Abstract
A quantitative analytical method for PFAS determination in airborne particulate matter (PM) has been developed using liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS), allowing for the determination of 33 compounds. The procedure was applied to ambient PM10 with limits of quantification for PFAS in the fg m−3 range. PM10 samples collected during a year-long campaign conducted in an urban site in Umbria (Central Italy) have been characterized for their PFAS content. Among the seven detected PFASs, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) were the most abundant compounds. Furthermore, this work allowed us to obtain the first seasonal trend of airborne PFASs in Central Italy. Seasonal trend analysis shows that PFAS concentration in the atmosphere peaks in summertime. A comparison with trends of other relevant primary and secondary air pollutants determined at the sampling site suggests a secondary nature of the observed PFAS, which are formed in the atmosphere directly from gaseous precursors and can contribute to worsen the urban air quality in summertime.
1. Introduction
Perfluroalkyl and polyfluoroalkyl Substances (PFAS) are a broad group of more than 14,000 compounds [1] defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e., any chemical with at least a perfluorinated methyl group (−CF3) or a perfluorinated methylene group (−CF2−) (with a few noted exceptions) [2]. Fluorine atoms, instead of hydrogen, make these substances thermally stable and chemically inert. Thus, they can be used to produce paper and food contact materials, cables and wiring, tapes, paints and varnishes, household products, lubricants and sealing agents, electronics and rechargeable batteries, textiles, and more. However, these properties make PFASs highly persistent in the environment and mobile across soil, water, and air compartments due to their volatile/semi-volatile behavior and amphiphilic properties. Moreover, some of those compounds can bioaccumulate in biota and induce adverse effects on living organisms [3,4,5]. Therefore, some were recently inserted into the Stockholm Convention on persistent organic pollutants [6,7], and interlaboratory tests were performed and reported in the scientific literature [8]. Several different sources of PFASs in the environment have been individuated and classified as direct sources, including industrial production of PFAS or PFAS-related materials and indirect sources, which are related to the formation of PFAS in the environment from precursor molecules, such as, for example, fluorotelomer substances [9]. Different monitoring plans and guidelines were outlined to evaluate the diffusion in environmental and food matrices and the exposure and effects of these substances on human health. The United Nations published guidance to provide an overview of challenges regarding the analysis of perfluorpalkyl and polyfluoroalkyl substances, focusing on PFOS (perfluoro octane sulfonic acid) in the water column and on setting guidelines for international monitoring programs [10]. The U.S. Environmental Protection Agency drafted a PFAS strategic roadmap to safeguard communities from PFAS contamination [11] and, in this context, also included a study to increase the understanding of the fate and transport of PFAS air emissions. The exposure to PFASs through airborne dust [12,13] or atmospheric transport from production plant emissions to urban areas [14,15] is well-documented, and some regular air-monitoring plans [16,17] were prepared as a consequence. Perfluorooctanoic acid (PFOA) and PFOS are legacy long-chain PFAs and have been gradually eliminated in most developed countries due to their toxic effects on wildlife and human beings. Surprisingly, PFOA and PFOS are still the most abundant compounds observed in atmospheric aerosols, even if their concentration levels are lower than those observed near an industrial site in China, where production is ongoing [18]. One possible explanation is that neutral perfluorinated acids can be formed directly in the atmosphere from acyl halides that, in turn, may be derived from the hydrolysis of a series of anthropogenic compounds, which include surfactants, fluoropolymers, coolants, and fire suppressors [19,20]. Clearly, more research is needed to advance an exhaustive understanding of PFASs’ role in air quality, including, for example, the mechanism of atmospheric formation and the role of precursors [19,20]. From an analytical point of view, the large number of compounds and low atmospheric concentrations pose a challenge to determining the total amount of PFASs (see i.e., [21,22]), and additional analytical methods are also desirable. Finally, long-term monitoring of PFASs in PM should be expanded to include more measurement sites in undersampled regions worldwide.
The main aims of this work were (1) to optimize an analytical method for PFAS determination in air filter samples, (2) to apply the protocol to PM10 samples collected in an urban environment in Central Italy, an area with very scarce datasets on PFAS atmospheric concentration, and (3) to perform an analysis of PFAS seasonal trends and correlations with some other relevant air-quality parameters in the context of a year-long monitoring campaign.
2. Materials and Methods
2.1. Reagents and Materials
LC-MS grade methanol and concentrated ammonia (30%) were purchased from Honeywell (Charlotte, North Carolina, USA). LC-MS grade acetonitrile (ACN) was supplied by Carlo Erba (Milano, Italy). Acetic acid and sodium acetate were purchased from VWR International Srl (Radnor, Pennsylvania, USA). n-nonane, ammonium acetate, and ENVI-Carb™ SPE Bulk were purchased from Sigma–Aldrich (St. Louis, MO, USA). Ultra-pure water was produced using a Milli-Q purification apparatus (Millipore, Bedford, MA, USA). SPE Strata-X-AW (200 mg, 6 mL) was from Phenomenex (Torrance, CA, USA), as well as the LC column (Kinetex XB-C18 100A, 100 × 3 mm 2.6 μm). Oasis HLB (2.1 × 20 mm, five μm, code 186002034) and Oasis WAX (2.1 × 20 mm, five μm code 1860022508) were obtained from Waters (Milford, MA, USA). PFAS native and labeled standards (internal standards, ISs) were supplied by Wellington Laboratories (Guelph, ON, Canada) (Table 1).

Table 1.
List of the 33 analytes and their Internal Standards (IS).
2.2. Sample Collection
The PM10 sampling campaign started in November 2015 and lasted until December 2016 in the city of Terni (Central Italy), a well-known environmental hot spot [23,24,25,26] due to the historical presence of many chemical and steel-production plants in an urban environment. A high-volume PM10 impactor (TISCH, TE6001), operated at 1440 L min−1, was deployed on the roof (3 m above the ground) of an urban air-quality cabin (Carrara, latitude 42°33′38″ longitude 12°39′3″). Seventy-five daily PM10 samples were collected on quartz filters (Whatman, Marlborough, MA, USA, QMA 20.3 ×25.4 cm), uniformly covering the four seasons. A set of ancillary meteorological data and air-quality parameters, including NOX (Teledyne T200), O3 (Teledyne T400), CO (Teledyne T300), benzene, toluene, and xylene (VOC BTX analyzer, Chromatotec) concentrations were also recorded.
2.3. Organic Carbon and Elemental Carbon Analysis in PM10
A substantial amount of organic matter, OM, deposited on PM10 filters can influence the PFAS determination [22]. Therefore, the PM10 samples were analyzed using an Organic Carbon/Elemental Carbon (OC/EC) Laboratory Instrument (Model 5L, Sunset Laboratory Inc.) to apply the EUSAAR_2 protocol [27,28]. A punch of 1 cm2 was used for the EC/OC analysis, and the accuracy of the measurements was tested using a sucrose solution and found to be within ±10%. OM has been evaluated from OC utilizing a factor of 1.6 for urban sites in Italy [29].
2.4. Sample Treatment
A portion of 4 cm2 (2 × 2 cm) was cut from a quartz filter and spiked at 12.5 pg cm−2 with the mixture of ISs (5 μL of a solution at 10 ng mL−1). After adding 3 mL of UP water, the sample was extracted with 7 mL of ACN for 15 min, sonicated for 15 min, and centrifuged at 10,000 rpm for 10 min. The supernatant was transferred to a 15 mL tube, and the extraction was repeated using 5 mL of ACN containing 0.75% acetic acid, sonicated, and centrifuged. The reunited supernatants were evaporated until about 5 mL under a nitrogen stream (40 °C). The clean-up and instrumental conditions were the same as described by Barola et al. 2020 [30]. Briefly, 40 mL of UP water were added to each sample. Strata-X-AW SPE cartridges were conditioned with 6 mL of MeOH and 6 mL of water and rinsed three times with the eluting mixture (3 × 6 mL of 2% NH4OH in MeOH). The SPE cartridges were then reconditioned with 6 mL of MeOH and 6 mL of water. After sample loading, the cartridges were washed with 6 mL of sodium acetate 25 mM (pH = 4), 4 mL of MeOH 40%, and 6 mL MeOH and dried under vacuum. The analytes were eluted with 6 mL of 2% NH4OH in MeOH into a 15 mL tube containing 80 mg of d-SPE ENVI-CarbTM and 100 μL of acetic acid. After shaking and centrifugation, the supernatant was transferred into a 15 mL tube containing 50 μL of n-nonane. After evaporation, 0.2 mL of 80:20 MeOH/ammonium acetate four mM were added, and the sample was injected into the LC-HRMS system.
2.5. Chromatographic and MS Conditions
The analyte separation was performed on a Thermo Ultimate 3000 HPLC system (Thermoscientific, San Jose, CA, USA) equipped with a Kinetex XB C18 column (100 × 3.0 mm; 2.6 μm). Between the pump and injector, an Oasis WAX (20 × 2.1 mm) and Oasis HLB (20 × 2.1 mm) were installed. Mobile phases were UP water (A) and methanol (B), containing two mM of ammonium acetate. The gradient started with 100% eluent A at 0.05 mL min−1. In 1 min, the flow increased to 0.3 mL min−1, and the eluent B increased to 20%. This condition was maintained for 2 min and followed by a linear increase of B up to 70% in 8 min. This condition was maintained for 4 min. A successive linear increase to 100% of the mobile phase B followed for the next 6 min. After 2 min, the flow returned to 0.05 mL min−1, and the system returned to 100% A in 5 min. A re-equilibration step of 2 min followed for a total run time of 30 min. The column and sample temperatures were set at 40 °C and 16 °C, respectively. The injection volume was 20 μL. The mass analyzer Q-Orbitrap (Thermo Scientific, Waltham, MA, USA) was equipped with a heated electrospray ionization source (HESI-II), operating in negative polarity mode. The optimized HESI-II temperature was set at 320 °C, the capillary temperature was set at 300 °C, the electrospray voltage was set at 3.5 kV, and the S-lens value was adjusted at 50 V. Sheath and auxiliary gas were 40 and 15 arbitrary units, respectively. The extraction mass window was five ppm. The method allowed for the identification and quantification of the 33 PFAS reported in Table 1. Further details are reported in Table 2.

Table 2.
Monitored ion species and retention times (RT).
2.6. Quality Control (QC)
In each analytical batch, along with the filter samples collected at the urban site, one reagent blank, one field blank filter, two fortified samples (6.25 and 12.5 pg cm−2), and one matrix-matched standard and solvent standard curve were injected. Calibration curve was built in solvent (MeOH/ammonium acetate 4 mM 80/20 v/v) at 5, 25, 125, 500, and 2500 pg/mL (concentration of ISs: 500 pg/mL). Reagent blank (procedural blank) and field blank filter were used to monitor the possible laboratory contamination due to solvents, consumable parts (e.g., tubes, SPE cartridges, etc), mobile phases, and sampling materials. The two spiked samples (6.25 pg cm−2 and 12.5 pg cm−2) included in each batch were used as quality control (QC) and as an ongoing assessment of method performances. In addition, to estimate LOQs (limits of quantification), three spiked samples at 0.25, 0.5, and 1 pg cm−2 were analyzed in triplicate. LOQs were set at the lowest level of spiking with trueness (apparent recovery) included in the range 65–135% and precision less or equal to 25% (intermediate precision) according to Ref. [31]. Trueness and CV% in Table 3 were obtained from QCs.

Table 3.
Analyte and related labeled internal standard, LOD (pg/cm2), LOQ (pg/cm2), trueness (%), and CV%.
2.7. Statistical Analysis
All the statistical analyses were conducted using the R 4.2.2 statistical framework (R Core Team) within the RStudio integrated development environment
3. Results and Discussion
3.1. Optimization of Sample Preparation
In the optimization procedure, we aimed to reduce the filter’s fraction dedicated to the PFAS determination as much as possible because we aimed to use the rest of the filter for other analyses. The total loaded surface of the air filter samples was 405 cm2. A filter portion of 4 cm2 was chosen for the present analysis, which provided a satisfactory sensitivity. For the sample extraction, ACN was used to prevent the esterification of carboxylate substances that could react in an acidic condition in the presence of methanol to form the related methyl ester compound. The two purification steps guaranteed the selective extraction of acidic compounds (weak anion exchange solid phase extraction—SPE) and the removal of aromatic compounds through a pi–pi interaction with graphitized carbon (dispersive EnviCarb bulk) [32]. In order to prevent or at least minimize the contamination from the sampling preparation, SPE cartridges were washed three times before sample loading with methanol (2% of ammonium hydroxide) to remove traces of PFAS contained in the plastic support and the stationary phase of the WAX column [30]. Moreover, the final solution composition (MeOH/AA 4 mM 80/20 v/v) makes the most lipophilic substances (C16-C18) stable in this condition. Table 3 reports method LOQs, trueness, and precision (CVs evaluated in lab reproducibility conditions). Linearity was verified from 0.25 pg/cm2 to 2500 pg/cm2. The matrix effect, evaluated by comparing analyte signals in matrix-matched standards and solvent standards, was negligible for all the analytes. Considering that the typical sampled air volume was 1600 m3, LOQ ranged from 60 to 120 fg m−3, depending on the analyte.
3.2. PFAS Concentration and Composition Profiles
Seventy-five PM10 filter samples from the Terni site were analyzed for PFAS determination, as described above. Among the 33 searched PFASs listed in Table 1, only seven compounds were identified—specifically PFBA, PFHxA, PFHpA, PFOA, PFDA, 6:2FTS, and PFOS—and six quantified above LOQ, which is consistent with previous studies’ results [21], except for PFBA, for which the LOQ was outside the desired precision range. Concentrations are provided in Table 4, which also includes the PFAS sum, along with the OM contents. Due to the presence of measurable background concentrations of some PFASs (PFBA, PFBS, PFHxA, PFHpA, 6:2FTS, PFOA, PFNA, PFOS, and PFDA) in laboratory and field blank filters, the PFAS concentrations are furnished after the subtraction of the mean background concentrations, plus three times their standard deviation. The average background is of the order of 25% of the measured concentrations.

Table 4.
PM10, OM, and PFAS concentrations (n = 75 samples) from Terni in 2016.
A descriptive statistic of compound concentrations is presented in Table 4, including the total concentration of PFAS, calculated as the sum of the detectable compounds and the organic matter content. PFAS distributions are slightly positively skewed. It is known [33] that, for aqueous samples, OM may produce an interference connected with the retention performances of the SPE cartridge, which may affect more hydrophilic compounds. This effect occurs for OM levels much higher than the PFAS concentration, i.e., in the order of ppm. To exclude this potential artifact from the present atmospheric samples, we determined the OM abundance in the PM10 filters. The obtained OM abundances are reported in Table 4 and were found to be in a low concentration range, i.e., ppb, which is only slightly higher with respect to the concentration of the targeted PFAS compounds. The fluorotelomer sulfonate 6:2FTS was only detected in three samples restricted to springtime. Therefore, it is not reported in Table 4 because it is statistically not significant on a yearly based dataset.
3.3. General Trends of Air Quality in Terni during the Sampling Campaign
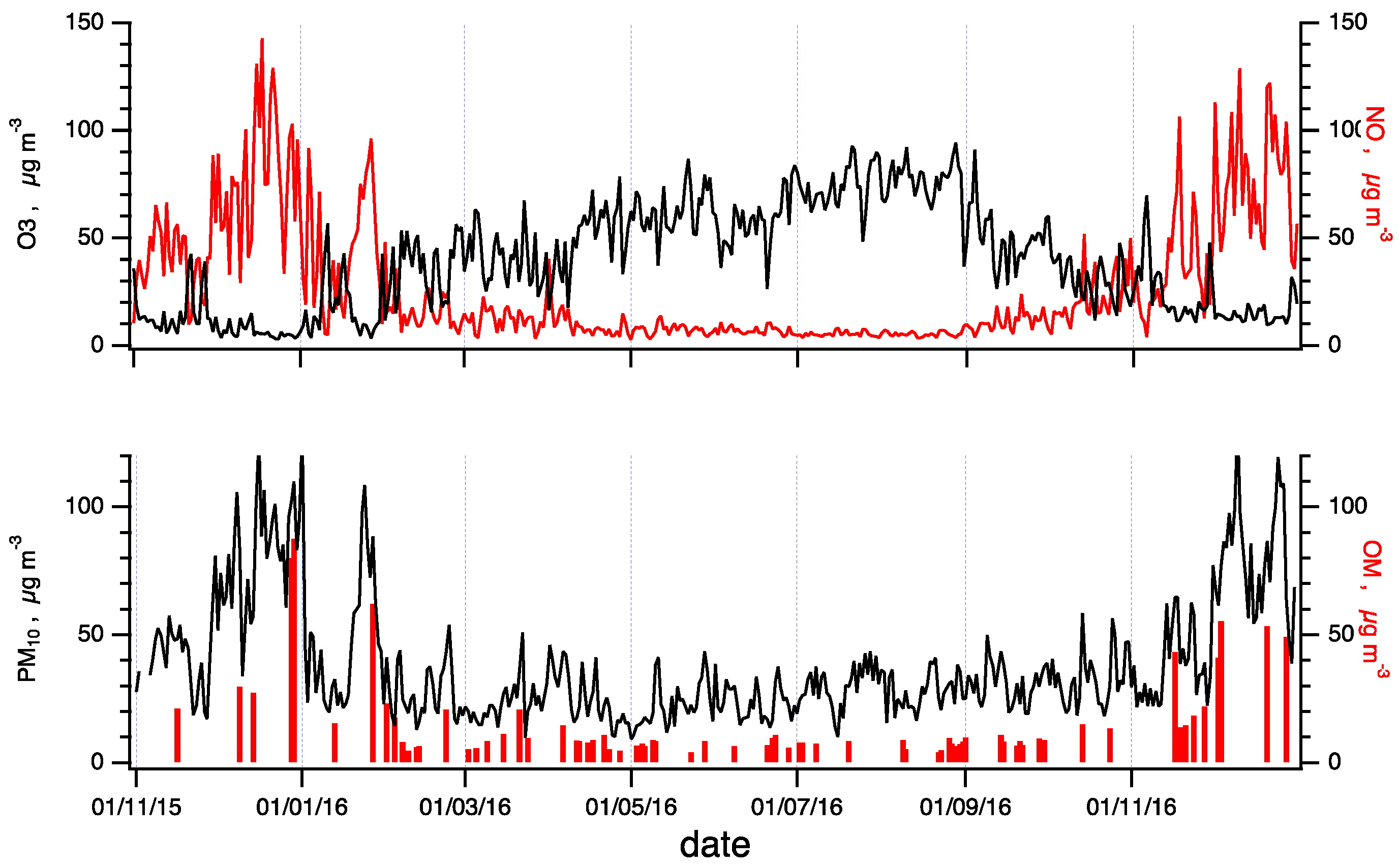
Timelines of PM10, the mass concentration of particulate matter, and OM, the organic matter fraction of PM10, are shown in the lower panel of Figure 1. They both show wintertime maxima, aligning with a general phenomenology of PM10 in Italy [34]. This trend is typical of basin valleys enclosed by mountain ranges, where cities and industries are often established. These valleys are territories featuring low wind speeds and atmospheric stability conditions, which may promote the formation of strong vertical aerosol gradients in the troposphere and a very shallow atmospheric boundary layer [23,24,25].
Figure 1.
General air-quality trends in the city of Terni in the observation period. Upper panel: timelines of ozone, O3 (black), nitrogen monoxide, and NO (red) concentrations; lower panel: PM10 (black) and its organic matter content (OM; red).
Timelines of two important gaseous pollutants monitored at the sampling site, ozone (O3) and nitric oxide (NO), are plotted in the upper panel of Figure 1. NO is a primary pollutant directly emitted from combustion sources such as vehicular traffic, domestic heating systems, and industrial plants. The NO concentration shows a wintertime maximum and a substantial decline in the summertime, mainly related to the effective oxidation cycle that promotes the O3 formation, which is a secondary photochemical pollutant that accumulates in the summertime.
Therefore, the air quality in the Terni basin in the wintertime is dominated by meteorological conditions and by photochemistry in the summertime.
3.4. PFAS Concentration and Composition Profiles
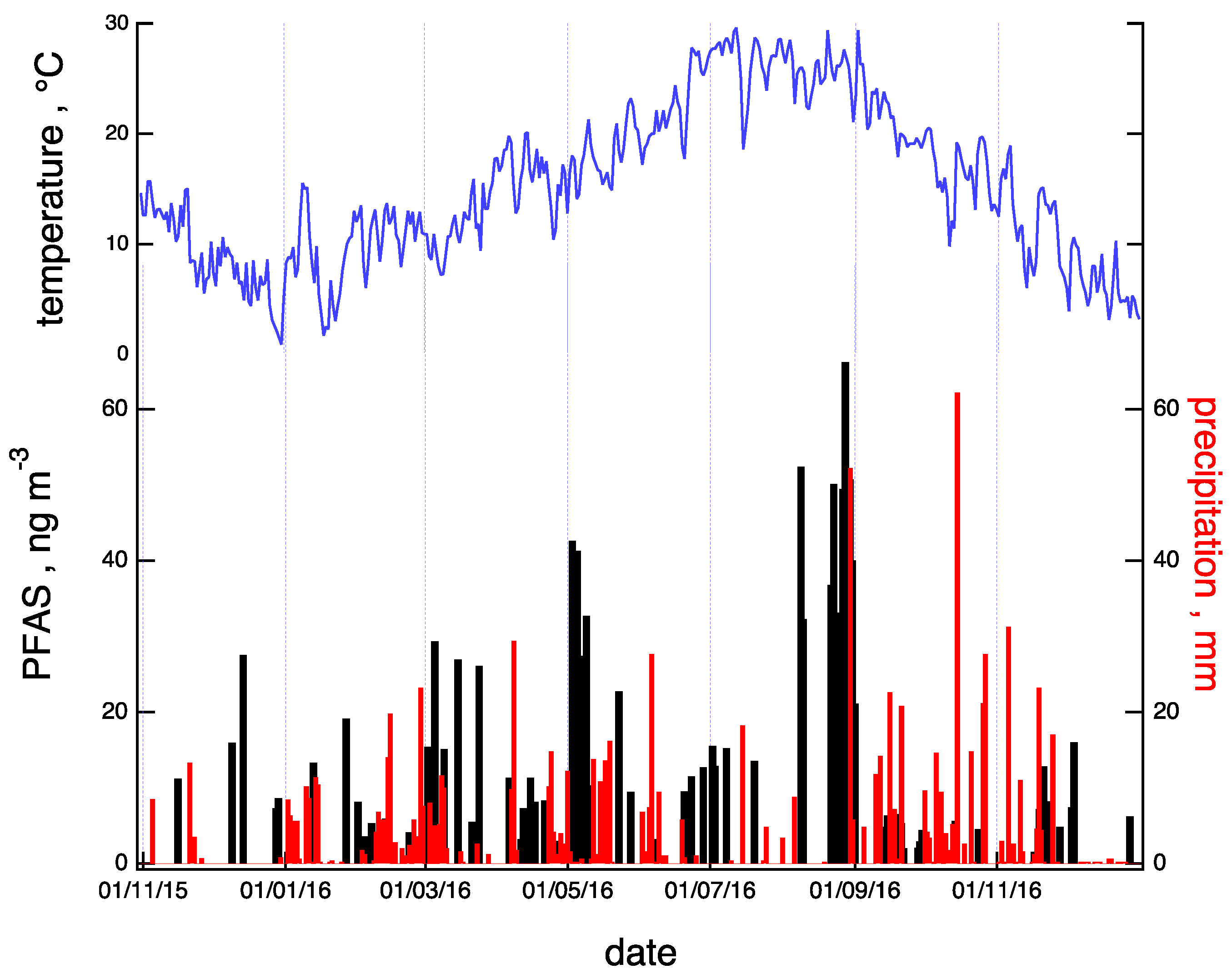
PFOA was the most abundant compound with concentrations ranging from 0.2 to 26.1 pg m−3 (median: 4.8 pg m−3). These results are comparable to those recorded at other urban sites such as Manchester (15.7 pg m−3), Beijing (12.5 pg m−3), Zurich (7.7 pg m−3), Tsukuba (2.6 pg m−3), and Kjeller (1.54 pg m−3), but they are much lower than the values measured near fluorochemical manufacturing plants such as Hazelrigg (552 pg m−3), Manchester (341 pg m−3 ), and Changshu (556 pg m−3) [35,36,37,38]. Slightly lower concentrations were measured for PFOS: from 0.2 to 22.6 pg m−3 (median: 1.2 pg m−3), values within the range of those were recently measured worldwide [15]. The timeline of the total PFAS concentration, expressed as the sum of the seven detected compounds (see Section 3.2), is shown in the upper panel of Figure 2 (black bars) for the observation period (November 2015–December 2016). The value of precipitation (red sticks) registered at the site is also reported in the same plot. The PFAS concentration shows an increasing trend from winter to summer and a decline in the successive autumn and winter periods. Indeed, a marked decline in PFAS concentration appeared after prolonged precipitation periods (such as those registered in May and September), suggesting an efficient role of rain as a scavenger of neutral PFAS, both in the gas and particulate phases [39].
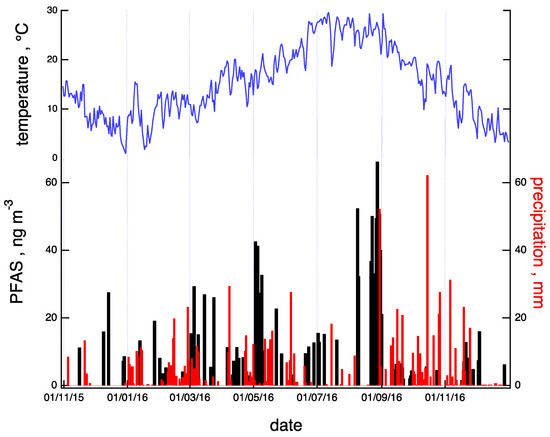
Figure 2.
Timelines of total PFAS concentration (PFAS; black) and precipitation (red) for the observation period. In the upper panel (blue), the yearly trend of temperature at the sampling site is reported.
3.5. PFAS Daily and Seasonal Trends
The timeline of the total PFAS concentration, expressed as the sum of the seven detected compounds (see Section 3.2), is shown in the lower panel of Figure 2 (black bars) for the observation period (November 2015–December 2016). The values of precipitation (red sticks) and temperature (blue line) registered at the site are also reported in the same plot. The PFAS concentration shows an increasing trend from winter to summer and a decline in the successive autumn and winter periods. The PFAS concentration is moderately positively correlated (R = 0.4) with the temperature. Indeed, a marked decline in PFAS concentration appeared after prolonged precipitation periods (such as those registered in May and September), suggesting an efficient role of rain as a scavenger of neutral PFAS, both in the gas and particulate phases [39].
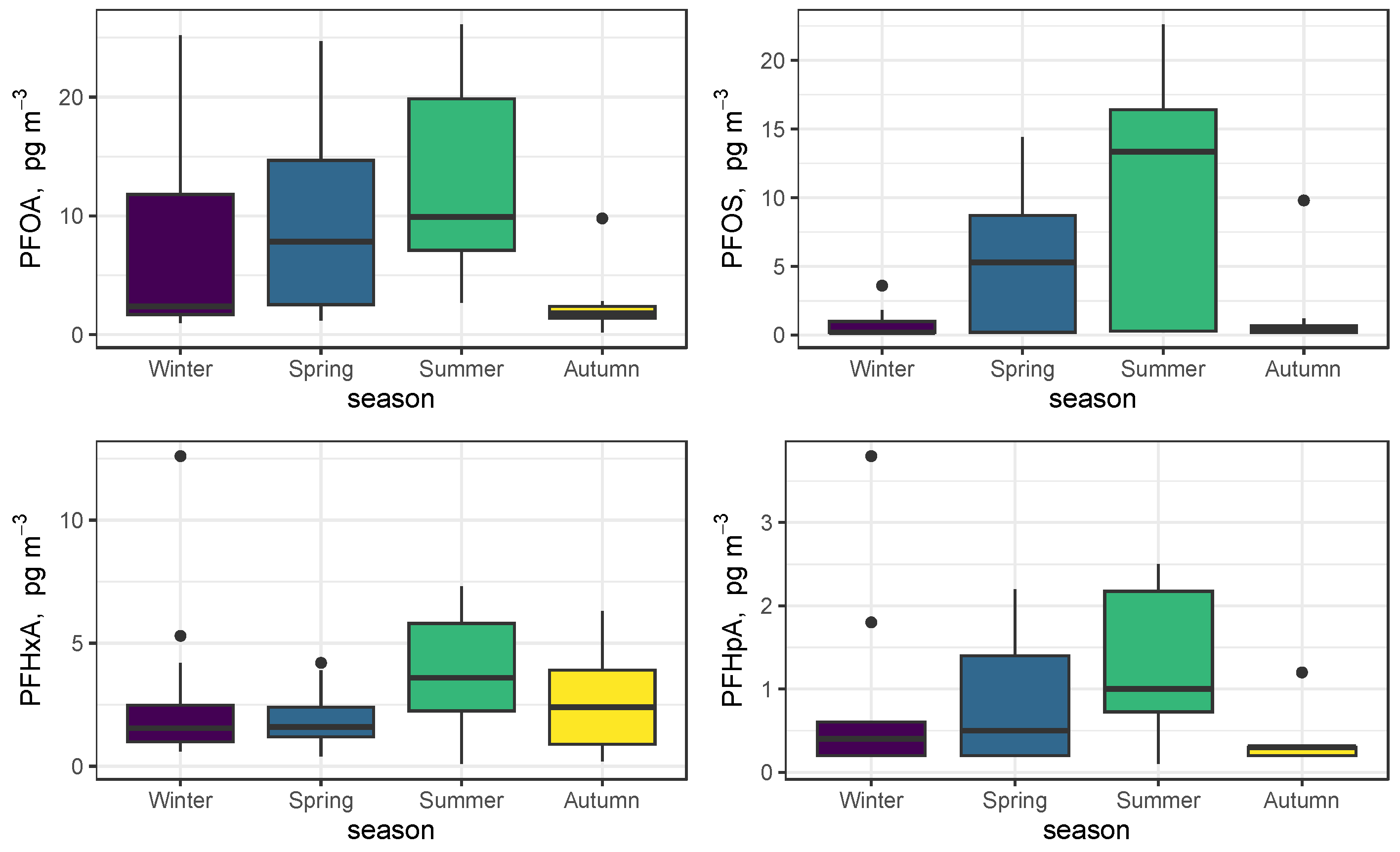
The seasonal trends at the site are better summarized as seasonal boxplots and are shown in Figure 3 for the four most abundant PFASs (PFOA, PFOS, PFHxA, and PFHpA). Conventional meteorological seasons have been used. All four compounds have a summer maximum and show an increasing trend from winter to summer, with a decline in autumn [40]. This trend is more pronounced for PFOS than for PFOA, which shows greater variability in the winter period. As a consequence, the ratio of the median values of the two most important C8 compounds, PFOA and PFOS, reads 9.5 in winter and declines to 1.3 in the summertime.
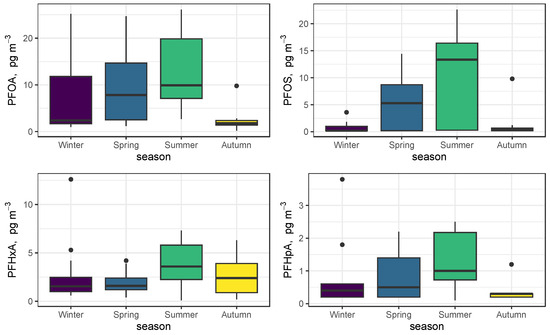
Figure 3.
Seasonal trends of PFOA, PFOS, PFHxA, and PFHpA.
We noticed that the seasonal trend of PFAS in PM10 is opposite to that of PM10 itself, which results in one order of magnitude enhancement of the mass-to-mass concentration of PFAS in PM10 in the summertime. PFASs are positively correlated (R = 0.46) with O3., a typical secondary pollutant of a photochemical nature. Moreover, the most abundant PFOAs and PFOSs are not correlated with the typical vehicular traffic markers, such as benzene (R = −0.03 and R = −0.21, respectively), toluene (R = 0.01 and R = −0.16), CO (R = −0.1 and R = −0.27), and NO (R = −0.06 and R = −0.20). Altogether, these results agree with a secondary nature of the observed neutral PFAS, particularly in the summertime. This is consistent with a photochemical formation in the atmosphere from precursors emitted at the sources [20,39,40]. These precursors include fluorotelomer alcohols, which oxidatively degrade in the atmosphere to yield PFOA and have been, and continue to be, used extensively in the industry for various purposes, from stain repellents to firefighting foams. Heat waves, wildfires, and photochemical processes may impact summertime air quality [41,42,43] and are currently of great concern because significant gaps in knowledge are present. As a result of the present study, we suggest that, in addition to what is mentioned above, the elevated concentrations of PFAS may also contribute to lowering the air quality in the summertime.
4. Conclusions
A method for PFAS determination in airborne particulate matter sampled using quartz filters has been optimized, allowing for the determination of 33 compounds with quantification limits mainly ranging from 0.25 to 1 pg cm−2. The procedure was then applied to ambient PM10 samples collected during a year-long campaign conducted in an urban site in Central Italy. PFOA and PFOS were the most abundant PFASs. Even if their production was phased out in the U.S. and Europe, these compounds and their precursors are still manufactured in some countries, and there is no regulation about their release in the environment. Seasonal atmospheric trends showed that PFAS concentrations peak in summer, suggesting a secondary nature of PFOA and PFOS, probably due to the transformation of precursors directly into the atmosphere. The mass-to-mass concentration of PFAS in PM10 in the summertime is 10 times higher than in the wintertime.
Author Contributions
Conceptualization, D.C. and S.M.; methodology, R.G., S.M. and D.C.; investigation, S.M., S.C., C.B., E.B., C.P., R.S. and M.G.; resources, R.G. and D.C.; data curation, D.C.; writing—original draft preparation, D.C.; writing—review and editing, R.G., S.M. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data presented in the present paper are available upon request.
Acknowledgments
We acknowledge the support of ARPA Umbria in the management of the sampling station in Terni.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Available online: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm (accessed on 17 January 2024).
- Available online: https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/terminology-per-and-polyfluoroalkyl-substances.pdf (accessed on 17 January 2024).
- Contaminants of Emerging Concern in the Marine Environment, 1st ed.; Leon, V.M., Bellas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–228. ISBN 9780323902977. [Google Scholar]
- Keller, J.M.; Ngai, L.; McNeill, J.B.; Wood, L.D.; Stewart, K.R.; O’Connell, S.G.; Kucklick, J.R. Perfluoroalkyl contaminants in plasma of five sea turtle species: Comparisons in concentration and potential health risks. Environ. Toxicol. Chem. 2012, 31, 1223. [Google Scholar] [CrossRef]
- Zodrow, J.; Vedagiri, U.; Sorell, T.; McIntosh, L.; Larson, E.; Hall, L.; Dourson, M.; Dell, L.; Cox, D.; Barfoot, K.; et al. PFAS Experts Symposium 2: PFAS Toxicology and Risk Assessment in 2021—Contemporary issues in human and ecological risk assessment of PFAS. Remediation 2022, 32, 29–44. [Google Scholar] [CrossRef]
- UNEP. 2021 Proposal to List Long-Chain Perfluorocarboxylic Acids, Their Salts and Related Compounds in Annexes A, B And/or C to the Stockholm Convention on Persistent Organic Pollutants, UNEP/POPS/POPRC.17/7. Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland. 2021. Available online: http://chm.pops.int/Convention/POPsReviewCommittee/Chemicals/tabid/243/Default.aspx (accessed on 17 January 2024).
- UNEP. 2022 Decision SC-10/13. Perfluorohexane Sulfonic Acid (PFHxS), its Salts and PFHxS-Related Compounds, United Nations Environment Programme (UNEP), ed. SC-10/13. Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland. 2022. Available online: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 17 January 2024).
- van der Veen, I.; Fiedler, H.; de Boer, J. Assessment of the per- and polyfluoroalkyl substances analysis under the Stockholm Convention–2018/2019. Chemosphere 2023, 313, 137549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Buck, R.C.; Hungerbühler, K. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environ. Int. 2014, 70, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://wedocs.unep.org/handle/20.500.11822/29682;jsessionid=56C148634575FC7B83A02D6695EB18B0 (accessed on 17 January 2024).
- Paris-Davila, T.; Gaines, L.G.T.; Lucas, K.; Nylander-French, L.A. Occupational exposures to airborne per- and polyfluoroalkyl substances (PFAS)—A review. Am. J. Ind. Med. 2023, 66, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Timshina, A.S.; Sobczak, W.J.; Griffin, E.K.; Lin, A.M.; Townsend, T.G.; Bowden, J.A. Up in the air: Polyfluoroalkyl phosphate esters (PAPs) in airborne dust captured by air conditioning (AC) filters. Chemosphere 2023, 325, 138307. [Google Scholar] [CrossRef]
- Liu, L.S.; Guo, Y.T.; Wu, Q.Z.; Zeeshan, M.; Qin, S.J.; Zeng, H.X.; Lin, L.Z.; Chou, W.C.; Yu, Y.J.; Dong, G.H.; et al. Per- and polyfluoroalkyl substances in ambient fine particulate matter in the Pearl River Delta, China: Levels, distribution and health implications. Environ. Pollut. 2023, 334, 122138. [Google Scholar] [CrossRef]
- Dauchy, X. Evidence of large-scale deposition of airborne emissions of per- and polyfluoroalkyl substances (PFASs) near a fluoropolymer production plant in an urban area. Chemosphere 2023, 337, 139407. [Google Scholar] [CrossRef]
- Saini, A.; Chinnadurai, S.; Schuster, J.K.; Eng, A.; Harner, T. Per- and polyfluoroalkyl substances and volatile methyl siloxanes in global air: Spatial and temporal trends. Environ. Pollut. 2023, 323, 121291. [Google Scholar] [CrossRef]
- Available online: https://www.pca.state.mn.us/sites/default/files/tdr-g1-23.pdf (accessed on 17 January 2024).
- Available online: https://www.diva-portal.org/smash/get/diva2:1775705/FULLTEXT01.pdf (accessed on 17 January 2024).
- Faust, J.A. PFAS on atmospheric aerosol particles: A review. Environ. Sci. Process. Impacts 2023, 25, 133–150. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Young, C.J.; Mabury, S.A. Atmospheric Perfluorinated Acid Precursors: Chemistry, Occurrence, and Impacts. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 208. [Google Scholar]
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Kourtchev, I.; Hellebust, S.; Heffernan, E.; Wenger, J.; Towers, S.; Diapouli, E.; Eleftheriadis, K. A new on-line SPE LC-HRMS method for the analysis of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in PM2.5 and its application for screening atmospheric particulates from Dublin and Enniscorthy, Ireland. Sci. Total Environ. 2022, 835, 155496. [Google Scholar] [CrossRef]
- Ferrero, L.; Cappelletti, D.; Moroni, B.; Sangiorgi, G.; Perrone, M.G.; Crocchianti, S.; Bolzacchini, E. Wintertime aerosol dynamics and chemical composition across the mixing layer over basin valleys. Atmos. Environ. 2012, 56, 143–153. [Google Scholar] [CrossRef]
- Moroni, B.; Cappelletti, D.; Marmottini, F.; Scardazza, F.; Ferrero, L.; Bolzacchini, E. Integrated single particle-bulk chemical approach for the characterization of local and long range sources of particulate pollutants. Atmos. Environ. 2012, 50, 267–277. [Google Scholar] [CrossRef]
- Tositti, L.; Moroni, B.; Dinelli, E.; Morozzi, P.; Brattich, E.; Sebastiani, B.; Petroselli, C.; Crocchianti, S.; Selvaggi, R.; Goretti, E.; et al. Deposition processes over complex topographies: Experimental data meets atmospheric modeling. Sci. Total Environ. 2020, 744, 140974. [Google Scholar] [CrossRef]
- Massimi, L.; Simonetti, G.; Buiarelli, F.; Di Filippo, P.; Pomata, D.; Riccardi, C.; Ristorini, M.; Astolfi, M.L.; Canepari, S. Spatial distribution of levoglucosan and alternative biomass burning tracers in atmospheric aerosols, in an urban and industrial hot-spot of Central Italy. Atmos. Res. 2020, 239, 104904. [Google Scholar] [CrossRef]
- Moroni, B.; Crocchianti, S.; Petroselli, C.; Selvaggi, R.; Becagli, S.; Traversi, R.; Cappelletti, D. Potential source contribution function analysis of long-range transported aerosols in the Central Mediterranean: A comparative study of two background sites in Italy. Rend. Lincei. 2019, 30, 337–349. [Google Scholar] [CrossRef]
- Cavalli, F.; Viana, M.; Yttri, K.E.; Genberg, J.; Putaud, J.P. Toward a standardised thermal–optical protocol for measuring atmospheric organic and elemental carbon: The EUSAAR protocol. Atmos. Meas. Tech. 2010, 3, 79–89. [Google Scholar] [CrossRef]
- Sandrini, S.; Fuzzi, S.; Piazzalunga, A.; Prati, P.; Bonasoni, P.; Cavalli, F.; Bove, M.C.; Calvello, M.; Cappelletti, D.; Colombi, C.; et al. Spatial and seasonal variability of carbonaceous aerosol across Italy. Atmos. Environ. 2014, 99, 587–598. [Google Scholar] [CrossRef]
- Barola, C.; Moretti, S.; Giusepponi, D.; Paoletti, F.; Saluti, G.; Cruciani, G.; Brambilla, G.; Galarini, R. A liquid chromatography-high resolution mass spectrometry method for the determination of thirty-three per- and polyfluoroalkyl substances in animal liver. J. Chromatog. A 2020, 1628, 461442. [Google Scholar] [CrossRef]
- EURL for Halogenated POPs in Feed and Food (2022): Guidance Document on Analytical Parameters for the Determination of Per- and Polyfluoroalkyl Substances (PFAS) in Food and Feed, Version 1.2 of 11 May 2022. Available online: https://eurl-pops.eu/core-working-groups#_pfas (accessed on 17 January 2024).
- Powley, C.R.; George, S.W.; Ryan, T.W.; Buck, R.C. Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal. Chem. 2005, 77, 6353. [Google Scholar] [CrossRef]
- Sanan, T.; Magnuson, M. Analysis of per- and polyfluorinated alkyl substances in sub-sampled water matrices with online solid phase extraction/isotope dilution tandem mass spectrometry. J. Chromatog. A 2020, 1626, 461324. [Google Scholar] [CrossRef]
- Pietrodangelo, A.; Bove, M.C.; Forello, A.C.; Crova, F.; Bigi, A.; Brattich, E.; Riccio, A.; Becagli, S.; Bertinetti, S.; Calzolai, G.; et al. A PM10 chemically characterized nation-wide dataset for Italy. Geographical influence on urban air pollution and source apportionment. Sci. Total Environ. 2024, 908, 167891. [Google Scholar] [CrossRef]
- Yao, Y.; Chang, S.; Zhao, Y.; Tang, J.; Sun, H.; Xie, Z. Per- and poly-fluoroalkyl substances (PFASs) in the urban, industrial, and background atmosphere of Northeastern China coast around the Bohai Sea: Occurrence, partitioning, and seasonal variation. Atmos. Environ. 2017, 167, 150–158. [Google Scholar] [CrossRef]
- Barber, J.L.; Berger, U.; Chaemfa, C.; Huber, S.; Jahnke, A.; Temme, C.; Jones, K.C. analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J. Environ. Monit. 2007, 9, 530–541. [Google Scholar] [CrossRef]
- Yu, N.; Guo, H.; Yang, J.; Jin, L.; Wang, X.; Shi, W.; Zhang, X.; Yu, H.; Wei, S. Non-target and suspect screening of per- and polyfluoroalkyl substances in airborne particulate matter in China. Environ. Sci. Technol. 2018, 52, 8205–8214. [Google Scholar] [CrossRef]
- Müller, C.E.; Gerecke, A.C.; Bogdal, C.; Wang, Z.; Scheringer, M.; Hungerbuhler, K. Atmospheric fate of poly- and perfluorinated alkyl substances (PFASs): I. Day-night patterns of air concentrations in summer in Zurich, Switzerland. Environ. Pollut. 2012, 169, 196–203. [Google Scholar] [CrossRef]
- Casas, G.; Martinez-Varela, A.; Vila-Costa, M.; Jiménez, B.; Dachs, J. Rain Amplification of Persistent Organic Pollutants. Environ. Sci. Technol. 2021, 55, 12961–12972. [Google Scholar]
- Zhou, J.; Baumann, K.; Mead, R.N.; Skrabal, S.A.; Kieber, R.J.; Avery, G.B.; Shimizu, M.; DeWitt, J.C.; Sun, M.; Vance, S.A.; et al. PFOS dominates PFAS composition in ambient fine particulate matter (PM2.5) collected across North Carolina nearly 20 years after the end of its US production. Environ. Sci. Process. Impacts 2021, 23, 580–587. [Google Scholar] [CrossRef]
- Wilmot, T.Y.; Mallia, D.V.; Hallar, A.G.; Lin, J.C. Wildfire activity is driving summertime air quality degradation across the western US: A model-based attribution to smoke source regions. Environ. Res. Lett. 2022, 17, 114014. [Google Scholar] [CrossRef]
- Shen, L.; Mickley, L.J. Effects of El Nino on Summertime Ozone air quality in the Eastern United States. Geophys. Res. Lett. 2017, 44, 12543. [Google Scholar] [CrossRef]
- Chan, K.L.; Wang, S.; Liu, C.; Zhou, B.; Wenig, M.O.; Saiz-Lopez, A. On the summertime air quality and related photochemical processes in the megacity Shanghai, China. Sci. Total Environ. 2017, 580, 974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).