Abstract
Oxidative stress degrades skin collagen and elastin and causes inflammatory reactions that affect mitochondrial DNA leading to aging. In the present study, a potential cosmetic nano-emulsion (o/w) of seven substances (chlorogenic acid, caffeine, rutin, hesperidin, quercetin, α-tocopherol and retinol) with antioxidant and anti-aging properties was prepared and analyzed. The lipophilic components were entrapped in the dispersed nanoparticles (jojoba) of the emulsion while the hydrophilic ones dissolved in the aqueous phase (glycerol/water). Suitable excipients were selected using an experimental design methodology with two mixtures and two responses (particle size and zeta potential). The quantitative extraction of chlorogenic acid and caffeine from Crithmum maritimum L. plant and coffee beans (Coffea arabica L.) and their stability were also studied. The analysis of the substances was carried out on an HPLC-DAD, with a phenyl column and gradient elution system (solvent A: water with 0.2% formic acid and B: acetonitrile with 0.2% formic acid). Validation of the method was performed in terms of linearity (r2 > 0.998), precision and repeatability (%RSD < 2) while the limits of detection (LLODs) and quantification (LLOQs) were also determined. The antioxidants were quantified after being extracted from the substrate (%recovery 96.7–102.5, %RSD < 2).
1. Introduction
The deterioration of skin morphology and physiology is the first and most obvious symptom of the aging process which occurs progressively with age. Aging can be described as the gradual accumulation of lesions over time, which disrupts the function of cells, tissues and organs, ultimately leading to disease and mortality. This process is complex and is influenced by several indicators, such as genetic predisposition, epigenetic factors and environmental influences. Intrinsic or chronological aging occurs naturally over time, while extrinsic aging results from human exposure to external factors such as UV radiation [1], smoking, air pollution and stress [2]. Mechanisms for skin aging include cell oxidation, the actions of reactive oxygen species (ROS), autophagy, mitochondrial DNA (mtDNA) mutations, telomere shortening and hormonal changes. Thus, in the anti-aging industry, the need for the preparation of safe, effective and approved formulations with antioxidant properties arises effortlessly.
In the present study, a nano-emulsion (o/w) [3] with cosmetic, anti-aging properties for dermal use was formulated and evaluated. There are two preparation approaches for nano-emulsions, that of high energy and low energy. The former can be carried out by high-pressure homogenization, micro-fluidization and ultrasonication. The low-energy approach is performed by membrane emulsification, spontaneous emulsification and phase inversion emulsification [4].
Chlorogenic acid, caffeine, rutin, hesperidin and quercetin (hydrophilic) were incorporated into the nano-emulsion by dissolution in the aqueous phase while vitamins A and E, by nano-particle dispersion in the oily phase. The main actions of vitamin A (retinol) are related to vision, cell growth, antioxidant capacity, immune improvement, and epithelial reproduction and integrity [4]. The biological activity of vitamin E (α-tocopherol) is also intertwined with its antioxidant properties (it inhibits the propagation of lipid peroxidation by reacting with the lipid peroxyl radical on follicular membranes), which makes it an excellent candidate for anti-aging formulations [5]. Rutin acts therapeutically on many systems of the human body (CNS, endocrine, immune, cardiovascular) and on the skin by suppressing aging-related inflammation [6]. Quercetin is a flavone with numerous beneficial effects while at the same time, as an anti-aging product ingredient, it stands out for its antioxidant properties. Chlorogenic acid is also a water-soluble polyphenolic phenyl acrylate compound found in green coffee, Crithmum maritimum L. and tea extracts [7] with antioxidant, antibacterial and anti-cancer activity [8]. A similar alkaloid is caffeine (1,3,7-trimethylxanthine), which is considered the main active ingredient of Coffea arabica L. and in recent years has often been found in cosmetic formulations. In addition to its stimulant action, caffeine can prevent carcinogenesis by protecting the skin from UV radiation (antioxidant) and by enhancing the apoptosis of DNA-damaged cells [9]. Finally, hesperidin is a secondary plant metabolite, which apart from its anti-inflammatory and anti-cancer activity, has antioxidant properties (inhibits the activities of the enzymes hyaluronidase, collagenase and elastase) that delay the aging process and alleviate the skin pathologies associated with it [10,11]. The amounts of active ingredients incorporated into the emulsion were chlorogenic acid and vitamin E 4% w/w, hesperidin and vitamin A 3% w/w and caffeine, quercetin, and rutin 2% w/w, which were determined based on the compositions of similar commercial preparations.
The aim of the present study was to develop a reliable and flexible HPLC method for the determination of seven active ingredients in a nano-emulsion, which will be a tool for the qualitative and quantitative evaluation of a formulation at all stages of its preparation (extraction, synthesis, stability and control of the final product).
After an extensive literature search on chromatographic methods for the simultaneous determination of seven active pharmaceutical ingredients (APIs) on different substrates, it was found that there is no corresponding reference. Of course, some of the polar compounds (chlorogenic acid, rutin, quercetin, hesperidin and caffeine) have been analyzed in various combinations, but not together with the two lipophilic vitamins (α-tocopherol and retinol) [12]. Pascale and Papadoyannis used separate extracts of the chicory plant to determine, with different HPLC methods, caffeine and chlorogenic acid in a fraction, with phenolic derivatives, rutin, hesperidin and quercetin in the flavonoid fraction and vitamins A and E in the group of vitamins [13]. The two vitamins A and E were also determined in the presence of seven other water-soluble and non-polar vitamins after solid-phase extraction (SPE) and RP-HPLC analysis in pharmaceutical preparations and biological fluids [14].
In the present work, the two active compounds, caffeine and chlorogenic acid, were quantitatively obtained from their plant extracts and then incorporated into the emulsion. Therefore, the extraction efficiency and stability of their solutions were tested by applying the proposed HPLC method. Next, the determination of the best nano-emulsion composition was studied by an experimental design methodology, using the “Design-Expert 11” program, with two mixtures and responses (particle size and zeta potential). Finally, a method was developed to extract and quantify the active ingredients from the final product. The proposed HPLC method is reliable, robust and can be used as a tool to study and control for a nano-emulsion at all stages of its production.
2. Experimental Methods
2.1. Materials and Solutions
Acetonitrile (ACN) and methanol both of an HPLC gradient grade were obtained from VWR Chemicals (Radnor, PA, USA). High-purity water (18.2 MΩ·cm resistivity) produced by a B30 water purification system was used during this study (Adrona SIA, Riga, Latvia). Formic acid (Sigma-Aldrich, Burlington, MA, USA) ≥ 96%, acetic acid ≥ 99.8% (Fluka, Charlotte, NC, USA), trifluoroacetic acid 99% (Merck, Darmstadt, Germany), potassium dihydrogen phosphate of a quality level, MQ500 (KH2PO4) (Merck) and phosphoric acid (H3PO4 ≥ 85%) (Merck) were utilized in the mobile phase. Glycerin (AROMA LAB), coconut oil, jojoba oil, peanut oil (Chemco; Syndesmos) and the surfactants Tween (20, 80) and Span 20 (Chemco; Syndesmos) were used as emulsion excipients.
Reference standards, vitamin E (α-tocopherol), vitamin A (retinol), rutin, hesperidin and chlorogenic acid were purchased from the company Sigma-Aldrich, while caffeine from Boehringer, Ingelheim and quercetin were from Fluka Biochemika (Figure S1, Supplementary Materials).
Two of the active ingredients, caffeine and chlorogenic acid, were obtained from Crithmum maritimum L. and coffee beans (Arabica variety) using an extraction procedure. Leaves of Crithmum maritimum were collected from a coastal area of Chalkidiki, washed with deionized water and then left to dry for 2 h at 70 °C. After drying, the leaves were cut with a cutting mill and stored in special containers. Similarly, about 5 g of coffee beans (Coffea arabica L.) were heated in a suitable fireproof vessel for 8 min to undergo the extraction process.
Potassium phosphate buffer, (KH2PO4) 20 mM and 40 mM at pH 7: The solution was prepared by weighing 2.72 g (20 mM) or 5.44 g (40 mM) of KH2PO4, in a 1000 mL volumetric flask, which was filled with HPLC water up to the mark. The pH of the solution was adjusted to 7 by adding drops of 1 M NaOH solution under magnetic stirring. This was followed by filtration with a 0.45 µm filter.
Sodium phosphate buffer (NaH2PO4–H2O), 20 mM at pH 2.5: An amount of 1.38 g of sodium monobasic phosphate hydrated was accurately weighed and dissolved in 500 mL of HPLC water. The buffer was acidified using phosphoric acid solution until the pH value reached 2.5. The solution was then filtered with a 0.45 µm filter.
Sodium phosphate buffer (NaH2PO4–H2O), 20 mM at pH 6.7: An amount of 1.38 g of hydrated sodium monobasic phosphate was accurately weighed and dissolved in 500 mL of HPLC water. The pH value was adjusted to 6.7 with 0.2 M NaOH. The solution was then filtered with a 0.45 µm filter.
Standard solutions: Total amounts of 11.10 mg quercetin, 11.20 mg rutin, 12.60 mg hesperidin, 9.30 mg caffeine, 9.30 mg chlorogenic acid, 9.50 mg vitamin A and 31.0 mg vitamin E were accurately weighed in separate 10 mL volumetric flasks. The flasks were filled to the mark with 0.2% formic acid methanolic solution. By appropriate dilutions, an intermediate mixed stock (mix1) and then seven standard solutions (diluent: methanol with 0.2% formic acid) were prepared and analyzed by HPLC (Table S1, Supplementary Materials).
Standard solutions for spiking: In a similar way, two intermediate mixed standard solutions mix2 and mix3 (diluent: ethanol) were also prepared to disperse the APIs into the oil and water phases of the emulsion. The intermediate mix2 contained the two vitamins A and E (300 μg/mL vitamin A and 400 μg/mL vitamin E), while mix3 contained the remaining active ingredients (200 μg/mL quercetin, 200 μg/mL rutin, 300 μg/mL hesperidin, 200 μg/mL caffeine and 400 μg/mL chlorogenic acid).
Spiked placebo: For the preparation of the spiked placebo, 1 mL of both mixed solutions (mix2 and mix3) were transferred to two separate tubes and evaporated (with nitrogen). After adding 1 mL of jojoba to mix2 and 8 mL of water–glycerin 1:1.3 to mix3, the two mixtures as well as 1 mL of Spam 20 were mixed (vortexed) and subjected to sonication (10 min). At the extraction procedure, to 1 mL of spiked placebo sample were added 9 mL of extractant diluent (methanol/0.2% formic acid) and the cleaning process followed.
Control standard solution: Amounts of 1 mL of mix2 and 1 mL of mix3 solutions were added to 8 mL of diluent (methanol/0.2% formic acid) and subjected to the same processing (ultrasonication, stirring, cooling and centrifugation) as the unknown sample, in order to be analyzed by HPLC.
Emulsion base: Total volumes of 3.50 mL of water, 4.50 mL of glycerol, 0.90 mL of jojoba oil and 1.10 mL of Span 20 were used to prepare the nano-emulsion (o/w) based on ultrasonic methodology and according to the procedure described below (Section 3.1).
Drug-free (blank): For the blank solution, 1 mL of the emulsion base was added to 9 mL of methanol with formic acid 0.2%. The mixture was subjected to the same processing as that of the standard and unknown sample.
2.2. Instrumentation and Chromatographic Conditions
The chromatographic determination of the active ingredients was carried out by means of a Shimadzu 220 series high-performance liquid chromatography (HPLC) system, consisting of two pumps (LC-20AD), a degasser (DGU-14A), an automatic sampling system (SIL-10AD), a column oven (CTO-6A) and a UV detector with photodiode array (SPD-M20A). The detector was set up at two wavelengths (326 nm and 280 nm). LC-Solution software, version 1.25, was then used.
The stationary phase was a phenyl column (4.6 mm × 150 mm, 5 µm), thermostable at 40 °C, while the composition of the initial mobile phase was the following: phase A, 90% water (with 0.2% formic acid) and phase B, 10% acetonitrile with 0.2% formic acid. According to the gradient elution program applied, the mobile phase increased (with respect to phase B) linearly as follows. In the first 4 min from 10% to 20%, in the next minute, phase B becomes 40% where it remains for 2 min (t = 7 min). Subsequently, at t = 8 min, phase B becomes 50% and in the next two minutes (t = 10 min) 60%. Then, every two minutes the content of phase B rises linearly by 20% until it reaches 100% (t = 14 min). After one minute (t = 15 min), phase B returns to 10% and remains until the end of the analysis (t = 20 min). The flow rate of the mobile phase was 1 mL/min while the sample injection volume was 50 µL.
To study the nano-emulsions and their behavior, a Zetasizer, Malvern, ZEN3600, was used.
3. Results and Discussion
3.1. Formation Study of the Nano-Emulsion
For the preparation of the nano-emulsion (o/w), an important problem to be solved was to ensure the stability of caffeine and chlorogenic acid, in their aqueous extracts. Therefore, based on related reports [15,16], glycerin (1:1 v/v) was added in order to stabilize both APIs. Then, to determine the ratio of the two phases and the emulsifier (o/w/surfactant) to be used in the nano-emulsion, a preliminary study was carried out with the following combinations: 50:40:10, 60:30:10, 70: 20:10 and 80:10:10:10 (w/w/w). According to the results, the best combination was 80:10:10, due to the better stability and texture of the emulsion. Defining the type of emulsion (o/w), the ratio of the two phases (80% aqueous, 10% oil, 10% emulsifier) and the composition of the aqueous phase (water, glycerol 1:1), further research was conducted to determine the remaining excipients. Thus, coconut, peanut and jojoba oils were tested for the oleic phase [17], while Tween 20, Tween 80 and Span 20 were tested as surfactants [18]. The excipients were evaluated at 15 different combinations (Table S2, Supplementary Materials) and the emulsions were prepared by two different techniques: (a) the phase inversion technique where both aqueous and oleic phases were heated separately at 40 °C. Under continuous stirring and keeping the temperature constant, the oleic phase and surfactant were mixed in a beaker. Subsequentially, the aqueous phase was added dropwise (of 1 mL/min) under continuous stirring. (b) An ultrasonic process was used where low- or high-frequency sound waves were used to form emulsion droplets from two immiscible liquids in the presence of a surfactant. The mixing process of the two phases and the surfactant was analogous to that of phase inversion. The operating conditions were amplitude = 45%, timer = 4 min and pulse = 40 06. Having as an emulsion stability criterion the non-separation of the two phases, four combinations were considered optimal. In these, different amounts of surfactants were further tested at concentration levels of 0.5 mL (5.26%) and 1.5 mL (14.28%), respectively. Hence, it was found that the emulsion with jojoba oil and Span 20 showed less tendency to separate. Subsequently, the proportions of these ingredients were studied by the experimental design methodology program. Since the aqueous phase (glycerol/water) constituted 80% of the formulation composition while the other two ingredients constituted 20%, a two-mixture design (mixture1 × mixture2) with two responses (particle size, zeta potential) was modeled (Table S3, Supplementary Materials). The proposed 11 experiments were performed in duplicate (a) by the phase inversion (epi) and (b) the ultrasonic (sonics) technique where the latter was considered as the best. The significance of the considered factors (A: ml glycerin, B: mL H2O, C: mL jojoba, D: mL Span 20) of the two mixtures on the emulsion quality was assessed using the analysis of variance (ANOVA). The overall evaluation of the models was determined by the values of specific statistical parameters presented in Table 1.

Table 1.
ANOVA results for the best fit models by ultrasonic technique.
The optimal emulsion composition was found by applying (a) the graphical method of superimposing isometric curves on an overlay plot and locating the region of optimal solutions and (b) a desirability function (Figures S2 and S3, Supplementary Materials). According to the experimental results, the samples prepared with the ultrasonic process presented smaller particle sizes and higher zeta potential values, while the ideal emulsion composition was 45% glycerol, 35% water, 9% jojoba and 11% Span 20.
To further study the stability of the proposed nano-emulsion, three identical samples were prepared and measured (zeta potential and particle size) over a period of one month (Figures S4 and S5, Supplementary Materials). According to the results, the particle size of the dispersion ranged between 178 and 222 nm, which allowed it to be classified as a “nano-emulsion” [19], while the absolute value of zeta potential was >25, indicating its stability [20].
3.2. HPLC-DAD Method Optimization
Under the present experimental conditions, it was extremely difficult to find a chromatographic method for the determination of seven compounds in an emulsion substrate (o/w), which exhibited significantly different degrees of lipophilicity. Both vitamins A and E are highly lipophilic (XlogPmax = 10.7), while rutin, caffeine, quercetin, chlorogenic acid and hesperidin are characterized as hydrophilic (XlogPmin = −1.3) [21]. For the investigation of the chromatographic conditions, the influence of various factors was studied considering the shape of the peaks, their resolution (Rs) as well as the elution times of the analytes.
Regarding the stationary phase and its interaction with the analytes, reversed-phase (RP) chromatographic columns with different dimensions and mechanisms such as C18: Supelco Discovery HS (150 mm × 4.6 mm, 5 μm), C8: Supelco (250 mm × 4.6 mm, 5 μm), anionic column SAX: 5 μm (125 mm × 4 mm, 5 μm), CN column Waters Spherisorb® 5 μm (250 mm× 4.6 mm, 5 μm) and phenyl ACE-ACT (150 mm × 4.6 mm, 5 μm) were studied. For the anionic column, the problem was mainly found in the chlorogenic acid, as it eluted with the solvent front. In other words, the k’ of the chlorogenic acid was close to zero. The CN column was considered unsuitable as the resolution between chlorogenic acid and caffeine was equal to 1.3, while the k’ values were 0.1 for chlorogenic and 0.4 for caffeine. Furthermore, it should be mentioned that the chlorogenic acid showed a double peak and tailing. Finally, as for the C8 and C8 columns, the problem mainly appeared in chlorogenic acid. At the base of its peak, in both columns, a double peak was presented, which dramatically increased the resolution with caffeine. Changes in the mobile phase (different ratios, flow rates and temperatures) did not ameliorate the results. The best performance was obtained with the phenyl column, which was chosen as the most suitable.
Since the lipophilicity of the analyzed ingredients was significantly different, their elution was achieved with different organic/water solvent ratios. Thus, as a first step, under isocratic conditions, we investigated which solvents (acetonitrile: water or methanol with water) were the most effective.
However, the “tailing” of the analyte peaks (especially in the methanol–water mobile phase) led to the use of buffered solutions. Mixtures of NaH2PO4–H2O or KH2PO4 acetonitrile–aqueous solutions at different concentrations (20 mM, 40 mM) and a pH = 2.5 and 6.7 were tested as the mobile phase, but none of them was good since chlorogenic acid’s peak was double. This problem was faced by using as a mobile phase a binary system of 0.2% formic acid aqueous solution and acetonitrile with 0.2% formic acid. Decreasing the concentration of formic acid to 0.1% did not give satisfactory results. The same method was tested by replacing the formic acid with trifluoroacetic and acetic acid but there was no improvement in the chromatographic behavior of the analytes. By studying all the active ingredients separately and isocratically, it became evident that chlorogenic acid and caffeine had good k’ values with a lower percentage of aqueous mobile phase, while rutin, quercetin and the two vitamins had good k’ values with a higher percentage of organic mobile phase. Therefore, the use of a gradient system of increasing eluting power was necessary.
A factor that also had to be taken into consideration was the diluent of the analytes. The behavior of quercetin and chlorogenic acid which usually gave double peaks played a decisive role. Chlorogenic acid is not soluble in acetonitrile and is completely soluble in water or in a mixture of the two solvents containing at least 25% water. However, at this ratio, chlorogenic acid gave double peaks while an increase in the solvent in water created solubility problems mainly in lipophilic active ingredients (vitamins A, E). Thus, acetonitrile was replaced by methanol, in which chlorogenic is soluble, and combinations were studied as the following: (a) methanol, 100%; (b) methanol–phosphate buffer, 20 mM or 40 mM, at pH = 7, in a ratio of 2:1 v/v; (c) methanol–acetonitrile, in a ratio of 2:1 v/v; and (d) methanol with formic acid, 0.1–1% v/v After investigation, it was found that the best result was obtained in the case of methanol solution containing formic acid. The ratio of formic acid from 0.2% to 1% did not alter the result and 0.2% was chosen for column protection reasons (Figure 1).
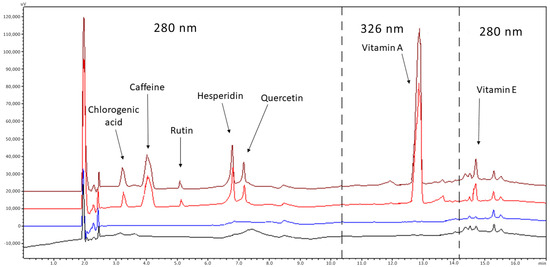
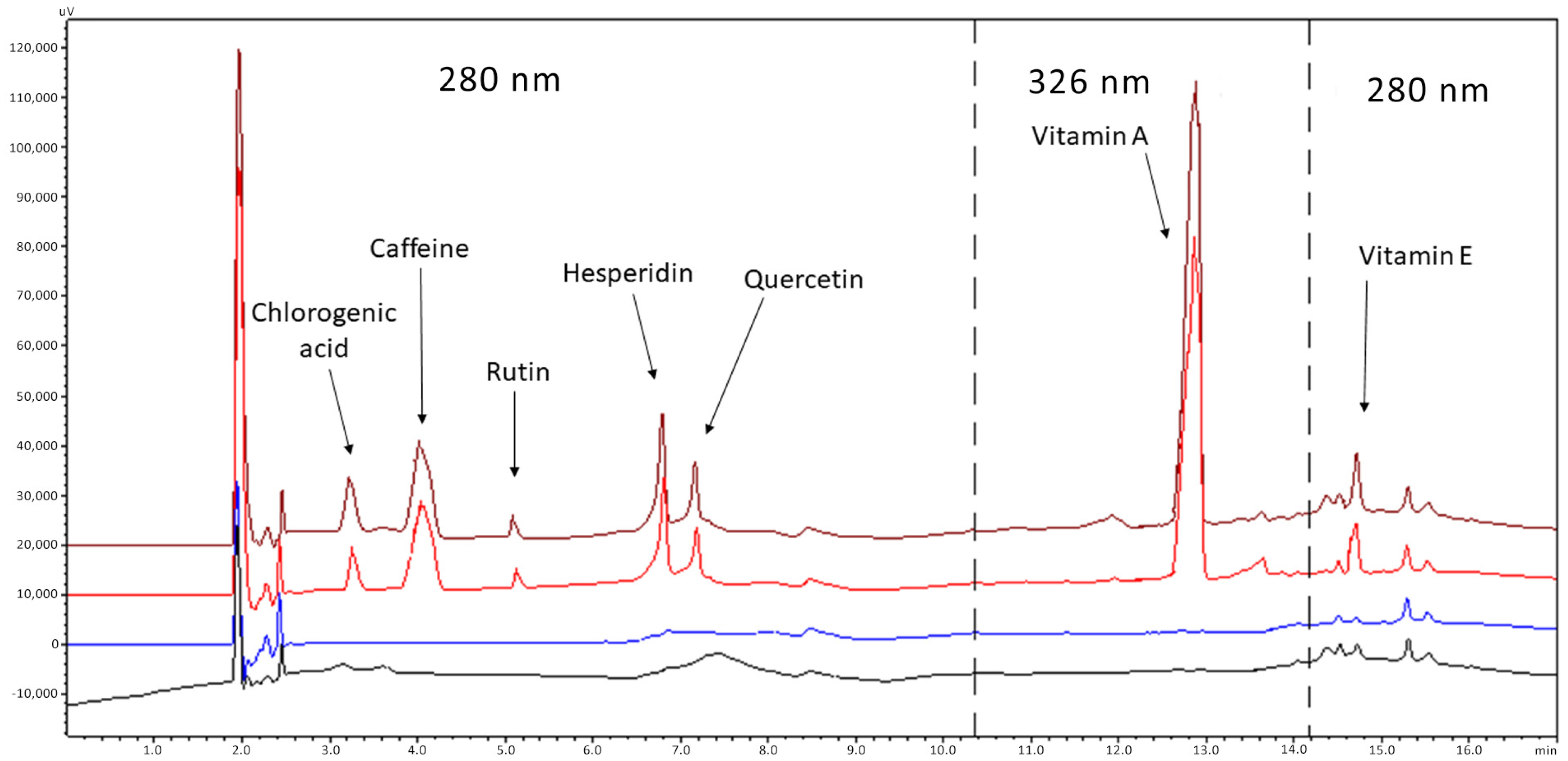
Figure 1.
Chromatograms of blank emulsion (black line), blank diluent (blue line) of a standard solution (red line) and spiked sample (brown line). Concentrations: 4 μg/mL chlorogenic acid, 2 μg/mL caffeine, 2 μg/mL rutin, 3 μg/mL hesperidin, 2 μg/mL quercetin, 3 μg/mL vitamin A, 4 μg/mL vitamin E. Analytes between the dashed lines are detected at 326 nm, while outside at 280 nm.
Finally, regarding the choice of wavelengths (λ) and for practical reasons, API detection was carried out at two wavelengths (280 and 326 nm). These were not the maximum (λmax: chlorogenic acid 330 nm, caffeine 273 nm, rutin 271 nm, hesperidin 283 nm, quercetin 258/380 nm, α-tocopherol 326 nm and retinol 292 nm) but gave a satisfactory signal for all analytes.
3.3. HPLC-DAD Method Validation
Validation of the HPLC method was performed according to the International Conference on Harmonization of Technical Requirements for the Registration of Medicinal Products for Human Use (ICH Q2) guidelines [22]. Before validation, system suitability experiments were performed (Table 2).

Table 2.
System suitability tests.
3.3.1. Specificity
To evaluate the specificity, a blank sample containing all the excipients of the preparation was compared with the corresponding sample, spiked with the seven analytes at the concentrations of 40 μg/mL for chlorogenic acid, 20 μg/mL for caffeine, 20 μg/mL for quercetin, 30 μg/mL for hesperidin, 20 μg/mL for rutin, 30 μg/mL for vitamin A and 30 μg/mL for vitamin E. Figure 1 depicts the typical chromatograms of drug-free (blank) and spiked placebo samples. The method proved to be specific for the determination of antioxidants since no peak was observed in the chromatograms of the blank samples at the retention time of the analytes.
Unexpected peaks due to carryover were calculated by applying the following equation: %carryover = (post-challenge blank peak area)/(peak area of the highest concentration standard) ×100. In all cases, the areas of unexpected peaks were <2% LLOQ.
3.3.2. Linearity, LLODs and LLOQs
Linearity was investigated in seven different concentration ranges. Each standard was analyzed in triplicate. The results of the linear regression, correlation coefficient, and limits of detection (LLODs) and quantitation (LLOQs) for each analyte are presented in Table 3. For the calculation of the LLOD and LLOQ values, the signal-to-noise ratio was used [23].

Table 3.
Data on the linear regression equations and LLOD and LLOQ values of the seven analyzed antioxidants.
3.3.3. Accuracy and Precision
The accuracy of the proposed method was studied by analyzing samples at three concentration levels (low, medium and high). Evaluation of results was based on %recovery and %relative standard deviation (%RSD) values. As shown in Table 4, satisfactory recoveries were obtained in a range of (99–101% ± 2).

Table 4.
Accuracy and precision results of the proposed method.
Precision was investigated in terms of repeatability and intermediate precision (Table 4). More specifically, the repeatability (intra-day precision) was assessed by analyzing standards at three concentration levels (three replicates) by one analyst, on the same day. On the other hand, intermediate precision was calculated by analyzing standards at three concentration levels (three replicates) by one analyst, on three consecutive days.
3.3.4. Robustness
For robustness testing, small deliberate changes were investigated. In detail, the column temperature was tested within the limits of 40 ± 1 to ± 2 °C, the initial composition of mobile phase B at 10 ± 1% to ± 2%, the injection volume at 50 ± 1 to ± 2 μL, and the detection wavelength λ at ± 1 to ± 2 nm. Based on the results, the tolerance of the system was satisfactory and ensured good quality of the chromatograms.
3.4. Extraction of Chlorogenic Acid and Caffeine
For the quantitative extraction of both caffeine and chlorogenic acid, two different procedures were followed, the first of strong heating for a short time and the second of mild heating for a long time. In applying the methods, 8.01 g of powdered Crithmum maritimum L. or 1.00 g of coffee beans were weighed in a 500 mL volumetric flask and 150 mL of water–glycerol at 50:50% v/v were added. This was followed by (a) heating in a water bath at 70 °C for 3 h with stirring and (b) heating at 30 °C for 21 h with stirring. The two techniques were compared in terms of recovery efficiency and stability of the active ingredients.
The extracted samples were analyzed by HPLC, and their results were qualitatively compared to choose the most efficient method (A or B). In the case of Crithmum maritimum L., it was found that the concentration of chlorogenic acid obtained by method B was three times lower than the corresponding of method A (chlorogenic acid 22.79 μg/mL), while the quality of the peaks was clearly better (sharp peaks with good separation).
Respectively, for the case of Coffea arabica L., it was found that with method B, the signals of the chromatographic peaks of both chlorogenic acid and caffeine were five times lower in intensity than those obtained with method A (chlorogenic acid 71.07 µg/mL and caffeine 59.59 µg/mL). According to the above, method A was chosen as the most suitable for both cases.
As the nano-emulsion is intended for dermal use (face and neck application), the solvents for extraction and subsequent preservation of the formulation must not be irritating or toxic. Water was considered as the solvent of choice, since both active substances are soluble in it, but it did not ensure the stability of the APIs. Glycerol is widely recommended in industry as a maintenance solvent because it is biodegradable (green solvent) and can be used without safety concerns [15].
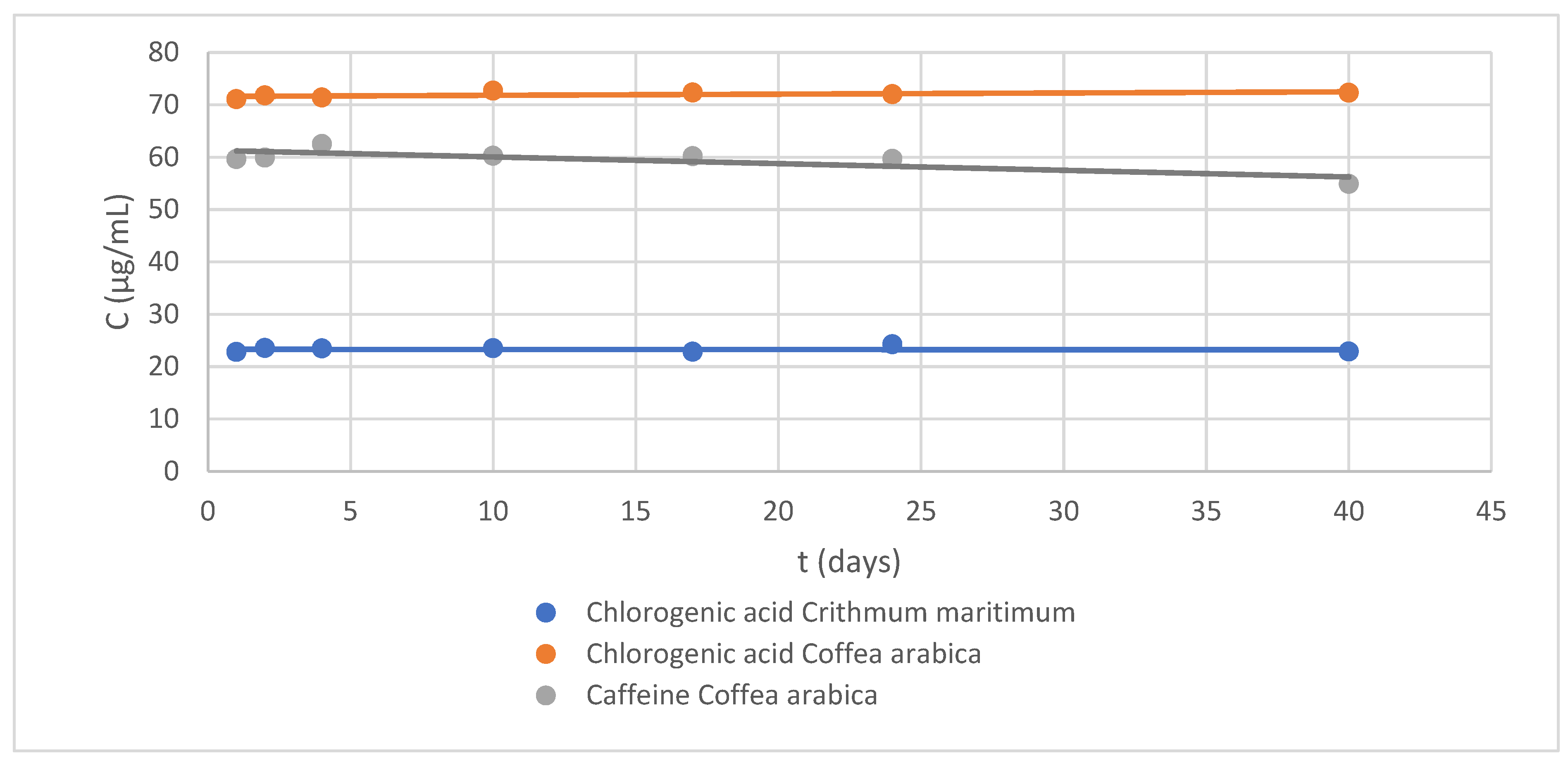
Thus, after obtaining the aqueous extracts of both APIs, an equal amount of glycerol (glycerol:water 50% v/v) was added and the solution was kept at 2–4 °C. Next, the stability of the chlorogenic acid and caffeine was studied over 40 days (Figure 2). According to their stability profile, the potential of glycerin as a preservative was confirmed [16].
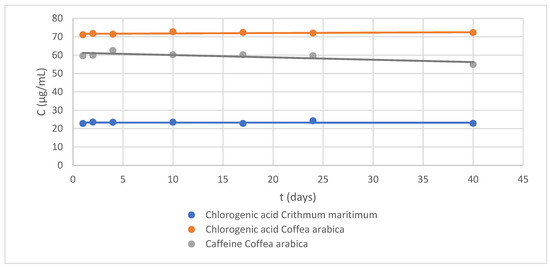
Figure 2.
Stability control chart at 40 days: (blue) chlorogenic acid, 22.79 µg/mL, in Crithmum maritimum extract; (orange) chlorogenic acid, 71.07 µg/mL, in Coffea arabica L.; and (gray) caffeine, 59.59 µg/mL, in Coffea arabica L. extract.
3.5. Sample Purification Method
Subsequently, a second sample processing method was developed to perform purification and quantitative recovery of APIs from the nano-emulsion. The optimization of the extraction procedure was performed using a 1 mL spiked placebo sample. Chloroform and ether were tested as lipophilic extraction solvents and water, acetonitrile and methanol, as hydrophilic. Regarding chloroform or ether, they were used in a liquid/liquid extraction method in combination with water–formic acid, 0.2%, in a ratio of 5:4 v/v. Both non-polar solvents can mainly pick up the two lipophilic vitamins (A and E) while water was used to collect the hydrophilic active substances. After the two phases were separated, the organic one was collected in a tube A and evaporated, while the aqueous one was subjected to solid-phased extraction technique with a Bond Elut C18 cartridge (conditioning, 2 mL MeOH; equilibration, 2 mL H2O– formic acid, 0.2%; loading, 3 mL sample in water–formic acid, 0.2%). Elution of the active compounds was carried out with 1 mL diluent (methanol–formic acid, 0.2%) in tube A. The samples were centrifuged and analyzed. As sample recoveries were not satisfactory, more flexible and convenient techniques were explored.
Thus, an attempt to find a solvent with broader extractive activity was carried out using methanol, acetonitrile and/or water in various proportions and buffers. In all cases, the purification procedure included the following steps: 9 mL of diluent was added to 1 mL of placebo sample, the mixture was treated for 15 min in an ultrasonic bath and then it was placed in the freezer for 20 min. This was followed by centrifugation for 5 min at 5000 rpm and the samples were analyzed. After thorough investigation, methanol with 0.2% formic acid was considered as the most suitable.
The treatment of the samples was further investigated by (a) varying the type and the time of the mixing procedure (simple mixing for 20 s in a Vortex device or 30 min stirring with a magnetic stirrer or 10 min magnetic stirrer and 5 min ultrasonic bath for one cycle, or repeating the first cycle for an additional 2–3 cycles) and (b) using the freezing technique at different times (20 min–3 h) [24] and centrifugation of the sample (2–10 min at 5000 rpm). The %recovery values were calculated based on a standard solution subjected to the same treatment. Considering the overall %recovery values of the APIs, the two-cycle procedure (20 min stirring, 10 min in ultrasonic bath, 20 min freezing and 5 min centrifugation) was selected as the most suitable (Figure S6, Supplementary Materials).
The %recovery of the active compounds applying the optimal processing conditions were calculated on five spiked samples which were analyzed by HPLC (2 replicates) under gradient elution conditions (Figure 1). According to the results, the mean %recovery values of the five samples for chlorogenic acid were the following: 101.5% (%RSD = 1.3%); 98.7% (RSD% = 1.2%) for caffeine; 102.5% (%RSD = 1.7%) for quercetin; 98.4% (%RSD = 1.5%) for hesperidin; 96.7% (%RSD = 1.3) for rutin; 99.7% (%RSD = 1.9%) for vitamin A; and 97.3% (%RSD = 1.2%) for vitamin E.
4. Conclusions
In the context of the present study, an emulsion was prepared with seven active substances (chlorogenic acid, rutin, quercetin, caffeine, caffeine, hesperidin, retinol and α-tocopherol), which could be a candidate cosmetic formulation with anti-aging properties. After determining the ingredients and composition of the emulsion (water, glycerin, jojoba oil and Span 20 in a ratio of 3.5:4.5:0.9:1.1), the formulation was prepared and its stability was studied for 30 days, based on the zeta potential (high charge values) and particle size (<222 nm).
For the quantitative determination of the active substances, a chromatographic method (RP-HPLC) was developed and validated, suitable for the evaluation of the quality of the formulation. Emphasis was placed on the pretreatment of the samples in order to achieve quantitative recovery of the seven antioxidants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11020043/s1, Figure S1: Chemical structures of antioxidants; Table S1. Concentrations of the standard solutions; Table S2: Emulsion samples with different compositions; Table S3: Design summary, limits and requirements used; Figure S2: Isometric curves of the overlay plot; Figure S3: Optimal quantities and expected responses, based on the desirability function; Figure S4: Emulsion stability study chart over a period of one month; Figure S5. Visual representation of stable emulsions after one month; Figure S6: Emulsion sample after purification procedure.
Author Contributions
Conceptualization, C.K.M. and G.K.; methodology, G.K., A.D. and C.K.M.; software, C.K.M.; validation, G.K. and C.K.M.; formal analysis, G.K., A.D. and C.K.M.; investigation, G.K., A.D. and C.K.M.; data curation, G.K. and C.K.M.; writing—original draft preparation, C.K.M.; writing—review and editing, G.K. and C.K.M.; visualization, G.K. and C.K.M.; supervision, C.K.M.; project administration, C.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article or supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Quan, T. Molecular insights of human skin epidermal and dermal aging. J. Dermatol. Sci. 2023, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kevin, M.G.; Taylor; Aulton, M.E. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 4th ed.; Churchill Livingstone: Edinburgh, Scotland, 2013. [Google Scholar]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Bora, J.; Tongbram, T.; Mahnot, N.; Mahanta, C.L.; Badwaik, L.S. Tocopherol. In Nutraceuticals and Health Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–278. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic Acids and Caffeine from Coffee By-Products: A Review on Skincare Applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Stanisic, D.; Costa, A.F.; Cruz, G.; Durán, N.; Tasic, L. Applications of Flavonoids, With an Emphasis on Hesperidin, as Anticancer Prodrugs: Phytotherapy as an Alternative to Chemotherapy. Stud. Nat. Prod. Chem. 2018, 58, 161–212. [Google Scholar] [CrossRef]
- Novotná, R.; Škařupová, D.; Hanyk, J.; Ulrichová, J.; Křen, V.; Bojarová, P.; Brodsky, K.; Vostálová, J.; Franková, J. Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents. Molecules 2023, 28, 1728. [Google Scholar] [CrossRef] [PubMed]
- Irondi, E.A.; Akintunde, J.K.; Agboola, S.O.; Boligon, A.A.; Athayde, M.L. Blanching influences the phenolics composition, antioxidant activity, and inhibitory effect of Adansonia digitata leaves extract on α-amylase, α-glucosidase, and aldose reductase. Food Sci. Nutr. 2017, 5, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hasnaa M., A.T.; El Aziz Hanan A., A.; Azza, A.E.L.H.; El Deen, K. Utilization of Chicory Plant for Supplementing Some Products. Curr. Sci. Int. 2023, 6, 777–787. Available online: https://www.curresweb.com/csi/csi/2017/777-787.pdf (accessed on 26 September 2023).
- Papadoyannis, I.N.; Tsioni, G.K.; Samanidou, V.F. Simultaneous Determination of Nine Water and Fat Soluble Vitamins After SPE Separation and RP-HPLC Analysis in Pharmaceutical Preparations and Biological Fluids. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 3203–3231. [Google Scholar] [CrossRef]
- Johnson, W. Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of trilaurin, triarachidin, tribehenin, tricaprin, tricaprylin, trierucin, triheptanoin, triheptylundecanoin, triisononanoin, triisopalmitin, triisostearin, trilinolein, trimyristin, trioctanoin, triolein, tripalmitin, tripalmitolein, triricinolein, tristearin, triundecanoin, glyceryl triacetyl hydroxystearate, glyceryl triacetyl ricinoleate, and glyceryl stearate diacetate. Int. J. Toxicol. 2001, 20 (Suppl. 4), 61–94. [Google Scholar] [PubMed]
- Fougère, B.J.; Wynn, S.G. Herb Manufacture, Pharmacy, and Dosing. In Veterinary Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2007; pp. 221–236. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 371–384. [Google Scholar] [CrossRef]
- Krstić, M.; Medarević, Đ.; Đuriš, J.; Ibrić, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; Elsevier Science: Amsterdam, The Netherlands, 2018; pp. 473–508. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Q2(R2) Guideline on Validation of Analytical Procedures. December 2023. Available online: www.ema.europa.eu/contact (accessed on 19 January 2024).
- Lister, A.S. 7 Validation of HPLC methods in pharmaceutical analysis. Sep. Sci. Technol. 2005, 6, 191–217. [Google Scholar] [CrossRef]
- Synaridou, M.S.; Tsamis, V.; Tsanaktsidou, E.; Ouranidis, A.; Kachrimanis, K.; Markopoulou, C.K. Response Surface and Freezing-Out Methodologies for the Extraction, Separation, and Validation of Seven Vitamins in a Novel Supplement with Determination by High-Performance Liquid Chromatography. Anal. Lett. 2023, 56, 1241–1255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).