Improving the Current European Pharmacopoeia Enantio-Selective HPLC Method for the Determination of Enantiomeric Purity in Atorvastatin Calcium Salt Drug Substance
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
2.3. HPLC Operating Conditions and Partial Method Validation
3. Results
3.1. Enantioseparation under Normal-Phase Conditions
3.2. Chemo- and Diastereo-Selectivity of the Normal-Phase Enantio-Selective Method
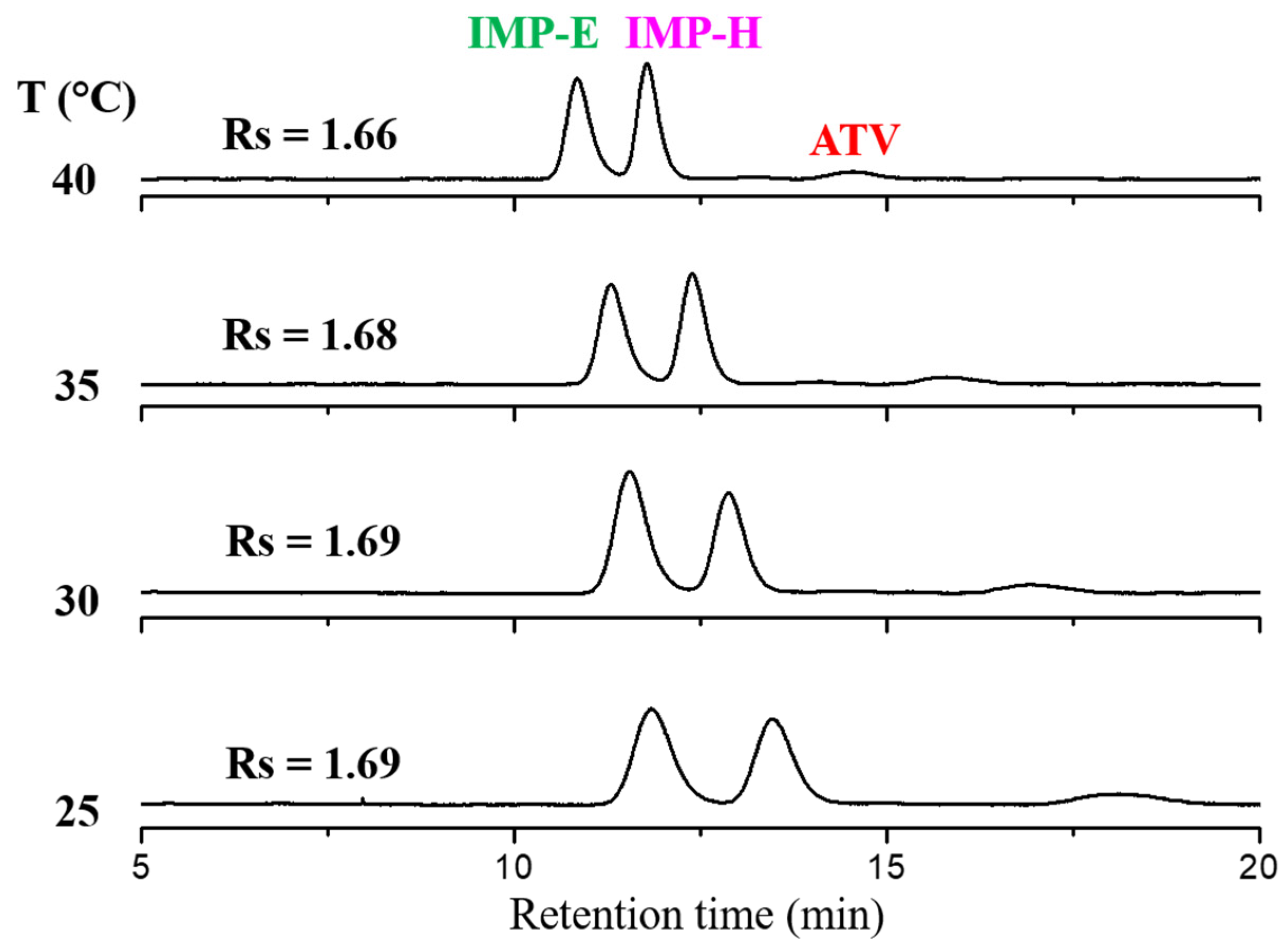
3.3. Optimization of the Normal-Phase Enantio-Selective EP Method
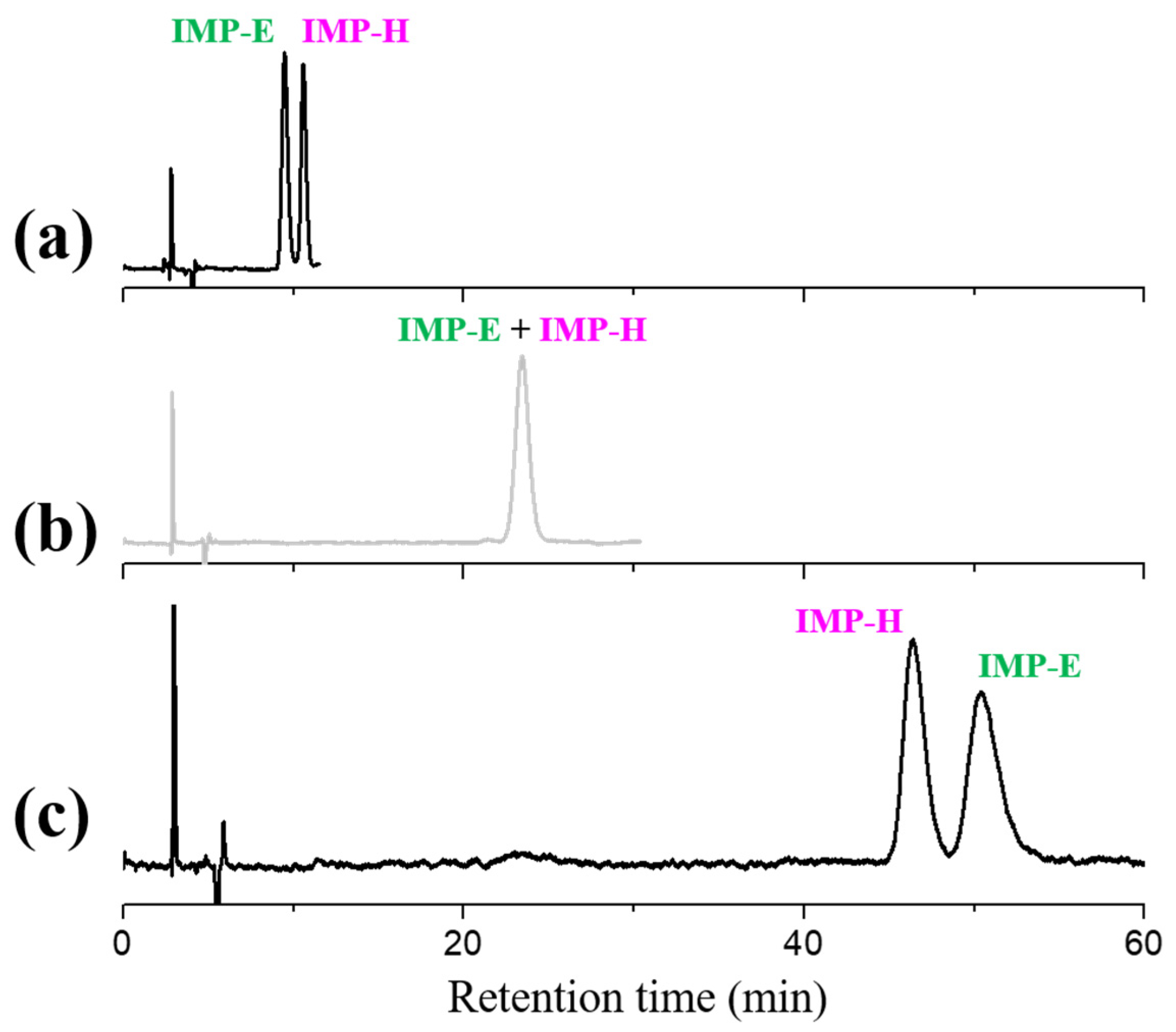
3.4. Influence of Ethanol Percentage on Elution Order of Impurities E and H
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natalija, N.; Jelena, A.; Katerina, B.; Zoran, K.; Aneta, D. Green Strategies toward eco-friendly HPLC methods in pharma analysis. In High-Performance Liquid Chromatography—Recent Advances and Applications; IntechOpen: London, UK, 2023; Chapter 5. [Google Scholar] [CrossRef]
- Monograph: Atorvastatinum Calcicum 04/2022: 2191. In The European Pharmacopoeia; Corrected 11.1; Council of Europe: Strasbourg, France, 2022.
- Lea, A.P.; McTavish, D. A Review of its Pharmacology and Therapeutic Potential in the Management of Hyperlipidaemias. Atorvastatin. Drugs 1997, 53, 828–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Yu, L.; Sun, C.; Kang, Y.; Zeng, S. Direct differentiation of stereoisomers of ezetimibe/ambrisentan/atorvastatin and their mechanism study by electrospray ionization quadrupole time-of-flight mass spectrometry. J. Mass Spectrom. 2018, 53, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Vishnumurthy, M.; Srinivas, K.; Kumar, R.; Mukkanti, K. A validated LC method for determination of the enantiomeric purity of atorvastatin in bulk drug and dosage forms. Rasayan J. Chem. 2009, 2, 836–841. [Google Scholar]
- Daicel Chiral Technologies, Atorvastatin Application Note. 2014. Available online: https://chiraltech.com/wp-content/uploads/2014/06/05-Chiralpak-IA-3-for-Measurement-of-Atorvastatin.pdf (accessed on 2 April 2024).
- Thunberg, L.; Hashemi, J.; Andersson, S. Comparative study of coated and immobilized polysaccharide-based chiral stationary phases and their applicability in the resolution of enantiomers. J. Chromatogr. B 2008, 875, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Aboul-Enein, H.Y. Comparison, applications, advantages, and limitations of immobilized and coated amylose tris-(3,5-dimethylphenylcarbamate) chiral stationary phases in HPLC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2669–2680. [Google Scholar] [CrossRef]
- Patole, S.; Gosar, A.; Shaik, T. Impurities characterization in pharmaceuticals: A review. Int. J. Pharm. Res. 2019, 15, 46–64. [Google Scholar]
- Kątny, M.; Frankowski, M. Impurities in drug products and active pharmaceutical ingredients. Crit. Rev. Anal. Chem. 2017, 47, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mammone, F.R.; Zanitti, L.; Puxeddu, M.; La Regina, G.; Silvestri, R.; Borioni, A.; Cirilli, R. A novel validated UHPLC method for the estimation of Rosuvastatin and its complete impurity profile in tablet formulations. Molecules 2023, 28, 431. [Google Scholar] [CrossRef]
- Cantatore, C.; Bertocchi, P.; De Orsi, D.; Panusa, A.; Cirilli, R. Enantioselective HPLC analysis of escitalopram oxalate and its impurities using a cellulose-based chiral stationary phase under normal- and green reversed-phase conditions. J. Sep. Sci. 2022, 45, 1059–1066. [Google Scholar] [CrossRef]
- Rosetti, A.; Ferretti, R.; Zanitti, L.; Casulli, A.; Villani, C.; Cirilli, R. Single-run reversed-phase HPLC method for determining sertraline content, enantiomeric purity, and related substances in drug substance and finished product. J. Pharm. Anal. 2020, 10, 610–616. [Google Scholar] [CrossRef]
- Papp, L.A.; Szabó, Z.I.; Hancu, G.; Farczádi, L.; Mircia, E. Comprehensive review on chiral stationary phases in single-column simultaneous chiral–achiral HPLC separation methods. Molecules 2024, 29, 1346. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.A.; Hancu, G.; Szabó, Z.I. Simultaneous determination of enantiomeric and organic impurities of vildagliptin on a cellulose tris(3-chloro-4-methylphenylcarbamate) column under revered-phase conditions. J. Pharm. Biomed. Anal. 2023, 234, 115495. [Google Scholar] [CrossRef] [PubMed]
- Umstead, W.; Onishi, T.; Franco, P. Polysaccharides. In Chiral Separations and Stereochemical Elucidation: Fundamentals, Methods, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; Chapter 6; pp. 187–245. [Google Scholar] [CrossRef]
- Zhang, T.; Franco, P. 3 μm particle-based chiral stationary phases for the fast and efficient resolution of enantiomers. LCGC Eur. 2008, 21, 430–437. [Google Scholar]
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Płotka, J.; Tobiszewski, M.; Sulej, A.M.; Kupska, M.; Górecki, T.; Namieśnik, J. Green chromatography. J. Chromatogr. A 2013, 1307, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Buddrick, O.; Jones, O.A.H.; Morrison, P.D.; Small, D.M. Heptane as a less toxic option than hexane for the separation of vitamin E from food products using normal phase HPLC. RSC Adv. 2013, 3, 24063–24068. [Google Scholar] [CrossRef]



| CSP | Mobile Phase | k1 | k2 | α | Rs |
|---|---|---|---|---|---|
| Chiralpak IA-3 | n-Hexane-EtOH-TFA (90:10:0.1) | 2.52 | 3.10 | 1.23 | 2.62 |
| n-Hexane-IPA-TFA (90:10:0.1) | 3.70 | - | 1.00 | - | |
| Chiralpak AD-3 | n-Hexane-EtOH-TFA (90:10:0.1) | 4.11 | 7.36 | 1.79 | 4.10 |
| n-Hexane-EtOH-TFA (80:20:0.1) | 0.72 | 1.27 | 1.75 | 1.99 | |
| n-Hexane-IPA-TFA (90:10:0.1) | 5.98 | - | 1.00 | - | |
| Chiralpak ID-3 | n-Hexane-EtOH-TFA (90:10:0.1) | 3.57 | - | 1.06 | - |
| n-Hexane-IPA-TFA (90:10:0.1) | 7.14 | - | 1.06 | - | |
| Chiralpak IG-3 | n-Hexane-EtOH-TFA (90:10:0.1) | 4.87 | 5.49 | 1.13 | 1.20 |
| n-Hexane-IPA-TFA (90:10:0.1) | 11.1 | - | 1.00 | - | |
| Chiralpak IH-3 | n-Hexane-EtOH-TFA (90:10:0.1) | 2.89 | - | 1.00 | - |
| n-Hexane-IPA-TFA (90:10:0.1) | 4.13 | - | 1.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammone, F.R.; Sadutto, D.; D’Ettorre, G.; Mosca, A.; Cirilli, R. Improving the Current European Pharmacopoeia Enantio-Selective HPLC Method for the Determination of Enantiomeric Purity in Atorvastatin Calcium Salt Drug Substance. Separations 2024, 11, 154. https://doi.org/10.3390/separations11050154
Mammone FR, Sadutto D, D’Ettorre G, Mosca A, Cirilli R. Improving the Current European Pharmacopoeia Enantio-Selective HPLC Method for the Determination of Enantiomeric Purity in Atorvastatin Calcium Salt Drug Substance. Separations. 2024; 11(5):154. https://doi.org/10.3390/separations11050154
Chicago/Turabian StyleMammone, Francesca Romana, Daniele Sadutto, Giulia D’Ettorre, Antonina Mosca, and Roberto Cirilli. 2024. "Improving the Current European Pharmacopoeia Enantio-Selective HPLC Method for the Determination of Enantiomeric Purity in Atorvastatin Calcium Salt Drug Substance" Separations 11, no. 5: 154. https://doi.org/10.3390/separations11050154
APA StyleMammone, F. R., Sadutto, D., D’Ettorre, G., Mosca, A., & Cirilli, R. (2024). Improving the Current European Pharmacopoeia Enantio-Selective HPLC Method for the Determination of Enantiomeric Purity in Atorvastatin Calcium Salt Drug Substance. Separations, 11(5), 154. https://doi.org/10.3390/separations11050154






