Impact of Cumin and Green Tea on Amlodipine Pharmacodynamics and Pharmacokinetics in Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monitoring Rat Blood Pressure and Pharmacokinetic Study
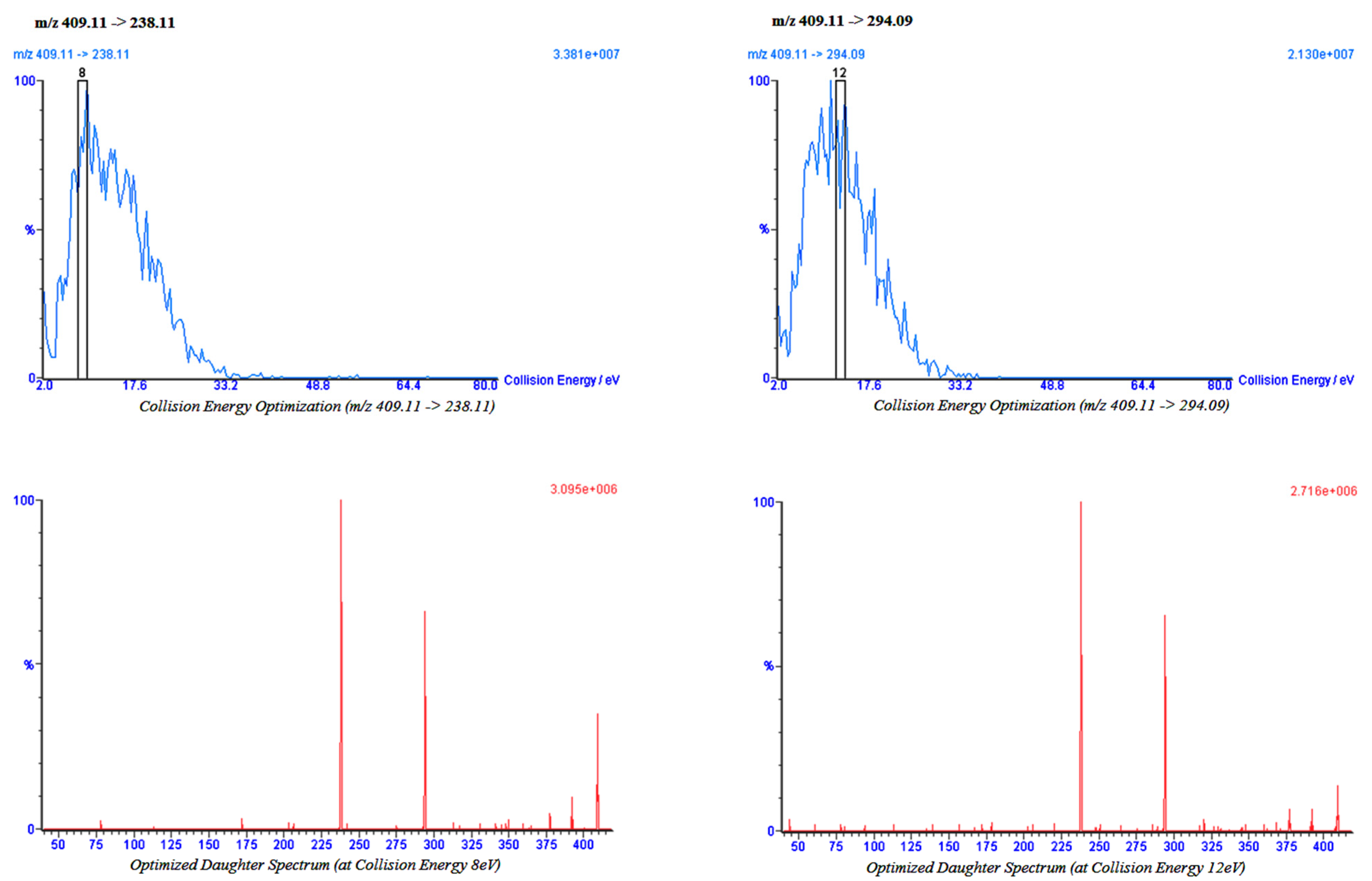
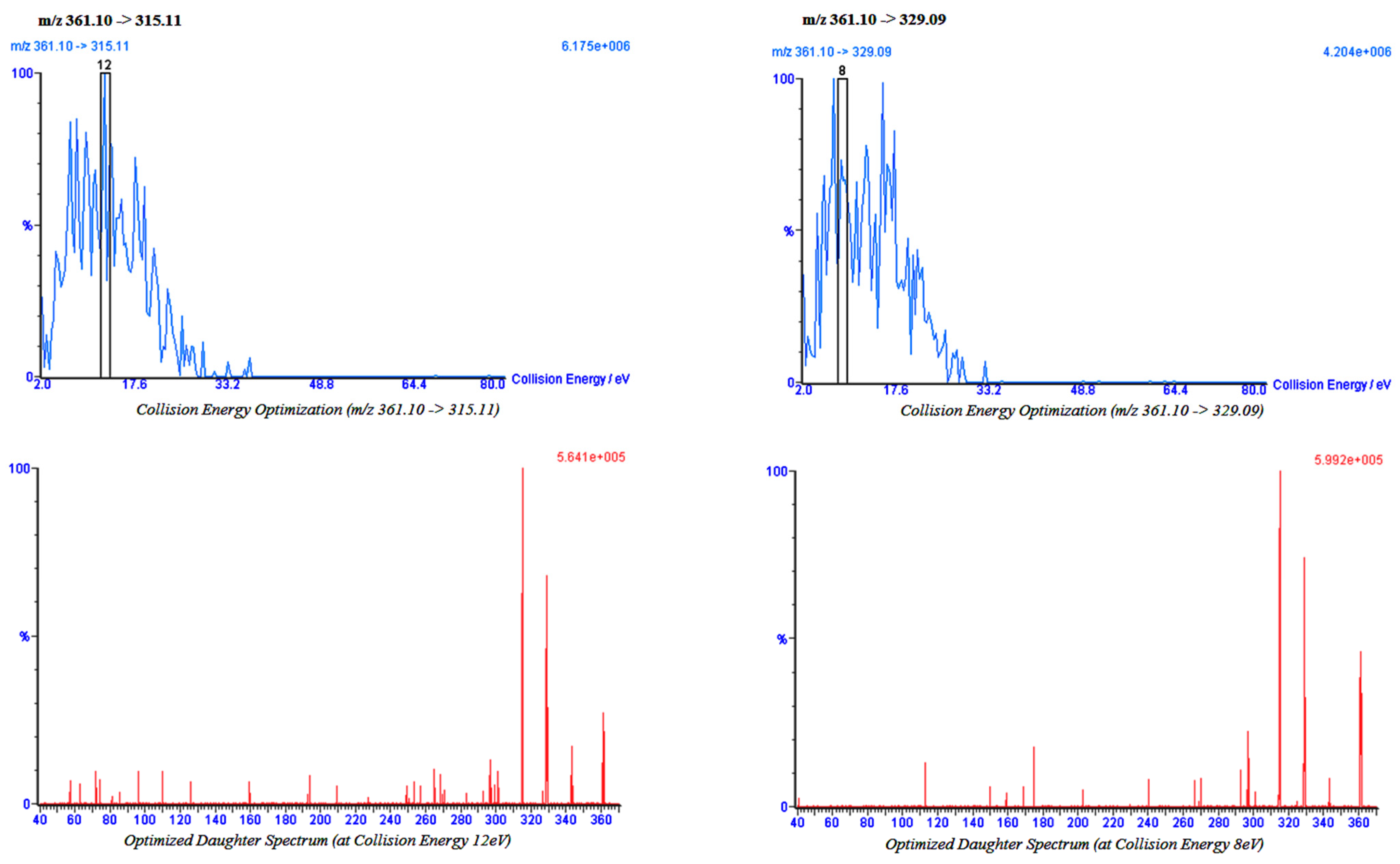
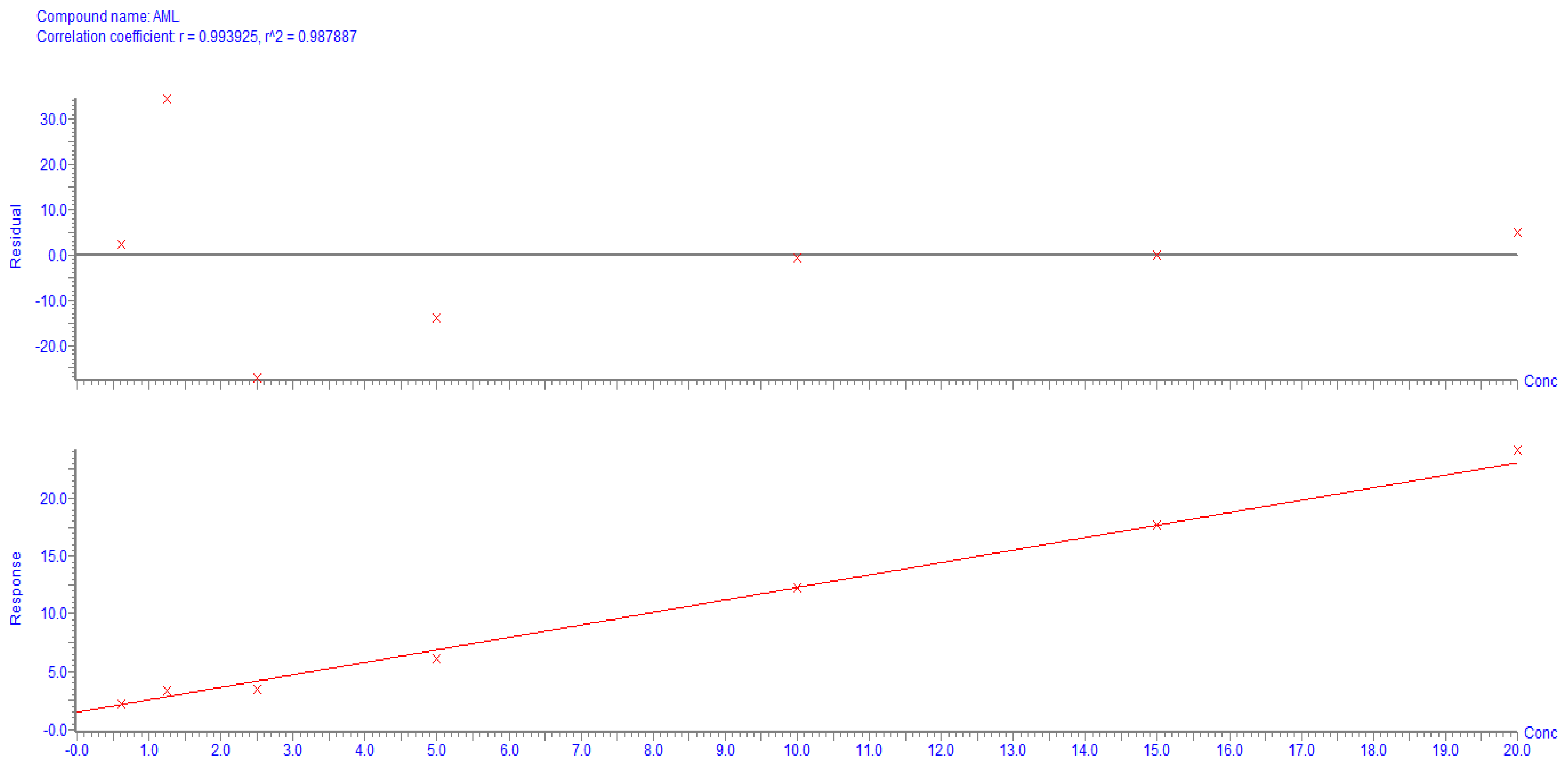
2.2. Bio-Analytical Method
3. Statistical Analysis
4. Results
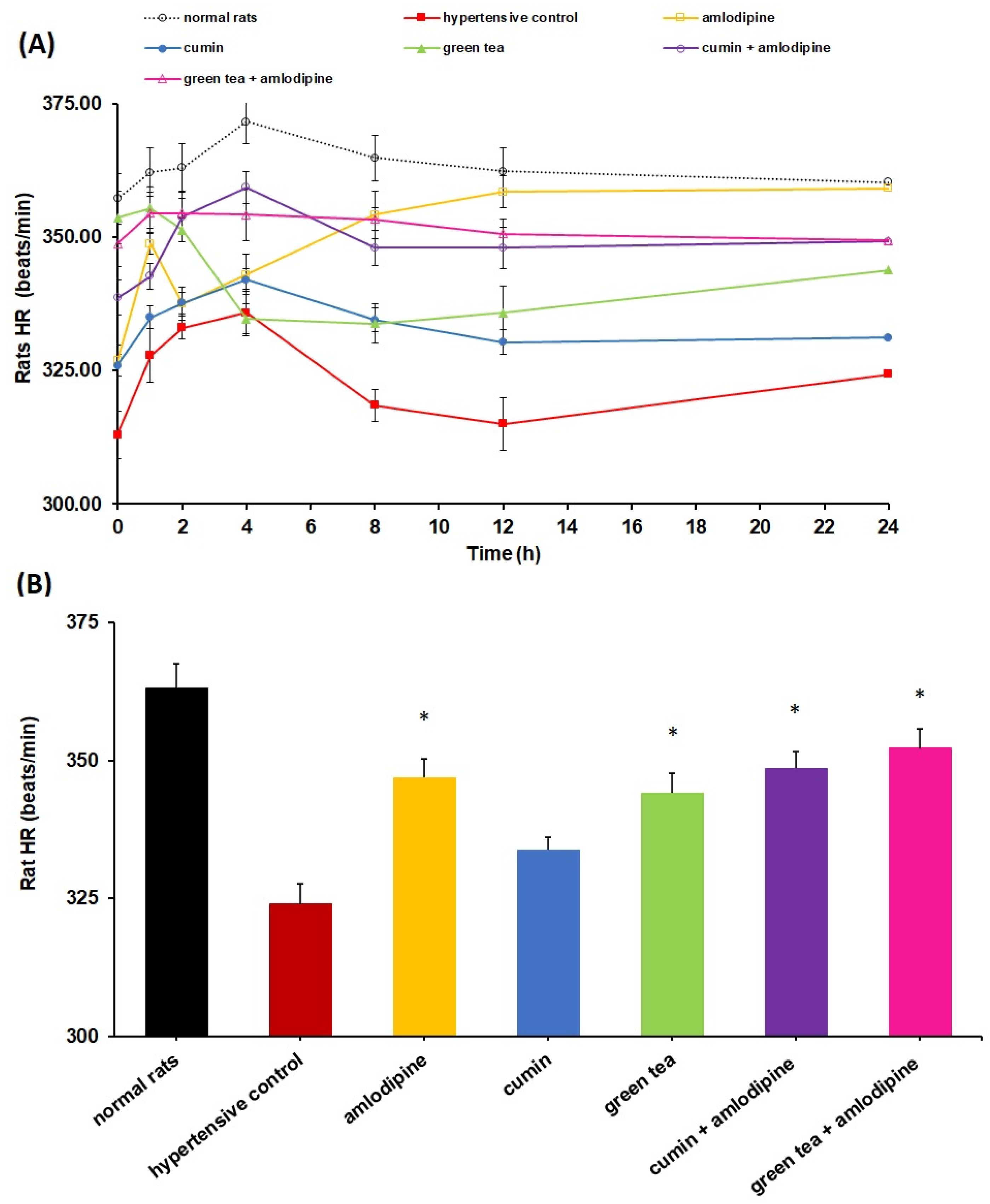
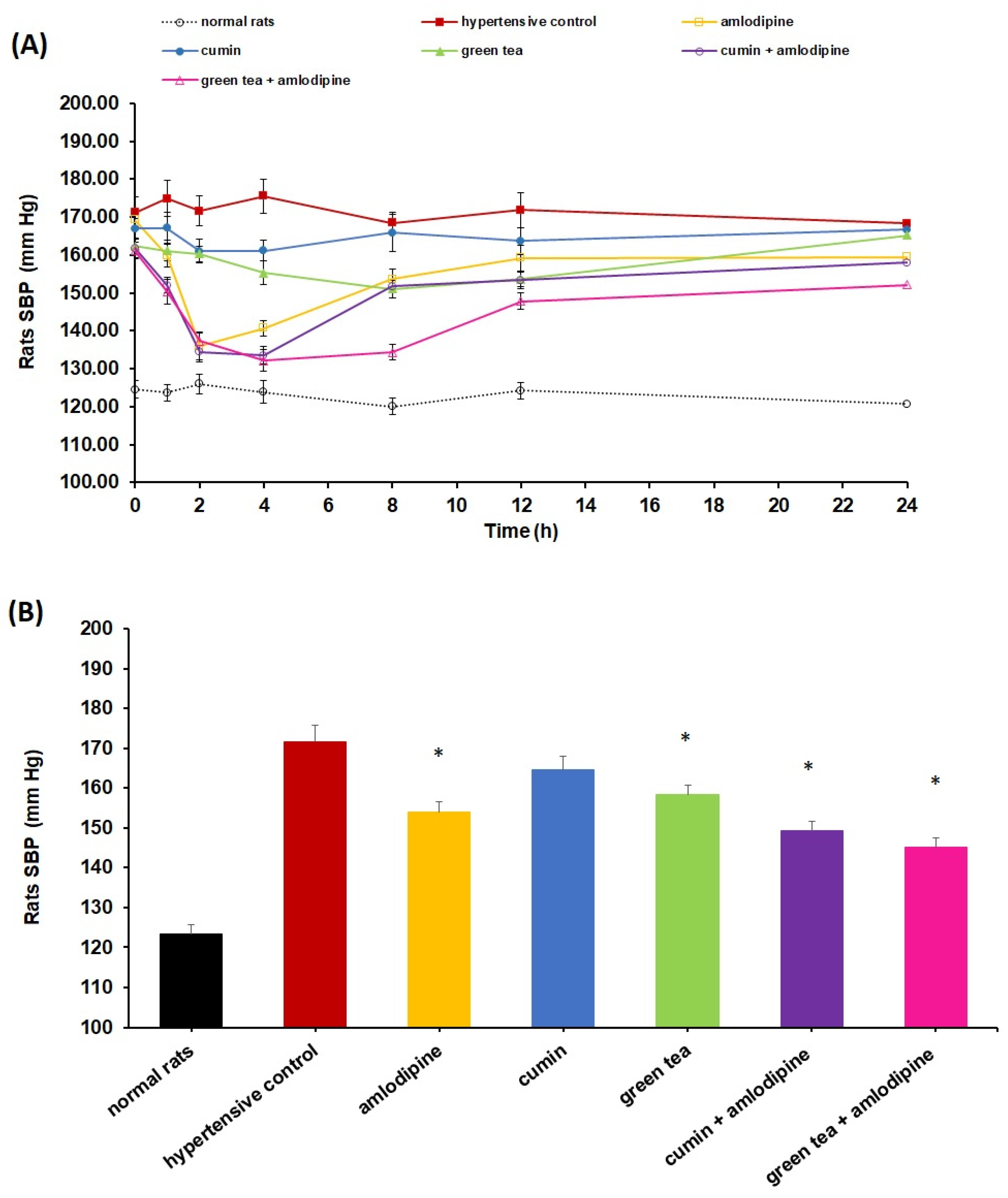
4.1. Impact of Cumin and Green Tea on Amlodipine Pharmacodynamics
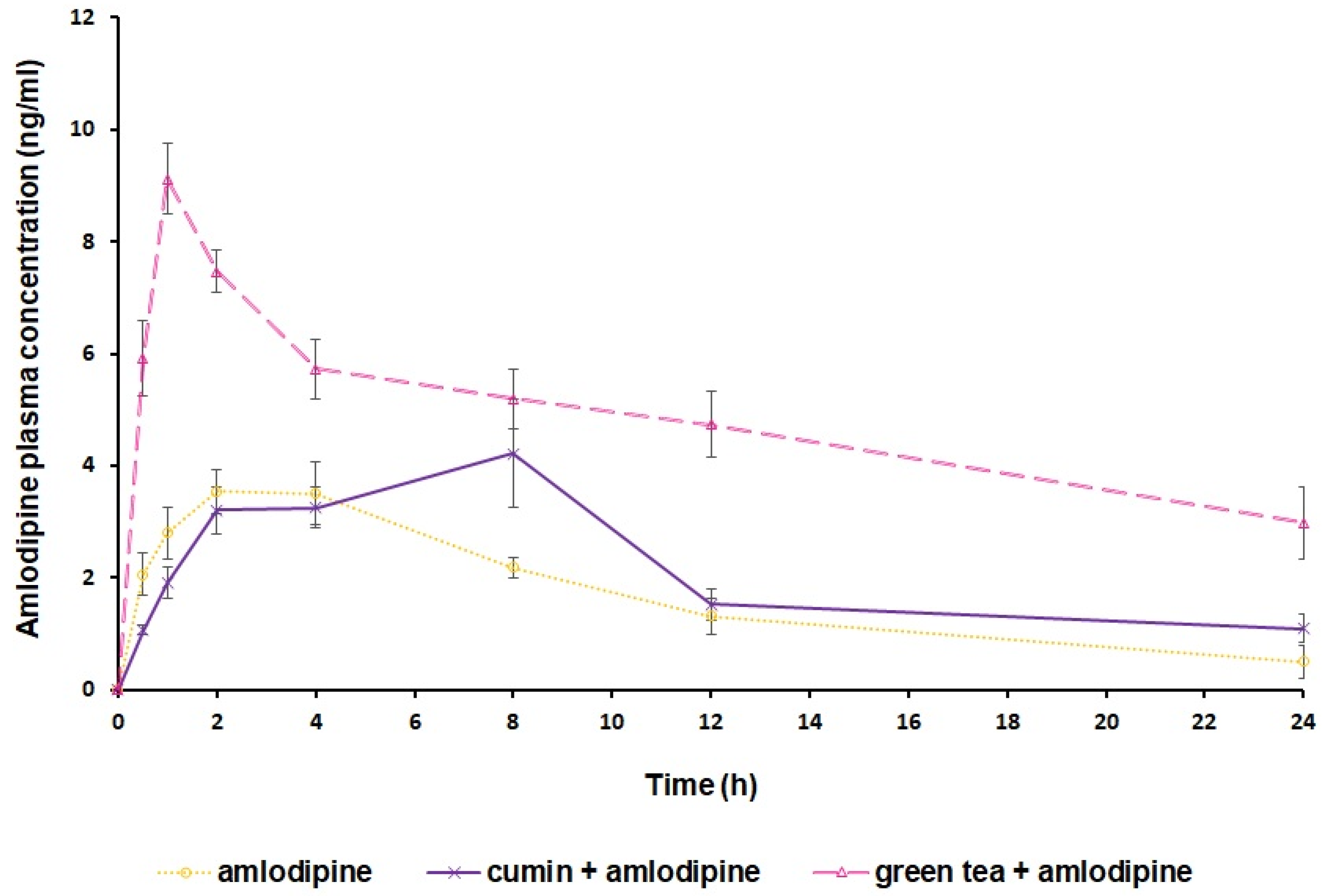
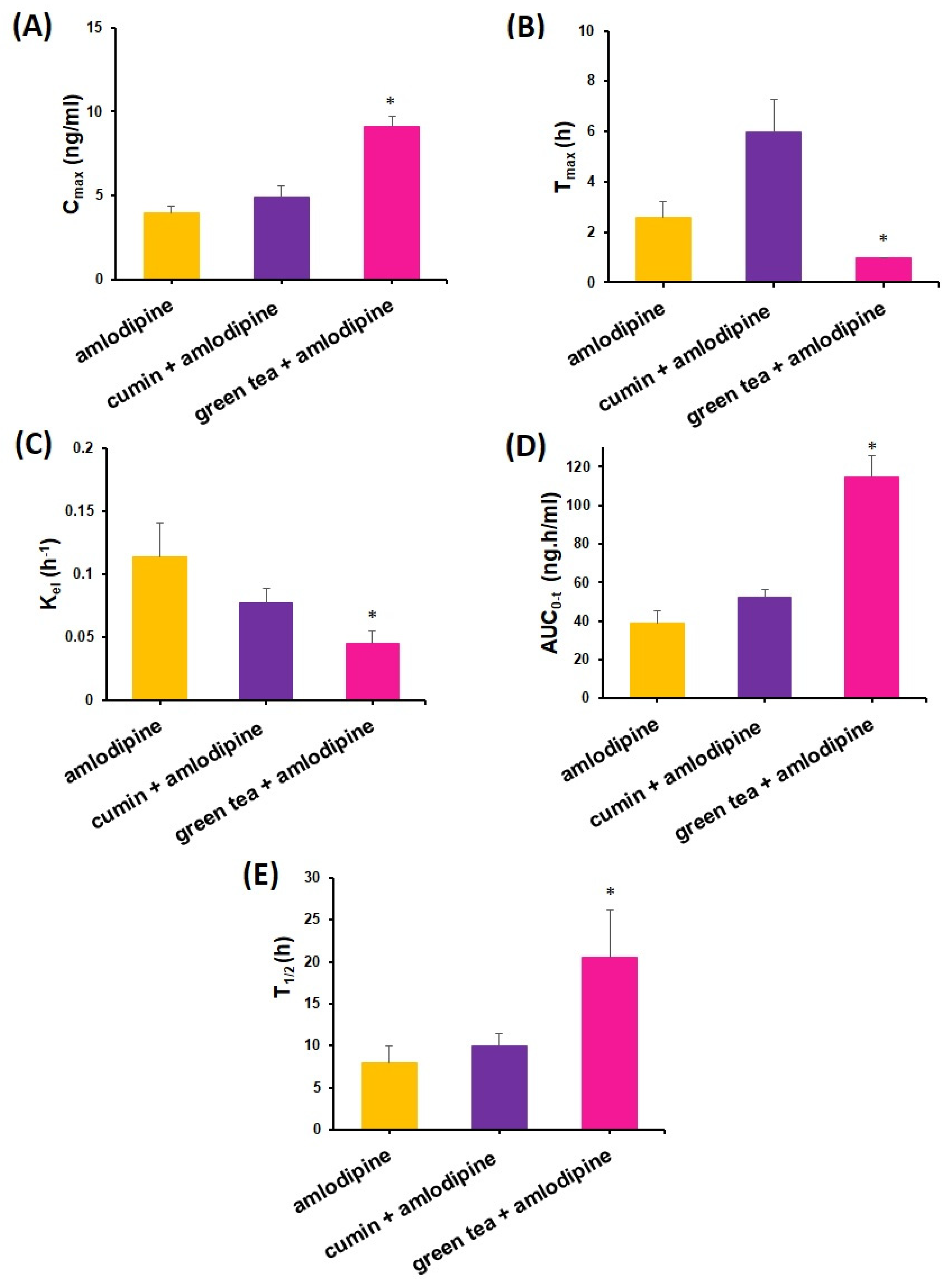
4.2. Impact of Cumin and Green Tea on Amlodipine Pharmacokinetics
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Abu Zar, M.; Kamal, A.; Faquih, A.E.; Bhan, C.; Iftikhar, W.; Malik, M.B.; Ahmad, M.Q.; Ali, N.S.; Sami, S.A.; et al. American Heart Association High Blood Pressure Protocol 2017: A Literature Review. Cureus 2018, 10, e3230. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Potential pharmacodynamic and pharmacokinetic interactions of Nigella Sativa and Trigonella Foenum-graecum with losartan in L-NAME induced hypertensive rats. Saudi J. Biol. Sci. 2020, 27, 2544–2550. [Google Scholar] [CrossRef]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Al-Makki, A.; DiPette, D.; Whelton, P.K.; Murad, M.H.; Mustafa, R.A.; Acharya, S.; Beheiry, H.M.; Champagne, B.; Connell, K.; Cooney, M.T.; et al. Hypertension Pharmacological Treatment in Adults: A World Health Organization Guideline Executive Summary. Hypertension 2022, 79, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Al-Hanawi, M.K.; Keetile, M. Socio-Economic and Demographic Correlates of Non-communicable Disease Risk Factors Among Adults in Saudi Arabia. Front. Med. 2021, 8, 605912. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Karam, S. Hypertension in the Middle East: Current state, human factors, and barriers to control. J. Hum. Hypertens. 2022, 36, 428–436. [Google Scholar] [CrossRef] [PubMed]
- El Bcheraoui, C.; Memish, Z.A.; Tuffaha, M.; Daoud, F.; Robinson, M.; Jaber, S.; Mikhitarian, S.; Al Saeedi, M.; Almazroa, M.A.; Mokdad, A.H.; et al. Hypertension and Its Associated Risk Factors in the Kingdom of Saudi Arabia, 2013: A National Survey. Int. J. Hypertens. 2014, 2014, 564679. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Bukvicki, D.; Gottardi, D.; Prasad, S.; Novaković, M.; Marin, P.; Tyagi, A. The Healing Effects of Spices in Chronic Diseases. Curr. Med. Chem. 2020, 27, 4401–4420. [Google Scholar] [CrossRef] [PubMed]
- El-Dahiyat, F.; Rashrash, M.; Abuhamdah, S.; Abu Farha, R.; Babar, Z.-U.-D. Herbal medicines: A cross-sectional study to evaluate the prevalence and predictors of use among Jordanian adults. J. Pharm. Policy Pract. 2020, 13, 2. [Google Scholar] [CrossRef]
- Alkhamaiseh, S.I.; Aljofan, M. Prevalence of use and reported side effects of herbal medicine among adults in Saudi Arabia. Complement. Ther. Med. 2020, 48, 102255. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Mohammed, A.A.; Alfahaid, F.; Albshabshe, A. Herbal medicine use by Saudi patients with chronic diseases: A cross-sectional study (experience from Southern Region of Saudi Arabia). J. Health Spec. 2018, 6, 77. [Google Scholar] [CrossRef]
- Brantley, S.J.; Argikar, A.A.; Lin, Y.S.; Nagar, S.; Paine, M.F. Herb-drug interactions: Challenges and opportunities for improved predictions. Drug Metab. Dispos. 2014, 42, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Effect of Hibiscus sabdariffa and Zingiber officinale on the antihypertensive activity and pharmacokinetic of losartan in hypertensive rats. Xenobiotica 2020, 50, 847–857. [Google Scholar] [CrossRef]
- Abernethy, D.R. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology 1992, 80 (Suppl. S1), 31–36. [Google Scholar] [CrossRef]
- Hocht, C.; Bertera, F.M.; Santander Plantamura, Y.; Parola, L.; Del Mauro, J.S.; Polizio, A.H. Factors influencing hepatic metabolism of antihypertensive drugs: Impact on clinical response. Expert Opin. Drug Metab. Toxicol. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Mukherjee, D.; Zha, J.; Menon, R.M.; Shebley, M. Guiding dose adjustment of amlodipine after co-administration with ritonavir containing regimens using a physiologically-based pharmacokinetic/pharmacodynamic model. J. Pharmacokinet. Pharmacodyn. 2018, 45, 443–456. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: Traditional uses, chemical constituents, and nutraceutical effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Kalaivani, P.; Saranya, R.B.; Ramakrishnan, G.; Ranju, V.; Sathiya, S.; Gayathri, V.; Thiyagarajan, L.K.; Venkhatesh, J.R.; Babu, C.S.; Thanikachalam, S. Cuminum cyminum, a dietary spice, attenuates hypertension via endothelial nitric oxide synthase and NO pathway in renovascular hypertensive rats. Clin. Exp. Hypertens. 2013, 35, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Samani, K.G.; Farrokhi, E. Effects of cumin extract on oxLDL, paraoxanase 1 activity, FBS, total cholesterol, triglycerides, HDL-C, LDL-C, Apo A1, and Apo B in in the patients with hypercholesterolemia. Int. J. Health Sci. (Qassim) 2014, 8, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, C.; Tang, W.; Zhang, X.; Jiang, X.; Duoji, Z.; Kangzhu, Y.; Zhao, X.; Xu, X.; Hong, F.; et al. The association between tea consumption and blood pressure in the adult population in Southwest China. BMC Public Health 2023, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Yildirim Ayaz, E.; Dincer, B.; Mesci, B. Effect of Green Tea on Blood Pressure in Healthy Individuals: A Meta-Analysis. Altern. Ther. Health Med. 2023, 29, 66–73. [Google Scholar] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Hao, H.; Li, H.; Guo, X.; Zhang, D. Effect Of epigallocatechin-3-gallate on the pharmacokinetics of amlodipine in rats. Xenobiotica 2019, 49, 970–974. [Google Scholar] [CrossRef]
- Oga, E.F.; Sekine, S.; Shitara, Y.; Horie, T. Pharmacokinetic Herb-Drug Interactions: Insight into Mechanisms and Consequences. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 93–108. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Cordova, C.A.; Chew, W.M.; Xu, M.J.; Hsu, C.H.; Ranger-Moore, J.; Alberts, D.S. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2473–2476. [Google Scholar] [CrossRef]
- Meyboodi, M.; Mohammadpour, A.; Emami, S.A.; Karbasforooshan, H. Drug Interactions of Green Tea. J. Pharm. Care 2021, 8, 196–203. [Google Scholar] [CrossRef]
- Misaka, S.; Miyazaki, N.; Fukushima, T.; Yamada, S.; Kimura, J. Effects of green tea extract and (-)-epigallocatechin-3-gallate on pharmacokinetics of nadolol in rats. Phytomedicine 2013, 20, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Choi, D.K. Cuminum cyminum extract attenuates scopolamine-induced memory loss and stress-induced urinary biochemical changes in rats: A noninvasive biochemical approach. Pharm. Biol. 2011, 49, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Al-Saaedi, A.M.; Khudhair, I.H. Antipyretic activity of the aqueous extract of cumin (Cuminum cyminum L.) with yeast induced pyrexia in female rats. Univ. Thi-Qar J. Sci. 2021, 8, 33–35. [Google Scholar]
- Haidari, F.; Omidian, K.; Rafiei, H.; Zarei, M.; Mohamad Shahi, M. Green Tea (Camellia sinensis) Supplementation to Diabetic Rats Improves Serum and Hepatic Oxidative Stress Markers. Iran. J. Pharm. Res. 2013, 12, 109–114. [Google Scholar]
- Yenzeel Al-Hilfy, J.H. Effect of green tea aqueous extract on body weight, glucose level, and kidney functions in diabetic male albino rats. J. Al-Nahrain Univ. Sci. 2012, 15, 116–166. [Google Scholar]
- Abd El-Baky, A. Clinicopathological Effect of Camellia sinensis Extract on Streptozotocin-Induced Diabetes in Rats. World J. Med. Sci. 2013, 8, 205–211. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, M.; Meng, X.; Sun, B. Effects of curcumin on the pharmacokinetics of amlodipine in rats and its potential mechanism. Pharm. Biol. 2020, 58, 465–468. [Google Scholar] [CrossRef]
- Abdelrahman, I.A.; Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Jenoobi, F.I. Cinnamon modulates the pharmacodynamic & pharmacokinetic of amlodipine in hypertensive rats. Saudi Pharm. J. 2023, 31, 101737. [Google Scholar] [CrossRef]
- Alam, M.A.; Bin Jardan, Y.A.; Raish, M.; Al-Mohizea, A.M.; Ahad, A.; Al-Jenoobi, F.I. Effect of Nigella sativa and Fenugreek on the Pharmacokinetics and Pharmacodynamics of Amlodipine in Hypertensive Rats. Curr. Drug Metab. 2020, 21, 318–325. [Google Scholar] [CrossRef]
- Alam, M.A.; Bin Jardan, Y.A.; Raish, M.; Al-Mohizea, A.M.; Ahad, A.; Al-Jenoobi, F.I. Herb-drug interaction: Pharmacokinetics and pharmacodynamics of anti-hypertensive drug amlodipine besylate in presence of Lepidium sativum and Curcuma longa. Xenobiotica 2022, 52, 177–185. [Google Scholar] [CrossRef]
- Alam, M.A.; Bin Jardan, Y.A.; Alzenaidy, B.; Raish, M.; Al-Mohizea, A.M.; Ahad, A.; Al-Jenoobi, F.I. Effect of Hibiscus sabdariffa and Zingiber officinale on pharmacokinetics and pharmacodynamics of amlodipine. J. Pharm. Pharmacol. 2021, 73, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Han, P.; Yang, C.B.; Gu, X.W.; Zhang, W.; Xu, L.P.; Fu, S.T.; Su, D.F.; Xie, H.H. Synergism of irbesartan and amlodipine on hemodynamic amelioration and organ protection in spontaneously hypertensive rats. Acta Pharmacol. Sin. 2011, 32, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Geng, G.; Sen, S.; Li, P.; Li, X. Effects of berberine on pharmacokinetics of amlodipine in rats and its potential mechanism. Lat. Am. J. Pharm. 2018, 37, 1354–1360. [Google Scholar]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; Huang, J.; Cai, Q.; Yang, C.S.; Wan, X.; Xie, Z. Effects and Mechanisms of Tea Regulating Blood Pressure: Evidences and Promises. Nutrients 2019, 11, 1115. [Google Scholar] [CrossRef]
- Szulińska, M.; Stępień, M.; Kręgielska-Narożna, M.; Suliburska, J.; Skrypnik, D.; Bąk-Sosnowska, M.; Kujawska-Łuczak, M.; Grzymisławska, M.; Bogdański, P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl-induced hypertensive rat model. Food Nutr. Res. 2017, 61, 1295525. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, I.A.; Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Jenoobi, F.I. Impact of Cumin and Green Tea on Amlodipine Pharmacodynamics and Pharmacokinetics in Hypertensive Rats. Separations 2023, 10, 514. https://doi.org/10.3390/separations10090514
Abdelrahman IA, Ahad A, Raish M, Bin Jardan YA, Alam MA, Al-Jenoobi FI. Impact of Cumin and Green Tea on Amlodipine Pharmacodynamics and Pharmacokinetics in Hypertensive Rats. Separations. 2023; 10(9):514. https://doi.org/10.3390/separations10090514
Chicago/Turabian StyleAbdelrahman, Ibrahim Abdelsalam, Abdul Ahad, Mohammad Raish, Yousef A. Bin Jardan, Mohd Aftab Alam, and Fahad I. Al-Jenoobi. 2023. "Impact of Cumin and Green Tea on Amlodipine Pharmacodynamics and Pharmacokinetics in Hypertensive Rats" Separations 10, no. 9: 514. https://doi.org/10.3390/separations10090514
APA StyleAbdelrahman, I. A., Ahad, A., Raish, M., Bin Jardan, Y. A., Alam, M. A., & Al-Jenoobi, F. I. (2023). Impact of Cumin and Green Tea on Amlodipine Pharmacodynamics and Pharmacokinetics in Hypertensive Rats. Separations, 10(9), 514. https://doi.org/10.3390/separations10090514






