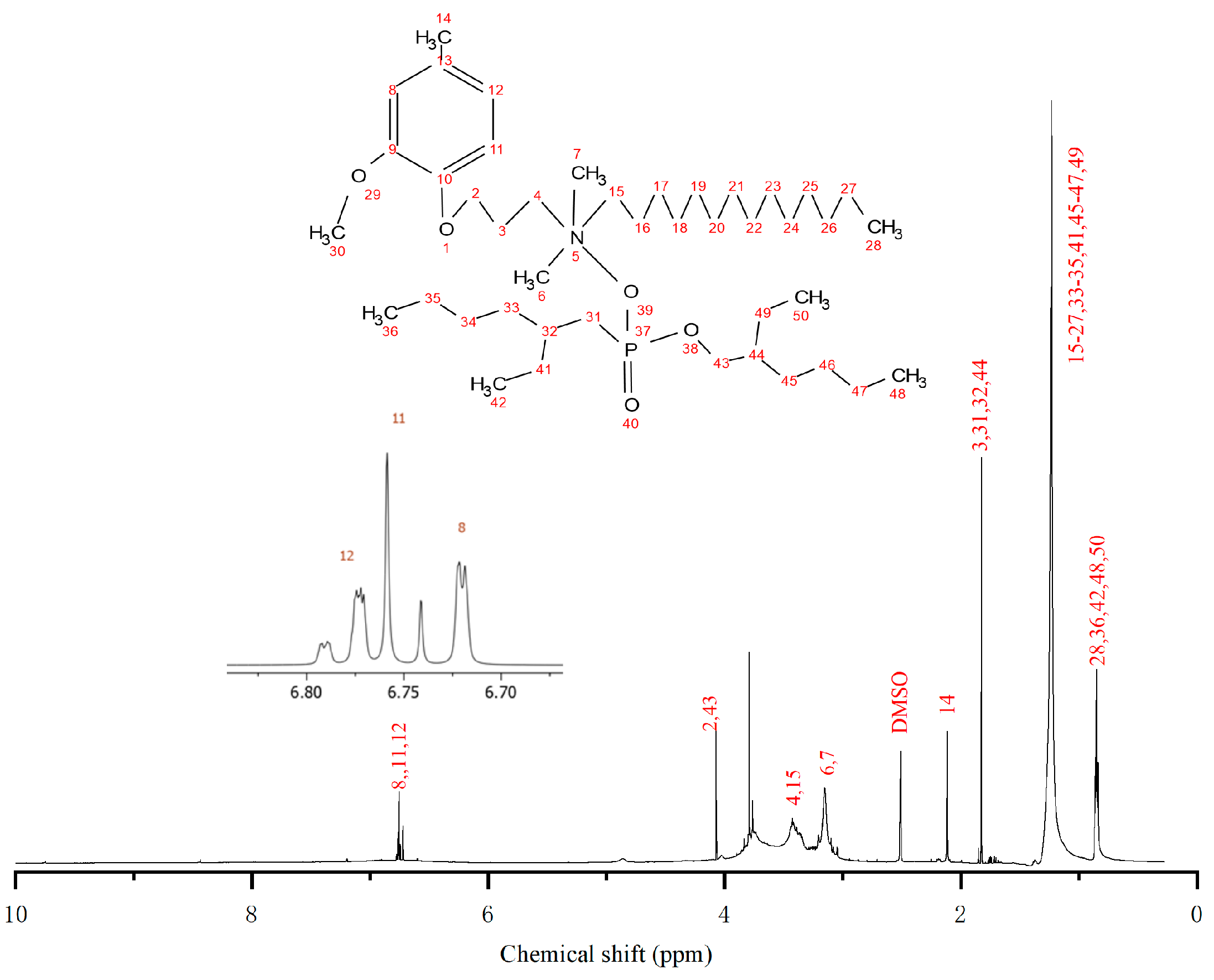
3.2. NMR Spectroscopic Characterization of QP–IL
3.3. Effect of Different Factors on Extraction for Co and Ni
3.3.1. Influence of Time on the Extraction
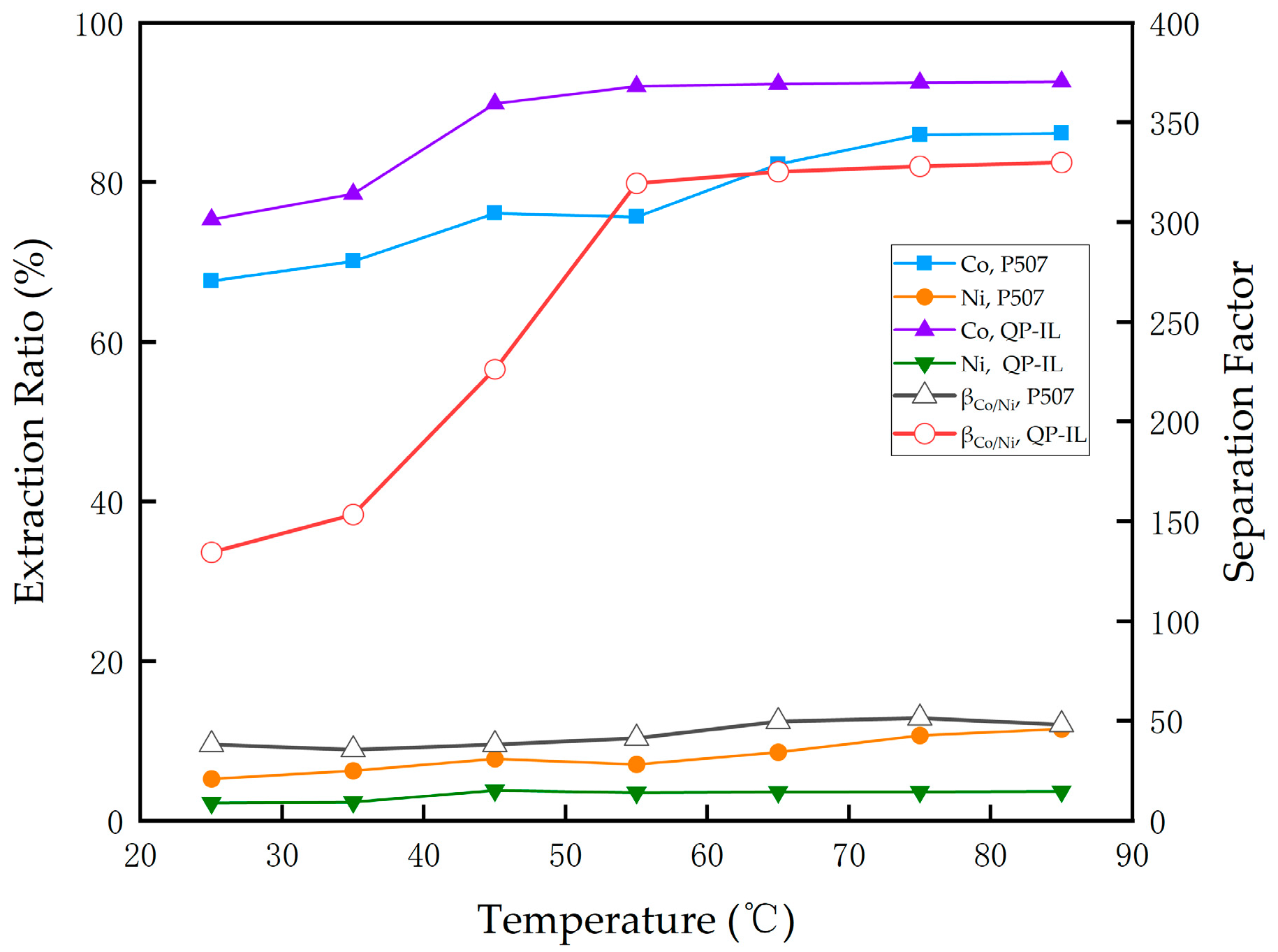
3.3.2. Effect of Temperature on Extraction
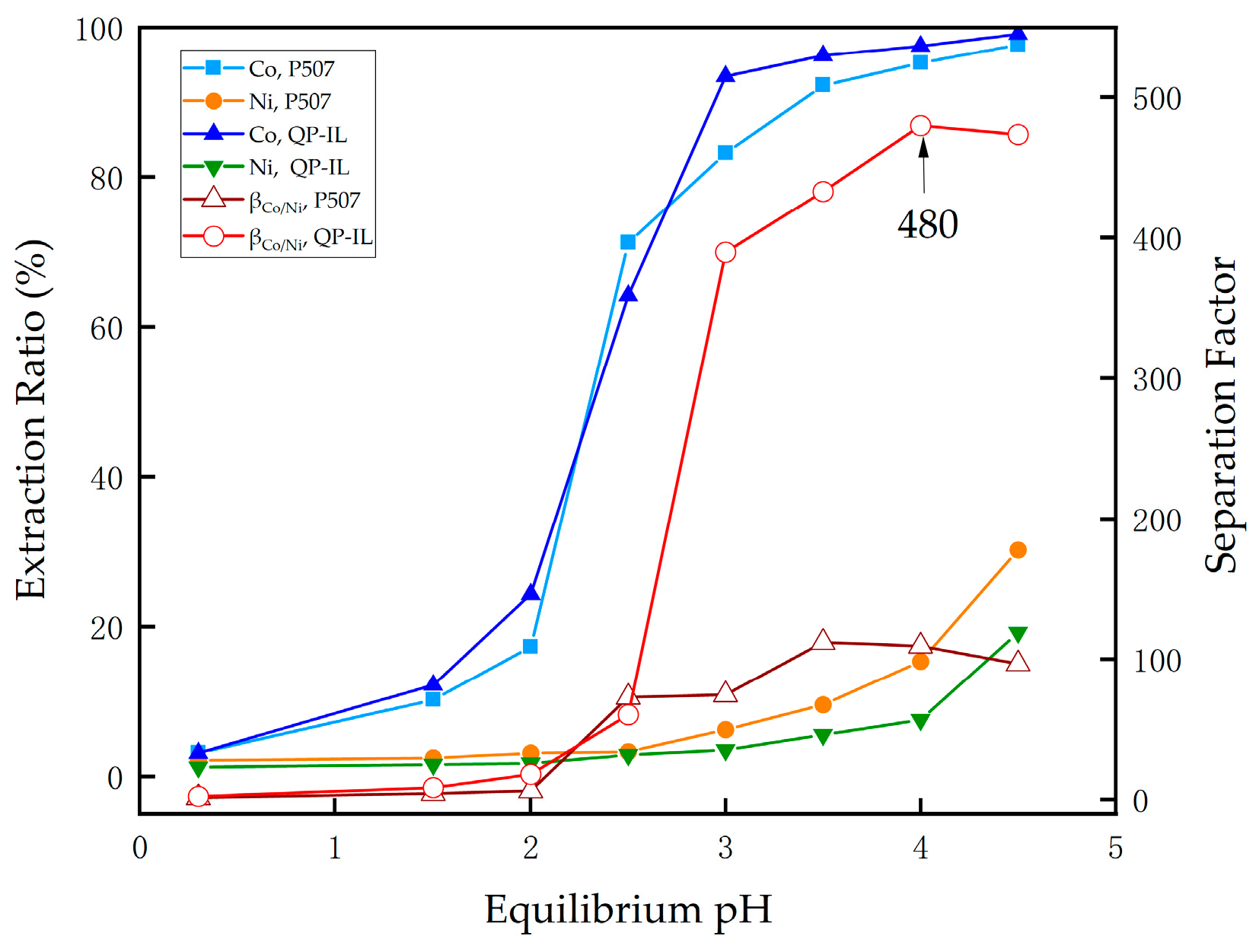
3.3.3. Effect of Equilibrium pH on Extraction Behavior
3.3.4. Effect of Saponification on Extraction of Co and Ni
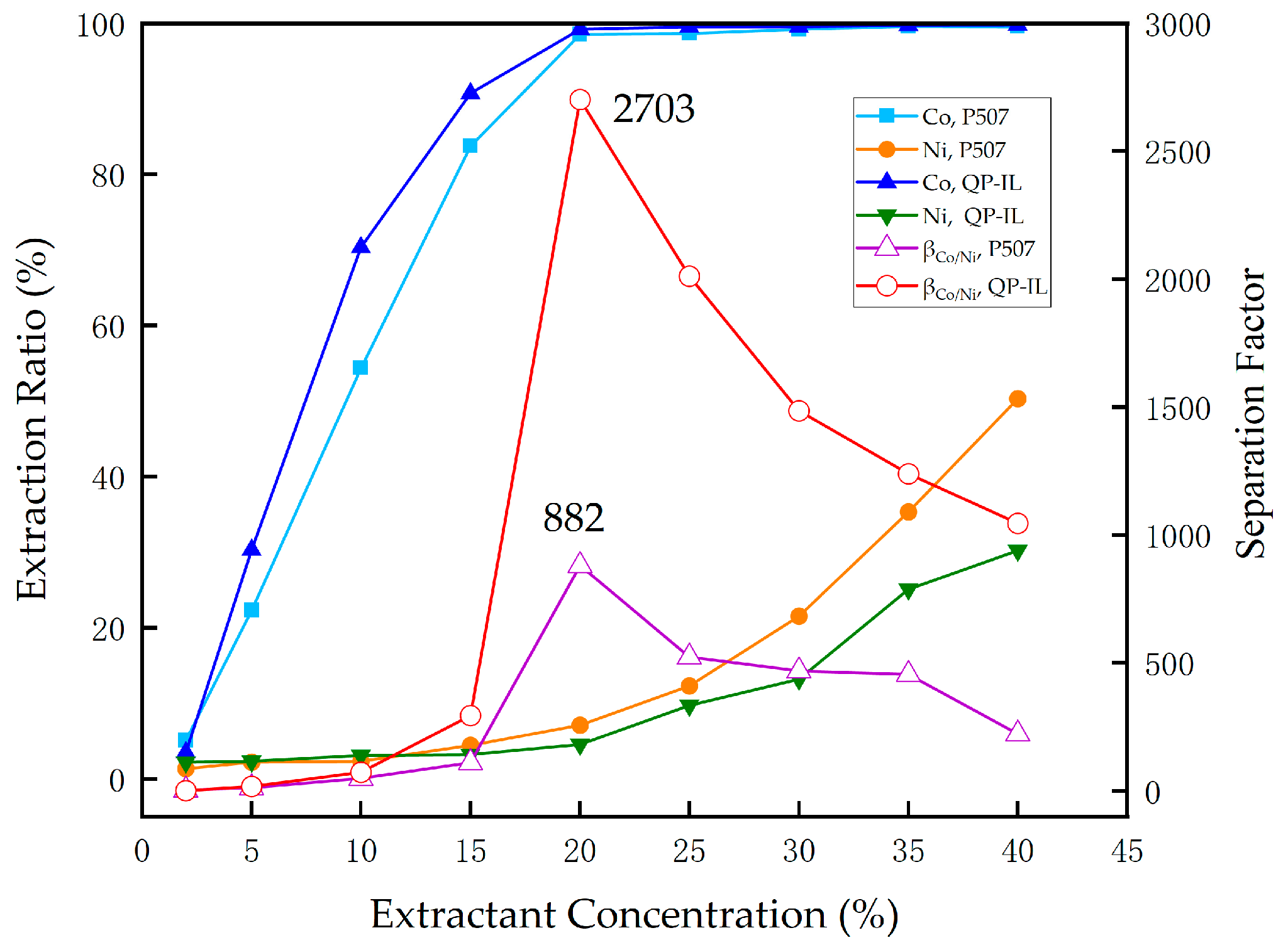
3.3.5. Effect of Concentration on Extraction of Co and Ni
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nasrollahzadeh, M.; Ghasemzadeh, M.; Gharoubi, H.; Nezafat, Z. Progresses in polysaccharide and lignin-based ionic liquids: Catalytic applications and environmental remediation. J. Mol. Liq. 2021, 342, 117559. [Google Scholar] [CrossRef]
- Das, A.K.; Mitra, K.; Conte, A.J.; Sarker, A.; Chowdhury, A.; Ragauskas, A.J. Lignin-A green material for antibacterial application—A review. Int. J. Biol. Macromol. 2024, 261, 129753. [Google Scholar] [CrossRef]
- Yang, G.; Gong, Z.; Zhou, B.; Luo, X.; Liu, J.; Du, G.; Zhao, C.; Shuai, L. Hydrodeoxygenation of condensed lignins followed by acid-mediated methylolation enables preparation of lignin-based wood adhesives. Green Chem. 2024, 26, 753–759. [Google Scholar] [CrossRef]
- Beaucamp, A.; Muddasar, M.; Culebras, M.; Collins, M.N. Sustainable lignin-based carbon fibre reinforced polyamide composites: Production, characterisation and life cycle analysis. Compos. Commun. 2024, 45, 101782. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, H.; Qian, K.; Li, L.; Shi, D.; Zhang, R.; Li, L.; Yu, H.; Zheng, C.; Xie, S.; et al. Wood structure-inspired injectable lignin-based nanogels as blood-vessel-embolic sustained drug-releasing stent for interventional therapies on liver cancer. Biomaterials 2023, 302, 122324. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shim, J.; Choi, J.-W.; Jin Suh, D.; Park, Y.-K.; Lee, U.; Choi, J.; Ha, J.-M. Continuous-flow production of petroleum-replacing fuels from highly viscous Kraft lignin pyrolysis oil using its hydrocracked oil as a solvent. Energy Convers. Manag. 2020, 213, 112728. [Google Scholar] [CrossRef]
- Sun, S.; Bai, R.; Gu, Y. From waste biomass to solid support: Lignosulfonate as a cost-effective and renewable supporting material for catalysis. Chemistry 2014, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgoli, N.S.S. Recent progresses in the application of lignin derived (nano)catalysts in oxidation reactions. Mol. Catal. 2020, 489, 110942. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Burzynska, L.; Gumowska, W. Hydrometallurgical recovery of copper and cobalt from reduction-roasted copper converter slag. Miner. Eng. 2009, 22, 88–95. [Google Scholar] [CrossRef]
- Prakash, U.; Bhagat, L.; Abhilash; Zhao, H.; van Hullebusch, E.D. Processing of Waste Copper Converter Slag Using Organic Acids for Extraction of Copper, Nickel, and Cobalt. Minerals 2020, 10, 290. [Google Scholar] [CrossRef]
- Ziyadanogullari, B. Recovery of Copper and Cobalt from Concentrate and Converter Slag. Sep. Sci. Technol. 2000, 35, 1963–1971. [Google Scholar] [CrossRef]
- Arslan, F. Recovery of copper, cobalt, and zinc from copper smelter and converter slags. Hydrometallurgy 2002, 67, 1–7. [Google Scholar] [CrossRef]
- Gumowska, W.; Rudnik, E.; Partyka, J. Mechanism of the anodic dissolution of Cu70-Co4-Fe14-Pb7 alloy originated from reduced copper converter slag in an ammoniacal solution: Recovery of copper and cobalt. Hydrometallurgy 2008, 92, 34–41. [Google Scholar] [CrossRef]
- Das, C.; Pandey, B.D. Leaching of copper, nickel and cobalt from Indian Ocean manganese nodules by Aspergillus niger. Hydrometallurgy 2010, 105, 89–95. [Google Scholar] [CrossRef]
- Verma, J.K. Extraction of copper, nickel and cobalt from the leach liquor of manganese-bearing sea nodules using LIX 984N and ACORGA M5640. Miner. Eng. 2011, 24, 959–962. [Google Scholar] [CrossRef]
- Dreano, A.; Fouvry, S.; Guillonneau, G. Understanding and formalization of the fretting-wear behavior of a cobalt-based alloy at high temperature. Wear 2020, 452, 203297. [Google Scholar] [CrossRef]
- Amsarajan, S.; Jagirdar, B.R. Air-stable magnetic cobalt-iron (Co7Fe3) bimetallic alloy nanostructures via co-digestive ripening of cobalt and iron colloids. J. Alloys Compd. 2020, 816, 152632. [Google Scholar] [CrossRef]
- Agarwal, A.; Jo, Y.T.; Rana, M.; Shin, D.; Park, J.-H. Chlorinated polyvinyl chloride (CPVC) assisted leaching of lithium and cobalt from spent lithium-ion battery in subcritical water. J. Hazard. Mater. 2020, 393, 122367. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.; Chen, Y.; Wang, C. Efficient and economical recovery of lithium, cobalt, nickel, manganese from cathode scrap of spent lithium-ion batteries. J. Clean. Prod. 2018, 204, 437–446. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. Investigation on liquid emulsion membrane (LEM) prepared with hydrodynamic cavitation process for cobalt (II) extraction from wastewater. Sep. Purif. Technol. 2020, 237, 116385. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Yen, C.H.; Lin, J.-L.; Xu, R.-B. Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J. Mater. Cycles Waste Manag. 2019, 21, 300–307. [Google Scholar] [CrossRef]
- Biswal, B.K.; Jadhav, U.U.; Madhaiyan, M.; Ji, L.; Yang, E.-H.; Cao, B. Biological Leaching and Chemical Precipitation Methods for Recovery of Co and Li from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 12343–12352. [Google Scholar] [CrossRef]
- Feng, X.N.; Sun, J.; Ouyang, M.G.; He, X.M.; Lu, L.U.; Han, X.B.; Fang, M.; Peng, H. Characterization of large format lithium ion battery exposed to extremely high temperature. J. Power Sources 2014, 272, 457–467. [Google Scholar] [CrossRef]
- Fu, G.; Wang, Z.; Hall, P. Recovering lithium cobalt oxide, aluminium, and copper from spent lithium-ion battery via attrition scrubbing. J. Clean. Prod. 2020, 260, 120869. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; Mubarok, M.Z.; Magnalita, A.; Vaughan, J.; Sugiarto, A.T. Processing mixed nickel-cobalt hydroxide precipitate by sulfuric acid leaching followed by selective oxidative precipitation of cobalt and manganese. Hydrometallurgy 2020, 191, 105185. [Google Scholar] [CrossRef]
- Wen, J.; Tran, T.T.; Lee, M.S. Comparison of separation of Mn(II), Co(II), and Ni(II) by oxidative precipitation between chloride and sulfate solutions. Physicochem. Probl. Miner. Process. 2024, 60, 183029. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Han, B.; Li, S.; Chang, D.; Li, J.; Li, T.; Yang, S.; Chen, Y.; Zhang, H. Efficient phenyl phosphate ester extractant synthesis and solvent extraction performance evaluation for transition metals. Sep. Purif. Technol. 2024, 336, 126251. [Google Scholar] [CrossRef]
- Abo Atia, T.; Deferm, C.; Machiels, L.; Khoshkhoo, M.; Riaño, S.; Binnemans, K. Solvent Extraction Process for Refining Cobalt and Nickel from a “Bulk Hydroxide Precipitate” Obtained by Bioleaching of Sulfidic Mine Tailings. Ind. Eng. Chem. Res. 2023, 62, 17947–17958. [Google Scholar] [CrossRef]
- Rodrigues, I.R.; Deferm, C.; Binnemans, K.; Riaño, S. Separation of cobalt and nickel via solvent extraction with Cyanex-272: Batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction. Sep. Purif. Technol. 2022, 296, 121326. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- Zioui, D.; Arous, O.; Mameri, N.; Kerdjoudj, H.; Sebastian, M.S.; Vilas, J.L.; Nunes-Pereira, J.; Lanceros-Mendez, S. Membranes based on polymer miscibility for selective transport and separation of metallic ions. J. Hazard. Mater. 2017, 336, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W.; Ferrier, J.; Liu, F.; Csetenyi, L.; Gadd, G.M. Biorecovery of cobalt and nickel using biomass-free culture supernatants from Aspergillus niger. Appl. Microbiol. Biotechnol. 2020, 104, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Cho, S.-S.; Lee, G.H.; Lee, C.G.; Uhm, S. Extraction/Separation of Cobalt by Solvent Extraction: A Review. Appl. Chem. Eng. 2015, 26, 631–639. [Google Scholar] [CrossRef]
- Kumari, A.; Panda, R.; Lee, J.Y.; Thriveni, T.; Jha, M.K.; Pathak, D.D. Extraction of rare earth metals (REMs) from chloride medium by organo-metallic complexation using D2EHPA. Sep. Purif. Technol. 2019, 227, 115680. [Google Scholar] [CrossRef]
- Pavon, S.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Solvent extraction modeling of Ce/Eu/Y from chloride media using D2EHPA. Aiche J. 2019, 65, e16627. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, L.; Peng, J.; Li, S.; Ju, S.; Zhang, L. Microfluidic solvent extraction of La (III) with 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (P507) by a microreactor. Chem. Eng. Process. Process Intensif. 2015, 91, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Hessel, V.; Peng, J.; Wang, Q.; Zhang, L. Co and Ni extraction and separation in segmented micro-flow using a coiled flow inverter. Chem. Eng. J. 2017, 307, 1–8. [Google Scholar] [CrossRef]
- Irannajad, M.; Haghighi, H.K.; Nasirpour, Z. New Solvent Extraction Process of Nickel and Copper by D2EHPA in the Presence of Carboxylates. Trans. Indian Inst. Met. 2020, 73, 1053–1063. [Google Scholar] [CrossRef]
- Nadimi, H.; Haghshenas Fatmehsari, D.; Firoozi, S. Separation of Ni and Co by D2EHPA in the Presence of Citrate Ion. Metall. Mater. Trans. B 2017, 48, 2751–2758. [Google Scholar] [CrossRef]
- Abu Elgoud, E.M.; Ismail, Z.H.; El-Nadi, Y.A.; Aly, H.F. Separation of cerium(IV) and yttrium(III) from citrate medium by solvent extraction using D2EHPA in kerosene. Chem. Pap. 2020, 74, 2461–2469. [Google Scholar] [CrossRef]
- Abu Elgoud, E.M.; Ismail, Z.H.; El-Nadi, Y.A.; Abdelwahab, S.M.; Aly, H.F. Extraction of Some Rare Earth Elements (La, Pr and Er) from Citrate Medium Using D2EHPA in kerosene. Arab. J. Nucl. Sci. Appl. 2019, 52, 74–85. [Google Scholar] [CrossRef]
- Jafari, H.; Abdollahi, H.; Gharabaghi, M.; Balesini, A.A. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. Sep. Purif. Technol. 2018, 197, 210–219. [Google Scholar] [CrossRef]
- Habibpour, R.; Dargahi, M.; Kashi, E.; Bagherpour, M. Comparative study on Ce (III) and La (III) solvent extraction and separation from a nitric acid medium by D2EHPA and Cyanex272. Metall. Res. Technol. 2018, 115, 207. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Raut, D.R.; Sengupta, A. Extraction of uranyl ion from nitric acid medium using solvent containing TOPO and its mixture with D2EHPA in room temperature ionic liquids. Sep. Purif. Technol. 2014, 133, 69–75. [Google Scholar] [CrossRef]
- Torkaman, R.; Asadollahzadeh, M.; Torab-Mostaedi, M.; Maragheh, M.G. Reactive extraction of cobalt sulfate solution with D2EHPA/TBP extractants in the pilot plant Oldshue-Rushton column. Chem. Eng. Res. Des. 2017, 120, 58–68. [Google Scholar] [CrossRef]
- Sousa, C.D.; Nascimento, M.; Ferreira, I.L.S. Modeling of nickel extraction by D2EHPA in sulfuric media. Rem-Rev. Esc. Minas 2011, 64, 447–452. [Google Scholar]
- Lin, L.; Wei, J.-H.; Wu, G.-Y.; Toyohisa, F.; Atsushi, S. Extraction studies of cobalt (II) and nickel (II) from chloride solution using PC88A. Trans. Nonferrous Met. Soc. China 2006, 16, 687–692. [Google Scholar]
- Zhang, Y.; Man, R.-l.; Wang, H.; Liang, Y.-h. Extraction of cobalt with P507 and preparation of cobalt oxalate powders by ethane diacid stripping. J. Cent. South Univ. (Sci. Technol.) 2011, 42, 317–322. [Google Scholar]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef]
- Qiu, L.N.; Pan, Y.H.; Zhang, W.W.; Gong, A.J. Application of a functionalized ionic liquid extractant tributylmethylammonium dibutyldiglycolamate ([A336][BDGA]) in light rare earth extraction and separation. PLoS ONE 2018, 13, e0201405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.D.; Ding, Y.; Chen, J.; Liu, Y. Microcapsules containing ionic liquid [A336][P507] for La3+/Sm3+/Er3+ recovery from dilute aqueous solution. J. Rare Earths 2016, 34, 1260–1268. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Dutra, A.J.B. Separation of nickel(II), cobalt(II) and lanthanides from spent Ni-MH batteries by hydrochloric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2013, 133, 37–43. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Bourdot Dutra, A.J. Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. J. Power Sources 2012, 220, 286–291. [Google Scholar] [CrossRef]
- Sarangi, K. Separation of copper, zinc, cobalt and nickel ions by supported liquid membrane technique using LIX 84I, TOPS-99 and Cyanex 272. Sep. Purif. Technol. 2008, 59, 169–174. [Google Scholar] [CrossRef]
- Parija, C.; Reddy, B.; Sarma, P.B. Recovery of nickel from solutions containing ammonium sulphate using LIX 84-I. Hydrometallurgy 1998, 49, 255–261. [Google Scholar] [CrossRef]
- Guimardes, A.S.; Silva, L.A.; Pereira, A.M.; Correia, J.C.G.; Mansur, M.B. Purification of concentrated nickel sulfuric liquors via synergistic solvent extraction of calcium and magnesium using mixtures of D2EHPA and Cyanex 272. Sep. Purif. Technol. 2020, 239, 116570. [Google Scholar] [CrossRef]
- van Stee, J.; Hermans, G.; John, J.J.; Binnemans, K.; Van Gerven, T. Cobalt/nickel purification by solvent extraction with ionic liquids in millifluidic reactors: From single-channel to numbered-up configuration with solvent recycle. Chem. Eng. J. 2024, 479, 147234. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of cobalt, lithium and nickel from the “black mass” of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Boudesocque, S.; Viau, L.; Dupont, L. Selective Cobalt over Nickel separation using neat and confined ionic liquids. J. Environ. Chem. Eng. 2020, 8, 104319. [Google Scholar] [CrossRef]
- Diabate, P.D.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Separation of cobalt, nickel and copper with task-specific amido functionalized glycine-betaine-based ionic liquids. Sep. Purif. Technol. 2020, 244, 116782. [Google Scholar] [CrossRef]
- Sun, X.; Ji, Y.; Liu, Y.; Chen, J.; Li, D. An engineering-purpose preparation strategy for ammonium-type ionic liquid with high purity. AIChE J. 2009, 56, 989–996. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, Y.; Huang, J.; Bao, S.; Yang, X.; Shen, C. High-efficient and selective extraction of vanadium (V) with N235-P507 synergistic extraction system. Chem. Eng. Res. Des. 2017, 120, 284–290. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Bian, X.; Zeng, W. Synergistic extraction and separation of lanthanum (III) and cerium (III) using a mixture of 2-ethylhexylphosphonic mono-2-ethylhexyl ester and di-2-ethylhexyl phosphoric acid in the presence of two complexing agents containing lactic acid and citric acid. Hydrometallurgy 2014, 149, 238–243. [Google Scholar] [CrossRef]
- Rao, M.J.; Zhang, T.; Li, G.H.; Zhou, Q.; Luo, J.; Zhang, X.; Zhu, Z.P.; Peng, Z.W.; Jiang, T. Solvent Extraction of Ni and Co from the Phosphoric Acid Leaching Solution of Laterite Ore by P204 and P507. Metals 2020, 10, 545. [Google Scholar] [CrossRef]


| Parameter | Optima Condition |
|---|---|
| Plasma gas flow rate (Ar)/(L/min) | 15 |
| Auxiliary gas flow (Ar)/(L/min) | 0.15 |
| Atomizer gas flow (Ar)/(L/min) | 0.50 |
| Pump injection volume/(mL/min) | 1.5 |
| Ni and Co analysis line/(nm) | 216.5, 213.6 |
| Wave Numbers | Characteristic Groups | |
|---|---|---|
| a | 3430 cm−1 | -OH (benzene ring) |
| b c | 2926 cm−1 2856 cm−1 | C-H of long-chain alkyl group |
| d | 721 cm−1 | C-N bond |
| e | 972 cm−1 | P-OH |
| f | 1196 cm−1 | P=O |
| g | 1462 cm−1 | phenolic ring |
| 1000~910 cm−1 | quaternary ammonium salt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xu, W. Synthesis of Quaternary-Ammonium-Lignin-Based Ionic Liquids and Comparison of Extraction Behavior of Co(II) and Ni(II) with 2-Ethylhexyl Phosphoric Acid Mono-2-Ethylhexyl Ester. Separations 2024, 11, 116. https://doi.org/10.3390/separations11040116
Li G, Xu W. Synthesis of Quaternary-Ammonium-Lignin-Based Ionic Liquids and Comparison of Extraction Behavior of Co(II) and Ni(II) with 2-Ethylhexyl Phosphoric Acid Mono-2-Ethylhexyl Ester. Separations. 2024; 11(4):116. https://doi.org/10.3390/separations11040116
Chicago/Turabian StyleLi, Guijiang, and Wenze Xu. 2024. "Synthesis of Quaternary-Ammonium-Lignin-Based Ionic Liquids and Comparison of Extraction Behavior of Co(II) and Ni(II) with 2-Ethylhexyl Phosphoric Acid Mono-2-Ethylhexyl Ester" Separations 11, no. 4: 116. https://doi.org/10.3390/separations11040116
APA StyleLi, G., & Xu, W. (2024). Synthesis of Quaternary-Ammonium-Lignin-Based Ionic Liquids and Comparison of Extraction Behavior of Co(II) and Ni(II) with 2-Ethylhexyl Phosphoric Acid Mono-2-Ethylhexyl Ester. Separations, 11(4), 116. https://doi.org/10.3390/separations11040116







