Mineral Heterostructures for Simultaneous Removal of Lead and Arsenic Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Standard Materials, and Equipment
2.2. MOHs–Physico-Chemical Properties
2.3. Adsorption Experiments
2.3.1. Optimization Methods
2.3.2. Adsorption Behaviors
2.3.3. Single Metal Adsorption
2.3.4. Competitive Adsorption Studies
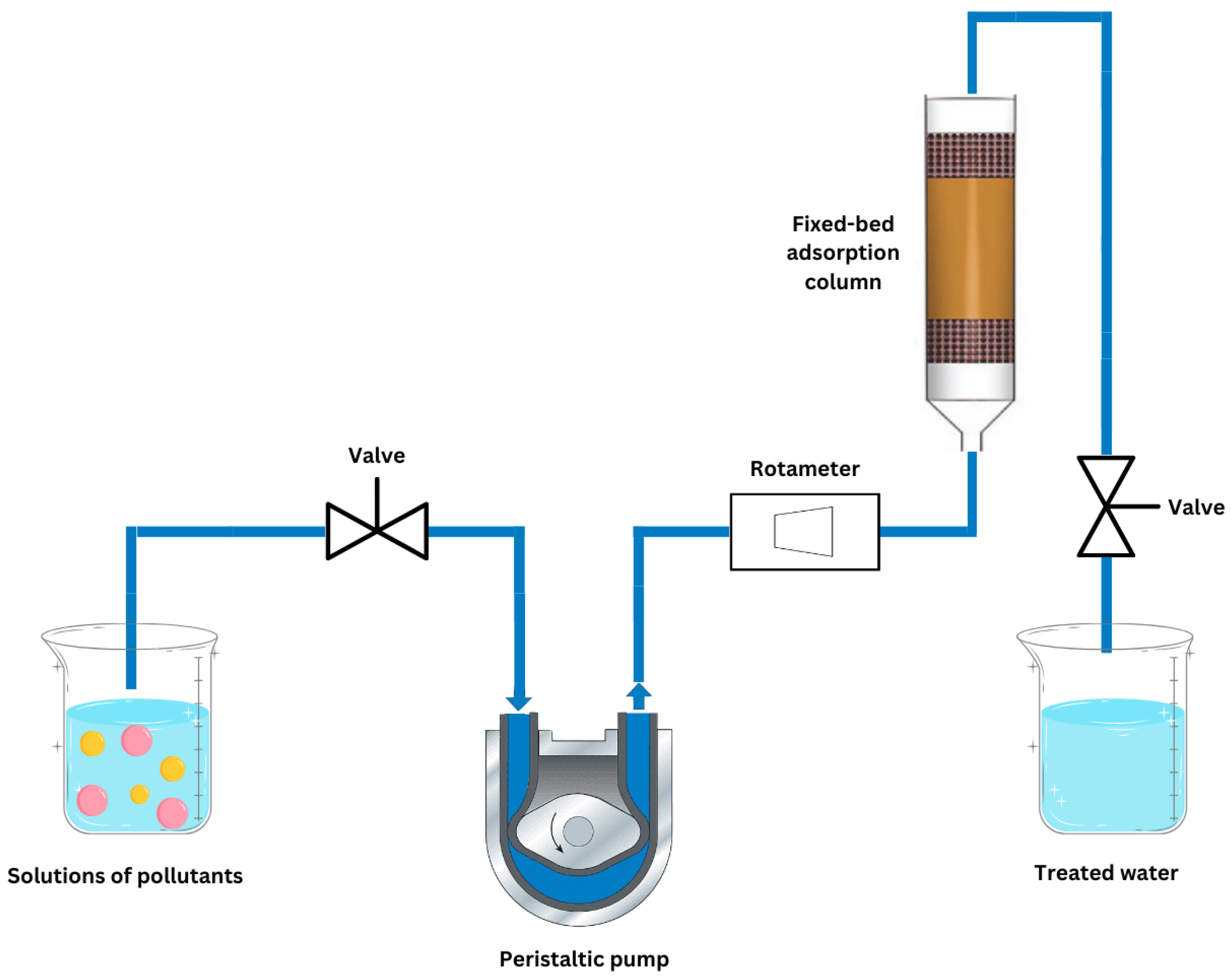
2.3.5. Bed Column Experiments
2.4. Regeneration
2.5. Material Characterization
3. Results
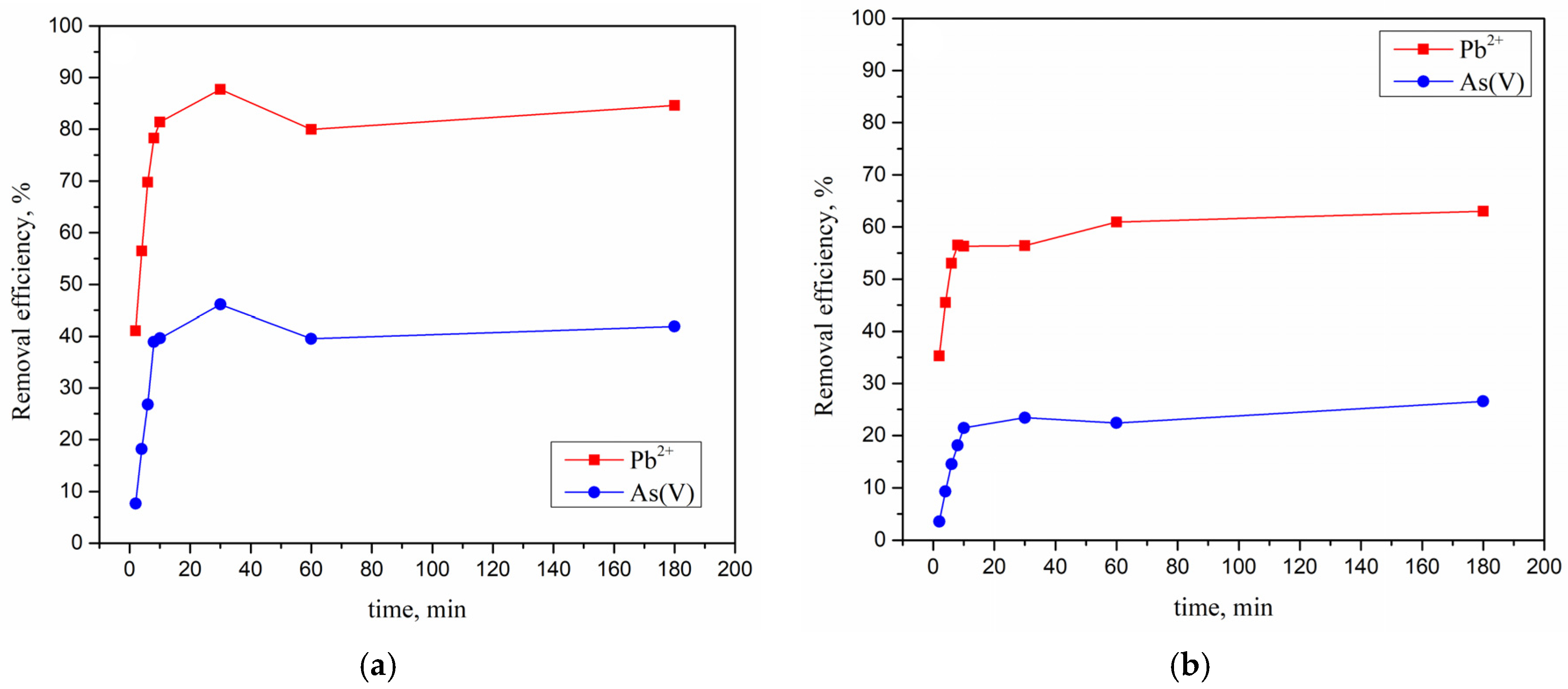
3.1. Optimization of Process Parameters
3.2. The Influence of pH Value
3.3. Batch Adsorption Experiments
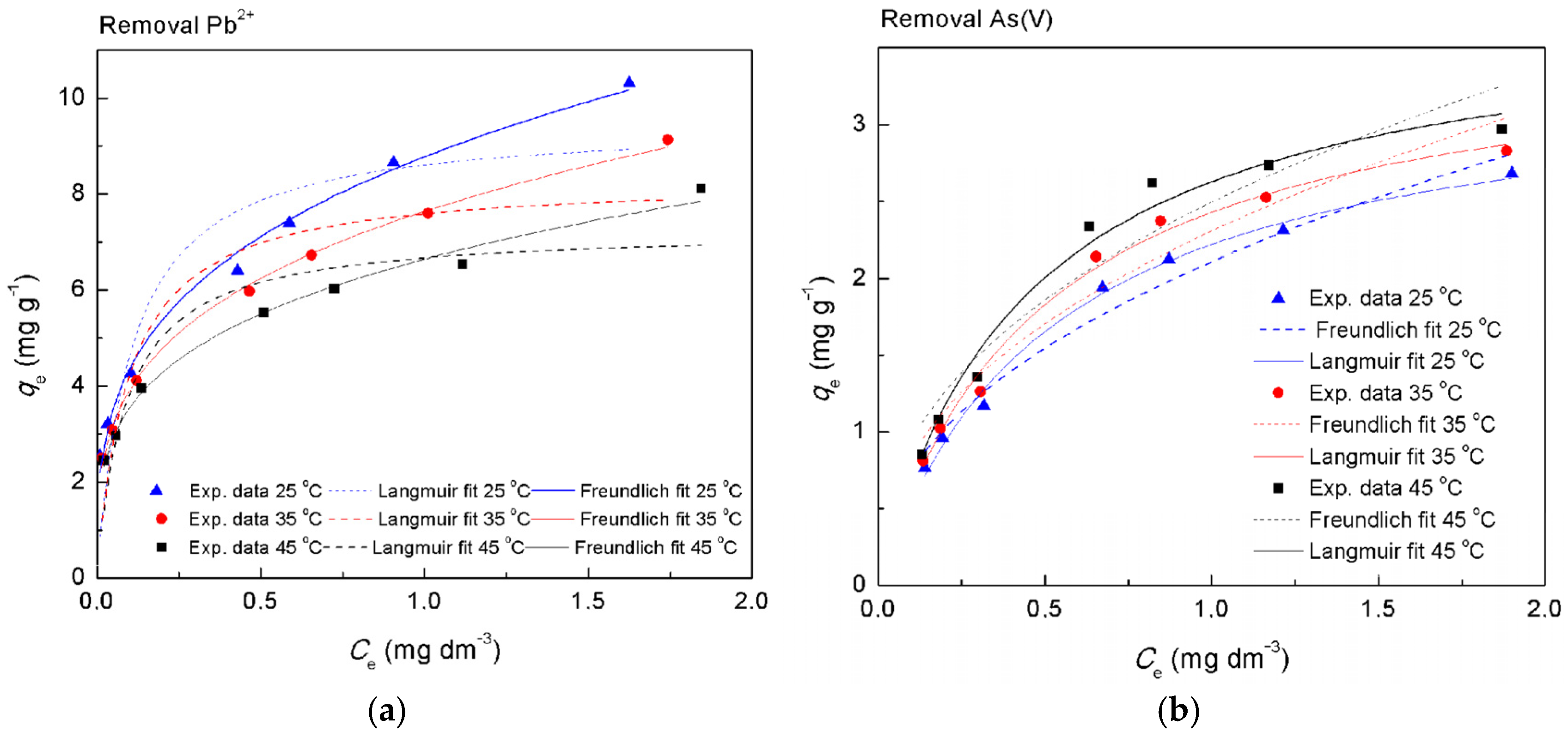
3.3.1. Single Metal Adsorption—Isotherm Studies
3.3.2. Thermodynamic Studies
3.3.3. Kinetic Study
3.4. Simultaneous Adsorption of Pb2+ and As(V) Ions
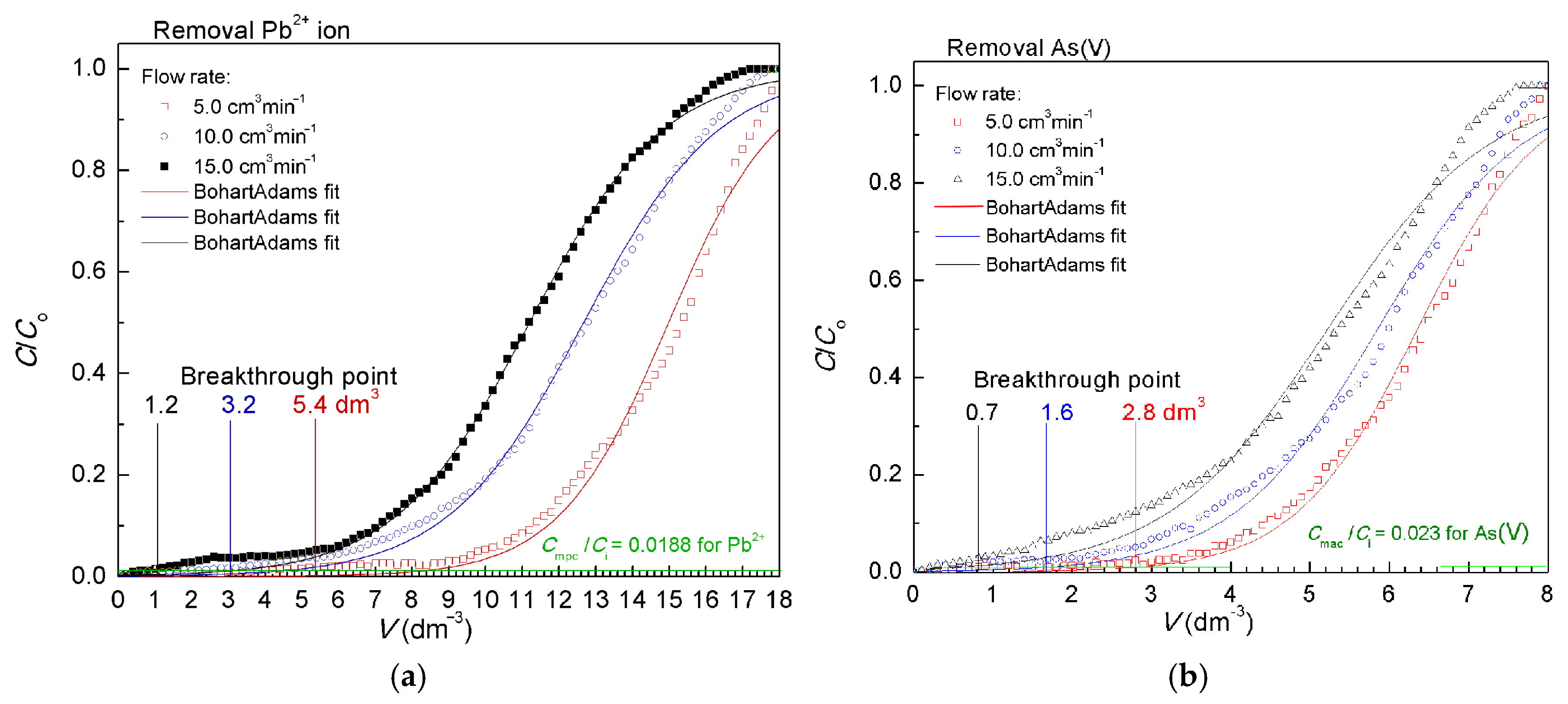
3.5. Bed Column Experiments
3.6. Regeneration and Desorption
3.6.1. Desorption of Ions After Simultaneous Adsorption
3.6.2. Regeneration of MOHs in Flow System
3.7. Structural and Morphological Characteristics
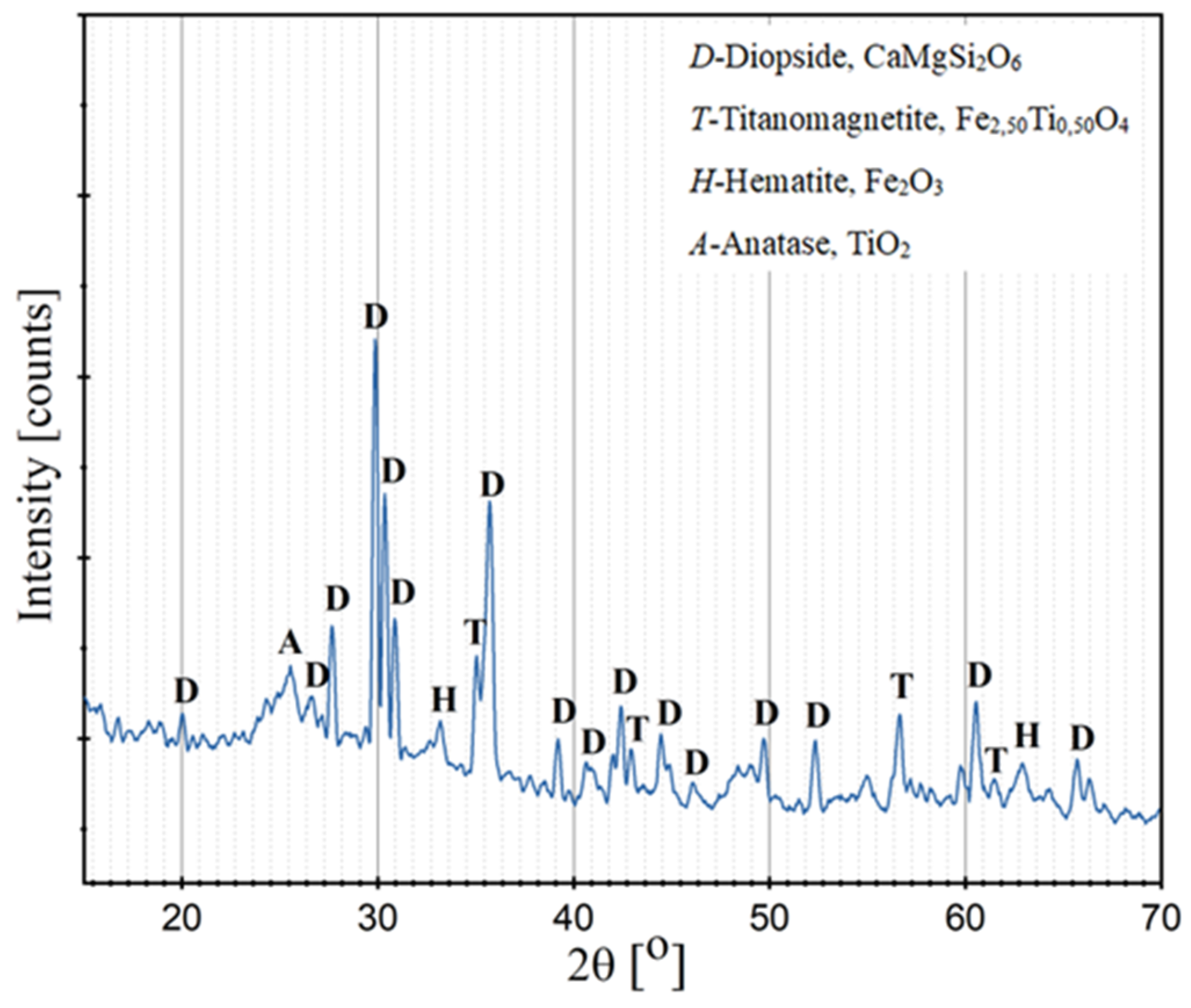
3.7.1. X-Ray Diffraction
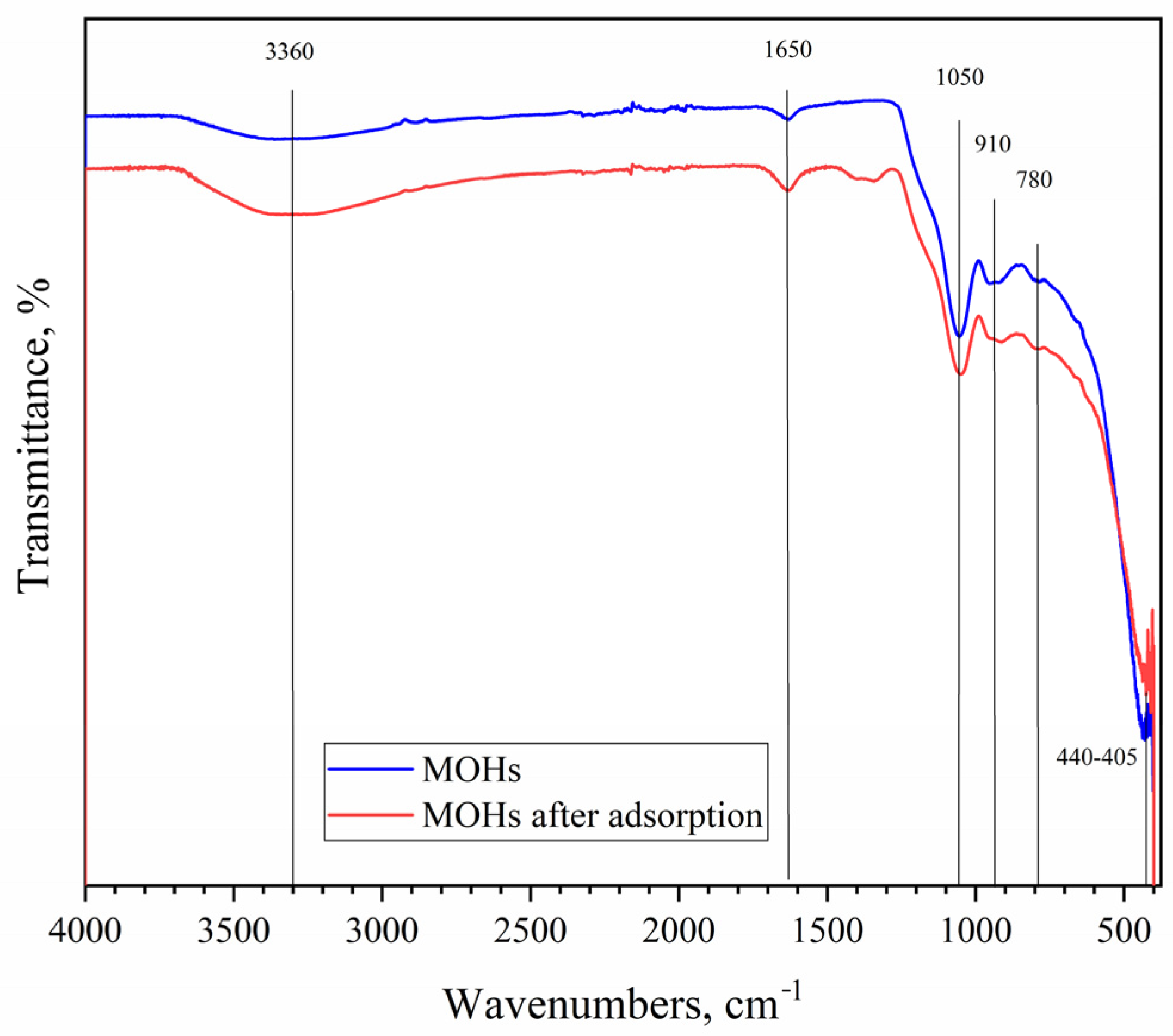
3.7.2. FT-IR Analysis
3.7.3. SEM and EDS Analysis
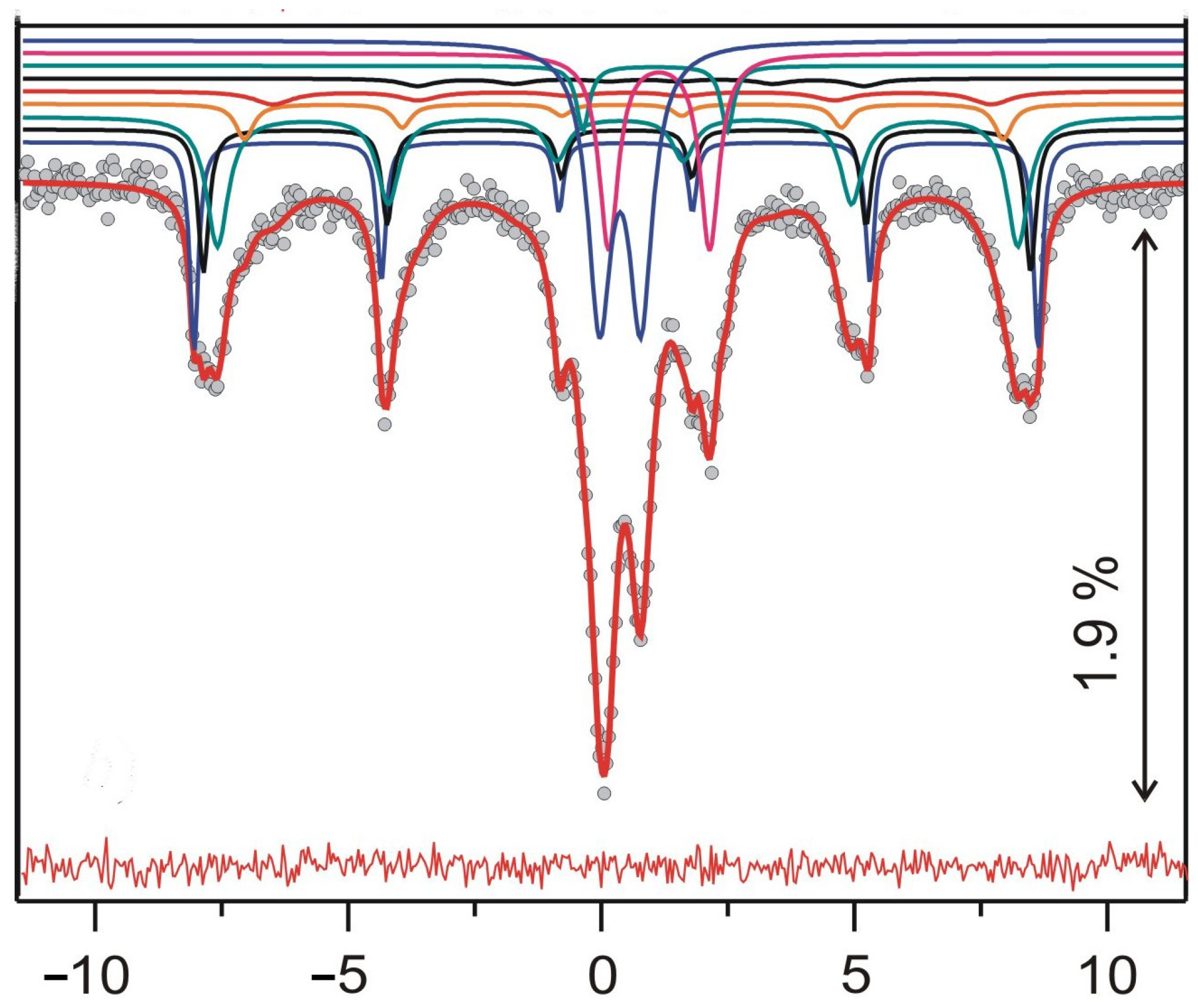
3.7.4. Mossbauer Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Facile Synthesis of Xanthan Biopolymer Integrated 3D Hierarchical Graphene Oxide/Titanium Dioxide Composite for Adsorptive Lead Removal in Wastewater. Bioresour. Technol. 2020, 309, 123296. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.J. Arsenic in the Geo-Environment: A Review of Sources, Geochemical Processes, Toxicity and Removal Technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A Review of Toxicity and Mechanisms of Individual and Mixtures of Heavy Metals in the Environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Yang, H.H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U.F. Cross Talk between Synthetic Food Colors (Azo Dyes), Oral Flora, and Cardiovascular Disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, Selenium, Boron, Lead, Cadmium, Copper, and Zinc in Naturally Contaminated Rocks: A Review of Their Sources, Modes of Enrichment, Mechanisms of Release, and Mitigation Strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water Cleaning Adsorptive Membranes for Efficient Removal of Heavy Metals and Metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive Removal of Lead (II) Ion from Water and Wastewater Media Using Carbon-Based Nanomaterials as Unique Sorbents: A Review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; ISBN 9789241548151. [Google Scholar]
- Akpomie, K.G.; Dawodu, F.A.; Adebowale, K.O. Mechanism on the Sorption of Heavy Metals from Binary-Solution by a Low Cost Montmorillonite and Its Desorption Potential. Alex. Eng. J. 2015, 54, 757–767. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Gupta, G.; Khan, J.; Singh, N.K. Application and Efficacy of Low-Cost Adsorbents for Metal Removal from Contaminated Water: A Review. Mater. Today Proc. 2021, 43, 2958–2964. [Google Scholar] [CrossRef]
- Wagle, D.; Shipley, H.J. Adsorption of Arsenic (V) to Titanium Dioxide Nanoparticles: Effect of Particle Size, Solid Concentration, and Other Metals. Environ. Eng. Sci. 2016, 33, 299–305. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent Advances in Adsorptive Removal of Heavy Metal and Metalloid Ions by Metal Oxide-Based Nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Arora, R. Adsorption of Heavy Metals-a Review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Ray, P.Z.; Shipley, H.J. Inorganic Nano-Adsorbents for the Removal of Heavy Metals and Arsenic: A Review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and Application of Fe3O4@SiO2@TiO2 for Photocatalytic Decomposition of Organic Matrix Simultaneously with Magnetic Solid Phase Extraction of Heavy Metals Prior to ICP-MS Analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of Chitosan-Based Adsorbents Used in Heavy Metal Adsorption: A Review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Novack, A.M.; dos Reis, G.S.; Hackbarth, F.V.; Marinho, B.A.; Ðolić, M.B.; Valle, J.A.B.; Sampaio, C.H.; Lima, E.C.; Dotto, G.L.; Ulson de Souza, A.A.; et al. Facile Fabrication of Hybrid Titanium(IV) Isopropoxide/Pozzolan Nanosheets (TnS-Pz) of High Photocatalytic Activity: Characterization and Application for Cr(VI) Reduction in an Aqueous Solution. Environ. Sci. Pollut. Res. 2020, 28, 23568–23581. [Google Scholar] [CrossRef]
- Lin, Y.P.; Mehrvar, M. Photocatalytic Treatment of an Actual Confectionery Wastewater Using Ag/TiO2/Fe2O3: Optimization of Photocatalytic Reactions Using Surface Response Methodology. Catalysts 2018, 8, 409. [Google Scholar] [CrossRef]
- Đolić, M.; Karanac, M.; Radovanović, D.; Umićević, A.; Kapidžić, A.; Veličković, Z.; Marinković, A.; Kamberović, Ž. Closing the Loop: As(V) Adsorption onto Goethite Impregnated Coal-Combustion Fly Ash as Integral Building Materials. J. Clean. Prod. 2021, 303, 126924. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Milosavljević, M.; Marinović-Cincović, M.; Rusmirović, J.; Ristić, M.; Marinković, A. Efficient Arsenic Removal by Cross-Linked Macroporous Polymer Impregnated with Hydrous Iron Oxide: Material Performance. Chem. Eng. J. 2015, 279, 66–78. [Google Scholar] [CrossRef]
- Pantić, K.; Bajić, Z.J.; Veličković, Z.S.; Djokić, V.R.; Rusmirović, J.D.; Marinković, A.D.; Perić-Grujić, A. Adsorption Performances of Branched Aminated Waste Polyacrylonitrile Fibers: Experimental versus Modelling Study. Desalin. Water Treat. 2019, 171, 223–249. [Google Scholar] [CrossRef]
- Sharifipour, F.; Hojati, S.; Landi, A.; Faz Cano, A. Kinetics and Thermodynamics of Lead Adsorption from Aqueous Solutions Onto Iranian Sepiolite and Zeolite. Int. J. Environ. Res. 2015, 9, 1001–1010. [Google Scholar]
- Thanos, A.G.; Katsou, E.; Malamis, S.; Drakopoulos, V.; Paschalakis, P.; Pavlatou, E.A.; Haralambous, K.J. Cr(VI) Removal from Aqueous Solutions Using Aluminosilicate Minerals in Their Pb-Exchanged Forms. Appl. Clay Sci. 2017, 147, 54–62. [Google Scholar] [CrossRef]
- Wahab, N.; Saeed, M.; Ibrahim, M.; Munir, A.; Saleem, M.; Zahra, M.; Waseem, A. Synthesis, Characterization, and Applications of Silk/Bentonite Clay Composite for Heavy Metal Removal From Aqueous Solution. Front. Chem. 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bajda, T.; Szala, B.; Solecka, U. Removal of Lead and Phosphate Ions from Aqueous Solutions by Organo-Smectite. Environ. Technol. 2015, 36, 2872–2883. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Fijałkowska, G.; Wiśniewska, M.; Wójcik, G. Chromium(VI) Reduction and Accumulation on the Kaolinite Surface in the Presence of Cationic Soil Flocculant. J. Soils Sediments 2020, 20, 3688–3697. [Google Scholar] [CrossRef]
- Bozorgi, M.; Abbasizadeh, S.; Samani, F.; Mousavi, S.E. Performance of Synthesized Cast and Electrospun PVA/Chitosan/ZnO-NH2 Nano-Adsorbents in Single and Simultaneous Adsorption of Cadmium and Nickel Ions from Wastewater. Environ. Sci. Pollut. Res. 2018, 25, 17457–17472. [Google Scholar] [CrossRef]
- Seo, D.C.; Yu, K.; DeLaune, R.D. Comparison of Monometal and Multimetal Adsorption in Mississippi River Alluvial Wetland Sediment: Batch and Column Experiments. Chemosphere 2008, 73, 1757–1764. [Google Scholar] [CrossRef]
- Demiral, I.; Samdan, C.A.; Demiral, H. Kinetics & Equilibrium Adsorption Study of Lead(II) onto Activated Carbon Prepared from Pumpkin Seed Shell. Fresenius Environ. Bull. 2017, 26, 4484–4494. [Google Scholar]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Perendija, J.; Veličković, Z.S.; Cvijetić, I.; Lević, S.; Marinković, A.D.; Milošević, M.; Onjia, A. Bio-Membrane Based on Modified Cellulose, Lignin, and Tannic Acid for Cation and Oxyanion Removal: Experimental and Theoretical Study. Process Saf. Environ. Prot. 2021, 147, 609–625. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. A Review on Graphene Oxide and Its Composites Preparation and Their Use for the Removal of As3+and As5+ from Water under the Effect of Various Parameters: Application of Isotherm, Kinetic and Thermodynamics. Process Saf. Environ. Prot. 2018, 119, 138–163. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wu, H.; Pan, S.; Cheng, X.; Sun, Y.; Xu, Y. Effective and Simultaneous Removal of Organic/Inorganic Arsenic Using Polymer-Based Hydrated Iron Oxide Adsorbent: Capacity Evaluation and Mechanism. Sci. Total Environ. 2020, 742, 140508. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.S.; Diebold, U. Adsorption on Metal Oxide Surfaces. Surf. Interface Sci. 2016, 6, 793–817. [Google Scholar] [CrossRef]
- Reczek, L.; Michel, M.M.; Trach, Y.; Siwiec, T.; Tytkowska-Owerko, M. The Kinetics of Manganese Sorption on Ukrainian Tuff and Basalt—Order and Diffusion Models Analysis. Minerals 2020, 10, 1065. [Google Scholar] [CrossRef]
- Taleb, K.A.; Rusmirović, J.D.; Rančić, M.P.; Nikolić, J.B.; Drmanić, S.; Veličković, Z.S.; Marinković, A.D. Efficient Pollutants Removal by Amino-Modified Nanocellulose Impregnated with Iron Oxide. J. Serbian Chem. Soc. 2016, 81, 1199–1213. [Google Scholar] [CrossRef]
- Lalhmunsiama; Pawar, R.R.; Hong, S.M.; Jin, K.J.; Lee, S.M. Iron-Oxide Modified Sericite Alginate Beads: A Sustainable Adsorbent for the Removal of As(V) and Pb(II) from Aqueous Solutions. J. Mol. Liq. 2017, 240, 497–503. [Google Scholar] [CrossRef]
- Ren, C.; Ding, X.; Fu, H.; Li, W.; Wu, H.; Yang, H. Core-Shell Superparamagnetic Monodisperse Nanospheres Based on Amino-Functionalized CoFe2O4@SiO2 for Removal of Heavy Metals from Aqueous Solutions. RSC Adv. 2017, 7, 6911–6921. [Google Scholar] [CrossRef]
- Yin, J.; Deng, C.; Yu, Z.; Wang, X.; Xu, G. Effective Removal of Lead Ions from Aqueous Solution Using Nano Illite/Smectite Clay: Isotherm, Kinetic, and Thermodynamic Modeling of Adsorption. Water 2018, 10, 210. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W. Evaluating the Adsorption of Shanghai Silty Clay to Cd(II), Pb(II), As(V), and Cr(VI): Kinetic, Equilibrium, and Thermodynamic Studies. Environ. Monit. Assess. 2021, 193, 131. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Afshang, M.; Gilvari, T.; Aghabarari, B.; Mozaffari, S. Rapid and Effective Removal of Heavy Metal Ions from Aqueous Solution Using Nanostructured Clay Particles. Results Surf. Interfaces 2023, 10, 100097. [Google Scholar] [CrossRef]
- Zbair, M.; Anfar, Z.; Ahsaine, H.A. Reusable Bentonite Clay: Modelling and Optimization of Hazardous Lead and p-Nitrophenol Adsorption Using a Response Surface Methodology Approach. RSC Adv. 2019, 9, 5756–5769. [Google Scholar] [CrossRef]
- Awwad, A.M.; Amer, M.W.; Al-aqarbeh, M.M. TiO2-Kaolinite Nanocomposite Prepared from the Jordanian Kaolin Clay: Adsorption and Thermodynamics of Pb(II) and Cd(II) Ions in Aqueous Solution. Chem. Int. 2020, 6, 168–178. [Google Scholar] [CrossRef]
- Amer, M.W.; Awwad, A.M. Removal of As(V) from Aqueous Solution by Adsorption onto Nanocrystalline Kaolinite: Equilibrium and Thermodynamic Aspects of Adsorption. Environ. Nanotechnol. Monit. Manag. 2018, 9, 37–41. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Wu, Y.; Li, Y.; Zhao, J.; Na, P. Synthesis of Magnetic Orderly Mesoporous A-Fe2O3 Nanocluster Derived from MIL-100(Fe) for Rapid and Efficient Arsenic(III,V) Removal. J. Hazard. Mater. 2018, 343, 304–314. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-Supported Nanoscale Zero-Valent Iron: New Findings on Simultaneous Adsorption of Cd(II), Pb(II), and As(III) in Aqueous Solution and Soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Hira, N.E.; Lock, S.S.M.; Arshad, U.; Asif, K.; Ullah, F.; Farooqi, A.S.; Yiin, C.L.; Chin, B.L.F.; Huma, Z.E. Screening of Metal Oxides and Hydroxides for Arsenic Removal from Water Using Molecular Dynamics Simulations. ACS Omega 2023, 8, 48130–48144. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous Removal of Arsenic, Cadmium, and Lead from Soil by Iron-Modified Magnetic Biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef]
- Liao, Q.; Tu, G.; Yang, Z.; Wang, H.; He, L.; Tang, J.; Yang, W. Simultaneous Adsorption of As(III), Cd(II) and Pb(II) by Hybrid Bio-Nanocomposites of Nano Hydroxy Ferric Phosphate and Hydroxy Ferric Sulfate Particles Coating on Aspergillus Niger. Chemosphere 2019, 223, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, M.; Koopal, L.K. Surfactant Adsorption to Soil Components and Soils. Adv. Colloid Interface Sci. 2016, 231, 59–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, C.; Pan, L.; Zhang, X.; Zou, J.J. Rational Design, Synthesis, Adsorption Principles and Applications of Metal Oxide Adsorbents: A Review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of Adsorbents and Recovery of Heavy Metals: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Zhang, X.; Yang, H.; Xue, X.; Niu, X. The Effect of Strong Magnetic Field on the Microstructure of Pure Diopside and Diopside Doped with Fe3+ or Mn2+. J. Alloys Compd. 2016, 657, 152–156. [Google Scholar] [CrossRef]
- Choudhary, R.; Vecstaudza, J.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Sasikumar, S.; Locs, J. In-Vitro Bioactivity, Biocompatibility and Dissolution Studies of Diopside Prepared from Biowaste by Using Sol-Gel Combustion Method. Mater. Sci. Eng. C 2016, 68, 89–100. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Li, X.; Lin, Q.; Xiao, R.; Zhang, M.; Yin, G.; Zhou, Y. Effect of Lignosulfonate on the Adsorption Performance of Hematite for Cd(II). Sci. Total Environ. 2020, 738, 139952. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel Insight into the Adsorption of Cr(VI) and Pb(II) Ions by MOF Derived Co-Al Layered Double Hydroxide @hematite Nanorods on 3D Porous Carbon Nanofiber Network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Lai, L.; Zhou, H.; Zhang, H.; Ao, Z.; Pan, Z.; Chen, Q.; Xiong, Z.; Yao, G.; Lai, B. Activation of Peroxydisulfate by Natural Titanomagnetite for Atrazine Removal via Free Radicals and High-Valent Iron-Oxo Species. Chem. Eng. J. 2020, 387, 124165. [Google Scholar] [CrossRef]
- Zanatta, A.R. A Fast-Reliable Methodology to Estimate the Concentration of Rutile or Anatase Phases of TiO2. AIP Adv. 2017, 7. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, L.; Li, Q.; Chen, Y.; Yan, S.; Jiao, J.; Liu, G.; Mei, L. Epitaxial Rutile TiO2 Film Based on MgF2 Substrate for Ultraviolet Detector. J. Alloys Compd. 2016, 683, 439–443. [Google Scholar] [CrossRef]
- Perelomov, L.; Sarkar, B.; Rahman, M.M.; Goryacheva, A.; Naidu, R. Uptake of Lead by Na-Exchanged and Al-Pillared Bentonite in the Presence of Organic Acids with Different Functional Groups. Appl. Clay Sci. 2016, 119, 417–423. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 01, 206–210. [Google Scholar] [CrossRef]
- Alizadeh Eslami, P.; Kamboh, M.A.; Rashidi Nodeh, H.; Wan Ibrahim, W.A. Equilibrium and Kinetic Study of Novel Methyltrimethoxysilane Magnetic Titanium Dioxide Nanocomposite for Methylene Blue Adsorption from Aqueous Media. Appl. Organomet. Chem. 2018, 32, 1–13. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly Efficient Adsorption of Strontium Ions by Carbonated Mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Zou, C.; Cao, J.; Zhao, M.; Wang, Z.; Lu, J. Combined Sodium and Fluorine Promote Diopside Continuous Growth to Achieve One-Step Crystallization in CaO-Al2O3-SiO2-Fe2O3 Glass-Ceramics. J. Eur. Ceram. Soc. 2019, 39, 4979–4987. [Google Scholar] [CrossRef]
- Srinath, P.; Abdul Azeem, P.; Venugopal Reddy, K.; Chiranjeevi, P.; Bramanandam, M.; Prasada Rao, R. A Novel Cost-Effective Approach to Fabricate Diopside Bioceramics: A Promising Ceramics for Orthopedic Applications. Adv. Powder Technol. 2021, 32, 875–884. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, M.K. Pb2+ Doped Diopside CaMgSi2O6: New UV Luminescent Phosphor. Optik 2020, 202, 163542. [Google Scholar] [CrossRef]
- Nwosu, F.O.; Ajala, O.J.; Owoyemi, R.M.; Raheem, B.G. Preparation and Characterization of Adsorbents Derived from Bentonite and Kaolin Clays. Appl. Water Sci. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ünügül, T.; Nigiz, F.U.; Karayünlü Bozbaş, S. Application of Response Surface Methodology for Optimization of Copper Removal Using a Novel Polymeric Adsorbent. J. Polym. Environ. 2022, 30, 4887–4901. [Google Scholar] [CrossRef]
- Melak, F.; Alemayehu, E.; Ambelu, A.; Van Ranst, E.; Du Laing, G. Evaluation of Natural Quartz and Zeolitic Tuffs for As(V) Removal from Aqueous Solutions: A Mechanistic Approach. Int. J. Environ. Sci. Technol. 2018, 15, 217–230. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J. Synthesis of Magnetically Separable and Recyclable Magnetic Nanoparticles Decorated with β-Cyclodextrin Functionalized Graphene Oxide an Excellent Adsorption of As(V)/(III). J. Mol. Liq. 2017, 237, 387–401. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rančić, M.; Pavlović, V.; Marinković, A. Arsenic Removal by Magnetite-Loaded Amino Modified Nano/Microcellulose Adsorbents: Effect of Functionalization and Media Size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef]
- Đolić, M.B.; Rajaković, V.N.; Marković, J.P.; Janković-Mandić, L.J.; Mitrić, M.M.; Onjia, A.E.; Rajaković, L.V. The Effect of Different Extractants on Lead Desorption from a Natural Mineral. Appl. Surf. Sci. 2015, 324, 221–231. [Google Scholar] [CrossRef]
- Mihajlović, S.; Vukčević, M.; Pejić, B.; Grujić, A.P.; Ristić, M. Application of Waste Cotton Yarn as Adsorbent of Heavy Metal Ions from Single and Mixed Solutions. Environ. Sci. Pollut. Res. 2020, 27, 35769–35781. [Google Scholar] [CrossRef]






| Run | A t (min) | B m (mg) | C T (°C) | D pH | Y qe (mol g−1) × 10−5 |
|---|---|---|---|---|---|
| 1. | 16 | 11 | 45 | 12 | 0.359 |
| 2. | 16 | 2 | 45 | 7 | 4.36 |
| 3. | 2 | 11 | 25 | 7 | 0.957 |
| 4. | 16 | 20 | 25 | 7 | 1.13 |
| 5. | 30 | 11 | 25 | 7 | 2.02 |
| 6. | 2 | 11 | 45 | 7 | 0.998 |
| 7. | 30 | 11 | 45 | 7 | 1.94 |
| 8. | 30 | 11 | 35 | 12 | 0.396 |
| 9. | 2 | 11 | 35 | 2 | 0.367 |
| 10. | 16 | 2 | 35 | 12 | 0.89 |
| 11. | 16 | 11 | 35 | 7 | 1.83 |
| 12. | 16 | 11 | 35 | 7 | 1.83 |
| 13. | 16 | 11 | 35 | 7 | 1.83 |
| 14. | 2 | 2 | 35 | 7 | 2.20 |
| 15. | 16 | 11 | 35 | 7 | 1.83 |
| 16. | 2 | 11 | 35 | 12 | 0.204 |
| 17. | 30 | 11 | 35 | 2 | 0.713 |
| 18. | 30 | 2 | 35 | 7 | 4.40 |
| 19. | 16 | 20 | 35 | 12 | 0.221 |
| 20. | 16 | 20 | 45 | 7 | 1.08 |
| 21. | 2 | 20 | 35 | 7 | 0.61 |
| 22. | 16 | 2 | 25 | 7 | 4.54 |
| 23. | 16 | 11 | 35 | 7 | 1.83 |
| 24. | 16 | 11 | 25 | 12 | 0.374 |
| 25. | 16 | 20 | 35 | 2 | 0.399 |
| 26. | 16 | 2 | 35 | 2 | 1.6 |
| 27. | 16 | 11 | 25 | 2 | 0.673 |
| 28. | 16 | 11 | 45 | 2 | 0.646 |
| 29. | 30 | 20 | 35 | 7 | 1.2 |
| Isotherm Model | Ion | Pb2+ | As(V) | ||||
|---|---|---|---|---|---|---|---|
| Temperature | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | |
| Langmuir isotherm | qm (mg g−1) | 9.504 | 8.3122 | 7.2543 | 3.378 | 3.613 | 3.813 |
| KL (dm3 mg−1) | 8.312 | 9.5707 | 11.304 | 1.913 | 2.049 | 2.217 | |
| R2 | 0.816 | 0.838 | 0.840 | 0.991 | 0.991 | 0.981 | |
| Freundlich isotherm | KF (mg g−1)(dm3 mg−1)1/n | 6.643 | 7.637 | 8.776 | 2.107 | 2.307 | 2.494 |
| n | 3.668 | 3.4439 | 3.2886 | 2.232 | 2.289 | 2.360 | |
| R2 | 0.991 | 0.997 | 0.992 | 0.967 | 0.940 | 0.910 | |
| Temkin isotherm | AT (dm3 g−1) | 286.17 | 240.04 | 240.02 | 18.20 | 18.82 | 19.63 |
| bT | 1.51 | 1.38 | 1.21 | 0.75 | 0.81 | 0.87 | |
| B (J mol−1) | 1643 | 1860 | 2185 | 3287 | 3146 | 3039 | |
| R2 | 0.931 | 0.952 | 0.958 | 0.990 | 0.985 | 0.973 | |
| Dubinin–Radushkevich isotherm | qm (mg g−1) | 7.95 | 7.24 | 6.51 | 2.52 | 2.77 | 3.01 |
| Kad (mol kJ−2) | 10.17 | 10.26 | 10.37 | 10.30 | 10.21 | 10.12 | |
| Ea (kJ mol−1) | 7.012 | 6.980 | 6.944 | 6.968 | 6.999 | 7.028 | |
| R2 | 0.859 | 0.878 | 0.888 | 0.961 | 0.969 | 0.974 | |
| Ion | ΔGΘ (kJ mol−1) | ΔHΘ (kJ mol−1) | ΔSΘ (J mol−1 K−1) | R2 | ||
|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | ||||
| Pb2+ | −45.55 | −47.44 | −49.42 | 12.11 | 193.33 | 0.996 |
| As(V) | −39.39 | −40.88 | −42.42 | 5.80 | 151.54 | 0.996 |
| Pollutant | Eq. Parameter | PFO | PSO | Second-Order |
|---|---|---|---|---|
| Pb2+ | qe | 1.941 | 6.493 | 6.493 |
| k (k1, k2) | 0.189 | 0.141 | 0.00464 | |
| R2 | 0.891 | 0.999 | 0.572 | |
| As(V) | qe | 3.484 | 6.888 | 6.888 |
| k (k1, k2) | 0.164 | 0.060 | 0.0009 | |
| R2 | 0.944 | 0.998 | 0.659 |
| Sorbate | Temperature | qe (mg g−1) | k2 (g (mg min)−1) | R2 | Ea (kJ mol−1) |
|---|---|---|---|---|---|
| Pb2+ | 25 °C | 6.493 | 0.14064 | 0.999 | 2.151 |
| 35 °C | 6.552 | 0.14454 | 0.999 | ||
| 45 °C | 6.611 | 0.14853 | 0.999 | ||
| As(V) | 25 °C | 6.475 | 0.05771 | 0.997 | 2.216 |
| 35 °C | 6.888 | 0.05973 | 0.998 | ||
| 45 °C | 7.310 | 0.06095 | 0.997 |
| Model | Eq. Parameters | Pb2+ | As(V) |
|---|---|---|---|
| Weber–Morris (Step 1) | kp1 (mg g−1 min−0.5) | 1.623 | 1.609 |
| C (mg g−1) | 1.563 | 0.782 | |
| R2 | 0.998 | 0.997 | |
| Weber–Morris (Step 2) | kp2 (mg g−1 min−0.5) | 0.102 | 0.135 |
| C (mg g−1) | 5.736 | 5.571 | |
| R2 | 0.999 | 0.994 | |
| Weber–Morris (Step 3) | kp3 (mg g−1 min−0.5) | 0.00597 | - |
| C (mg g−1) | 6.177 | - | |
| R2 | 0.999 | - | |
| Dunwald–Wagner | K | 0.698 | 0.0567 |
| R2 | 0.693 | 0.820 | |
| Homogenous Solid Diffusion Model | Ds | 7.44 × 10−11 | 6.58 × 10−11 |
| R2 | 0.680 | 0.799 |
| Sorbent | pHpzc | Experimental Conditions | qmax (mg g−1) | Adosrption Isotherm | Kinetic Model | Thermodynamic Parameters | Reference |
|---|---|---|---|---|---|---|---|
| Iron oxide-modified sericite alginate beads | 4.9 | m = 0.8 g; V = 50 mL; pH = 5; T = 25 °C; t = 15 h. | qPb = 133.73 qAs = 21.61 | Freundlich (Pb2+ and As(V)) | PFO (Pb2+) PSO (As(V))- chemisorptions | - | [40] |
| CoFe2O4@SiO2-NH2 | - | m/V = 0.4 g L−1; Co(Pb2+) = 80 mg L−1; pH = 7; T = 35 °C, t = 0–780 min. | qPb = 181.6 | Langmuir | PSO | ΔG° < 0 spontaneous ΔH° > 0 endothermic ΔS° > 0 | [41] |
| Illite–smectite clay | - | m/V = 0.625–12.5 g L−1; Co(Pb2+) = 1 mg L−1; T = 298 K; t = 60 min. | qPb = 0.256 | Langmuir | PSO | ΔG° < 0 spontaneous ΔH° > 0 endothermic ΔS° > 0 | [42] |
| Shanghai silty clay | - | m/V = 15 g L−1 (Pb2+); m/V = 40 g L−1 (As(V)); Co(Pb2+) = 100 mg L−1; Co(As(V)) = 50 mg L−1; pHAs = 7; pHPb = 6; T = 298 K; t = 24 h. | qAs = 2.8 qPb = 26.46 | Langmuir (Pb2+) Freundlich (As(V)) | PSO- chemisorption | ΔG° < 0 spontaneous ΔH° > 0 (Pb2+) endothermic ΔH° < 0 As(V) exothermic ΔS° > 0 | [43] |
| Clay | - | m = 1 g; V = 100 mL; Co(Pb2+) = 100 mg L−1; pH = 7; T = 25 °C t = 120 min. | qPb = 36.23 | Langmuir | PSO | - | [44] |
| Bentonite clay (BC) calcined at 500 °C | 5.89 | m = 100 mg; V = 200 mL; pH = 5; T = 20 °C; t = 0– 500 min. | qPb = 94.01 | Langmuir | PFO | ΔG° < 0 spontaneous ΔH° > 0 endothermic ΔS° > 0 | [45] |
| TiO2/kaolinit | - | m = 0.5 g; V = 20 mL; Co(Pb2+) = 5–80 mg L−1; pH = 6; T = 30 °C; t = 80 min. | qPb = 333.33 | Langmuir | - | ΔG° < 0 spontaneous ΔH° > 0 endothermic ΔS° > 0 | [46] |
| Nanocrystalline kaolinite | - | m/V = 0.5 g L−1; Co(As(V)) = 30 mg L−1; pH = 8; T = 303 K; t = 120 min. | qAs = 43.67 | Langmuir | - | ΔG° < 0 spontaneous ΔH° > 0 endothermic ΔS° > 0 | [47] |
| α -Fe2O3 nanoclusters | - | m = 3 mg; Co(As(V)) = 2–150 mg L−1; pH = 3; T = 25 °C, t = 12 h. | qAs = 181.82 | Langmuir | PSO (intraparticle) diffusion rather | - | [48] |
| Zeolite-supported nanoscale zero-valent iron | 5.6 | m/V = 0.5 g L−1; Co(Pb2+) = 100 mg L−1; Co(As(III)) = 5 mg L−1; pHPb = 5.5; pHAs = 7; T = 25 °C; t = 10 h. | qPb = 85.90 qAs = 12.84 | Langmuir As(III)- complexation, Pb2+ -reduction | PSO | - | [49] |
| MOHs | 6.6 | m/V = 200–2000 mg L−1;Co(Pb2+) = 5 mg L−1; Co(As(V)) = 5 mg L−1; pHPb = 5; pHAs =6, T = 25 °C; t = 30 min. | qPb = 9.50 qAs =3.81 | Freundlich (Pb2+) Langmuir (As(V)) | PSO | ΔG° < 0 spontaneous ΔH° > 0 endothermic ΔS° > 0 | This study |
| Model | Pb2+ | As(V) | ||||||
|---|---|---|---|---|---|---|---|---|
| Q | cm3 min−1 | 5.0 | 10.0 | 15.0 | 5.0 | 10.0 | 15.0 | |
| Bohart–Adams | kBA | dm3 mg−1 min−1 | 6.277 | 10.08 | 15.53 | 29.86 | 37.89 | 33.90 |
| qo | mg g−1 | 8.204 | 7.001 | 6.132 | 2.841 | 2.616 | 2.335 | |
| R2 | 0.988 | 0.996 | 0.998 | 0.992 | 0.990 | 0.987 | ||
| Yoon–Nelson | kYN | min−1 | 0.667 | 0.541 | 0.550 | 1.295 | 1.095 | 0.979 |
| θ | min | 14.98 | 12.67 | 11.19 | 6.355 | 5.853 | 5.224 | |
| R2 | 0.988 | 0.995 | 0.997 | 0.991 | 0.989 | 0.986 | ||
| Thomas | kTh | L min−1 mg−1 | 1.255 | 1.018 | 1.035 | 2.986 | 2.526 | 2.529 |
| qo | mg g−1 | 22.97 | 19.42 | 17.17 | 8.01 | 7.38 | 6.60 | |
| R2 | 0.947 | 0.957 | 0.951 | 0.991 | 0.989 | 0.985 | ||
| Adsorbate | Pb2+ | As(V) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | Ʃ3 | I | II | III | IV | V | Ʃ3 | |
| Adsorption (mg g−1) 1 | 8.20 | 8.04 | 7.85 | 7.53 | 7.13 | 2.84 | 2.64 | 2.44 | 2.20 | 1.99 | ||
| Desorption (mg g−1) 1 | 8.10 | 7.88 | 7.53 | 7.15 | 6.69 | 2.68 | 2.48 | 2.24 | 2.00 | 1.75 | ||
| C (ppm) 2 | 81.0 | 78.8 | 75.3 | 71.5 | 66.9 | 26.8 | 24.8 | 22.48 | 20.03 | 17.51 | ||
| Δq (mg g−1) 3 | 0.10 | 0.16 | 0.31 | 0.38 | 0.44 | 1.39 | 0.16 | 0.16 | 0.20 | 0.20 | 0.24 | 0.95 |
| Spectrum Label | O | Mg | Al | Si | Ca | Ti | Fe | As | Pb | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 11 | 55.38 | - | - | 16.64 | 0.47 | 24.53 | 1.96 | 0.47 | 0.56 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spasojević, T.; Ćujić, M.; Marjanović, V.; Veličković, Z.; Kokunešoski, M.; Grujić, A.P.; Đolić, M. Mineral Heterostructures for Simultaneous Removal of Lead and Arsenic Ions. Separations 2024, 11, 324. https://doi.org/10.3390/separations11110324
Spasojević T, Ćujić M, Marjanović V, Veličković Z, Kokunešoski M, Grujić AP, Đolić M. Mineral Heterostructures for Simultaneous Removal of Lead and Arsenic Ions. Separations. 2024; 11(11):324. https://doi.org/10.3390/separations11110324
Chicago/Turabian StyleSpasojević, Tijana, Mirjana Ćujić, Vesna Marjanović, Zlate Veličković, Maja Kokunešoski, Aleksandra Perić Grujić, and Maja Đolić. 2024. "Mineral Heterostructures for Simultaneous Removal of Lead and Arsenic Ions" Separations 11, no. 11: 324. https://doi.org/10.3390/separations11110324
APA StyleSpasojević, T., Ćujić, M., Marjanović, V., Veličković, Z., Kokunešoski, M., Grujić, A. P., & Đolić, M. (2024). Mineral Heterostructures for Simultaneous Removal of Lead and Arsenic Ions. Separations, 11(11), 324. https://doi.org/10.3390/separations11110324







