Abstract
This work presents a biological remediation process for molybdenum-bearing wastewater which may lead to the fabrication of biogenic Mo chalcogenide particles with (photo)catalytic properties. The process is based on dissimilatory sulphate reduction, utilising sulphate-reducing bacteria (SRB), and reductive precipitation of molybdate which is the predominant species of molybdenum in oxygenated water/wastewater. The SRB culture was established in a biofilm reactor which was fed with synthetic solutions containing sulphate (17.7 mM), molybdate molybdenum (2 mM), divalent iron (1.7 mM) and ethanol as the carbon/electron donor. The performance of the bioreactor was monitored in terms of pH, sulphate and molybdenum (Mo(VI) and total) content. The presence of thiomolybdate species was studied by scanning UV-Vis absorbance of samples from the reactor outflow while the reactor precipitates were studied via electron microscopy coupled with energy dispersive spectrometry, X-ray diffractometry and laser light scattering. A molar molybdate/sulphate ratio of 1:12.5 proved effective for molybdate reduction and recovery by 76% in 96 h, whereas sulphate was reduced by 57%. Molybdenum was immobilised in the sulphidic precipitates of the bioreactor, presumably via two principal mechanisms: (i) microbially mediated reduction and precipitation, and (ii) thiomolybdate formation and sorption/incorporation into iron sulphides.
1. Introduction
Molybdenum (Mo) is a transition metal of growing economic importance which occurs naturally as molybdenite (MoS2) and is recovered as a by-product of copper and tungsten mining operations. Mo, which is used mainly in steel and chemicals but also in industrial lubrication and catalysis applications [1], is needed in all technologies in the upcoming green energy transition [2]. As the European Union (EU) is totally dependent on Mo imports [3], particular attention has been paid to improve Mo recovery rates from waste and wastewater.
In addition to mining wastewater and metallurgical slags, additional secondary Mo sources have recently been identified. For example, molybdenum-containing catalysts are widely used in the petroleum refining industry for mild hydrogenation and removal of sulphur (hydrodesulfurization, HDS), nitrogen and oxygen. Spent HDS catalysts usually consist of molybdenum sulphide mixed with sulphides of vanadium, cobalt and nickel on an alumina carrier. The composition of such a spent catalyst is 2–10% Mo, 0–12% V, 0.5–4% Co, 0.5–10% Ni, 10% S and 10% C, with the balance being Al2O3 [1]. In addition, Mo-containing solutions may originate from the wastewater generated during the hydrometallurgical treatment of waste electronic equipment, such as thin-film photovoltaic (PV) panels [4] for the recovery of critical metals [5]. Based on the installed and expected PV capacity, global PV waste is estimated to reach 1.7–8 million tonnes by 2030 and 60–78 million tonnes by 2050 [6]. Thus, end-of-life thin-film PV panels could also serve as a significant potential secondary source of Mo.
Molybdenum, which is found as Mo(VI) in such wastewater and leachates, can be recovered via various methods [7,8], such as precipitation [9,10], adsorption [11,12] and solvent extraction [13,14]. Among the precipitation methods, sulphide precipitation is generally advantageous due to the lower solubility of metal sulphide precipitates, faster reaction rates, better settling properties and potential for selective metal removal and further valorisation of sulphide precipitates by smelting or hydrometallurgical treatment [15]. For molybdenum in particular, its potential selective recovery from leachates by adding biogenic H2S [16] and aqueous Na2S [17,18] has been experimentally evaluated. Vemic et al. [18] identified MoO3 in the precipitates and Cibati et al. [16] reported molybdenum recovery as a sulphide/oxide precipitate, whereas Hamza et al. [17] produced a pure MoS2 solid material after a multi-stage leaching/precipitation process.
Molybdenum (di)sulphide (MoS2), a chalcogenide with a two-dimensional layered structure, has recently received much attention due to its wide range of environmental and energy applications [19], such as hydrogen production [20,21] and CO2 reduction [22]. In addition to various conventional synthesis methods [23], several efforts have been made to fabricate metal chalcogenide particles via microbially mediated pathways [24]. Among these, methods based on biogenic sulphide and dissimilatory sulphate reduction [25,26] have driven research to produce metal sulphide nanoparticles.
Biological sulphate reduction, utilising sulphate-reducing bacteria (SRB) [27], has been demonstrated in lab- and pilot-scale studies [28] for the treatment of wastewater with a significant sulphate and metal content. This process occurs anaerobically via the oxidation of organic carbon sources (or H2) and the reduction of sulphate (SO42−) to sulphide (H2S, HS−) by SRB [29]. Sulphide and bicarbonate ions, which are formed during sulphate reduction and carbon source oxidation, buffer the solution pH around neutral to slightly alkaline values. Therefore, sulphate-reducing bioreactors are considered advantageous for metal sequestering from wastewater by bioprecipitation [30] and/or other secondary mechanisms [31], such as reductive precipitation [32]. Molybdate (), which is a structural analogue of sulphate, can be reduced by SRB into Mo(V) and finally into Mo(IV) which can then precipitate as sulphide [33,34,35,36]. However, this remediation approach has not been extensively evaluated due to the reported inhibitory effects of molybdate on microbial activity [37,38,39,40].
This work demonstrates the capacity of a biofilm reactor to treat solutions containing Mo(VI), simulating wastewater generated upon leaching with H2SO4, for the recovery of sulphidic particles of molybdenum and presents a remediation process for Mo-laden wastewater which may lead to the fabrication of biogenic Mo chalcogenide particles.
2. Materials and Methods
2.1. Sulphate-Reducing Bioreactor
The sulphate-reducing bioreactor used in this study has been set up and run for the remediation of acidic industrial wastewater [41]. In short, the reactor was a plexiglass tube (length: 50 cm; I.D.: 9.5 cm) which was packed with porous, sintered-glass cylindrical pieces (length: 2.5–3.5 cm; diam.: 1 cm—Biohome® Ultimate Marine [42]), resulting in a bed height of 40 cm and reactor effective volume of 1.7 L. The reactor was inoculated by transferring sufficient packing material with already grown microbial biomass from a previously operated bioreactor with ethanol as carbon/electron source. The bacterial culture was initially dominated by Desulfobacter postgatei [43], an acetate-utilising species.
The reactor operated at constant room temperature (25 °C) in fed-batch upflow mode; it was fed from a 2 L bottle via a peristaltic pump (2 L/h). The feed solution was renewed periodically, without emptying the reactor, at intervals of four days. The reactor was fed with synthetic solutions based on a modified Postgate’s medium (DSMZ GmbH, Desulfovibrio medium no.63), where lactate was replaced with ethanol. The basal medium contained 0.5 g/L K2HPO4, 1 g/L NH4Cl and 0.1 g/L CaCl2·2H2O. The feed solution also contained divalent iron (100 mg/L, added as FeSO4·7H2O), molybdenum (200 mg/L added as Na2MoO4·2H2O) and sulphate (1700 mg/L, added as Na2SO4 and MgSO4·7H2O).
Ethanol is proposed as an alternative carbon source/electron donor for sulphate-reducing bacteria for several reasons, including ease of availability and low cost. Moreover, White and Gadd demonstrated that ethanol was more effective in stimulating sulphide production than lactate which, however, produced the greatest biomass [44]. Ethanol, like lactate, can be incompletely oxidised to acetate (reaction (1)) or completely oxidised to CO2 (reaction (2)) via the oxidation of acetate (reaction (3)), depending on the SRB species [29]. Acetate oxidation (reaction (3)), which depends on the presence of acetate-utilising SRB in the microbial consortium, has been proven to be the critical step, as it controls the generation of alkalinity and the residual organic content of the effluent. Acetate may also inhibit sulphate reduction at a high concentration and low pH [45], being highly toxic to SRB in undissociated forms [46]. Thus, avoiding acetate accumulation via its oxidation is considered a key factor for the optimisation of the entire process.
Ethanol was supplied with 20% surplus over the stoichiometric quantity required for the reduction of sulphate (Reaction (2)) and molybdate, considering (i) the complete oxidation of ethanol based on the capacity of the dominant species to metabolise acetate and (ii) the assimilation of carbon for the growth and preservation of biomass. For the reduction of molybdate, which progresses enzymatically and not merely chemically [36], the half reaction was considered for the complete oxidation of ethanol into carbon dioxide (Reaction (4)) [47].
2.2. Experimental Procedure and Analytical Determinations
The bioreactor performance was monitored in terms of pH, sulphate and molybdenum (Mo(VI) and total) content. Sampling was performed at the feeding bottle each time the solution was renewed as well as at the reactor outlet after 1, 2, 3, 4, 5, 24, 48, 72 and 96 h.
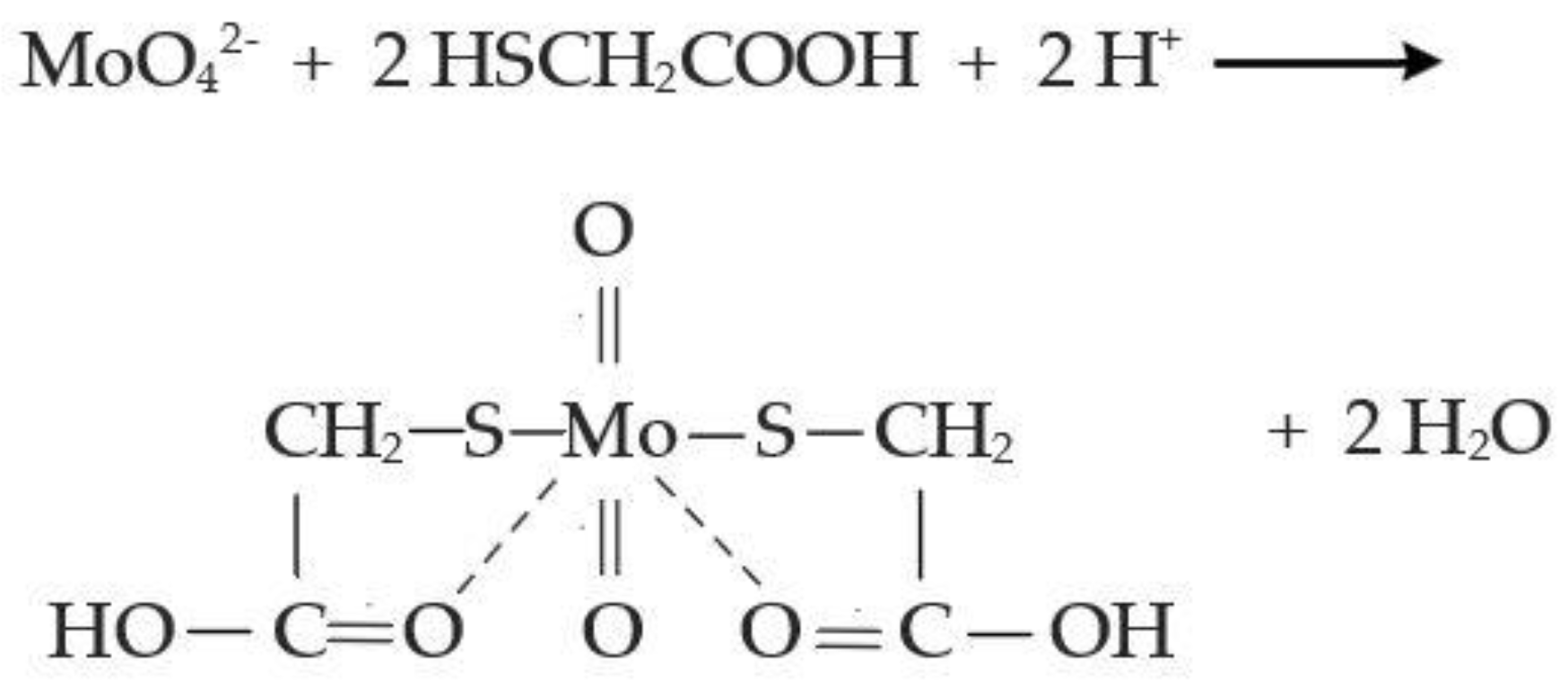
Solution pH was determined in unfiltered samples, which were then vacuum-filtered through 0.2 μm sterile membranes (Whatman® ME24/21) before any other chemical determination. The presence of any intermediate thiomolybdate species was identified by scanning the UV-Vis absorbance of the reactor outflow, immediately after sampling and filtering, in the 250–500 nm range (Hach DR/6000). Sulphate concentration was determined via turbidimetry at 450 nm after formation of BaSO4 (Hach DR/6000, Method 8051). Molybdate molybdenum concentration was determined at 420 nm after a reaction with mercaptoacetic acid (Hach DR/6000, Method 8036). This method involves pH buffering and the prevention of Mo(VI) reduction into Mo(V) before the addition of mercaptoacetic acid. The Mo(VI) concentration is proportional to the yellow colour, which is formed when mercaptoacetic acid reacts with Mo(VI) (Figure 1). Therefore, this colorimetric method allows for the quantitative determination of Mo(VI), which is found in molybdate and thiomolybdate species. The concentration of total molybdenum was determined by inductively coupled plasma optical emission spectroscopy (Leeman Labs, Inc., Mason, OH, USA).
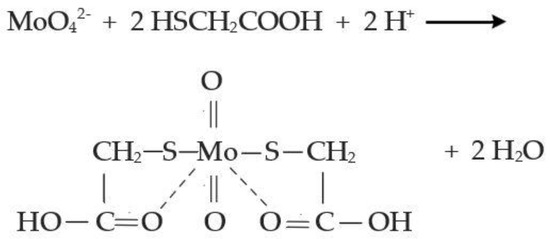
Figure 1.
Reaction (5).
All experiments were carried out in duplicate; the presented results are mean values of the results obtained after operating the reactor with the aforementioned feed solutions for six months. For comparison, at the end of the six-month period, the reactor was run with a feed solution without molybdate.
All concentration results are presented in dimensionless form to the initial concentration determined after 1 h of solution circulation, when the new feed solution is assumed to have been completely mixed with the reactor content.
The initial concentration values determined after 1 h of solution circulation were also input into Hydra-Medusa [48], a chemical speciation software, in order to calculate the concentration fraction of the various Mo species at the equilibrium established at selected redox conditions as well as to predict the predominant aqueous species and solid phases. Mo speciation was determined at 25 °C, at ESHE = 500 mV (corresponding to the oxic feed solution) and at ESHE = −250 mV (corresponding to the anoxic conditions established in the sulphate-reducing bioreactor).
2.3. Sampling and Physical–Chemical Analysis of the Reactor Precipitates
For the physical–chemical analysis of the reactor precipitates, a sludge sample was collected from the reactor bottom, centrifuged (for 10 min at 4000 rpm) and decanted immediately after centrifugation. In order to prevent oxidation and remobilisation, or redistribution among fractions, of the metals contained therein, the solid material was transferred into a desiccator, where it was kept under a nitrogen atmosphere, at room temperature (approx. 25 °C) [49]. The dehydrated material was then homogenised. All subsequent tests were conducted under ambient air conditions.
The particle size distribution (PSD) of the reactor precipitates was determined using laser light scattering (Horiba Partica LA-960V2). This method uses the volume of the particle to measure its size based on the Mie theory for light scattering by spherical particles and it was selected due to the extremely fine grain size of the sludge [49,50]. The sludge sample was analysed in an aqueous suspension.
For the identification of the various mineral phases, powder X-ray diffraction (XRD) analysis was performed using a Bruker D8 Focus X-Ray Diffractometer (Bruker, Germany) with nickel-filtered CuKa radiation (λ = 1.5406 Å) at 40 kV and 40 mA. The samples were slowly step-scanned from 10° to 80° (2θ), at a step of 0.02° and step time of 6 s. This low scan rate was selected in order to detect the precipitated Mo phases accurately.
Microstructural and morphological observation of the reactor precipitates was carried out on polished sections using a scanning electron microscope (JEOL JSM 6380-LV) in low-vacuum mode (accelerating voltage: 20 kV). Microanalyses were carried out with an Oxford INCA (Oxford Instruments, UK) energy-dispersive spectrometer (EDS) connected to the microscope. Polished sections were prepared following typical procedures for metallographic specimens: sludge samples, after being impregnated in a low-viscosity epoxy resin (Akasel Denmark) under vacuum, were cut via micro saw, then ground down to 2000 SiC paper grit and polished through three stages (6 μm and 1 μm diamond paste, followed by colloidal silica gel) on a lapping disk.
The nanoscale investigation of the reactor precipitates was carried out with a high-resolution JEOL 2100 LaB6 transmission electron microscope (HRTEM) operating at 200 kV. Prior to the analysis, a sample suspension (about 0.1 g of sample in 100 mL ethanol) was prepared and cured with ultrasound to disaggregate any agglomerated particles. A drop from the suspension was then placed on a 300-mesh carbon coated copper grid and air-dried overnight. The grain microstructure was also studied using a bright-field detector in scanning (STEM) mode. Elemental analysis was performed using an Oxford X-Max 100 Silicon Drift energy-dispersive X-ray spectrometer (EDS) in connection with TEM, with a probe size ranging from 2 to 5 nm in STEM mode.
3. Results and Discussion
3.1. Mo Speciation
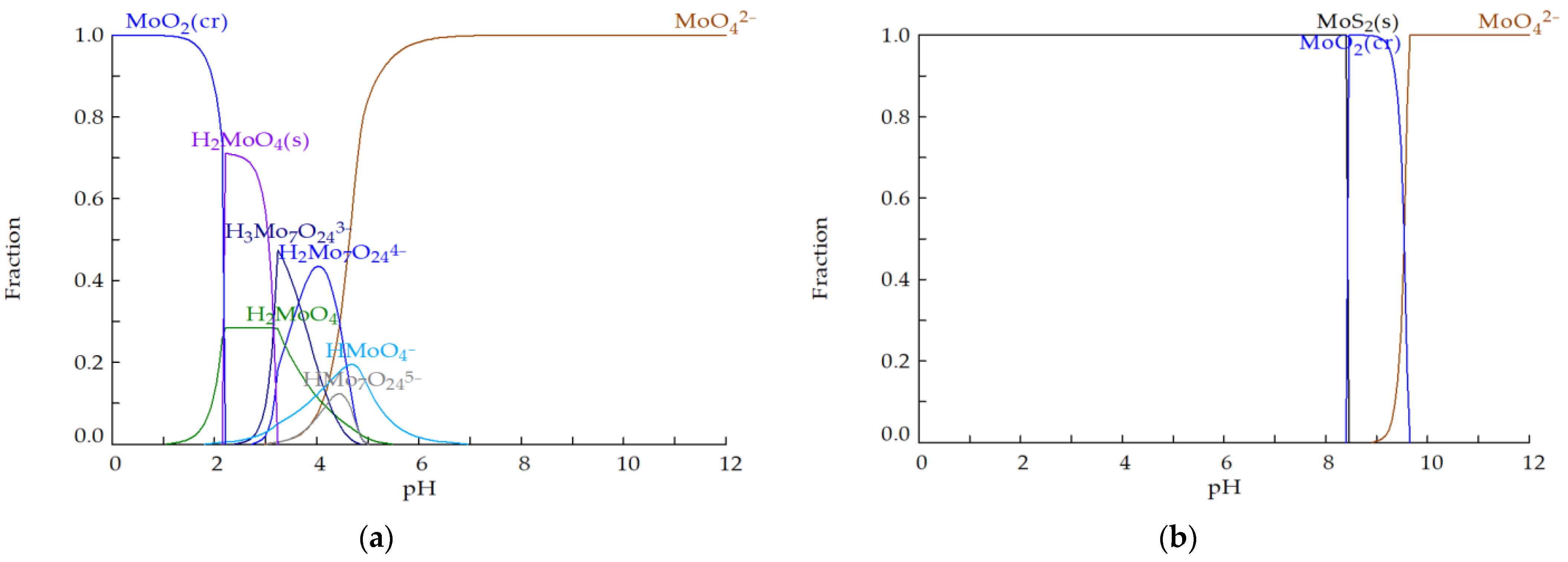
Figure 2 depicts the simulation results of molybdenum speciation given by Hydra-Medusa. Under oxic conditions (Figure 2a), molybdenum occurs predominantly as , as protonation occurs significantly only below pH 5. However, under anoxic and sulphidic conditions (typical conditions found in a sulphate-reducing bioreactor, Figure 2b), Mo is found as MoS2 up to a pH of 8.5. Above a pH of 8.5, under anoxic conditions, the dominant stable phases are MoO2 when 8.5 < pH < 9, and when pH > 9.
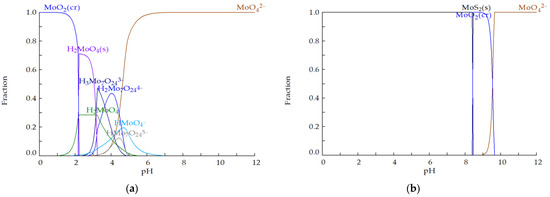
Figure 2.
Concentration fraction of Mo species in (a) oxic (E = 500 mV) and (b) anoxic (E = −250 mV) conditions for the Mo–Fe–O–H–S system at 25 °C for = 15.65 mM, = 1.70 mM and = 1.25 mM (calculated with Hydra-Medusa [48]; “cr” stands for crystalline solid and “s” for solid of unknown crystallinity).
Moreover, under anoxic and sulphidic conditions, molybdate undergoes sulphidation forming thiomolybdate species (; i.e., → → → → ) which are stable when the pH is near neutral and alkaline values [51]. A study of Mo speciation in anaerobic, weakly sulphidic ([H2S] < 11 μΜ) natural waters [52] has reported that the molybdate sulphidation reaction is faster at a lower pH; at a pH of 6.24, the reaction went nearly to completion in less than three days, whereas at a pH of 8.96 little to no was present after 12 days, and the dominant species was . It has also been reported [53] that a 3-fold change in [H2S] produces a 100-fold change in while each successive sulphidation reaction is one order of magnitude slower than the previous one.
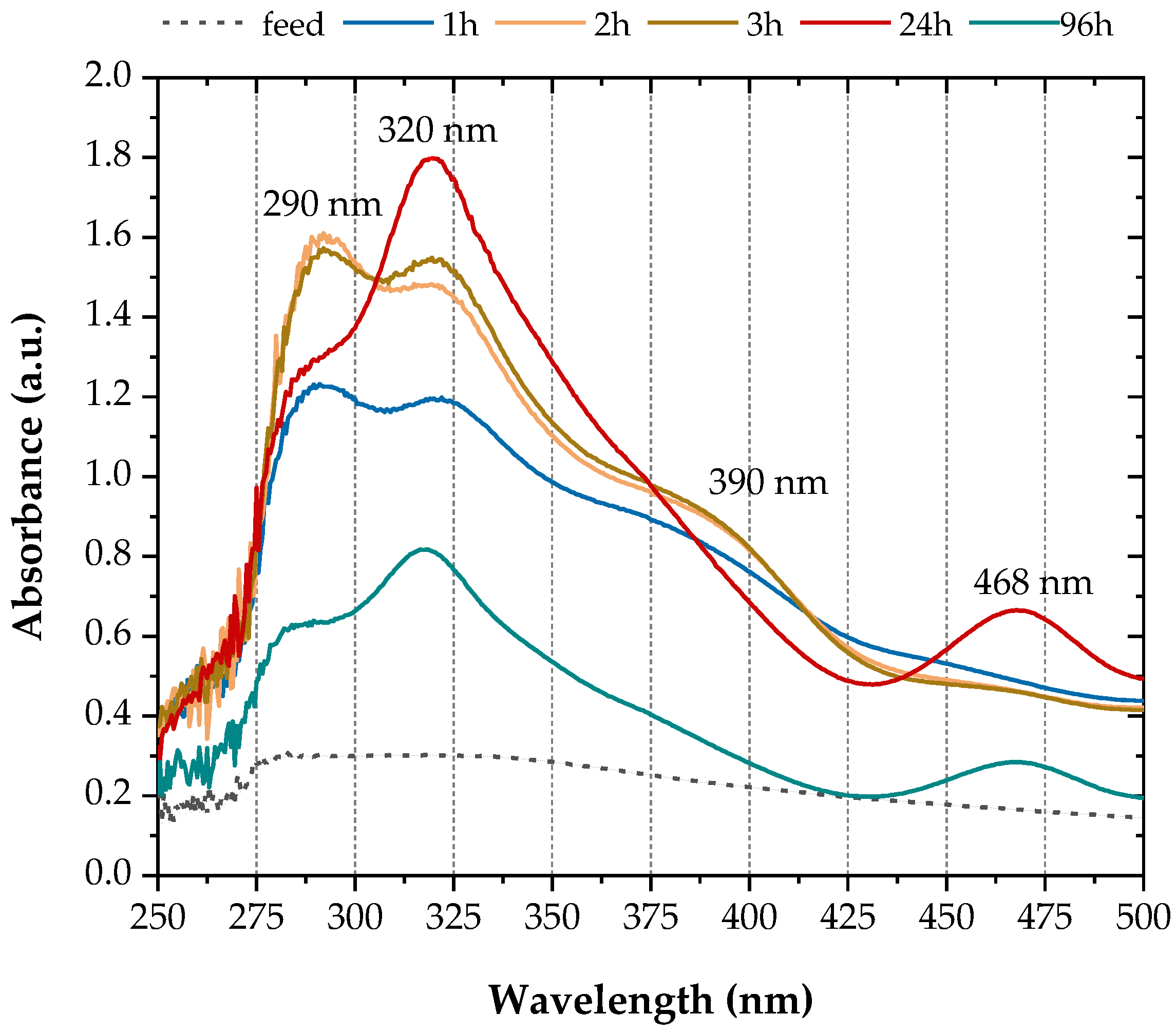
Spectral analysis of the reactor outflow, which had a characteristic purple colour, revealed absorbance peaks at 290, 320, 390 and 468 nm (Figure 3). Similar absorbance peaks were identified after scanning the centrifugate of the growth medium when D. vulgaris was grown in media containing 0.1 mM sodium molybdate [33]. Based on the literature (Table 1), these spectra indicate the formation of intermediate thiomolybdate species and ultimately after 24 h. Figure 3 also shows that the concentration of was reduced by 55% by the end of the experiments (96 h).
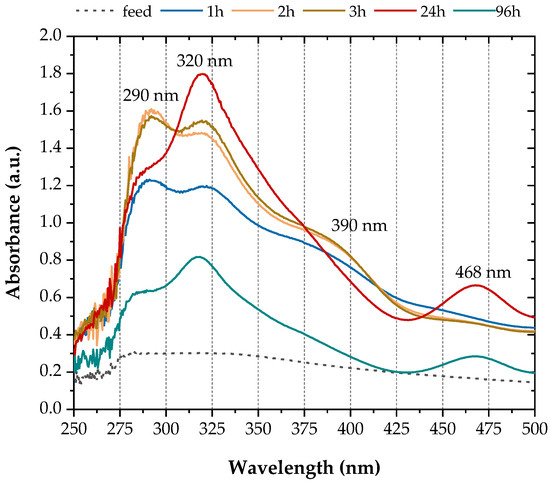
Figure 3.
UV-Vis absorbance spectra of the reactor outflow.

Table 1.
Wavelengths of UV-Vis absorbance maxima for thiomolybdate species.
3.2. Reduction of Sulphate and Molybdate
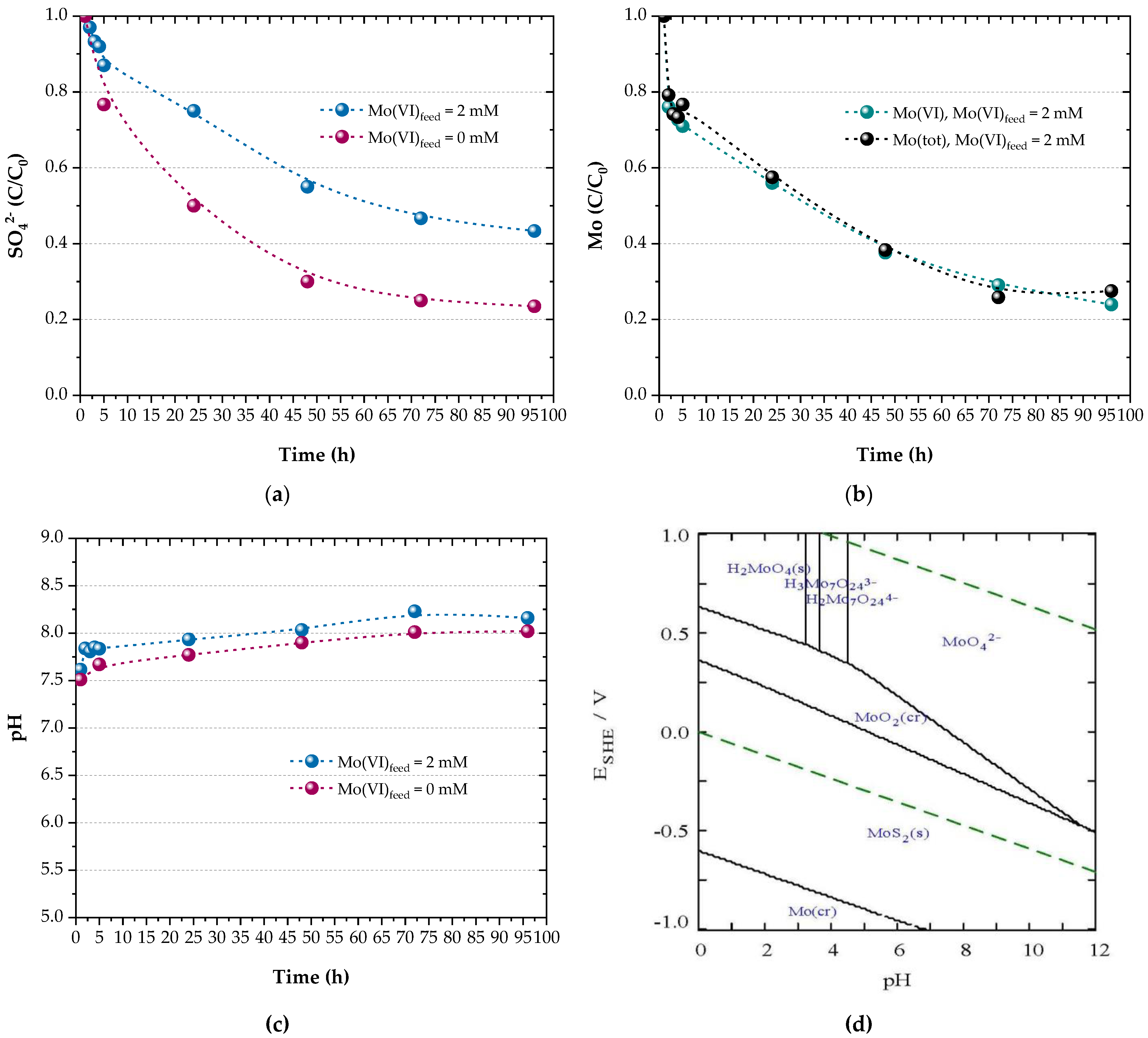
Figure 4 shows that sulphate reduction (Figure 4a) proceeded along with molybdate sequestering (Figure 4b) and a pH increase (Figure 4c), validating the concept of the proposed process.
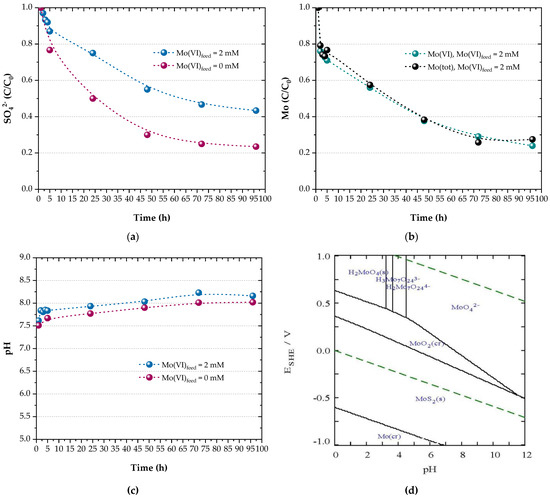
Figure 4.
Profiles of (a) sulphate, (b) molybdenum (VI and total) and (c) pH during bioreactor runs. (d) E–pH diagram for the Mo–Fe–O–H–S system at 25 °C for = 15.65 mM, = 1.70 mM and = 1.25 mM (calculated with Hydra-Medusa [48]).
Sulphate reduction in the presence of 1.25 mM molybdate reached 57% after 96 h, whereas sulphate was reduced by approximately 76% during a control experiment which was carried out without adding molybdate into the feed solution (Figure 4a). The limited electron exchange in the presence of molybdate indicates a potential inhibitory effect on the SRB metabolic activity which, however, needs to be further verified in conjunction with the oxidation of the electron donor by the microorganisms established in the reactor biofilm. Nevertheless, similar results were reported for pure SRB cultures when 0.5–2 mM molybdate was added to solutions containing 20 mM sulphate [33]; Desulfovibrio gigas and D. vulgaris were more sensitive to molybdate than D. desulfuricans, which was inhibited by 50% at 1 mM molybdate. Above 2 mM molybdate, sulphate reduction was completely inhibited and the corresponding ratio of molybdate/sulphate was calculated as 1:10. In this study, a molar molybdate/sulphate ratio of 1:12.5 also proved effective for molybdate recovery.
When comparing sulphate reduction in the absence of molybdate with the previously reported efficiency of the biofilm reactor a year ago [41], or the initial microbial culture [43], it is less efficient as 25% of the initial sulphate content remains in the reactor outflow after 96 h. Sulphate reduction is even lower than the results attained in the presence of molybdate four months ago [58]. This finding may be connected with a possible alteration of the initial microbial population [43] or long-term exposure (over six months) to feed solutions containing molybdate, which stimulates the growth of methanogens or other anaerobes. As these microbes compete with SRB for the electron donor, the species comprising the microbial population of the reactor biofilm need to be further identified.
The concentration of molybdate molybdenum and total molybdenum in the reac-tor outflow is depicted in Figure 4b. It is indicated that Mo(VI) is reduced and recovered by 76% in 96 h. This finding is consistent with Figure 3 and indicates that all residual Mo is present as , meaning that Mo recovery is limited by Mo(VI) reduction. The fact that Mo recovery is not restricted by the available sulphide is also indicated by the stoichiometry of the precipitation reactions: the biogenic sulphide which is produced by the end of the experiments due to sulphate reduction (15.65 mM × 0.57 = 8.9 mM) is theoretically sufficient for the quantitative precipitation of iron (1.7 mM) and molybdenum (1.25 mM) as sulphides (3.4 mM sulphide for FeS2 + 2.5 mM sulphide for MoS2 = 5.9 mM).
The attained recovery rate is lower than the preliminary results attained after feeding the bioreactor with molybdate-containing solutions for two months (presented in [58]). This also underlines the strong link between molybdenum recovery and microbial activity in the reactor.
3.3. Characterisation of the Reactor Precipitates
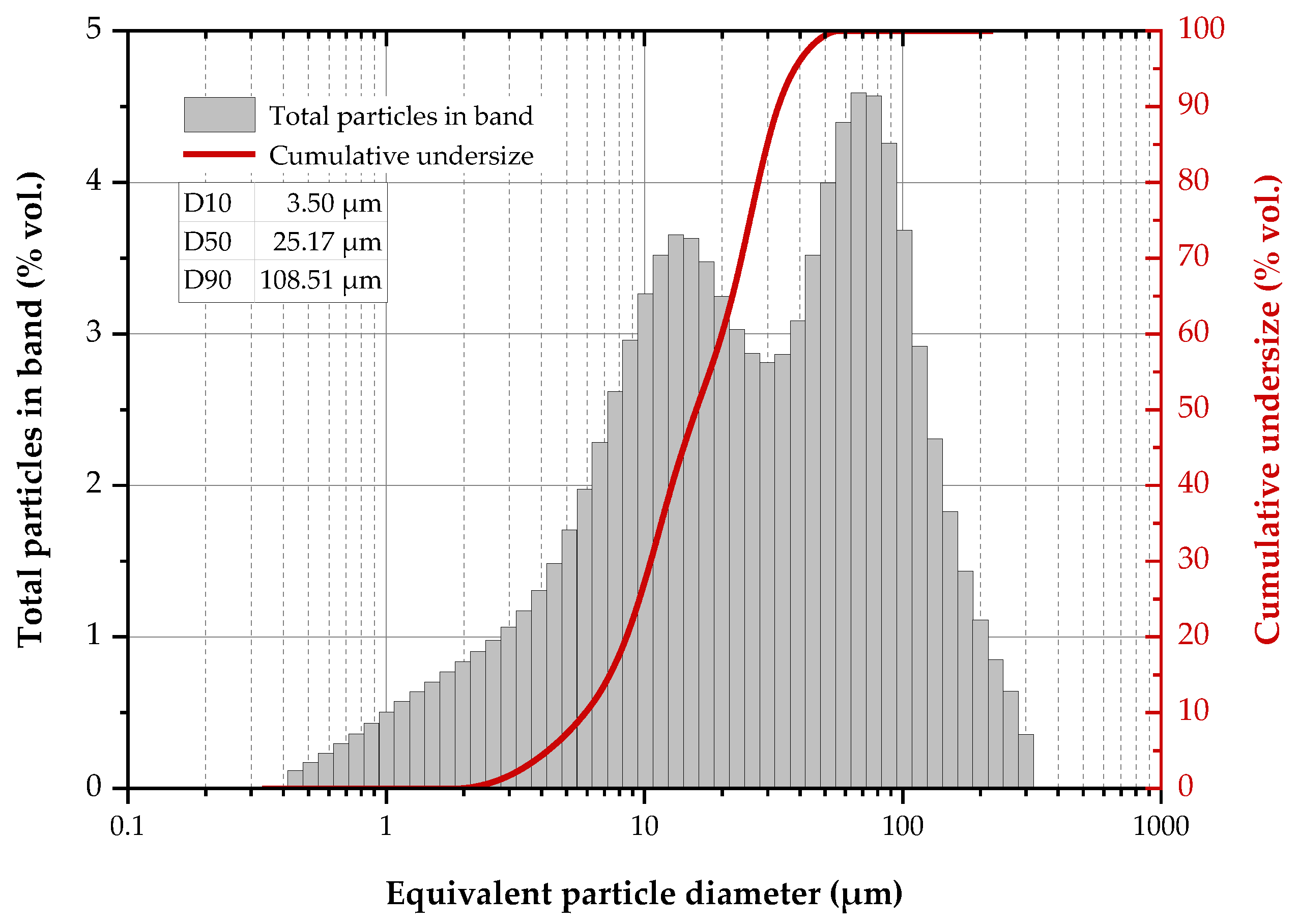
The particle size distribution of the reactor precipitates (Figure 5) revealed two discrete grain size distributions in the 0.4–300 μm range, namely 0.4–12 μm (peak value at 10 μm) for the fine fraction and 12–300 μm (peak value: 70 μm) for the coarser and possibly aggregated particles. Moreover, it is shown that the particle diameter is smaller than 108.5 μm (by 90%), 25.2 μm (by 50%) and 3.5 μm (by 10%).
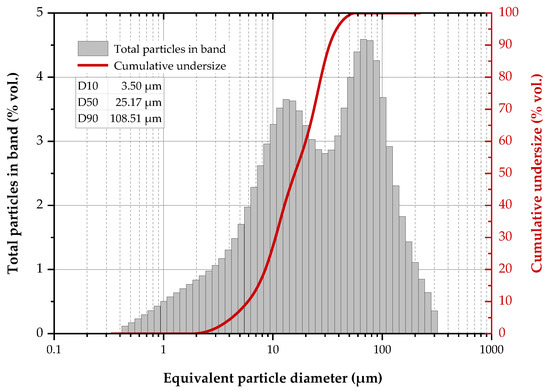
Figure 5.
Particle size distribution of the reactor precipitates.
The grain size of the precipitates is larger than previously reported results [49] and this may be due to the limited sulphate reduction shown in Figure 4a. It has been demonstrated that PSD is affected by the sulphide concentration as, under a sulphide surplus, the precipitated metal phases are characterised by smaller particles with worse settling properties [59,60]. Nevertheless, if the produced solid material is intended to be utilised in applications where the grain size is critical (as for example, in photocatalytic applications where nanoscale materials exhibit enhanced catalytic properties [19]), the grain size of the reactor precipitates can be further optimised via a strict control of the reactor operating conditions.
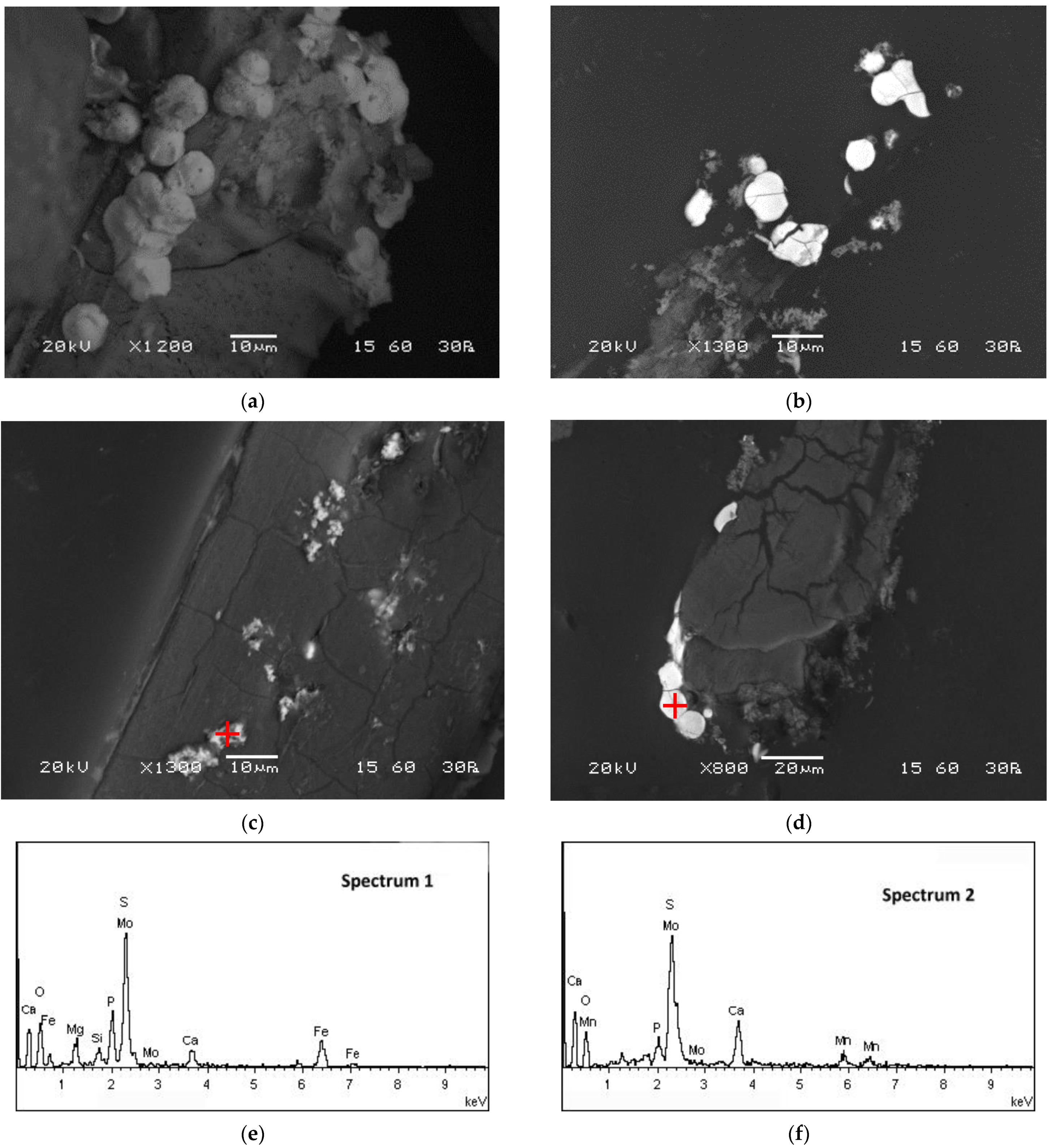
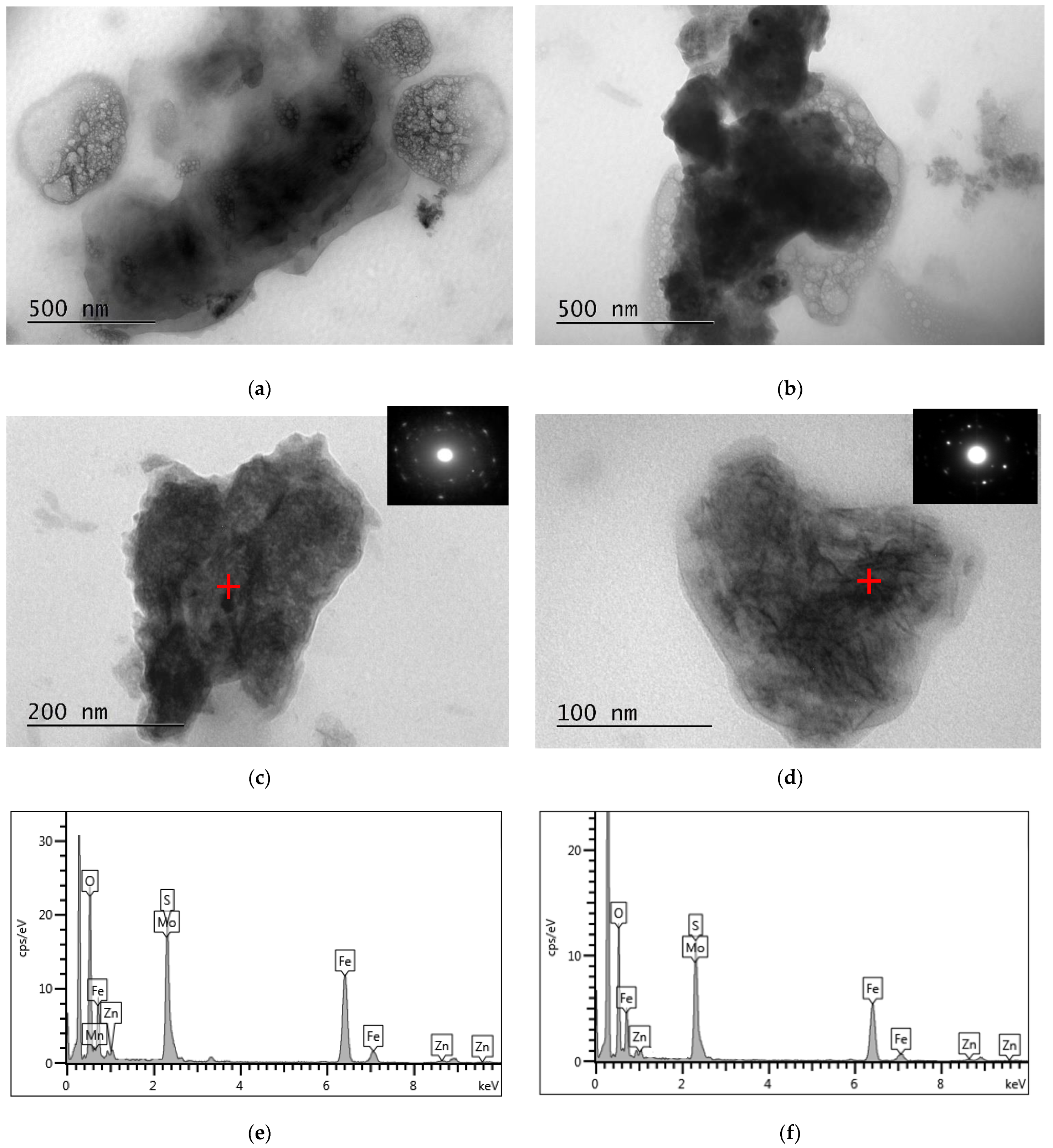
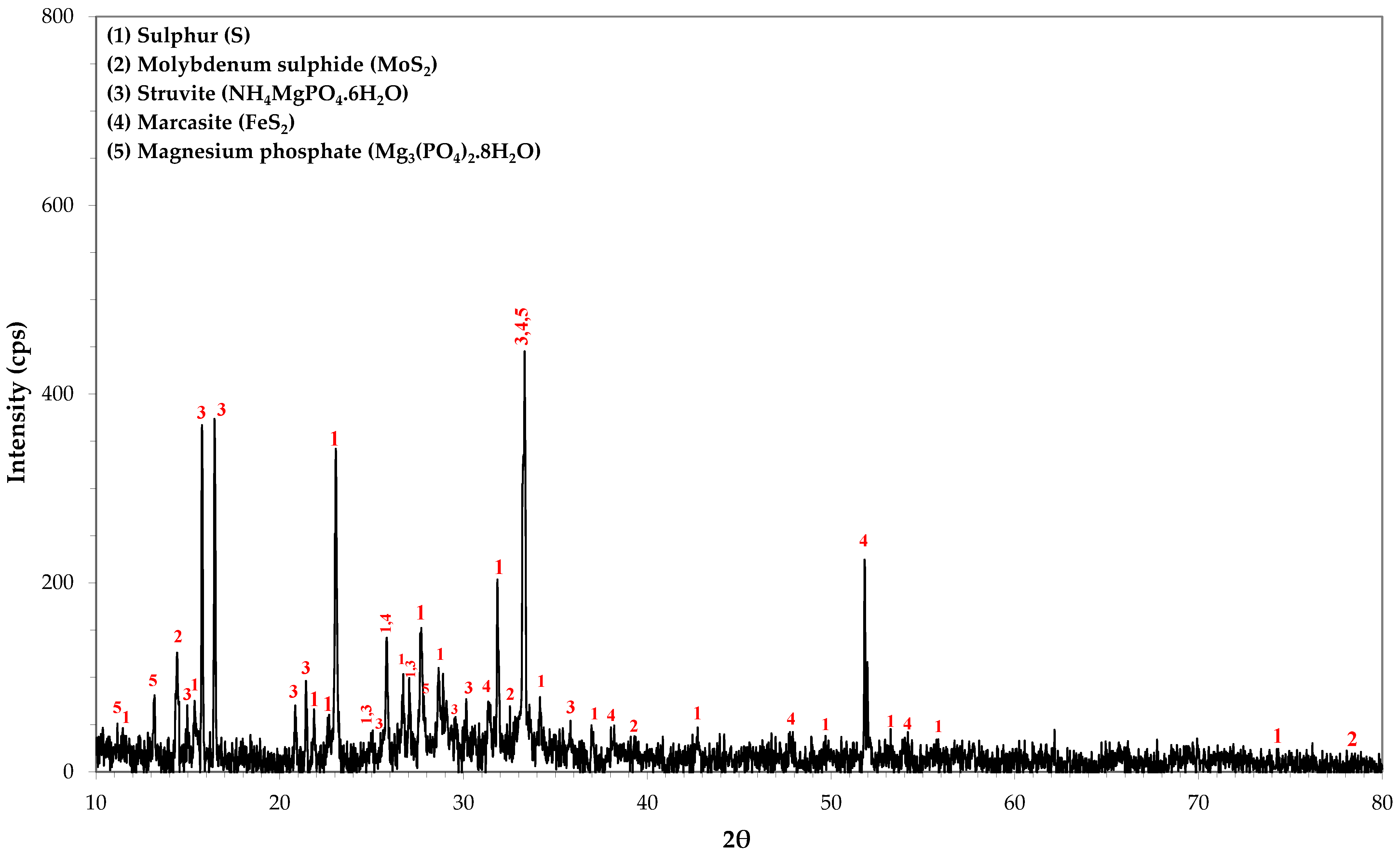
SEM-EDS and TEM-EDS results for the reactor precipitates are depicted in Figure 6 and Figure 7, respectively. Most of the particles have been deposited on the surface of the packing material and present spherical development (Figure 6a; SEM micrograph taken before impregnation of the sample in the resin). EDS spectra indicate that molybdenum is generally immobilised in microcrystalline sulphidic phases that also contain Fe (Figure 6e and Figure 7e,f), Mn (Figure 6f and Figure 7e,f) and Zn (Figure 7e,f). The X-ray diffractogram (Figure 8) also indicates the main mineral phases identified in the reactor precipitates: sulphur (S), molybdenum sulphide (MoS2), iron sulphide (FeS2), struvite (NH4MgPO4·6H2O) and magnesium phosphate (Mg3(PO4)2·8H2O).
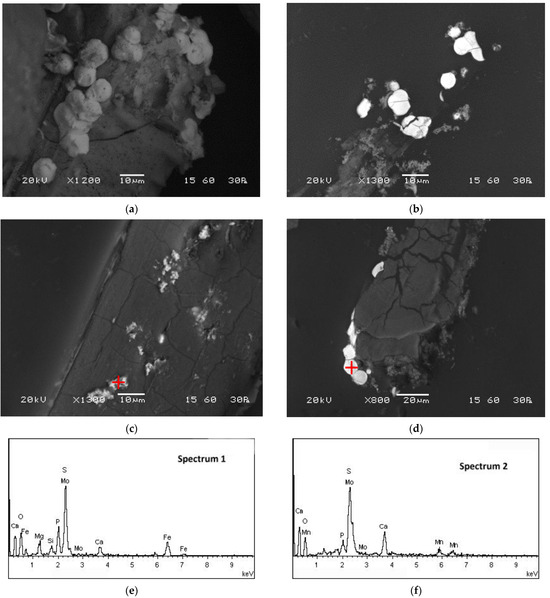
Figure 6.
SEM micrographs of the reactor precipitates (a) before impregnation in the resin and (b–d) on polished sections after impregnation in the resin (dark areas correspond to the surface of the inert packing material of the reactor). (e,f) EDS spectra on spots marked with a red cross on the relevant micrographs (spectrum 1 corresponds to (c) and spectrum 2 corresponds to (d)).
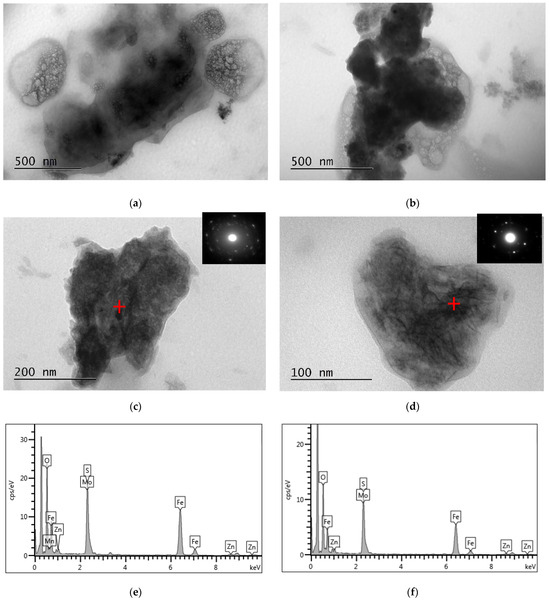
Figure 7.
(a–d) Bright-field TEM micrographs of the reactor precipitates and selected area diffraction patterns (SAED) of mixed (c) FeS2 and (d) MoS2 particles; (e,f) EDS spectra on spots marked with a red cross on the relevant micrographs (spectrum (e) corresponds to (c) and spectrum (f) corresponds to (d)).
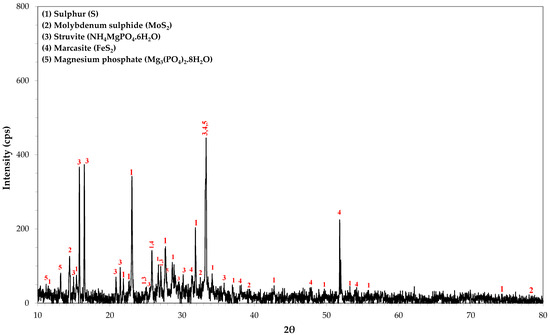
Figure 8.
XRD analysis of the reactor precipitates.
Divalent iron is generally added in the solutions fed to the reactor and precipitates as FeS or FeS2 in the presence of biogenic sulphide [43,49]; thus, sulphidic phases of iron are typically abundant in sulphate-reducing bioreactors. Zinc and manganese were found in acid mine drainage feed which was treated in the reactor during previous reported runs [41]. As most divalent metals under sulphidic conditions, Zn precipitates as sulphide (Zn precipitation as wurtzite and sphalerite was already demonstrated [43,49]). Manganese could have precipitated as sulphide upon reduction or oxide under the alkaline reactor conditions. Calcium, magnesium and phosphorus detected in EDS spectra are contained in the basal medium and may have precipitated as phosphate phases [50], whereas the presence of Si in the reactor sludge is attributed to the abrasion of the inert packing material.
Molybdenum is expected to have precipitated as MoS2 in the reactor bed, as molybdenum sulphide is the predominant phase depicted in the E-pH diagram (Figure 4d) for alkaline-reducing conditions. Molybdenum disulphide was also identified via energy dispersive X-ray analysis of the deposits found extracellularly on the surface and the periplasm of D. gigas and D. desulfuricans cells grown in media containing 0.1 mM sodium molybdate [33]. Moreover, the analysis of the electron diffraction pattern of the black precipitate formed in cell suspensions of D. desulfuricans in the presence of Mo(VI) and sulphate revealed that the Mo(IV) phase was nanoscale MoS2 [36].
In this study, although the EDS spectra cannot unambiguously indicate the presence of MoS2 due to the intense overlap of the L series peaks of Mo and the S Kα peak, MoS2 was identified in the electron diffraction pattern (Figure 7d) and in the X-ray diffractogram (Figure 8) which verified the reduction of Mo(VI) into Mo(IV) and its subsequent precipitation as sulphide. The X-ray diffraction pattern showed that MoS2 had a hexagonal structure and the detected diffraction peaks at 14.39°, 32.69° and 39.49° corresponded to the (002), (100), (103) crystal planes. Further study of the nature and the crystalline structure of the precipitate via HRTEM revealed that MoS2 had a layer-like structure and was developed in nanosheets and aggregated layers. The bioreactor precipitate, consisting of Mo, S and small amounts of iron and oxygen, had a nano flower-like structure assembled from nanosheets. The nanosheets were detected being slightly stuck around each other, or with bubble-like structures, with very sharp and thin sheets of MoS2.
In addition to the thermodynamically favoured MoS2 formation upon the interaction of Mo(IV) with biogenic sulphide, another mechanistic approach was proposed for the immobilisation of Mo in sediments under anoxic and sulphidic conditions, i.e., the Fe sulphide pathway. It has been suggested that the latter is initiated with the formation of thiomolybdate [61], which interacts with Fe sulphide leading to the adsorption of Mo onto pyrite, or the incorporation of Mo into Mo-Fe-S cubane structures, such as FeMoS2(S2) [52,62,63,64,65]. These mechanisms may explain the presence of molybdenum in sulphidic phases where the electron diffraction pattern corresponds to FeS2 (Figure 7c).
As a result, microbial activity promoted the development and precipitation of mixed Mo and Fe sulphides. Further research will clarify the mechanism of molybdenum immobilisation in such a sulphate-reducing system. This understanding will facilitate process design for the selective precipitation of Mo and control of the purity of the biogenic Mo precipitates.
4. Conclusions
This work demonstrates a microbially mediated process for the fabrication of molybdenum chalcogenides from wastewater containing sulphate and molybdate. A molar molybdate/sulphate ratio of 1:12.5 proved effective for molybdate recovery by 76 % from solutions containing 2 mM Mo as . Molybdenum was retained in the sulphidic precipitates of the bioreactor, presumably via the following two principal mechanisms: (i) microbially mediated reduction and precipitation and (ii) thiomolybdate formation and sorption/incorporation into iron sulphides.
Further investigation of the mechanism regulating the immobilisation of molybdenum may tune the entire process in order to fabricate a valuable material, i.e., MoS2, with desirable properties for a wide range of environmental and energy applications.
Author Contributions
Conceptualization, P.K., A.H. and E.R.; methodology, P.K., A.H. and P.E.T.; investigation, D.-A.S.; writing—original draft preparation, P.K., A.H. and P.E.T.; writing—review and editing, P.K., A.H., P.E.T. and E.R.; supervision, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The experimental data reported and discussed in this work are available upon request.
Acknowledgments
The authors would like to thank D. Sparis (Laboratory of Metallurgy, School of Mining and Metallurgical Engineering, NTUA, Greece) for his kind help in the particle size distribution tests. The authors also acknowledge the donation of the packing material used for setting up the sulphate-reducing bioreactor described in this work. The packing material was kindly provided by FilterPro (Durham, UK).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mitchell, P.C.H.; Outteridge, T.; Kloska, K.; McMahon, S.; Epshteyn, Y.; Sebenik, R.F.; Burkin, A.R.; Dorfler, R.R.; Laferty, J.M.; Leichtfried, G.; et al. Molybdenum and molybdenum compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2020; pp. 1–63. [Google Scholar]
- Hund, K.; La Porta, D.; Fabregas, T.P.; Laing, T.; Drexhage, J. Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition; Climate-Smart Mining Initiative of the World Bank Group: Washington, DC, USA, 2020. [Google Scholar]
- EC. Study on the Critical Raw Materials for the EU 2023—Final Report; Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs: Brussels, Belgium, 2023. [Google Scholar]
- Ong, K.H.; Agileswari, R.; Maniscalco, B.; Arnou, P.; Kumar, C.C.; Bowers, J.W.; Marsadek, M.B. Review on substrate and molybdenum back contact in CIGS thin film solar cell. Int. J. Photoenergy 2018, 2018, 9106269. [Google Scholar] [CrossRef]
- Theocharis, M.; Tsakiridis, P.E.; Kousi, P.; Hatzikioseyian, A.; Zarkadas, I.; Remoundaki, E.; Lyberatos, G. Hydrometallurgical treatment for the extraction and separation of indium and gallium from end-of-life CIGS photovoltaic panels. Mater. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- Weckend, S.; Wade, A.; Heath, G. End-of-Life Management: Solar Photovoltaic Panels; International Renewable Energy Agency (IRENA) and International Energy Agency—Photovoltaic Power Systems (IEA-PVPS): Paris, France, 2016; p. 100. [Google Scholar]
- Abejón, R. An overview to technical solutions for molybdenum removal: Perspective from the analysis of the scientific literature on molybdenum and drinking water (1990–2019). Water 2022, 14, 2108. [Google Scholar] [CrossRef]
- Zeng, L.; Yong Cheng, C. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part II: Separation and purification. Hydrometallurgy 2009, 98, 10–20. [Google Scholar] [CrossRef]
- Swinkels, P.L.J.; van der Weijden, R.D.; Ajah, A.N.; Arifin, Y.; Loe, H.L.; Manik, M.H.; Siriski, I.; Reuter, M.A. Conceptual process design as a prerequisite for solving environmental problems; a case study of molybdenum removal and recovery from wastewater. Miner. Eng. 2004, 17, 205–215. [Google Scholar] [CrossRef]
- Freedman, M.L. Precipitation of molybdenum (VI) in strongly acid solutions. J. Chem. Eng. Data 1963, 8, 113–116. [Google Scholar] [CrossRef]
- Vollprecht, D.; Plessl, K.; Neuhold, S.; Kittinger, F.; Öfner, W.; Müller, P.; Mischitz, R.; Sedlazeck, K.P. Recovery of molybdenum, chromium, tungsten, copper, silver, and zinc from industrial waste waters using zero-valent iron and tailored beneficiation processes. Processes 2020, 8, 279. [Google Scholar] [CrossRef]
- Moret, A.; Rubio, J. Sulphate and molybdate ions uptake by chitin-based shrimp shells. Miner. Eng. 2003, 16, 715–722. [Google Scholar] [CrossRef]
- Valenzuela, F.R.; Andrade, J.P.; Sapag, J.; Tapia, C.; Basualto, C. The solvent extraction separation of molybdenum and copper from acid leach residual solution of Chilean molybdenite concentrate. Miner. Eng. 1995, 8, 893–904. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, H.; Suzuki, H. Liquid-liquid extraction of molybdenum(VI) from aqueous acid solutions by TBP and TOPO. Hydrometallurgy 1990, 23, 297–308. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Cibati, A.; Cheng, K.Y.; Morris, C.; Ginige, M.P.; Sahinkaya, E.; Pagnanelli, F.; Kaksonen, A.H. Selective precipitation of metals from synthetic spent refinery catalyst leach liquor with biogenic H2S produced in a lactate-fed anaerobic baffled reactor. Hydrometallurgy 2013, 139, 154–161. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.-C.; Guibal, E. Metal valorization from the waste produced in the manufacturing of Co/Mo catalysts: Leaching and selective precipitation. J. Mater. Cycles Waste Manag. 2019, 21, 525–538. [Google Scholar] [CrossRef]
- Vemic, M.; Bordas, F.; Comte, S.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Recovery of molybdenum, nickel and cobalt by precipitation from the acidic leachate of a mineral sludge. Environ. Technol. 2016, 37, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Gadore, V. MoS2 based nanocomposites: An excellent material for energy and environmental applications. J. Environ. Chem. Eng. 2021, 9, 105836. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H.S. Performance evaluation of molybdenum dichalcogenide (MoX2; X= S, Se, Te) nanostructures for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 17878–17886. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, R.; Ng, Y.H.; Zhang, P.; Xiang, Q.; Li, X. A review on 2D MoS2 cocatalysts in photocatalytic H2 production. J. Mater. Sci. Technol. 2020, 56, 89–121. [Google Scholar] [CrossRef]
- Meier, A.J.; Garg, A.; Sutter, B.; Kuhn, J.N.; Bhethanabotla, V.R. MoS2 nanoflowers as a gateway for solar-driven CO2 photoreduction. ACS Sustain. Chem. Eng. 2019, 7, 265–275. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, V.; Kumar, R. A comprehensive review on synthesis and applications of molybdenum disulfide (MoS2) material: Past and recent developments. Inorg. Chem. Commun. 2020, 121, 108200. [Google Scholar] [CrossRef]
- Jacob, J.M.; Lens, P.N.L.; Balakrishnan, R.M. Microbial synthesis of chalcogenide semiconductor nanoparticles: A review. Microb. Biotechnol. 2016, 9, 11–21. [Google Scholar] [CrossRef]
- Nelson, D.; Amedea, B.S. Microbial syntheses of metallic sulfide nanoparticles: An overview. Curr. Biotechnol. 2012, 1, 287–296. [Google Scholar] [CrossRef]
- Yanchatuña Aguayo, O.P.; Mouheb, L.; Villota Revelo, K.; Vásquez-Ucho, P.A.; Pawar, P.P.; Rahman, A.; Jeffryes, C.; Terencio, T.; Dahoumane, S.A. Biogenic sulfur-based chalcogenide nanocrystals: Methods of fabrication, mechanistic aspects, and bio-applications. Molecules 2022, 27, 458. [Google Scholar] [CrossRef]
- Postgate, J.R. The Sulphate-Reducing Bacteria; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]
- Bijmans, M.F.M.; Buisman, C.J.N.; Meulepas, R.J.W.; Lens, P.N.L. Sulfate reduction for inorganic waste and process water treatment. In Comprehensive Biotechnology, 2nd ed.; Murray, M.-Y., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 435–446. [Google Scholar]
- Rabus, R.; Hansen, T.A.; Widdel, F. Dissimilatory sulfate- and sulfur-reducing prokaryotes. In The Prokaryotes: Ecophysiology and Biochemistry, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2007; Volume 2, pp. 659–768. [Google Scholar]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Hockin, S.L.; Gadd, G.M. Bioremediation of metals and metalloids by precipitation and cellular binding. In Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Barton, L.L., Hamilton, W.A., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 405–434. [Google Scholar]
- Lovley, D.R. Dissimilatory metal reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef]
- Biswas, K.C.; Woodards, N.A.; Xu, H.; Barton, L.L. Reduction of molybdate by sulfate-reducing bacteria. BioMetals 2009, 22, 131–139. [Google Scholar] [CrossRef]
- Chen, G.; Ford, T.E.; Clayton, C.R. Interaction of sulfate-reducing bacteria with molybdenum dissolved from sputter-deposited molybdenum thin films and pure molybdenum powder. J. Colloid Interface Sci. 1998, 204, 237–246. [Google Scholar] [CrossRef]
- Tucker, M.D.; Barton, L.L.; Thomson, B.M. Removal of U and Mo from water by immobilized Desulfovibrio desulfuricans in column reactors. Biotechnol. Bioeng. 1998, 60, 88–96. [Google Scholar] [CrossRef]
- Tucker, M.D.; Barton, L.L.; Thomson, B.M. Reduction and immobilization of molybdenum by Desulfovibrio desulfuricans. J. Environ. Qual. 1997, 26, 1146–1152. [Google Scholar] [CrossRef]
- Hasnain Isa, M.; Anderson, G.K. Molybdate inhibition of sulphate reduction in two-phase anaerobic digestion. Process Biochem. 2005, 40, 2079–2089. [Google Scholar] [CrossRef]
- Newport, P.J.; Nedwell, D.B. The mechanisms of inhibition of Desulfovibrio and Desulfotomaculum species by selenate and molybdate. J. Appl. Bacteriol. 1988, 65, 419–423. [Google Scholar] [CrossRef]
- Tanaka, S.; Lee, Y.-H. Control of sulfate reduction by molybdate in anaerobic digestion. Water Sci. Technol. 1997, 36, 143–150. [Google Scholar] [CrossRef]
- Zane, G.M.; Wall, J.D.; De León, K.B. Novel mode of molybdate inhibition of Desulfovibrio vulgaris Hildenborough. Front. Microbiol. 2020, 11, 610455. [Google Scholar] [CrossRef]
- Strongyli, D.-A.; Kousi, P.; Hatzikioseyian, A.; Remoundaki, E. A sulphate-reducing bioreactor for treating acidic industrial wastewater. In Proceedings of the 8th International Conference on Research Frontiers in Chalcogen Cycle Science and Technology, Galway, Ireland, 17–18 November 2022; pp. 30–33. [Google Scholar]
- Mendrinou, P.; Hatzikioseyian, A.; Kousi, P.; Oustadakis, P.; Tsakiridis, P.; Remoundaki, E. Simultaneous removal of soluble metal species and nitrate from acidic and saline industrial wastewater in a pilot-scale biofilm reactor. Environ. Process. 2021, 8, 1481–1499. [Google Scholar] [CrossRef]
- Kousi, P.; Remoundaki, E.; Hatzikioseyian, A.; Battaglia-Brunet, F.; Joulian, C.; Kousteni, V.; Tsezos, M. Metal precipitation in an ethanol-fed, fixed-bed sulphate-reducing bioreactor. J. Hazard. Mater. 2011, 189, 677–684. [Google Scholar] [CrossRef]
- White, C.; Gadd, G.M. A comparison of carbon/energy and complex nitrogen sources for bacterial sulphate-reduction: Potential applications to bioprecipitation of toxic metals as sulphides. J. Ind. Microbiol. Biotechnol. 1996, 17, 116–123. [Google Scholar] [CrossRef]
- Reis, M.A.M.; Almeida, J.S.; Lemos, P.C.; Carrondo, M.J.T. Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol. Bioeng. 1992, 40, 593–600. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sen, A.M.; Kimura, S.; Rowe, O.F.; Hallberg, K.B. Novel biosulfidogenic system for selective recovery of metals from acidic leach liquors and waste streams. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2006, 115, 19–24. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Puigdomenech, I. Chemical Equilibrium Diagrams Hydra-Medusa; Jan 2016; KTH, School of Chemical Science and Engineering, Department of Chemistry: Stockholm, Sweden, 2009. [Google Scholar]
- Kousi, P.; Remoundaki, E.; Hatzikioseyian, A.; Korkovelou, V.; Tsezos, M. Fractionation and leachability of Fe, Zn, Cu and Ni in the sludge from a sulphate-reducing bioreactor treating metal-bearing wastewater. Environ. Sci. Pollut. Res. 2018, 25, 35883–35894. [Google Scholar] [CrossRef]
- Remoundaki, E.; Kousi, P.; Joulian, C.; Battaglia-Brunet, F.; Hatzikioseyian, A.; Tsezos, M. Characterization, morphology and composition of biofilm and precipitates from a sulphate-reducing fixed-bed reactor. J. Hazard. Mater. 2008, 153, 514–524. [Google Scholar] [CrossRef]
- Ryzhenko, B.N. Technology of groundwater quality prediction: 1. Eh-pH diagram and detention coefficient of molybdenum and tungsten in aqueous solutions. Geochem. Int. 2010, 48, 407–414. [Google Scholar] [CrossRef]
- Wagner, M.; Chappaz, A.; Lyons, T.W. Molybdenum speciation and burial pathway in weakly sulfidic environments: Insights from XAFS. Geochim. Cosmochim. Acta 2017, 206, 18–29. [Google Scholar] [CrossRef]
- Erickson, B.E.; Helz, G.R. Molybdenum(VI) speciation in sulfidic waters:: Stability and lability of thiomolybdates. Geochim. Cosmochim. Acta 2000, 64, 1149–1158. [Google Scholar] [CrossRef]
- Reid, R.S.; Clark, R.J.; Quagraine, E.K. Accurate UV–visible spectral analysis of thiomolybdates. Can. J. Chem. 2007, 85, 1083–1089. [Google Scholar] [CrossRef]
- McDonald, J.W.; Friesen, G.D.; Rosenhein, L.D.; Newton, W.E. Syntheses and characterization of ammonium and tetraalkylammonium thiomolybdates and thiotungstates. Inorg. Chim. Acta 1983, 72, 205–210. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sykes, A.G. Kinetics of the interconversion of sulfido- and oxomolybdate(VI) species MoOxS4−x2− in aqueous solutions. Inorg. Chem. 1980, 19, 2881–2885. [Google Scholar] [CrossRef]
- Tsigdinos, G.A. Inorganic sulfur compounds of molybdenum and tungsten. In Aspects of Molybdenum and Related Chemistry; Tsigdinos, G.A., Moh, G.H., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1978; pp. 65–105. [Google Scholar]
- Strongyli, D.-A.; Kousi, P.; Hatzikioseyian, A.; Remoundaki, E. Molybdenum recovery in a sulphate-reducing bioreactor. In Proceedings of the 2nd International Conference on Sustainable Chemical and Environmental Engineering, Limassol, Cyprus, 14–18 June 2023; pp. 6–7. [Google Scholar]
- Bijmans, M.F.M.; van Helvoort, P.-J.; Buisman, C.J.N.; Lens, P.N.L. Effect of the sulfide concentration on zinc bio-precipitation in a single stage sulfidogenic bioreactor at pH 5.5. Sep. Purif. Technol. 2009, 69, 243–248. [Google Scholar] [CrossRef]
- Mokone, T.P.; van Hille, R.P.; Lewis, A.E. Effect of solution chemistry on particle characteristics during metal sulfide precipitation. J. Colloid Interface Sci. 2010, 351, 10–18. [Google Scholar] [CrossRef]
- Phillips, R.; Xu, J. A critical review of molybdenum sequestration mechanisms under euxinic conditions: Implications for the precision of molybdenum paleoredox proxies. Earth-Sci. Rev. 2021, 221, 103799. [Google Scholar] [CrossRef]
- Bostick, B.C.; Fendorf, S.; Helz, G.R. Differential adsorption of molybdate and tetrathiomolybdate on pyrite (FeS2). Environ. Sci. Technol. 2003, 37, 285–291. [Google Scholar] [CrossRef]
- Helz, G.R.; Bura-Nakić, E.; Mikac, N.; Ciglenečki, I. New model for molybdenum behavior in euxinic waters. Chem. Geol. 2011, 284, 323–332. [Google Scholar] [CrossRef]
- Helz, G.R.; Miller, C.V.; Charnock, J.M.; Mosselmans, J.F.W.; Pattrick, R.A.D.; Garner, C.D.; Vaughan, D.J. Mechanism of molybdenum removal from the sea and its concentration in black shales: EXAFS evidence. Geochim. Cosmochim. Acta 1996, 60, 3631–3642. [Google Scholar] [CrossRef]
- Vorlicek, T.P.; Kahn, M.D.; Kasuya, Y.; Helz, G.R. Capture of molybdenum in pyrite-forming sediments: Role of ligand-induced reduction by polysulfides. Geochim. Cosmochim. Acta 2004, 68, 547–556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).