Abstract
Utilizing natural plant extracts as food additives represents a promising strategy for enhancing the quality, nutritional value, and safety of food products, benefiting both consumers and the environment. Therefore, the primary objective of this study was to develop an environmentally sustainable process for the production of solid powder derived from Allium ursinum liquid extract, with the intent of utilizing it as a natural food additive. To address the challenge of instability and enhance the solubility of bioactive compounds in A. ursinum extracts obtained through subcritical water extraction, this study employed the spray drying process. Notably, the results demonstrated the remarkable efficiency of the spray drying process, with maltodextrin as a carrier, yielding uniformly encapsulated particles with an average size of approximately 4 µm, spherical shape with smooth, intact surfaces. The most optimal conditions for achieving the highest content of total phenolics (23.10 mg GAE/g) and total flavonoids (4.92 mg CE/g) in the A. ursinum extract were identified, involving an inlet temperature of 120 °C and an 80% maltodextrin concentration. The encapsulated powders showed excellent stability, with minimal loss of total phenolics (12.64%) and total flavonoids (10.52%) after three months of storage. Physicochemical analysis confirmed the successful preservation of bioactive compounds through microencapsulation using maltodextrin, suggesting its potential for application in innovative food or pharmaceutical products.
1. Introduction
The application of natural plant extracts as bioactive ingredients into new formulations of health-promoting properties has become an increasing trend, especially in the functional food sector. The addition of herbal extracts or bioactive compounds into highly consumed products is an attractive method for creating modern nutrition and a promising approach for improving the delivery of bioactive compounds [1]. Besides the improved nutritional and health-promoting character of food, natural plant extracts can also enhance the flavor, color, and aroma. As a versatile ingredient that can be used in a wide range of food products, natural plant extracts can provide unique food flavor profiles, providing consumers with a more enjoyable eating experience that cannot be achieved with synthetic additives [2,3].
However, the efficiency of plant extracts as functional and health-promoting additives depends on various factors. To produce safer and high-quality extracts, the origin of plant material used, the extraction method applied, encapsulation process parameters, and the stability of the extract/encapsulate should be considered [4]. Before applying extract as an additive, comprehensive scientific studies should be conducted to determine the quality, safety, and efficacy of the plant extract. To satisfy the increasing consumer demand for greener products and cleaner labels, herbal extracts prepared by sustainable processes and “green” extraction techniques using “green” solvents are attracting the attention of various sectors. Extracts prepared in this way can be used as safe substitutes for generally applied synthetic additives.
In this respect, Allium species have been widely used as edible and medicinal plants. One of the Allium species, Allium ursinum or wild garlic, is the focus of scientific research nowadays as it represents a rich source of phenolic and sulfur compounds [5]. In particular, A. ursinum has been proven to possess good antibacterial and antioxidant activity by several authors [6,7]. Special attention to its extracts is given as the development of bacterial resistance is several times slower in the case of A. ursinum extracts compared to certain antibiotics [8]. The high antimicrobial activity of A. ursinum extract obtained by water, as a completely safe solvent, in its subcritical conditions has already been confirmed against foodborne bacteria [7]. According to the authors, the antimicrobial activity of the aqueous subcritical extract is more related to the phenolic compounds present in the subcritical aqueous extract rather than the sulfur ones. It has been proven that polyphenolic compounds, due to their abundant structural diversity and variations in chemical composition, exhibit antibacterial activity against a wide range of microorganisms [9]. However, the application of polyphenols as natural additives, functional ingredients, and supplements may be limited due to their low stability caused by environmental and processing conditions [10]. The incorporation of herbal extract rich in bioactive compounds in food systems necessitates integrated extraction and encapsulation processes to ensure the stability of the extract and the final products.
Due to the instability of a wide range of bioactive compounds, encapsulation has been found to be a practical method of preserving bioactivity, with a good potential for use in functional and nutraceutical products [11]. Encapsulation of herbal extract improves the final product quality, maintains the chemical integrity of the bioactive compounds, and prevents them from possible chemical degradation and modification during storage. In particular, spray drying is often a preferred method of encapsulation due to its capacity to convert a liquid extract into a powder with accurate specifications in a continuous way, with easy scale-up and relatively economical operation. Spray drying is a powerful particle engineering technique that enables the production of encapsulates/powders of micro- or nano-size particles with homogenous distribution in particle size, spherical shape, and surface characteristics. Through precision in creating particles with controlled attributes, spray drying holds the potential to enhance delivery, consequently improving the availability and accessibility of bioactive compounds. Moreover, using spray drying, powders with great handling and storage stability are obtained, which are more suitable for packing and transporting than the liquid forms [12]. Besides increased stability, mitigation of unpleasant tastes or flavors, controlled release, improved aqueous solubility, and bioavailability can be achieved. Additionally, dried extract powders can be used in the manufacture of solid dosage forms for food supplements or medicines [11].
During spray drying, different parameters need to be carefully adjusted to provide the required quality of dry powdered herbal extract production. The physicochemical properties of spray-dried powders and the process yield depend on processing conditions, product design, and formulation, hence representing critical aspects in production [13]. The spray-drying conditions, especially the inlet and outlet temperatures, are important factors contributing to the efficiency of the encapsulation process, and attention must be paid to balance the energy consumption of the drying process and the quality of final products in terms of physical and chemical properties of the encapsulated extract [14]. Additionally, for the formulation of dry herbal extracts with good physiochemical properties suitable for successful incorporation and commercial exploitation, an appropriate carrier is required. The carrier protects present bioactive compounds during storage, enables easier handling and addition to foods/pharmaceuticals or cosmetics, and possibly controls the release of bioactive compounds in the final product. Maltodextrin is widely used as an encapsulating carrier for its low price, high water solubility, and low viscosity, even at high concentrations, which makes it suitable for atomization in spray drying. It also has high water solubility that could reduce stickiness and agglomeration drawbacks that occur during spray drying [15].
Wild garlic and its extracts have a great potential to be used as food additives; however, wild garlic is a seasonal plant that is not cultivated, and it is only available for a short time in the spring. Additionally, the poor stability of A. ursinum aqueous extracts, rich in bioactive compounds with antimicrobial potential, requires a solution for a stable form of extract that will enable its use throughout the year. Incorporating encapsulated wild garlic extract into functional foods can contribute to improved nutritional profiles and added health-promoting characteristics, including antioxidant and antimicrobial properties. Further exploration of its applications within functional food formulations could reveal its full potential [16].
Therefore, this research offers a solution in the form of dry A. ursinum extract obtained through spray drying. To the best of our knowledge, no study has been carried out regarding the effect of spray drying conditions on the physical and chemical properties of encapsulated A. ursinum aqueous extract. Accordingly, the purpose of the current study was to determine appropriate spray drying conditions and composition of the feed solution (mixture of liquid extract and carrier) for encapsulating A. ursinum aqueous subcritical extract as well as to evaluate the effect of process parameters on the physiochemical properties of the obtained encapsulated A. ursinum extract powder. Another reason for the encapsulation of A. ursinum extract is its pungency and unpleasant taste, which limits the application of liquid extracts and requires a more suitable form to be implemented into food or pharmaceutical products.
2. Material and Methods
2.1. Chemicals and Reagents
Folin—Ciocalteu reagent—and 1,1-Diphenyl-2-picryl-hydrazyl-hydrate (DPPH) were purchased from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). Gallic acid was purchased from Sigma (Sigma, St. Luis, MO, USA). Maltodextrins (MD) classified using dextrose equivalent (DE) as DE 19.7 were purchased from Brenntag (Mülheim, Germany). All other chemicals and reagents were of analytical reagent grade.
2.2. Extraction Process
Dried A. ursinum levees used for the extract preparation were donated by a local tea factory (Fructus doo, Bačka Palanka, Serbia). For the liquid extract preparation, an eco-friendly technique called subcritical water extraction was used. The extraction process was conducted on a laboratory-scale high-pressure extraction unit (Parr Instrument Company, Moline, IL, USA) using the optimized subcritical water extraction conditions reported by [17] at a temperature of 181 °C, an extraction time of 10 min, and an added acidifier at 1.1%. The pressure was kept at 20 bar. The extract was filtered through filter paper (4–12 μm pore size, Schleicher and Schuell, Taufkirchen, Germany).
2.3. Encapsulation Process
For producing a dry powdered extract of Allium ursinum, a semi-industrial Anhydro spray dryer (APV Anhydro AS, Søborg, Denmark) was used. Maltodextrin (DE 19.7) was the drying agent and carrier at concentrations of 0%, 10%, 40%, and 80% relative to the dry residue of the liquid extract. Prior to drying, carrier materials were dissolved in distilled water, and carrier solutions were added to the prepared liquid extract and mixed continuously with a magnetic stirrer at a temperature of 30 °C. The liquid feed was pumped into the spray drying chamber at a constant flow rate of 1.36 L/h using a peristaltic pump (Thermo Scientific, Thermo FH100 digital, Rugby, England)). The speed of the atomizer was set as 20,000–21,000 rpm. The inlet air temperature (Ti) was set at 120 ± 5 °C and 140 ± 5 °C, while the outlet temperature (To) was 80 ± 5 °C for each individual drying. The obtained powder/encapsulate was separated from the air via a cyclone. All powders were packed in airtight containers and kept at 4 °C in the dark until analyses.
The efficiency of powder production (weight percentage) was determined by comparing the theoretically calculated powder that should be produced from the applied liquid feed and the weight of the powder obtained, where powder particles adhered to the walls of the drying chamber or cyclone were not considered.
2.4. Powder Characterization
2.4.1. Bulk Density
The bulk density was measured by determining the volume that the powder of the defined mass occupied in the cylinder. Dry extract/encapsulate (5 g) was placed into a 25 mL graduated glass cylinder and exposed to vibration for 3 min. Subsequently, bulk density was expressed as mg of powder extract per mL [18]. All experiments were performed in three replicates, and the results are expressed as mean values.
2.4.2. Moisture Content
Moisture content was determined gravimetrically by drying the extract at a temperature of 105 °C until a constant mass was achieved [18]. All measurements were carried out in triplicates, and the results are expressed as mean values.
2.4.3. Hygroscopicity
Hygroscopicity was measured by monitoring the change in mass of dry extracts over time due to water absorption from the surroundings. The experiment was conducted in a desiccator with a sodium chloride-saturated solution, achieving 70% relative humidity. Approximately 1 g of dried extract powder was measured, and hygroscopicity was monitored after 24 h, 48 h, and 5 days. The results were expressed as grams of absorbed water per 100 g of dry extract powder [19]. All measurements were carried out in triplicates, and the results are expressed as mean values.
2.4.4. Rehydration
The time (expressed in seconds) needed for the dry extract powder to completely rehydrate was determined by adding 2 g of dry extract powder into 50 mL of distilled water at room temperature. The dry extract powder was mixed with water via a magnetic stirrer in a glass flask until it was completely dissolved. All measurements were carried out in triplicates, and the results are expressed as mean values [20].
2.4.5. Water Solubility Index and Water Absorption Index
Water solubility index (WSI) and water absorption index (WAI) were determined by mixing 1.0 g of dry extract powder with 10 mL of distilled water in a centrifuge tube and incubating it for 15 min at 30 °C. After centrifugation, the supernatant and remaining solid particles were dried at 105 °C. WSI was calculated as the ratio of dried supernatant mass to the dry sample (1.0 g), expressed as a percentage. WAI was calculated as the mass of solid particles remaining after eliminating the supernatant from the total dry matter in the original sample (1.0 g), expressed as a percentage [21]. Measurements were conducted in triplicates, and the results are expressed as mean values.
2.4.6. Color
The color of the dry extract powder/encapsulate was measured using a Chroma meter (CR-300, Konica Minolta Sensing, Inc., Tokyo, Japan) calibrated with a white standard plate. The color was interpreted in the CIELAB color system. All measurements were carried out in triplicates, and the results are expressed as mean values [17].
2.4.7. Particle Size Distribution
Particle size distribution of the dry extract powder/encapsulate was determined using the Mastersizer 2000 laser diffraction particle size analyzer (Malvern Instruments, Malvern, UK). The Scirocco dispersion unit was used for dispersing encapsulate in the air. The sample was added until an adequate obscuration was obtained (5–10% for dry samples). The results were quantified as the volume-based particle size distribution by means of the Mastersizer 2000 software version 5. The diameters d(0.1), d(0.5), d(0.9) (the diameters of particles at 10, 50, and 90% cumulative volume, respectively), D[4,3] (volume mean diameter), and Span value (distribution width) were obtained [22]. All measurements were carried out in triplicates, and the results are expressed as mean values.
2.5. Scanning Electron Microscopy (SEM)
The morphology (shape and surface) of the dry extract powder/encapsulate was analyzed using scanning electric microscopy (SEM) with an input voltage of 2.0 kV. A small amount of the encapsulated powder was fixed onto the surface of a double-coated metallic adhesive tape adhered to a metallic stub. The stub was then coated with a fine layer of Ag/Pd under an argon atmosphere using high-vacuum conditions [23].
2.6. Differential Scanning Calorimetry Analysis (DSC)
The Mettler Toledo DSC 821e thermal analysis system with the STARe thermal analysis program V6.0 (Mettler Inc., Schwerzenbach, Switzerland) was used for DSC measurements. The sample (approx. 3 mg) was examined in the temperature range between 20 °C and 200 °C. The heating rate was 10 °C min−1. During the DSC investigation, argon was used as an inert carrier gas at a flow rate of 10 L/h [23]. All measurements were carried out in triplicates, and the results are expressed as mean values.
2.7. Analysis of Bioactive Compounds
2.7.1. Total Phenolic Content (TPC)
The contents of total phenolic compounds in A. ursinum dry extract powders were determined using the Folin–Ciocalteu procedure. Gallic acid was used as the standard compound for the preparation of the calibration curve, and the absorbance of the samples was measured at 750 nm after 1 h of incubation (6300 Spectrophotometer, Jenway, Dunmow, UK). Total phenolic contents were expressed in mg of gallic acid equivalents (GAE) per gram of encapsulate (mg GAE/g). All experiments were performed in three replicates, and the results are expressed as mean values.
2.7.2. Total Flavonoid Content (TFC)
The total flavonoid content was determined using the aluminum chloride colorimetric assay. Catechin was used as a standard for the creation of the calibration curve, and absorbance was measured at 510 nm. The content of flavonoids in dry extract powders was expressed as mg CE per g of dry extract (mg CE/g). All experiments were performed in triplicate, and the results were expressed as mean values.
2.7.3. Radical Scavenging Activity Assay
Radical Scavenging Activity assay, specifically the DPPH assay, was carried out following the method of [24]. Different amounts of extract were mixed with methanol (95%) and 90 μM 2,2-diphenyl-1-picryl-hydrazyl (DPPH) to achieve different final concentrations of the extract in the test solution. After 60 min at room temperature, the absorbance was measured at 515 nm. The activity was expressed as the inhibitory concentration (IC50) at an RSC value of 50% (the concentration of the test solution required to obtain 50% of radical scavenging capacity).
2.8. Storage Stability
The samples of A. ursinum dry extract powder were stored in vials at room temperature (25 ± 2 °C), protected from light and humidity for 90 days. The total phenolic content, total flavonoid content, and antioxidant capacity of the encapsulated extract were determined after encapsulation and evaluated every 15 days. Water activity, color, mean diameter, and particle size were determined at the beginning (time 0) and the end (after 90 days) of storage [25].
2.9. Statistical Analysis
One-way analysis of variance (ANOVA) was performed using SPSS statistical software (version 23, IBM, Crop., Armonk, NY, USA) at p < 0.05. The means were compared with Duncan’s test at p < 0.05. The results were expressed as the mean ± standard deviation. Each experiment was conducted in triplicate.
3. Results and Discussion
3.1. Physical Characteristics of Obtained Extract Powders
Spray drying is commonly used to obtain powdered herbal extracts, efficiently drying various bioactive components. However, improper optimization can lead to issues like extract stickiness, low-quality extracts, and degradation of bioactive compounds. Analyzing the powder’s physical properties, such as particle size, moisture content, and morphology, is vital for ensuring quality control and determining solubility, shelf stability, bioactivity retention, bioavailability, and bioaccessibility. To consider the encapsulation process successful, the dry extract must be obtained without any stickiness or adhesion to the walls of the spray dryer chamber. Additionally, it should exhibit a high encapsulation yield while minimizing any reduction in biological activity.
The efficiency of the spray drying process varied between 26.45% and 73.36% in our study (Table 1). To effectively obtain dry extracts of A. ursinum, it was crucial to add maltodextrin (MD) at a concentration higher than 40%. This aligns with previous research that has shown MD to enhance powder yield during spray drying of various herbal extracts. For instance, studies on mountain tea, sage, willow gentian, and Aronia have reported improved yield with MD addition [26,27,28,29]. The impact of MD addition highly varies depending on the herbal material used. For instance, a concentration of MD as a carrier over 40 was also significant for Aronia melanocarpa, resulting in sample recovery in range of 50% up to 75%, [28], while in the case of Marrubium vulgare, the addition of only 10% MD increased the efficiency from 58.36% (without MD) to 77.07% [18]. Notably, in our research, an 80% MD concentration resulted in 2–3 times higher efficiency compared to no MD or low 10% MD concentration. A higher drying inlet temperature (140 °C) with 80% MD led to a slight, non-significant decrease in efficiency compared to a lower temperature (120 °C), while, for example, Aronia melanocarpa extract temperature of 140 °C showed to be the one with the highest yield.

Table 1.
Physical characteristics of obtained extract powders.
In the assessment of extract adherence to equipment surfaces, our investigation revealed that the inclusion of 40% maltodextrin (MD) resulted in notably enhanced efficiency at 57.81%, accompanied by a substantial reduction in stickiness. Furthermore, employing 80% MD yielded the most favorable outcomes, achieving an efficiency of 73.36%, coupled with a significant decrease in stickiness. Extract sickness is commonly attributed to the high content of sugars (glucose, fructose, and sucrose), organic acids (citric, malic, and tartaric acid), or other bioactive compounds (such as polyphenolics) present in the liquid extract being dried. These components contribute to the stickiness due to their low glass transition temperature (Tg) (which is, for example, for glucose and fructose Tg = 14.31 °C). Consequently, when their concentration in the liquid feed is high, they can induce a decrease in the Tg of the entire batch [30]. To reduce stickiness, the glass transition temperature must be increased, which is mostly achieved by the addition of carrier additives. The addition of MD proved to be highly beneficial, significantly enhancing both the yield and properties of the obtained dry extract [31].
Differential scanning calorimetry (DSC) was used to determine the glass transition temperature, monitor physical and chemical transformations, and study interactions between components and the carrier. Pure maltodextrin had a Tg of 66.6 °C (Table 1), and higher amounts of maltodextrin increased Tg, improving drying efficiency and reducing stickiness. The thermograms presented in Figure 1 display a series of thermal events represented as peaks during the sample heating process. DSC curves showed differences in peak positions and intensity due to water content, moisture levels, and material structure variations caused by interactions with the carrier [32]. Upon comparing the DSC curves of the tested samples, it is evident that an increase in the proportion of MD correlates with an increase in the Tg value. The results indicate that the most notable change occurred with the addition of 10% MD as a carrier, but concentrations above 10% did not significantly raise the glass transition temperature.
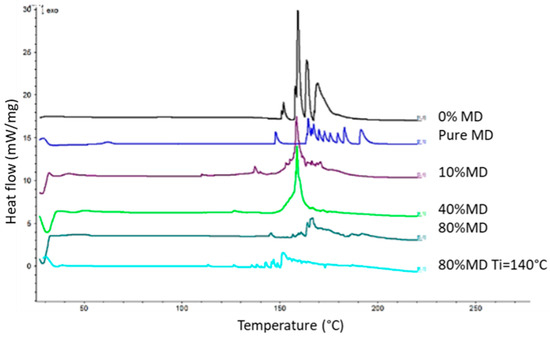
Figure 1.
DSC thermograms of dry extract powders.
In the experimental study, we analyzed the impact of using maltodextrin (MD) as a drying agent or carrier on the efficiency and physicochemical properties of the dry extracts. As shown in Table 1, the L*, a*, and b* values were measured to quantify the color parameters of the dry extract powders. The L* parameter represents the lightness of the color, while the a* and b* parameters denote the green-red and blue-yellow color components, respectively. The yellow-brown color of the dry extract powders varied based on the amount of maltodextrin added, primarily affecting the a* and b* parameters rather than L*. Increasing the MD content generally resulted in a decrease in the a* and b* values, indicating a shift towards less red and less yellow colors. Furthermore, increasing the drying temperature at a higher MD percentage exacerbated this shift. These results provide valuable insights into the relationship between processing conditions and the color characteristics of dry extracts, which can be utilized to optimize production processes and ensure the desired color attributes for consumer acceptance and industrial applications. As the MD percentage increased from 0 to 80%, a noticeable trend in the color parameters was observed. In terms of L*, there was an increase from 64.95 to 79.76, indicating a shift towards lighter shades.
The moisture content of the produced dry extract samples is presented in Table 1, ranging from 5.02% to 15.38%. The addition of MD effectively reduced the moisture content in the dry extracts, even at an 80% concentration with varying inlet temperatures. The higher concentration of MD as a carrier led to a significant reduction in moisture content, resulting in the formation of a well-performing carrier. This finding is consistent with other studies that have also highlighted the effectiveness of using MD as a carrier in reducing moisture content. Previous research indicated that powders prepared with MD as a carrier exhibited the lowest moisture content and hygroscopicity compared to other carriers used [21]. Higher inlet temperatures improved heat transfer, resulting in the lowest moisture content (5.02%). The differences in moisture content were statistically significant, highlighting the importance of drying temperature for the product’s stability. Considering the high moisture content observed in samples with low MD addition, their suitability for further use in the food or pharmaceutical industry may be limited, emphasizing the importance of using 80% MD for further applications.
The hygroscopicity of the plant extracts can be attributed to the presence of components with a hydrophilic character, such as carbohydrates, glycosides, organic acids, phenols, amino acids, and proteins, which may affect particle–particle interactions and contributes to their poor flow and compatibility properties [33]. Based on research conducted by Vladić et al. [19], it was expected that the addition of MD would reduce the hygroscopicity of dry extracts. The lowest hygroscopicity was noticed when the highest concentration of carrier was applied [26]. However, upon analyzing the results presented in Table 1, it was observed that there were no statistically significant differences in hygroscopicity for samples produced with 10%, 40%, or 80% MD after the first and seventh day of production. This suggests that the addition of MD did not have a significant impact on the hygroscopicity of the dry extracts in this particular study. In addition to carrier concentration, the drying temperature also has a great influence on the hygroscopicity of the dry extract, and it is expected that the dry extracts obtained at higher temperatures have higher hygroscopicity; this is because higher temperatures result in more porous particles, enabling greater absorption of water [34]. Several studies have reported that the physical and chemical properties of encapsulated powder can be affected by both excessively high or low drying temperatures; therefore, finding the optimal drying temperature is crucial to minimize hygroscopicity and maintain the desired properties of the dry extracts [35]. The observed differences in hygroscopicity were more closely related to the drying temperature rather than the amount of carrier used. After two days, the hygroscopicity of the dry extracts increased by approximately 1.2–1.4 times compared to the initial measurement, and after seven days, it increased by approximately 1.5–2.3 times. This indicates the ability of dry extracts to absorb moisture from the surrounding environment. The higher moisture content can be attributed to the specific chemical structure of MD DE 19.7, which has greater water binding capabilities compared to MDs with lower dextrose equivalent (DE) values. To mitigate the impact of hygroscopicity, it is recommended to store dry extracts under controlled conditions with reduced moisture presence. This will help maintain the quality and stability of the dry extracts over time.
Given that powder forms are predominantly present in the production of various pharmaceutical and food products, bulk density is an important feature from both functional and economic aspects, as it is important for further product processing, storage, packaging, and distribution. The bulk mass of the obtained dry extracts of A. ursinum ranged from 230–620 mg/mL. Similar results were obtained in other research where spray drying was applied in order to obtain dried extract [35]. However, besides the amount of added carrier, the bulk volume is also influenced by the type of carrier, the inlet temperatures inside the spray dryer chamber, the speed of spraying, and the flow of the feed. For example, whey protein (WP) at higher concentrations exhibited higher bulk density compared to formulations containing MD, while the 100% MD powder displayed the lowest bulk density among other WP and MD powders [21].
The water solubility index is also a crucial parameter for dried extract powders, as it indicates their ability to rapidly dissolve for further utilization in food or pharmaceutical products. The results obtained from analyzing the dry extracts (Table 1) demonstrated high values of the solubility index in water, ranging from 77.13% to 85.05%. The addition of MD in spray drying for extract powder production has been found to increase the water solubility index (WSI). This consistent finding across multiple research studies suggests that a higher mass fraction of MD leads to improved solubility properties in the extract powders. This highlights the potential of MD as an effective carrier in spray drying processes for obtaining extract powders with enhanced solubility characteristics [28]. A higher solubility index value signifies better dissolution, indicating that the obtained dry extracts readily dissolve in water. The addition of a larger quantity of MD did not have a statistically significant impact on the WSI. However, it did affect the dissolution rate. Dry extracts with a higher content of MD exhibited faster dissolution in water. In contrast to the WSI, the WAI assesses the ability of powdered products, such as dry extracts, to absorb water. In this case, lower values are desirable as they indicate less water absorption. Upon analyzing the results presented in Table 1, it was observed that an increase in the amount of MD leads to a reduction in WAI in dry extracts. Consequently, it can be inferred that the addition of MD has a favorable impact on these specific properties of the obtained extracts.
The spray drying process of the subcritical aqueous extract of A. ursinum resulted in the formation of micro-sized particles. The particle size distributions of the encapsulate can be observed in Figure 2. It is evident that the encapsulate particles exhibited a monomodal distribution, with a D[4,3] (volume mean diameter) of 4.799 µm. The low Span value of 1.906 indicates a narrow size distribution and good uniformity of the encapsulated extract particles. The median volume diameter, d(0.5), reveals that 50% of the encapsulated volume comprises particles larger than 4.012 μm. Additionally, 10% of the encapsulated volume consists of particles larger than 9.147 μm (d(0.9)), while 10% of the encapsulated volume contains particles smaller than 1.502 μm (d(0.1)).
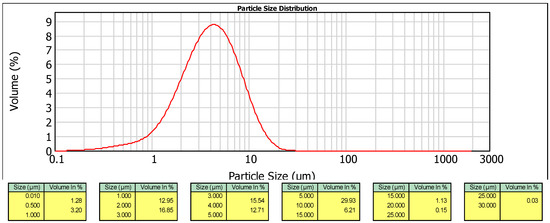
Figure 2.
Particle size distribution analysis of A. ursinum dried extract obtained using 80% of MD at 120 °C.
The morphology of the spray-dried powders was investigated using SEM (Scanning Electron Microscopy). Figure 3 presents the size and morphology of the encapsulated powder obtained at a temperature of 140 °C with an 80% MD content. The SEM micrographs reveal the typical characteristics of spray-dried powders, with the presence of various sizes of micropowder particles, all measuring less than 10 µm. The obtained powders exhibited an almost spherical shape with minor surface indentations, as depicted in Figure 3. These surface irregularities are commonly observed in samples prepared through the spray-drying technique. They can be attributed to shrinkage during the drying process caused by solvent evaporation and cooling [36]. Previous research has demonstrated that the spray-drying process can effectively transform large-sized raw maltodextrin (MD) particles, which have an irregular needle shape, into small individual particles that are nearly spherical and have a smooth surface, which indicates that spray drying is a suitable method for modifying the morphology and characteristics of MD particles [19]. Nevertheless, the absence of wall cracks and cavities on the surface of the microcapsule confirmed that the encapsulation had been performed effectively.

Figure 3.
Micrographs of the particles using the SEM of A. ursinum dried extract obtained using 80% of MD at 120 °C.
3.2. Polyphenol Content and Antioxidant Capacity of the Produced Encapsulates
According to a study by Stupar et al. [7], the antimicrobial activity of subcritical water wild garlic extract was found to be primarily dependent on phenolic compounds rather than sulfuric compounds. Therefore, this research focused on evaluating the TPC, TFC, and antioxidant activity, considering the impact of carrier material concentration and drying temperature on bioactive compound content (Table 2).

Table 2.
Total phenolic content, total flavonoid content, and antioxidant activity of obtained powders.
The total phenolic content of the dry extracts ranged from 23.00 to 28.21 mg GAE/g. Increasing MD concentration resulted in decreased bioactive component content compared to extracts without MD. While MD addition did not cause drastic changes in TPC, the results were significantly different across the range. Similar observations were made for TFC, with the highest content of flavonoids (6.33 mg CE/g) found in the sample with 10% MD addition. However, higher MD concentrations led to a decrease in TFC. Notably, the addition of 40% and 80% MD did not show statistically significant differences in terms of TFC and antioxidant activity.
Larger amounts of carrier (MD) may lead to a reduction in phenols and flavonoids due to a “diluting” effect in the feeding mixture. Increasing the inlet drying temperature can cause phenolic component degradation and particle surface cracking, potentially decreasing the bioactive compound content [37]. However, the increase in drying temperature from 120 to 140 °C did not affect TPC, TFC, or antioxidant activity. Despite the diluting effect, MD as a carrier plays a protective role, reducing the degradation of phenolic compounds and preserving antioxidant potential.
3.3. Storages Stability
The chemical and physical stability of extracts is crucial for determining the shelf life not only of the extract itself but also of products containing natural extracts. Therefore, it is important to determine whether the quality of the powder is affected during storage by environmental factors, such as temperature, oxygen, and relative humidity, to determine the best conditions for the storage of the powder. Furthermore, during storage, stickiness can occur; therefore, it is of high importance to produce the powder with the lowest water content and ensure proper packaging and storage conditions in terms of adequate moisture and temperature [18].
Encapsulated powders with favorable physicochemical characteristics were studied for stability during three months of storage at room temperature. Visual observation showed the powders remained yellowish and loose, without sticking or lumps throughout the storage period. Physical analysis confirmed these observations, with no statistical changes detected compared to freshly prepared powders. The stable physical characteristics are presented in Table 3.

Table 3.
Physicochemical characteristics of powders after 3 months of storage.
During the storage period, the chemical composition, specifically TPC and TFC, as well as antioxidant activity, were monitored at two-week intervals. Gradual decreases in TPC and TFC were observed as storage time increased, with total reductions of 10.64% in TPC and 8.52% in TFC after three months. Exposure to light and air at the surface led to an 11.82% reduction in antioxidant activity in the powders. However, no significant difference was observed between powders produced at different spray-drying temperatures (Table 3). These findings highlight the importance of encapsulation in protecting bioactive compounds from undesired chemical degradation reactions, essential for successful incorporation into real food systems.
4. Conclusions
This research highlights the immense value of Allium ursinum as a rich and potent source of bioactive compounds. The innovative approach of combining subcritical water extraction and spray drying encapsulation offers not only an environmentally friendly solution but also one that is highly applicable to industrial settings, enabling the preservation of these valuable bioactive compounds found in A. ursinum. By demonstrating the feasibility of subcritical water extraction and spray drying encapsulation, this research represents a significant step toward the practical utilization of herbal extracts in everyday products.
The conducted storage stability tests yielded highly encouraging results, suggesting that the overall quality and nutritional benefits of A. ursinum extract are well-preserved, offering crucial insights for the establishment of efficient processes and the production of dry Allium ursinum extract as a natural additive or pharmaceutical ingredient.
These findings provide valuable insights to researchers and the industry for setting up processes and establishing efficient production processes and the widespread utilization of dry Allium ursinum extract. The incorporation of this extract into various products holds the promise of significant improvements in color, nutritional value, and bioactive properties. This not only enhances the sensory attributes and nutritional profiles of the products but also drives innovation, addresses evolving consumer needs, and sets the stage for integrating Allium ursinum extract, contributing to the continued development of natural and sustainable solutions.
Author Contributions
Conceptualization, A.S. and S.V.; Data curation, A.S., J.V. and T.R.; Formal analysis, A.S., J.V. and T.R.; Methodology, A.S.; Supervision, S.V. and A.M.; Validation, S.V. and J.V.; Writing—original draft, A.S. and T.R.; Writing—review and editing, S.V. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia under the Agreements on the Implementation and Financing of Research (nos. 451-03-47/2023-01/200222).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Elsorady, M.E.; Elgindy, A.A. Effect of Ginger Extract (Zingiber Officinale) as a Natural Antioxidant on Sunflower Oil Oxidation. Food Feed Res. 2022, 49, 173–182. [Google Scholar] [CrossRef]
- Vrgović, P.; Pojić, M.; Teslić, N.; Mandić, A.; Kljakić, A.C.; Pavlić, B.; Stupar, A.; Pestorić, M.; Škrobot, D.; Mišan, A. Communicating Function and Co-Creating Healthy Food: Designing a Functional Food Product Together with Consumers. Foods 2022, 11, 961. [Google Scholar] [CrossRef]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and Nano Bio-Based Delivery Systems for Food Applications: In Vitro Behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef]
- Vidović, S.; Tomšik, A.; Vladić, J.; Jokić, S.; Aladić, K.; Pastor, K.; Jerković, I. Supercritical Carbon Dioxide Extraction of Allium Ursinum: Impact of Temperature and Pressure on the Extracts Chemical Profile. Chem. Biodivers. 2021, 18, e2100058. [Google Scholar] [CrossRef] [PubMed]
- Krivokapic, M.; Jakovljević, V.; Sovilić, M.; Bradić, J.; Petković, A.; Radojević, I.; Branković, S.; Čomić, L.; Anđić, M.; Kcović, A.; et al. Biological Activities of Different Extracts from Allium Ursinum Leaves. Acta Pol. Pharm.-Drug Res. 2020, 77, 121–129. [Google Scholar] [CrossRef]
- Stupar, A.; Šarić, L.; Vidović, S.; Bajić, A.; Kolarov, V.; Šarić, B. Antibacterial Potential of Allium Ursinum Extract Prepared by the Green Extraction Method. Microorganisms 2022, 10, 1358. [Google Scholar] [CrossRef]
- Mašková, L.; Janská, P.; Klimša, V.; Knejzlík, Z.; Tokárová, V.; Kašpar, O. Development of Compartmentalized Antibacterial Systems Based on Encapsulated Alliinase. Adv. Powder Technol. 2021, 32, 2720–2732. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2011, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, X.; Shen, J.; Li, Z.; Tan, S.; Liu, W.; Cheng, Z. Choosing the Appropriate Wall Materials for Spray-Drying Microencapsulation of Natural Bioactive Ingredients: Taking Phenolic Compounds as Examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunovi´c, J.Š.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Šaponjac, V.T.; Vulić, J.; Stajčić, S. Encapsulation and Degradation Kinetics of Bioactive Compounds from Sweet Potato Peel During Storage Running Title: Encapsulation of Sweet Potato Peel Bioactive Compounds. Food Technol. Biotechnol. 2020, 58, 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Selomulya, C. Spray Drying Strategy for Encapsulation of Bioactive Peptide Powders for Food Applications. Adv. Powder Technol. 2020, 31, 409–415. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the Spray-Drying Temperatures on the Physiochemical Properties of an Encapsulated Bitter Melon Aqueous Extract Powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Mousavi Kalajahi, S.E.; Ghandiha, S. Optimization of Spray Drying Parameters for Encapsulation of Nettle (Urtica dioica L.) Extract. LWT 2022, 158, 113149. [Google Scholar] [CrossRef]
- Filipčev, B.; Kojić, J.; Miljanić, J.; Šimurina, O.; Stupar, A.; Škrobot, D.; Travičić, V.; Pojić, M.; Kojić, J.; Miljanić, J.; et al. Wild Garlic (Allium ursinum) Preparations in the Design of Novel Functional Pasta. Foods 2023, 12, 4376. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical Water Extraction of Wild Garlic (Allium ursinum L.) and Process Optimization by Response Surface Methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Gavarić, A.; Vladić, J.; Ambrus, R.; Jokić, S.; Szabó-Révész, P.; Tomić, M.; Blažić, M.; Vidović, S. Spray Drying of a Subcritical Extract Using Marrubium Vulgare as a Method of Choice for Obtaining High Quality Powder. Pharmaceutics 2019, 11, 523. [Google Scholar] [CrossRef]
- Vladić, J.; Ambrus, R.; Szabó-Révész, P.; Vasić, A.; Cvejin, A.; Pavlić, B.; Vidović, S. Recycling of Filter Tea Industry By-Products: Production of A. Millefolium Powder Using Spray Drying Technique. Ind. Crops Prod. 2016, 80, 197–206. [Google Scholar] [CrossRef]
- Hogekamp, S.; Schubert, H. Rehydration of Food Powders. Food Sci. Technol. Int. 2003, 9, 223–235. [Google Scholar] [CrossRef]
- Šavikin, K.; Nastić, N.; Janković, T.; Bigović, D.; Miličević, B.; Vidović, S.; Menković, N.; Vladić, J. Effect of Type and Concentration of Carrier Material on the Encapsulation of Pomegranate Peel Using Spray Drying Method. Foods 2021, 10, 1968. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Tumbas Šaponjac, V.; Petrović, J.; Vulić, J.; Fišteš, A.; Jovanović, P. Physical, Sensorial and Bioactive Characteristics of White Chocolate with Encapsulated Green Tea Extract. J. Sci. Food Agric. 2019, 99, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Šarić, L.; Bertoni, S.; Protti, M.; Albertini, B.; Mercolini, L.; Passerini, N. Encapsulations of Wild Garlic (Allium ursinum L.) Extract Using Spray Congealing Technology. Food Res. Int. 2019, 119, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Koca, I. Optimization and Stabilization of the Antioxidant Properties from Alkanet (Alkanna tinctoria) with Natural Deep Eutectic Solvents. Arab. J. Chem. 2020, 13, 6437–6450. [Google Scholar] [CrossRef]
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjeković, A.; Poklar Ulrih, N.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- Jovanović, M.; Ćujić-Nikolić, N.; Drinić, Z.; Janković, T.; Marković, S.; Petrović, P.; Šavikin, K. Spray Drying of Gentiana asclepiadea L. Root Extract: Successful Encapsulation into Powders with Preserved Stability of Bioactive Compounds. Ind. Crops Prod. 2021, 172, 114044. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Vidaković, A.; Vidović, S.; Velićanski, A.; Versari, A.; Radosavljević, R.; Zeković, Z. Sage Processing from By-Product to High Quality Powder: I. Bioactive Potential. Ind. Crops Prod. 2017, 107, 81–89. [Google Scholar] [CrossRef]
- Vidović, S.; Ramić, M.; Ambrus, R.; Vladić, J.; Szabó-Révész, P.; Gavarić, A. Aronia Berry Processing by Spray Drying: From Byproduct to High Quality Functional Powder. Food Technol. Biotechnol. 2019, 57, 513. [Google Scholar] [CrossRef]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a Carrier of Health Benefit Compounds in Satureja Montana Dry Powder Extract Obtained by Spray Drying Technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Celli, G.B.; Dibazar, R.; Ghanem, A.; Brooks, M.S.-L. Degradation Kinetics of Anthocyanins in Freeze-Dried Microencapsulates from Lowbush Blueberries (Vaccinium angustifolium Aiton) and Prediction of Shelf-Life. Dry. Technol. 2016, 34, 1175–1184. [Google Scholar] [CrossRef]
- Caliskan, G.; Nur Dirim, S. The Effects of the Different Drying Conditions and the Amounts of Maltodextrin Addition during Spray Drying of Sumac Extract. Food Bioprod. Process. 2013, 91, 539–548. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; Macêdo, R.O.; Medeiros, A.C.D. Thermal Characterization of Dried Extract of Medicinal Plant by DSC and Analytical Techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Gallo, L.; Ramírez-Rigo, M.V.; Piña, J.; Bucalá, V. A Comparative Study of Spray-Dried Medicinal Plant Aqueous Extracts. Drying Performance and Product Quality. Chem. Eng. Res. Des. 2015, 104, 681–694. [Google Scholar] [CrossRef]
- de Souza, V.B.; Thomazini, M.; de Carvalho Balieiro, J.C.; Fávaro-Trindade, C.S. Effect of Spray Drying on the Physicochemical Properties and Color Stability of the Powdered Pigment Obtained from Vinification Byproducts of the Bordo Grape (Vitis labrusca). Food Bioprod. Process. 2015, 93, 39–50. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Sampaio, F.C.; de Faria, J.T.; Pitangui, C.G.; Lovaglio, F.; Casazza, A.A.; Converti, A.; Perego, P. Optimization of Spray Drying Microencapsulation of Olive Pomace Polyphenols Using Response Surface Methodology and Artificial Neural Network. LWT 2018, 93, 220–228. [Google Scholar] [CrossRef]
- Kurek, M.A.; Pratap-Singh, A. Plant-Based (Hemp, Pea and Rice) Protein–Maltodextrin Combinations as Wall Material for Spray-Drying Microencapsulation of Hempseed (Cannabis sativa) Oil. Foods 2020, 9, 1707. [Google Scholar] [CrossRef] [PubMed]
- Kanakidi, L.-D.; Tsimogiannis, D.; Kiokias, S.; Oreopoulou, V. Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification. Nutraceuticals 2022, 2, 1–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).