Photocatalytic Removal of Crystal Violet Dye Utilizing Greenly Synthesized Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Plant Extract
2.2. Green Fabrication of the Phytosynthesized IONPs
2.3. Characterization of the Biogenic IONPs
2.3.1. UV Optical Spectroscopy
2.3.2. Transmission Electron Microscopy (TEM) Analysis
2.3.3. Energy Dispersive X-ray (EDX) Analysis
2.3.4. FTIR (Fourier Transform Infrared) Analysis
2.3.5. XRD Analysis
2.3.6. Brunauer–Emmett–Teller (BET)/Barrett–Joyner–Halenda (BJH) Analysis
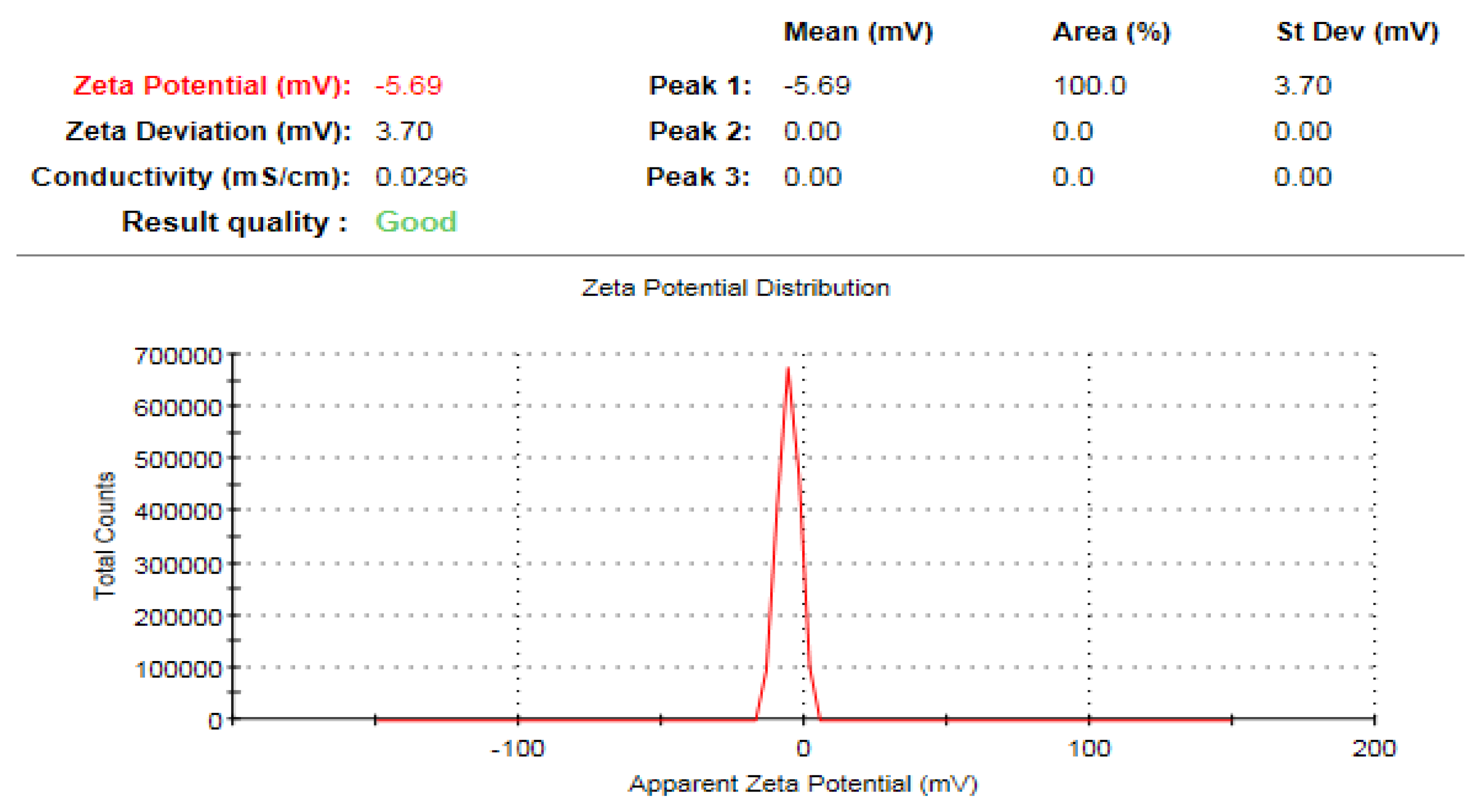
2.3.7. Zeta Potential Analysis
2.4. Photoctalytic Degradation of Synthetic Dyes Using IONPs
2.5. Determination of Photocatalysis Reaction at Different pH Values
2.6. Cycling Test of the Biogenic IONPs
2.7. Antioxidant Assay
2.8. Statistical Analysis
3. Results and Discussion
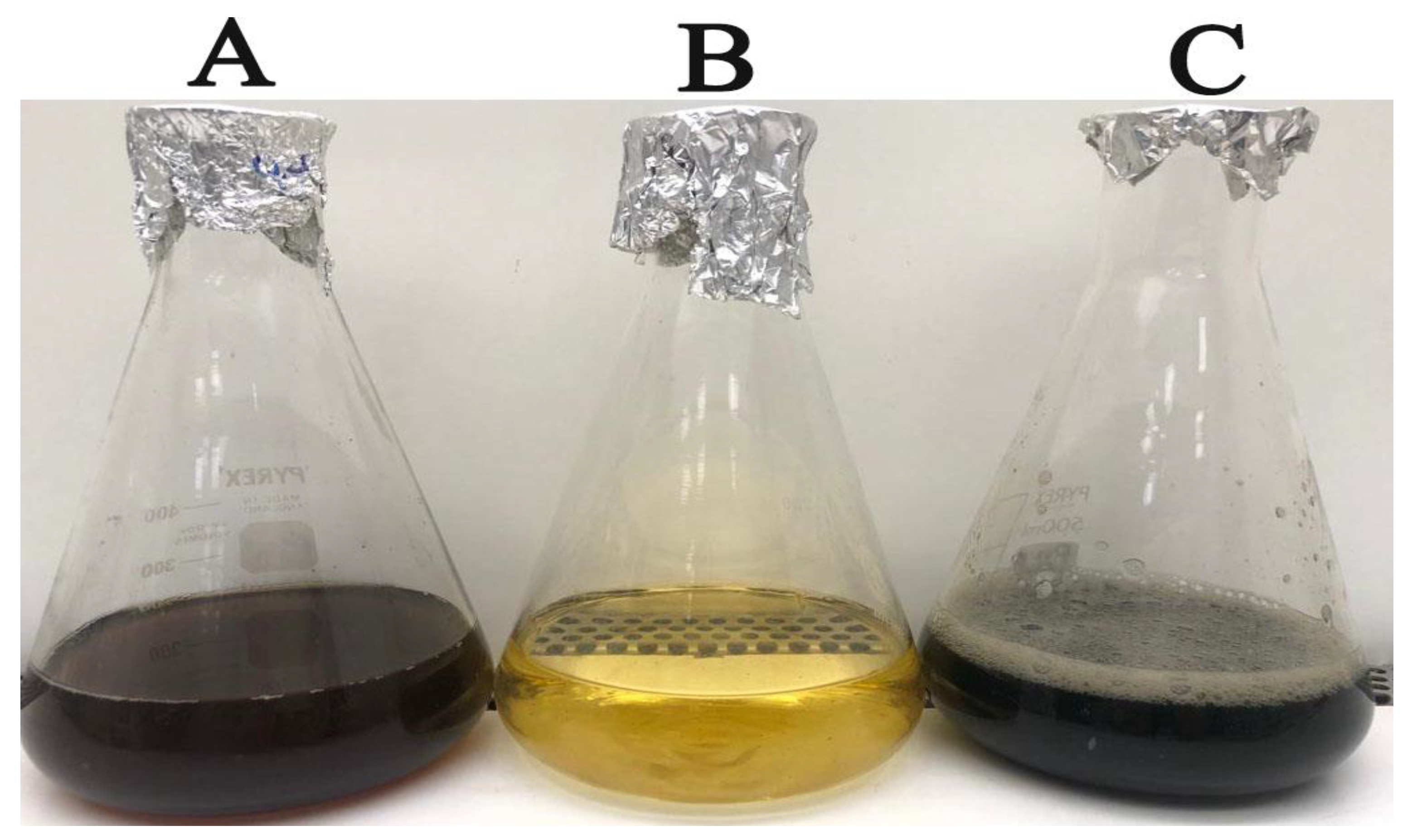
3.1. Green Synthesis of Bioinspired IONPs
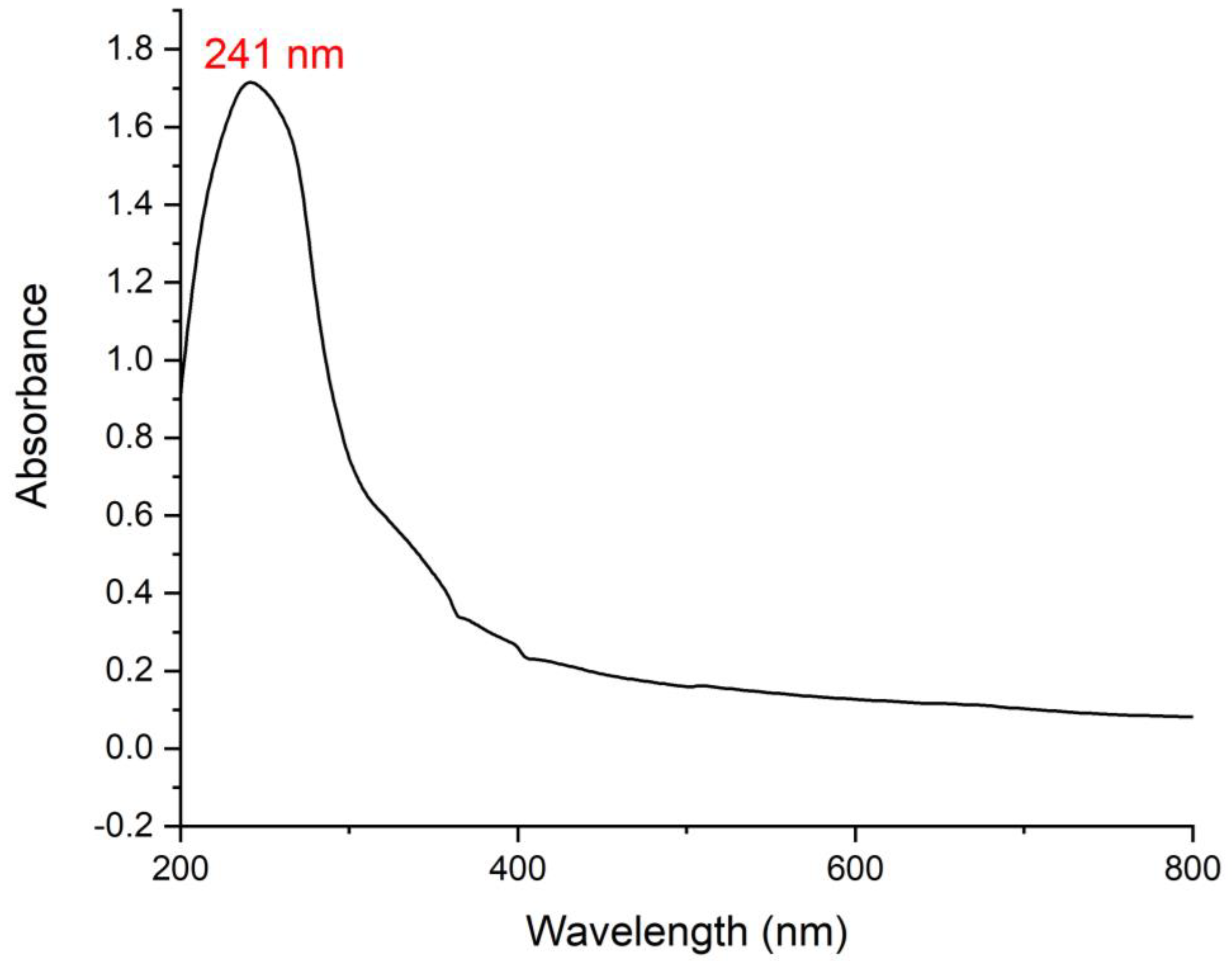
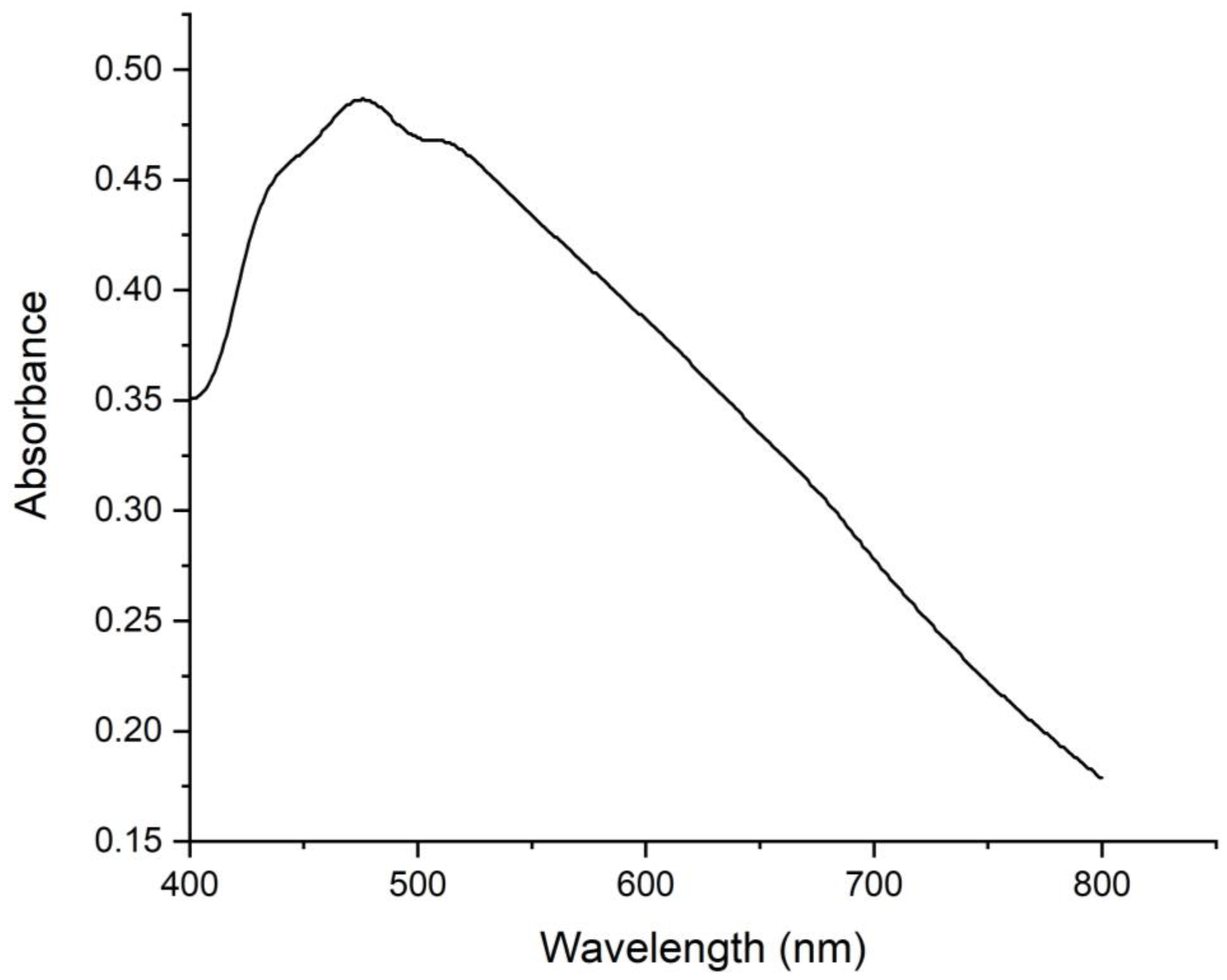
3.2. UV Analysis of the Bioinspired IONPs
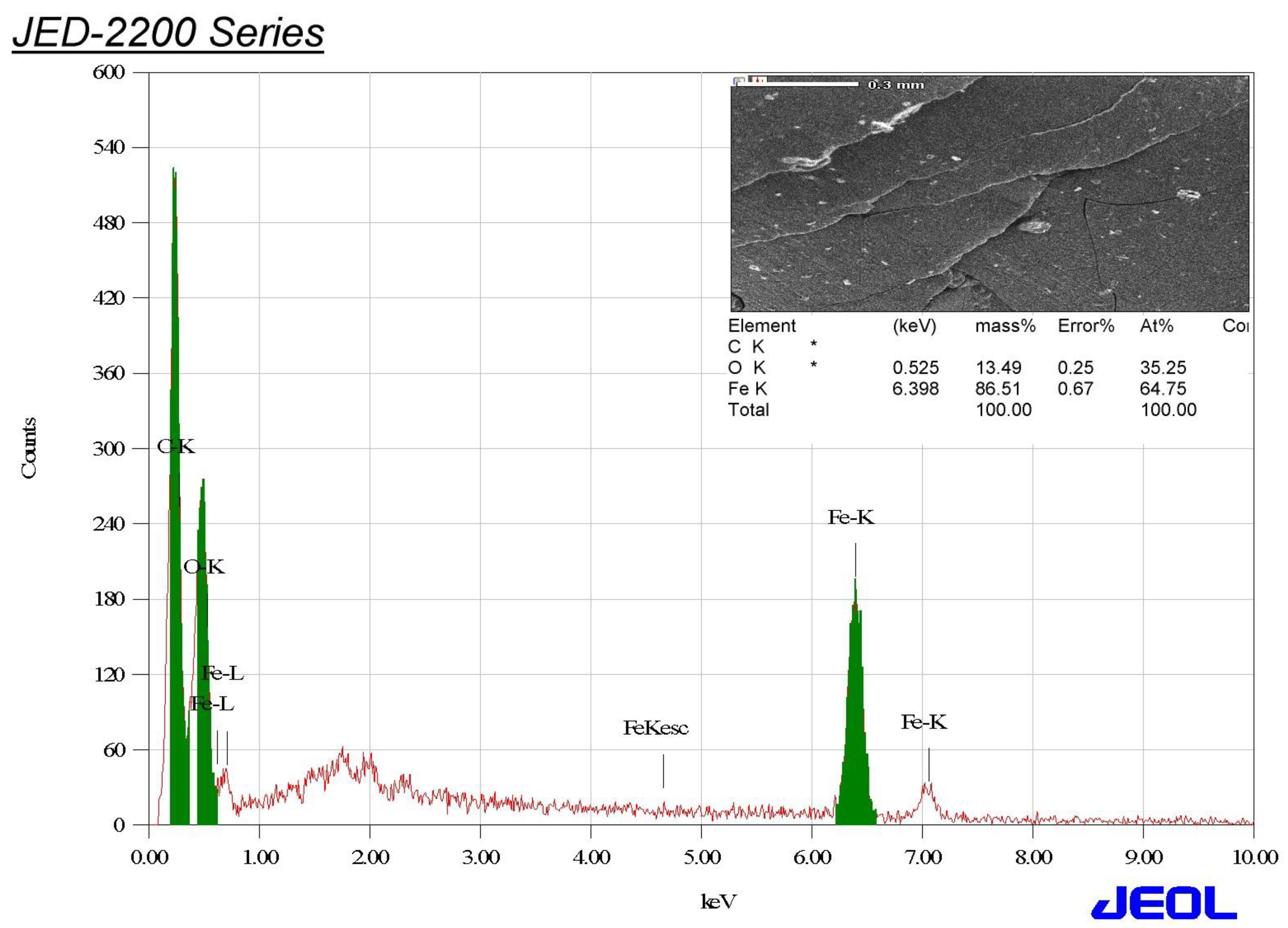
3.3. EDX Analysis of the Biogenic IONPs
3.4. FTIR Analysis of the Biogenic IONPs
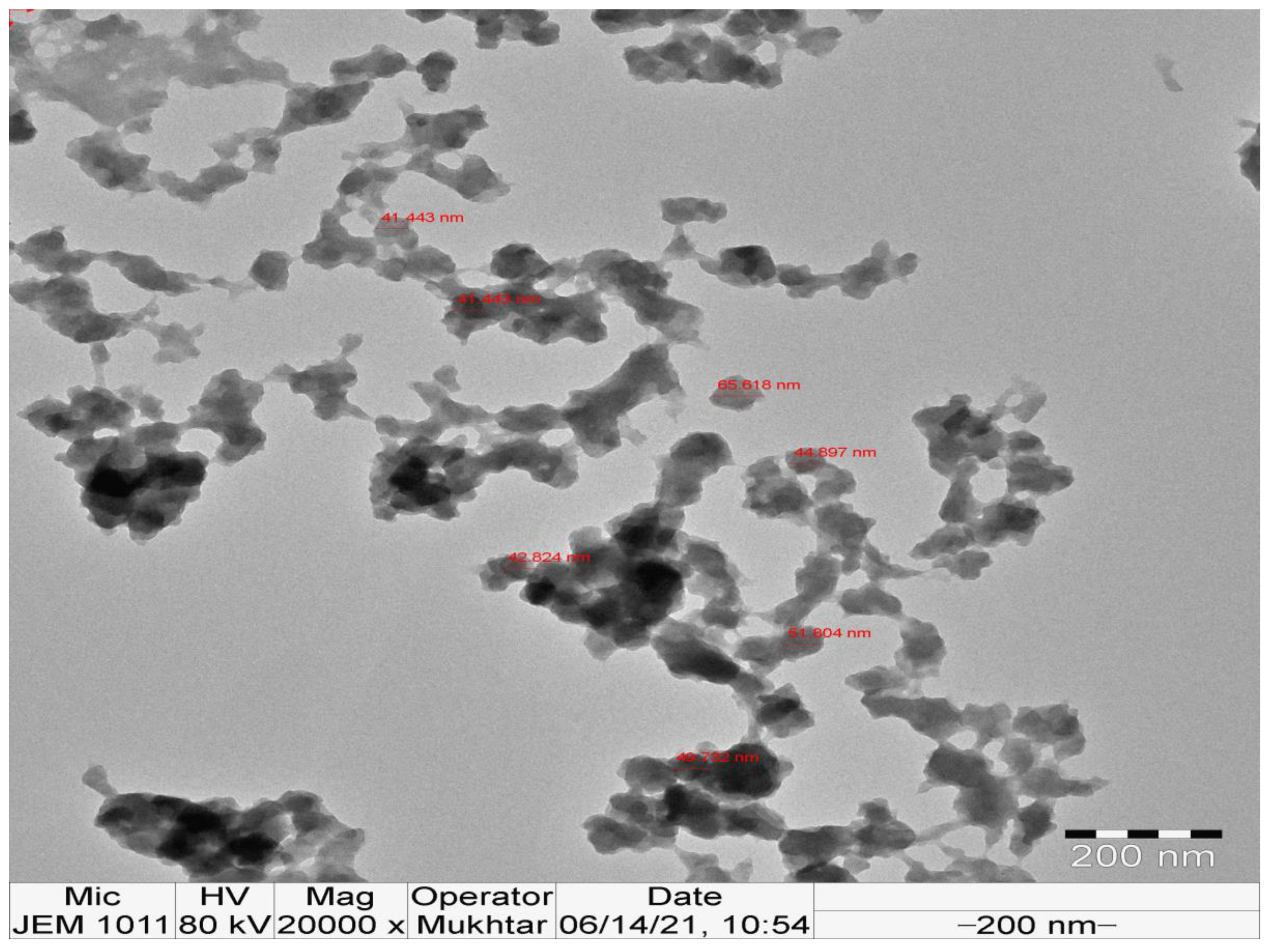
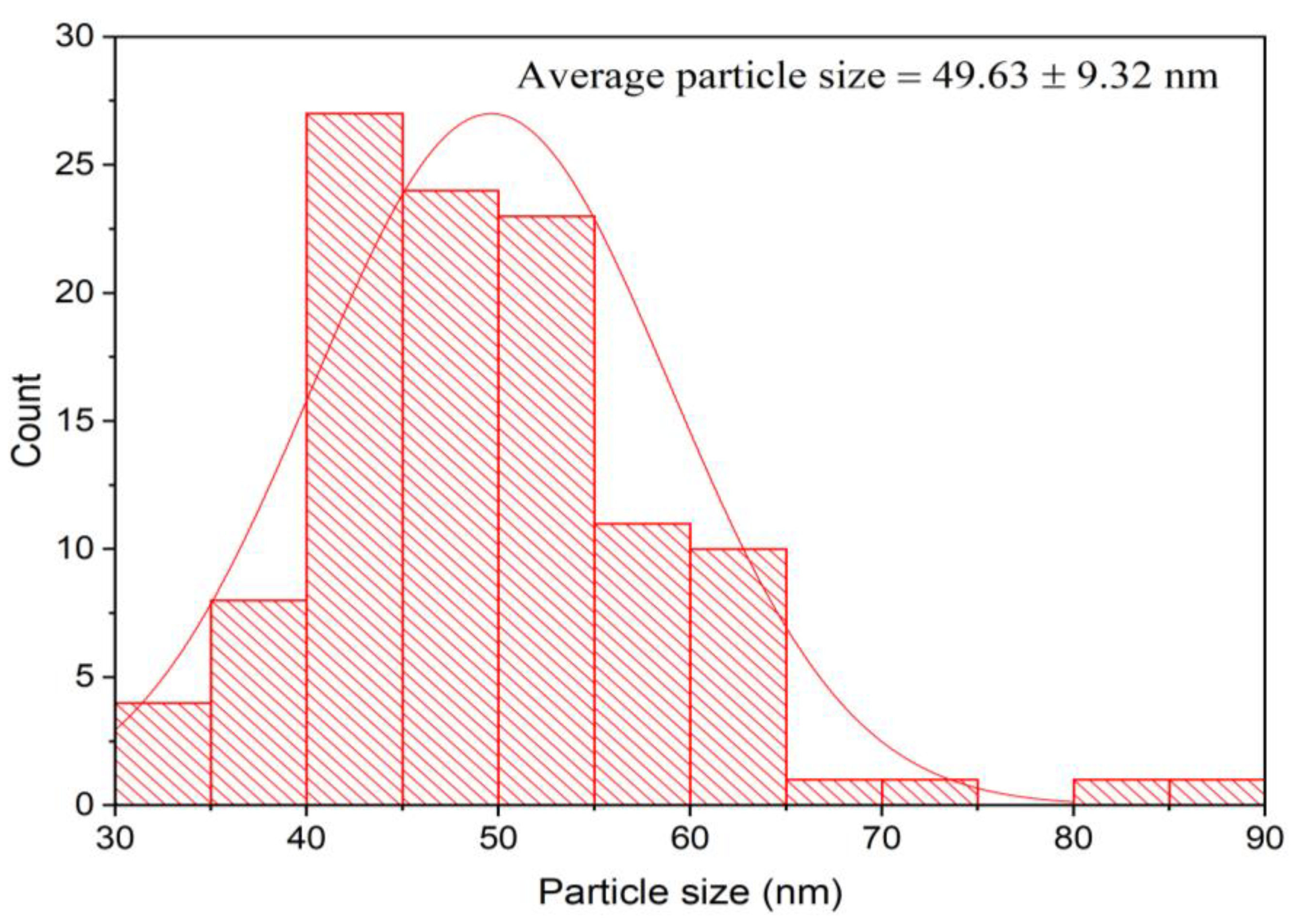
3.5. TEM Investigation of the Bioinspired IONPs
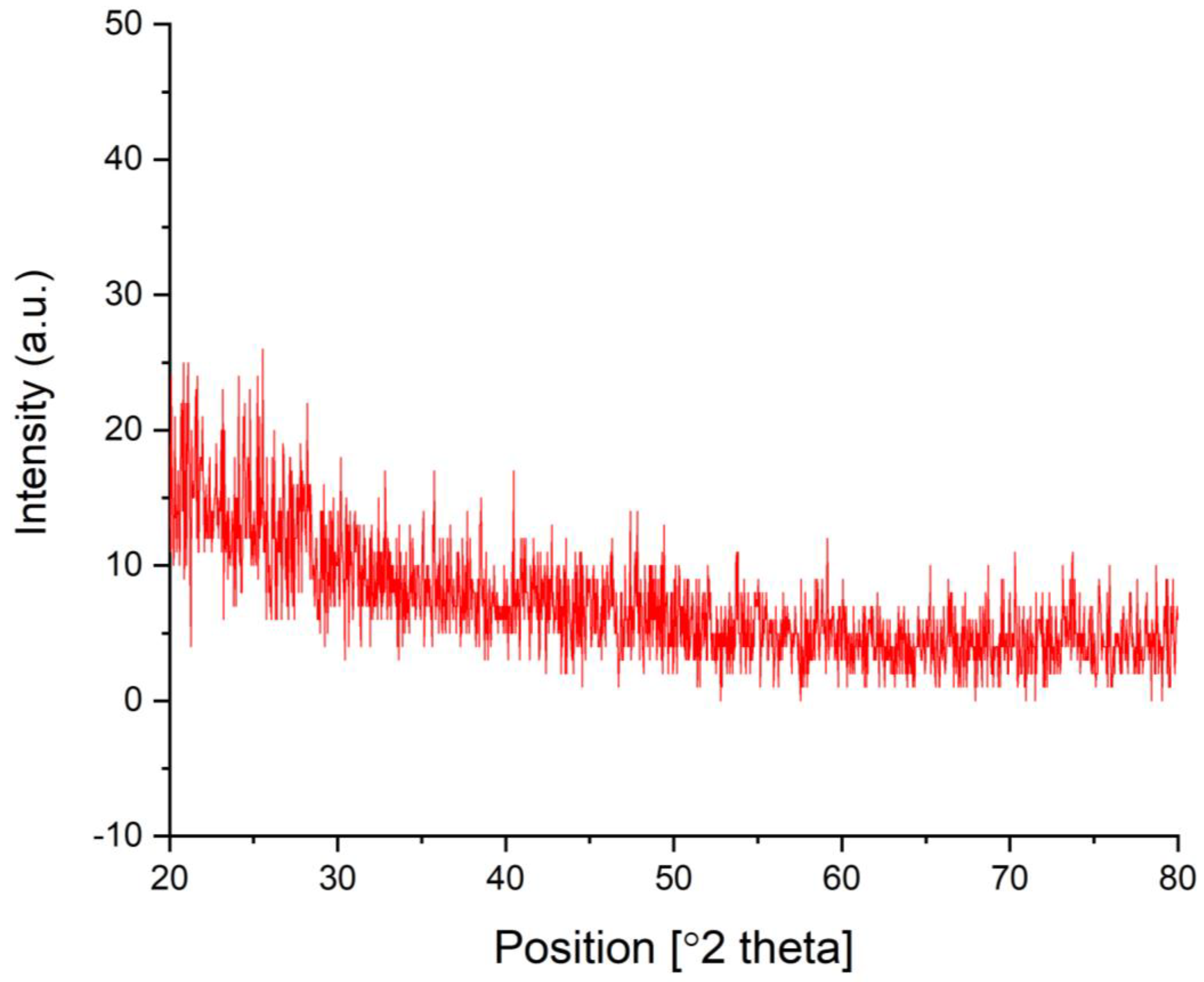
3.6. XRD Analysis of the Biogenic IONPs
3.7. Specific Surface Area, Pore Size, and Pore Volume of the Biogenic IONPs
3.8. Zeta Potential Analysis
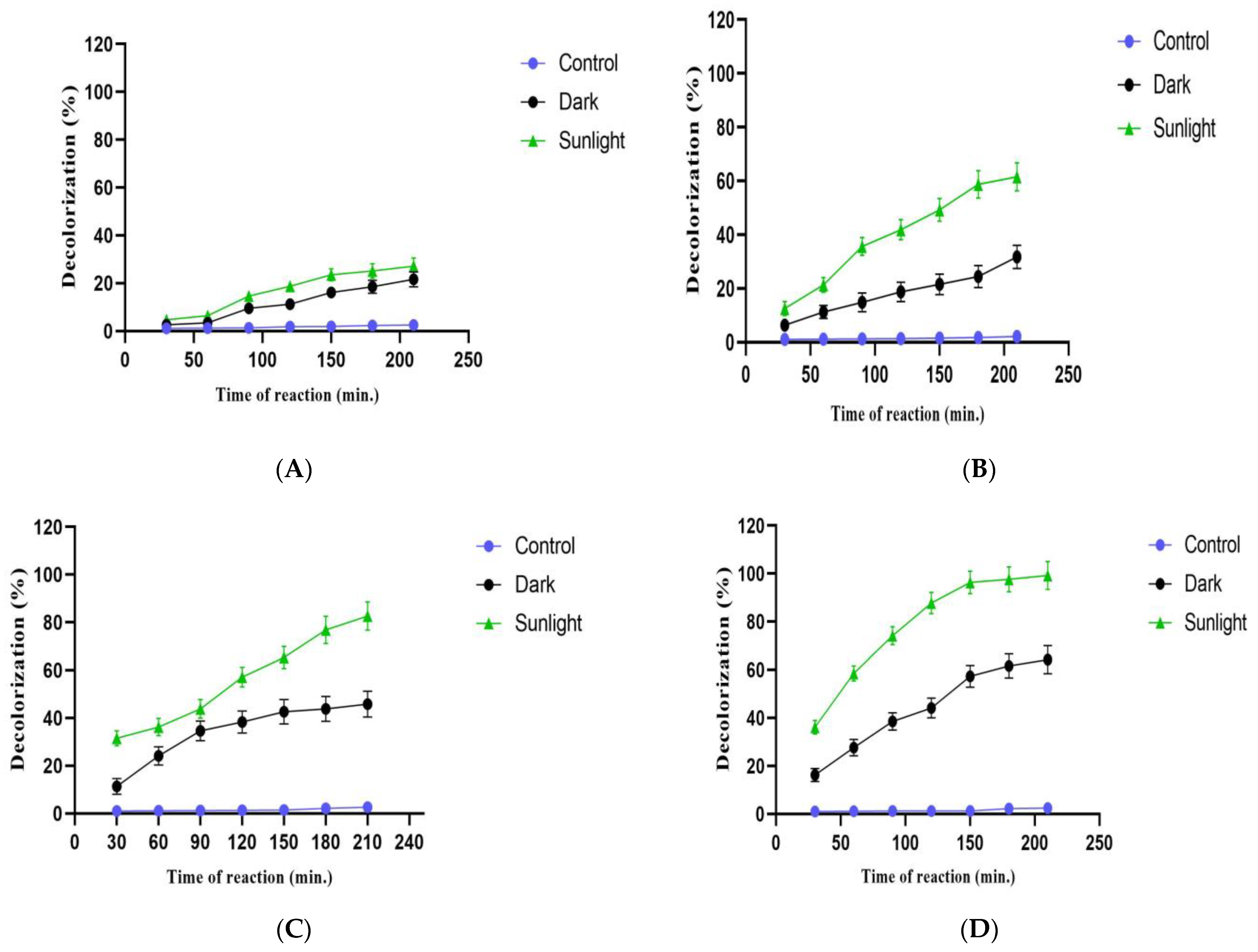
3.9. Photocatalytic Degradation of Crystal Violet Dye Using IONPs
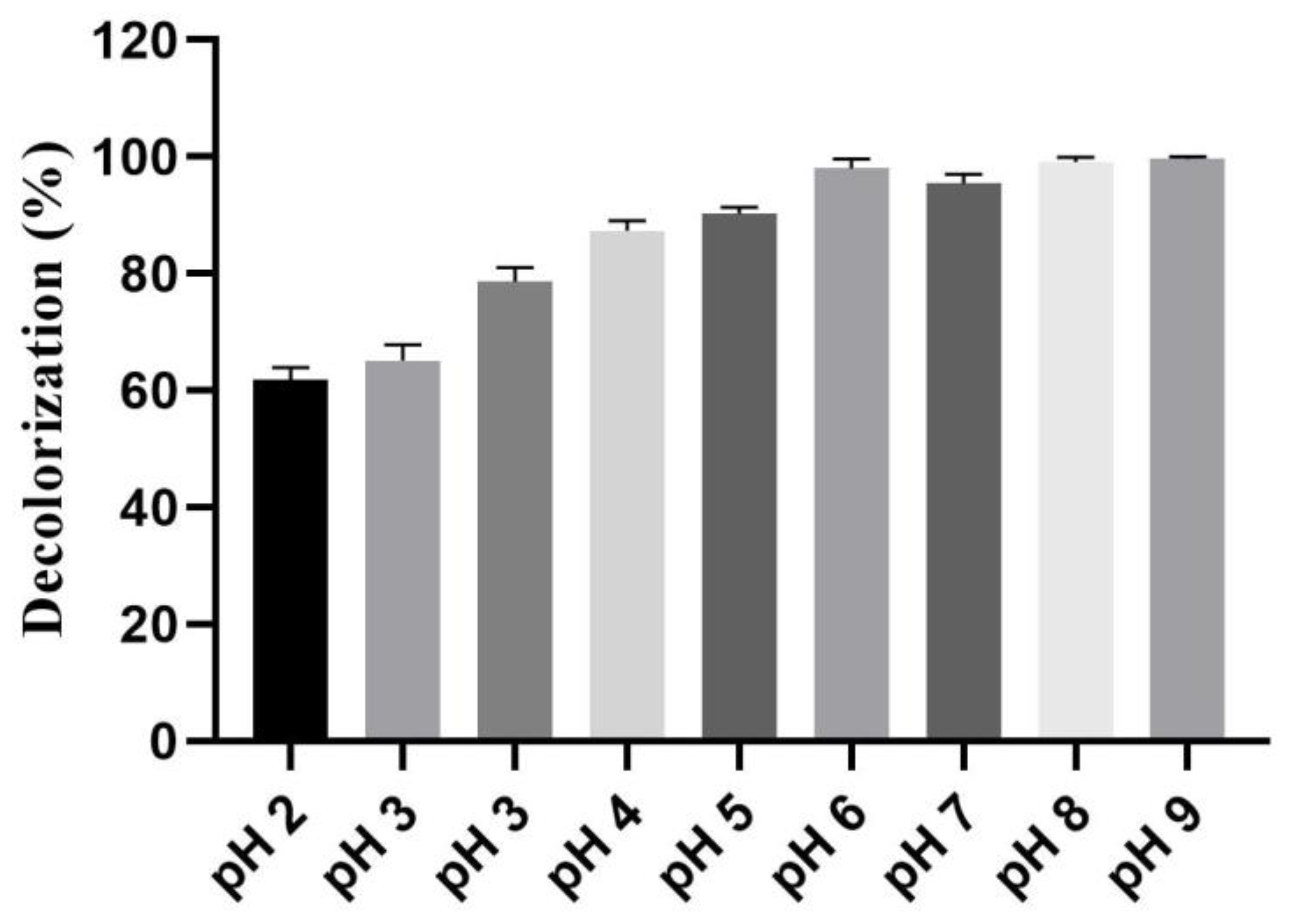
3.10. Detection of Photocatalytic Activity of the Biogenic IONPs at Different pH Values
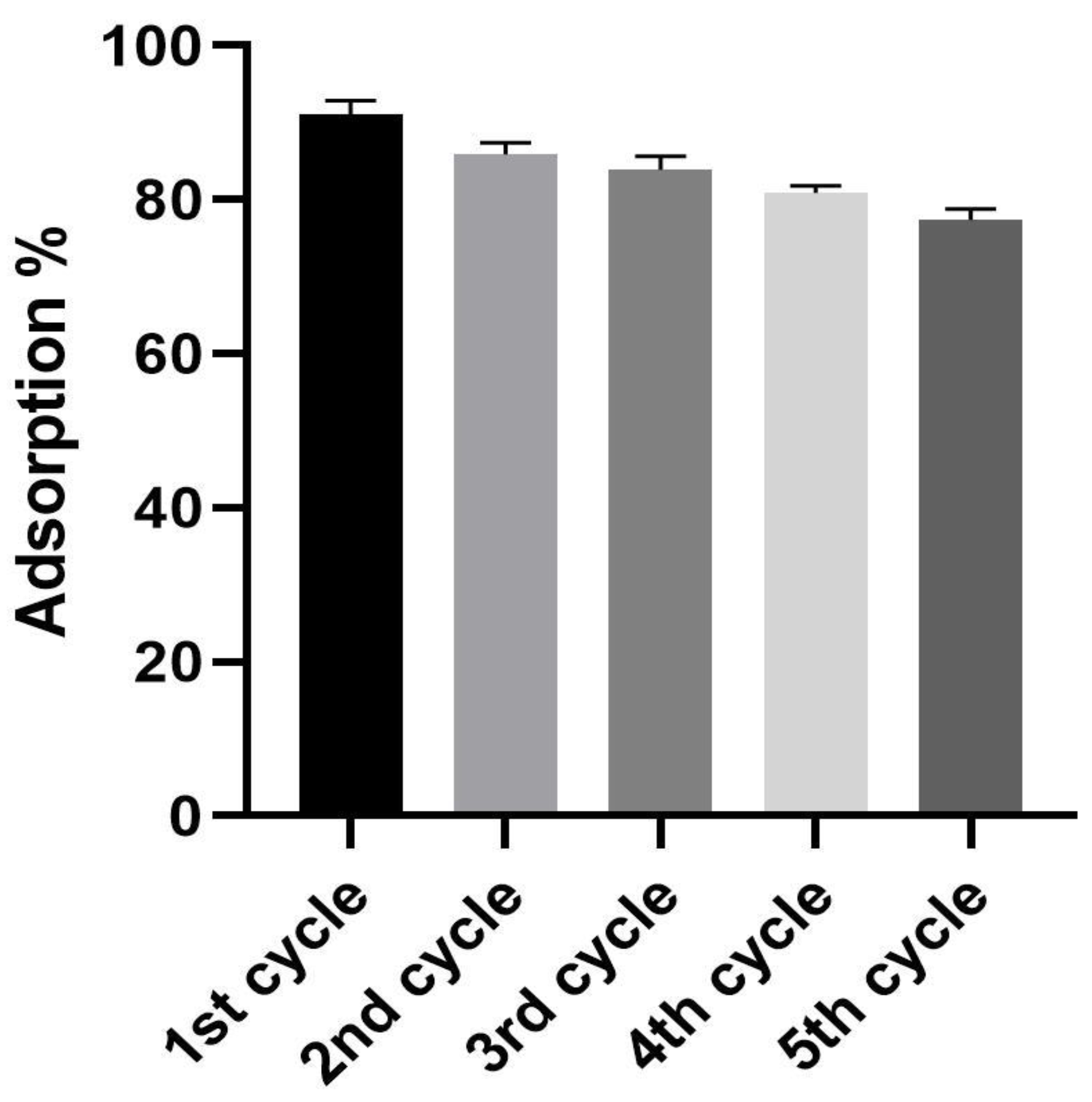
3.11. Cycling Test of the Biogenic IONPs
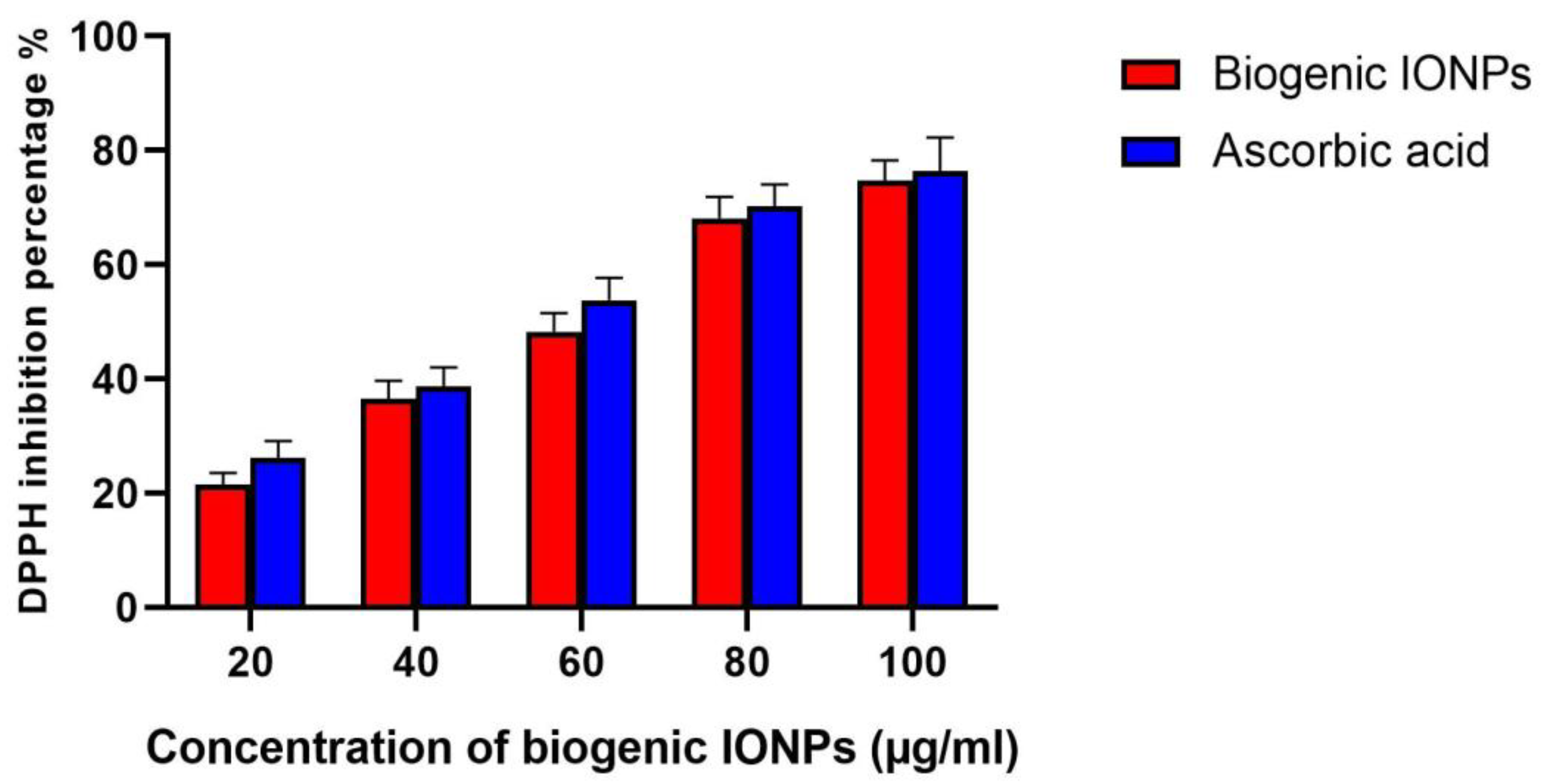
3.12. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective Water/Wastewater Treatment Methodologies for Toxic Pollutants Removal: Processes and Applications towards Sustainable Development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and Its Adverse Effects on Environment and Human Health. In Environmental Concerns and Sustainable Development: Volume 1: Air, Water and Energy Resources; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; pp. 235–256. ISBN 9789811358890. [Google Scholar]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent Advances in Carbon Nanomaterial-Based Adsorbents for Water Purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Farhan Hanafi, M.; Sapawe, N. A Review on the Water Problem Associate with Organic Pollutants Derived from Phenol, Methyl Orange, and Remazol Brilliant Blue Dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Gao, Y.; Yang, W.; Li, Y.; Guo, C. Porous Metalloporphyrinic Nanospheres Constructed from Metal 5,10,15,20-Tetraksi(4′-Ethynylphenyl)Porphyrin for Efficient Catalytic Degradation of Organic Dyes. RSC Adv. 2018, 8, 7330–7339. [Google Scholar] [CrossRef]
- Karri, R.R.; Ravindran, G.; Dehghani, M.H. Chapter 1-Wastewater—Sources, Toxicity, and Their Consequences to Human Health. In Soft Computing Techniques in Solid Waste and Wastewater Management; Karri, R.R., Ravindran, G., Dehghani, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–33. ISBN 978-0-12-824463-0. [Google Scholar]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and Health Concerns of Persistent Coloring Pollutants of Textile Industry Wastewater and Treatment Approaches for Environmental Safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Vandana; Priyadarshanee, M.; Mahto, U.; Das, S. Chapter 2-Mechanism of Toxicity and Adverse Health Effects of Environmental Pollutants. In Microbial Biodegradation and Bioremediation, 2nd ed.; Das, S., Dash, H.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–53. ISBN 978-0-323-85455-9. [Google Scholar]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Ahmad, A.; Hamidah Mohd-Setapar, S.; Sing Chuong, C.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent Advances in New Generation Dye Removal Technologies: Novel Search for Approaches to Reprocess Wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Shahadat, M.; Isamil, S. Regeneration Performance of Clay-Based Adsorbents for the Removal of Industrial Dyes: A Review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Q.; Li, H.; Wang, J.; Tai, G.; Wang, F.; Han, J.; Zhu, Y.; Wu, G. Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption. Int. J. Mol. Sci. 2022, 23, 5900. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological Treatment of Real Textile Effluent Using Aspergillus Flavus and Fusarium Oxysporium and Their Consortium along with the Evaluation of Their Phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Jiang, Q.; Reddy, N.; Yang, Y. Biodegradable Hollow Zein Nanoparticles for Removal of Reactive Dyes from Wastewater. J. Environ. Manag. 2013, 125, 33–40. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Karri, R.R.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Nizamuddin, S. Magnetic Nanoparticles Incorporation into Different Substrates for Dyes and Heavy Metals Removal—A Review. Environ. Sci. Pollut. Res. 2020, 27, 43526–43541. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed Res. Int. 2014, 2014, e498420. [Google Scholar] [CrossRef]
- Chouke, P.B.; Shrirame, T.; Potbhare, A.K.; Mondal, A.; Chaudhary, A.R.; Mondal, S.; Thakare, S.R.; Nepovimova, E.; Valis, M.; Kuca, K. Bioinspired Metal/Metal Oxide Nanoparticles: A Road Map to Potential Applications. Mater. Today Adv. 2022, 16, 100314. [Google Scholar] [CrossRef]
- Saied, E.; Salem, S.S.; Al-Askar, A.A.; Elkady, F.M.; Arishi, A.A.; Hashem, A.H. Mycosynthesis of Hematite (α-Fe2O3) Nanoparticles Using Aspergillus Niger and Their Antimicrobial and Photocatalytic Activities. Bioengineering 2022, 9, 397. [Google Scholar] [CrossRef]
- Shafey, A.M.E. Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Prabhakar Vattikuti, S.V.; Devarayapalli, K.C.; Yoo, K.; Shim, J.; Sreekanth, T.V.M. Green Synthesis: Photocatalytic Degradation of Textile Dyes Using Metal and Metal Oxide Nanoparticles-Latest Trends and Advancements. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2617–2723. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Nagra, U.; Shabbir, M.; Zaman, M.; Mahmood, A.; Barkat, K. Review on Methodologies Used in the Synthesis of Metal Nanoparticles: Significance of Phytosynthesis Using Plant Extract as an Emerging Tool. Curr. Pharm. Des. 2020, 26, 5188–5204. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Nanotechnology Applications in Pollution Sensing and Degradation in Agriculture: A Review. Environ. Chem. Lett. 2009, 7, 191–204. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, W.; Wang, S. Photocatalytic Oxygen Evolution. In Solar-to-Chemical Conversion; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 129–162. ISBN 978-3-527-82507-3. [Google Scholar]
- Nande, A.; Raut, S.; Michalska-Domanska, M.; Dhoble, S.J. Green Synthesis of Nanomaterials Using Plant Extract: A Review. Curr. Pharm. Biotechnol. 2021, 22, 1794–1811. [Google Scholar] [CrossRef] [PubMed]
- Vinu, R.; Madras, G. Renewable Energy via Photocatalysis. Curr. Org. Chem. 2013, 17, 2538–2558. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Wang, C.; Lin, W.; Li, S. Novel Cd0.5Zn0.5S/Bi2MoO6 S-Scheme Heterojunction for Boosting the Photodegradation of Antibiotic Enrofloxacin: Degradation Pathway, Mechanism and Toxicity Assessment. Sep. Purif. Technol. 2023, 304, 122401. [Google Scholar] [CrossRef]
- Su, R. Photocatalysis for Pollution Remediation. In UV-Visible Photocatalysis for Clean Energy Production and Pollution Remediation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 267–283. ISBN 978-3-527-83799-1. [Google Scholar]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Shao, W.; Cheng, M.; Luo, X.; Zhou, M.; Li, S.; Ma, T.; Cheng, C.; Zhao, C. High-Valence Transition Metal Modified FeNiV Oxides Anchored on Carbon Fiber Cloth for Efficient Oxygen Evolution Catalysis. Adv. Fiber Mater. 2022, 4, 774–785. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y. Ta3N5/CdS Core–Shell S-Scheme Heterojunction Nanofibers for Efficient Photocatalytic Removal of Antibiotic Tetracycline and Cr(VI): Performance and Mechanism Insights. Adv. Fiber Mater. 2023, 5, 994–1007. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Zhang, Y.; Cai, J.; He, M.; Li, M.; Chen, Z.; Zhang, L. Growth of BiOBr/ZIF-67 Nanocomposites on Carbon Fiber Cloth as Filter-Membrane-Shaped Photocatalyst for Degrading Pollutants in Flowing Wastewater. Adv. Fiber Mater. 2022, 4, 1620–1631. [Google Scholar] [CrossRef]
- Li, S.; Yan, R.; Cai, M.; Jiang, W.; Zhang, M.; Li, X. Enhanced Antibiotic Degradation Performance of Cd0.5Zn0.5S/Bi2MoO6 S-Scheme Photocatalyst by Carbon Dot Modification. J. Mater. Sci. Technol. 2023, 164, 59–67. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Li, C.; Li, B.; Tao, B.; Gu, S.; Wang, G.; Chang, H. Redox Regulation of Photocatalytic Nitrogen Reduction Reaction by Gadolinium Doping in Two-Dimensional Bismuth Molybdate Nanosheets. Appl. Surf. Sci. 2022, 600, 154105. [Google Scholar] [CrossRef]
- Bharathi, D.; Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K. Bio-Inspired Synthesis of Flower Shaped Iron Oxide Nanoparticles (FeONPs) Using Phytochemicals of Solanum Lycopersicum Leaf Extract for Biomedical Applications. Biocatal. Agric. Biotechnol. 2020, 27, 101698. [Google Scholar] [CrossRef]
- Yassin, M.T.; Elgorban, A.M.; Al-Askar, A.A.; Sholkamy, E.N.; Ameen, F.; Maniah, K. Synergistic Anticandidal Activities of Greenly Synthesized ZnO Nanomaterials with Commercial Antifungal Agents against Candidal Infections. Micromachines 2023, 14, 209. [Google Scholar] [CrossRef]
- Mirza, A.U.; Kareem, A.; Nami, S.A.A.; Khan, M.S.; Rehman, S.; Bhat, S.A.; Mohammad, A.; Nishat, N. Biogenic Synthesis of Iron Oxide Nanoparticles Using Agrewia Optiva and Prunus Persica Phyto Species: Characterization, Antibacterial and Antioxidant Activity. J. Photochem. Photobiol. B 2018, 185, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, D.; Cui, Z.; Zhang, Q.; Gong, X.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. Optimizing the Reaction Pathway by Active Site Regulation in the CdS/Fe2O3 Z-Scheme Heterojunction System for Highly Selective Photocatalytic Benzylamine Oxidation Integrated with H2 Production. ACS Catal. 2022, 12, 12386–12397. [Google Scholar] [CrossRef]
- Rajendran, A.; Alsawalha, M.; Alomayri, T. Biogenic Synthesis of Husked Rice-Shaped Iron Oxide Nanoparticles Using Coconut Pulp (Cocos Nucifera L.) Extract for Photocatalytic Degradation of Rhodamine B Dye and Their in Vitro Antibacterial and Anticancer Activity. J. Saudi Chem. Soc. 2021, 25, 101307. [Google Scholar] [CrossRef]
- Batool, T.; Shah, Z.H.; Ashraf, H.; Ali, D.; Shamaila, S.; Anjum, T.; Naseem, S.; Riaz, S. Solar Energy Driven Photo Catalytic Action and Antimicrobial Activities of Iron Oxide Nanoparticles. J. Sol-Gel Sci. Technol. 2023, 1–17. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. Research Progress on Iron Oxide-Based Magnetic Materials: Synthesis Techniques and Photocatalytic Applications. Ceram. Int. 2016, 42, 9–34. [Google Scholar] [CrossRef]
- Al-Tamimi, S.A. Biogenic Green Synthesis of Metal Oxide Nanoparticles Using Oat Biomass for Ultrasensitive Modified Polymeric Sensors. Green Chem. Lett. Rev. 2021, 14, 166–179. [Google Scholar] [CrossRef]
- Dejen, K.D.; Zereffa, E.A.; Murthy, H.C.A.; Merga, A. Synthesis of ZnO and ZnO/PVA Nanocomposite Using Aqueous Moringa Oleifeira Leaf Extract Template: Antibacterial and Electrochemical Activities. Rev. Adv. Mater. Sci. 2020, 59, 464–476. [Google Scholar] [CrossRef]
- Omran, B.A.; Aboelazayem, O.; Nassar, H.N.; El-Salamony, R.A.; El-Gendy, N.S. Biovalorization of Mandarin Waste Peels into Silver Nanoparticles and Activated Carbon. Int. J. Environ. Sci. Technol. 2021, 18, 1119–1134. [Google Scholar] [CrossRef]
- Ranjani, S.; Priya, P.S.; Veerasami, M.; Hemalatha, S. Novel Polyherbal Nanocolloids to Control Bovine Mastitis. Appl. Biochem. Biotechnol. 2022, 194, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Yadav, K.K.; Gnanamoorthy, G.; Choudhary, N.; Khan, S.H.; Gupta, N.; Kamyab, H.; Bach, Q.-V. A Novel Synthesis and Characterization of Polyhedral Shaped Amorphous Iron Oxide Nanoparticles from Incense Sticks Ash Waste. Environ. Technol. Innov. 2020, 20, 101089. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gnanamoorthy, G.; Ali, D.; Bera, S.P.; Roy, A.; Kumar, G.; Choudhary, N.; Kalasariya, H.; Basnet, A. Cytotoxicity, Removal of Congo Red Dye in Aqueous Solution Using Synthesized Amorphous Iron Oxide Nanoparticles from Incense Sticks Ash Waste. J. Nanomater. 2022, 2022, e5949595. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef]
- Hasany, S.F.; Abdurahman, N.H.; Sunarti, A.R.; Jose, R. Magnetic Iron Oxide Nanoparticles: Chemical Synthesis and Applications Review. Curr. Nanosci. 2013, 9, 561–575. [Google Scholar] [CrossRef]
- Yan, S.; Abhilash, K.P.; Tang, L.; Yang, M.; Ma, Y.; Xia, Q.; Guo, Q.; Xia, H. Research Advances of Amorphous Metal Oxides in Electrochemical Energy Storage and Conversion. Small 2019, 15, 1804371. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional Magnetic Iron Oxide Nanoparticles: Diverse Synthetic Approaches, Surface Modifications, Cytotoxicity towards Biomedical and Industrial Applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Dash, A.; Ahmed, M.T.; Selvaraj, R. Mesoporous Magnetite Nanoparticles Synthesis Using the Peltophorum Pterocarpum Pod Extract, Their Antibacterial Efficacy against Pathogens and Ability to Remove a Pollutant Dye. J. Mol. Struct. 2019, 1178, 268–273. [Google Scholar] [CrossRef]
- Ekwumemgbo, P.A.; Shallangwa, G.A.; Okon, I.E. Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Prosopis Africana Leaf Extract. Commun. Phys. Sci. 2023, 9, 125–136. [Google Scholar]
- Mishra, A.K.; Ramaprabhu, S. Nano Magnetite Decorated Multiwalled Carbon Nanotubes: A Robust Nanomaterial for Enhanced Carbon Dioxide Adsorption. Energy Environ. Sci. 2011, 4, 889–895. [Google Scholar] [CrossRef]
- Vinayagam, R.; Pai, S.; Varadavenkatesan, T.; Narasimhan, M.K.; Narayanasamy, S.; Selvaraj, R. Structural Characterization of Green Synthesized α-Fe2O3 Nanoparticles Using the Leaf Extract of Spondias Dulcis. Surf. Interfaces 2020, 20, 100618. [Google Scholar] [CrossRef]
- Alqarni, L.S.; Alghamdi, M.D.; Alshahrani, A.A.; Nassar, A.M. Green Nanotechnology: Recent Research on Bioresource-Based Nanoparticle Synthesis and Applications. J. Chem. 2022, 2022, e4030999. [Google Scholar] [CrossRef]
- Martins, E.S.; Espindola, A.; Britos, T.N.; Chagas, C.; Barbosa, E.; Castro, C.E.; Fonseca, F.L.A.; Haddad, P.S. Potential Use of DMSA-Containing Iron Oxide Nanoparticles as Magnetic Vehicles against the COVID-19 Disease. ChemistrySelect 2021, 6, 7931–7935. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Ogawa, K.; Aoyagi, H. Biosynthesis of Silver Nanoparticles Mediated by Extracellular Pigment from Talaromyces Purpurogenus and Their Biomedical Applications. Nanomaterials 2019, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Marchetto, D.; Paganelli, S.; Piccolo, O. Bio-Generated Metal Binding Polysaccharides as Catalysts for Synthetic Applications and Organic Pollutant Transformations. New Biotechnol. 2011, 29, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Aljeldah, M.M.; Yassin, M.T.; Mostafa, A.A.-F.; Aboul-Soud, M.A. Synergistic Antibacterial Potential of Greenly Synthesized Silver Nanoparticles with Fosfomycin Against Some Nosocomial Bacterial Pathogens. Infect. Drug Resist. 2023, 16, 125–142. [Google Scholar] [CrossRef]
- Khan, A.; Roy, A.; Bhasin, S.; Emran, T.B.; Khusro, A.; Eftekhari, A.; Moradi, O.; Rokni, H.; Karimi, F. Nanomaterials: An Alternative Source for Biodegradation of Toxic Dyes. Food Chem. Toxicol. 2022, 164, 112996. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, J.; Ye, J. Structural, Photocatalytic, and Photophysical Properties of Perovskite MSnO3 (M = Ca, Sr, and Ba) Photocatalysts. J. Mater. Res. 2007, 22, 1859–1871. [Google Scholar] [CrossRef]
- Bryukhanov, V.V.; Minaev, B.M.; Tsibul’nikova, A.V.; Slezhkin, V.A. The Effect of Gold Nanoparticles on Exchange Processes in Collision Complexes of Triplet and Singlet Oxygen Molecules with Excited Eosin Molecules. Opt. Spectrosc. 2015, 119, 29–38. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a Photocatalytic Material: A Review. Appl. Catal. Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Muthukumar, H.; Chandrasekaran, N.I.; Mohammed, S.N.; Pichiah, S.; Manickam, M. Iron Oxide Nano-Material: Physicochemical Traits and in Vitro Antibacterial Propensity against Multidrug Resistant Bacteria. J. Ind. Eng. Chem. 2017, 45, 121–130. [Google Scholar] [CrossRef]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile Synthesis of Magnetic Iron Oxide Nanoparticles Using Inedible Cynometra Ramiflora Fruit Extract Waste and Their Photocatalytic Degradation of Methylene Blue Dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; El-Sharkawy, R.M.; Ibrahim, G.A.A. A Novel Bionanocomposite from Doped Lipase Enzyme into Magnetic Graphene Oxide-Immobilized-Cellulose for Efficient Removal of Methylene Blue and Malachite Green Dyes. J. Mol. Liq. 2022, 368, 120676. [Google Scholar] [CrossRef]
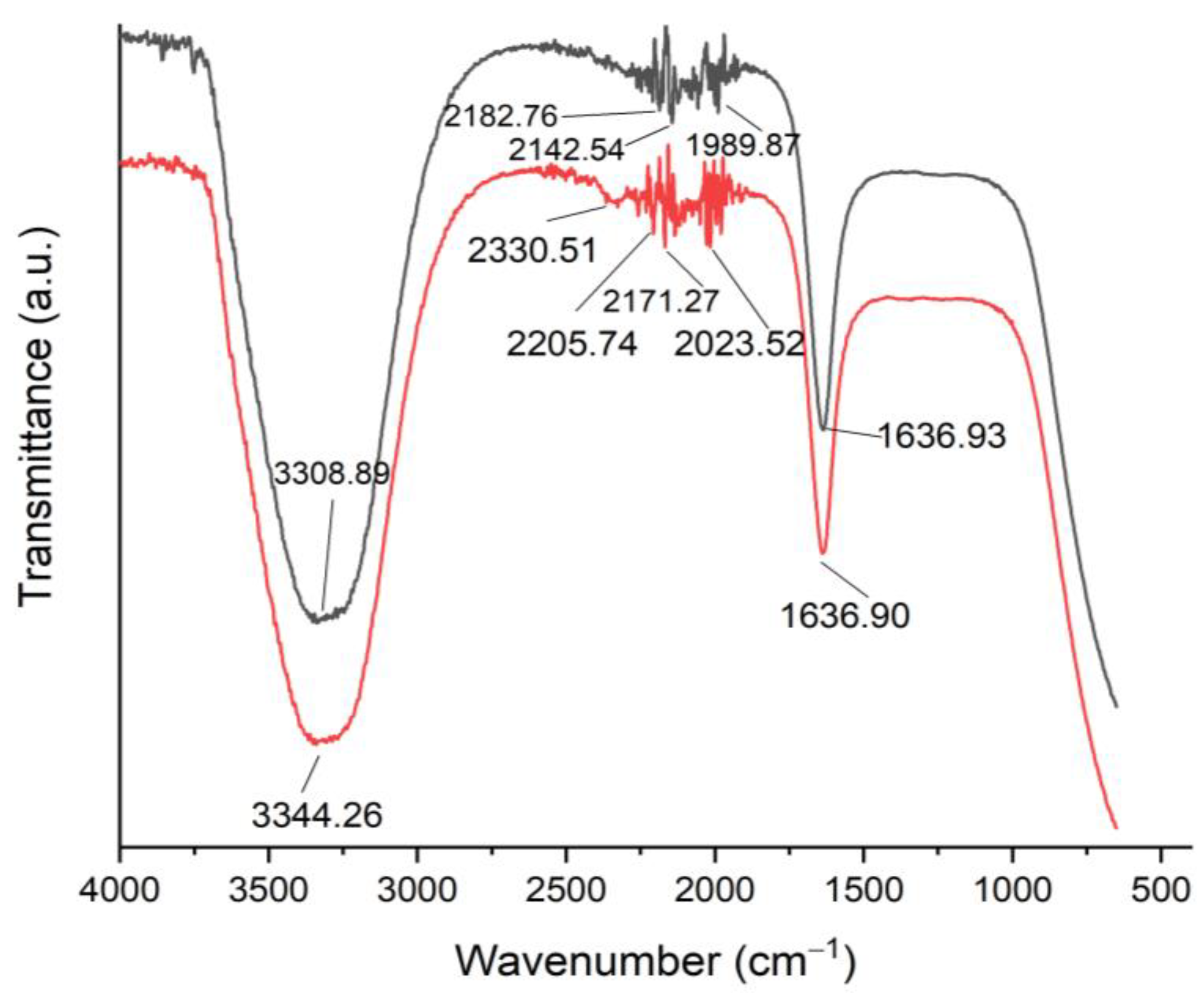


| Functional Groups of C. sinensis Extract | ||||
|---|---|---|---|---|
| No. | Absorption Peak (cm−1) | Appearance | Functional Groups | Molecular Motion |
| 1 | 3308.89 | Strong, broad | Alcohols and phenols | O-H stretching |
| 2 | 2182.76 | weak | Alkynes | C≡Cstretching |
| 3 | 2142.54 | weak | Thiocyanate | S-C≡N stretching |
| 4 | 1989.87 | weak | Aromatic compound | C-H bending |
| 5 | 1636.93 | Medium | Alkenes | C=C stretching |
| Functional groups of biogenic IONPs | ||||
| 1 | 3344.26 | Strong, broad | Alcohols and phenols | O-H stretching |
| 2 | 2330.51 | Weak | Carbon dioxide | O=C=O stretching |
| 3 | 2205.74 | Weak | Alkynes | C≡Cstretching |
| 4 | 2171.27 | Weak | Thiocyanate | S-C≡N stretching |
| 5 | 2023.52 | Weak | Isothiocyanate | N=C=S stretching |
| 6 | 1636.90 | Medium | Alkenes | C=C stretching |
| Parameter | Value |
|---|---|
| Specific surface area (m2/g) BET | 48.3 |
| pore size (nm) BJH | 1.74 |
| pore volume (cm3/g) BJH | 0.022 |
| Reaction Time (min.) | Decolorization Percentages (%) | |
|---|---|---|
| Sunlight | Dark | |
| 30 | 36.17 | 16.24 |
| 60 | 58.54 | 27.63 |
| 90 | 74.12 | 38.54 |
| 120 | 87.76 | 44.17 |
| 150 | 96.32 | 57.32 |
| 180 | 97.63 | 61.63 |
| 210 | 99.23 | 64.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, M.T.; Al-Otibi, F.O.; Al-Askar, A.A. Photocatalytic Removal of Crystal Violet Dye Utilizing Greenly Synthesized Iron Oxide Nanoparticles. Separations 2023, 10, 513. https://doi.org/10.3390/separations10090513
Yassin MT, Al-Otibi FO, Al-Askar AA. Photocatalytic Removal of Crystal Violet Dye Utilizing Greenly Synthesized Iron Oxide Nanoparticles. Separations. 2023; 10(9):513. https://doi.org/10.3390/separations10090513
Chicago/Turabian StyleYassin, Mohamed Taha, Fatimah O. Al-Otibi, and Abdulaziz A. Al-Askar. 2023. "Photocatalytic Removal of Crystal Violet Dye Utilizing Greenly Synthesized Iron Oxide Nanoparticles" Separations 10, no. 9: 513. https://doi.org/10.3390/separations10090513
APA StyleYassin, M. T., Al-Otibi, F. O., & Al-Askar, A. A. (2023). Photocatalytic Removal of Crystal Violet Dye Utilizing Greenly Synthesized Iron Oxide Nanoparticles. Separations, 10(9), 513. https://doi.org/10.3390/separations10090513





