Exploring Humic Acid as an Efficient and Selective Adsorbent for Lead Removal in Multi-Metal Coexistence Systems: A Review
Abstract
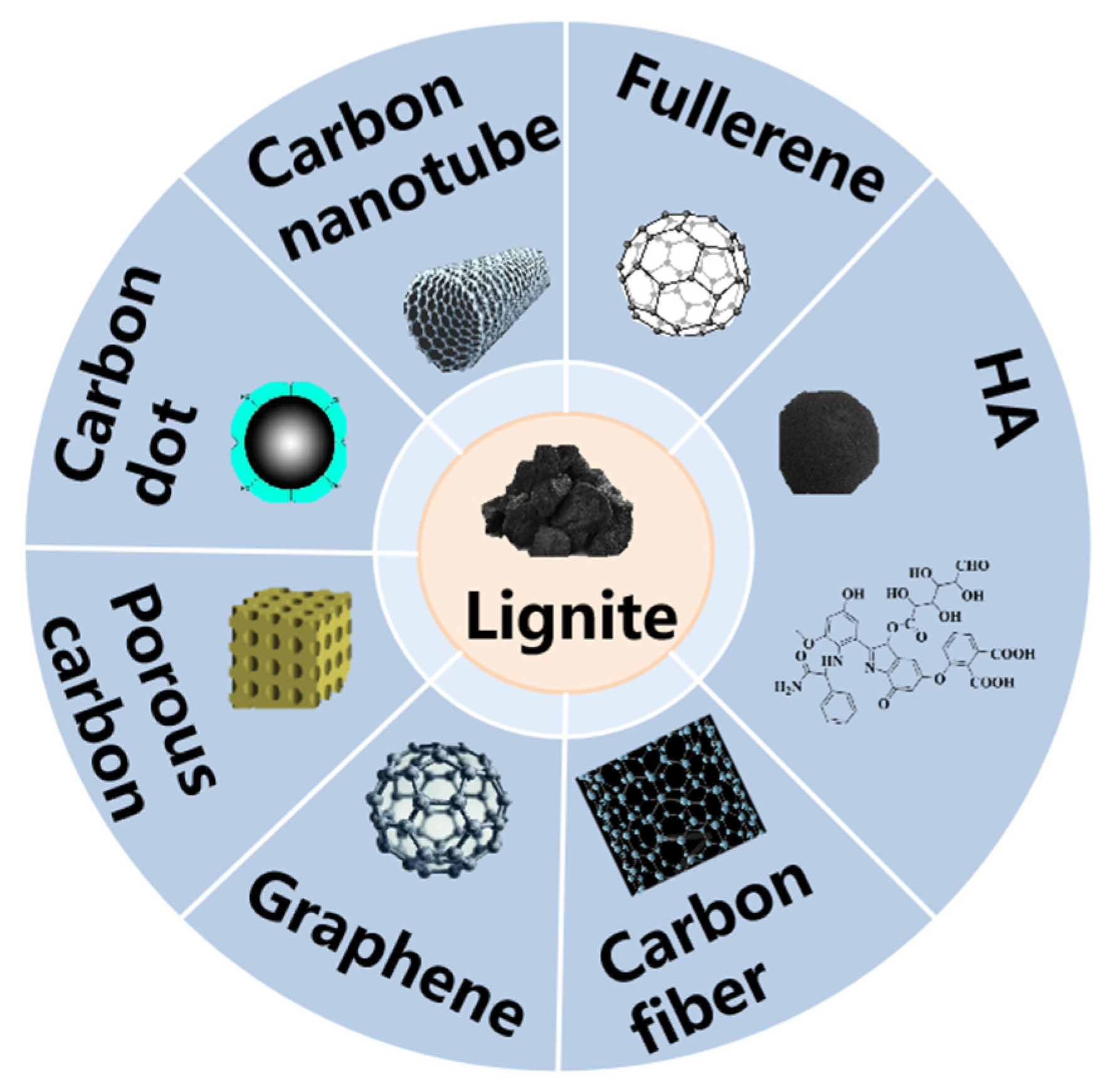
1. Introduction
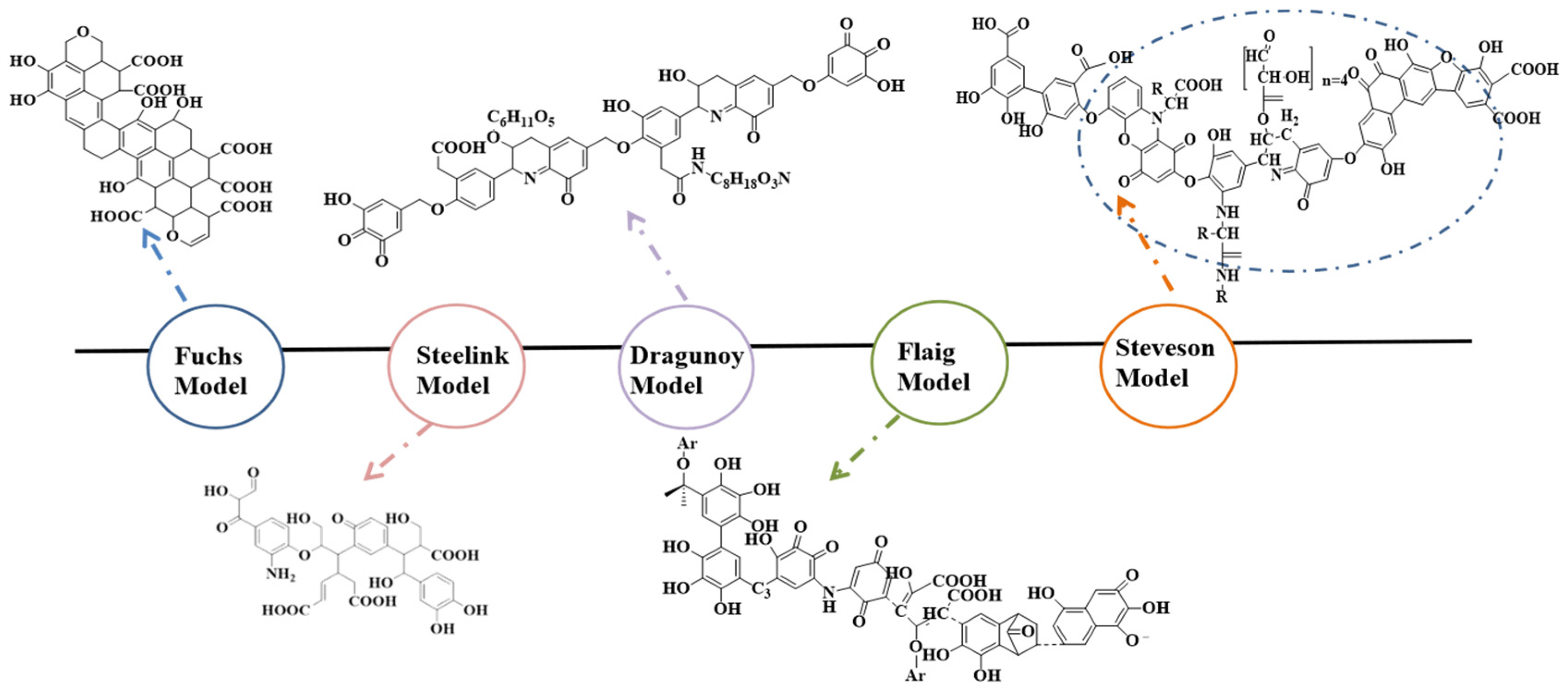
2. Research Status of Molecular Structure of HA
2.1. Characterization Methods of HA Structure
2.1.1. Characterization of HA Elemental Composition
2.1.2. Characterization of HA Functional Groups
2.2. HA Fractionation
2.2.1. Methods of HA Fractionation
2.2.2. The Potential Impact of HA Fractionation on the Environment
2.3. The Interaction between Typical Structures of HA and Heavy Metal Ions
- (1)
- Electrostatic interaction: HA exhibits diverse pKa values, functioning as a weak acid and a strong acidic ion-exchange as a polyelectrolyte. This characteristic allows for the formation of diffuse double layers around charged particles, facilitating heavy metal adsorption [55,56]. HISS standards suggest that the content of –COOH in HA significantly exceeds that of hydroxyl groups, and their dissociation occurs at pH > 4.4, resulting in a predominantly negatively charged surface on HA [57]. This facilitates electrostatic reactions with heavy metals. At higher pH values (>9.5), –OH become more prone to metal complexation as they are easily protonated. The increase in pH effectively deprotonates carboxylic groups, enhancing their binding with positively charged metals [58]. Studies have shown that hydroxyl and carboxyl groups in HA chelate with heavy metals, enhancing the adsorption rate [59]. Zhao et al. [44] proposed that phenolic and carboxylic groups are the primary adsorption sites for Pb2+, while, according to Kinnibrugh’s research [60], different functional groups exhibit varying binding affinities with metal ions, with carboxyl groups showing significant binding capacities: Pb2+ > Cu2+ > Cd2+. Beyond active sites, structural features also influence the complexation of heavy metals by HA [61].
- (2)
- Carboxyl Coordination and Phenolic Hydroxyl Coordination: The carboxyl functional groups (–COOH) in HA are important structures involved in the complexation with heavy metal ions. Heavy metal ions typically form coordination bonds with hydroxy oxygen in carboxyl groups or oxygen in carboxyl groups, resulting in the formation of stable complexes. This coordination interaction forms the basis for the selective adsorption of heavy metal ions by HA. The phenolic hydroxyl functional groups (–OH) in HA are also key structures involved in the complexation with heavy metal ions. The oxygen atoms in these functional groups can form coordination bonds, facilitating the coordination interaction with heavy metal ions and promoting the adsorption and fixation of heavy metals.
- (3)
- Ion Exchange: Functional groups in HA possess certain ion exchange capabilities, allowing adsorption through ion exchange with heavy metal ions. This interaction is often influenced by factors such as solution pH and ion concentration.
- (4)
- π electrons: The π electrons of aromatic functional groups play a crucial role in interacting with cations, serving as π electron donors for the adsorption of heavy metals [62]. The cation–π electron interaction is dependent on the aromaticity degree of the HA surface. Higher C/H ratios indicate higher aromaticity, resulting in greater electron donation capacity, reduction capacity, and adsorption capability. Li et al. [63] observed heavy metal adsorption involving cation–π interactions, especially with corn stover biochar.
- (5)
- Precipitation: The interaction between carbonate (CO32−) and sulfate groups (SO42−) in HA and heavy metals is one of the significant behaviors of HA in the environment. These interactions generally occur through mechanisms such as coordination, ion exchange, and adsorption. (1) Coordination: The carboxyl and phenolic functional groups in HAs can form coordination bonds with heavy metals, resulting in the formation of complexes. The carboxyl groups on carbonate and sulfate can provide coordination sites, forming complexes with heavy metal ions, stabilizing their forms of presence, and reducing their toxicity. (2) Ion exchange: The carbonate and sulfate groups in Hs carry negative charges and can participate in ion exchange reactions. In the presence of heavy metal ions in the environment, they can undergo ion exchange with the carbonate and sulfate groups in HAs, being adsorbed onto the surface of HAs, thus reducing the activity and toxicity of heavy metal ions in the environment. (3) Precipitation: Under the influence of high concentrations of carbonate or sulfate, as well as factors such as the concentration of heavy metal ions and pH values, precipitation reactions of heavy metals with carbonate or sulfate may occur. Some heavy metal ions (such as Ca2+, Pb2+) can form carbonate precipitates with carbonate, especially under alkaline conditions. Similarly, some heavy metal ions (such as Cd2+, Pb2+, Hg2+) can also form sulfate precipitates with sulfate, particularly under acidic conditions.
3. Selective Adsorption of Lead Ion by Coexisting System of Multiple Metals
3.1. Types and Physicochemical Characteristics of Heavy Metals in Aqueous Solution
3.2. Application of Selective Adsorbents in Lead-Containing Wastewater
3.2.1. Electrostatic Interaction
3.2.2. Specific Chelation Interaction
3.2.3. Ion Exchange
3.2.4. Pore Structure and Matching with Heavy Metal Size
3.2.5. The pH Values
3.2.6. Chemical Precipitation
3.3. The Limitations, Challenges, and Future Prospects of Selective Adsorption Mechanisms for Pb2+
3.3.1. Limitations and Challenges of Electrostatic Interactions
3.3.2. Limitations and Challenges of Chelation
3.3.3. Limitations and Challenges of Ion Exchange
3.3.4. Limitations and Challenges of Pore Structure and Heavy Metal Size Matching
3.3.5. Limitations and Challenges of pH Values
3.3.6. Limitations and Challenges of Chemical Precipitation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chooto, P.; Wararatananurak, P.; Innuphat, C. Determination of trace levels of Pb(II) in tap water by anodic stripping voltammetry with boron-doped diamond electrode. Sci. Asia 2010, 36, 150–156. [Google Scholar] [CrossRef]
- Mansoor, L.; Erkan, Y.; Basak, S. Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta Int. J. Pure Appl. Anal. Chem. 2016, 146, 130–137. [Google Scholar]
- Özdemir, C.; Saçmacı, Ş.; Kartal, Ş. A coprecipitation procedure for the determination of some metals in food and environmental samples by flame atomic absorption spectroscopy. Anal. Methods 2013, 5, 3977–3983. [Google Scholar] [CrossRef]
- Igawa, M.; Kanamori, H.; Nanzai, B. Gel-phase Extraction for the Removal of Heavy-metal Ions. Chem. Lett. 2010, 39, 996–997. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; Feng, Q.; Wei, N.; Zhang, Y. Reed hemicellulose-based hydrogel prepaped by glow discharge electrolysis plasma and its adsorption propertion for heavy metal ions. Fresenius Environ. Bull. 2016, 25, 1791–1798. [Google Scholar]
- Jin, K.; Lin, X.; Li, A. Porosint-Supported PVDF Derivatives with Good Adsorption Performance and Recyclability for the Removal of Aqueous Heavy Metal Ions. Chem. Sel. 2023, 8, 2–9. [Google Scholar] [CrossRef]
- Mazumder, A.; Bhattacharya, S.; Bhattacharjee, C. Role of nano-photocatalysis in heavy metal detoxification. In Nanophotocatalysis and Environmental Applications: Detoxification and Disinfection; Springer: Cham, Switzerland, 2019; pp. 1–33. [Google Scholar]
- Chen, J.W. Research Progress of Membrane Technology Application in Heavy Metal Wastewater Treatment. Guangdong Chem. Ind. 2009, 36, 132–136. [Google Scholar]
- Yu, H.; Qiu, C.; Wang, C.; Jie, J.; Sun, L.; Luo, S.; Liu, F.; Chen, J. Influence of Fenton pretreatment on heavy metal speciation and bioleaching efficiency in municipal sludge. Chin. J. Environ. Eng. 2019, 13, 725–731. [Google Scholar]
- Mo, Z.; Zhang, H.; Shahab, A.; Khan, F.A.; Chen, J.; Huang, C. Functionalized metal-organic framework UIO-66 nanocomposites with ultra-high stability for efficient adsorption of heavy metals: Kinetics, thermodynamics, and isothermal adsorption. J. Taiwan Inst. Chem. Eng. 2023, 146, 104778. [Google Scholar] [CrossRef]
- Petranovska, A.L.; Abramov, N.V.; Turanska, S.P.; Gorbyk, P.P.; Kaminskiy, A.N.; Kusyak, N.V. Adsorption of cis -dichlorodiammineplatinum by nanostructures based on single-domain magnetite. J. Nanostruct. Chem. 2015, 5, 275–285. [Google Scholar] [CrossRef]
- Sudhakar, A. An Efficient, Uncatalyzed, and Rapid Synthesis of Thiazoles and Aminothiazoles Under Microwave Irradiation and Investigation of Their Biological Activity. Phosphorus 2010, 185, 103–109. [Google Scholar]
- Zhan, K.; Wen, X.; Wang, X.; Kong, C. A method for characterization of stress concentration degree of coal mine roadway surrounding rock. J. Geophys. Eng. 2023, 4, 699–711. [Google Scholar] [CrossRef]
- Lai, Y.; Hao, L.; Dong, L.; Yu, S.; Liu, J. Coating zirconium oxide-nanocomposite with humic acid for recovery of mercury and chromium in hazardous waste of chemical oxygen demand test. J. Environ. Sci. 2023, 126, 40–47. [Google Scholar] [CrossRef]
- Zhou, L.P.; Yuan, L.; Zhang, S.Q. Advances in humic acid structures and their regulatory role in maize roots. J. Plant Nutr. Fertil. 2022, 28, 334–343. [Google Scholar]
- Plechanov, N. Studies of molecular weight distributions of fulvic and humic acids by gel permeation chromatography. Examination of the solute molecular composition using RI, UV, fluorescence and weight measurement as detection techniques. Org. Geochem. 1983, 5, 143–149. [Google Scholar] [CrossRef]
- Parsons, J.W. Humus Chemistry—Genesis, Composition, Reactions. Soil Sci. 1983, 135, 129–130. [Google Scholar] [CrossRef]
- Zhao, X.; Xi, B.; Tan, W.; Li, X.; Dang, Q.; Li, R. On the applicability of the ‘humic acids’ nomenclature from natural ecosystems to engineering sciences. Environ. Sci. Ecotechnol. 2021, 6, 100082. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Xiong, L.; Xu, N.; Ni, J. Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes. Chem. Eng. J. 2013, 215–216, 366–374. [Google Scholar] [CrossRef]
- Kort, J.; Kyasaram, R.; Cao, S.; Fu, P.; Driscoll, J.; Malek, E. Prognostic Value of Dynamic Monoclonal Protein Pattern on Probability of Myeloma Progression from the Precursor State. Blood 2021, 138, 3781. [Google Scholar] [CrossRef]
- Tan, K.H.; Giddens, J.E. Molecular weights and spectral characteristics of humic and fulvic acids. Geoderma 1972, 8, 221–229. [Google Scholar] [CrossRef]
- Zheng, E.; Zhu, Y.; Hu, J.; Zhang, Z.; Xu, T. Effects of humic acid on japonica rice production under different irrigation practices and a TOPSIS-based assessment on the Songnen Plain, China. Irrig. Sci. 2022, 40, 87–101. [Google Scholar] [CrossRef]
- Fan, X.J.; Yu, X.F.; Cao, T. Light Absorption and Fluorescence Characteristics of Atmospheric Water-soluble Organic Compounds and Humic-like Substances During the Winter Season in Guangzhou. Environ. Sci. 2019, 40, 532–539. [Google Scholar]
- Datta, A.; Choudhury, M.; Sharma, P.C.; Jat, H.S.; Jat, M.L. Stability of humic acid carbon under conservation agriculture practices. Soil Tillage Res. 2022, 216, 105240. [Google Scholar] [CrossRef]
- Fatima, N.; Jamal, A.; Huang, Z.; Liaquat, R.; Ahmad, B.; Haider, R.; Ali, M.I.; Shoukat, T.; ALOthman, Z.A.; Ouladsmane, M.; et al. Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples. Sustainability 2021, 13, 8969. [Google Scholar] [CrossRef]
- Peuravuori, J.; Simpson, A.J.; Lam, B. Structural features of lignite humic acid in light of NMR and thermal degradation experiments. J. Mol. Struct. 2007, 826, 131–142. [Google Scholar] [CrossRef]
- Verheyen, T.V.; Johns, R.B. Structural investigations of Australian coals—III. A 13C-NMR study on the effects of variation in rank on coal humic acids. Geochim. Cosmochim. Acta 1982, 46, 2061–2067. [Google Scholar] [CrossRef]
- Ricca, G.; Federico, L.; Astori, C.; Gallo, R. Structural investigations of humic acid from leonardite by spectroscopic methods and thermal analysis. Geoderma 1993, 57, 263–274. [Google Scholar] [CrossRef]
- Balkas, T.I.; Bastürk, O.; Gaines, A.F.; Salihoǧlu, I.; Yilmaz, A. Comparison of five humic acids. Fuel 1983, 62, 373–379. [Google Scholar] [CrossRef]
- Novk, F.; Estauberov, M.; Hrabal, R.; Zhang, S.Q.; Yuan, L.; Zhao, B.Q. Structural Features of Lignohumic Acids. Humic Acid 2016, 01, 24–32. [Google Scholar]
- Peuravuori, J.; Zbankova, P.; Pihlaja, K. Aspects of structural features in lignite and lignite humic acids. Fuel Process. Technol. 2006, 87, 829–839. [Google Scholar] [CrossRef]
- Clemow, L.M.; Favas, G.; Jackson, W.R.; Marshall, M.; Redlich, P.J. Humic acids and methylated humic acids as models for reactions of brown coal with CO/H2O and with H2. Fuel 1999, 78, 567–572. [Google Scholar] [CrossRef]
- Qian, S.; Ding, W.; Li, Y.; Liu, G.; Sun, J.; Ding, Q. Characterization of humic acids derived from Leonardite using a solid-state NMR spectroscopy and effects of humic acids on growth and nutrient uptake of snap bean. Chem. Speciat. Bioavailab. 2015, 27, 156–161. [Google Scholar] [CrossRef]
- Chi, X.Y.; Zeng, L.X.; Du, Y.J.; Huang, J.Q.; Kang, Y.; Luo, J.W.; Zhang, Q.Y. Adsorption of levofloxacin on natural zeolite: Effects of ammonia nitrogen and humic acid. Water Sci. Technol. 2022, 85, 2928–2944. [Google Scholar] [CrossRef]
- Xing, Z.B. Sorption of phenanthrene by nanosized alumina coated with sequentially extracted humic acids. Environ. Sci. Pollut. Res. 2010, 2, 17–27. [Google Scholar]
- Dong, W.X.; Tian, H.T.; Ping, Z.Y. Complexation characteristics of humic acids with Fe2+ and their fractions from soil with different fertilization II—The complexation of humic acid fractions with Fe2+. Plant Nutr. Fertil. Sci. 2001, 7, 139–144. [Google Scholar]
- Sievers, D.A.; Kuhn, E.M.; Tucker, M.P.; Mcmillan, J.D. Effects of Dilute-Acid Pretreatment Conditions on Filtration Performance of Corn Stover Hydrolyzate. Bioresour. Technol. 2017, 243, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qi, Z.; Liu, C. Inhibition mechanisms of humic acid and protein on the degradation of sulfamethazine by horseradish peroxidase. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127473. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Guo, L.; Wang, P.; He, C.; Liu, D.; Bian, H.; Sheng, L. Effects of polystyrene nanoplastics with different functional groups on rice (Oryza sativa L.) seedlings: Combined transcriptome, enzymology, and physiology. Sci. Total Environ. 2022, 834, 155092. [Google Scholar] [CrossRef]
- Fujitake, N.; Kusumoto, A.; Tsukamoto, M.; Noda, Y.; Suzuki, T.; Otsuka, H. Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs. Soil Sci. Plant Nutr. 1999, 45, 349–358. [Google Scholar] [CrossRef]
- Hardy, E.; Rodriguez, C.; Trujillo, L.E. Lipopolysaccharide (LPS) and Protein-LPS complexes: Detection and Characterization by Gel Electrophoresis, Mass Spectrometry and Bioassays. Biol. Med. 2016, 8, 2–7. [Google Scholar] [CrossRef]
- Klučáková, M. Characterization of pH-fractionated humic acids with respect to their dissociation behaviour. Environ. Sci. Pollut. Res. 2016, 23, 7722–7731. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Characterization of pH-fractionated humic acids derived from Chinese weathered coal. Chemosphere 2017, 166, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Li, Y.; Zhang, K.; Zhang, H.; Li, Y. Effects of humic acid on sludge performance, antibiotics resistance genes propagation and functional genes expression during Cu(II)-containing wastewater treatment via metagenomics analysis. Bioresour. Technol. 2021, 323, 124575. [Google Scholar] [CrossRef]
- Hermosin, B.; Trubetskoj, O.A.; Trubetskaya, O.E.; Saiz-Jimenez, C. Thermally assisted hydrolysis and methylation of humic fractions obtained by polyacrylamide gel electrophoresis. J. Anal. Appl. Pyrolysis 2001, 58, 341–347. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Hermosin, B.; Trubetskaya, O.E.; Reznikova, O.I.; Trubetskoj, O.A. Thermochemolysis of genetically different soil humic acids and their fractions obtained by tandem size exclusion chromatography–polyacrylamide gel electrophoresis. Geoderma 2006, 131, 22–32. [Google Scholar] [CrossRef]
- Ouatmane, A.; D’Orazio, V.; Hafidi, M.; Senesi, N. Chemical and Physicochemical Characterization of Humic Acid-like Materials from Composts. Compost Sci. Util. 2002, 1, 39–46. [Google Scholar] [CrossRef]
- Drosos, M.; Leenheer, J.A.; Avgeropoulos, A.; Deligiannakis, Y. H-binding of size-and polarity-fractionated soil and lignite humic acids after removal of metal and ash components. Environ. Sci. Pollut. Res. 2014, 21, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.N.; Bryan, N.D. Colloidal properties of humic substances. Adv. Colloid Interface Sci. 1998, 78, 1–48. [Google Scholar] [CrossRef]
- And, S.K.; Xing, B. Humic Acid Fractionation upon Sequential Adsorption onto Goethite. Langmuir 2008, 24, 2525–2531. [Google Scholar]
- Monsallier, J.M. Influence of Humic Acid Size on Actinide Complexation; The Florida State University: Tallahassee, FL, USA, 1998. [Google Scholar]
- Tanaka, T. Functional groups and reactivity of size-fractionated Aldrich humic acid. Thermochim. Acta 2012, 532, 60–64. [Google Scholar] [CrossRef]
- Masoodi, H.R.; Bagheri, S.; Mohammadi, M.; Zakarianezhad, M.; Makiabadi, B. The influence of cation-π and anion-π interactions on some NMR data of s-triazine… HF hydrogen bonding: A theoretical study. Chem. Phys. Lett. 2013, 588, 4–10. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, Z.A.; Mahurin, S.M.; Jiang, X.; Chai, S.H.; Lu, H.; Nelson, K.; Dai, S. Hypercrosslinked Phenolic Polymers with Well-Developed Mesoporous Frameworks. Angew. Chem. Int. Ed. 2015, 54, 4582–4586. [Google Scholar] [CrossRef]
- Vorov, O.K.; Livesay, D.R.; Jacobs, D.J. Nonadditivity in Conformational Entropy upon Molecular Rigidification Reveals a Universal Mechanism Affecting Folding Cooperativity. Biophys. J. 2011, 100, 1129–1138. [Google Scholar] [CrossRef]
- Chappaz, A.; Curtis, P.J. Integrating empirically dissolved organic matter quality for WHAM VI using the DOM optical properties: A case study of Cu-Al-DOM interactions. Environ. Sci. Technol. 2013, 47, 2001–2007. [Google Scholar] [CrossRef]
- El-Wakeel, S.T.; Radwan, E.K.; Ghafar, H.H.A.; Moursy, A.S. Humic acid-carbon hybrid material as lead(II) ions adsorbent. Desalination Water Treat. 2017, 74, 216–223. [Google Scholar] [CrossRef]
- Christl, I.; Kretzschmar, R. Relating Ion Binding by Fulvic and Humic Acids to Chemical Composition and Molecular Size. Environ. Sci. Technol. 2001, 35, 2505–2511. [Google Scholar] [CrossRef]
- Wang, Y.; Envelope, Z.H.P.; Sheng, L.; Zhao, M.; Feng, J. Adsorption of lead ions from aqueous solution using NH4H2PO4 modified humic acid residue. Environ. Technol. Innov. 2022, 28, 102920. [Google Scholar] [CrossRef]
- Kinniburgh, D.G.; Milne, C.J.; Benedetti, M.F.; Pinheiro, J.P.; Filius, J.; Koopal, L.K.; Van Riemsdijk, W.H. Metal ion binding by humic acid: Application of the NICA-Donnan model. Environ. Sci. Technol. 1996, 30, 1687–1698. [Google Scholar] [CrossRef]
- Qiong, Z. Study on the Preparation of Activated Carbon from Coal and Its Adsorption Properties; Shanxi Chemical Industry: Taiyuan, China, 2018. [Google Scholar]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol. 2018, 261, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Mao, N.; Shuai, Q. Efficient removal of tetracycline from aqueous solution by covalent organic frameworks derived porous carbon. J. Environ. Chem. Eng. 2021, 9, 104842. [Google Scholar] [CrossRef]
- Jana, A.; Roy, O.; Ravuru, S.S.; De, S. Tuning of graphene oxide intercalation in magnesium aluminium layered double hydroxide and their immobilization in polyacrylonitrile beads by single step mussel inspired phase inversion: A super adsorbent for lead. Chem. Eng. J. 2019, 391, 123587. [Google Scholar] [CrossRef]
- Chen, D.; Shen, W.; Wu, S.; Chen, C.; Luo, X.; Guo, L. Ion exchange induced removal of Pb(ii) by MOF-derived magnetic inorganic sorbents. Nanoscale 2016, 8, 7172–7179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Xu, J.; Cao, Z.; Fu, R.; Zhou, C.; Wang, Z.; Xu, X. Adsorption behavior and mechanism of Pb(II) and complex Cu(II) species by biowaste-derived char with amino functionalization. J. Colloid Interface Sci. 2020, 559, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Wang, L.-M.; Li, Y.-H. Preparation of a Novel Bi-imprinted Adsorbent for Pb(II) and Its Application to FAAS Determination of Trace Amount of Lead in Water Sample. Ptca (Part B Chem. Anal.) 2014, 50, 589–594. [Google Scholar]
- Cimino, R.; Cychosz, K.A.; Thommes, M.; Neimark, A.V. Experimental and theoretical studies of scanning adsorption–desorption isotherms. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 76–89. [Google Scholar] [CrossRef]
- Song, L.; Zhao, R.; Yun, D.; Lu, P.; He, J.; Wang, X. Influence of Co(II), Ni(II), tartrate, and ethylenediaminetetraacetic acid on Cu(II) adsorption onto a polyvinylidene fluoride-based chelating membrane. Toxicol. Environ. Chem. 2014, 96, 362–378. [Google Scholar] [CrossRef]
- Fu, L.; Liu, F.; Ma, Y.; Tao, X.; Ling, C.; Li, A.; Shuang, C.; Li, Y. High-efficient technique to simultaneous removal of Cu(II), Ni(II) and tannic acid with magnetic resins: Complex mechanism behind integrative application. Chem. Eng. J. 2015, 63, 83–91. [Google Scholar] [CrossRef]
- Lv, J.; Chen, J. Synthesis of Core-Shell Nanoscale Zero Valent Iron under Air Atmosphere and Its Enlightenment for Groundwater In-Situ Remediation. Geol. Sci. Technol. Inf. 2017, 3, 242–248. [Google Scholar]
- Wang, N.; Xu, X.; Li, H.; Yuan, L.; Yu, H. Enhanced Selective Adsorption of Pb(II) from Aqueous Solutions by One-Pot Synthesis of Xanthate-Modified Chitosan Sponge: Behaviors and Mechanisms. Ind. Eng. Chem. Res. 2016, 55, 1222–1231. [Google Scholar] [CrossRef]
- Dragan, E.S.; Loghin, D.F.A. Fabrication and characterization of composite cryobeads based on chitosan and starches-g-PAN as efficient and reusable biosorbents for removal of Cu2+, Ni2+, and Co2+ ions. Int. J. Biol. Macromol. 2018, 120, 1872–1883. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.K.; Shahi, V.K. Selective Adsorption of Pb(II) from Aqueous Medium by Cross-Linked Chitosan-Functionalized Graphene Oxide Adsorbent. ACS Sustain. Chem. Eng. 2019, 7, 1427–1436. [Google Scholar] [CrossRef]
- Neiber, R.R.; Galhoum, A.A.; Sayed, E.T.E.; Guibal, E.; Xin, J.; Lu, X. Selective lead (II) sorption using aminophosphonate-based sorbents: Effect of amine linker, characterization and sorption performance. Chem. Eng. J. 2022, 442, 136300–136317. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Ly, Q.S. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef]
- Bassam, R.; Alouani, M.E.; Jarmouni, N.; Maissara, J.; Chbihi, M.E.M.; Belaaouad, S. Investigation of competitive adsorption and desorption of heavy metals from aqueous solution using raw rock: Characterization kinetic, isotherm, and thermodynamic. Mater. Today Proc. 2021, 52, 158–165. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-Inspired Synthesis of Polydopamine-Functionalized Graphene Hydrogel as Reusable Adsorbents for Water Purification. ACS Appl. Mater. Interfaces 2013, 5, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Gao, L.; Fu, X.; Siyar, S.H.; Sui, G.; Yang, X. Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal. Appl. Surf. Sci. 2019, 467–468, 1122–1133. [Google Scholar] [CrossRef]
- Xue, S.; Xiao, Y.; Wang, G.; Fan, J.; Wan, K.; He, Q.; Gao, M.; Miao, Z. Adsorption of heavy metals in water by modifying Fe3O4 nanoparticles with oxidized humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126333–126342. [Google Scholar] [CrossRef]
- Parajuli, D.; Adhikari, C.R.; Kuriyama, M.; Kawakita, H.; Ohto, K.; Inoue, K.; Funaoka, M. Selective recovery of gold by novel lignin-based adsorption gels. Ind. Eng. Chem. 2006, 45, 8–14. [Google Scholar] [CrossRef]
- Zou, L.; Shao, P.; Zhang, K.; Yang, L.; You, D.; Shi, H.; Pavlostathis, S.G.; Lai, W.; Liang, D.; Luo, X. Tannic acid-based adsorbent with superior selectivity for lead(II) capture: Adsorption site and selective mechanism. Chem. Eng. J. 2019, 364, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Fu, L.; Liu, H.; Zhang, D.; Xiong, C.; Wang, S.; Zhang, L. Design of Zr-MOFs by Introducing Multiple Ligands for Efficient and Selective Capturing of Pb(II) from Aqueous Solutions. ACS Appl. Mater. Interfaces 2023, 15, 5974–5989. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; Zhao, M.; Wang, S.; Zhang, L. Phenylthiosemicarbazide-functionalized UiO-66-NH2 as highly efficient adsorbent for the selective removal of lead from aqueous solutions. J. Hazard. Mater. 2021, 413, 125289. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, C.; An, Y.; Xiong, Z.; Hu, X.; Zhang, G.; Zheng, H. Magnetic phosphorylated chitosan composite as a novel adsorbent for highly effective and selective capture of lead from aqueous solution. J. Hazard. Mater. 2020, 405, 124195. [Google Scholar] [CrossRef]
- Nuzahat, H.; Wei, C. Structural response of humic acid upon binding with lead: A spectroscopic insight. Sci. Total Environ. 2018, 43, 479–485. [Google Scholar]
- Kadlubanski, P.; Calderón-Mojica, K.; Rodriguez, W.A.; Majumdar, D.; Roszak, S.; Leszczynski, J. Role of the Multipolar Electrostatic Interaction Energy Components in Strong and Weak Cation-pi Interactions. J. Phys. Chem. A 2013, 117, 7989–8000. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L.Q.; Rhue, R.D.; Reve, W. Selectivities of Potassium-Calcium and Potassium-Lead Exchange in Two Tropical Soils. Soil Sci. Soc. Am. J. 2003, 67, 1707–1714. [Google Scholar] [CrossRef]
- Teppen, B.J.; Miller, D.M. Hydration Energy Determines Isovalent Cation Exchange Selectivity by Clay Minerals. Soil Sci. Soc. Am. J. 2006, 70, 31–40. [Google Scholar] [CrossRef]
- Charles, S.; Teppen, B.J.; Li, H.; Laird, D.A.; Boyd, S.A. Exchangeable Cation Hydration Properties Strongly Influence Soil Sorption of Nitroaromatic Compounds. Soil Sci. Soc. Am. J. 2006, 70, 1470–1479. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J. Mater. Chem. A 2016, 4, 10885–10892. [Google Scholar] [CrossRef]
- Liu, J.T.; Xiao, G. 3D graphene/delta-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. [Google Scholar] [CrossRef]
- Sabaté, J.; Labanda, J.; Llorens, J. Influence of coion and counterion size on multi-ionic solution nanofiltration. J. Membr. Sci. 2009, 345, 298–304. [Google Scholar] [CrossRef]
- Wang, D.X.; Su, M.; Yu, Z.Y.; Wang, X.L.; Ando, M.; Shintani, T. Separation performance of a nanofiltration membrane influenced by species and concentration of ions. Desalination 2005, 175, 219–225. [Google Scholar] [CrossRef]
- Gao, L.Y.; Deng, J.H.; Huang, G.F.; Li, K.; Cai, K.Z.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, X.X.; Shi, L.N.; Li, J.F.; Li, S.J.; Lü, J.H.; Li, Y.M. Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef]
- Li, A.Y.; Deng, H.; Jiang, Y.H.; Ye, C.H.; Yu, B.G.; Zhou, X.L.; Ma, A.Y. Superefficient Removal of Heavy Metals from Wastewater by Mg-Loaded Biochars: Adsorption Characteristics and Removal Mechanisms. Langmuir 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Ni, F.; Liu, Q.; Yang, Y.; Wang, M.; Ao, T.; Chen, W. Synthesis of Magnesium Modified Biochar for Removing Copper, Lead and Cadmium in Single and Binary Systems from Aqueous Solutions: Adsorption Mechanism. Water 2021, 13, 599. [Google Scholar] [CrossRef]
- Kalinichev, A.G. Complexation with metal ions and colloidal aggregation of natural organic matter in aqueous solutions: A computational molecular modeling perspective (invited talk). In Proceedings of the ANDRA Workshop on Organic Matter in Clay Rock, Nantes, France, 20–24 September 2010. [Google Scholar]
- Kinniburgh, D.G.; van Riemsdijk, W.H.; Koopal, L.K.; Borkovec, M.; Benedetti, M.F.; Avena, M.J. Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf. A 1999, 151, 147–166. [Google Scholar] [CrossRef]
- Yan, M.; Lu, Y.; Gao, Y.; Benedetti, M.F.; Korshin, G.V. In-Situ Investigation of Interactions between Magnesium Ion and Natural Organic Matter. Environ. Sci. Technol. 2015, 9, 8323–8329. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Mota, A.M.; Benedetti, M.F. Effect of aluminum competition on lead and cadmium binding to humic acids at variable ionic strength. Environ. Sci. Technol. 2000, 34, 5137–5143. [Google Scholar] [CrossRef]
- Marsac, R.; Davranche, M.; Gruau, G.; Dia, A.; Coz, B.L. Aluminium competitive effect on rare earth elements binding to humic acid. Geochim. Cosmochim. Acta 2012, 89, 1–9. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Bo, W.; Wan, K.; Miao, Z. Calcium-doped magnetic humic acid nano particles for the efficient removal of heavy metals from wastewater: The role of Ca. Environ. Technol. 2023, 233, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Dipal, K. Response of plant species grown on central verge area of nh-8 near Navsari, India. J. Environ. Res. Dev. Environ. Res. Dev. 2012, 7, 1016–1020. [Google Scholar]
- Qiao, X.X.; Liu, G.F.; Wang, J.T.; Zhang, Y.Q.; Lü, J. Highly Efficient and Selective Removal of Lead Ions from Aqueous Solutions by Conjugated Microporous Polymers with Functionalized Heterogeneous Pores. Cryst. Growth Des. 2019, 20, 337–344. [Google Scholar] [CrossRef]
- Wang, L.; Hung, Y.T.; Shammas, N. Environmental Engnieering. In Advanced Physicochemical Treatment Processes; Humana: Totowa, NJ, USA, 2006; pp. 1–44. [Google Scholar]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Zidan, A.A.; El Zokm, G.M.; Okbah, M.A. Humic acid and nano-zeolite NaX as low cost and eco-friendly adsorbents for removal of Pb (II) and Cd (II) from water: Characterization, kinetics, isotherms and thermodynamic studies. Biomass Convers. Biorefin. 2024, 14, 3615–3632. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Xue, S.; Fan, J.; Wan, K.; Wang, G.; Xiao, Y.; Bo, W.; Gao, M.; Miao, Z. Calcium-Modified Fe3O4 Nanoparticles Encapsulated in Humic Acid for the Efficient Removal of Heavy Metals from Wastewater. Langmuir 2021, 37, 10994–11007. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.W.; Miao, Z.Y.; Gao, M.Q.; Wan, K.J. Structural analysis of lignite-derived humic acid and its microscopic interactions with heavy metal ions in aqueous solution. Sci. Total Environ. 2023, 897, 165385. [Google Scholar] [CrossRef]
- Nassar, N.N. Kinetics, equilibrium and thermodynamic studies on the adsorptive removal of nickel, cadmium and cobalt from wastewater by superparamagnetic iron oxide nanoadsorbents. Can. J. Chem. Eng. 2012, 90, 1231–1238. [Google Scholar] [CrossRef]
- Nassar, N.N. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.U.; Su, X.M.; Zhao, Y.; Liao, R. Interfaces, Preparation of hydroxypropyl-cyclodextrin-graphene/Fe3O4 and its adsorption properties for heavy metals. Surf. Interfaces 2019, 16, 43–49. [Google Scholar] [CrossRef]

| Ions | Radius (Å) | Hydration Radius (Å) | Hydration Energy (kJ mol−1) | Diffusion Coefficient (10−9 m2 s−1) | Stokes Radius (nm) |
|---|---|---|---|---|---|
| K+ | 1.33 | 3.31 | −295 | 1.957 | 0.124 |
| Na+ | 0.95 | 3.58 | −365 | 1.333 | 0.183 |
| Ca2+ | 0.99 | 4.12 | −1505 | 0.718 | 0.307 |
| Mg2+ | 0.65 | 4.28 | −1830 | 0.706 | 0.345 |
| Functional Groups/Ions | –COOH | –OH | –NH2 | –SH | References |
|---|---|---|---|---|---|
| Na | + | N/A | N/A | N/A | [101] |
| Ca | ++ (low pH) | + (high pH) | + | N/A | [102] |
| Mg | ++ | + | N/A | N/A | [103] |
| Al | ++ | + | N/A | N/A | [104,105] |
| References | Years | Modification Methods | Adsorption Capacity (mg/g) | Conclusions |
|---|---|---|---|---|
| [111] | 2022 | - | Pb2+: 23.11 Cd2+: 12.00 | HA has an affinity for Pb2+ |
| [112] | 2021 | Magnetic HA nanoparticles | Pb2+: 105.60 Cu2+: 67.43 Cd2+: 65.23 | HA exhibits selectivity for Pb2+ |
| [113] | 2021 | Calcium-modified Fe3O4 nanoparticles encapsulated in HA | Pb2+: 208.33 Cu2+: 98.33 Cd2+: 99.01 | Ca-ion exchange is the main mechanism for the selective adsorption of Pb2+ |
| [106] | 2023 | Calcium-doped magnetic HA nano particles | Pb2+: 278.65 Cu2+: 154.31 Cd2+: 145.55 | Ca activates the functional groups of HA |
| [114] | 2023 | - | Pb2+: 67.67 | Carboxylic acid is the key functional group affecting the selective adsorption of HA |
| [115] | 2012 | Fe3O4 | Pb2+: 29.00 Cd2+: 18.60 | - |
| [116] | 2010 | Resin microspheres | Pb2+: 99.19 Cu2+: 45.80 Cd2+: 13.75 | - |
| [117] | 2019 | Hydroxypropyl-cyclodextrin-graphene | Pb2+: 99.19 Cu2+: 45.80 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, S.; Hu, Y.; Wan, K.; Miao, Z. Exploring Humic Acid as an Efficient and Selective Adsorbent for Lead Removal in Multi-Metal Coexistence Systems: A Review. Separations 2024, 11, 80. https://doi.org/10.3390/separations11030080
Xue S, Hu Y, Wan K, Miao Z. Exploring Humic Acid as an Efficient and Selective Adsorbent for Lead Removal in Multi-Metal Coexistence Systems: A Review. Separations. 2024; 11(3):80. https://doi.org/10.3390/separations11030080
Chicago/Turabian StyleXue, Shuwen, Yunhu Hu, Keji Wan, and Zhenyong Miao. 2024. "Exploring Humic Acid as an Efficient and Selective Adsorbent for Lead Removal in Multi-Metal Coexistence Systems: A Review" Separations 11, no. 3: 80. https://doi.org/10.3390/separations11030080
APA StyleXue, S., Hu, Y., Wan, K., & Miao, Z. (2024). Exploring Humic Acid as an Efficient and Selective Adsorbent for Lead Removal in Multi-Metal Coexistence Systems: A Review. Separations, 11(3), 80. https://doi.org/10.3390/separations11030080








