Structure Elucidation and Immunoactivity Study of Armillaria mellea Fruiting Body Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AMPN and AMPA
2.3. Separation and Purification of AMPN and AMPA
2.4. Determination of Physicochemical Properties and Structure
2.4.1. Chemical Composition
2.4.2. Molecular Weight and Homogeneity
2.4.3. Monosaccharide Composition
2.4.4. FTIR Analysis
2.4.5. Methylation Analysis
2.4.6. NMR Spectroscopy Analysis
2.4.7. Congo Red Experiment
2.5. Immune Activities of Polysaccharides
2.5.1. Animals and Experimental Design
2.5.2. Effect of the AMPN and AMPA on the Weight of Immune Organs
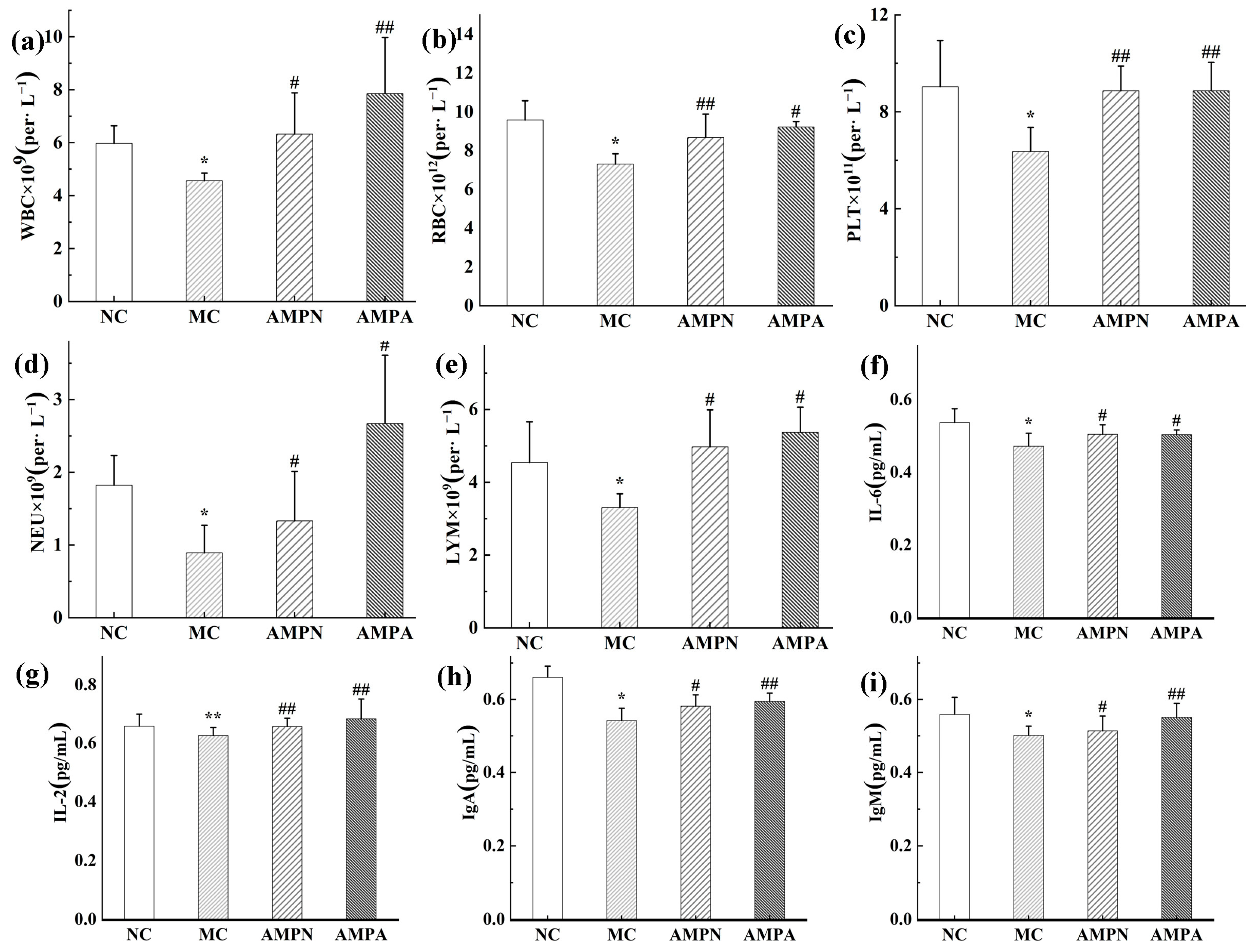
2.5.3. Determination of Blood Cell Count
2.5.4. Determination of Cytokines
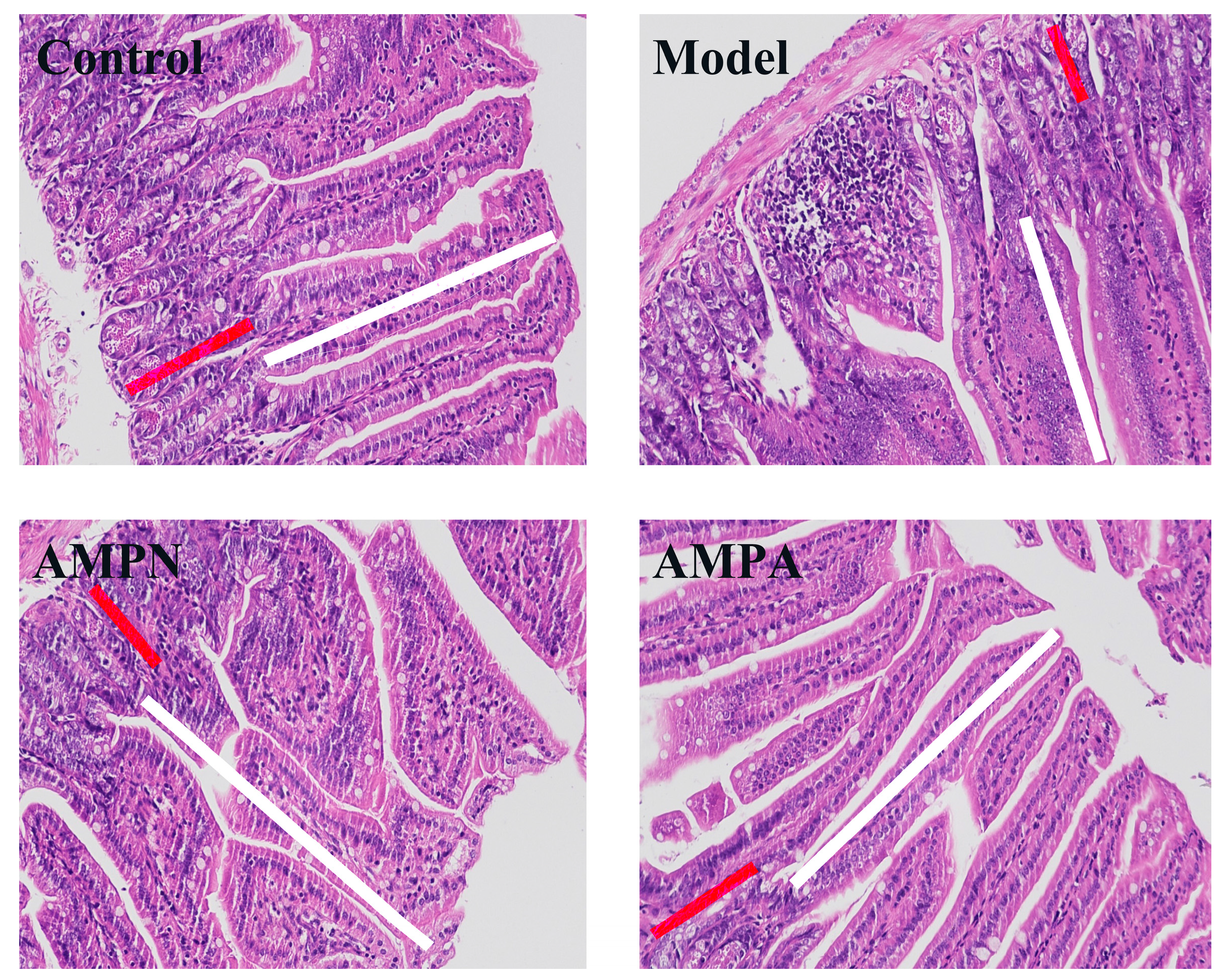
2.5.5. Colon Histopathological Examination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Physicochemical Properties and Structure
3.1.1. Chemical Composition Analysis of AMPN and AMPA
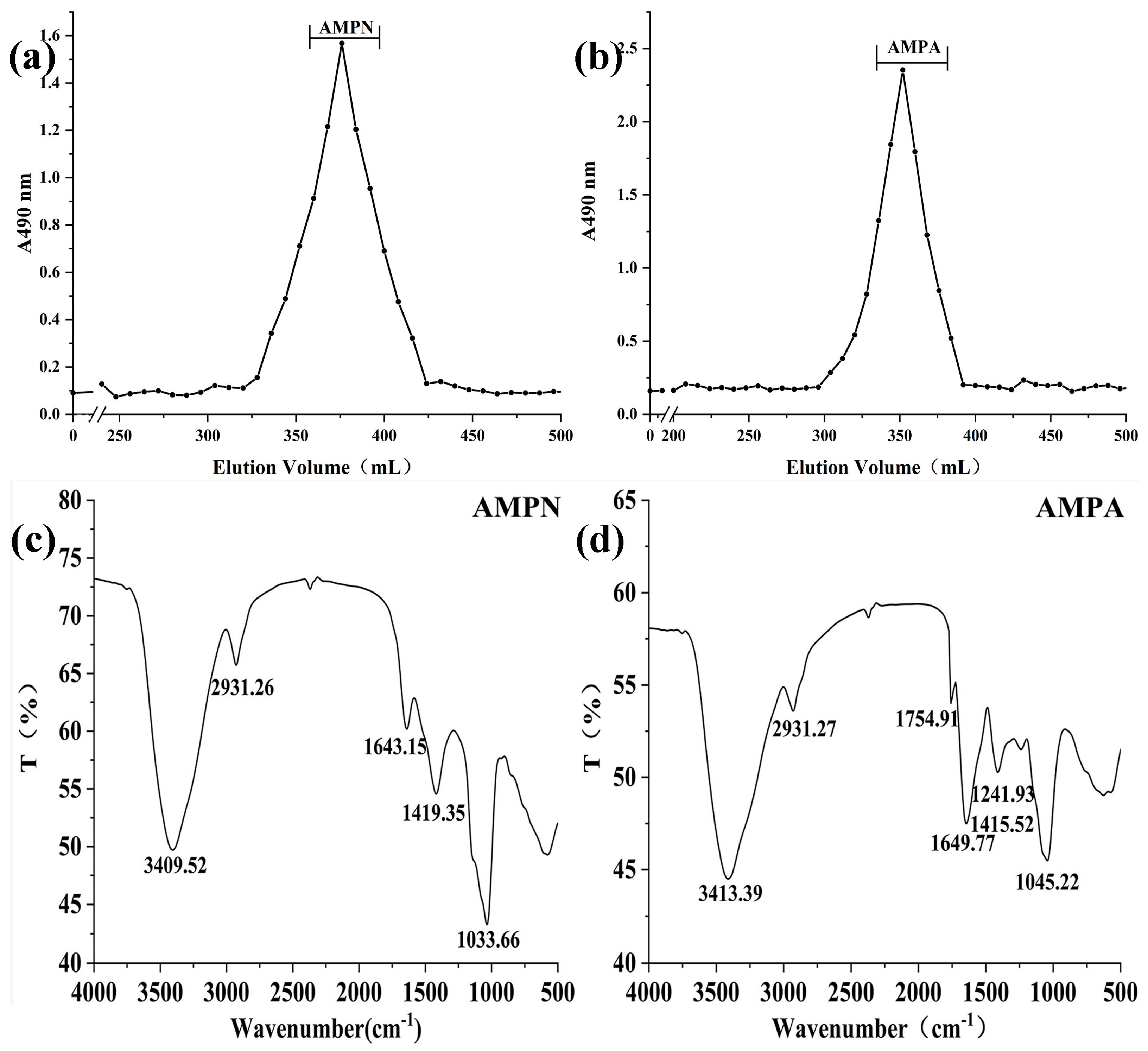
3.1.2. Molecular Weight Detection
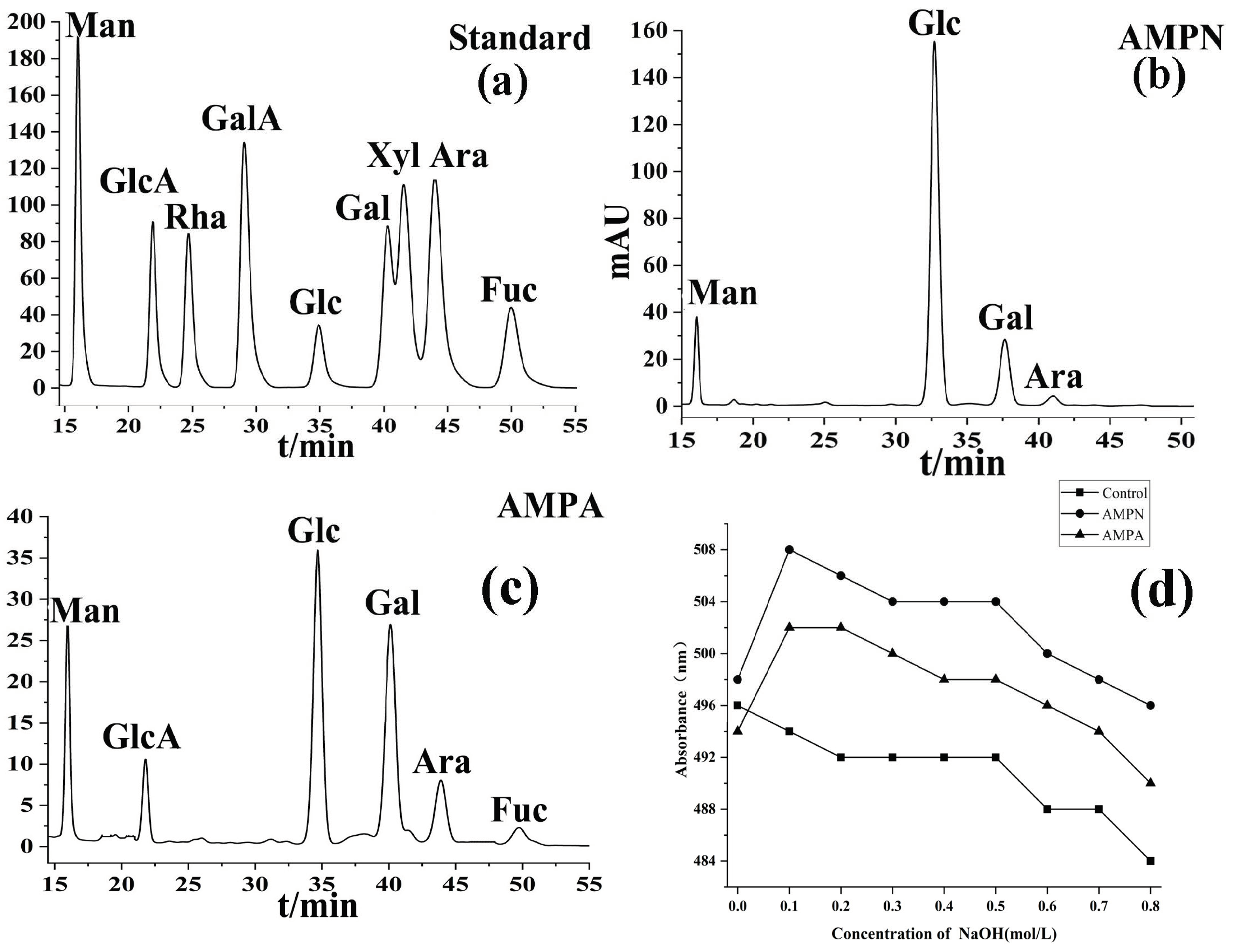
3.1.3. Monosaccharide Composition
3.1.4. IR Spectra Analysis
3.1.5. Methylation Analysis
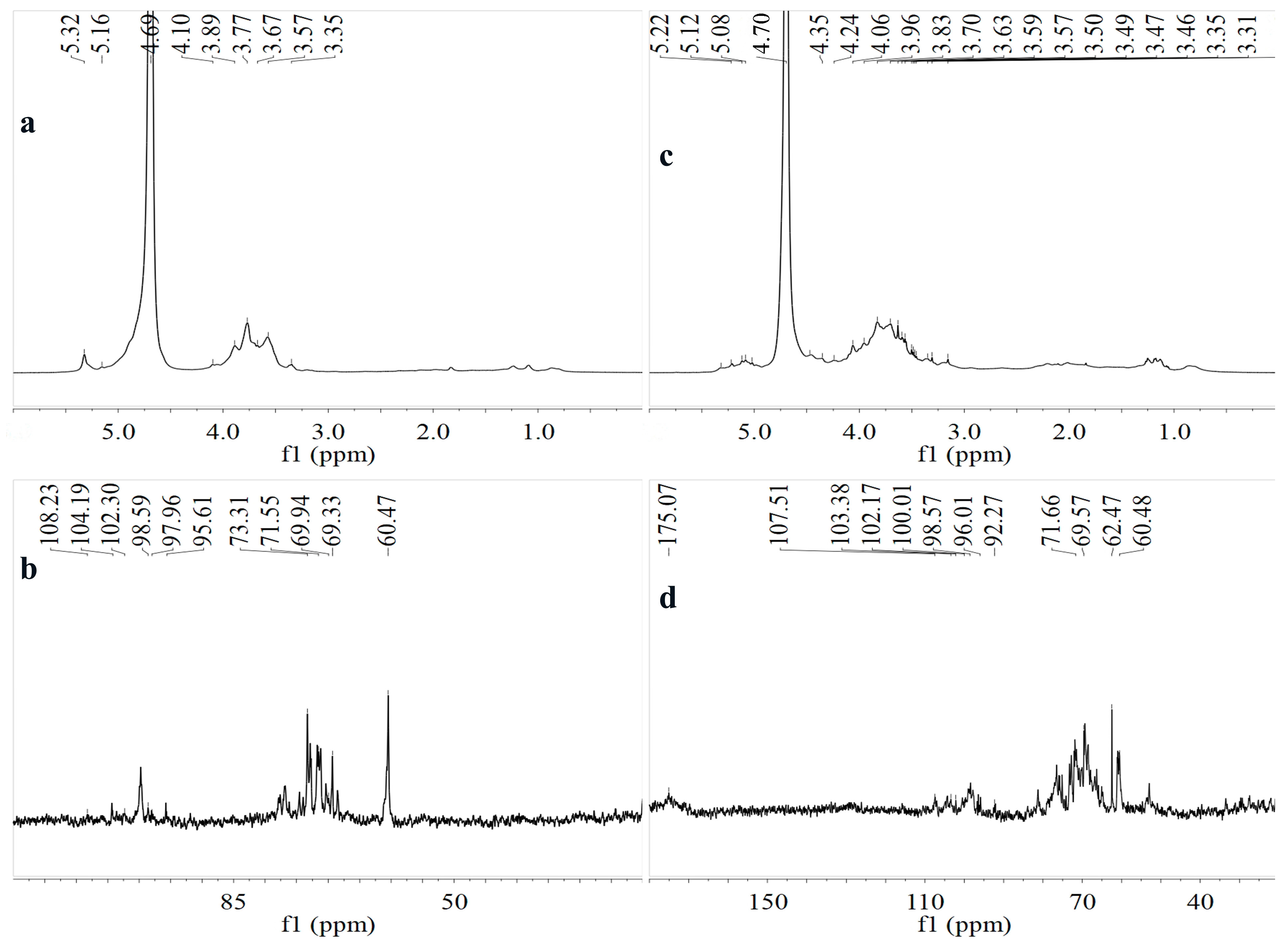
3.1.6. NMR Spectroscopy Analysis
3.1.7. Congo Red Analysis of AMPN and AMPA
3.2. Effects of AMPN and AMPA in Immunosuppressive Mice
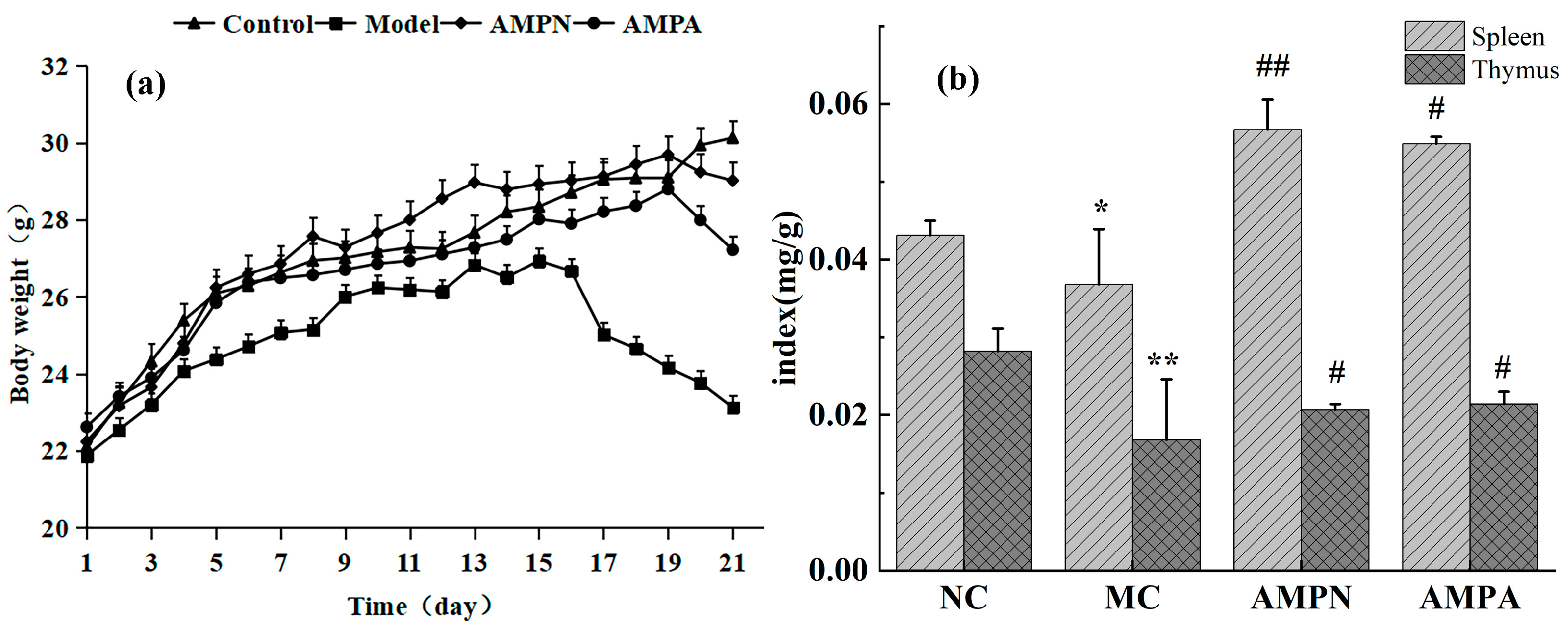
3.2.1. Effects of the AMPN and AMPA on Body Weight
3.2.2. Effects of the AMPN and AMPN on Immune Organ Index
3.2.3. Effect of AMPN and AMPN on Clinical Biochemical Parameters
3.2.4. Effects of AMP on Colon Tissue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.-E.; Wang, H.-L.; Lu, K.-H.; Huang, Y.-J.; Panyod, S.; Liu, W.-T.; Yang, S.-H.; Chen, M.-H.; Lu, Y.-S.; Sheen, L.-Y. Water extract of Armillaria mellea (Vahl) P. Kumm. Alleviates the depression-like behaviors in acute- and chronic mild stress-induced rodent models via anti-inflammatory action. J. Ethnopharmacol. 2021, 265, 113395. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Yan, L.; Zhang, Q.; Zhu, J.; Huang, N.; Wang, Z. Chemical compositions and antioxidant activities of polysaccharides from the sporophores and cultured products of Armillaria mellea. Molecules 2015, 20, 5680–5697. [Google Scholar] [CrossRef]
- Strapáč, I.; Baranová, M.; Smrčová, M.; Bedlovičová, Z. Antioxidant Activity of Honey Mushrooms (Armillaria mellea). Folia Vet. 2016, 60, 37–41. [Google Scholar] [CrossRef]
- Dörfer, M.; Gressler, M.; Hoffmeister, D. Diversity and bioactivity of Armillaria sesquiterpene aryl ester natural products. Mycol. Prog. 2019, 18, 1027–1037. [Google Scholar] [CrossRef]
- Huang, J.-W.; Lai, C.-J.-S.; Yuan, Y.; Zhang, M.; Zhou, J.-H.; Huang, L.-Q. Correlative analysis advance of chemical constituents of Polyporusumbellatus and Armillaria mellea. Zhongguo Zhong Yao Za Zhi 2017, 42, 2905–2914. [Google Scholar]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Wołkowska, M.; Ekiert, H. Chemical, pharmacological, and biological characterization of the culinary-medicinal honey mushroom, Armillaria mellea (Vahl) P. Kumm. (Agaricomycetideae): A review. Int. J. Med. Mushrooms 2011, 13, 167–175. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, S.; Lu, Z.; Xu, H.; Shi, J.-S.; Xu, Z.-H. Anti-inflammatory activity of mycelial extracts from medicinal mushrooms. Int. J. Med. Mushrooms 2014, 16, 319–325. [Google Scholar] [CrossRef]
- Ren, S.; Gao, Y.; Li, H.; Ma, H.; Han, X.; Yang, Z.; Chen, W. Research Status and Application Prospects of the Medicinal Mushroom Armillaria mellea. Appl. Biochem. Biotechnol. 2023, 195, 3491–3507. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Kanak, S.; Moczulski, M.; Martyna, A.; Kubiński, K.; Masłyk, M.; Szpakowska, N.; Kaczyński, Z.; Nowak, R.; Olech, M. Crude Polysaccharides from Wild-Growing Armillaria mellea—Chemical Composition and Antidiabetic, Anti-Inflammatory, Antioxidant, and Antiproliferative Potential. Appl. Sci. 2023, 13, 3853. [Google Scholar] [CrossRef]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef]
- Han, S.-S.R.; Cho, C.-K.; Lee, Y.-W.; Yoo, H.-S. Antimetastatic and immunomodulating effect of water extracts from various mushrooms. J. Acupunct. Meridian Stud. 2009, 2, 218–227. [Google Scholar] [CrossRef]
- Wei, X.; Yao, J.; Wang, F.; Wu, D.; Zhang, R. Extraction, isolation, structural characterization, and antioxidant activity of polysaccharides from elderberry fruit. Front. Nutr. 2022, 9, 947706. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, J.; Lang, Y.; Yao, L.; Xu, H.; Shi, H.; Xu, S. A polysaccharide from Armillaria mellea exhibits strong in vitro anticancer activity via apoptosis-involved mechanisms. Int. J. Biol. Macromol. 2012, 51, 663–667. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Li, H.; Xu, G.; Yuan, G. Effects of an Armillaria mellea Polysaccharide on Learning and Memory of D-Galactose-Induced Aging Mice. Front. Pharmacol. 2022, 13, 919920. [Google Scholar] [CrossRef]
- Yan, J.; Han, Z.; Qu, Y.; Yao, C.; Shen, D.; Tai, G.; Cheng, H.; Zhou, Y. Structure elucidation and immunomodulatory activity of a β-glucan derived from the fruiting bodies of Amillariellamellea. Food Chem. 2018, 240, 534–543. [Google Scholar] [CrossRef]
- Zhang, T.; Du, Y.; Liu, X.; Sun, X.; Cai, E.; Zhu, H.; Zhao, Y. Study on antidepressant-like effect of protoilludane sesquiterpenoid aromatic esters from Armillaria mellea. Nat. Prod. Res. 2021, 35, 1042–1045. [Google Scholar] [CrossRef]
- Yao, L.; Lv, J.; Duan, C.; An, X.; Zhang, C.; Li, D.; Li, C.; Liu, S. Armillaria mellea fermentation liquor ameliorates p-chlorophenylalanine-induced insomnia associated with the modulation of serotonergic system and gut microbiota in rats. J. Food Biochem. 2022, 46, e14075. [Google Scholar] [CrossRef]
- Li, I.-C.; Lin, T.-W.; Lee, T.-Y.; Lo, Y.; Jiang, Y.-M.; Kuo, Y.-H.; Chen, C.-C.; Chang, F.-C. Oral Administration of Armillaria mellea Mycelia Promotes Non-Rapid Eye Movement and Rapid Eye Movement Sleep in Rats. J. Fungi 2021, 7, 371. [Google Scholar] [CrossRef]
- An, S.; Lu, W.; Zhang, Y.; Yuan, Q.; Wang, D. Pharmacological Basis for Use of Armillaria mellea Polysaccharides in Alzheimer’s Disease: Antiapoptosis and Antioxidation. Oxid. Med. Cell Longev. 2017, 2017, 4184562. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic activity of Armillaria mellea polysaccharides: Joint ultrasonic and enzyme assisted extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar] [CrossRef]
- Yang, S.; Yan, J.; Yang, L.; Meng, Y.; Wang, N.; He, C.; Fan, Y.; Zhou, Y. Alkali-soluble polysaccharides from mushroom fruiting bodies improve insulin resistance. Int. J. Biol. Macromol. 2019, 126, 466–474. [Google Scholar] [CrossRef]
- Chen, R.; Ren, X.; Yin, W.; Lu, J.; Tian, L.; Zhao, L.; Yang, R.; Luo, S. Ultrasonic disruption extraction, characterization and bioactivities of polysaccharides from wild Armillaria mellea. Int. J. Biol. Macromol. 2020, 156, 1491–1502. [Google Scholar] [CrossRef]
- Jing, Y.; Yan, M.; Zhang, H.; Liu, D.; Qiu, X.; Hu, B.; Zhang, D.; Zheng, Y.; Wu, L. Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatumsibiricum. Foods 2023, 12, 2088. [Google Scholar] [CrossRef]
- Lung, M.-Y.; Chang, Y.-C. Antioxidant properties of the edible Basidiomycete Armillaria mellea in submerged cultures. Int. J. Mol. Sci. 2011, 12, 6367–6384. [Google Scholar] [CrossRef]
- Latgé, J.-P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef]
- Fu, J.; Li, J.; Sun, Y.; Liu, S.; Song, F.; Liu, Z. In-depth investigation of the mechanisms of Schisandra chinensis polysaccharide mitigating Alzheimer’s disease rat via gut microbiota and feces metabolomics. Int. J. Biol. Macromol. 2023, 232, 123488. [Google Scholar] [CrossRef]
- Zhao, G.; Hou, X.; Li, X.; Qu, M.; Tong, C.; Li, W. Metabolomics analysis of alloxan-induced diabetes in mice using UPLC–Q-TOF-MS after Crassostrea gigas polysaccharide treatment. Int. J. Biol. Macromol. 2018, 108, 550–557. [Google Scholar] [CrossRef]
- Molaei, H.; Jahanbin, K. Structural features of a new water-soluble polysaccharide from the gum exudates of Amygdalus scoparia Spach (Zedo gum). Carbohydr. Polym. 2018, 182, 98–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Mei, N.; Li, W.; Yang, T.; Xie, J. Three acidic polysaccharides derived from sour jujube seeds protect intestinal epithelial barrier function in LPS induced Caco-2 cell inflammation model. Int. J. Biol. Macromol. 2023, 240, 124435. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Xie, N.; Cheng, J.; Wang, Y.; Yuan, S.; Li, Q.; Shi, R.; He, L.; Chen, M. Molecular Characterization and Bioactivities of a Novel Polysaccharide from Phyllostachys pracecox Bamboo Shoot Residues. Foods 2023, 12, 1758. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-C.; Xu, L.; Chen, X.; Wu, J.-Y. Molecular properties and antioxidant activities of polysaccharides isolated from alkaline extract of wild Armillaria ostoyae mushrooms. Carbohydr. Polym. 2016, 137, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Sun, L.; Shen, D.; Ren, A.; Ma, F.; Tai, G.; Fan, L.; Zhou, Y. Beta-1,6 glucan converts tumor-associated macrophages into an M1-like phenotype. Carbohydr. Polym. 2020, 247, 116715. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Cheng, J.-J.; Lee, I.-J.; Lu, M.-K. Purification, structural elucidation, and anti-inflammatory activity of xylosylgalactofucan from Armillaria mellea. Int. J. Biol. Macromol. 2018, 114, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, N.; Feng, Q.; Li, H.; Wang, D.; Ma, L.; Liu, S.; Chen, C.; Wu, W.; Jiao, L. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int. J. Biol. Macromol. 2019, 123, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, H.; Zhang, X.; Tong, H.; Liu, J. Structural elucidation and immunological activity of a polysaccharide from the fruiting body of Armillaria mellea. Bioresour. Technol. 2009, 100, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar]
- Kumar, V.; Rana, V.; Soni, P.L. Molecular weight determination and correlation analysis of Dalbergia sissoo polysaccharide with constituent oligosaccharides. Phytochem. Anal. 2013, 24, 75–80. [Google Scholar] [CrossRef]
- Yu, G.; Yue, C.; Zang, X.; Chen, C.; Dong, L.; Liu, Y. Purification, characterization and in vitro bile salt-binding capacity of polysaccharides from Armillaria mellea mushroom. Czech J. Food Sci. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Jiao, L.; Li, H.; Li, J.; Bo, L.; Zhang, X.; Wu, W.; Chen, C. Study on structure characterization of pectin from the steamed ginseng and the inhibition activity of lipid accumulation in oleic acid-induced HepG2 cells. Int. J. Biol. Macromol. 2020, 159, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-P.; Li, C.-Y.; Peng, X.; Zou, Y.-F.; Rise, F.; Paulsen, B.S.; Wangensteen, H.; Inngjerdingen, K.T. Polysaccharides from Aconitum carmichaelii leaves: Structure, immunomodulatory and anti-inflammatory activities. Carbohydr. Polym. 2022, 291, 119655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Gu, S.; Pan, L.; Sun, H.; Gong, E.; Zhu, Z.; Wen, T.; Daba, G.M.; Elkhateeb, W.A. Structure analysis and antioxidant activity of polysaccharide-iron (III) from Cordyceps militaris mycelia. Int. J. Biol. Macromol. 2021, 178, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, J.; Qi, J.; Ding, M.; Liu, W.; Shao, T.; Han, J.; Wang, G. Konjac Glucomannan from Amorphophallus konjac enhances immunocompetence of the cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Liu, J.; He, Y.; Liu, L.; Xu, Z.; Lin, X.; Liu, N.; Kai, G. Systems pharmacology reveals the mechanism of Astragaloside IV in improving immune activity on cyclophosphamide-induced immunosuppressed mice. J. Ethnopharmacol. 2023, 313, 116533. [Google Scholar] [CrossRef]
- Su, L.; Li, X.; Guo, Z.; Xiao, X.; Chen, P.; Zhang, J.; Mao, C.; Ji, D.; Mao, J.; Gao, B.; et al. Effects of different steaming times on the composition, structure and immune activity of Polygonatum Polysaccharide. J. Ethnopharmacol. 2023, 310, 116351. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, T.; He, X. Characterization, sulfated modification and bioactivity of a novel polysaccharide from Millettiadielsiana. Int. J. Biol. Macromol. 2018, 117, 108–115. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Makarova, E.N.; Belyy, V.A. Structural studies of biologically active pectin-containing polysaccharides of pomegranate Punica granatum. Int. J. Biol. Macromol. 2019, 122, 29–36. [Google Scholar] [CrossRef]
- Yao, H.-Y.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Ren, T.; Xu, M.; Zhou, S.; Ren, J.; Li, B.; Jiang, P.; Li, H.; Wu, W.; Chen, C.; Fan, M.; et al. Structural characteristics of mixed pectin from ginseng berry and its anti-obesity effects by regulating the intestinal flora. Int. J. Biol. Macromol. 2023, 242, 124687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.; Guo, L.; Huang, L.; Gao, W. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Du, H.; Tan, H.; Li, J.; Wang, L.; Li, L.; Zhang, Y.; Liu, Y. Optimization of protein removal process of Lonicera japonica polysaccharide and its immunomodulatory mechanism in cyclophosphamide-induced mice by metabolomics and network pharmacology. Food Sci. Nutr. 2023, 11, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.M.; Mendoza, J.; Diven, J.; Denton, T.; Union, N.; Lajiness, M. Age-related changes in the immune system of mice of eight medium and long-lived strains and hybrids. I. Organ, cellular, and activity changes. Mech. Ageing Dev. 1979, 11, 295–346. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, H.; Chen, L.; Fang, Y.; Chen, J. Immunoregulatory function of Dictyophoraechinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide. Open Life Sci. 2021, 16, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Chang, Y.-Z.; Ji, B.; Zhao, H.-Y.; Shen, C.-Y.; Chang, R. Efficacy of gecko polysaccharide on suppressed immune response induced by cyclophosphamide in mice. J. Tradit. Chin. Med. 2021, 41, 539–545. [Google Scholar]
- Lee, J.-S.; Oka, K.; Watanabe, O.; Hara, H.; Ishizuka, S. Immunomodulatory effect of mushrooms on cytotoxic activity and cytokine production of intestinal lamina propria leukocytes does not necessarily depend on β-glucan contents. Food Chem. 2011, 126, 1521–1526. [Google Scholar] [CrossRef][Green Version]
- Zhang, N.; Liu, J.; Guo, X.; Li, S.; Wang, F.; Wang, M. Armillaria luteo-virens Sacc Ameliorates Dextran Sulfate Sodium Induced Colitis through Modulation of Gut Microbiota and Microbiota-Related Bile Acids. Nutrients 2021, 13, 3926. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Tian, Y.; Wang, Y.; Du, J.; Ye, X.; Lu, L.; Sun, C. Dietary supplementation with Dendrobium officinale leaves improves growth, antioxidant status, immune function, and gut health in broilers. Front. Microbiol. 2023, 14, 1255894. [Google Scholar] [CrossRef] [PubMed]
- Vornewald, P.M.; Forman, R.; Yao, R.; Parmar, N.; Lindholm, H.T.; Lee, L.S.K.; Martín-Alonso, M.; Else, K.J.; Oudhoff, M.J. Mmp17-deficient mice exhibit heightened goblet cell effector expression in the colon and increased resistance to chronic Trichuris muris infection. Front. Immunol. 2023, 14, 1243528. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Echeverry, H.; Yitbarek, A.; Camelo-Jaimes, G.; Sharif, S.; Guenter, W.; House, J.D.; Rodriguez-Lecompte, J.C. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 2012, 91, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
| Group | Total Sugar (%) | Protein (%) | Monosaccharide Composition (mol%) | |||||
|---|---|---|---|---|---|---|---|---|
| Man | Glc | Ara | Gal | GlcA | Fuc | |||
| Armillaria mellea neutral polysaccharide (AMPN) | 47.21 | 0.11 | 16.6 | 68.3 | 2.4 | 12.7 | - | - |
| Armillaria mellea acidic polysaccharide (AMPA) | 57.93 | 0.17 | 26.9 | 31.3 | 7.5 | 22.4 | 8.9 | 3 |
| Methylated Sugar | Type of Linkage | Molar (%) | |
|---|---|---|---|
| AMPN | AMPA | ||
| 2,3,4,6-Me4-Gal | Gal-(1→ | 3 | 26.7 |
| 2,3,4,6-Me4-Man | Man-(1→ | 16.2 | 22.4 |
| 2,3,4-Me3-Gal | →6)-Gal-(1→ | 0.2 | - |
| 2,3,4-Me3-Man | →6)-Man-(1→ | 0.3 | - |
| 2,3,5-Me3-Ara | Ara-(1→ | 1.4 | 1.1 |
| 2,3,6-Me3-Glc | →4)-Glc-(1→ | 6.2 | - |
| 2,3-Me2-Ara | →5)-Ara-(1→ | 1.1 | 4.7 |
| 2,4,6-Me3-Gal | →3)-Gal-(1→ | 7.9 | - |
| 3,6-Me2-Glc | →2,4)-Glc-(1→ | 62 | 33.9 |
| 3-Me-Fuc | →2,4)-Fuc-(1→ | - | 2.2 |
| 2,3,4-Me3-Fuc | Fuc-(1→ | - | 1.4 |
| 2,4,6-Me3-Man | →2)-Man-(1→ | - | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, Y.; Niu, H.; Wang, E.; Jiao, L.; Li, H.; Wu, W. Structure Elucidation and Immunoactivity Study of Armillaria mellea Fruiting Body Polysaccharides. Separations 2024, 11, 3. https://doi.org/10.3390/separations11010003
Li Q, Li Y, Niu H, Wang E, Jiao L, Li H, Wu W. Structure Elucidation and Immunoactivity Study of Armillaria mellea Fruiting Body Polysaccharides. Separations. 2024; 11(1):3. https://doi.org/10.3390/separations11010003
Chicago/Turabian StyleLi, Qingqing, Ying Li, Huazhou Niu, Enhui Wang, Lili Jiao, Hui Li, and Wei Wu. 2024. "Structure Elucidation and Immunoactivity Study of Armillaria mellea Fruiting Body Polysaccharides" Separations 11, no. 1: 3. https://doi.org/10.3390/separations11010003
APA StyleLi, Q., Li, Y., Niu, H., Wang, E., Jiao, L., Li, H., & Wu, W. (2024). Structure Elucidation and Immunoactivity Study of Armillaria mellea Fruiting Body Polysaccharides. Separations, 11(1), 3. https://doi.org/10.3390/separations11010003






