Eco-Friendly Green Approach to the Biosorption of Hazardous Dyes from Aqueous Solution on Ragweed (Ambrosia artemisiifolia) Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Sample Collection and Pretreatment of A. artemisiifolia Biomass
2.3. Preparation of Magnetic Biosorbent (Fe3O4/RWB)
2.4. Biosorption Studies
3. Results and Discussion
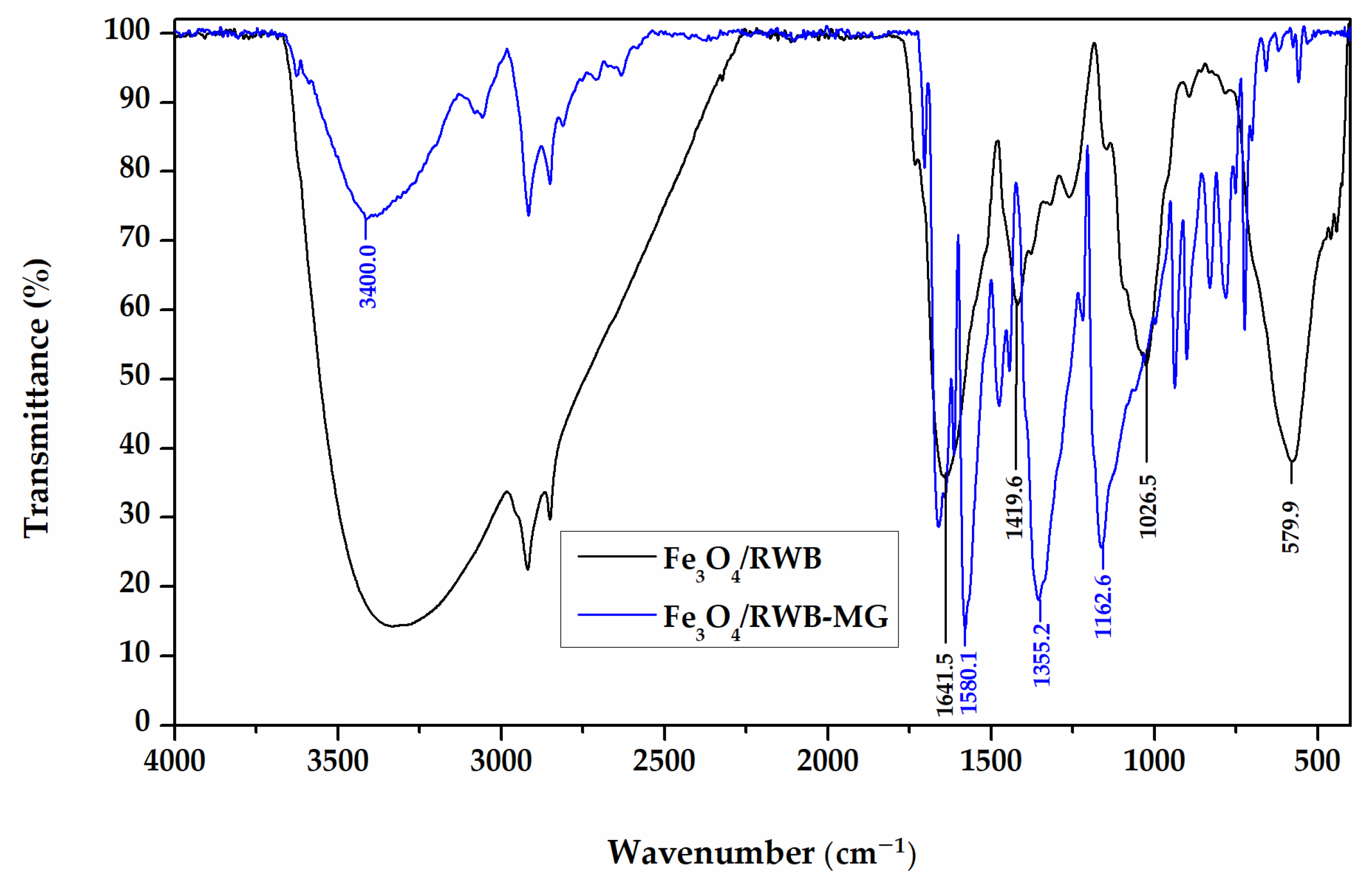
3.1. Characterization of Fe3O4/RWB
3.2. Batch Biosorption Study
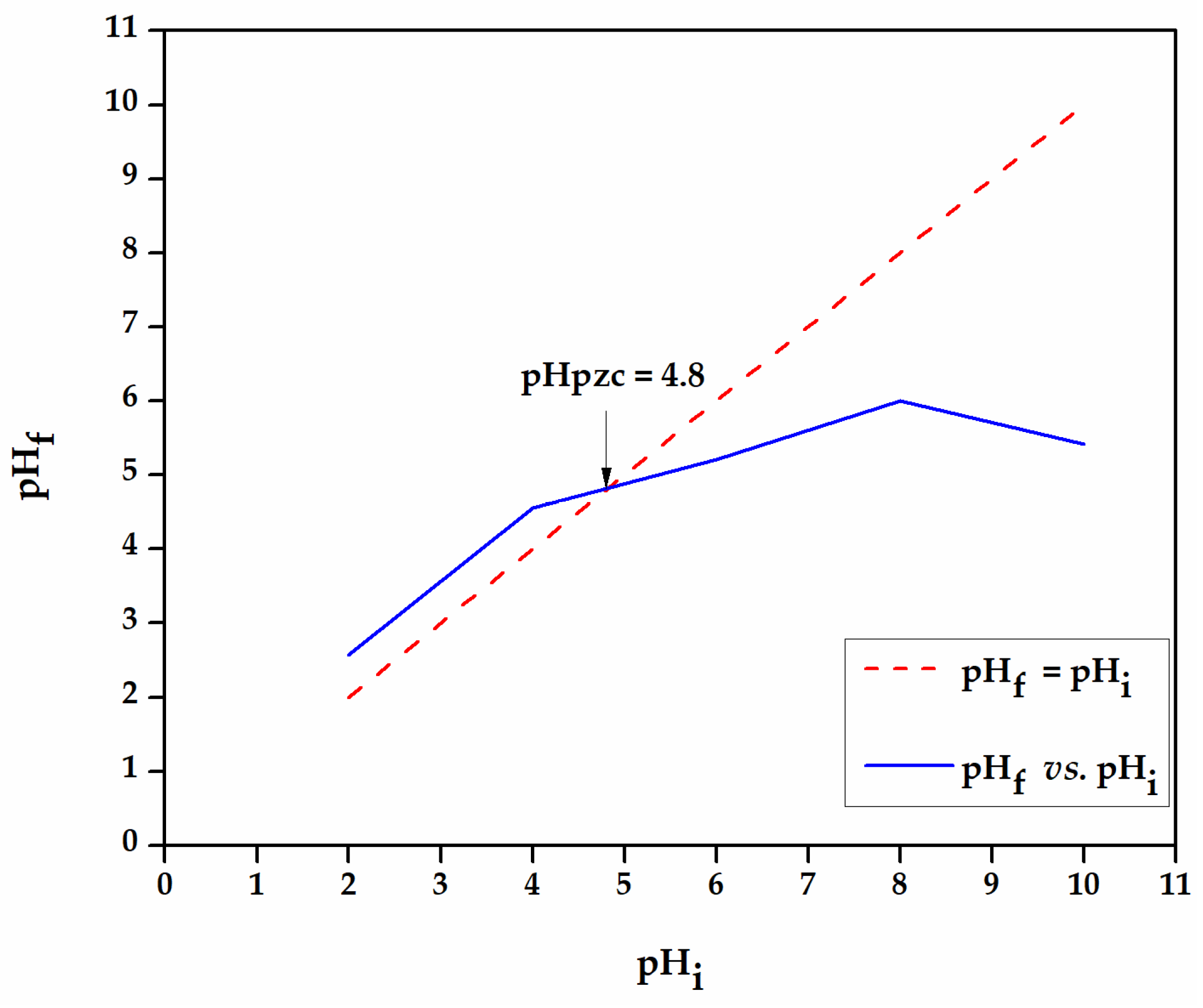
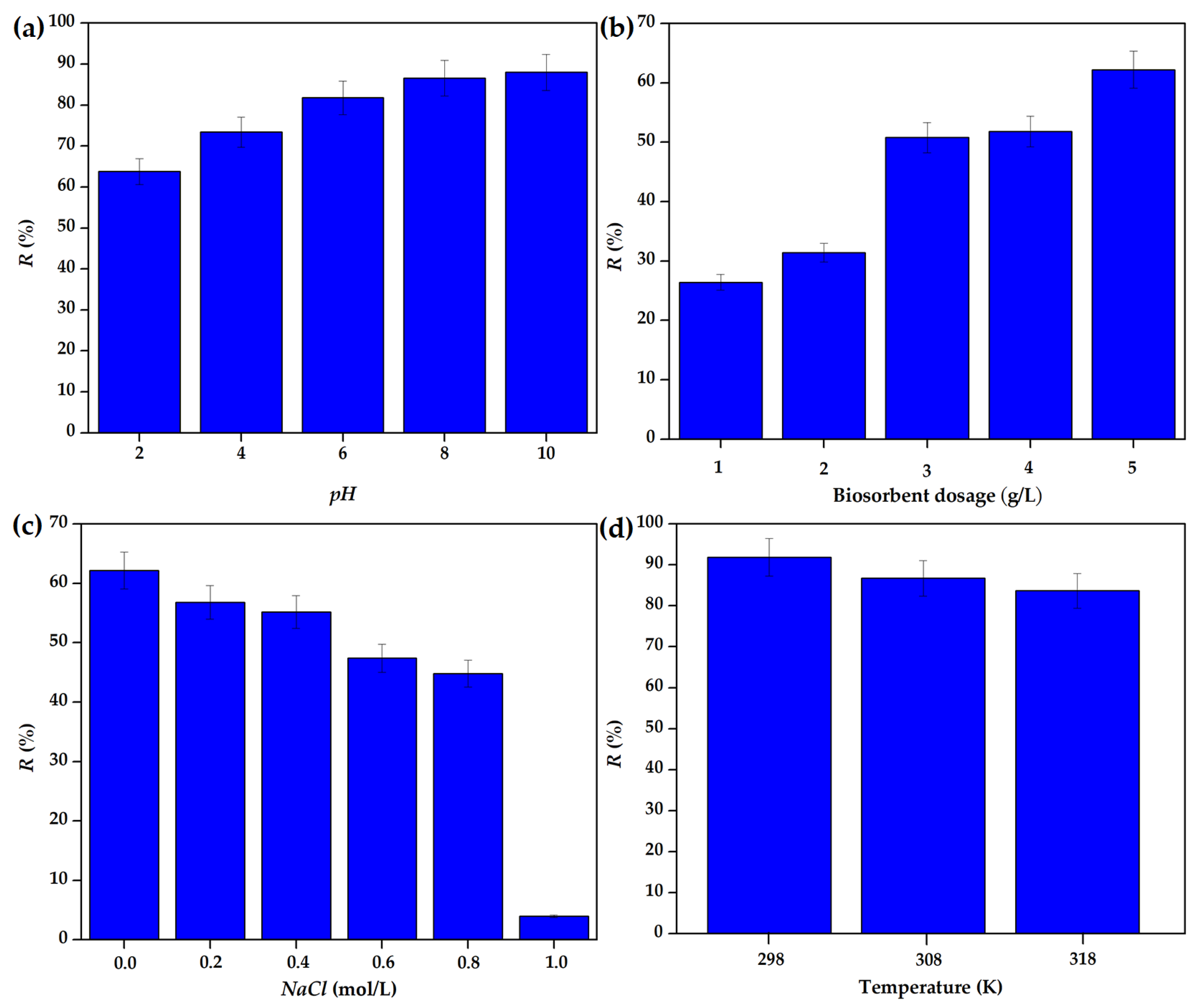
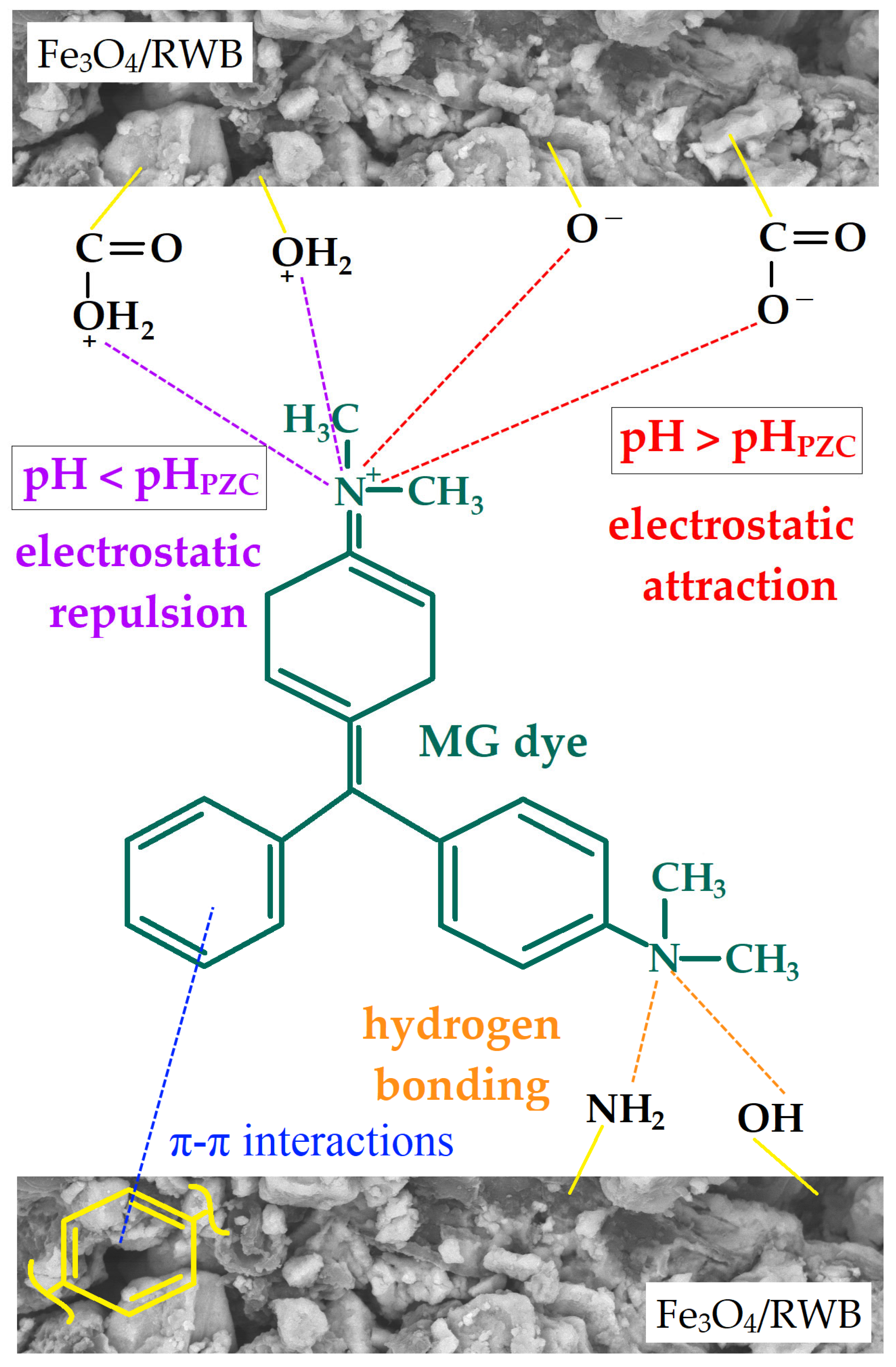
3.2.1. Effect of pH
3.2.2. Effect of Sorbent Dose
3.2.3. Effect of Ionic Strength
3.2.4. Biosorption Thermodynamics
3.2.5. Biosorption Kinetic
3.2.6. Biosorption Isotherms
3.2.7. Comparation Studies
3.3. Reusability of Fe3O4/RWB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Hadj Ali, Y.A.; Hejji, L.; Seddik, N.B.; Azzouz, A.; Pérez-Villarejo, L.; Stitou, M.; Sonne, C. Remediation of malachite-green dye from textile wastewater using biosorbent almond shell-based cellulose. J. Mol. Liq. 2024, 399, 124435. [Google Scholar] [CrossRef]
- Yunusa, U.; Bishir, U.; Ibrahim, M.B. Kinetic and thermodynamic studies of malachite green adsorption using activated carbon prepared from desert date seed shell. Alger. J. Eng. Technol. 2020, 2, 37–45. [Google Scholar]
- Li, S.; Tao, M. Removal of cationic dyes from aqueous solutions by a low-cost biosorbent: Longan shell. Desal. Water Treat. 2016, 57, 5193–5199. [Google Scholar] [CrossRef]
- Gao, Y.; Chang, H.; Liang, D.; Yang, X.Y.; Chen, Z.Q.; Liu, X. Harnessing Prunella vulgaris L. residues for enhanced biosorption of dyes in wastewater. Chem. Eng. Res. Des. 2023, 200, 469–480. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Biosorption of methylene blue and malachite green from single and binary solutions by Pinus pinaster bark. Maderas-Cienc. Tecnol. 2024, 26, 1–12. [Google Scholar] [CrossRef]
- Pathy, A.; Krishnamoorthy, N.; Chang, S.X.; Paramasivan, B. Malachite green removal using algal biochar and its composites with kombucha SCOBY: An integrated biosorption and phytoremediation approach. Surf. Interfaces 2022, 30, 101880. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Shen, X.; Sotto, A.; Gao, C.; Shen, J. Polyethyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar] [CrossRef]
- Miyar, H.K.; Pai, A.; Goveas, L.C. Adsorption of Malachite Green by extracellular polymeric substance of Lysinibacillus sp. SS1: Kinetics and isotherms. Heliyon 2021, 7, e07169. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Althomali, R.H.; Adachi, A.; Saleh, E.A.M.; Husain, K.; Lhassani, A.; Lahkimi, A. Nitrogen and phosphorus co-doped carbocatalyst for efficient organic pollutant removal through persulfate-based advanced oxidation processes. J. Saudi Chem. Soc. 2023, 27, 101648. [Google Scholar] [CrossRef]
- Perendija, J.; Ljubić, V.; Popović, M.; Milošević, D.; Arsenijević, Z.; Đuriš, M.; Cvetković, S. Assessment of waste hop (Humulus Lupulus) stems as a biosorbent for the removal of malachite green, methylene blue, and crystal violet from aqueous solution in batch and fixed-bed column systems: Biosorption process and mechanism. J. Mol. Liq. 2024, 394, 123770. [Google Scholar] [CrossRef]
- Li, Z.; Han, Q.; Zong, Z.; Xu, Q.; Wang, K. Exploring on the optimal preparation conditions of activated carbon produced from solid waste produced from sugar industry and Chinese medicine factory. Waste Dispos. Sustain. Energy. 2020, 2, 65–77. [Google Scholar] [CrossRef]
- Nath, J.; Ray, L. Biosorption of Malachite green from aqueous solution by dry cells of Bacillus cereus M116 (MTCC 5521). J. Environ. Chem. Eng. 2015, 3, 386–394. [Google Scholar] [CrossRef]
- Deniz, F.; Kepekci, R.A. Bioremoval of Malachite green from water sample by forestry waste mixture as potential biosorbent. Microchem. J. 2017, 132, 172–178. [Google Scholar] [CrossRef]
- Chen, L.; Mi, B.; He, J.; Li, Y.; Zhou, Z.; Wu, F. Functionalized biochars with highly-efficient malachite green adsorption property produced from banana peels via microwave-assisted pyrolysis. Bioresour. Technol. 2023, 376, 128840. [Google Scholar] [CrossRef]
- Knolmajer, B.; Jócsák, I.; Taller, J.; Keszthelyi, S.; Kazinczi, G. Common Ragweed-Ambrosia artemisiifolia L.: A Review with Special Regards to the Latest Results in Biology and Ecology. Agronomy 2024, 14, 497. [Google Scholar] [CrossRef]
- Battlay, P.; Wilson, J.; Bieker, V.C.; Lee, C.; Prapas, D.; Petersen, B.; Hodgins, K.A. Large haploblocks underlie rapid adaptation in the invasive weed Ambrosia artemisiifolia. Nat. Commun. 2023, 14, 1717. [Google Scholar] [CrossRef]
- Janaćković, P.; Rajčević, N.; Gavrilović, M.; Novaković, J.; Radulović, M.; Miletić, M.; Marin, P.D. Essential oil composition of Ambrosia artemisiifolia and its antibacterial activity against phytopathogens. Biol. Life Sci. Forum. 2022, 15, 22. [Google Scholar]
- The Ministry of Environmental Protection of the Republic of Serbia; Environmental Protection Agency. Air Quality in the Republic of Serbia in 2023; The Ministry of Environmental Protection of the Republic of Serbia, Environmental Protection Agency: Belgrade, Serbia, 2024; p. 98. [Google Scholar]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conserv. Recycl. 2021, 164, 105204. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Tran, T.V.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.V.N. Invasive plants as biosorbents for environmental remediation: A review. Environ. Chem. Lett. 2022, 20, 1421–1451. [Google Scholar] [CrossRef]
- Osman, A.I.; Ayati, A.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Karimi-Maleh, H.; Sillanpaä, M. Advanced adsorbents for ibuprofen removal from aquatic environments: A review. Environ. Chem. Lett. 2024, 22, 373–418. [Google Scholar] [CrossRef]
- Medina-Zazueta, L.; Miranda-Castro, F.C.; Romo-Garcia, F.; Martínez-Gil, M.; Esparza-Ponce, H.E.; Encinas-Basurto, D.; Ibarra, J. Development of sustainable magnetic biosorbent using aqueous leaf extract of Vallesia glabra for methylene blue removal from wastewater. Sustainability 2023, 15, 4586. [Google Scholar] [CrossRef]
- Sharma, R.; Sarswat, A.; Pittman, C.U.; Mohan, D. Cadmium and lead remediation using magnetic and non-magnetic sustainable biosorbents derived from Bauhinia purpurea pods. RSC Adv. 2017, 7, 8606–8624. [Google Scholar] [CrossRef]
- Hamri, N.; Imessaoudene, A.; Hadadi, A.; Cheikh, S.; Boukerroui, A.; Bollinger, J.C.; Mouni, L. Enhanced adsorption capacity of methylene blue dye onto kaolin through acid treatment: Batch adsorption and machine learning studies. Water 2024, 16, 243. [Google Scholar] [CrossRef]
- Tadić, T.; Vuković, Z.; Pavlović, V.; Nastasović, A.; Onjia, A.; Marković, B. Preparation of magnetic surface molecularly imprinting polymers based on glycidyl methacrylate as selective sorbents for aniline removal from aqueous medium. Sci. Sinter. 2024, 00, 8. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Dinari, M. Bio-sorbent alginate/citric acid-sawdust/Fe3O4 nanocomposite beads for highly efficient removal of malachite green from water. Int. J. Biol. Macromol. 2022, 222, 2683–2696. [Google Scholar] [CrossRef]
- Sadiq, A.C.; Rahim, N.Y.; Suah, F.B.M. Adsorption and desorption of malachite green by using chitosan-deep eutectic solvents beads. Int. J. Biol. Macromol. 2020, 164, 3965–3973. [Google Scholar] [CrossRef]
- Jasińska, A.; Bernat, P.; Paraszkiewicz, K. Malachite green removal from aqueous solution using the system rapeseed press cake and fungus Myrothecium roridum. Desalination Water Treat. 2013, 51, 7663–7671. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Américo-Pinheiro, J.H.P. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Convers. Biorefin. 2024, 14, 11739–11756. [Google Scholar] [CrossRef]
- Farias, K.C.; Guimarães, R.C.; Oliveira, K.R.; Nazário, C.E.; Ferencz, J.A.; Wender, H. Banana peel powder biosorbent for removal of hazardous organic pollutants from wastewater. Toxics 2023, 11, 664. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. Usability of activated carbon with well-developed mesoporous structure for the decontamination of malachite green from aquatic environments: Kinetic, equilibrium and regeneration studies. J. Porous Mater. 2018, 25, 477–488. [Google Scholar] [CrossRef]
- Melhi, S.; Alqadami, A.A.; Alosaimi, E.H.; Ibrahim, G.M.; El-Gammal, B.; Bedair, M.A.; Elnaggar, E.M. Effective Removal of Malachite Green Dye from Water Using Low-Cost Porous Organic Polymers: Adsorption Kinetics, Isotherms, and Reusability Studies. Water 2024, 16, 1869. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, H.; Chen, C.; Yang, Y.; Xu, H. Biosorption of malachite green from aqueous solutions by Pleurotus ostreatus using Taguchi method. J. Environ. Health Sci. Eng. 2014, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Muinde, V.; Onyari, J.M.; Wamalwa, B.M.; Wabomba, J. Adsorption of malachite green from aqueous solutions onto rice husks: Kinetic and equilibrium studies. J. Environ. Prot. 2017, 8, 215–230. [Google Scholar] [CrossRef]
- Smiri, M.; Guey, F.; Chemingui, H.; Dekhil, A.B.; Elarbaoui, S.; Hafiane, A. Remove of humic acid from water using magnetite nanoparticles. Eur. J. Adv. Chem. Res. 2020, 1. [Google Scholar] [CrossRef]
- Olukanni, O.D.; Adenopo, A.; Awotula, A.O.; Osuntoki, A.A. Biodegradation of malachite green by extracellular laccase producing Bacillus thuringiensis RUN1. J. Basic Appl. Sci. 2013, 9, 543–549. [Google Scholar] [CrossRef]
- Merrad, S.; Abbas, M.; Trari, M. Adsorption of malachite green onto walnut shells: Kinetics, thermodynamic, and regeneration of the adsorbent by chemical process. Fibers Polym. 2023, 24, 1067–1081. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Mohamed, H.A.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Chen, F.; Wang, X.; Zhu, Q.; Ao, Q. Surface characterization of corn stalk superfine powder studied by FTIR and XRD. Colloids Surf. B Biointerfaces 2013, 104, 207–212. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M. Binary adsorption of chromium and cadmium metal ions by hemp (Cannabis sativa) based adsorbents. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100683. [Google Scholar] [CrossRef]
- Ouyang, Z.W.; Chen, E.C.; Wu, T.M. Thermal stability and magnetic properties of polyvinylidene fluoride/magnetite nanocomposites. Materials 2015, 8, 4553–4564. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Hussain, S.; Ghosh, A.K.; Pal, A.K. Studies on cellulose nanocrystals extracted from Musa sapientum: Structural and bonding aspects. Cellul. Chem. Technol. 2018, 52, 729–739. [Google Scholar]
- Lemos, E.S.; Fiorentini, E.F.; Bonilla-Petriciolet, A.; Escudero, L.B. Malachite Green removal by grape stalks biosorption from natural waters and effluents. Adsorpt. Sci. Technol. 2023, 2023, 6695937. [Google Scholar] [CrossRef]
- Carvalho, J.T.T.; Milani, P.A.; Consonni, J.L.; Labuto, G.; Carrilho, E.N.V.M. Nanomodified sugarcane bagasse biosorbent: Synthesis, characterization, and application for Cu (II) removal from aqueous medium. Environ. Sci. Pollut. Res. 2021, 28, 24744–24755. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Mauler, R.S.; Daitx, T.S.; Krindges, I.; Cemin, A.; Bonetto, L.R.; Giovanela, M. Removal of malachite green dye from aqueous solutions by a magnetic adsorbent. Sep. Sci. Technol. 2020, 55, 1089–1101. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Wu, S.; Jia, G.; Cui, X.; Zhao, P.; Li, Y. Enhanced adsorption of malachite green on hydroxyl functionalized coal: Behaviors and mechanisms. Process Saf. Environ. Prot. 2022, 163, 48–57. [Google Scholar] [CrossRef]
- Nastasović, A.; Marković, B.; Suručić, L.; Onjia, A. Methacrylate-based polymeric sorbents for recovery of metals from aqueous solutions. Metals 2022, 12, 814. [Google Scholar] [CrossRef]
- Sorour, F.H.; Aboeleneen, N.M.; Abd El-Monem, N.M.; Ammar, Y.A.; Mansour, R.A. Removal of malachite green from wastewater using date seeds as natural adsorbent; isotherms, kinetics, Thermodynamic, and batch adsorption process design. Int. J. Phytoremediation 2024, 26, 1321–1335. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Saberikhah, E.; Emami, M.S.; Sotudeh, M. Study of biosorption parameters: Isotherm, kinetics and thermodynamics of basic blue 9 biosorption onto foumanat tea waste. Cellulose Chem. Technol. 2014, 48, 735–743. [Google Scholar]
- Parlayıcı, Ş.; Pehlivan, E. Biosorption of methylene blue and malachite green on biodegradable magnetic Cortaderia selloana flower spikes: Modeling and equilibrium study. Int. J. Phytoremediation 2021, 23, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.Y.; Alene, A.N.; Habte, A.T.; Getahun, D.M. Adsorptive removal of malachite green dye from aqueous solution onto activated carbon of Catha edulis stem as a low cost bio-adsorbent. Environ. Syst. Res. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Youssef, E.E.; Beshay, B.Y.; Tonbol, K.; Makled, S.O. Biological activities and biosorption potential of red algae (Corallina officinalis) to remove toxic malachite green dye. Sci. Rep. 2023, 13, 13836. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Köksal, E.; Göde, F.; Pehlivan, E. Decolorization of water through removal of methylene blue and malachite green on biodegradable magnetic Bauhinia variagata fruits. Int. J. Phytoremediation 2022, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Săcară, A.M.; Indolean, C.; Cristea, V.M.; Mureşan, L.M. Application of adaptive neuro-fuzzy interference system on biosorption of malachite green using fir (Abies nordmanniana) cones biomass. Chem. Eng. Commun. 2019, 206, 1249–1263. [Google Scholar] [CrossRef]
- Djelloul, C.; Ghodbane, H. Removal of malachite green using ultrasound-assisted and conventional batch adsorption based on Paracentrotus lividus shells as biosorbent. Desalination Water Treat. 2022, 267, 201–214. [Google Scholar] [CrossRef]
- Deniz, F.; Ersanli, E.T. Purification of malachite green as a model biocidal agent from aqueous system by using a natural widespread coastal biowaste (Zostera marina). Int. J. Phytoremediation 2021, 23, 772–779. [Google Scholar] [CrossRef]
- Ouettar, L.; Guechi, E.K.; Hamdaoui, O.; Fertikh, N.; Saoudi, F.; Alghyamah, A. Biosorption of triphenyl methane dyes (malachite green and crystal violet) from aqueous media by alfa (Stipa tenacissima L.) leaf powder. Molecules 2023, 28, 3313. [Google Scholar] [CrossRef]





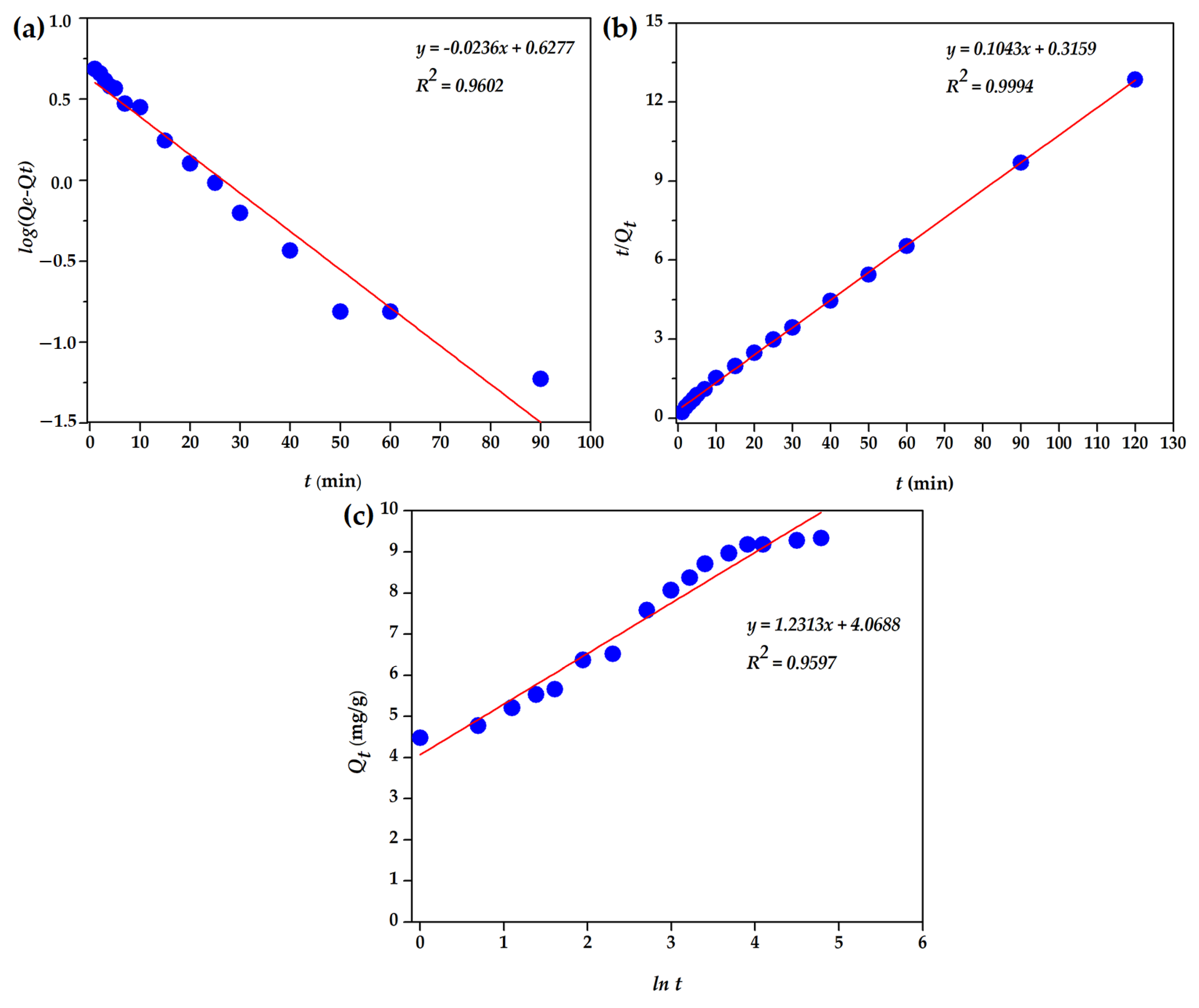

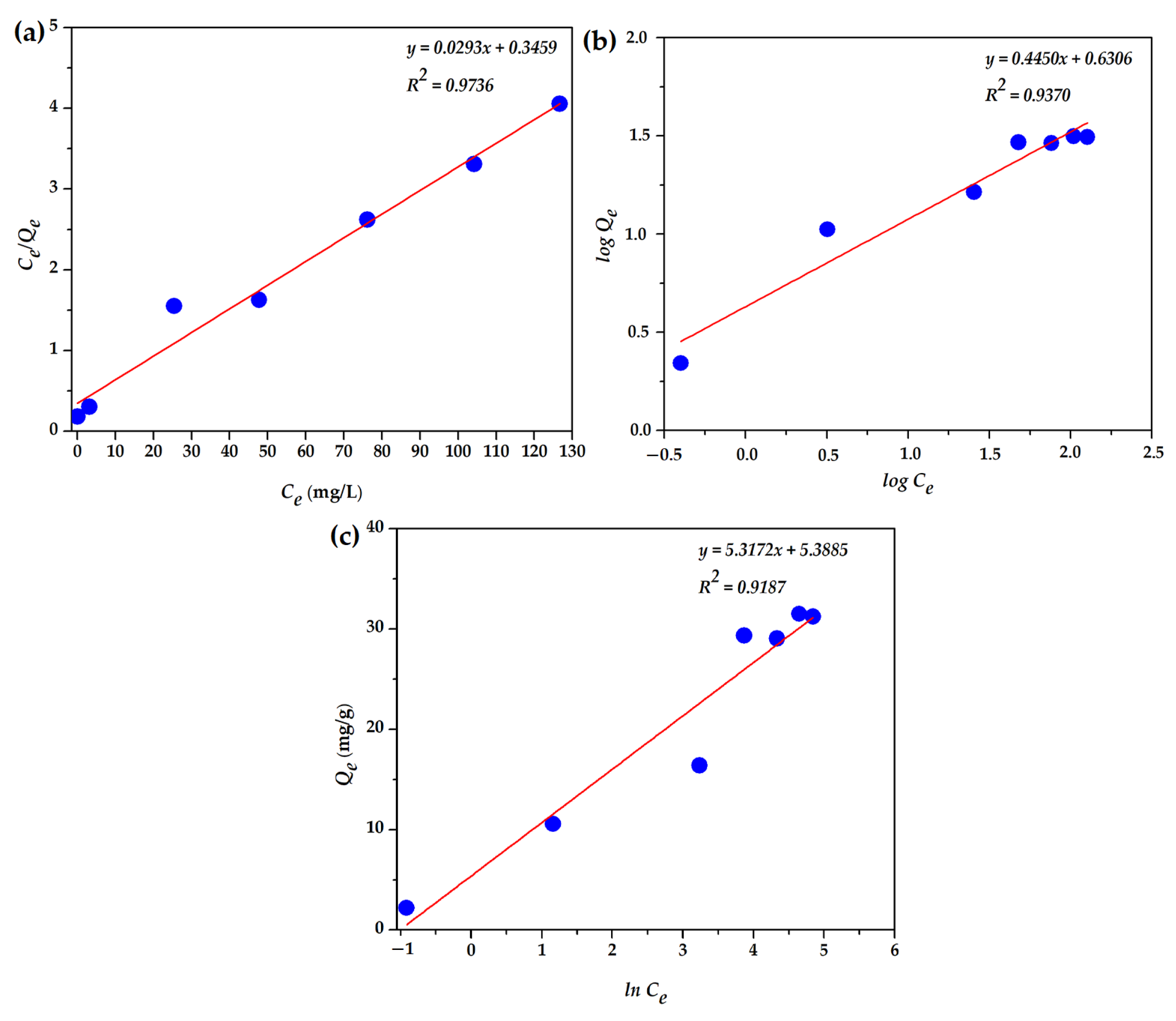
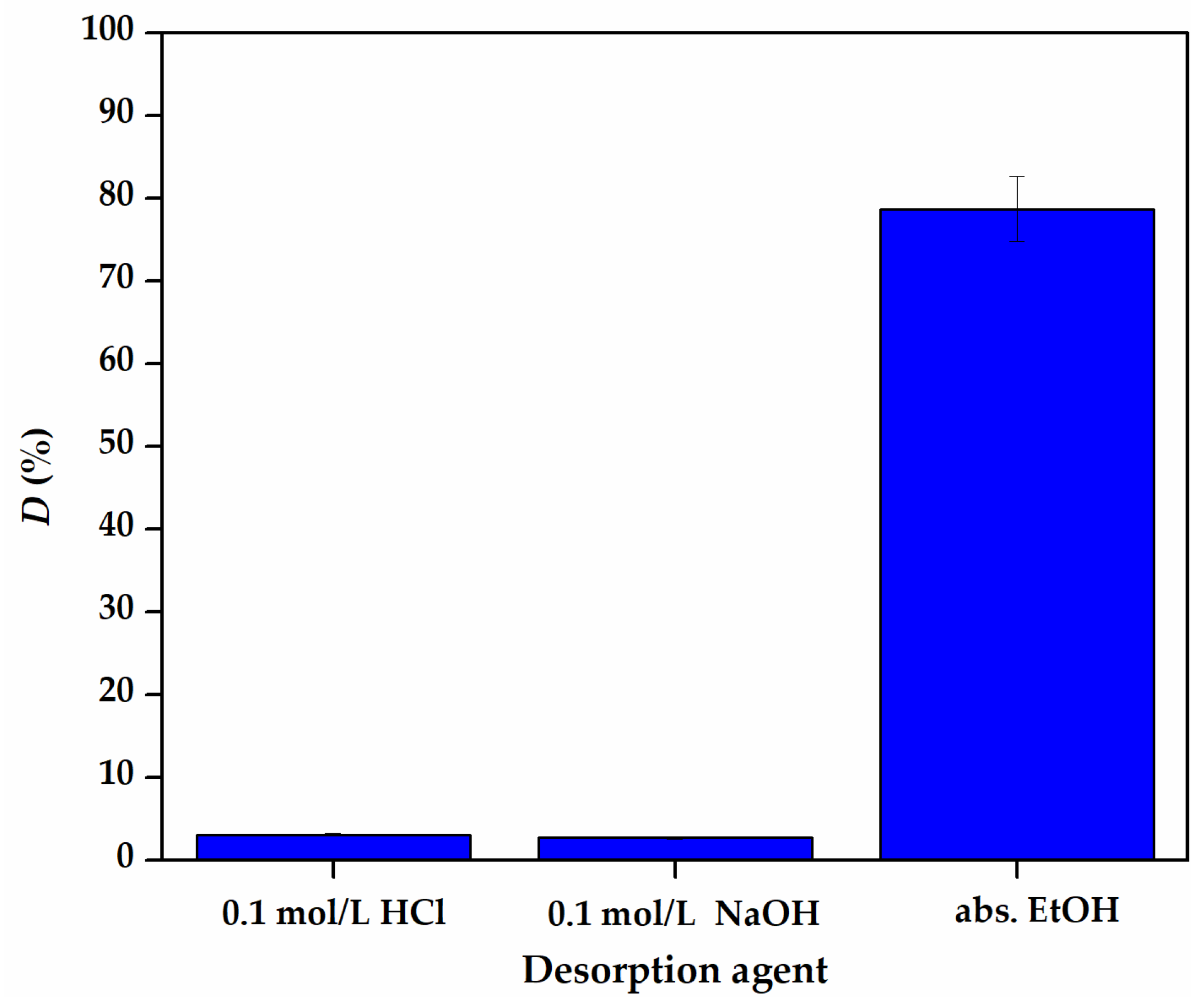
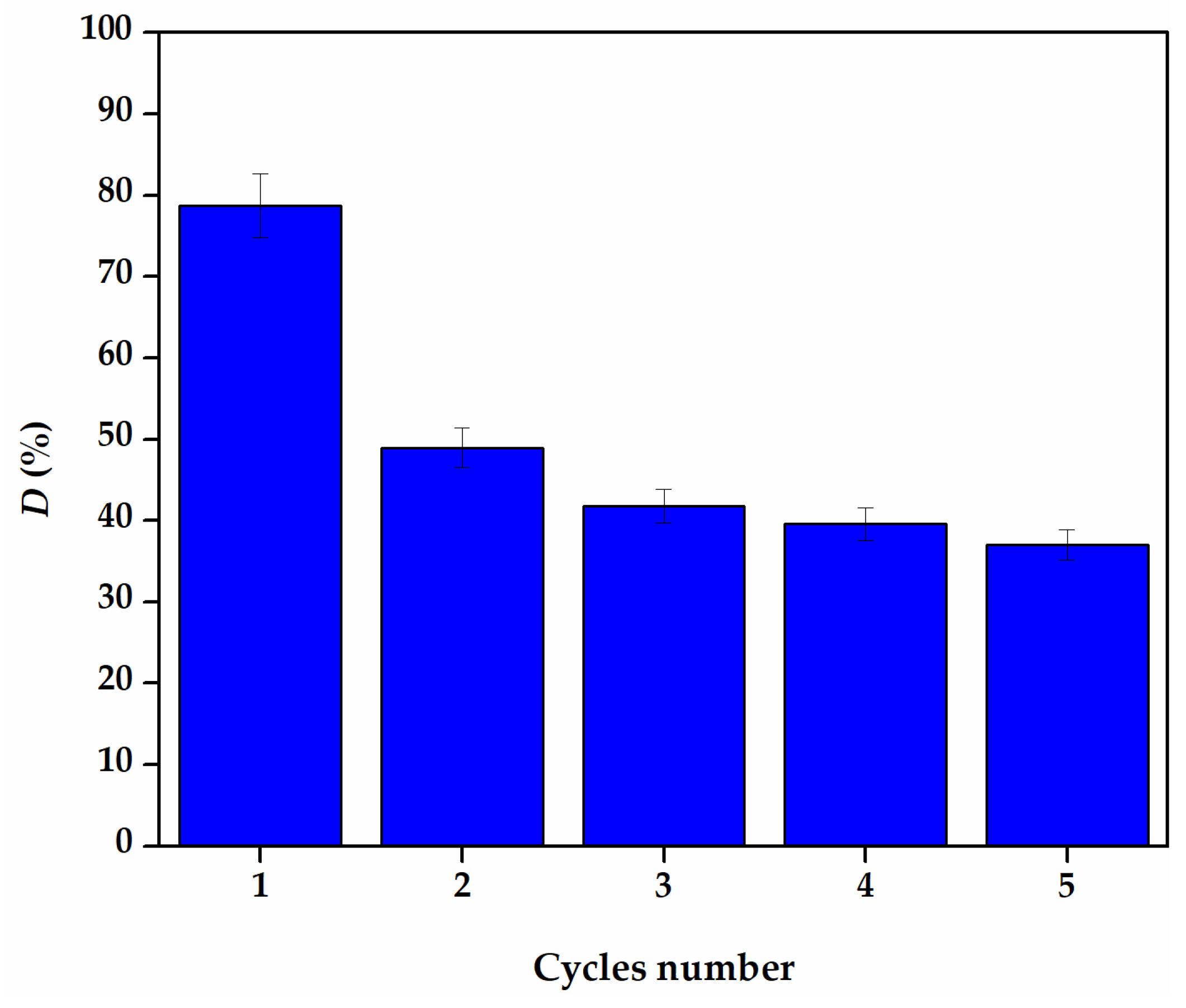
| Temperature (K) | ΔG° (J/mol) | ΔH° (J/mol) | ΔS° (J/mol·K) |
|---|---|---|---|
| 298 | −2010 | −31,097 | −98 |
| 308 | −691 | ||
| 318 | −60 |
| Parameter | Values |
|---|---|
| Qeexp, mg/g | 9.3 |
| (PFO) | |
| Qecal, mg/g | 4.24 |
| k1, min−1 | 0.054 |
| R2 | 0.960 |
| (PSO) | |
| Qecal, mg/g | 9.59 |
| k2, g/mg min | 0.034 |
| R2 | 0.999 |
| Elovich model | |
| αE, mg/g | 72.02 |
| βE, g/mg | 0.81 |
| R2 | 0.960 |
| Parameter | Value |
|---|---|
| Langmuir model | |
| Qm, mg/g | 34.1 |
| KL, L/mg | 0.085 |
| R2 | 0.997 |
| Freundlich model | |
| nF | 2.25 |
| KF, L/g | 4.54 |
| R2 | 0.937 |
| Temkin model | |
| AT, L/mg | 10.31 |
| R2 | 0.9187 |
| Biosorbent | Concetration (mg/L) | pH | Biosorbent Dosage (g/L) | Time (min) | Temperature (K) | Qm (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4/RWB | 50 | 8.0 | 5.00 | 120 | 298 | 34.1 | This study |
| Mag. Cortaderia selloana flower spikes | 25–350 | 6.0 | 4.00 | 45 | 298 | 56.5 | [52] |
| Catha edulis stem | 10–50 | 10.0 | 10.00 | 60 | / | 5.6 | [53] |
| Red algae Corallina ofcinalis | 20 | 6.0 | 1.00 | 120 | 300 | 101.3 | [54] |
| Mag. Bauhinia variagata fruits | 150 | 8.0 | 0.20 | 60 | 298 | 30.1 | [55] |
| Fir (Abies nordmanniana) cones | 110 | 3.3 | 50.00 | 8760 | 294 | 11.0 | [56] |
| Paracentrotus lividus shells | 10 | initial | 4.00 | 180 | 294 | 7.2 | [57] |
| Coastal biowaste (Zostera marina) | 15 | 4 | 0.01 | 360 | 298 | 97.6 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedić, N.; Tadić, T.; Marković, B.; Nastasović, A.; Popović, A.; Bulatović, S. Eco-Friendly Green Approach to the Biosorption of Hazardous Dyes from Aqueous Solution on Ragweed (Ambrosia artemisiifolia) Biomass. Separations 2024, 11, 310. https://doi.org/10.3390/separations11110310
Nedić N, Tadić T, Marković B, Nastasović A, Popović A, Bulatović S. Eco-Friendly Green Approach to the Biosorption of Hazardous Dyes from Aqueous Solution on Ragweed (Ambrosia artemisiifolia) Biomass. Separations. 2024; 11(11):310. https://doi.org/10.3390/separations11110310
Chicago/Turabian StyleNedić, Natalija, Tamara Tadić, Bojana Marković, Aleksandra Nastasović, Aleksandar Popović, and Sandra Bulatović. 2024. "Eco-Friendly Green Approach to the Biosorption of Hazardous Dyes from Aqueous Solution on Ragweed (Ambrosia artemisiifolia) Biomass" Separations 11, no. 11: 310. https://doi.org/10.3390/separations11110310
APA StyleNedić, N., Tadić, T., Marković, B., Nastasović, A., Popović, A., & Bulatović, S. (2024). Eco-Friendly Green Approach to the Biosorption of Hazardous Dyes from Aqueous Solution on Ragweed (Ambrosia artemisiifolia) Biomass. Separations, 11(11), 310. https://doi.org/10.3390/separations11110310









