Abstract
An evaluation of the possible hepatotoxicity/hepatoprotective effects of a defatted extract of the above ground parts of Phlomis russeliana was conducted in vitro and in vivo. The extract was tested in vitro on hepatocytes alone and in a carbon tetrachloride (CCl4)-bioactivation model. The same toxic substance was used for the in vivo evaluation on old Wistar rats. The extract was standardised via high performance liquid chromatography (HPLC) by the quantification of total flavonoids and verbascoside. Gallic acid equivalents were used to express the content of total phenolic compounds. The identification of flavonoids in this species was undertaken for the first time. The extract was not statistically hepatotoxic in vitro on the isolated rat hepatocytes. In the CCl4-induced hepatotoxicity model, the extract had a hepatoprotective effect, which was concentration-dependant (the highest at 50 µg/mL). An in vivo study on old rats confirmed the observed antioxidant and hepatoprotective effects. The histological findings were favourable for the rats, given the extract and CCl4 in combination. They had an unchanged organ structure, which is commensurable with these animals, treated with a combination of CCl4 and silymarin.
1. Introduction
More than 15,000 plant species are utilised in traditional medicine; these species could also be exploited as sources of raw materials to create novel medications [1]. In industrialised nations, chemical synthesis has supplanted plant synthesis as the primary source of pharmaceuticals due to advancements in modern medicine and drug research. However, the majority of people on the planet use plant-based medications because they cannot afford pharmaceuticals. Due to the possibility of creating novel medicinal products through the bioactive components of traditional medicinal herbs, there has been a lot of interest in these plants [1].
The liver is the primary site of biotransformation for the deactivation, bioactivation, and detoxification of several xenobiotics. To assess this process, various setups of experiments are available (in vitro and in vivo). Isolated hepatocytes in cell cultures or suspensions are a common object in experimental pharmacology and toxicology. Following isolation and in vitro incubation under appropriate circumstances, the hepatocytes maintain their primary function, and consequently, their biotransformation capacities, linked to phases I and II of xenobiotic biotransformation. For a variety of in vitro investigations on the cytotoxicity, metabolism, as well as other properties of novel, promising drugs of both synthetic and natural origin, isolated rat hepatocytes provide a stable system. Two major criteria accepted and advised by the European Centre for the Validation of Alternative Methods (ECVAM) for evaluating the functional metabolic status of hepatocytes are the trypan blue exclusion (for viability) and lactate dehydrogenase enzyme leakage (for the disruption of the cell membrane). Changes in the levels of tripeptide glutathione, one of the most significant naturally occurring variables involved in cell protection, and malondialdehyde, which is produced by the peroxidation of membrane lipids, can be used to assess the harmful alterations that occur at different levels in cells due to the bioactivation/biotransformation of the substances under investigation. In a similar manner, the ingestion of CCl4 is considered the most valuable model for hepatotoxicity. The consequences are renal failure and encephalopathy, leading to overall organ insufficiency. This experimental model in rodents has proved its qualities through numerous studies [2].
Phlomis L. is a genus that may also be of interest for the development of new plant-based pharmaceuticals. Throughout Europe, Asia, and North Africa, there are over 105 species in this genus, which is a member of the Lamiaceae family. They are widely applied, as the aerial parts are consumed as infusions against intestinal issues and as a hepatoprotective aid, which involves protecting the kidneys and the cardiovascular system as well.
There are several Phlomis L. species that are known to have antidiabetic qualities, including P. ocymifolia (Burm.f.) and P. aurea Decne. Their capacity to lower oxidative stress in diabetes or to promote the biosynthesis of enzymes, which are included in the metabolism of glucose, is considered the primary cause of this action, which shields the pancreas and liver. The P. anisodonta Boiss. methanol extract was shown to have antihyperglycaemic effects in a rat model of diabetes produced by streptozotocin [3]. Pancreatic cells are irreversibly damaged by streptozotocin, which results in degranulation and decreased insulin output. Severe weight loss and the prevalence of diabetic comorbidities, including oxidative stress-related cardiac, cardiovascular, gastrointestinal, neurological, renal, and bladder dysfunction, are hallmarks of streptozotocin-induced diabetes. When the P. anisodonta Boiss. methanol extract (400 mg/kg) was given to rats treated with streptozotocin for ten days, there was a considerable glucose level reduction. The insulin amount in the plasma was increased, and the loss of body weight was reduced. The capacity of the P. anisodonta Boiss. methanol extract to increase Fe2+ levels in the plasma, to decrease the rate of lipid peroxidation in the liver, and to induce hepatic antioxidant enzymes was the cause for the antihyperglycaemic effect that was observed. Rats given the P. anisodonta Boiss. methanol extract showed a substantial increase in enzyme activity (catalase, glutathione peroxidase, and superoxide dismutase) in the liver [3]. Extracts from the above ground parts of P. fruticosa L. and P. lanata Willd. had antioxidant effects in vitro [4].
Several isolated compounds and/or extracts from Phlomis L. plants (P. nissolii L., P. herba-venti L., P. tuberosa L., etc.) have been reported to possess antioxidant activity. Using different models (iron chelating, reducing ability, inhibition of xanthine oxidase, scavenging of superoxide radical) and methods (2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), etc.), this action was proved in vitro [5]. Verbascoside (acteoside) and forsytoside B are the main phenylethanoid glycosides in the species from Phlomis L. The compounds were isolated from P. caucasica’s overground parts and had strong in vitro antioxidant abilities [6]. A previous study on the hepatoprotective properties of a defatted extract of P. tuberosa L. proved its in vitro/in vivo effectiveness against CCl4 toxicity [7]. The results gained provide the basis for further research on the hepatoprotection topic of other representatives of the genus Phlomis L.
P. russeliana Lag. ex Benth. accumulates some essential oil, with the main components of sesquiterpene (germacrene D, β-caryophyllene) and aliphatic compounds (hexadecenoic acid, pentacosane) [8]. A review of this species’ biological action, phytochemistry, as well as its traditional use was previously published [5]. The plant accumulates mainly phenolic compounds, such as verbascoside, isoverbascoside, forsythoside B, samioside, leucosceptosides A and B, etc. [9]. Recent studies have shown that P. russeliana Lag. ex Benth. extracts exhibit wound-healing [10], antibacterial, and anticancer activities [11]. The plant’s overground parts have been used in traditional medicine as an infusion for gastrointestinal discomfort, to stimulate the appetite, to relief indigestion, etc. [12]. There are, however, no reports on the possible hepatoprotective effect of this species, although it is widely used for other gastrointestinal ailments. A recent report on the DPPH radical scavenging capacity of an extract from its aerial parts [10] suggests it could have potential antioxidant effects as well.
In context with the evidence presented in the literature, the aims here were to obtain a defatted extract from Phlomis russeliana’s aerial parts, to analyse its content and to study its potential hepatoprotective and antioxidant properties in in vitro/in vivo models of hepatotoxicity induced by carbon tetrachloride (CCl4).
2. Materials and Methods
2.1. Plant Material
The overground parts of P. russeliana Lag. ex Benth. were harvested from the University of Sofia’s Botanical Garden (Sofia, Bulgaria) in June 2023. The identity was confirmed by one of the authors (I. K.) A specimen was kept at the Herbarium of the Faculty of Pharmacy at Medical University of Sofia (#FF-100). The material was collected as required for herbae [13] and had a leaves/flowers/stems ratio of 2:2:1.
2.2. Extraction and Isolation of Verbascoside
The plant substance (300 g) was dried, milled, and sieved (3 mm); then, the lipophilic constituents were removed by percolation with dichloromethane (9 ×1.5 L). After this, the material was aired in the fume cupboard and then percolated exhaustively with 80% methanol (MeOH) (20 × 1.5 L). The solvent was removed, then the viscous residue was lyophilised (0.160 mbar, −105 °C, 98 h) and named EPhR. To the obtained verbascoside, twenty grams of EPhR was used. This was separated over a column filled with Diaion HP-20 (elutents 100% H2O, and 50%, 85% and 100% MeOH). Then, the fractions collected were dried and analysed. The phenolic compounds were in the fraction, which was eluted with 50% MeOH according to HPLC. It was purified and separated by repetitive column chromatography (Sephadex LH-20, MeOH) to produce verbascoside (41 mg). The identity of the compound was confirmed by HPLC and its UV absorbance (see Section 3).
2.3. Phytochemical Analysis
The standard substances (see Table 1), acetonitrile, methanol (both HPLC grade, gradient), sodium carbonate, ortho-phosphoric acid, the Folin–Ciocalteu reagent and pyrogallol were obtained from Sigma Aldrich (Taufkirchen, Germany). A Young Lin 9100 (Hogye-dong, Anyang, Republic of Korea) HPLC system, including a vacuum degasser (model 9101), a quaternary pump (model 9110), a column thermostat (model 9131), a PDA detector (model 9160), and a manual injector Rheodyne 7725i, was used. The software for data acquisition and analysis was Clarity Chromatography Software (v. 2. DataApex, Prague, Czech Republic). A known European Pharmacopoeia method [14], which uses quercetin as the reference, was deployed to determine the total flavonoid content of EPhR. After acid hydrolysis at 100 °C for half an hour, the sample was injected in the chromatograph. The apparatus, the stationary and mobile phases, as well as the other parameters of the analysis were as those described in [14]. The analytical wavelength was 370 nm. The results were calculated as the percentage of flavonol glycosides, with reference to quercetin.

Table 1.
Compounds identified in EPhR.
For the identification of flavonoids and for the quantitation of verbascoside, an aliquot (0.20 g) from EPhR were dissolved and diluted with MeOH in a 10.0 mL volumetric flask. After centrifugation at 10,000× g and membrane filtration (PVDF, 0.45 µm), 20 µL of the aliquot was injected to the HPLC. Separations were made on a RP-C18 Luna® column (100Å, 250 × 4.6 mm, 5 µm, Phenomenex, Torrance, CA, USA) with an ODS cartridge Security Guard® (Phenomenex, Torrance, CA, USA) maintained at 35 °C. The solvents were (A) H2O + 0.1% H3PO4 and (B) acetonitrile (MeCN). Before use, they were filtered through a 0.45 µm PVDF membrane and sonicated. The flow was 1 mL/min. The wavelength was set at 330 nm.
For identification purposes, the gradient was 5% B (initial); 5 min→13 min, 5%→15% B (linear); 13 min→45 min, 15%→50% B (linear); 45 min→50 min, 50%→95% B (linear); 50 min→55 min, 95% B (isocratic); and 55 min→60 min 95%→5% B (linear). Standard solutions in MeOH (0.20 mg/mL) of each compound were made. The identification was performed on the basis of the coincidence of the retention time to that of the chemical reference substance (CRS). Validation was performed [15]. To support the identification, the UV-vis spectra of the compounds in the sample, recorded by the DAD detector, were compared to that of the reference substances [16].
Another gradient was used for the quantification of verbascoside as follows: initial 10% B; 5 min→25 min, 10%→100% B (linear); 25 min→28 min, isocratic 100% B; 28 min→30 min, and 100→10% B (linear). Solutions (0.3, 0.6, 0.9, 1.2, 1.5 and 1.8 mg/mL) of verbascoside CRS were prepared in MeOH. The method was validated, as described by the International Conference of Harmonization (ICH 2005) [17].
A spectrophotometric method [18] with the Folin–Ciocalteu reagent (FCR) was applied to measure the total content of phenolics in EPhR. A solution of EPhR in water (1 mg/mL) was prepared. The reference substance was pyrogallol (0.1 mg/mL in H2O). In total, 5 mL of FCR diluted ten-fold was combined with a 1 mL sample solution and 4 mL 7.5% solution of Na2CO3 in water before being incubated for 30 min (in the dark). The absorbance of the mixture was recorded at 765 nm (blank H2O). Gallic acid solutions (100, 150, 200, 250, 300, 350, 400, and 450 µg/mL in H2O), treated by the same protocol, were used to construct the calibration curve. It gave the equation y = 0.0082x + 0.0894 with r2 = 0.9941. The results for the total phenols were expressed as mg/g gallic acid equivalents (GAE).
Each analysis was repeated three times; the statistical program MedCalc 12.2 (MedCalc Software, Ostend, Belgium) was used, and the results were presented as the mean ± SD.
2.4. Animals
Thirty-five-year-old (2.5 years) male Wistar rats were used. Five rats were used to isolate hepatocytes, and thirty of them to perform the in vivo experiment. They were purchased from the National Breeding Centre (at Bulgarian Academy of Sciences). Ordinance No. 17, 2006, was used to determine the minimum requirements for the protection and humane treatment of experimental animals and the European Ordinance guidelines for working with experimental animals were strictly followed. The rodents were housed in Plexiglas cages under conventional conditions, with free access to food and water and a 12-h light/dark cycle at a temperature of 20–25 °C. The animals were denied food for twelve hours before the study. The experiment’s commitment was approved by the Medical University of Sofia’s (KENIMUS) Institutional Ethics Committee. In addition, permit No. 301 (until 20 July 2025) was granted for the animal studies by the Ministry of Agriculture and Foods’ Bulgarian Food Safety Agency.
2.5. In Vitro Experiments on Isolated Rat Hepatocytes
An two step in situ collagenase perfusion [19] with optimisations made by us [20] was performed to obtain the primary hepatocyte suspension. A pre-incubation with EPhR or silymarin (5, 10 and 50 µg/mL) for 30 min was performed, followed by incubation in combination with CCl4 (1 h). The hepatocytes’ viability was determined [19]. The activity of lactate dehydrogenase (LDH) was assessed by a kinetic method. Briefly, the hepatocytes were incubated with the substances for 1.5 h. The enzyme was determined in the supernatant, which was left after the centrifugation of the hepatocytes at 400× g for 4 min. An LDH kit (Anticel, USA) was used. The extinction was determined spectrophotometrically (340 nm) at 30 s, 1, 2, and 3 min. The level of reduced glutathione (GSH) and the production of malonedialdehyde (MDA) were measured following the procedures in [19].
2.6. In Vivo Experimental Design
Five rats (n = 5) were randomly allocated in six groups, as follows:
First: Control (physiological saline for 14 days, p.o.).
Second: Phlomis russeliana extract (EPhR) (100 mg/kg for 14 days, p.o.) [21].
Third: Silymarin (100 mg/kg for 14 days, p.o.) [22].
Fourth: 10% solution of CCl4 (once on day 7, 1.25 mL/kg, p.o.) [23].
Fifth: EPhR (100 mg/kg for 14 days, p.o.), with a 10% solution of CCl4 (given once, on day 7, 1.25 mL/kg, p.o.).
Sixth: Silymarin (100 mg/kg for 14 days, p.o.), with a 10% solution of CCl4 (given once, on day 7, 1.25 mL/kg, p.o.).
At the end, blood was collected from the tail vein, and then the rats were decapitated. The liver, kidneys and the brain were taken from each animal.
2.6.1. GSH and MDA Determination in Liver Tissue
A small portion of each liver was taken for pathological examination (see Histopathological examination). The organs were kept refrigerated. They were homogenised with the appropriate buffers by a tissue homogeniser, Polytron (Kinematica, Malters, Switzerland). The production of MDA and level of GSH in the liver homogenates were determined spectrophotometrically (for MDA, 532 nm and for GSH, 412 nm) according to [24].
2.6.2. Assay of Aspartate Aminotransferase (ASAT) and Alanine Aminotransferase (ALAT) in Blood Serum
A tube with EDTA was used to collect the blood, which was then centrifuged at 10,000× g for 10 min. The activity of ASAT and ALAT was measured in the resulting serum with the corresponding kits on a BS-120 automatic biochemical analyser (Mindray, Shenzhen, China).
2.6.3. Histopathological Examination
Ten percent buffered neural formalin was used to fix the histological samples. They were cut, diaphonised, mounted on microscope slides and stained with Haematoxylin-Eosin (H&E), as described in [7,25]. The prepared slides were observed, and pictures were taken with a D740T microscope (Levenhuk, Tampa, FL, USA).
2.7. Statistical Analysis
The statistical program ‘MEDCALC’ processed the results with the non-parametric Mann–Whitney method (p < 0.05; p < 0.01 and p < 0.001 significance levels).
3. Results
3.1. Obtaining EPhR and Chemical Analysis
A defatted extract (31.05 g) from the aerial parts of P. russeliana (EPhR) was obtained. The main phenolic compound—verbascoside—was isolated by repetitive CC over different sorbents. It was isolated with 98.7% purity (41 mg). Its retention time and UV-maxima (recorded on the PDA of the HPLC) coincided with the values for verbascoside CRS. In order to analyse the phytochemical composition, three HPLC methods were used. The EPhR was standardised by two parameters—the total flavonoid content and quantity of verbascoside. The total phenolics were also determined.
3.1.1. Flavonoid Identification in EPhR
A total of 13 reference substances was used for HPLC identification (Figure 1, Table 1). For the first time naringin, luteolin-3′-glucuronide, luteolin-7-glucuronide and quercetin-3-glucoside were identified in the plant. As seen from the total flavonoid content, their quantity was significant.
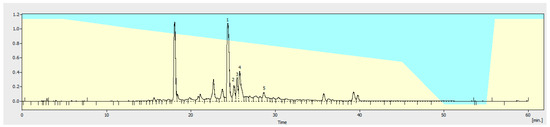
Figure 1.
HPLC chromatogram of EPhR used to identify the compounds. 1. Verbascoside (24.470 min); 2. Quercetin-3-glucoside (25.157 min); 3. Luetolin-7-glucuronide (25.470 min); 4. Naringin (25.820 min); and 5. Luteolin-3′-glucuronide (28.810 min).
3.1.2. Assay of Total Flavonoids
EPhR had 5.84% total flavonoids according to the modified European Pharmacopoeia method.
3.1.3. Quantitation of Verbascoside
The quantity of verbascoside in EPhR was determined and validated according to the approved ICH Q2 (R1) [26] guidelines and HPLC-UV method. In the conditions used (see Section 2), verbascoside had a retention time of 13.18 min (Figure 2). During the method validation procedure, we examined the suitability of the system, the precision, the linearity, the accuracy, and the selectivity. The system was found to be suitable. No peaks interfering at tR (verbascoside) with AUC above 0.05% were detected in the blank baseline. A 0.12% verbascoside solution in MeOH showed a signal-to-noise (S/N) ratio equivalent to or more than ten. The agreement of this solution was ≤1.7%. The factor of resolution (peaks neighbouring) was determined as ≥1.2. Two injections had a difference of ≤1.8%.
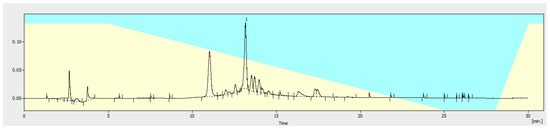
Figure 2.
HPLC chromatogram of EPhR used to preform quantitation. 1. Verbascoside (13.180 min).
Specificity. Peaks in a blank (MeOH) with AUC > 0.1% interfering at tR (verbascoside) were not found. No evidence of co-elution was found after peak purity analysis.
Limits of Detection and Quantitation. These were calculated using the S/N from the response’s slope and the standard deviation (SD). The limit of detection (LOD) was 0.001 mg/mL, and the limit of quantification (LOQ) was 0.01 mg/mL.
Accuracy and linearity. The linearity of the method was examined from 26% to 151% of the known concentration. The calibration data were processed by linear regression. A correlation coefficient (r2) of 0.9933 was calculated after the linear least-square analysis. This is indicative of the correlation between the concentration and the peaks’ AUC in the interval studied (Figure 3). There was no significant bias, and quantitation could be performed, as deduced from the y-intercept (≤0.5%) of the 100% level. According to ICH [26], the developed method for verbascoside was found to be linear/accurate.
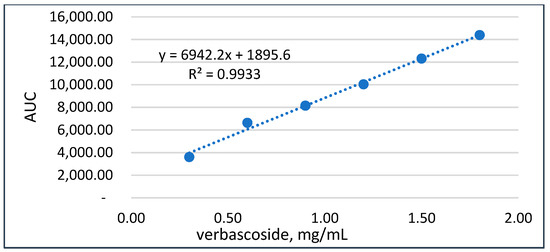
Figure 3.
Calibration curve of verbascoside.
Precision. At this step, six verbascoside standard solutions (level 100%) were analysed. The purity was 98.9% (average), and the relative standard deviation was 0.636% (n = 6), which is in accordance with the requirement of RSD ≤ 2%. The method was found to be precise.
The validated method was applied for EPhR to identify and quantify verbascoside (Figure 2) in it. The extract contained 8.08% (w/w) verbascoside.
3.1.4. Total Compounds Content
The quantity of phenolic compounds in EPhR was determined by FCR via a spectrophotometric assay. The total phenolic compounds were 388 mg/g, expressed as GAE [18]. The standard 0.11 mg/mL solution of pyrogallol had 333 mg/g GAE.
3.2. Evaluation of the Pharmacological Effects
3.2.1. In Vitro Investigations
Administered alone, EPhR and silymarin had no toxic effect (statistically significant) on isolated rat hepatocytes. The samples did not change statistically significantly the main functional metabolic parameters of hepatocytes, including the viability, activity of the LDH enzyme, GSH level and production of MDA (Table 2).

Table 2.
Hepatocytes’ viability and levels of LDH, MDA and GSH (administration alone).
In the CCl4-induced model of metabolic bioactivation, EPhR exhibited a pronounced statistically significant hepatoprotective and antioxidant effect, comparable to the effect of silymarin (a proved antioxidant and hepatoprotective agent). Administered alone, at a concentration of 86 µM, CCl4 exhibited a statistically significant pronounced pro-oxidant/cytotoxic action on isolated rat hepatocytes. The viability of the cells and the level of GSH were reduced (each by 50%), the LDH activity was increased by 217%, and production of MDA was increased by 262%, relative to the untreated hepatocytes (control) (Figure 4, Figure 5, Figure 6 and Figure 7). In this model, EPhR had a hepatoprotective effect on hepatocyte viability (concentration-dependent, statistically significant). The highest concentration of 50 µg/mL had the best activity (Figure 4).
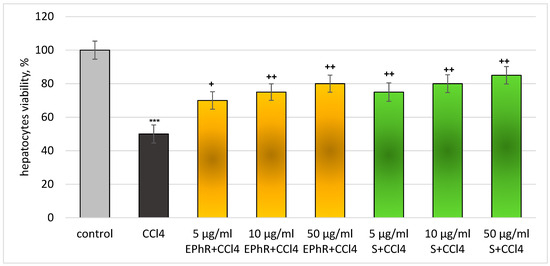
Figure 4.
Hepatocyte viability changes in a carbon tetrachloride model. *** p < 0.001 vs. control (untreated hepatocytes); + p < 0.05, ++ p < 0.01, vs. CCl4. EPhR—extract from P. russeliana; S—silymarin.
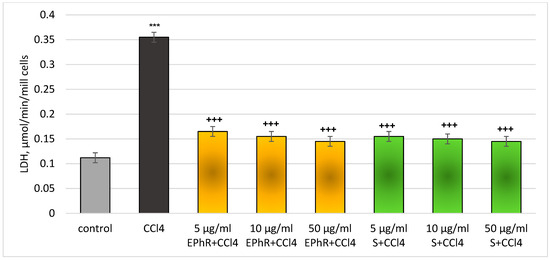
Figure 5.
LDH activity changes in rat hepatocytes in a carbon tetrachloride model. *** p < 0.001 vs. control (untreated hepatocytes); +++ p < 0.001 vs. CCl4. EPhR—extract from P. russeliana; S—silymarin.
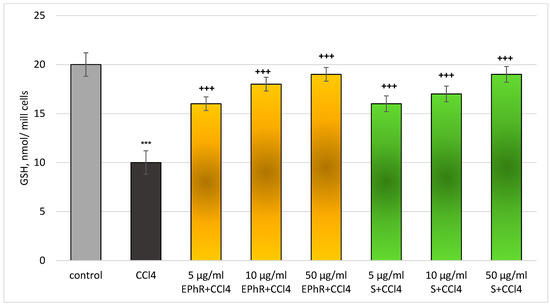
Figure 6.
Changes in levels of GSH in rat hepatocytes in a carbon tetrachloride model. *** p < 0.001 vs. control (untreated hepatocytes); +++ p < 0.001 vs. CCl4. EPhR—extract from P. russeliana; S—silymarin.
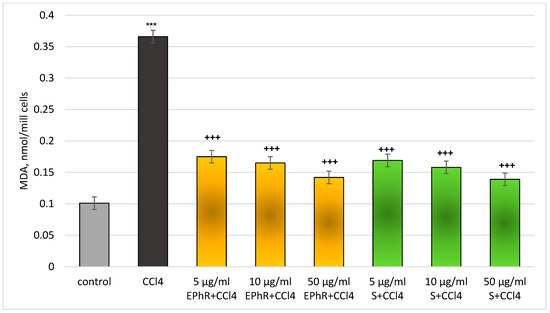
Figure 7.
Changes in levels of MDA in rat hepatocytes in a carbon tetrachloride model. *** p < 0.001 vs. control (untreated hepatocytes); +++ p < 0.001 vs. CCl4. EPhR—extract from P. russeliana; S—silymarin.
A 5 µg/mL extract preserves the vitality by 40%; 10 µg/mL by 50%; and 50 µg/mL by 60%, compared to the toxic agent. In total, 5 µg/mL of silymarin preserves the vitality by 50%; 10 µg/mL by 60%; and 50 µg/mL by 70%, compared to the toxic agent. The effects of the extract and silymarin on this indicator were comparable.
Upon the release of the LDH enzyme, in a model of CCl4-induced hepatotoxicity, EPhR showed a concentration-dependent, statistically significant, protective effect again. The effect was most pronounced at the highest concentration of 50 µg/mL (Figure 5). The release of LDH was lowered by 54% (from 5 mg/mL EPhR); by 56% (from 10 µg/mL EPhR); and by 59% (from 50 µg/mL EPhR) vs. CCl4. In total, 5 µg/mL of silymarin reduced LDH release by 56%; 10 µg/mL by 58%; and 50 µg/mL by 59% compared to the toxic agent. The action of the extract and silymarin on this parameter was commensurable.
The concentration-dependant and statistically significant hepatoprotective effect of EPhR was observed on the same model (CCl4-induced toxicity) on the levels of GSH. The highest concentration (50 µg/mL) of EPhR had the best activity (Figure 6).
The levels of GSH were preserved as follows: 60% (from 5 mg/mL EPhR); 70% (10 µg/mL EPhR); and 90% (50 µg/mL EPhR), vs. CCl4. The action of the extract and silymarin on this parameter was commensurable.
The MDA production (a parameter for lipid membrane oxidation) was lowered to a statistically significant level when EPhR was applied in the same model on hepatocytes. Again, the best effect was at the highest dose (50 µg/mL) (Figure 7).
The generation of MDA was reduced as follows: 52% (from 5 mg/mL EPhR); 55% (from 10 mg/mL EPhR); and 61% (from 50 mg/mL EPhR) vs. CCl4. In total, 5 µg/mL of silymarin reduced MDA production by 54%; 10 µg/mL by 57%; and 50 µg/mL by 62% compared to the toxic agent. The antioxidant effects of the extract and silymarin were comparable.
3.2.2. In Vivo Experiments with Old Wistar Rats
In vivo experiments (14-day treatment) on old rats were also performed to examine the potential hepatoprotective/antioxidant effects of EPhR and to compare it with the effect of silymarin in a CCl4-induced hepatotoxicity model. There was no mortality in the animals during treatment, according to the approved experimental model.
The assessment was made on the following liver biochemical parameters: the release of liver enzymes ALAT and ASAT, as well as levels of GSH and production of MDA. Administered alone, CCl4 exhibited a pronounced statistically significant hepatotoxic effect, increasing ASAT by 100%, ALAT by 150%, and MDA production by 200%, and decreasing the level of GSH by 50%, relative to the control (untreated rats) (Figure 8).
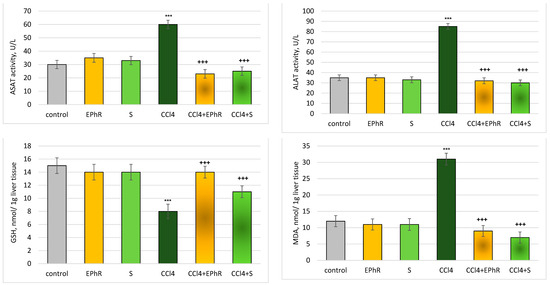
Figure 8.
Changes in the in vivo investigated biochemical parameters. *** p < 0.001 vs. control (non-treated rats); +++ p < 0.001 vs. CCl4. EPhR—extract from P. russeliana; S—silymarin.
When administered alone, the extract and silymarin showed no hepatotoxicity (statistically significant). The studied parameters were not altered (Figure 8). When combined with CCl4, the extract exhibited a pronounced statistically significant hepatoprotective/antioxidant effect, which was comparable to silymarin. Only for the level of GSH did the extract show a stronger action than silymarin (Figure 8). The extract significantly reduced CCl4-increased levels of ASAT by 60% and silymarin by 65% compared to the toxic agent. ALAT (elevated by the toxic agent) was reduced statistically significantly by the extract (by 64%) and by silymarin (by 68%) compared to pure carbon tetrachloride.
The level of GSH was preserved statistically significantly (by 80%) when EPhR was applied and after silymarin (by 40%) compared to the toxic agent. This finding is interesting, considering the well-established action of silymarin as a hepatoprotector and the age of the animals.
MDA production was reduced statistically significantly by the extract (by 73%) and by silymarin (by 77%) vs. rats given only CCl4.
Following euthanasia, the rats were subjected to pathomorphological evaluation. A normal anatomical structure was found (without visible alterations) during the gross pathology investigation of the liver of the control group rats. The organs were located within the normal topographical range, occupied the central cranial part of the abdominal cavity and were behind the diaphragm. The red-brown colour of the organs, with a smooth capsular surface and a well-visible lobar appearance was noted (Figure 9A).
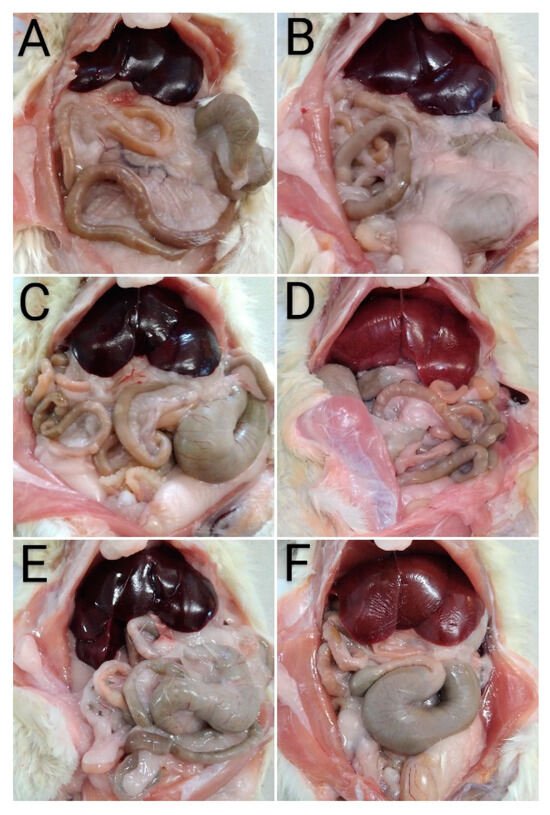
Figure 9.
Gross examination of the livers. (A) Control group—normal topographic location and macroscopic morphological structure of the liver; (B) a group treated with EPhR—unaltered topographical location and macroscopic morphological structure of the liver; (C) a group treated with S—normal topographic location and macroscopic morphological structure of the liver; (D) group treated with CCl4—liver with a brown-yellow colour; (E) group treated with CCl4 and EPhR—unaltered macroscopic morphological structure of the liver; and (F) group treated with CCl4 and S—normal macroscopic structure and pale brown liver colour.
A similar pattern was observed in the groups treated with EPhR and silymarin alone, as well as in those treated with CCl4 combined with EPhR (Figure 9B,C,E). Macroscopically, distinct changes were found in the group treated with CCl4 alone. The rat livers had a friable, greasy consistency and a brown-yellow colour (Figure 9D). In animals treated with CCl4 in combination with silymarin, a paler colour was found compared to the control group (Figure 9F).
The microscopic examination of the livers of rats in the control group and those treated with EPhR and silymarin revealed normal parenchymal architectonics and a lack of microscopically visible alterations (Figure 10A–C).
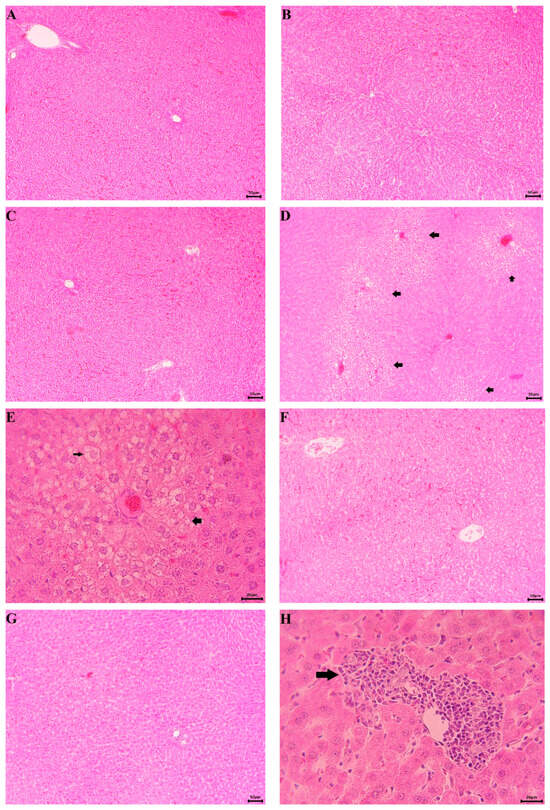
Figure 10.
Microscopic investigation of the animals’ livers (H&E staining). (A) Control group—normal histological structure of the parenchyma (×100); (B) EPhR-treated group—unaltered liver parenchyma (×100); (C) S-treated group—normal histological structure of the parenchyma (×100); (D) CCl4-treated group—lipid accumulation in the centrilobular areas (arrows) (×100); (E) CCl4-treated group—presence of microvesicular lipid accumulations (thick arrow) and lytic changes in hepatocytes (thin arrow) (×400); (F) CCl4 and EPhR-treated group—unaltered histological architecture (×100); (G) CCl4- and S-treated group—normal histological structure of the parenchyma (×100); and (H) CCl4 and S-treated group—mononuclear aggregations in portal tract area (arrow) (×400).
Clear changes were found in animals intoxicated with CCl4, expressed in microvesicular lipid accumulations and necrotic-degenerative changes in hepatocytes located in the central area of the lobules and near the terminal hepatic venules (Figure 10D,E). In rats treated with CCl4 combined with EPhR and with CCl4 combined with silymarin, a pronounced protective effect was found, resulting in a lack of morphological changes in the liver parenchyma (Figure 10F,G). In the CCl4 and silymarin-treated groups, mononuclear accumulations were found around the portal tracts (Figure 10H).
During the gross examination of the kidneys, no pathological changes were found in any of the experimental groups. The organs had a normal topographical arrangement. A well-defined cortex and medulla were observed after incision. Normal histological architecture was found during the microscopic examination of the kidneys of control group animals. Unaltered glomerular structures, tubules, interstitium and blood vessels were observed (Figure 11A).
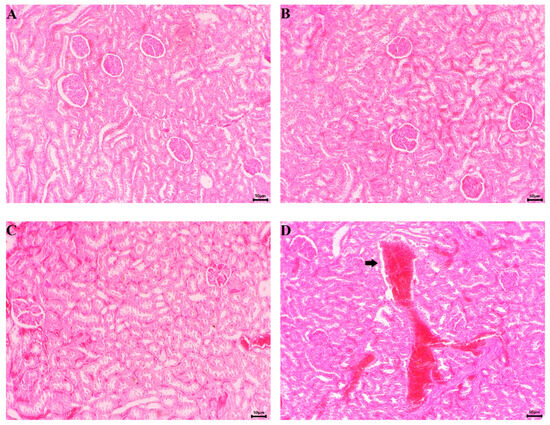
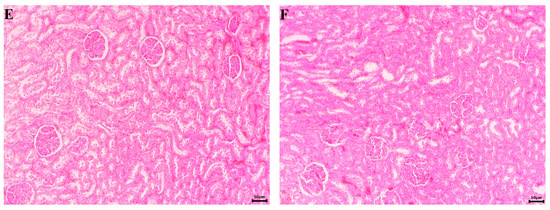
Figure 11.
Microscopic pictures of the animals’ kidneys (H&E staining, ×100). (A) Untreated control—normal histological structure of the parenchyma; (B) EPhR-treated group—unaltered microscopic structure of the parenchyma; (C) S-treated group—unchanged histoarchitecture; (D) CCl4-treated group—presence of extensive haemorrhage in the interstitial space (arrow); (E) CCl4- and EPhR-treated group—unaltered histological structure; and (F) CCl4- and S-treated group—normal microscopic structure.
Histological findings of the kidneys from the EPhR and silymarin treatment groups were commensurable to the control animals (Figure 11B,C). In CCl4-treated rats, the kidneys had extensive haemorrhages in the interstitium (Figure 11D). In the renal tubules’ epithelial cells, changes of a degenerative and necrotic nature were found, with the addition of desquamation and disintegration to some epithelial cells. A histological examination of kidneys from animals treated with CCl4 and EPhR combined, as well as those CCl4 and silymarin, showed an unaltered parenchymal structure (Figure 11E,F).
In the brain tissue, no significant macro- and microscopically visible changes were found in the animals of all groups, except in those treated alone with CCl4, where slight degenerative changes in the Purkinje cells were detected. In the histopathological examination of the cerebellums, it was found that EPhR had no neurotoxic properties and did not lead to morphologically visible changes (Figure 12).
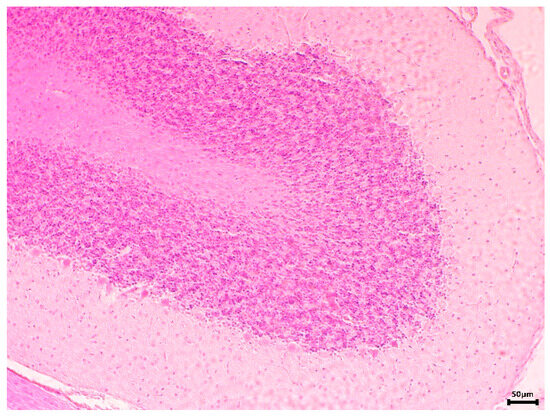
Figure 12.
Cerebellum of an EPhR-treated rat. Histological section through the apical part of a cerebellar folia. A normal layered structure without visible changes is shown. (H&E; 100×).
Treated animals had normal grey and white matter microscopic structure. Three well distinguished layers—molecular, Purkinje and granular—were observed in the cortex without signs of a toxic effect.
4. Discussion
As a continuation of our efforts to unveil the antioxidant and hepatoprotective activity of Phlomis L. species distributed in Bulgaria, a purified extract from the aerial parts of P. russeliana Lag. ex Benth. was prepared, analysed by two HPLC and one spectrophotometric method, and examined in in vitro/in vivo models of hepatic injury.
To our knowledge, there are no previous reports on the flavonoid composition of P. russeliana Lag. ex Benth. It is known that other species from the genus accumulate mainly flavone, flavonol, and flavanone glycosides [5]. Previous research reported only iridoids and phenylethanoids in the plant [5]. Using a qualitative HPLC analysis with authentic compounds, luteolin-3′-glucuronide, luteolin-7-glucuronide, quercetin-3-glucoside and naringin are reported in the species. This is in agreement with the data on the flavonoid content of other representatives. Unlike the study on P. tuberosa L. [7], where the flavonoids were only confirmed, here we report their presence for the first time. The quantitative analysis of total flavonoids proves that P. ruselliana’s overground parts are richer in these compounds compared to the herbs of P. tuberosa L.
A notable compound found in all Phlomis L. species is verbascoside. As a phenylethanoid glycoside, it contributes a lot to the effects [27] of various preparations from these plants. The results of the qualitative and quantitative analyses of P. russeliana Lag. ex Benth. were consistent with the reports on phenyletanoid glycosides in the overground parts of these plants [5]. Verbascoside was identified using a reference substance, and its quantity in EPhR was determined. A previous report quantified verbascoside in the aerial parts of P. tuberosa L. Here, we find another difference between these two species—the quantity of verbascoside in P. russeliana Lag. ex Benth. was nearly twice that in P. tuberosa L. aerial parts. Within the field of toxicology, experimental in vitro models play a crucial part in investigating the metabolism of compounds and determining the potential mechanism of toxicity and its potential impacts on an organism. When assessing the harmful or cytoprotective properties of some intriguing biologically active compounds, whether they are newly synthesised or have a biological origin, isolated hepatocytes provide an appropriate solution.
Determining the viability of a hepatocyte is one of the most crucial metrics to assess its metabolic potential. The trypan blue dye method provides a quick response to the physiological state of a cell without the need for specialised equipment. A visual evaluation of cell damage also takes advantage of nuclear proteins’ capacity to adsorb the dye. Additionally, even the weakest nucleus staining suggests that a cell membrane is compromised. Because of membrane-retained trypan blue, undamaged parenchymal cells show a distinct shape and yellow colour. They can be easily distinguished from dead cells tinted blue [19].
The cell release its soluble enzymes when the plasma membrane is compromised. The most recognisable examples of the liberated enzymes in a cytotoxic membrane injury are lactate dehydrogenase and liver transaminases. Therefore, another crucial indicator for determining the functioning status of the cell membrane is the hepatocytes’ capacity to release these enzymes. Either the conversion of pyruvic acid to lactic acid or the oxidation of lactate to pyruvate is the basis for calculating LDH activity [19].
MDA is amongst the primary indicators of membrane lipid oxidation. It is produced as a result of lipid oxidation, which is achieved through the disintegration of hydroperoxides (created when polyunsaturated fatty acids oxidise). The aldehydes that are most extensively researched during lipid peroxidation include MDA, 4-hydroxynonenal, and 4-hydroxyhexenal. As a highly reactive metabolite, MDA combines with free amino groups from proteins and amino acids to generate Schiff bases. It is more persistent than free radicals, diffuses readily, and makes up around 2% of the results of lipid peroxidation [19].
The synthesis of MDA is correlated with the amount of GSH present in the cell. Non-lipid oxidation can occasionally result in a drop in GSH. On the other hand, when a reactive metabolite produces a GSH shortfall in the cell, lipid peroxidation and a rise in MDA are seen [19]. The nucleophile GSH is one of the most significant protective mechanisms, and aids in the scavenging of reactive electrophilic compounds. GSH can be found in any cell compartment, and the liver has the highest amount of this antioxidant in all the body [19]. The defatted extract, when applied alone, did not show hepatotoxic effects on any of the criteria that are indicative of the functioning and the metabolic capacity of hepatocytes.
If we compare the hepatotoxicity of CCl4 to other harmful xenobiotics, it is arguably the most extensive. Liver necrosis and centrilobular fatty degeneration are caused by this hazardous substance. Since it dissolves in fat, it is found all over the body. Chronic use damages the kidneys and develops liver cirrhosis and tumours. Only fatty degeneration and cytochrome P450 (CYP) degradation are caused by modest dosages of CCl4, and these effects are primarily seen in the liver’s centrilobular zone [28]. CYP isoforms (1A2, 2E1, 2B1/B2, and 3A) bioactivate CCl4. It produces a trichloromethyl radical (CCl3•) [29], which can take part in multiple reactions. The endoplasmic reticulum and mitochondria, two extremely critical organelles for cells, as well as the cell membrane, are destroyed by this metabolite.
Lipid peroxidation produces some by products that harm cells as well. These products include conjugated dienes, 4-hydroxynonenal, MDA, and a few additional hydroxyalkenes. They also block the activities of glucose-6-phosphatase and protein synthesis, and lower the level of GSH [29]. In vitro research revealed an increase in membrane permeability. Following the p.o. application of CCl4, the endoplasmic reticulum exhibits the majority of the alterations. It binds covalently to phospholipids and the protein in microsomes one minute after delivery. After five minutes, conjugated dienes, which are markers of lipid peroxidation, can be found. After half an hour, electron imaging revealed reduced protein synthesis as well as modifications to the ribosomes and endoplasmic reticulum [28]. There is also less CYP activity and content. Triglycerides build up as globules in hepatocytes one-to-three hours after CCl4 administration. There is an increase in the endoplasmic reticulum’s loss of enzyme activity. Increased cytosolic calcium levels, granular endoplasmic reticulum vacuolisation, and ribosome rupture are observed. The lysosomes are discharged as the plasma membrane ruptures and distorts [28].
It has been discovered that phenolic compounds inhibit both human 3A4 and 2C9 isoforms of CYPs. Also, the major liver glucuronosyl-transferases are supressed [30]. The cytoprotective impact of EPhR in the CCl4 toxic model on isolated hepatocytes is probably due to a synergistic action that alters the activity of certain CYP isoforms implicated in CCl4 activation. Because silymarin possesses antioxidant properties that alter the cholesterol and phospholipid composition of the hepatocyte cell membrane, it has been proved to shield the cell’s membrane from the damage caused by CCl4 [31]. It was established that human microsomes could break down silybinin (silybin A and B) into a number of metabolites. Monohydroxy- and dihydroxy-silybinin are the other two metabolites; demethylated silybinin is the primary metabolite. Strong anti-inflammatory, cytoprotective, antioxidant, and anti-carcinogenic properties are exhibited by silymarin [32]. Similar to the mechanism of silymarin, the hepatoprotective effect of EPhR could be assigned to membrane stabilisation; the preservation of GSH levels (which is the primary scavenger of reactive oxygen species); and the potential inhibitory effects on some isoforms of cytochrome that metabolise CCl4 (resulting in generating toxic reactive metabolites).
Pathological studies on rat livers confirmed the hepatoprotective effect of EPhR. The oral administration of CCl4 is known to induce lipid accumulation in hepatocytes, liver degeneration, varying degrees of centrilobular necrosis, and less commonly leads to a marked inflammatory response [33]. This toxic agent has been used by a number of authors for an in vivo model for the characterisation of the protective properties of biologically active substances of species of the genus Phlomis L. [34,35]. No gross or microscopic changes were observed in the experimental rats’ livers in the non-treated control or in both groups treated alone with EPhR and silymarin, which proved that there is a lack of toxic effects in the studied extract. In the group treated with CCl4 alone, a brown-yellow colour and friable consistency of the organ was grossly detected, which is characteristic of degenerative processes associated with lipid deposits [36]. Histologically, in the same group, typical changes in intoxication were found, like disseminated distribution, mainly in the perivascular areas, of microvesicular, lipid-droplet build-ups in the hepatocytes. The nuclei showed signs of degeneration and necrosis. Rats treated with CCl4 and EPhR (combined) had an unaltered liver histological structure. No microscopically visible changes were found in their hepatocytes. The histological appearance was comparable to that of the group treated with the combination of CCl4 and silymarin.
These findings are consistent with previous research on the P. tuberosa L. defatted extract [7]. Although the effects were statistically significant in these studies, the experiments were on young Wistar rats. In the present research, the results obtained in old (2.5 years) Wistar rats are even better, despite the unfavourable age of the animals. It is known that old animals have age-related organ changes, which result in biochemical parameters such as GSH, ASAT, ALAT, etc. [37]. The chemical basis of the observed stronger effect is that the extract of P. russeliana Lag. ex Benth. had nearly twice the amount of verbascoside and total flavonoids compared to P. tuberosa L. Another major difference is EPhR’s total phenolic content. Using a GAE assay, it was noted that this extract has more phenolics compared to the previously investigated one [7]. Reports on the in vitro radical-scavenging capabilities of an extract form P. russeilana Lag. ex Benth. are present [10]. The findings of the present study complement knowledge on this extracts antioxidant activity. The levels of GSH were even higher in the old rats treated with EPhR than in those given silymarin, despite the age of the rodents. Moreover, its levels in CCl4, challenged and curatively treated with EPhR animals, reached 80% of the non-treated group, far exceeding the silymarin group. It is clear that through the higher phenolic compounds content, P. russeliana Lag. ex Benth. is a better hepatoprotector than both P. tuberosa L. and silymarin.
During the histopathological studies, a well-expressed nephroprotective effect of the examined extract was found. The kidney is also a target organ of damage in CCl4 intoxication, although less affected than the liver [33]. Intoxication with CCl4 leads to pronounced hemodynamic disorders, expressed in hyperaemia and extensive haemorrhages. Signs of dystrophia and necrosis were visible in the proximal tubules’ epithelial cells. Verbascoside, contained in the extract, favourably affected renal function and had a clear protective effect in conditions of intoxication [38]. Others investigated the nephroprotective effect of plant-derived extracts in the CCl4 model of kidney damage [39,40]. One of the main criteria for the degree of kidney damage, are the histological changes in the renal parenchyma. The microscopic examination of kidneys from the control group and those treated with EPhR and silymarin alone revealed normal microscopic architectonics. In the animals treated with CCl4, dystrophic-necrotic changes in the epithelium of the proximal cortical tubules were found, accompanied by the desquamation and disintegration of cells. Extensive haemorrhage was observed in the interstitium. Microscopically, groups treated with CCl4 and EPhR had normal histological architectonics of the renal parenchyma, with the unaltered structure of the tubules and interstitium. An accumulation of mononuclear cells in the interstitial tissue was observed. The histological findings were similar and comparable to that of kidneys from the CCl4- and silymarin-treated groups.
Future studies for elucidating molecular and cellular pathways involved in hepatoprotection and the antioxidant activity of these phytochemicals are needed. Nevertheless, the radical-scavenging potential of phenolics in general (both phenylethanoids and flavonoids) has been reported. Thus, the most likely mechanism of the observed effects is antioxidation through radical scavenging [6].
5. Conclusions
A defatted extract from Phlomis russeliana Lag. ex Benth. was analysed via one qualitative and two quantitative HPLC methods. Authentic reference substances were used to identify verbascoside and several flavonoids. Two HPLC tests were conducted, one by total flavonoids and the other utilising verbascoside as a reference substance. The extract was then tested for hepatotoxicity and hepatoprotection in vitro and in vivo, both on its own and in a metabolic bio activation model caused by carbon tetrachloride (CCl4). It did not cause concentration-dependent toxicity in hepatocytes when applied alone. In vitro, the extract demonstrated a protective effect (dose-dependent) when CCl4 was present. Old Wistar rats were used in the in vivo experiment of hepatotoxicity induced by CCl4. Biochemical evaluations in liver homogenate (GSH, MDA) and blood plasma (ASAT, ALAT) demonstrated the statistically significant hepatoprotective effect of the defatted extract. This impact was verified by a histological examination of the livers, kidneys, and brains of the experimental animals, and it was more pronounced than the effect of a purified extract from P. tuberosa. The results were comparable to those of silymarin, which is a proved hepatoprotector.
Author Contributions
Conceptualisation, M.K.-B. and I.K.; methodology, A.S., V.M. and M.K.-B.; software, A.S.; validation, A.S., M.K.-B. and V.M.; formal analysis, A.S., M.K.-B. and G.P.; investigation, A.S., M.K.-B., G.P. and V.M.; resources, M.K.-B., I.K., A.S. and V.M.; data curation, M.K.-B. and A.S.; writing—original draft preparation, M.K.-B., G.P., I.K. and A.S.; writing—review and editing, A.S.; visualisation, V.M.; supervision, I.K.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0004-C01.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Medical University of Sofia (KENIMUS) and the Bulgarian Food Safety Agency at the Bulgarian Ministry of Agriculture and Foods (with Permission No. 273, valid until 20 July 2025).
Data Availability Statement
Data connected with this study are freely available from the corresponding author upon reasonable written request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, R.O.; Glende, E.A.; Dolak, J.A.; Waller, R.L. Mechanisms of Carbon Tetrachloride Toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Sarkhail, P.; Amin, G.; Surmaghi, M.H.S.; Shafiee, A. Composition of the Volatile Oils of Phlomis Lanceolata Boiss. & Hohen., Phlomis anisodonta Boiss. and Phlomis bruguieri Desf. from Iran. Flavour Fragr. J. 2005, 20, 327–329. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of Some Greek Aromatic Plants for Antioxidant Activity. Phytother. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Amor, I.L.-B.; Boubaker, J.; Sgaier, M.B.; Skandrani, I.; Bhouri, W.; Neffati, A.; Kilani, S.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Phytochemistry and Biological Activities of Phlomis Species. J. Ethnopharmacol. 2009, 125, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Delazar, A.; Sabzevari, A.; Mojarrab, M.; Nazemiyeh, H.; Esnaashari, S.; Nahar, L.; Razavi, S.M.; Sarker, S.D. Free-Radical-Scavenging Principles from Phlomis Caucasica. J. Nat. Med. 2008, 62, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Kondeva-Burdina, M.; Shkondrov, A.; Popov, G.; Manov, V.; Krasteva, I. In Vitro/In Vivo Hepatoprotective and Antioxidant Effects of Defatted Extract and a Phenolic Fraction Obtained from Phlomis Tuberosa. Int. J. Mol. Sci. 2023, 24, 10631. [Google Scholar] [CrossRef] [PubMed]
- Demirci, B.; Toyota, M.; Demirci, F.; Dadandi, M.Y.; Can Baser, K.H. Anticandidal Pimaradiene Diterpene from Phlomis Essential Oils. Comptes Rendus Chim. 2009, 12, 612–621. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Montoro, P.; Piacente, S.; Pizza, C.; Dönmez, A.; Çalış, İ. Identification by HPLC-PAD-MS and Quantification by HPLC-PAD of Phenylethanoid Glycosides of Five Phlomis Species. Phytochem. Anal. 2005, 16, 1–6. [Google Scholar] [CrossRef]
- Okur, M.E.; Karadağ, A.E.; Üstündağ Okur, N.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Demirci, B.; Demirci, F. In Vivo Wound Healing and In Vitro Anti-Inflammatory Activity Evaluation of Phlomis Russeliana Extract Gel Formulations. Molecules 2020, 25, 2695. [Google Scholar] [CrossRef]
- Alpay, M.; Dulger, G.; Sahin, I.E.; Dulger, B. Evaluating Antimicrobial and Antioxidant Capacity of Endemic Phlomis Russeliana from Turkey and Its Antiproliferative Effect on Human Caco-2 Cell Lines. An. Acad. Bras. Cienc. 2019, 91, e20180404. [Google Scholar] [CrossRef] [PubMed]
- Akaydin, G.; Şimşek, I.; Arituluk, Z.C.; Yeşilada, E. An Ethnobotanical Survey in Selected Towns of the Mediterranean Subregion (Turkey). Turkish J. Biol. 2013, 37, 230–247. [Google Scholar] [CrossRef]
- Europe Council. European Pharmacopoeia 9.0; Europe Council: London, UK, 2017; ISBN 978-3-7692-6633-7. [Google Scholar]
- Shkondrov, A.; Krasteva, I.; Pavlova, D.; Zdraveva, P. Determination of Flavonoids in Related Astragalus Species (Sect. Incani) Occurring in Bulgaria. Comptes Rendus L’Academie Bulg. Sci. 2017, 70, 363–366. [Google Scholar]
- López, M.I.; Callao, M.P.; Ruisánchez, I. A Tutorial on the Validation of Qualitative Methods: From the Univariate to the Multivariate Approach. Anal. Chim. Acta 2015, 891, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982; Volume 31. [Google Scholar]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric Determination of the Total Phenolic Content, Spectral and Fluorescence Study of the Herbal Unani Drug Gul-e-Zoofa (Nepeta Bracteata Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Fau, D.; Berson, A.; Eugene, D.; Fromenty, B.; Fisch, C.; Pessayre, D. Mechanism for the Hepatotoxicity of the Antiandrogen, Nilutamide. Evidence Suggesting That Redox Cycling of This Nitroaromatic Drug Leads to Oxidative Stress in Isolated Hepatocytes. J. Pharmacol. Exp. Ther. 1992, 263, 69–77. [Google Scholar]
- Mitcheva, M.; Kondeva, M.; Vitcheva, V.; Nedialkov, P.; Kitanov, G. Effect of Benzophenones from Hypericum Annulatum on Carbon Tetrachloride-Induced Toxicity in Freshly Isolated Rat Hepatocytes. Redox Rep. 2006, 11, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Abdelghffar, E.A.R.; El-Nashar, H.A.S.; Fayez, S.; Obaid, W.A.; Eldahshan, O.A. Ameliorative Effect of Oregano (Origanum Vulgare) versus Silymarin in Experimentally Induced Hepatic Encephalopathy. Sci. Rep. 2022, 12, 17854. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choo, G.-S.; Yoo, E.-S.; Woo, J.-S.; Han, S.-H.; Lee, J.-H.; Jung, J.-Y. Silymarin Induces Inhibition of Growth and Apoptosis through Modulation of the MAPK Signaling Pathway in AGS Human Gastric Cancer Cells. Oncol. Rep. 2019, 42, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.-H.; Yang, Y.-S.; Lee, J.-C.; Moon, C.-J.; Kim, S.-H.; Jun, W.; Park, S.-C.; Kim, J.-C. Ameliorative Effects of Pycnogenol® on Carbon Tetrachloride-Induced Hepatic Oxidative Damage in Rats. Phyther. Res. 2007, 21, 1015–1019. [Google Scholar] [CrossRef]
- Bai, X.; Qiu, A.; Guan, J.; Shi, Z. Antioxidant and Protective Effect of an Oleanolic Acid-Enriched Extract of A. Deliciosa Root on Carbon Tetrachloride Induced Rat Liver Injury. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 169–173. [Google Scholar] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Validation of Analytical Procedures: Text and Methodology Q2 (R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2023. [Google Scholar]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic Potential of Phenylethanoid Glycosides: A Systematic Review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef] [PubMed]
- Timbrell, J. Factors Affecting Metabolism and Disposition. Toxic Responses to Foreign Compounds - Direct Toxic Action: Tissue Lessions. In Principles of Biochemical Toxicology, 3rd ed.; Timbrell, J., Ed.; Taylor and Francis: Oxfordshire, UK, 2000; pp. 175–180. [Google Scholar]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and Mechanism of Action of Haloalkanes: Carbon Tetrachloride as a Toxicological Model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Sridar, C.; Goosen, T.C.; Kent, U.M.; Williams, J.A.; Hollenberg, P.F. Silybin Inactivates Cytochromes P450 3A4 and 2C9 and Inhibits Major Hepatic Glucuronosyltransferases. Drug Metab. Dispos. 2004, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P.; Mourelle, M. Prevention by Silymarin of Membrane Alterations in Acute CCl4 Liver Damage. J. Appl. Toxicol. 1990, 10, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-N.; Hsieh, Y.-S.; Chiou, H.-L.; Chu, S.-C. Silibinin Inhibits Cell Invasion through Inactivation of Both PI3K-Akt and MAPK Signaling Pathways. Chem. Biol. Interact. 2005, 156, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O. Toxicological Profile for Carbon Tetrachloride; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005. [Google Scholar]
- Gu, H.; Gu, X.; Xu, Q.; Kang, W. Antioxidant Activity in Vitro and Hepatoprotective Effect of Phlomis Maximowiczii In Vivo. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Usmanov, D.; Yusupova, U.; Syrov, V.; Ramazonov, N.; Rasulev, B. Iridoid Glucosides and Triterpene Acids from Phlomis Linearifolia, Growing in Uzbekistan and Its Hepatoprotective Activity. Nat. Prod. Res. 2021, 35, 2449–2453. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Van Wettere, A.J.; Cullen, J.M. Hepatobiliary System and Exocrine Pancreas1. In Pathologic Basis of Veterinary Disease; Zachary, J., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; Chapter 8; pp. 412–470. [Google Scholar]
- Kwon, A.J.; Morales, L.; Chatagnier, L.; Quigley, J.; Pascua, J.; Pinkowski, N.; Brasser, S.M.; Hong, M.Y. Effects of Moderate Ethanol Exposure on Risk Factors for Cardiovascular Disease and Colorectal Cancer in Adult Wistar Rats. Alcohol 2024. [Google Scholar] [CrossRef]
- Chen, X.; Shi, M.; Yang, L.; Guo, F.; Liang, Y.; Ma, L.; Fu, P.P. Phenylethanoid Glycoside Verbascoside Ameliorates Podocyte Injury of Diabetic Kidney Disease by Regulating NR4A1-LKB1-AMPK Signaling. Acta Mater. Medica 2023, 2, 72–83. [Google Scholar] [CrossRef]
- Slama, K.; Rouag, M.; Tichati, L.; Taibi, F.; Boumendjel, M.; Boumendjel, A.; Messarah, M. Nephroprotective Role and Antioxidant Capacity of Atriplex Halimus on Carbon Tetrachloride-Induced Kidney Damage in Rats. Comp. Clin. Path. 2021, 30, 75–87. [Google Scholar] [CrossRef]
- Gomaa, A.A.-R.; Samy, M.N.; Attia, E.Z.; Attya, M.E.; Fawzy, M.A.; Desoukey, S.Y.; Kamel, M.S. Antioxidant, Hepatoprotective and Nephroprotective Activities of Gazania Rigens against Carbon Tetrachloride-Induced Hepatotoxicity and Nephrotoxicity in Rats. Tradit. Med. Res. 2022, 7, 44. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).