Antimicrobial Profiling of Piper betle L. and Piper nigrum L. Against Methicillin-Resistant Staphylococcus aureus (MRSA): Integrative Analysis of Bioactive Compounds Based on FT-IR, GC-MS, and Molecular Docking Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Methods
2.3. FTIR Analysis
2.4. Anti-Methicillin-Resistant Staphylococcus aureus Activity
2.5. GC-MS Analysis
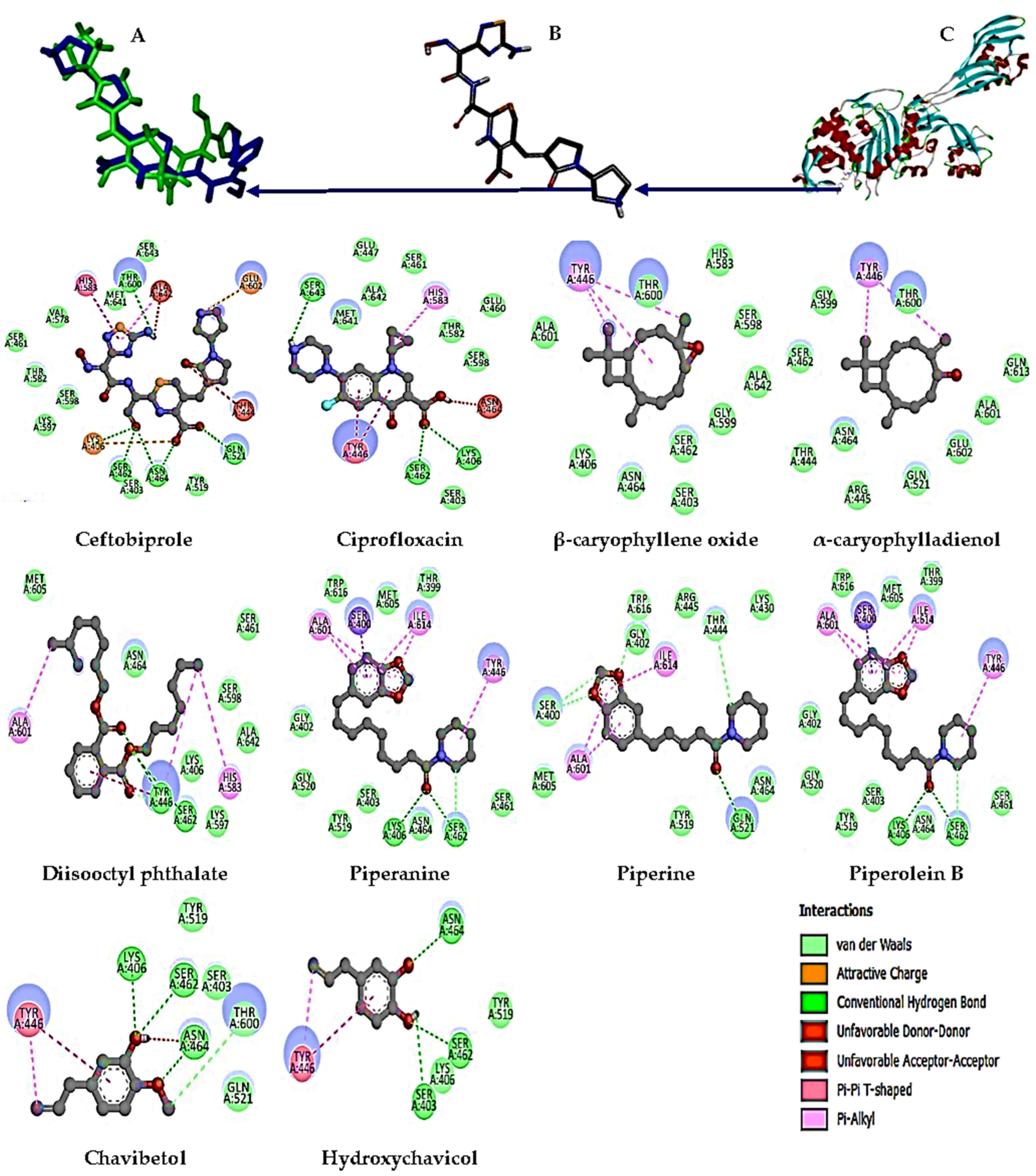
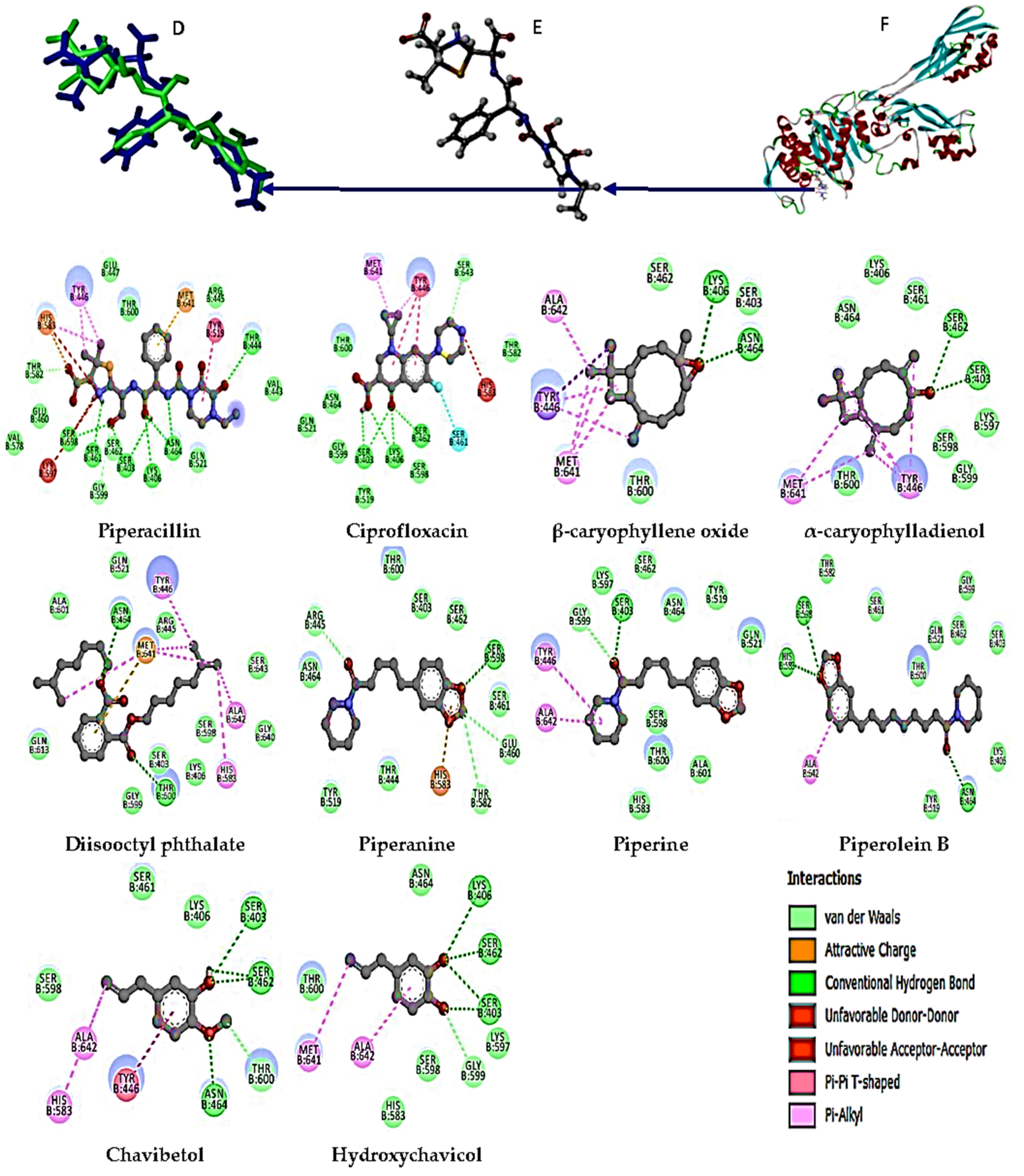
2.6. Molecular Docking Studies
2.6.1. Sample Preparation (Virtual Screening)
2.6.2. Molecular Docking
3. Results and Discussion
3.1. Extraction Optimization
3.2. FTIR Profiling Analysis
3.3. Anti-MRSA Activity
3.4. GC-MS Metabolite Characterization
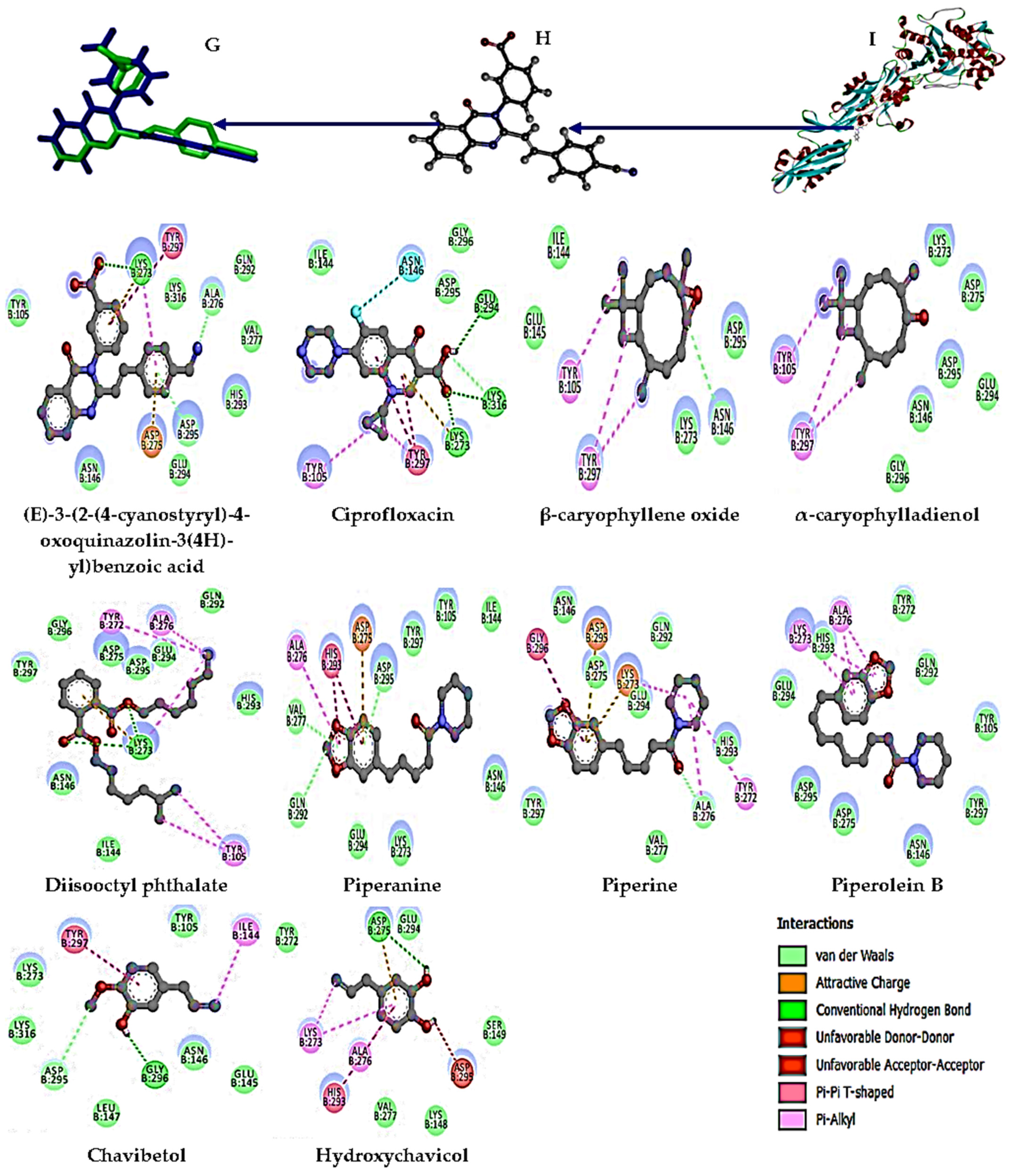
3.5. Molecular Docking Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashabsheh, R.H.; Al-Fawares, O.L.; Natsheh, I.; Bdeir, R.; Al-Khreshieh, R.O.; Bashabsheh, H.H. Staphylococcus aureus Epidemiology, Pathophysiology, Clinical Manifestations, and Application of Nano-Therapeutics as a Promising Approach to Combat Methicillin-Resistant Staphylococcus aureus. Pathogens Glob. Health 2024, 118, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G., Jr.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic Resistance in the Patient with Cancer: Escalating Challenges and Paths Forward. CA Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.J.; Aureden, K.; Ham, D.C.; Harris, A.D.; Hessels, A.J.; Huang, S.S.; Maragakis, L.L.; Milstone, A.M.; Moody, J.; Yokoe, D.; et al. SHEA/IDSA/APIC Practice Recommendation: Strategies to Prevent Methicillin-Resistant Staphylococcus aureus Transmission and Infection in Acute-Care Hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 1039–1067. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, L.E.; Gálvez-Acebal, J.; Rodríguez-Baño, J. Therapy of Staphylococcus aureus Bacteremia: Evidences and Challenges. Enferm. Infecc. Microbiol. Clin. 2020, 38, 489–497. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds Against Multidrug-Resistant Bacteria (MDR). Evid. Based Complement. Alternat. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef]
- Tran, V.T.; Nguyen, T.B.; Nguyen, H.C.; Do, N.H.; Le, P.K. Recent Applications of Natural Bioactive Compounds from Piper betle (L.) Leaves in Food Preservation. Food Control 2023, 154, 110026. [Google Scholar] [CrossRef]
- Ali, H.S.; Mishra, S. Natural Products as Antiparasitic, Antifungal, and Antibacterial Agents. In Drugs from Nature: Targets, Assay Systems and Leads; Springer: Singapore, 2024; pp. 367–409. [Google Scholar]
- Ueda, J.M.; Milho, C.; Heleno, S.A.; Soria-Lopez, A.; Carpena, M.; Alves, M.J.; Pires, T.; Prieto, M.A.; Simal-Gandara, J.; Calhelha, R.C.; et al. Emerging Strategies to Combat Methicillin-Resistant Staphylococcus aureus (MRSA): Natural Agents with High Potential. Curr. Pharm. Des. 2023, 29, 837–851. [Google Scholar] [CrossRef]
- Ali, R.A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar]
- Verma, N.; Gautam, B.S. Piper betle: Deep Insights into the Pharmacognostic and Pharmacological Perspectives. Int. J. Med. Phar. Sci. 2024, 13, 4. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Chandrakasan, G.; García-Trejo, J.F.; Feregrino-Pérez, A.A.; Aguirre-Becerra, H.; García, E.R.; Nieto-Ramírez, M.I. Preliminary Screening on Antibacterial Crude Secondary Metabolites Extracted from Bacterial Symbionts and Identification of Functional Bioactive Compounds by FTIR, HPLC, and Gas Chromatography–Mass Spectrometry. Molecules 2024, 29, 2914. [Google Scholar] [CrossRef]
- Noor, A.; Kamaraj, S.; Bhakti, M.A.; Kumari, C.; Kulshrestha, S.; Pandohee, J.; That, L.F.L.N. Analysis of Volatile Organic Compounds in Honey Using Gas Chromatography and Mass Spectrometry. In Advanced Techniques of Honey Analysis; Academic Press: Cambridge, MA, USA, 2024; pp. 287–307. [Google Scholar]
- Cucinotta, L.; Rotondo, A.; Coppolino, S.; Salerno, T.M. Gas Chromatographic Techniques and Spectroscopic Approaches for a Deep Characterization of Piper gaudichaudianum Kunth Essential Oil from Brazil. J. Chromatogr. A 2024, 1732, 465208. [Google Scholar] [CrossRef]
- Palermo, A. Metabolomics-and Systems-Biology-Guided Discovery of Metabolite Lead Compounds and Druggable Targets. Drug Discov. Today 2023, 28, 103460. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Ou-Yahia, D.; Dalli, M.; Bellaouchi, R.; Tikent, A.; Roubi, M.; Gseyra, N.; Asehraou, A.; Hano, C.; et al. Assessment of the Antioxidant and Antimicrobial Potential of Ptychotis verticillata Duby Essential Oil from Eastern Morocco: An In Vitro and In Silico Analysis. Antibiotics 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Yasir, B.; Astuti, A.D.; Raihan, M.; Natzir, R.; Subehan, S.; Rohman, A.; Alam, G. Optimization of Pagoda (Clerodendrum paniculatum L.) Extraction Based on Analytical Factorial Design Approach, Its Phytochemical Compound, and Cytotoxicity Activity. Egypt J. Chem. 2022, 65, 421–430. [Google Scholar] [CrossRef]
- Yasir, B.; Alam, G. Chemometrics-Assisted Fingerprinting Profiling of Extract Variation from Pagoda (Clerodendrum paniculatum L.) Using TLC-Densitometric Method. Egypt J. Chem. 2023, 66, 1589–1596. [Google Scholar] [CrossRef]
- Roshan, M.; Singh, I.; Vats, A.; Behera, M.; Singh, D.P.; Gautam, D.; Rajput, S.; Tarak, J.; Packirisamy, G.; De, S. Antimicrobial and Antibiofilm Effect of Cannabinoids from Cannabis sativa Against Methicillin-Resistant Staphylococcus aureus (MRSA) Causing Bovine Mastitis. Int. Microbiol. 2024, 1–14. [Google Scholar] [CrossRef]
- Martí, M.; Frígols, B.; Serrano-Aroca, A. Antimicrobial Characterization of Advanced Materials for Bioengineering Applications. J. Vis. Exp. 2018, 138, 57710. [Google Scholar]
- Alharthi, S.S. Bioactive Extracts of Plant Byproducts as Sustainable Solution of Water Contaminants Reduction. Arab. J. Chem. 2024, 17, 105864. [Google Scholar] [CrossRef]
- Sruthi, D.; Zachariah, T.J. Volatile and Non-Volatile Metabolite Profiling of Under-Explored Crop Piper colubrinum Link: With Chromatography Mass Spectrometry Approach and Its Biochemical Diversity from Medicinally Valued Piper Species. J. Chromatogr. B 2024, 1245, 124260. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chen, Z.; Zhang, N.; Jiang, P. High Throughput Virtual Screening and Validation of Plant-Based EGFR L858R Kinase Inhibitors Against Non-Small Cell Lung Cancer: An Integrated Approach Utilizing GC–MS, Network Pharmacology, Docking, and Molecular Dynamics. Saudi Pharm. J. 2024, 32, 102139. [Google Scholar] [CrossRef] [PubMed]
- Hendrarti, W.; Umar, A.H.; Syahruni, R.; Rafi, M.; Kusuma, W.A. Deciphering the Mechanism of Action Cosmos caudatus Compounds Against Breast Neoplasm: A Combination of Pharmacological Networking and Molecular Docking Approach with Bibliometric Analysis. Indones. J. Sci. Technol. 2024, 9, 527–556. [Google Scholar]
- Nguyen, L.T.T.; Nguyen, T.T.; Nguyen, H.N.; Bui, Q.T.P. Simultaneous Determination of Active Compounds in Piper betle Linn. Leaf Extract and Effect of Extracting Solvents on Bioactivity. Eng. Rep. 2020, 2, 10. [Google Scholar]
- Rajopadhye, A.A.; Namjoshi, T.P.; Upadhye, A.S. Rapid Validated HPTLC Method for Estimation of Piperine and Piperlongumine in Root of Piper longum Extract and Its Commercial Formulation. Rev. Bras. Farmacogn. 2012, 22, 1355–1361. [Google Scholar] [CrossRef]
- Luo, J.; Xiang, J.Y.; Yuan, H.Y.; Wu, J.Q.; Li, H.Z.; Shen, Y.H.; Xu, M. Isolation, Synthesis and Absolute Configuration of 4,5-Dihydroxypiperines Improving Behavioral Disorder in AlCl3-Induced Dementia. Bioorg. Med. Chem. Lett. 2021, 42, 128057. [Google Scholar]
- Nik, W.W.; Ravindran, K.; Al-Amiery, A.A.; Izionworu, V.O.; Fayomi, O.S.I.; Berdimurodov, E.; Daoudi, W.; Zulkifli, F.; Dhande, D.Y. Piper betle Extract as an Eco-Friendly Corrosion Inhibitor for Aluminium Alloy in Hydrochloric Acid Media. Int. J. Corros. Scale Inhib. 2023, 12, 948–960. [Google Scholar]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Fan, R.; Qin, X.W.; Hu, R.S.; Hu, L.S.; Wu, B.D.; Hao, C.Y. Studies on the Chemical and Flavour Qualities of White Pepper (Piper nigrum L.) Derived from Grafted and Non-Grafted Plants. Eur. Food Res. Technol. 2020, 246, 2601–2610. [Google Scholar]
- Ramesh, B.; Sarma, V.U.M.; Kumar, K.; Babu, K.S.; Devi, P.S. Simultaneous Determination of Six Marker Compounds in Piper nigrum L. and Species Comparison Study Using High-Performance Thin-Layer Chromatography–Mass Spectrometry. J. Planar Chromatogr. 2015, 28, 280–286. [Google Scholar]
- Sateesh Reddy, K.; Siva, B.; Divya Reddy, S.; Kumar, K.; Pratap, T.V.; Vidyasagar Reddy, K.; Venkateswara Rao, B.; Suresh Babu, K. Monitoring of Chemical Markers in Extraction of Traditional Medicinal Plants (Piper nigrum, Curcuma longa) Using In Situ ReactIR. J. AOAC Int. 2021, 104, 1181–1187. [Google Scholar] [CrossRef]
- Musa, T.A.; Sanagi, M.M.; Wan Ibrahim, W.A.; Ahmad, F.; Aboul-Enein, H.Y. Determination of 4-Allyl Resorcinol and Chavibetol from Piper betle Leaves by Subcritical Water Extraction Combined with High-Performance Liquid Chromatography. Food Anal. Methods 2014, 7, 893–901. [Google Scholar] [CrossRef]
- Rahmah, N.L.; Mustapa Kamal, S.M.; Sulaiman, A.; Taip, F.S.; Siajam, S.I. Subcritical Water Extraction of Total Phenolic Compounds from Piper betle L. Leaves: Effect of Process Conditions and Characterization. J. Food Meas. Charact. 2023, 17, 5606–5618. [Google Scholar] [CrossRef]
- Bouguettaya, A.; Yu, Q.; Liu, X.; Zhou, X.; Song, A. Efficient Agglomerative Hierarchical Clustering. Expert Syst. Appl. 2015, 42, 2785–2797. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Z.; Shang, Z.; Zhao, J. Classification and Identification of Rhodobryum roseum Limpr. and Its Adulterants Based on Fourier-Transform Infrared Spectroscopy (FTIR) and Chemometrics. PLoS ONE 2017, 12, e0170650. [Google Scholar] [CrossRef]
- Jantorn, P.; Tipmanee, V.; Wanna, W.; Prapasarakul, N.; Visutthi, M.; Sotthibandhu, D.S. Potential Natural Antimicrobial and Antibiofilm Properties of Piper betle L. Against Staphylococcus pseudintermedius and Methicillin-Resistant Strains. J. Ethnopharmacol. 2023, 317, 116820. [Google Scholar] [CrossRef]
- Khan, G.J.; Humma, Z.E.; Omer, M.O.; Sattar, A.; Altaf, I.; Chen, Z.; He, N. Effects of Curcuma longa L. and Piper nigrum L. Against Methicillin-Resistant Staphylococcus aureus and Infectious Angiogenesis. J. Biobased Mater. Bioenergy 2024, 18, 303–314. [Google Scholar] [CrossRef]
- Umapathy, V.R.; Dhanavel, A.; Kesavan, R.; Natarajan, P.M.; Bhuminathan, S.; Vijayalakshmi, P. Anticancer Potential of the Principal Constituent of Piper nigrum, Piperine: A Comprehensive Review. Cureus 2024, 16, e44287. [Google Scholar] [CrossRef]
- Das, S.; Malik, M.; Dastidar, D.G.; Roy, R.; Paul, P.; Sarkar, S.; Tribedi, P. Piperine, a Phytochemical, Prevents the Biofilm Formation of Methicillin-Resistant Staphylococcus aureus: A Biochemical Approach to Understand the Underlying Mechanism. Microb. Pathog. 2024, 189, 106601. [Google Scholar] [CrossRef]
- Ahirrao, P.; Tambat, R.; Chandal, N.; Mahey, N.; Kamboj, A.; Jain, U.K.; Singh, I.P.; Jachak, S.M.; Nandanwar, H.S. MsrA Efflux Pump Inhibitory Activity of Piper cubeba L.f. and Its Phytoconstituents Against S. aureus RN4220. Chem. Biodivers. 2020, 17, e2000144. [Google Scholar] [CrossRef]
- Driche, E.H.; Belghit, S.; Bijani, C.; Zitouni, A.; Sabaou, N.; Mathieu, F.; Badji, B. A New Streptomyces Strain Isolated from Saharan Soil Produces Di-(2-Ethylhexyl) Phthalate, a Metabolite Active Against Methicillin-Resistant Staphylococcus aureus. Ann. Microbiol. 2015, 65, 1341–1350. [Google Scholar] [CrossRef]
- Zamakshshari, N.; Ahmed, I.A.; Nasharuddin, M.N.; Hashim, N.M.; Mustafa, M.R.; Othman, R.; Noordin, M.I. Effect of Extraction Procedure on the Yield and Biological Activities of Hydroxychavicol from Piper betle L. Leaves. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100320. [Google Scholar] [CrossRef]
- Leesombun, A.; Sungpradit, S.; Bangphoomi, N.; Thongjuy, O.; Wechusdorn, J.; Riengvirodkij, S.; Boonmasawai, S. Effects of Piper betle Extracts Against Biofilm Formation by Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Dogs. Pharmaceuticals 2023, 16, 741. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, I.; Louis, H.; Ekpen, O.F.; Gber, T.E.; Gideon, M.E.; Ahmad, I.; Ejiofor, E.U. Modeling the Anti-Methicillin-Resistant Staphylococcus aureus (MRSA) Activity of (E)-6-Chloro-N2-Phenyl-N4-(4-Phenyl-5-(Phenyl Diazinyl)-2λ3,3λ2-Thiazol-2-yl)-1,3,5-Triazine-2,4-Diamine. Polycycl. Aromat. Compd. 2023, 43, 7942–7969. [Google Scholar] [CrossRef]
- Lovering, A.L.; Gretes, M.C.; Safadi, S.S.; Danel, F.; De Castro, L.; Page, M.G.; Strynadka, N.C. Structural Insights into the Anti-Methicillin-Resistant Staphylococcus aureus (MRSA) Activity of Ceftobiprole. J. Biol. Chem. 2012, 287, 32096–32102. [Google Scholar] [CrossRef]
- Janardhanan, J.; Bouley, R.; Martínez-Caballero, S.; Peng, Z.; Batuecas-Mordillo, M.; Meisel, J.E.; Chang, M. The Quinazolinone Allosteric Inhibitor of PBP 2a Synergizes with Piperacillin and Tazobactam Against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Bouley, R.; Kumarasiri, M.; Peng, Z.; Otero, L.H.; Song, W.; Suckow, M.A.; Mobashery, S. Discovery of Antibiotic (E)-3-(3-Carboxyphenyl)-2-(4-Cyanostyryl) Quinazolin-4(3H)-One. J. Am. Chem. Soc. 2015, 137, 1738–1741. [Google Scholar] [CrossRef]
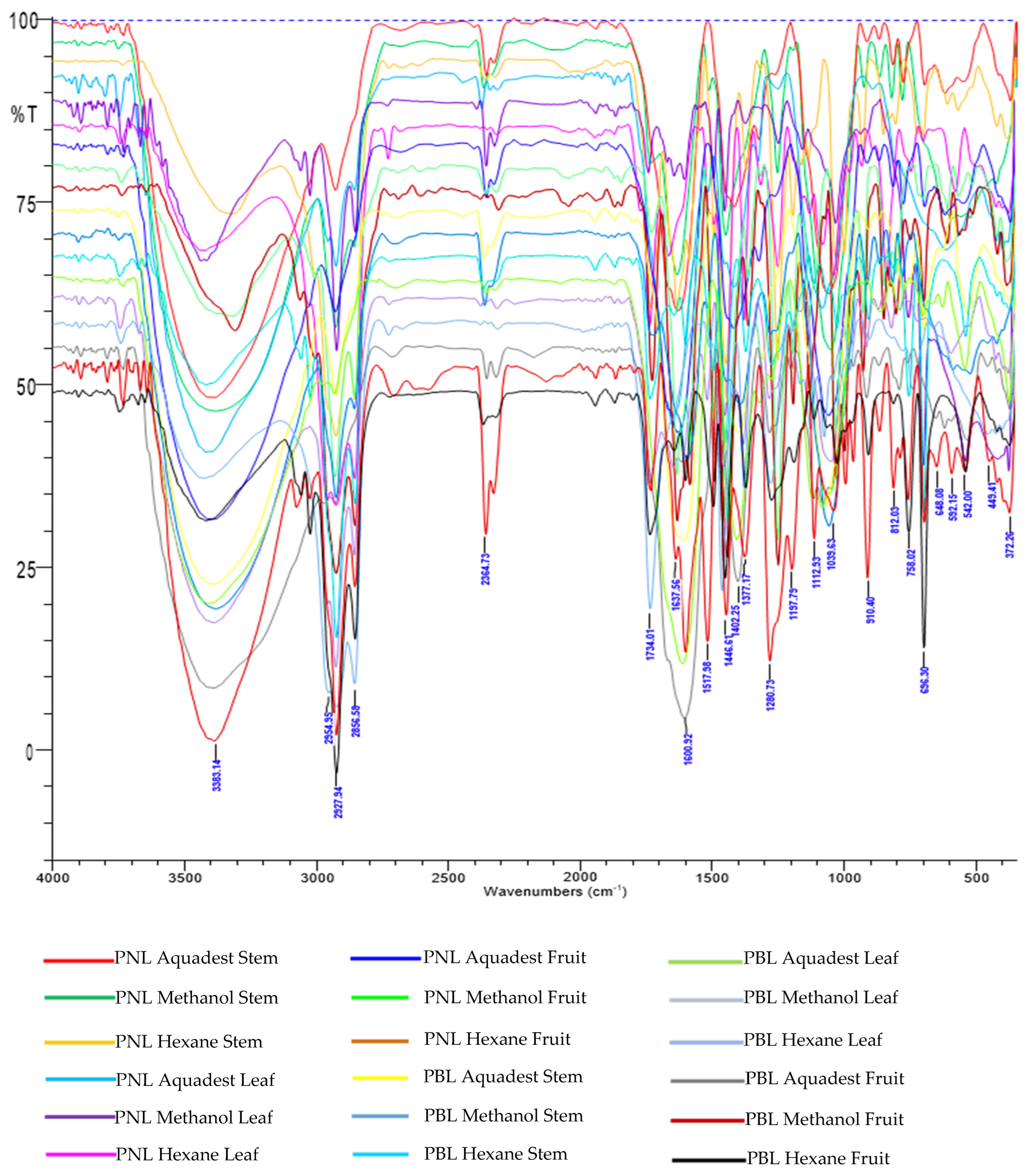
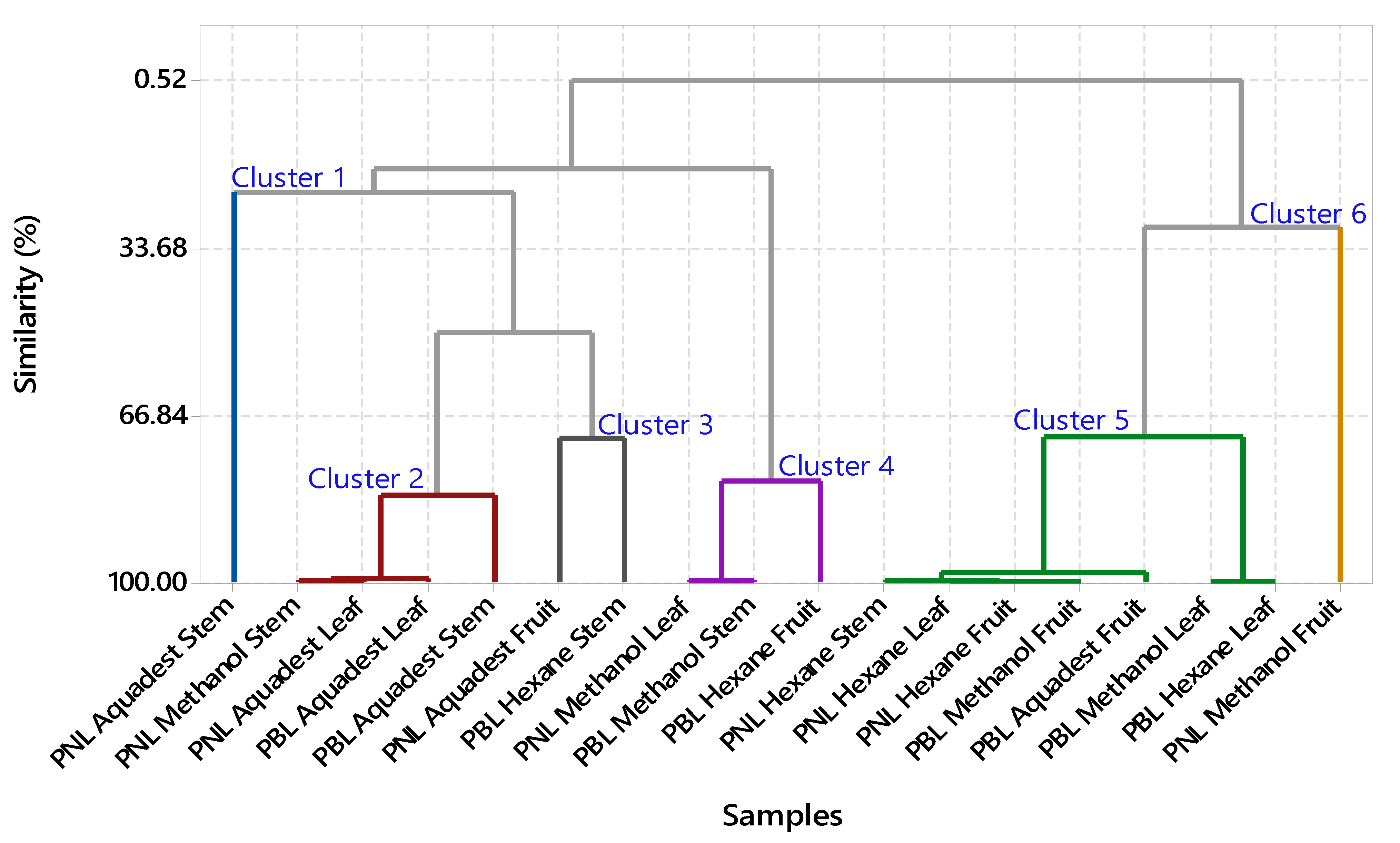
| Code | Sample | Plant Parts | Solvent | Extract (g) | Yield (%) |
|---|---|---|---|---|---|
| PNL | Piper nigrum | Stem | Aquadest | 0.83 | 4.15 |
| Methanol | 1.07 | 5.35 | |||
| Hexane | 0.13 | 0.65 | |||
| Leaf | Aquadest | 0.63 | 3.15 | ||
| Methanol | 0.43 | 2.15 | |||
| Hexane | 0.62 | 3.10 | |||
| Fruit | Aquadest | 0.33 | 1.65 | ||
| Methanol | 1.14 | 5.70 | |||
| Hexane | 0.4 | 2.00 | |||
| PBL | Piper betle | Stem | Aquadest | 0.33 | 1.65 |
| Methanol | 0.82 | 4.10 | |||
| Hexane | 0.11 | 0.55 | |||
| Leaf | Aquadest | 0.89 | 4.45 | ||
| Methanol | 0.67 | 3.35 | |||
| Hexane | 0.09 | 0.45 | |||
| Fruit | Aquadest | 1.36 | 6.80 | ||
| Methanol | 2.35 | 11.75 | |||
| Hexane | 0.54 | 2.70 |
| Sample | Inhibition Zone (mm) | Tukey Simultaneous Tests for Differences in Means | StDev | |||||
|---|---|---|---|---|---|---|---|---|
| Difference in Levels | Difference in Means | SE of Difference | 95% CI | T-Value | p-Value | |||
| PNL Aquadest Stem Extract | 8 | PNL Aquadest Leaf Extract—PNL Aquadest Stem Extract | −2.667 | 0.797 | (−5.388, 0.055) | −3.35 | 0.056 | 1.000 |
| 10 | PNL Aquadest Fruit Extract—PNL Aquadest Stem Extract | 4.667 | 0.797 | (1.945, 7.388) | 5.86 | 0.001 | ||
| 9 | PNL Methanol Fruit Extract—PNL Aquadest Stem Extract | 5.667 | 0.797 | (2.945, 8.388) | 7.11 | 0.000 | ||
| PNL Aquadest Leaf Extract | 6 | PBL Methanol Stem Extract—PNL Aquadest Stem Extract | 5.000 | 0.797 | (2.279, 7.721) | 6.27 | 0.000 | 0.577 |
| 6 | PBL Hexane Fruit Extract—PNL Aquadest Stem Extract | 10.333 | 0.797 | (7.612, 13.055) | 12.97 | 0.000 | ||
| 7 | Ciprofloxacin— PNL Aquadest Stem Extract | 16.667 | 0.797 | (13.945, 19.388) | 20.92 | 0.000 | ||
| PNL Aquadest Fruit Extract | 14 | PNL Aquadest Fruit Extract—PNL Aquadest Leaf Extract | 7.333 | 0.797 | (4.612, 10.055) | 9.20 | 0.000 | 0.577 |
| 14 | PNL Methanol Fruit Extract—PNL Aquadest Leaf Extract | 8.333 | 0.797 | (5.612, 11.055) | 10.46 | 0.000 | ||
| 13 | PBL Methanol Stem Extract—PNL Aquadest Leaf Extract | 7.667 | 0.797 | (4.945, 10.388) | 9.62 | 0.000 | ||
| PNL Methanol Fruit Extract | 14 | PBL Hexane Fruit Extract—PNL Aquadest Leaf Extract | 13.000 | 0.797 | (10.279, 15.721) | 16.31 | 0.000 | 0.577 |
| 15 | Ciprofloxacin— PNL Aquadest Leaf Extract | 19.333 | 0.797 | (16.612, 22.055) | 24.26 | 0.000 | ||
| 15 | PNL Methanol Fruit Extract—PNL Aquadest Fruit Extract | 1.000 | 0.797 | (−1.721, 3.721) | 1.25 | 0.861 | ||
| PBL Methanol Stem Extract | 14 | PBL Methanol Stem Extract—PNL Aquadest Fruit Extract | 0.333 | 0.797 | (−2.388, 3.055) | 0.42 | 0.999 | 0.000 |
| 14 | PBL Hexane Fruit Extract—PNL Aquadest Fruit Extract | 5.667 | 0.797 | (2.945, 8.388) | 7.11 | 0.000 | ||
| 14 | Ciprofloxacin— PNL Aquadest Fruit Extract | 12.000 | 0.797 | (9.279, 14.721) | 15.06 | 0.000 | ||
| PBL Hexane Fruit Extract | 20 | PBL Methanol Stem Extract—PNL Methanol Fruit Extract | −0.667 | 0.797 | (−3.388, 2.055) | −0.84 | 0.976 | 0.577 |
| 19 | PBL Hexane Fruit Extract—PNL Methanol Fruit Extract | 4.667 | 0.797 | (1.945, 7.388) | 5.86 | 0.001 | ||
| 19 | Ciprofloxacin— PNL Methanol Fruit Extract | 11.000 | 0.797 | (8.279, 13.721) | 13.80 | 0.000 | ||
| Ciprofloxacin | 24 | PBL Hexane Fruit Extract—PBL Methanol Stem Extract | 5.333 | 0.797 | (2.612, 8.055) | 6.69 | 0.000 | 2.080 |
| 28 | Ciprofloxacin— PBL Methanol Stem Extract | 11.667 | 0.797 | (8.945, 14.388) | 14.64 | 0.000 | ||
| 25 | Ciprofloxacin— PBL Hexane Fruit Extract | 6.333 | 0.797 | (3.612, 9.055) | 7.95 | 0.000 | ||
| Compounds Detected | Molecular Formula | MW (g/mol) | PubChem (CID) | RT (min) | Area (%) | SI (%) |
|---|---|---|---|---|---|---|
| Piper nigrum hexane fruit extract | ||||||
| β-caryophyllene oxide | C15H24O | 220 | 1742210 | 13.563 | 1.01 | 97 |
| α-caryophylladienol | C15H24O | 220 | 14524923 | 14.276 | 1.69 | 97 |
| Pellitorine | C14H25NO | 223 | 5318516 | 18.772 | 5.08 | 93 |
| n-Hexadecanoic acid | C16H32O2 | 256 | 985 | 19.279 | 2.51 | 96 |
| Octadecanoic acid | C18H36O2 | 284 | 5281 | 23.877 | 1.42 | 93 |
| Diisooctyl phthalate | C24H38O4 | 390 | 33934 | 31.081 | 14.67 | 95 |
| (2E,4E)-N-Isobutylhexadeca-2,4-dienamide | C20H37NO | 307 | 6442402 | 31.595 | 1.78 | 90 |
| Piperanine | C17H21NO3 | 287 | 5320618 | 32.804 | 4.03 | 94 |
| Piperine | C17H19NO3 | 285 | 638024 | 37.762 | 14.22 | 93 |
| Piperolein B | C21H29NO3 | 343 | 21580213 | 41.639 | 1.58 | 93 |
| Piper betle aquadest fruit extract | ||||||
| Chavibetol | C10H12O2 | 164 | 596375 | 10.420 | 12.01 | 97 |
| Hydroxychavicol | C9H10O2 | 150 | 70775 | 12.496 | 81.89 | 93 |
| Compounds | Protein Target | Bond-Free Energy (kcal/mol) | H-Bond Interaction |
|---|---|---|---|
| Ceftobiprole (Native ligand) | 4DKI | −9.8 | LYS 406, SER 462, ASN 464, GLN 521, THR 600 |
| Ciprofloxacin | −8.3 | LYS 406, SER 462, SER 643 | |
| β-caryophyllene oxid | −6.4 | NI | |
| α-caryophylladienol | −6.4 | NI | |
| Diisooctyl phthalate | −7.1 | SER 462, TYR 446 | |
| Piperanine | −7.6 | LYS 406, SER 462 | |
| Piperine | −7.7 | GLN 521, THR 444, GLY 402, SER 400 | |
| Piperolein B | −8.3 | LYS 406, SER 462, ASN 464 | |
| Chavibetol | −5.7 | THR 600, ASN 464, SER 462, LYS 406 | |
| Hydroxychavicol | −5.7 | SER 462, ASN 464, SER 403 | |
| Piperacillin (Native ligand) | 6H5O | −9.0 | THR 444, SER 598, SER 461, SER 403, LYS 406, ASN 464 |
| Ciprofloxacin | −8.1 | LYS 406, SER 403, SER 462, SER 643 | |
| β-Caryophyllene oxide | −6.9 | ASN 464, LYS 406 | |
| α-Caryophylladienol | −7.1 | SER 403, SER 462 | |
| Diisooctyl phthalate | −6.3 | ASN 464, THR 600 | |
| Piperanine | −7.3 | SER 598, THR 582, GLU 460, ARG 445 | |
| Piperine | −7.3 | SER 403, GLY 599 | |
| Piperolein B | −6.8 | ASN 464, SER 598, HIS 583 | |
| Chavibetol | −5.4 | SER 403, ASN 464, SER 462, THR 600 | |
| Hydroxychavicol | −5.6 | LYS 406, SER 403, SER 462, GLY 599 | |
| (E)-3-(2-(4-cyanostyryl)-4-oxoquinazolin-3(4H)-yl)benzoic acid (Native ligand) | 4CJN | −7.2 | LYS 273, ALA 276, ASP 295 |
| Ciprofloxacin | −6.2 | LYS 273, LYS 316, GLU 294 | |
| β-caryophyllene oxide | −5.2 | ASN 146 | |
| α-caryophylladienol | −5.2 | NI | |
| Diisooctyl phthalate | −5.0 | LYS 273 | |
| Piperanine | −6.0 | ASP 295, VAL 277, GLN 292 | |
| Piperine | −6.5 | ALA 276 | |
| Piperolein B | −5.9 | NI | |
| Chavibetol | −4.6 | ASP 295, GLY 296 | |
| Hydroxychavicol | −4.9 | ASP 275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasir, B.; Mus, S.; Rahimah, S.; Tandiongan, R.M.; Klara, K.P.; Afrida, N.; Nisaa, N.R.K.; Risna, R.; Jur, A.W.; Alam, G.; et al. Antimicrobial Profiling of Piper betle L. and Piper nigrum L. Against Methicillin-Resistant Staphylococcus aureus (MRSA): Integrative Analysis of Bioactive Compounds Based on FT-IR, GC-MS, and Molecular Docking Studies. Separations 2024, 11, 322. https://doi.org/10.3390/separations11110322
Yasir B, Mus S, Rahimah S, Tandiongan RM, Klara KP, Afrida N, Nisaa NRK, Risna R, Jur AW, Alam G, et al. Antimicrobial Profiling of Piper betle L. and Piper nigrum L. Against Methicillin-Resistant Staphylococcus aureus (MRSA): Integrative Analysis of Bioactive Compounds Based on FT-IR, GC-MS, and Molecular Docking Studies. Separations. 2024; 11(11):322. https://doi.org/10.3390/separations11110322
Chicago/Turabian StyleYasir, Budiman, Suwahyuni Mus, Sitti Rahimah, Rein Mostatian Tandiongan, Kasandra Putri Klara, Nurul Afrida, Nur Rezky Khairun Nisaa, Risna Risna, Agum Wahyudha Jur, Gemini Alam, and et al. 2024. "Antimicrobial Profiling of Piper betle L. and Piper nigrum L. Against Methicillin-Resistant Staphylococcus aureus (MRSA): Integrative Analysis of Bioactive Compounds Based on FT-IR, GC-MS, and Molecular Docking Studies" Separations 11, no. 11: 322. https://doi.org/10.3390/separations11110322
APA StyleYasir, B., Mus, S., Rahimah, S., Tandiongan, R. M., Klara, K. P., Afrida, N., Nisaa, N. R. K., Risna, R., Jur, A. W., Alam, G., & Rohman, A. (2024). Antimicrobial Profiling of Piper betle L. and Piper nigrum L. Against Methicillin-Resistant Staphylococcus aureus (MRSA): Integrative Analysis of Bioactive Compounds Based on FT-IR, GC-MS, and Molecular Docking Studies. Separations, 11(11), 322. https://doi.org/10.3390/separations11110322









