Copper–Chitosan-Modified Magnetic Textile as a Peroxidase-Mimetic Catalyst for Dye Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Textile Modified with Copper Chitosan
2.3. Decolorization of Model Dyes
2.4. Peroxidase-Like Activity
2.5. Further Characterization
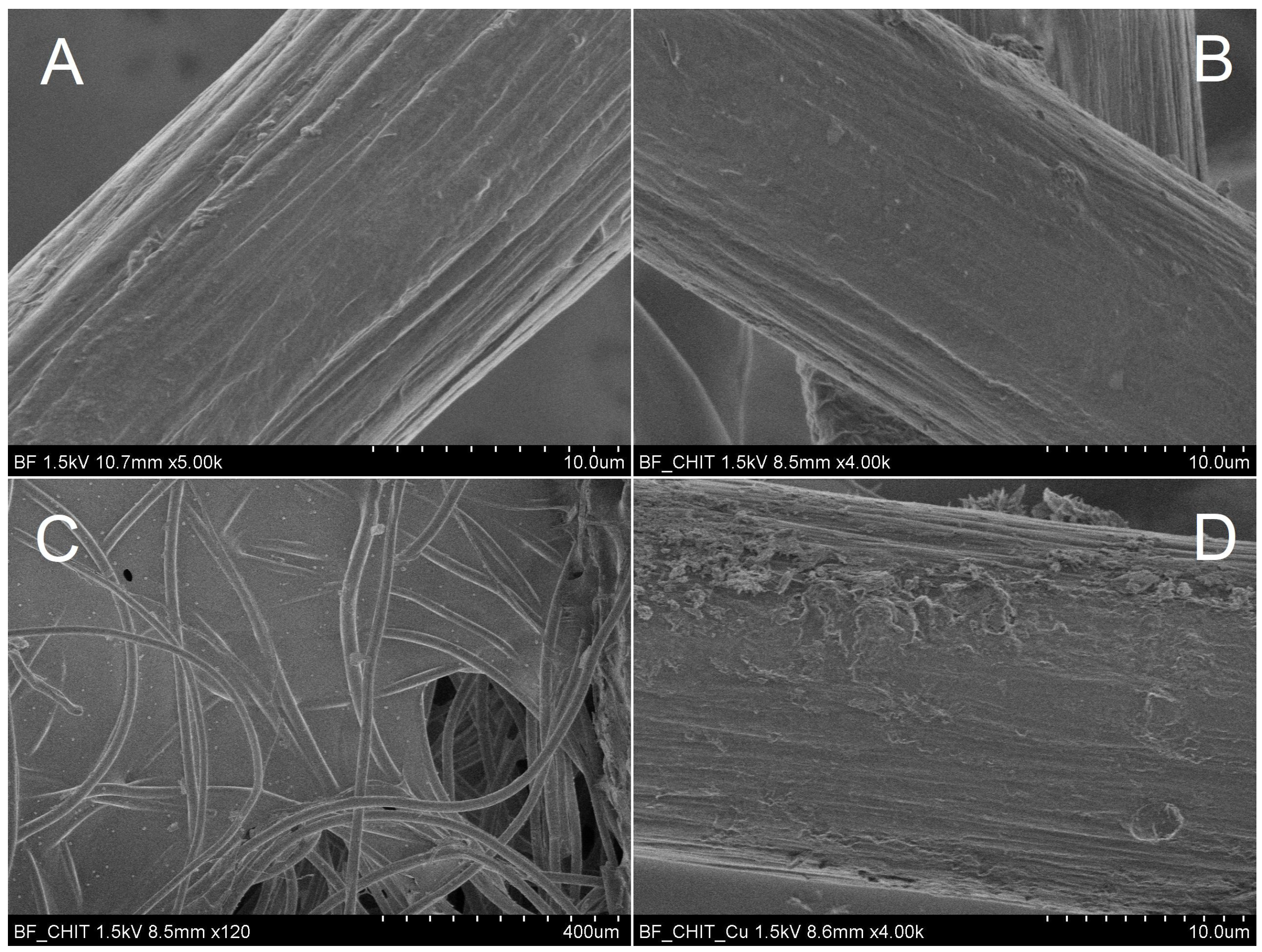
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Opwis, K.; Straube, T.; Kiehl, K.; Gutmann, J.S. Various strategies for the immobilization of biocatalysts on textile carrier materials. Chem. Engineer. Trans. 2014, 38, 223–228. [Google Scholar]
- Safarik, I.; Baldikova, E.; Safarikova, M.; Pospiskova, K. Magnetically responsive textile for a new preconcentration procedure: Magnetic textile solid phase extraction. J. Ind. Text. 2018, 48, 761–771. [Google Scholar] [CrossRef]
- Liu, S.; Mukai, Y. Selective adsorption and separation of proteins by ligand-modified nanofiber fabric. Polymers 2021, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Safarik, I.; Prochazkova, J.; Baldikova, E.; Pospiskova, K. Textile bound methyltrioctylammonium thiosalicylate ionic liquid for magnetic textile solid phase extraction of copper ions. J. Mol. Liq. 2019, 296, 111910. [Google Scholar] [CrossRef]
- Safarik, I.; Mullerova, S.; Pospiskova, K. Semiquantitative determination of food acid dyes by magnetic textile solid phase extraction followed by image analysis. Food Chem. 2019, 274, 215–219. [Google Scholar] [CrossRef]
- Kiehl, K.; Straube, T.; Opwis, K.; Gutmann, J.S. Strategies for permanent immobilization of enzymes on textile carriers. Eng. Life Sci. 2015, 15, 622–626. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Nierstrasz, V.A. An overview on biocatalysts immobilization on textiles: Preparation, progress and application in wastewater treatment. Chemosphere 2021, 279, 130481. [Google Scholar] [CrossRef]
- Li, H.Q.; Gong, J.X.; Li, Q.J.; Li, Z.; Zhang, J.F.; Chen, K.N.; Tang, Z.X.; Ding, G.Q.; Lin, S.Y. Cell Immobilization with textile carriers for TA biodegradation. Adv. Mater. Res. 2011, 331, 19–22. [Google Scholar] [CrossRef]
- Prochazkova, J.; Pospiskova, K.; Safarik, I. Magnetically modified electrospun nanotextile exhibiting peroxidase-like activity. J. Magn. Magn. Mater. 2019, 473, 335–340. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Stan, M.S.; Chirila, L.; Popescu, A.; Radulescu, D.M.; Radulescu, D.E.; Dinischiotu, A. Essential oil microcapsules immobilized on textiles and certain induced effects. Materials 2019, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Bunoiu, M.; Anitas, E.M.; Pascu, G.; Chirigiu, L.M.E.; Bica, I. Electrical and magnetodielectric properties of magneto-active fabrics for electromagnetic shielding and health monitoring. Int. J. Mol. Sci. 2020, 21, 4785. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, G.N.; Rtimi, S.; Assadi, A.A.; Hanna, K. Nano-sized iron oxides supported on polyester textile to remove fluoroquinolones in hospital wastewater. Environ. Sci.-Nano 2020, 7, 2156–2165. [Google Scholar] [CrossRef]
- Safarik, I.; Prochazkova, J.; Schroer, M.A.; Garamus, V.M.; Kopcansky, P.; Timko, M.; Rajnak, M.; Karpets, M.; Ivankov, O.I.; Avdeev, M.V.; et al. Cotton textile/iron oxide nanozyme composites with peroxidase-like activity: Preparation, characterization, and application. ACS Appl. Mater. Interfaces 2021, 13, 23627–23637. [Google Scholar] [CrossRef] [PubMed]
- Józefczak, A.; Kaczmarek, K.; Bielas, R.; Procházková, J.; Šafařík, I. Magneto-responsive textiles for non-invasive heating. Int. J. Mol. Sci. 2023, 24, 11744. [Google Scholar] [CrossRef]
- Safarik, I.; Pospiskova, K.; Baldikova, E.; Savva, I.; Vekas, L.; Marinica, O.; Tanasa, E.; Krasia-Christoforou, T. Fabrication and bioapplications of magnetically modified chitosan-based electrospun nanofibers. Electrospinning 2018, 2, 29–39. [Google Scholar] [CrossRef]
- Baldikova, E.; Pospiskova, K.; Safarikova, M.; Safarik, I. Non-woven fabric supported manganese dioxide microparticles as a low-cost, easily recoverable catalyst for hydrogen peroxide decomposition. Mater. Chem. Phys. 2018, 203, 280–283. [Google Scholar] [CrossRef]
- Bhandari, V.; Jose, S.; Badanayak, P.; Sankaran, A.; Anandan, V. Antimicrobial finishing of metals, metal oxides, and metal composites on textiles: A systematic review. Ind. Eng. Chem. Res. 2022, 61, 86–101. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Mukhtar, M.; Fényes, E.; Bartos, C.; Zeeshan, M.; Ambrus, R. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: A comprehensive review. Eur. Polym. J. 2021, 160, 110767. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and Its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-E.; Kan, C.-W.; Sun, C.; Du, J.; Xu, C. A review of chitosan textile applications. AATCC J. Res. 2019, 6, 8–14. [Google Scholar] [CrossRef]
- Safarik, I.; Mullerova, S.; Pospiskova, K. Magnetically responsive textile for preconcentration of acid food dyes. Mater. Chem. Phys. 2019, 232, 205–208. [Google Scholar] [CrossRef]
- Safarik, I.; Pospiskova, K. A simple extraction of blue fountain ink dye (Acid blue 93) from water solutions using Magnetic Textile Solid-Phase Extraction. Sep. Sci. PLUS 2018, 1, 48–51. [Google Scholar] [CrossRef]
- Su, C.; Berekute, A.K.; Yu, K.P. Chitosan@TiO composites for the adsorption of copper(II) and antibacterial applications. Sustain. Environ. Res. 2022, 32, 27. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Vårum, K.M.; Guibal, E.; Smidsrød, O. Binding of ions to chitosan—Selectivity studies. Carbohydr. Polym. 2003, 54, 471–477. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Peniche-Covas, C.; Alvarez, L.W.; Argüelles-Monal, W. The adsorption of mercuric ions by chitosan. J. Appl. Polym. Sci. 1992, 46, 1147–1150. [Google Scholar] [CrossRef]
- Hasan, S.; Krishnaiah, A.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M.; Smith, E.D. Adsorption of divalent cadmium (Cd(II)) from aqueous solutions onto chitosan-coated perlite beads. Ind. Eng. Chem. Res. 2006, 45, 5066–5077. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Kamari, A.; Fatinathan, S.; Ng, P.W. Adsorption of chromium from aqueous solution using chitosan beads. Adsorption 2006, 12, 249–257. [Google Scholar] [CrossRef]
- Cheung, W.; Ng, J.; Mckay, G. Kinetic analysis of the sorption of copper(II) ions on chitosan. J. Chem. Technol. Biotechnol. 2003, 78, 562–571. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, W.; Pan, B.; Zhang, Q.; Zhang, W.; Lv, L. Facile fabrication of magnetic chitosan beads of fast kinetics and high capacity for copper removal. ACS Appl. Mater. Interfaces 2014, 6, 3421–3426. [Google Scholar] [CrossRef]
- Podzus, P.E.; Debandi, M.V.; Daraio, M.E. Copper adsorption on magnetite-loaded chitosan microspheres: A kinetic and equilibrium study. Physica B 2012, 407, 3131–3133. [Google Scholar] [CrossRef]
- Verbych, S.; Bryk, M.; Chornokur, G.; Fuhr, B. Removal of copper(II) from aqueous solutions by chitosan adsorption. Sep. Sci. Technol. 2005, 40, 1749–1759. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of heavy metals by polysaccharide: A review. Polym.-Plast. Technol. Mater. 2020, 59, 1770–1790. [Google Scholar] [CrossRef]
- Zheng, A.X.; Zhang, X.L.; Gao, J.; Liu, X.L.; Liu, J.F. Peroxidase-like catalytic activity of copper ions and its application for highly sensitive detection of glypican-3. Anal. Chim. Acta 2016, 941, 87–93. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; Yang, G.; Yang, J.; Ding, D.; Zhang, M. Copper(II) ions enhance the peroxidase-like activity and stability of keratin-capped gold nanoclusters for the colorimetric detection of glucose. Microchim. Acta 2019, 186, 271. [Google Scholar] [CrossRef]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, H.J.; Zou, W.T.; Guo, R. Protein-mediated sponge-like copper sulfide as an ingenious and efficient peroxidase mimic for colorimetric glucose sensing. RSC Adv. 2020, 10, 28819–28826. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Xie, X.; Liang, Y.; Li, Z.; Yang, K.; Tao, J.; Zhang, H.; Wang, Z. Facile fabrication of a novel copper nanozyme for efficient dye degradation. ACS Omega 2021, 6, 6284–6291. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zheng, L.; Yao, W.; Liu, S.; Bu, Y.; Zeng, Q.; Zhang, X.; Deng, H.; Lin, X.; Chen, W. A facile route for constructing Cu–N–C peroxidase mimics. J. Mater. Chem. B 2020, 8, 8599–8606. [Google Scholar] [CrossRef]
- Liu, S.; Bilal, M.; Rizwan, K.; Gul, I.; Rasheed, T.; Iqbal, H.M.N. Smart chemistry of enzyme immobilization using various support matrices—A review. Int. J. Biol. Macromol. 2021, 190, 396–408. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Guo, Z.; Zhang, H.; Wang, J.; Jia, P.; Bu, T.; Liu, Y.; Li, L.; Wang, L. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int. J. Biol. Macromol. 2021, 167, 10–22. [Google Scholar] [CrossRef]
- Tummala, S.; Bandi, R.; Ho, Y.-P. Synthesis of Cu-doped carbon dot/chitosan film composite as a catalyst for the colorimetric detection of hydrogen peroxide and glucose. Microchim. Acta 2022, 189, 284. [Google Scholar] [CrossRef]
- Pospiskova, K.; Safarik, I. Textile-bound copper silicate as a new peroxidase-like nanozyme for organic dye decolorization. Chem. Eng. Technol. 2022, 45, 1207–1210. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Hamdaoui, M.; Harzallah, O. Chitosan: A biopolymer for textile processes and products. Text. Res. J. 2023, 93, 1456–1484. [Google Scholar] [CrossRef]
- Kamiński, W.; Modrzejewska, Z. Application of chitosan membranes in separation of heavy metal ions. Sep. Sci. Technol. 1997, 32, 2659–2668. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, Toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Nasab, S.G.; Semnani, A.; Teimouri, A.; Yazd, M.J.; Isfahani, T.M.; Habibollahi, S. Decolorization of crystal violet from aqueous solutions by a novel adsorbent chitosan/nanodiopside using response surface methodology and artificial neural network-genetic algorithm. Int. J. Biol. Macromol. 2019, 124, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeter. Biodegr. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- González-Rábade, N.; del Carmen Oliver-Salvador, M.; Salgado-Manjarrez, E.; Badillo-Corona, J.A. In vitro production of plant peroxidases—A review. Appl. Biochem. Biotechnol. 2012, 166, 1644–1660. [Google Scholar] [CrossRef]
- Bhunia, A.; Durani, S.; Wangikar, P.P. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Husain, Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Bio/Technol. 2010, 9, 117–140. [Google Scholar] [CrossRef]
- Kalsoom, U.; Bhatti, H.N.; Asgher, M. Characterization of plant peroxidases and their potential for degradation of dyes: A review. Appl. Biochem. Biotechnol. 2015, 176, 1529–1550. [Google Scholar] [CrossRef] [PubMed]
- Ulson de Souza, S.M.A.G.; Forgiarini, E.; Ulson de Souza, A.A. Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J. Hazard. Mater. 2007, 147, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Z.; Wang, T.-L.; Ji, L.-N. Enhanced dye decolorization efficiency by citraconic anhydride-modified horseradish peroxidase. J. Molec. Catal. B Enzym. 2006, 41, 81–86. [Google Scholar] [CrossRef]
- Attar, F.; Shahpar, M.G.; Rasti, B.; Sharifi, M.; Saboury, A.A.; Rezayat, S.M.; Falahati, M. Nanozymes with intrinsic peroxidase-like activities. J. Molec. Liq. 2019, 278, 130–144. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo red toxicity towards different living organisms: A Review. Processes 2023, 11, 807. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Martínez-Jerónimo, F. Congo red dye diversely affects organisms of different trophic levels: A comparative study with microalgae, cladocerans, and zebrafish embryos. Environ. Sci. Pollut. Res. 2019, 26, 11743–11755. [Google Scholar] [CrossRef]
- Soriano, J.J.; Mathieu-Denoncourt, J.; Norman, G.; de Solla, S.R.; Langlois, V.S. Toxicity of the azo dyes Acid Red 97 and Bismarck Brown Y to Western clawed frog (Silurana tropicalis). Environ. Sci. Pollut. Res. 2014, 21, 3582–3591. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.L.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, X.; Zhong, L.; Zhang, S.; Luo, X.; Liu, Q.; Tang, L.; Lu, Y. Recent advances in the applications of nanozymes for the efficient detection/removal of organic pollutants: A review. Environ. Sci. Nano 2022, 9, 1212–1235. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarik, I.; Prochazkova, J.; Zelena Pospiskova, K. Copper–Chitosan-Modified Magnetic Textile as a Peroxidase-Mimetic Catalyst for Dye Removal. Separations 2024, 11, 325. https://doi.org/10.3390/separations11110325
Safarik I, Prochazkova J, Zelena Pospiskova K. Copper–Chitosan-Modified Magnetic Textile as a Peroxidase-Mimetic Catalyst for Dye Removal. Separations. 2024; 11(11):325. https://doi.org/10.3390/separations11110325
Chicago/Turabian StyleSafarik, Ivo, Jitka Prochazkova, and Kristyna Zelena Pospiskova. 2024. "Copper–Chitosan-Modified Magnetic Textile as a Peroxidase-Mimetic Catalyst for Dye Removal" Separations 11, no. 11: 325. https://doi.org/10.3390/separations11110325
APA StyleSafarik, I., Prochazkova, J., & Zelena Pospiskova, K. (2024). Copper–Chitosan-Modified Magnetic Textile as a Peroxidase-Mimetic Catalyst for Dye Removal. Separations, 11(11), 325. https://doi.org/10.3390/separations11110325






