Recovery of Valuable Metals from Polymetallic Refractory Concentrate by a Sulfuric Acid Curing and Leaching Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Analysis
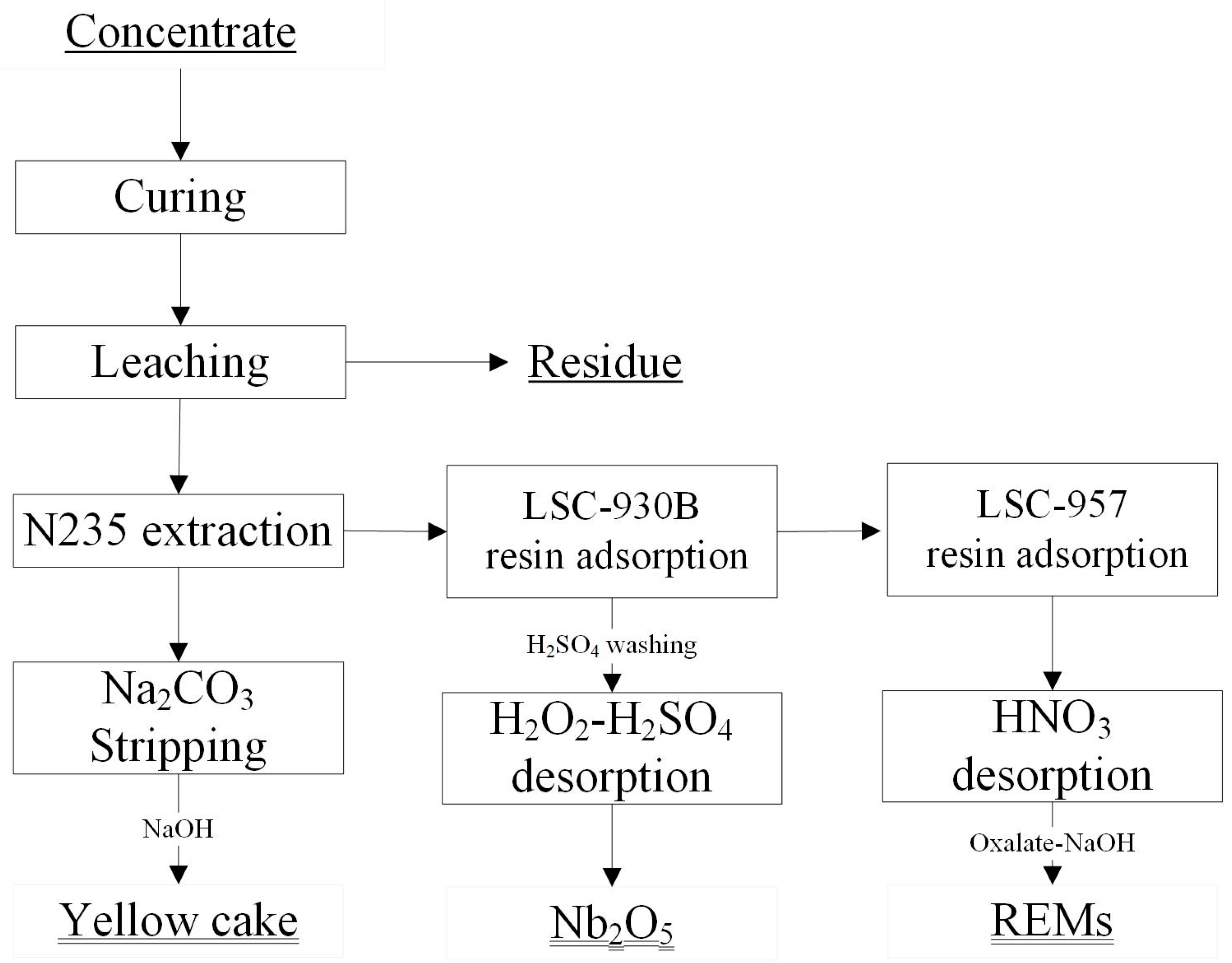
2.2. Curing and Leaching Process
- The leaching solution was treated with an extractant (type: N235) and synergic reagent 2-octanol diluted in kerosene. After two-stage uranium extraction, uranium was separated from other soluble sulfates, such as niobium, rare earth minerals, and iron. The loaded organic phase can form Na4UO2(CO3)3 through sodium carbonate stripping to enter the aqueous phase. The aqueous phase was then precipitated by caustic soda as a yellow cake (uranium content: 67.0%). At the same time, the carbonate ions in the complex were regenerated to form sodium carbonate, thereby enabling the recycling of sodium carbonate.
- The residual uranium extraction solution was exchanged through resin (type: LSC-930B), allowing niobium to enter the resin phase selectively. The resin loaded with niobium was washed with diluted sulfuric acid to remove iron and desorbed from the H2SO4 solution containing H2O2. The desorbed H2NbO4 solution was then hydrolyzed and precipitated at a high temperature (90–98 °C) to precipitate niobium hydrated oxide (Nb2O5·nH2O). Then, the oxide was sent for drying with a Nb2O5 content of 90.2%, and titanium could be further extracted from the hydrolyzed solution.
- After niobium adsorption, the solution went through resin (type: LSC-957) for REMs treatment, and the resin loaded with REMs went through a nitric acid solution for REMs desorption, oxalic acid precipitation, pH adjustment by NaOH, and roasting. The final REM oxide had a content of 82.5%.
3. Results and Discussion
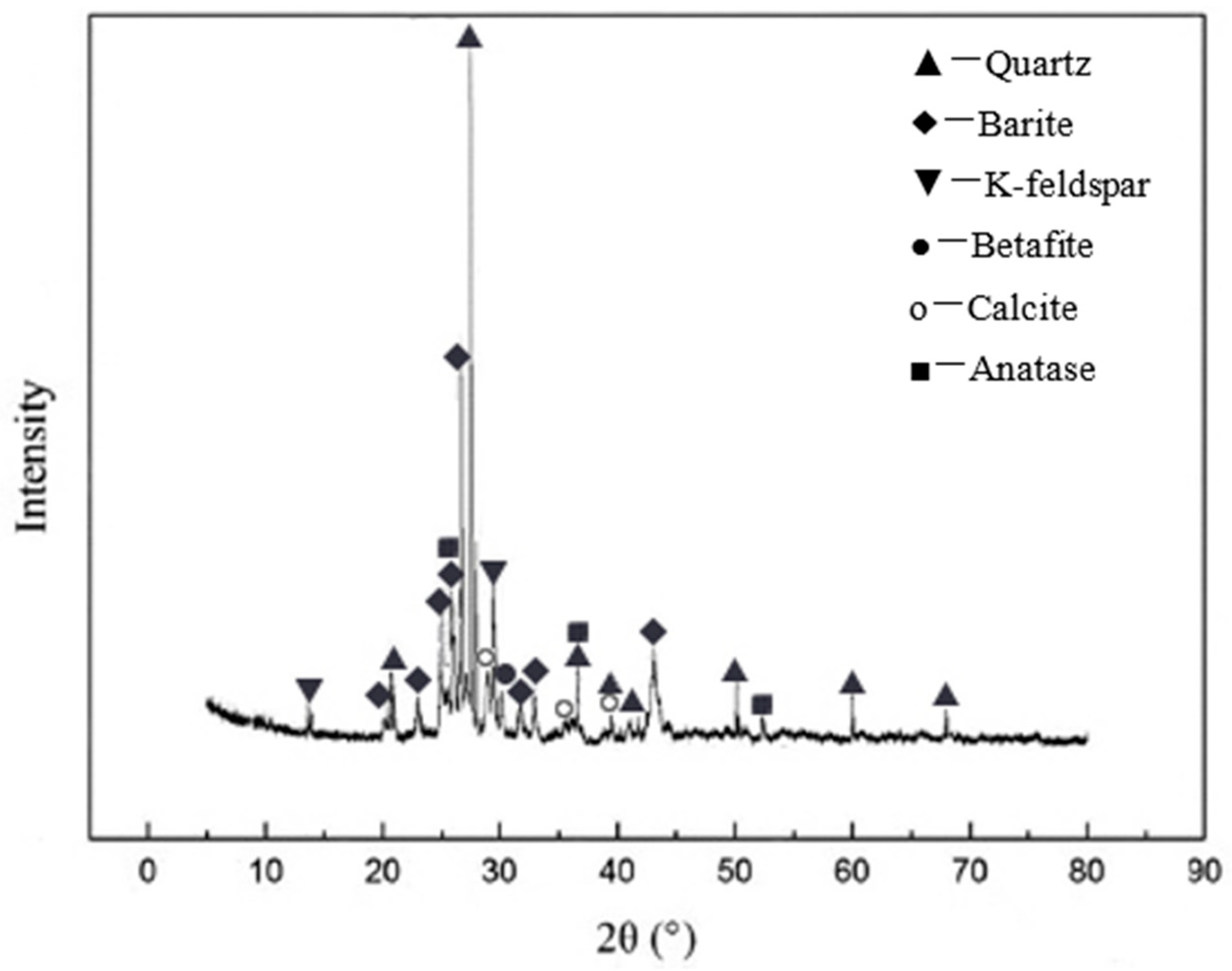
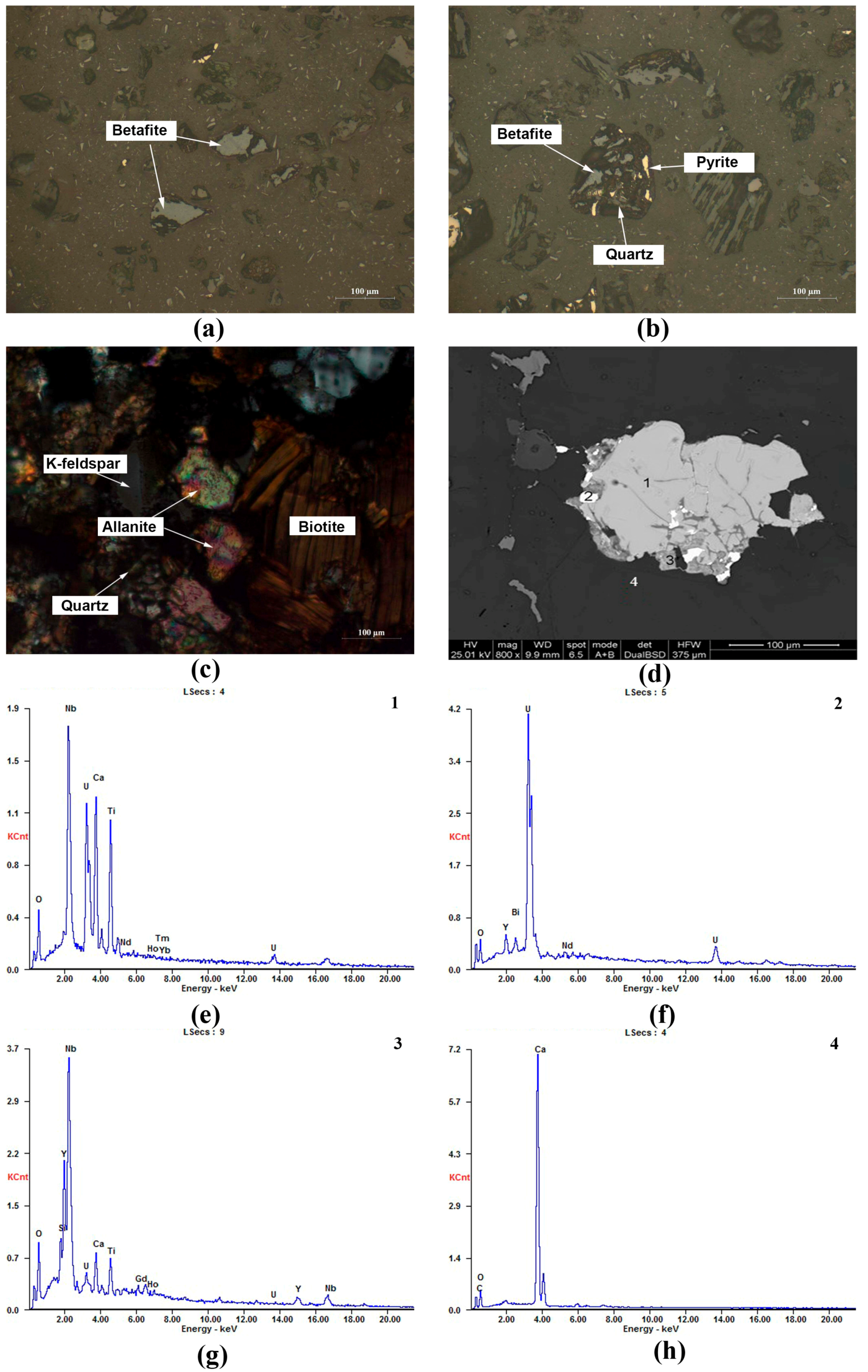
3.1. Concentrate Phase and Composition
3.2. Curing Process
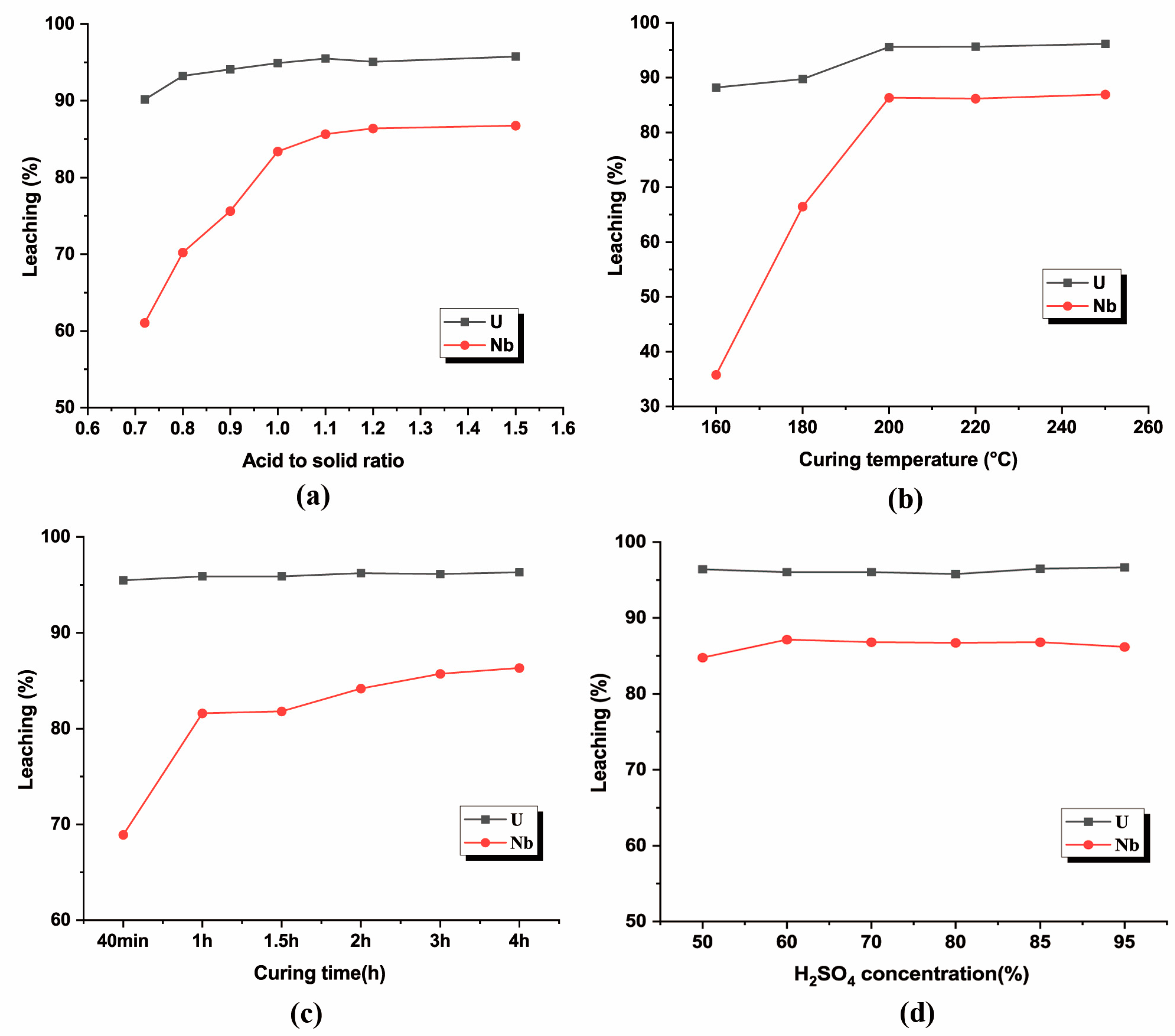
3.2.1. Effect of Acid-to-Solid Ratio
3.2.2. Effect of Curing Temperature
3.2.3. Effect of Curing Time
3.2.4. Effect of H2SO4 Concentration
3.3. Leaching Process
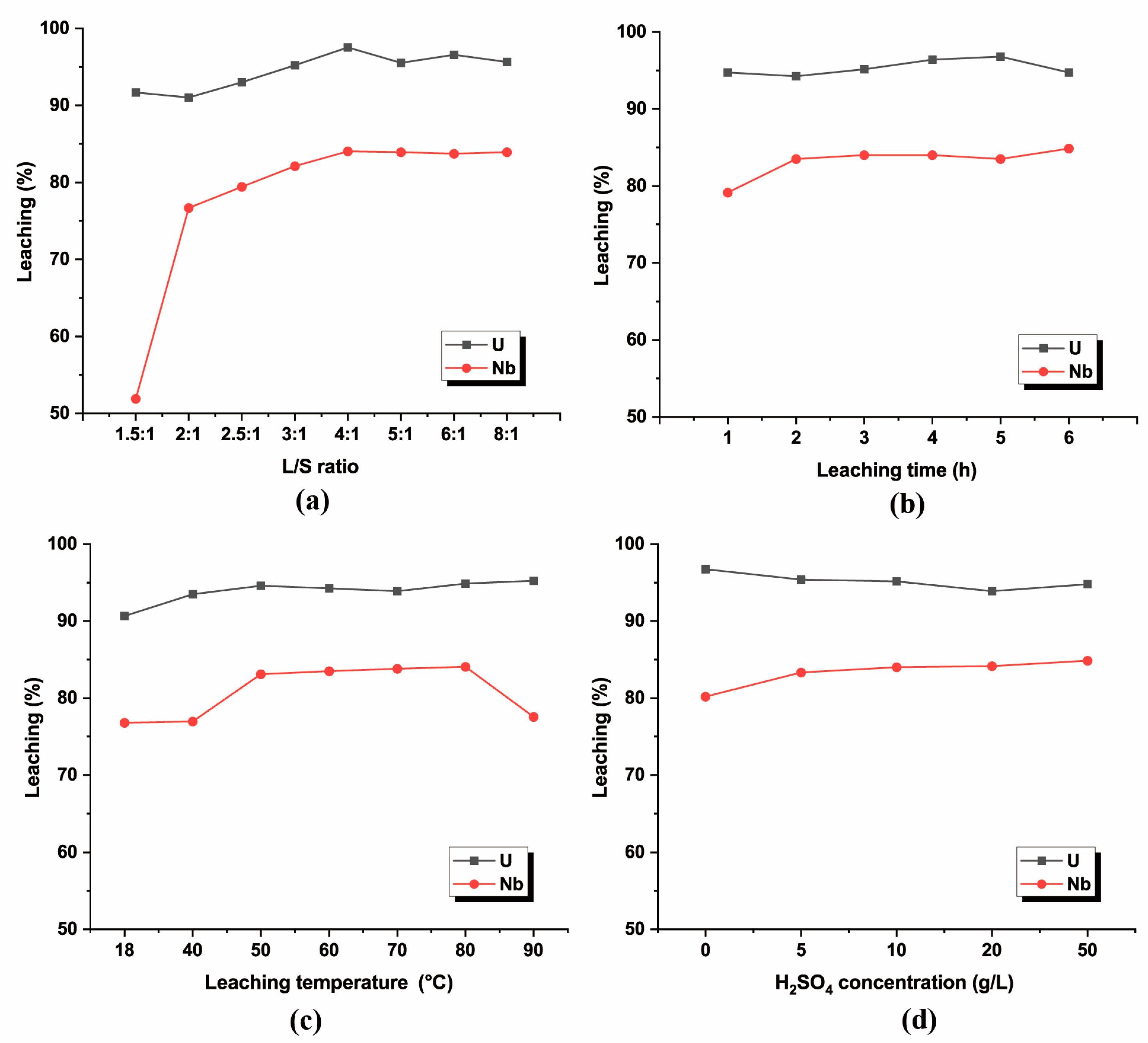
3.3.1. Effect of L/S Ratio
3.3.2. Effect of Leaching Time
3.3.3. Effect of Leaching Temperature
3.3.4. Effect of Leaching Solution Concentration
3.3.5. Validation of Optimal Conditions
3.4. Purification and Separation of Leaching Solution
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watling, H.R. Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 2014, 5, 1–60. [Google Scholar] [CrossRef]
- Aleksandrova, T.; Nikolaeva, N.; Afanasova, A.; Romashev, A.; Kuznetsov, V. Selective disintegration justification based on the mineralogical and technological features of the polymetallic ores. Minerals 2021, 11, 851. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Wawszczak, D.; Brykała, M. Possibility of uranium and rare metal recovery in the Polish copper mining industry. Hydrometallurgy 2016, 159, 12–18. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Mudd, G.M. Critical review of acid in situ leach uranium mining: 1. USA and Australia. Environ. Earth Sci. 2001, 41, 390–403. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Montenegro, M.R. Leaching behaviour and the solution consumption of uranium–vanadium ore in alkali carbonate–bicarbonate column leaching. Hydrometallurgy 2016, 161, 127–137. [Google Scholar] [CrossRef]
- Zhu, Z.; Pranolo, Y.; Cheng, C.Y. Separation of uranium and thorium from rare earths for rare earth production—A review. Miner. Eng. 2015, 77, 185–196. [Google Scholar] [CrossRef]
- Muñoz, J.; González, F.; Blázquez, M.; Ballester, A. A study of the bioleaching of a Spanish uranium ore. Part I: A review of the bacterial leaching in the treatment of uranium ores. Hydrometallurgy 1995, 38, 39–57. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M. The kinetics of the oxidation of iron(II) by chlorate in the leaching of uranium ores. Hydrometallurgy 2009, 100, 47–49. [Google Scholar] [CrossRef]
- Edwards, C.R.; Oliver, A.J. Uranium processing: A review of current methods and technology. JOM 2000, 52, 12–20. [Google Scholar] [CrossRef]
- Esmaeel, S.M.A. Sorption of uranium after carbonate leaching by low cost activated carbon–aluminum ferrisilicate composite. Int. J. Environ. Anal. Chem. 2022, 102, 2641–2660. [Google Scholar] [CrossRef]
- Santos, E.A.; Ladeira, A.C.Q. Recovery of uranium from mine waste by leaching with carbonate-based reagents. Environ. Sci. Technol. 2011, 45, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, D.; Bowell, R.; Geroni, J.; Penman, K.; Dey, M. Factors influencing the release rate of uranium, thorium, yttrium and rare earth elements from a low grade ore. Miner. Eng. 2012, 39, 165–172. [Google Scholar] [CrossRef]
- Deng, Y.; Hou, W.; Wei, X.; Wang, Q.; Wang, H.; Hu, E.; Lei, Z.; Hu, F.; Zhang, Y. Mechanism analysis on synergistic leaching of uranium from pyrite oxidized by Acidithiobacillus ferrivorans. J. Radioanal. Nucl. Chem. 2021, 330, 951–961. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Mattheiss, L.F. Electronic structure of niobium and tantalum. Phys. Rev. B 1970, 1, 373–380. [Google Scholar] [CrossRef]
- Shikika, A.; Sethurajan, M.; Muvundja, F.; Mugumaoderha, M.; Gaydardzhiev, S. A review on extractive metallurgy of tantalum and niobium. Hydrometallurgy 2020, 198, 105496. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S.; Xu, H.; Zhang, Y. Leaching of niobium and tantalum from a low-grade ore using a KOH roast–water leach system. Hydrometallurgy 2009, 98, 219–223. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Fang, X.; Qiu, T. Kinetics of pressure leaching of niobium ore by sulfuric acid. Int. J. Refract. Met. Hard Mater. 2014, 45, 218–222. [Google Scholar] [CrossRef]
- Makanyire, T.; Jha, A.; Sutcliffe, S. Kinetics of hydrochloric acid leaching of niobium from TiO2 residues. Int. J. Miner. Process. 2016, 157, 1–6. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Rosales, G.D.; Pinna, E.G.; Suarez, D.S. Extraction of niobium and tantalum from ferrocolumbite by hydrofluoric acid pressure leaching. Hydrometallurgy 2015, 156, 17–20. [Google Scholar] [CrossRef]
- Ghambi, S.; Sanchez-Segado, S.; Chipakwe, V.; Jha, A. An investigation on hydrofluoric (HF) acid-free extraction for niobium oxide (Nb2O5) and tantalum oxide (Ta2O5) from columbite/tantalite concentrates using alkali reductive roasting. Miner. Eng. 2021, 173, 107183. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Ma, S.; Zheng, S.; Sun, Q. Effect of mechanical activation on the leaching kinetics of niobium-bearing mineralisation in KOH hydrothermal system. Hydrometallurgy 2018, 181, 123–129. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, Y.; Ma, Z.; Lyu, X.; Zhou, J. Efficient recovery of niobium and tantalum from ferrocolumbium tantalum by a continuous leaching process using HF−H2SO4−HNO3 synergistic system. Min. Metall. Explor. 2022, 39, 1763–1770. [Google Scholar] [CrossRef]
- Rodríguez, O.; Alguacil, F.J.; Baquero, E.E.; García-Díaz, I.; Fernández, P.; Sotillo, B.; López, F.A. Recovery of niobium and tantalum by solvent extraction from Sn–Ta–Nb mining tailings. RSC Adv. 2020, 10, 21406–21412. [Google Scholar] [CrossRef] [PubMed]
- Allain, E.; Kanari, N.; Diot, F.; Yvon, J. Development of a process for the concentration of the strategic tantalum and niobium oxides from tin slags. Miner. Eng. 2019, 134, 97–103. [Google Scholar] [CrossRef]
- Gao, W.C.; Wen, J.K.; Wu, B.; Shang, H.; Liu, X. A novel approach to extract Nb, Y and Ce from a niobium-bearing ore of low grade by roasting KHSO4–H2SO4 system. Rare Met. 2021, 40, 1979–1986. [Google Scholar] [CrossRef]
- Yu, X.-L.; Bai, L.; Wang, Q.-C.; Liu, J.; Chi, M.-Y.; Wang, Z.-C. Recovery of rare earths, niobium, and thorium from the tailings of giant Bayan Obo ore in China. Met. Mater. Trans. B 2012, 43, 485–493. [Google Scholar] [CrossRef]
- Sanchez-Segado, S.; Makanyire, T.; Escudero-Castejon, L.; Hara, Y.; Jha, A. Reclamation of reactive metal oxides from complex minerals using alkali roasting and leaching—An improved approach to process engineering. Green Chem. 2015, 17, 2059–2080. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Orabi, A.H.; Falila, N.I.; Ismaiel, D.A.; Salem, H.M. Processing of the mineralized Black Mica for the recovery of uranium, rare earth elements, niobium, and tantalum. Hydrometallurgy 2020, 197, 105474. [Google Scholar] [CrossRef]
- Redkin, A.F.; Velichkin, V.I. Research of the Uranium, Niobium, and Tantalum Behavior in the Granite Melt–Chloride Fluid System at 750 °C and 1000 Bar. Geol. Ore Depos. 2020, 62, 372–382. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, D.; Sun, Q.; Liu, C.; Cheng, H.; Yang, F.; Zhang, B. Simultaneous extraction of uranium and niobium from a low-grade natural betafite ore. High Temp. Mater. Process. 2023, 42, 20220260. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Guo, X.; Zou, X. Study on dissolution mechanism of mineral/liquid interface during HF acid leaching niobium-tantalum ore. Miner. Eng. 2022, 188, 107835. [Google Scholar] [CrossRef]
- Jin, S. Extraction of uranium from coarse ore and acid-curing and ferric sulphate-trickle leaching process. Uranium Min. Metall. 1994, 13, 156–164. [Google Scholar]
- Radwan, H.A.; Faheim, A.A.; El-Sheikh, E.M.; El-Wahab, Z.H.A.; Gado, M.A. Optimization of the leaching process for uranium recovery and some associated valuable elements from low-grade uranium ore. Int. J. Environ. Anal. Chem. 2021, 103, 5259–5281. [Google Scholar] [CrossRef]
- Hu, E.; Lei, Z.; Wang, H.; Su, H.; Zhou, W.; Wan, Q.; Zhu, F.; Wang, Q. Extraction of uranium in caustic sludge from the production of nuclear fuel components by countercurrent dissolution and acid curing. J. Radioanal. Nucl. Chem. 2022, 331, 2445–2450. [Google Scholar] [CrossRef]
- Musowoya, M.D.; Hara, Y.R.S.; Chileshe, F.; Mundundu, J.; Parirenyatwa, S. Extraction of Tungsten, Yttrium, and Uranium from Tantalum—Niobium Ore from Muchinga Province in Zambia. In TMS Annual Meeting & Exhibition; Springer Nature: Cham, Switzerland, 2023; pp. 95–103. [Google Scholar]
- Van Lien, T.; Dinh, T.T.; Dung, N.T.K. Study on leaching systems and recovery for PALUA–PARONG low grade uranium sandstone ores. Hydrometallurgy 2020, 191, 105164. [Google Scholar] [CrossRef]
- El-Hussaini, O.M.; Mahdy, M.A. Sulfuric acid leaching of Kab Amiri niobium–tantalum bearing minerals, Central Eastern Desert, Egypt. Hydrometallurgy 2002, 64, 219–229. [Google Scholar] [CrossRef]
- El-Afandy, A.H.; Yousif, A.M.; Mubark, A.E. Subsequent Separation of Niobium (Nb), Thorium (Th), Rare Earth Elements (REEs), Zirconium (Zr), and Uranium (U) from Abu Rusheid Cataclastic Concentrate, South Eastern Desert, Egypt. Radiochemistry 2022, 64, 257–267. [Google Scholar] [CrossRef]
- Nettleton, K.C.; Nikoloski, A.N.; Da Costa, M. The leaching of uranium from betafite. Hydrometallurgy 2015, 157, 270–279. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, K.; Cao, Z.; Northwood, D.O.; Waters, K.E.; Wang, H.; Liu, S.; Zhu, K.; Ma, H. Agitation Leaching Behavior of Copper–Cobalt Oxide Ores from the Democratic Republic of the Congo. Minerals 2023, 13, 743. [Google Scholar] [CrossRef]
- Burns, P.C.; Finch, R.J. (Eds.) Uranium: Mineralogy, Geochemistry, and the Environment; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; Volume 38. [Google Scholar]
- Zheng, C.; Jiang, K.; Cao, Z.; Wang, H.; Liu, S.; Waters, K.E.; Ma, H. Pressure leaching behaviors of copper-cobalt sulfide concentrate from Congo. Sep. Purif. Technol. 2023, 309, 123010. [Google Scholar] [CrossRef]

| Element | CaO | MgO | TFe | TiO2 | Al2O3 | REMs | SiO2 | U | Nb |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 10.3 | 1.2 | 8.0 | 1.8 | 1.9 | 0.7 | 42.7 | 3191 ppm | 2135 ppm |
| Element | Fe | Al2O3 | REMs | CaO | MgO | TiO2 | U | Nb |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 3.0 | 1.8 | 0.2 | 12.8 | 1.5 | 1.2 | 185 ppm | 479 ppm |
| Element | U | Nb | REMs | TFe |
|---|---|---|---|---|
| Content (g/L) | 0.51 | 0.23 | 0.62 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Xue, J.; Jiang, K.; Jiang, X.; Wang, S.; Hu, J.; Northwood, D.O.; Waters, K.E.; Ma, H. Recovery of Valuable Metals from Polymetallic Refractory Concentrate by a Sulfuric Acid Curing and Leaching Method. Separations 2024, 11, 7. https://doi.org/10.3390/separations11010007
Jiang W, Xue J, Jiang K, Jiang X, Wang S, Hu J, Northwood DO, Waters KE, Ma H. Recovery of Valuable Metals from Polymetallic Refractory Concentrate by a Sulfuric Acid Curing and Leaching Method. Separations. 2024; 11(1):7. https://doi.org/10.3390/separations11010007
Chicago/Turabian StyleJiang, Wei, Jilai Xue, Kaixi Jiang, Xunxiong Jiang, Shengdong Wang, Jinping Hu, Derek O. Northwood, Kristian E. Waters, and Hao Ma. 2024. "Recovery of Valuable Metals from Polymetallic Refractory Concentrate by a Sulfuric Acid Curing and Leaching Method" Separations 11, no. 1: 7. https://doi.org/10.3390/separations11010007
APA StyleJiang, W., Xue, J., Jiang, K., Jiang, X., Wang, S., Hu, J., Northwood, D. O., Waters, K. E., & Ma, H. (2024). Recovery of Valuable Metals from Polymetallic Refractory Concentrate by a Sulfuric Acid Curing and Leaching Method. Separations, 11(1), 7. https://doi.org/10.3390/separations11010007






