Phytochemical Content and Anticancer Activity of Jamaican Dioscorea alata cv. White Yam Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Dioscorea Alata Tuber Samples
2.2. Preparation of Extracts
2.3. Phytochemical Analysis
2.3.1. Test for Phenols
2.3.2. Test for Alkaloids
2.3.3. Test for Tannins
2.3.4. Test for Glycosides
2.3.5. Test for Flavonoids
2.3.6. Test for Sterols
2.3.7. Test for Saponins
2.3.8. Test for Terpenoids
2.3.9. Test for Resins
2.3.10. Test for Carboxylic Acid
2.4. Cell Culture of DU145 Cells
2.5. Cell Viability of DU145 Cells
2.6. Antioxidant Capacity of Extracts Using 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
- AO: absorbance blank
- AS: sample absorbance
2.7. Polyphenol Quantification
- : Total phenolic content mg GAE/g dry extract
- : Concentration of gallic acid derived from calibration curve in mg/mL
- : Volume of extract in mL
- : Mass of extract in grams
2.8. High Performance Liquid Chromatography (HPLC)
2.9. Gas Chromatography Mass Spectrometry
3. Results
3.1. Dioscorea alata Solvent Extraction
3.2. Preliminary Phytochemical Assessment of Acetonic and Ethanoic Extracts of Dioscorea alata
3.3. Dioscorea alata Acetonic and Ethanolic Extracts Possess Antioxidant Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UWI Mona Discovers Anti Cancer Properties in Yams | Marketing and Communications Office, The University of West Indies at Mona. Available online: https://www.mona.uwi.edu/marcom/newsroom/entry/5775 (accessed on 6 January 2022).
- Lebot, V.; Lawac, F.; Legendre, L. The Greater Yam (Dioscorea Alata L.): A Review of Its Phytochemical Content and Potential for Processed Products and Biofortification. J. Food Compos. Anal. 2023, 115, 104987. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Kasai, T.; Kawabata, J. Nutritional Evaluation of Wild Yam (Dioscorea Spp.) Tubers of Nepal. Food Chem. 2003, 82, 619–623. [Google Scholar] [CrossRef]
- Srinivasan, S.; Koduru, S.; Kumar, R.; Venguswamy, G.; Kyprianou, N.; Damodaran, C. Diosgenin Targets Akt-Mediated Prosurvival Signaling in Human Breast Cancer Cells. Int. J. Cancer 2009, 125, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Cha, H.-J.; Hwang-Bo, H.; Kim, M.Y.; Kim, S.Y.; Ji, S.Y.; Cheong, J.; Park, C.; Lee, H.; Kim, G.-Y.; et al. Anti-Proliferative and Pro-Apoptotic Effects of Licochalcone A through ROS-Mediated Cell Cycle Arrest and Apoptosis in Human Bladder Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3820. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Dufie, W.-M.F.; Oduro, I.; Ellis, W.O.; Asiedu, R.; Maziya-Dixon, B. Potential Health Benefits of Water Yam (Dioscorea Alata). Food Funct. 2013, 4, 1496–1501. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Paradoxical Effects of Antioxidants on Cancer. Rejuvenation Res. 2014, 17, 306–311. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, E1544. [Google Scholar] [CrossRef]
- Singh, U.; Jialal, I. Oxidative Stress and Atherosclerosis. Pathophysiology 2006, 13, 129–142. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant Supplements for Preventing Gastrointestinal Cancers. Cochrane Database Syst. Rev. 2008, CD004183. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Tyagi, A.K.; Dhanalakshmi, S.; Agarwal, R.; Agarwal, C. Grape Seed Extract Inhibits Advanced Human Prostate Tumor Growth and Angiogenesis and Upregulates Insulin-like Growth Factor Binding Protein-3. Int. J. Cancer 2004, 108, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants Accelerate Lung Cancer Progression in Mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M. Unraveling the Truth about Antioxidants: Mitohormesis Explains ROS-Induced Health Benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Sheel, R.; Nisha, K. Qualitative Phytochemical Analysis for Isolation of Terpenes from Clerodendron Infortunatum Leaves. ISOR J. Appl. Chem. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.R.; Irfanullah; Sajid, M.; Zahra, Z. Phytochemical Investigation and Antimicrobial Appraisal of Parrotiopsis Jacquemontiana (Decne) Rehder. BMC Complement. Altern. Med. 2018, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Islam, M.E.; Jahan, N.; Islam, M.S.; Khan, A.; Islam, M.R.; Parvin, M.S. Antioxidant Activities of Ethanol Extracts and Fractions of Crescentia Cujete Leaves and Stem Bark and the Involvement of Phenolic Compounds. BMC Complement. Altern. Med. 2014, 14, 45. [Google Scholar] [CrossRef]
- Gizaw, A.; Marami, L.M.; Teshome, I.; Sarba, E.J.; Admasu, P.; Babele, D.A.; Dilba, G.M.; Bune, W.M.; Bayu, M.D.; Tadesse, M.; et al. Phytochemical Screening and In Vitro Antifungal Activity of Selected Medicinal Plants against Candida Albicans and Aspergillus Niger. in West Shewa Zone, Ethiopia. Adv. Pharmacol. Pharm. Sci. 2022, 2022, e3299146. [Google Scholar] [CrossRef]
- Shaikh, J.R.; Patil, M. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Bankole, A.E.; Adekunle, A.A.; Sowemimo, A.A.; Umebese, C.E.; Abiodun, O.; Gbotosho, G.O. Phytochemical Screening and in Vivo Antimalarial Activity of Extracts from Three Medicinal Plants Used in Malaria Treatment in Nigeria. Parasitol. Res. 2016, 115, 299–305. [Google Scholar] [CrossRef]
- Onwukaeme, D.N.; Ikuegbvweha, T.B.; Asonye, C.C. Evaluation of Phytochemical Constituents, Antibacterial Activities and Effect of Exudate of Pycanthus Angolensis Weld Warb (Myristicaceae) on Corneal Ulcers in Rabbits. Trop. J. Pharm. Res. 2007, 6, 725–730. [Google Scholar] [CrossRef]
- Kumar, S.; Venkateshwar, C.; Samuel, G.; Rao, S.G. Phytochemical Screening of Some Compounds from Plant Leaf Extracts of Holoptelea Integrifolia (Planch.) and Celestrus Emarginata (Grah.) Used by Gondu Tribes at Adilabad District, Andhrapradesh, India. Int. J. Eng. Sci. Invent. 2013, 2, 65–70. [Google Scholar]
- Siddique, A.B.; Rahman, S.M.M.; Hossain, M.A.; Hossain, M.A.; Rashid, M.A. Phytochemical Screening and Comparative Antimicrobial Potential of Different Extracts of Stevia Rebaudiana Bertoni Leaves. Asian Pac. J. Trop. Dis. 2014, 4, 275–280. [Google Scholar] [CrossRef]
- Wallace, K.; Asemota, H.; Gray, W. Acetone Extract of Dioscorea Alata Inhibits Cell Proliferation in Cancer Cells. Am. J. Plant Sci. 2021, 12, 300. [Google Scholar] [CrossRef]
- Wright, R.J.; Lee, K.S.; Hyacinth, H.I.; Hibbert, J.M.; Reid, M.E.; Wheatley, A.O.; Asemota, H.N. An Investigation of the Antioxidant Capacity in Extracts from Moringa Oleifera Plants Grown in Jamaica. Plants 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M. Extractable and Non-Extractable Antioxidants. Molecules 2019, 24, 1933. [Google Scholar] [CrossRef]
- Stennett, D.; Oladeinde, F.; Wheatley, A.; Bryant, J.; Dilworth, L.; Asemota, H. Effects of Dioscorea Polygonoides (Jamaican Bitter Yam) Supplementation in Normocholesterolemic and Genetically Modified Hypercholesterolemic Mice Species. J. Food Biochem. 2014, 38, 28–37. [Google Scholar] [CrossRef]
- Grindley, P.B.; Omoruyi, F.O.; Asemota, H.N.; St. A. Morrison, E.Y. Asemota Effect of Yam (Dioscorea cayenensis) and Dasheen (Colocassia esculenta) Extracts on the Kidney of Streptozotocin-Induced Diabetic Rats. Int. J. Food Sci. Nutr. 2001, 52, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Josheph Kumar, T. Roots and Tuber Crops as Functional Foods: A Review on Phytochemical Constituents and Their Potential Health Benefits. Available online: https://www.hindawi.com/journals/ijfs/2016/3631647/ (accessed on 14 September 2020).
- Das, A.; Chaudhuri, D.; Ghate, N.; Chatterjee, A.; Mandal, N. Phytochemical Analysis, Antioxidant and Anticancer Potential of Leaf Extracts from Edible Greater Yam, Dioscorea Alata L., from North-East India. Int. J. Phytopharm. 2014, 5, 109–119. [Google Scholar]
- Maithili, V.; Dhanabal, S.P.; Mahendran, S.; Vadivelan, R. Antidiabetic Activity of Ethanolic Extract of Tubers of Dioscorea Alata in Alloxan Induced Diabetic Rats. Indian J. Pharmacol. 2011, 43, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic Acid Inhibits Prostate Cancer Cell Proliferation and Metastasis by Suppressing the PI3K/Akt Pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef] [PubMed]
- Muzac-Tucker, I.; Asemota, H.N.; Ahmad, M.H. Biochemical Composition and Storage of Jamaican Yams (Dioscorea Sp). J. Sci. Food Agric. 1993, 62, 219–224. [Google Scholar] [CrossRef]
- Sakthidevi, G.; Mohan, V.R. Total Phenolic, Flavonoid Contents and In Vitro Antioxidant Activity of Dioscorea Alata l. Tuber. J. Pharm. Sci. Res. 2013, 5, 115. [Google Scholar]
- De Baere, S.; Eeckhaut, V.; Steppe, M.; De Maesschalck, C.; De Backer, P.; Van Immerseel, F.; Croubels, S. Development of a HPLC–UV Method for the Quantitative Determination of Four Short-Chain Fatty Acids and Lactic Acid Produced by Intestinal Bacteria during in Vitro Fermentation. J. Pharm. Biomed. Anal. 2013, 80, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, H.I.; Ganti, S.; Weiss, R.H. Chapter Fourteen-Metabolomic Profiling of Tumor-Bearing Mice. In Methods in Enzymology; Galluzzi, L., Kroemer, G., Eds.; Cell-Wide Metabolic Alterations Associated with Malignancy; Academic Press: Cambridge, MA, USA, 2014; Volume 543, pp. 275–296. [Google Scholar]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Anticarcinogenic Properties of Medium Chain Fatty Acids on Human Colorectal, Skin and Breast Cancer Cells in Vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Aiyelaagbe, O.O.; Negi, A.S.; Hamid, A.A.; Luqman, S.; Kumar, S.B.; Kaneez, F. Chemical Constituents from Alafia Barteri Oliv. Leaves with Cytotoxic Activity. J. Chin. Chem. Soc. 2015, 62, 751–755. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayı, H.; Hanoglu, A.; Yigit Hanoglu, D.; Ozkum Yavuz, D.; Ozek, T.; Vatansever, S. Fatty Acid Composition and Anticancer Activity in Colon Carcinoma Cell Lines of Prunus Dulcis Seed Oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Atasever-Arslan, B.; Yilancioglu, K.; Bekaroglu, M.G.; Taskin, E.; Cetiner, E.A.S. Cytotoxic Effect of Extract from Dunaliella Salina against SH-SY5Y Neuroblastoma Cells. Gen. Physiol. Biophys. 2015, 34, 201–207. [Google Scholar] [CrossRef]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric Acid Induce Cell Death in Colon Cancer Cells Mediated by the Epidermal Growth Factor Receptor Downregulation: An in Silico and in Vitro Study. Hum. Human. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef]
- Dey, P.; Chaudhuri, T.K. Phytochemical Characterization of Dioscorea alata Leaf and Stem by Silylation Followed by GC-MS Analysis. J. Food Biochem. 2016, 40, 630–635. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/jfbc.12235 (accessed on 24 January 2021).


| Time (min) | Flow Rate (mL/min) | Deionized Water (%) A | Acetonitrile (%) B |
|---|---|---|---|
| 0 | 1 | 75 | 25 |
| 5 | 1 | 50 | 50 |
| 10 | 1 | 25 | 75 |
| 20 | 1.5 | 0 | 100 |
| Fractions | Time (min) | Fractions | Time (min) |
|---|---|---|---|
| 0 | 3 | 6 | 2 |
| 1 | 3.5 | 7 | 2 |
| 2 | 3.5 | 8 | 2 |
| 3 | 2 | 9 | 2 |
| 4 | 2 | 10 | 2 |
| 5 | 2 | 11 | 5 |
| Phytochemicals | Acetone Extract (E-3) | Ethanol Extract (E-4) |
|---|---|---|
| Phenols | ++ | ++ |
| Alkaloids | + | + |
| Tannins | − | − |
| Sterols | + | ++ |
| Glycosides | ++ | ++ |
| Flavonoids | + | + |
| Saponins | ++ | + |
| Terpenoids | + | ++ |
| Carboxylic acid | ++ | + |
| Resins | − | + |
| Steroids | ++ | ++ |
| Extracts | Gallic Acid Equivalents (GAE) mg/g |
|---|---|
| E-3 | 31 ± 1.1 |
| E-4 | 72 ± 1.8 |
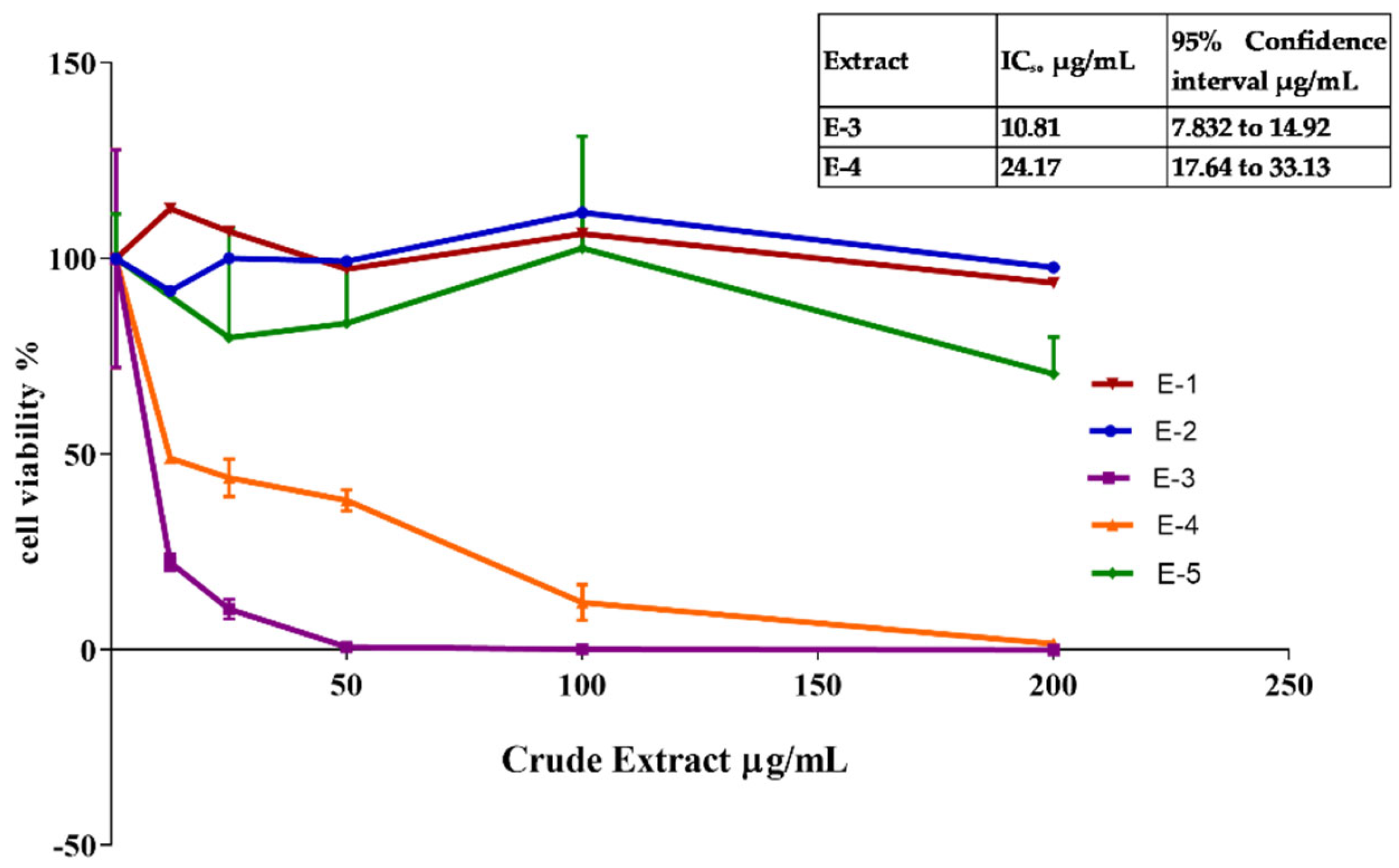
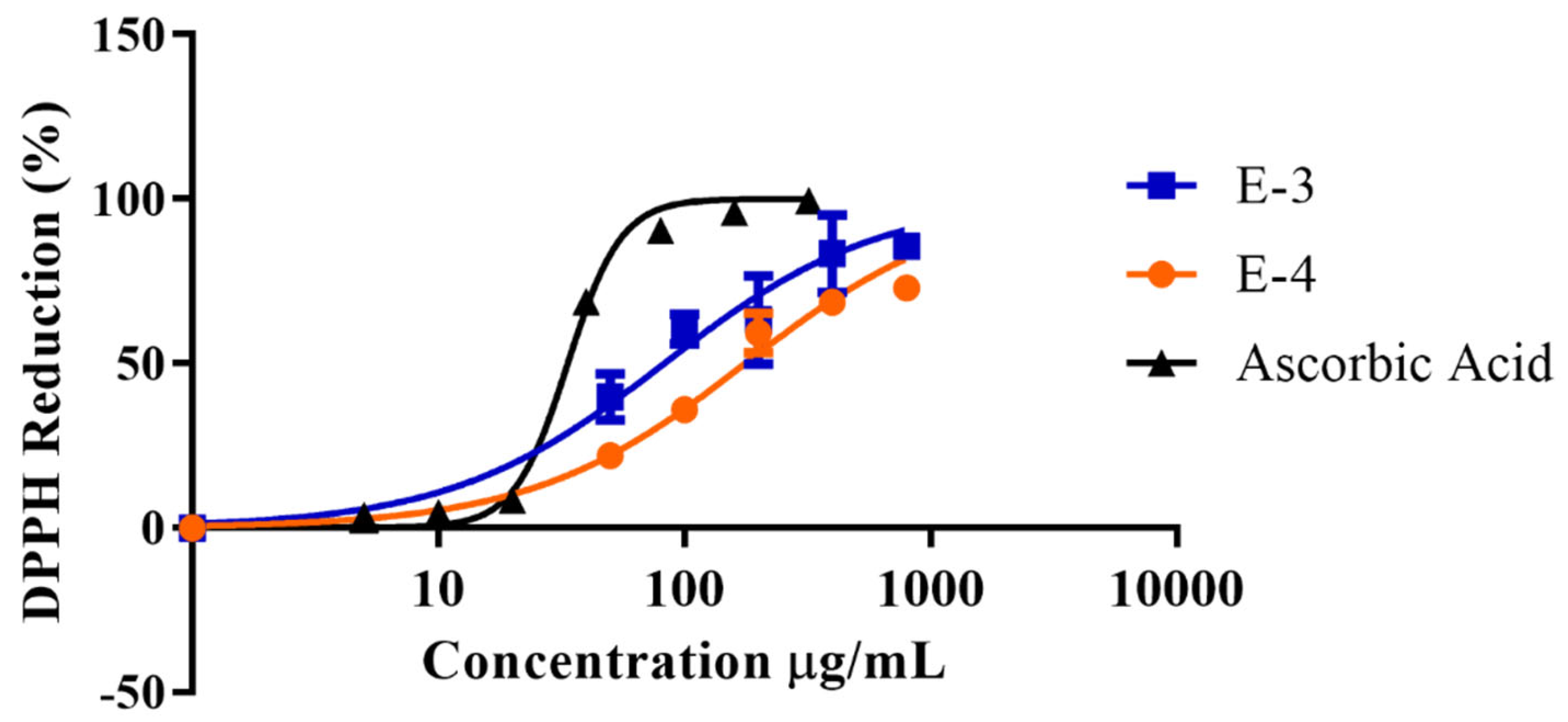
| Sample | IC50 (µg/mL) | 95% Confidence Interval |
|---|---|---|
| Ascorbic Acid | 33.6 | 31.98 to 35.34 |
| E-3 | 82.9 | 70.56 to 93.95 |
| E-4 | 166.9 | 140.4 to 204.3 |
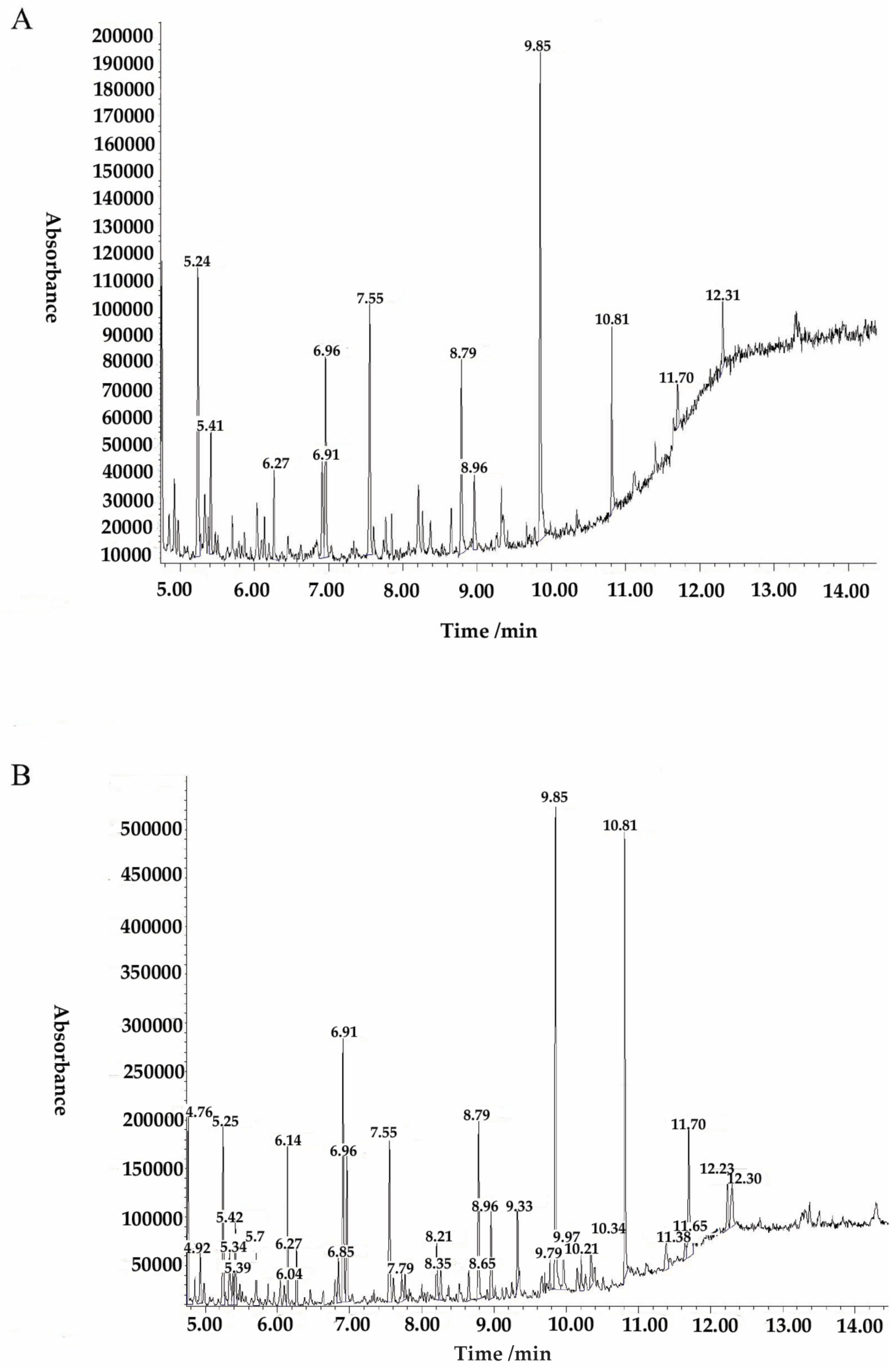
| Retention Time (min) | Abundance % | Name of Compounds | Molecular Mass g/mol |
|---|---|---|---|
| 5.24 | 9.59 | 1,2-Bis(trimethylsiloxy)ethane | 206.43 |
| 6.26 | 2.8 | Octanoic acid, trimethylsilyl ester | 216.39 |
| 6.91 | 3.62 | Butanedioic acid, bis(trimethylsilyl) ester | 262.45 |
| 6.96 | 6.37 | Nonanoic acid, trimethylsilyl ester | 230.42 |
| 7.55 | 8.57 | 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-methyl- | 192.17 |
| 8.79 | 5.82 | Dodecanoic acid, trimethylsilyl ester | 272.5 |
| 8.96 | 2.71 | Benzoic acid, 4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 282.48 |
| 9.85 | 16.18 | Azelaic acid, bis(trimethylsilyl)ester | 332.58 |
| 10.81 | 6.73 | Hexadecanoic acid, trimethylsilylester | 328.6 |
| 11.7 | 1.9 | Octadecanoic acid, trimethylsilylester | 356.7 |
| 12.31 | 2.74 | Dodecanamide | 199.33 |
| 11.7 | 1.9 | Heptadecanoic acid, trimethylsilylester | 342.6 |
| Retention Time (min) | Abundance % | Name of Compounds | Molecular g/mol |
|---|---|---|---|
| 4.76 | 3.34 | Propanoic acid, 2-(methoxyimino)-,trimethylsilyl ester | 189.28 |
| 5.25 | 3.24 | Ethanedioic acid, bis(trimethylsilyl) ester | 234.4 |
| 5.34 | 1.22 | Dithioerythritol, tetrakis(trimethylsilyl)- | 443 |
| 5.42 | 1.45 | Butanoic acid, 2-[(trimethylsilyl)amino]-, trimethylsilyl ester | 247.48 |
| 6.14 | 2.61 | Trimethylsilyl ether of glycerol | 308.64 |
| 6.26 | 0.92 | Octanoic acid, trimethylsilyl este | 216.39 |
| 6.91 | 4.96 | Butanedioic acid, bis(trimethylsilyl) ester | 262.45 |
| 6.96 | 3.04 | Nonanoic acid, trimethylsilyl este | 230.42 |
| 7.55 | 3.54 | Anthracene, 1-methyl- | 192.25 |
| 8.79 | 3.3 | Dodecanoic acid, trimethylsilyl ester | 272.5 |
| 8.96 | 1.73 | Benzoic acid, 4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 282.48 |
| 9.33 | 1.75 | Octanedioic acid, bis(trimethylsilyl) ester | 318.56 |
| 9.85 | 9.74 | Azelaic acid, bis(trimethylsily) ester | 332.58 |
| 9.96 | 1.55 | Arabinofuranose, 1,2,3,5-tetrakis-O-(trimethylsilyl)- | 150.13 |
| 10.21 | 0.98 | Trimethylsilyl ether of glycerol | 308.64 |
| 10.34 | 1.38 | .alpha.-D-Glucopyranoside, methyl2,3,4,6-tetrakis-O-(trimethylsilyl)- | 194.18 |
| 10.81 | 8.58 | Hexadecanoic acid, trimethylsilylester | 328.6 |
| 11.38 | 1.05 | Dodecanamide | 199.33 |
| 11.64 | 0.89 | Oleic acid | 282.5 |
| 11.7 | 4.03 | Octadecanoic acid, trimethylsilylester | 356.7 |
| 12.23 | 3.39 | .beta.-D-Galactopyranose, 1,2,3,4,6-pentakis-O-(trimethylsilyl)- | 541.1 |
| 12.3 | 3.54 | Benzeneethanamine, 2-fluoro-.beta.,3,4-trihydroxy-N-isopropyl- | 229.25 |
| 14.29 | 1.42 | 1H+R6:R27ido [1,2-a]quinoline-2-carboxylic acid, 5,6-dihydro-1-oxo-, methyl ester | 256.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, K.; Wright, R.; Williams-Longmore, M.; Wright, S.-G.; Asemota, H. Phytochemical Content and Anticancer Activity of Jamaican Dioscorea alata cv. White Yam Extracts. Separations 2024, 11, 44. https://doi.org/10.3390/separations11020044
Wallace K, Wright R, Williams-Longmore M, Wright S-G, Asemota H. Phytochemical Content and Anticancer Activity of Jamaican Dioscorea alata cv. White Yam Extracts. Separations. 2024; 11(2):44. https://doi.org/10.3390/separations11020044
Chicago/Turabian StyleWallace, Kenroy, Racquel Wright, Melisa Williams-Longmore, Sasha-Gay Wright, and Helen Asemota. 2024. "Phytochemical Content and Anticancer Activity of Jamaican Dioscorea alata cv. White Yam Extracts" Separations 11, no. 2: 44. https://doi.org/10.3390/separations11020044
APA StyleWallace, K., Wright, R., Williams-Longmore, M., Wright, S.-G., & Asemota, H. (2024). Phytochemical Content and Anticancer Activity of Jamaican Dioscorea alata cv. White Yam Extracts. Separations, 11(2), 44. https://doi.org/10.3390/separations11020044





