1. Introduction
Aminopyralid (AP) is a pyridine carboxylic acid group herbicide mimicking plant auxin hormone [
1]. It is used in both agricultural sites [
2] and uncultivated lands for excessive and selective control of noxious dicot weeds [
1,
3,
4]. In cultivated lands, AP is generally used in grain crop production to eliminate the weeds that compete with crops. Unlike 2,4-D, which has been used for weed control and has a long history, pyridine carboxylic acids are more effective at low doses, and AP toxicity in humans and animals is low [
5]. Hence, it is also widely used in pastures with grazing livestock. Therefore, AP remains intact during the digestion as well as post-harvest crops in free and conjugated forms [
6]. Consequently, when straw and its products are used in the farming of other plantations, AP demonstrates anti-dicot and anti-broad leaf activity on non-targeted plants, even at concentrations as low as 1 ppb to 10 ppb (tomatoes, beans) [
7]. Furthermore, unlike the manufacturer’s initial low-risk assessment (Dow AgroSciences, Indianapolis, IN, USA) [
6], AP is considered to be a persistent herbicide together with picloram and clopyralid, of which 90% dissipation in soil requires 116 days [
8]. It has been reported that AP exposure, even at low levels, leads to poor seed germination, twisted or cupped leaves, reduction in the flowering and fruit yield, and malformation of the fruit [
5,
9] in peas, beans, other legumes, carrots, parsnips, potatoes, tomatoes, and lettuce [
10].
Straw is a post-harvest byproduct of cereal farming and has multiple uses like fodder and mulching material in vegetable production. Straw mulch is an effective tool for sustainable soil management, considering the fact that there is an upcoming risk of water insufficiency. It provides a limitation of sunlight onto the ground surface, acts as a bio-control tool for weed growth, helps soil moisture maintenance with reduced evaporation and irrigation frequency [
11], and avoids surface soil loss [
12]. Furthermore, mulching promotes soil respiration, aids the overwintering of perennial plants [
13], and acts as a blanket that protects above-ground parts of the plants and fruits from soil-originated pathogens since it prevents water splashes from irrigation. In a study focusing on potato production, the use of straw mulch alone exhibited an efficacy of ≥ 90% in controlling toothed dock and demonstrated varied effectiveness against other weed species after 30 days, while combined straw and atrazine application resulted in > 90% anti-weed activity [
14]. However, straw can be also a source of persistent contaminants that may be released during mulch application and affect the target crops. AP-containing contaminated straw and manure led to severe sensitive plant injuries reported worldwide [
15,
16,
17,
18].
Multiresidue pesticide analysis methods have been developed for the rapid screening and quantification of pesticides for food safety, environmental monitoring, and biological exposure rates [
19]. Unfortunately, acidic herbicides are excluded from multi-compound analysis or are only involved for screening purposes due to their instability and poor solubility in the generic extraction solvent and the need for pH-sensitive extraction techniques. This is probably the reason why bio-assays with plants used as an indicator of AP residues are still recommended [
13,
14,
16,
20,
21], rather than its direct analytical determination.
Aminopyralid is found in the herbicidal end-use product as triisopropanolamine (TIPA) salt of aminopyralid that dissociates rapidly in water to the acid at environmental pH values greater than 2.56 [
21]. Base hydrolyzation to release the free AP from conjugates followed by SPE clean-up and ultimate butyl-derivatization have been the official method for aminopyralid and its conjugates from different matrices [
2,
22]. Commercially available specific picolinic acid group MIP-SPE cartridges have also been used as an alternative sample preparation method. Late studies on MIP-SPE cartridge development and testing on milk samples resulted in acceptable accuracy and precision [
23,
24].
Among other methods used for pesticide extraction and purification, the well-known QuEChERS method is applied for aminopyralid residual analysis in biological matrices. This method is suitable for a large group of pesticides, and it also can be easily modified based on the sample material characteristics. However, the QuEChERS method, including its modifications, is primarily intended for the purposes of multi-residue analyses involving a wide range of different, but mainly less polar, analytes [
25]. Thus, for aminopyralid, as a polar acidic compound easily soluble in water, the salting-out assisted liquid–liquid extraction (SALLE) may be a better alternative [
26]. According to European Union Reference Laboratories (EURLs), QuEChERS-based methodology provides a 0.05 mg/kg limit of quantitation (LOQ) for the majority of routinely analyzed matrices, which is sufficient efficiency for all maximum residue levels (MRLs) recommended by EFSA in common crop grains (with exception of rape seeds) [
2]. However, there is no information about AP residue levels in crop byproducts such as cereal straw.
The objective of this study was to create and optimize a cost-effective and concise approach for extracting and quantifying low levels of aminopyralid residues persisting in harvested cereal straw. This method is intended to be effective in cases where straw is employed as mulch, while also ensuring that it complies with the standard maximum residue level of 0.01 mg/kg [
2].
2. Materials and Methods
2.1. Material
Aminopyralid-free wheat bio-straw samples were provided by Kojátky Organic Farm (Czechia). Bio-straw samples were dried at 80 °C till constant weight, milled to a particle size of one millimeter, and used for analysis method optimization, validation tests, and matrix-match calibration.
The wheat straw samples from conventional production, which were treated with Mustang Forte® (containing 180 g of 2,4D, 10 g of aminopyralid, and 5 g of florasulam per liter) at a 1 L/ha dose on the crop field, mulched on the strawberry field, and wintered for a year were collected from a lot found at 50.3227972N, 14.3611767E (strawberry farm) in three replicates from different spots and used to test analytical extraction method efficiency.
2.2. Experimental Chemicals and Reagents
Aminopyralid (CAS No:150114-71-9, purity ≥ 98.0%) from Honeywell (Fluka, Offenbach, Germany) was used as an analytical standard. Liquid chromatography (LC) grade acetonitrile (MeCN) (Honeywell, Fluka, Germany) was used as the extraction solvent, whereas LC-grade methanol (MeOH) (Honeywell, Fluka, Germany) was used to prepare calibration standards and as the final solvent in extracted samples. The analytical grade trifluoroacetic acid and ethyl acetate (Sigma-Aldrich, St. Louis, MO, USA) were used for AP elution from AffiniSep SPE (Le Houlme, France) cartridges. LC/MS grade formic acid (97.5–98.5% purity, LC-MS Lichropur TM) was obtained from Supelco and used for solvent acidification. Ultrapure water was obtained from an in-house MilliQ ® purified water system.
The primary stock solution of AP was dissolved in MeCN and the sub-stock concentration was diluted to 100 µg/mL with MeCN. Prepared primary and sub-stocks were stored in 1.5 mL LC vials at −20 °C. A working concentration of 1 µg/mL was prepared by dilution from 100 µg/mL MeCN stock solution either in MeCN or MeOH containing 1% of FA depending on the experiment requirements.
2.3. Calibration Curve
Matrix-matched calibration curves were prepared using the blank straw with the same sample extraction method and in an identical final solvent as the samples. External calibration standards were prepared in the 1% formic acid in MeOH to calculate the matrix effects at the same concentrations of matrix-matched standards. The peak area of AP at the relevant concentration was recorded to use in the calibration curve with 1/x weighting. The standard curve coefficient of determination (R2) was accepted as valid if it was greater or equal to 0.99.
2.4. Validation Criteria for the Extraction Method
Validation parameters were set based on SANTE/12682/2019 guidelines [
27]. The linearity (sensitivity) of the calibration range was checked from eight concentration levels (1.0, 2.5, 5.0, 10.0, 25.0, 50.0, 75.0, and 100.0 ng/mL). Trueness (recovery%) was calculated based on the spiked blank sample’s practical concentration to theoretical concentration (70–120%) ratio. Precision (repeatability) was calculated as the % of the relative standard deviation (RSD%) of the same level spiked samples. The lowest spiking level was stated as the limit of quantification (LOQ) when the trueness (70–120%) and RSD% (<20%) met the acceptance criteria. The matrix effect was calculated as the ratio of slopes obtained from the matrix-matched calibration equation and solvent-based calibration standards. The retention time shift was calculated with the standard deviation of the AP spiked validation samples’ retention times.
2.5. Sample Preparation
2.5.1. Salting-Out Assisted Liquid–Liquid Extraction (SALLE)
Blank straw material (1 g) was spiked with AP with 50 ng/g concentration and mixed thoroughly. Then, the tube content was soaked with 10 mL of ultrapure water and held at 4 °C for 30 min. Samples were extracted with 10 mL of MeCN containing 1% formic acid using a vortex at high speed for one minute. QuEChERS salt pouch content (4 g of anhydrous MgSO4, and 1 g of NaCl; Bond Elute, Agilent, Hong Kong, China) was added into the tube and vortexed immediately in order to avoid clumping and minimize the temperature impact on the extract. Tubes were centrifuged (7806 RCF, +4 °C, 10 min) and the upper MeCN layer was transferred into a clean tube and stored at −21 °C for 30 min as a clean-up step, which was applied to aid the partition of residual saturated water. After the cooling step, the lower water fraction was discarded via a glass Pasteur pipette, and an upper MeCN layer was used in the LC-MS/MS analysis. In the meantime, 1 mL of the MeCN extract was pipetted into a microcentrifuge tube and evaporated under the stream of N2 on a dry-block heater at 37 °C. The dry extract was dissolved in 1 mL acidified MeOH (1% of formic acid, v/v) with a brief ultrasonication and centrifuged (44641 RCF, +4 °C, 5 min) to remove insoluble solids. The final sample (1 mL) corresponded to 0.1 g of extracted dry straw material. Validation samples, which were spiked with AP concentrations of 5, 10, 25, and 50 ng/g, were prepared in the same manner as the samples spiked with 50 ng/g, as described above.
2.5.2. Water Extraction and MIP Clean-Up
Blank straw samples (1 g) were spiked with AP at 50 ng/g and left to stand until they reached equilibrium. Before water extraction (20 mL), the tubes were covered with aluminum foil and placed horizontally onto the orbital shaker. The orbital shaker was set at 250 rpm, and 20 mL of MilliQ® water was used as an extractant. When the extraction was completed, the slurry was centrifuged and 10 mL of the water phase was filtered via a 0.2µm nylon filter into the 15 mL centrifuge tubes. An aliquot of 5 mL filtered extract was transferred into the pre-conditioned AFFINIMIP® molecularly imprinted polymer (MIP) picolinic acid cartridge (AffiniSep, France) in two portions (1 mL each), and AP was eluted with 2% trifluoroacetic acid in ethyl acetate. AP containing eluate was split into two equal portions (1 mL each) and dried under an N2 stream at 37 °C; dry block heater. Split portions were dissolved in 1 mL of a particular tested sample solvent.
2.5.3. UHPLC-MS/MS Conditions
All measurements were performed on an AB Sciex LC QTRAP 6500+ MS/MS mass spectrometer with a TurboV source (Framingham, MA, USA), equipped with a Shimadzu Exion HPLC (Kyoto, Japan), using positive electron spray ionization (ESI
+) in multiple reaction monitoring (MRM) mode. For MS/MS parameters optimization, AP solutions were applied into the ion source by direct infusion using a syringe pump (Hamilton OEM, Reno, NV, USA) operating at a flow rate of 7 µL/min, and the ion source parameters were set to curtain gas (CUR) at 35 L/min, IonSpray voltage at 5500 V, declustering and entrance potential at 80 and 10 V, respectively, and nebulizer gas (GS1) at 50 psi. The MS/MS parameters after optimization are presented in
Table 1.
Chromatographic separation of AP carried on Ace Excel 2 Super C18 column (100 × 2.1 mm, 3 µm, 90 Å) at 30 °C with a gradient elution of mobile phase A (MilliQ water containing 0.1% formic acid) and B (100% methanol). The flow rate was set at 300 µL/minute. The injection volume was 2 µL. The gradient programs specific to each separation method are listed in
Section 3.1 of the Results section. MS ESI
+ ion source parameters depended on the used method (see
Section 3.4 of Results). Compound fragmentation parameters were used as described at AP 1% formic acid in methanol in
Table 1.
2.6. Data Process and Statistical Evaluation
Compound optimization and peak integration were performed using Analyst 1.7.1 (AB Sciex, Darmstadt, Germany) software. Quantitation of the validation results were calculated in Microsoft Office Excel (2019). Method optimization results were evaluated using one-way ANOVA followed by Tukey’s honest significance test in SPSS 2019. For all comparisons, differences were considered significant at p < 0.01.
3. Results and Discussion
3.1. Sample Solvent Selection
The solvent of an injected sample is also considered a significant parameter for signal response quality. The first optimization step was the selection of the most suitable mobile phase and sample solvent for the final dissolution of the sample before analysis. Two organic solvents, acetonitrile (MeCN) and methanol (MeOH), in combination with formic acid (FA) and water were tested for the best signal response of analytical standard AP standard at a concentration of 50 ng/mL. The analysis was performed using electrospray ionization operating in positive mode (ESI
+), which provided the best yield of an AP [M + H]
+ precursor ion (Q1 ion) of 207.0
m/
z measured at optimal DP and EP values (see
Table 1). The highest ionization efficiency was reached using MeOH with 1% of FA, which corresponds with the common use of MeOH as a mobile phase solvent in LC/MS analysis of polar pesticides [
28]. Although MeCN has been widely used in residue analysis and is a generic extraction solvent in commonly used pesticide purification methods like QuEChERS, its aprotic nature usually suppresses the efficiency of ion formation by electrospray ionization. The reduction of signal response is particularly pronounced in the case of polar compounds such as AP. In the present study, AP samples prepared in acidified MeOH resulted in higher responses than samples in MeCN (
p < 0.01) (see
Table 2). Therefore, considering chromatographic compatibility (see
Section 2.5), the MeOH containing 1% of FA was selected as the optimal final sample solvent for LC/MS/MS analysis. This choice guided further optimization steps and the examination of real samples.
3.2. Optimization of MRM Parameters
The MRM mode of detection provides excellent sensitivity with good selectivity, making it an ideal method for trace analysis in complex matrices. For the method development, two MRM transitions from the initial five were selected. Two precursors [M + H]
+ (206.9
m/
z) and their isotopic M+2 ion (208.9
m/
z) were chosen. In the next step, the most intense fragmentation product ions (188.9
m/
z and 160.9
m/
z) were excluded from the [M+H]
+ transitions due to high interference with the background; instead, the third most abundant ion was used as a quantifier ion (133.9
m/
z,
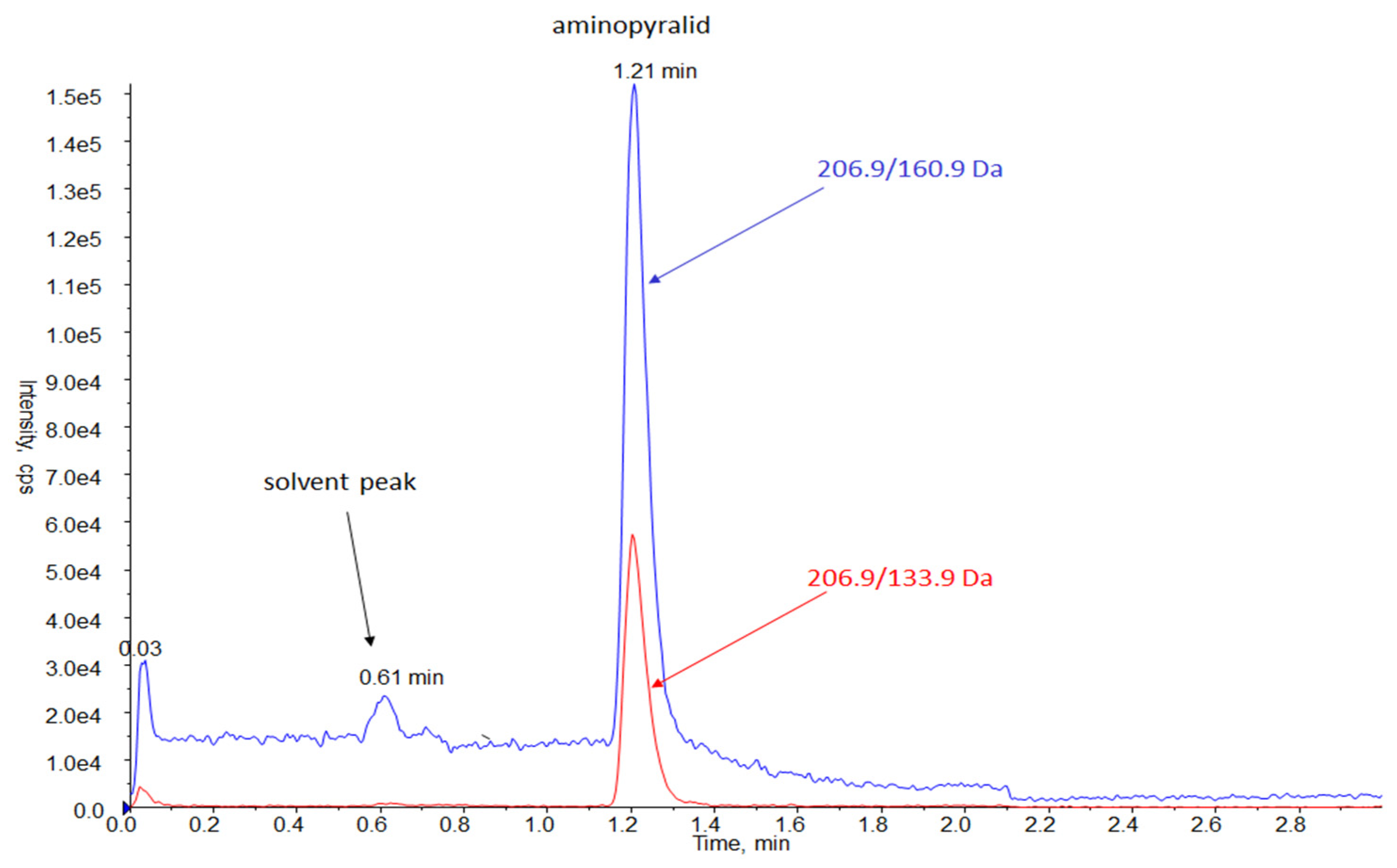
Figure 1). Besides interferences, the choice of sample solvents can directly impact the intensity of the generated fragment ions under identical instrumental conditions. The product ion for confirmatory transition was selected from AP M+2 isotopic precursor (transition 208.9/135.9), which occurs at an 80% abundance rate in nature due to the presence of two chlorine atoms in the AP molecule. A similar approach for AP and other halogenated compounds was followed in the other works [
28,
29]. Detailed MRM tuning parameters are described in
Section 2.5.3 of the Materials and Methods section (
Table 1).
3.3. Selection of Chromatographic Conditions
The composition of the mobile phase is an important factor influencing LC/MS analysis either directly due to the ionization process in the ion source, or indirectly via chromatographic separation (peak shape, coelution with interfering analytes and background, etc.). Two organic solvents, MeCN and MeOH, in combination with acidified (0.1% of FA) water were compared for their impact on the separation and signal intensity of AP. Acetonitrile was used only in one experimental method (Method 4), all other separations were performed with MeOH. Despite the better chromatographic properties of MeCN, the use of MeOH seems essential to achieve good sensitivity for AP analysis in ESI+ mode. For the chromatography, water, supplied with 0.1% of FA (phase A) and MeOH (phase B), was selected as the most appropriate mobile phase combination.
All gradient programs involved in the optimization of chromatographic parameters are shown in
Table 3. Initial chromatographic separation conditions were adapted from the preliminary experiments of this research (Method 1). The used mobile phase affected the retention time of AP, which was detected at 1.2 min for acidified MeOH and at 1.15 min for MeCN. This retention time was sufficient for the separation of AP from the solvent peak eluted in 0.6 min (see
Figure 1) due to the high chromatography resolution of the system.
However, a long run time was found to be inefficient and did not result in signal enhancement. Therefore, the gradient program was gradually shortened to 4.5 min (Method 2) and then to 3 min (Method 3), as is shown in
Table 3. None of these modifications led to a reduction in the peak area of aminopyralid or an enhancement in the signal quality of AP (see
Table 2). Method 4, containing MeCN as the mobile phase, had good results in terms of separation; however, it lagged behind Methods 2 and 3 in sensitivity as well as runtime length.
For Methods 2 and 3, the flow rate, injection volume, and column temperature were the same as in Method 1 and are described in
Section 2.5.3. The peak areas (signal response) of selected quantifier ions obtained by both optimized Methods 2 and 3 are shown in
Table 2. Finally, the optimized Method 3 was chosen for the AP monitoring in straw samples due to the shortest run time without a change in the response strength, which is an undeniable advantage for monitoring analyses containing a large number of samples.
3.4. Ion Source Parameters Optimization
Once optimal separation conditions were selected, ion source parameters were tuned to optimal MRM signal response. For these purposes, the optimized method with the shortest gradient (Method 3) was used. The values of ion source parameters and corresponding instrument responses are presented in
Table 4.
Initially, the effect of ion source temperature in combination with ion spray voltage (IS) on the AP peak area was evaluated. Four IS values (2500, 3500, 4500, and 5500 V) were tested at six temperature levels (350, 400, 450, 500, 550, and 600 °C) (see
Supplementary Materials). The IS voltage of 5500 V provided the best response at all tested temperature levels with the exception of the lowest (350 °C), where it was almost the same as at 4500 V. However, this temperature was already found to be too low for the mobile phase flow used (300 μL/min), resulting in high noise and unstable signal response. The signal increase was about 30% for 5500 V in comparison with 2500 V at a temperature of 550 °C. The response increased continuously with a growing ion source temperature up to 550 °C; after that, further increasing the temperature had no effect on the signal intensity (
Supplementary Materials). In this first step, the ion source temperature of 550 °C seems to be optimal for the analysis. After that, the temperature effect on the response was investigated in a narrow range from 500 °C to 600 °C at the optimal IS voltage of 5500 V (
Table 4). Similarly to the previous test, the response was growing up to 550 °C (about 11%), but the ion source temperature higher than 550 °C had no further effect on the AP peak intensity. Therefore, the source parameters were set to IS 5500 and the ion source temperature to 550 °C as optimal values for further analysis. A final change was made at nebulizer gas (GS1) and heating gas (GS2), which were set as 50 and 40 psi in accordance with the manufacturer’s recommendations to have 10 psi differences between each other and combined with the previously optimized parameters into a final analytical method.
3.5. Effect of Extraction Methods and Mobile Phase Combination on AP Detection in Spiked Straw
After the instrumental conditions were optimized using the AP standard in pure solvent, the developed method was tested by analyzing the sample extracts obtained by two different procedures to ensure the efficiency of the analytical and extraction processes in real straw samples to determine the influence of the matrix on the instrument response (matrix effect).
In comparison to other pesticides, a limited number of studies were conducted in terms of extraction methods of AP from plant materials. There are several workflows described for AP purification and extraction in plant matrices. AP residues from compost were extracted using NaOH, followed by hydrolysis with concentrated HCl and SPE [
31]. The method for determination of the picolinic acid group herbicides in fruit and vegetables has been published by Tian et al. [
32], using the QuEChERS non-buffered extraction. Another study focusing on multi-residue acid herbicide monitoring in cereal-based foods also employed a modified QuEChERS method for sample purification [
28]. However, all these methods based on QuEChERS are usually designed for multi-residue analysis purposes, and their applicability for sensitive monitoring of only the AP is disputable. On the other hand, the salting-out assisted liquid–liquid extraction (SALLE), a part of QuEChERS, could be of interest due to the need for effective phase separation. Another possibility is the use of specific SPE extraction methods such as molecularly imprinted cartridges (MIPs). MIPs were used for selective separation of AP in milk and water samples and achieved good recovery and precision [
23,
24].
Therefore, two of the most promising clean-up methods, SALLE and MIP, were compared for their AP extraction efficiency. Blank straw samples spiked with AP at a concentration of 50 ng/mg of dry weight were subjected to extraction using either acidified MeOH for SALLE or water extraction followed by MIP clean–up.
The testing of the final sample solvent effect on recovery was also included. After SALLE extraction, one portion of the organic phase (MeCN) was directly analyzed, whilst the second part was dried with an N
2 stream and resuspended in acidified MeOH. The eluate from MIP cartridges was dried by N
2 stream and the dried residue was dissolved in (i) a mixture of water and MeCN (50:50,
v/
v) acidified with 0.1% of FA or (ii) 1% formic acid in MeOH. Samples were analyzed by LC-MS/MS using separation Methods 3 and 4, as described above (
Table 3). To efficiently compare all three variables (extraction procedure, sample solvent, and analytical method), six combinations were analyzed, and AP concentration was calculated (
Table 5).
Mobile phase and sample solvent incompatibility lead to peak shape problems, especially in polar analytes. Injecting a sample dissolved in a solvent with higher elution strength than that of the mobile phase results in peak splitting or broadening, especially for early eluting analytes such as aminopyralid [
33]. Peak splitting was also recorded in our results. When dried MIP eluates were dissolved in acidified MeOH and analyzed with Method 3, AP was detected at two different retention times (at Rt 1.2 min and 1.5 min). When the recovery was calculated, AP concentration based on the peak with a retention time of 1.2 min yielded an unexpectedly low recovery at 71%. The AP peak measured at 1.5 min provided an average recovery of 101%. Such peak splitting does not allow reliable calculation of the real AP concentration in measured samples. Although the highest yield of AP was obtained in MIP-purified samples dissolved in water:MeCN, this was only when the most time-consuming separation Method 4 was used. Peak splitting, or the need to use a time-consuming separation method, led to the exclusion of the MIP method from further tests.
Thus, SALLE was chosen as a more promising extraction method for AP analysis on LC/MS/MS. When sample extracts were applied to LC/MS/MS after SALLE purification directly in the extraction solvent (acidified MeCN), they resulted in poor recovery and a response level below the detection limit. However, when the samples purified by SALLE were dried and extraction MeCN was replaced with acidified MeOH, the recovery of AP reached 84% and an acceptable RSD (17%) (see
Table 4). In comparison, the alternative MIP method combined with acidified MeOH as a sample solvent gave a lower recovery rate of 73%. Thus, the evaporation of the extraction solvent and re-dissolution of the sample in a solvent of similar composition to the mobile phase was included as a final step. Although it extends the total procedure, this step was found to be significant for AP detection, and solvent exchange did not lead to analyte loss.
Both compared extraction methods, MIP and SALLE, have benefits as well as disadvantages in comparison to each other. Nevertheless, if we take into account the run time length of tested chromatographic methods, SALLE combined with separation Method 3 seems to be the most optimal for routine analysis of AP in straw matrices.
Finally, the two most promising combinations of extraction (MIP and SALLE) and chromatographic separation methods (UHPLC) were compared in terms of cost-efficiency, used solvents at each step, and accessibility to the extraction consumables. Therefore, the salting-out assisted liquid–liquid extraction (SALLE) with acidified MeCN (MeCN+FA) and water in combination with chromatographic Method 3 and pure MeOH as a final solvent was chosen as the most appropriate for the straw matrices and further validation.
3.6. Extraction Method Validation
The validation of the extraction method was conducted by analyzing organic straw samples fortified with AP at four different concentration levels (5, 10, 25, and 50 ng/g) to assess the extraction efficiency. Three replicates for each concentration level were extracted with the SALLE method dissolved in acidified MeOH before the analysis. Validation scores are presented in
Table 6.
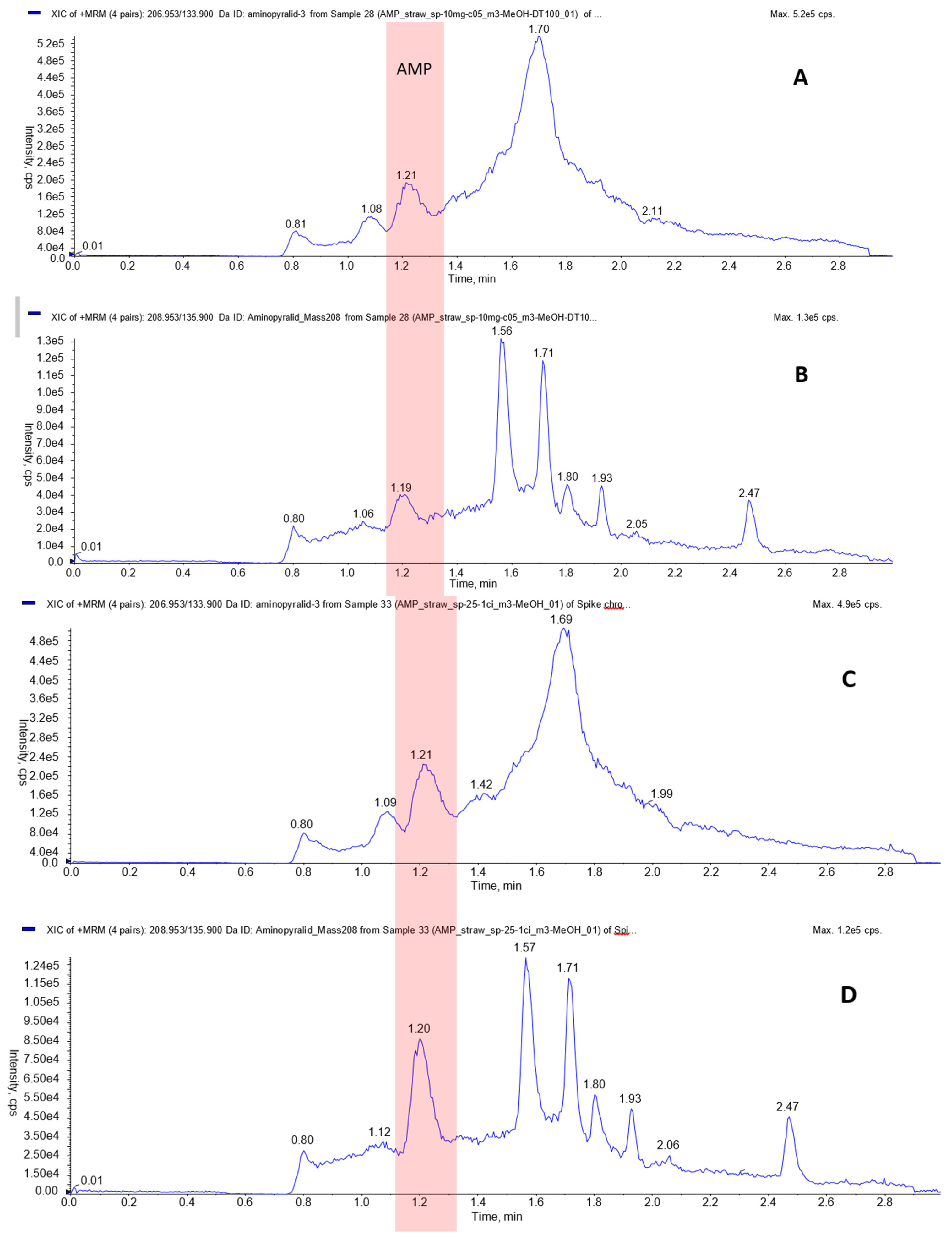
The lowest validated spike level corresponding to the 10 ng/g was set as the limit of quantitation (LOQ) for the developed SALLE method. The chromatograms of the two AP MRM transitions in the straw matrix are shown in
Figure 2. The confirmative transition (208.953/135.9) produced a more distinctive peak that aided in quantifying AP at lower concentrations.
3.7. Real Sample Analysis
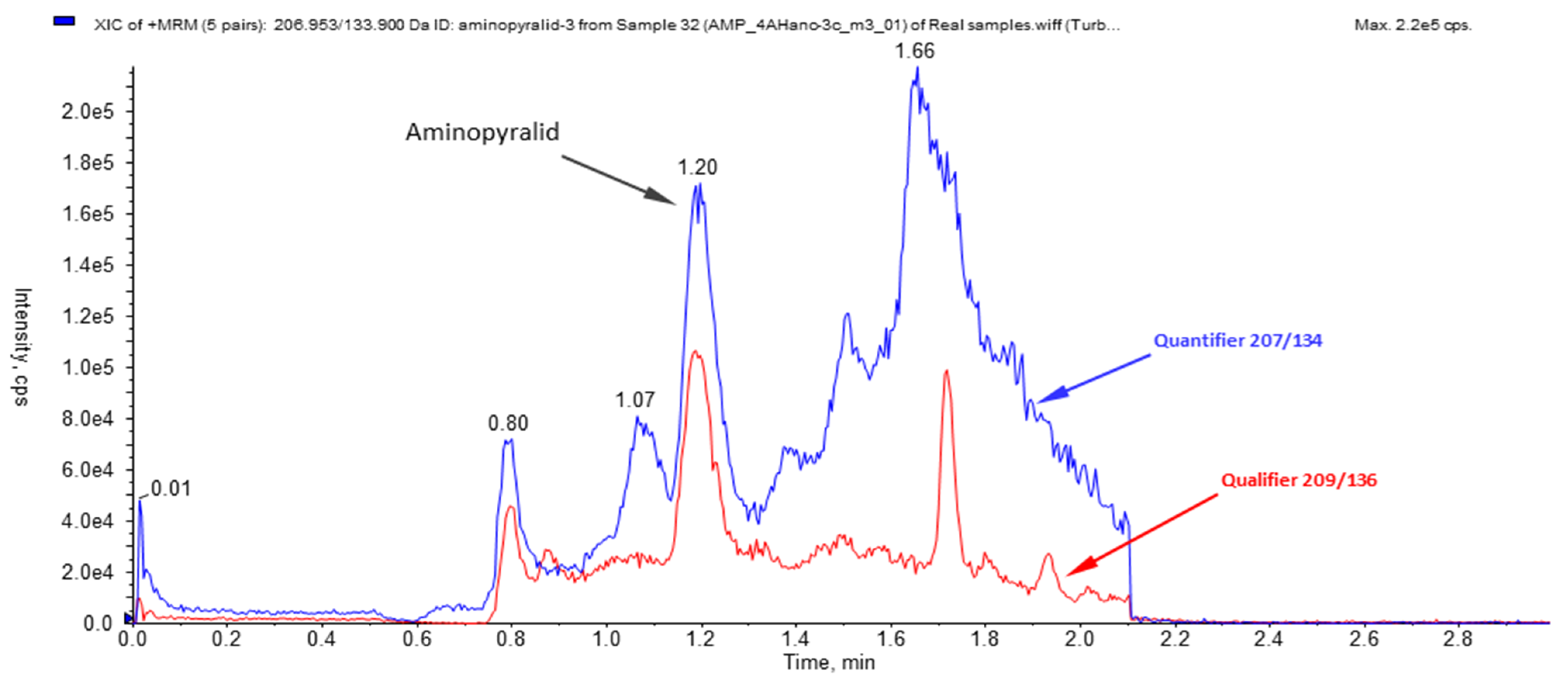
To check the method performance on real samples, straw samples from the mulched strawberry field and those overwintered were also tested with the validated method. The method performance was tested with four different real straw samples (two harvested straw samples and two mulches), each in three replicates. The results proved the presence of the aminopyralid residues in concentrations of 22.54 ± 0.80 ng/g (RSD of 4.14%) and 11.39 ± 0.56 ng/g (RSD of 4.90%) in one of the straw samples (
Figure 3) and one mulch, respectively. In the second two samples, the measured concentrations did not reach the LOQ limit.