Abstract
Lead is one of the most toxic heavy metals released into the environment through industrial sources. Its direct determination is often a problem due to the presence of relatively complex matrices as well as low content. Thus, the additional separation and preconcentration steps are necessary in the analytical procedures. Carbon nanotubes (CNTs) continue to attract significant interest for these purposes as they exhibit a high specific surface area, exceptional porosities, and numerous adsorption sites. The modified CNTs with active groups, reagents, or materials have been widely explored using more mutual interactions that can significantly improve their sorption capacity and selectivity. This paper summarizes the recent developments from 2017 in the application of carbon nanotubes for the separation of Pb(II) and its enrichment/removal from the matrix components. Attention is given to oxidized CNTs, their modification with complexing compounds, functionalization with metal oxides and polymers, new nanocomposites, and carbon nanotube membranes.
1. Introduction
Carbon nanotubes (CNTs), ever since their discovery by Iijma, have gained considerable sustained attention from the scientific community [1,2,3,4]. Their structure consists of a tubular sheet of graphene with a honeycomb structure of carbon atoms. They can be divided into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) according to the number of graphene sheets. The properties and the type of CNT structure are significantly influenced by the synthesis techniques and subsequent parameters. Their high surface-to-volume ratio, geometry, and hollow structure make them widely applicable as sorbents, filters, or membranes for separation and enrichment purposes. MWCNTs provide a greater surface in comparison to SWCNTs due to the presence of concentric graphene sheets, resulting in enhanced interaction with the analytes. CNTs strongly adsorb several pollutants through various interactions, such as π-π and electrostatic interactions, hydrophobic effect, and covalent bonding [5,6]. Many studies have been also carried out on their applications in device modelling, coating materials, thin-film electronics, actuators and capacitors, sensors and biosensors, and energy storage [7,8,9,10,11,12]. CNTs have also found several applications in biomedical science for medical diagnosis and drug delivery [13,14]. Their antibacterial and antifungal properties have been used to prevent bacterial adhesion on medical devices [15].
There are many methods for the synthesis of CNTs, but carbon arc discharge, laser ablation, and chemical vapor deposition (CVD) techniques are commonly applied [7,16,17,18]. The arc discharge method provides the growth of CNTs with minimum structural defects in comparison with other techniques, but needs the use of a high temperature (>1700 °C), low pressure, and expensive gases. The laser ablation method has the advantage of producing a high yield of CNTs of good quality and purity in a short time; however, it can be performed only in a vacuum with controlled inert gases, which results in high costs. The main advantages of different CVD techniques include the ease of controlling the reaction course, a high yield, small amount of impurities, and relatively low operating temperature (<800 °C). Taguchi orthogonal array technique was applied to investigate the interdependent effect of various parameters during the synthesis of CNTs using a catalytic CVD method, such as temperature, acetylene, and argon flow rates as well as time on the diameter of the final product [19]. The obtained smaller nanotubes with a diameter range of 5–15 nm revealed a larger BET surface area of 1306 ± 5 m2/g and higher maximum sorption capacity at pH 5 for Pb(II), equal to 215.38 ± 0.03 mg/g (at 50 °C, 60 min sorption time), in comparison to MWCNTs with a bigger diameter range of 16–25 nm (qmax = 201.35 ± 0.02 mg/g and surface area of 1245 ± 4 m2/g). The microwave irradiation method with low cost and high efficiency has been also employed [20,21,22]. It operates at low temperatures and is easy to control. The synthesis methods are quickly improved year by year to produce a large amount of size-controlled CNTs for different applications. Purification of CNTs from amorphous carbon and catalyst particles is required after synthesis. It is usually achieved using hydrochloric acid treatment and sonication of nanotubes in different media [20].
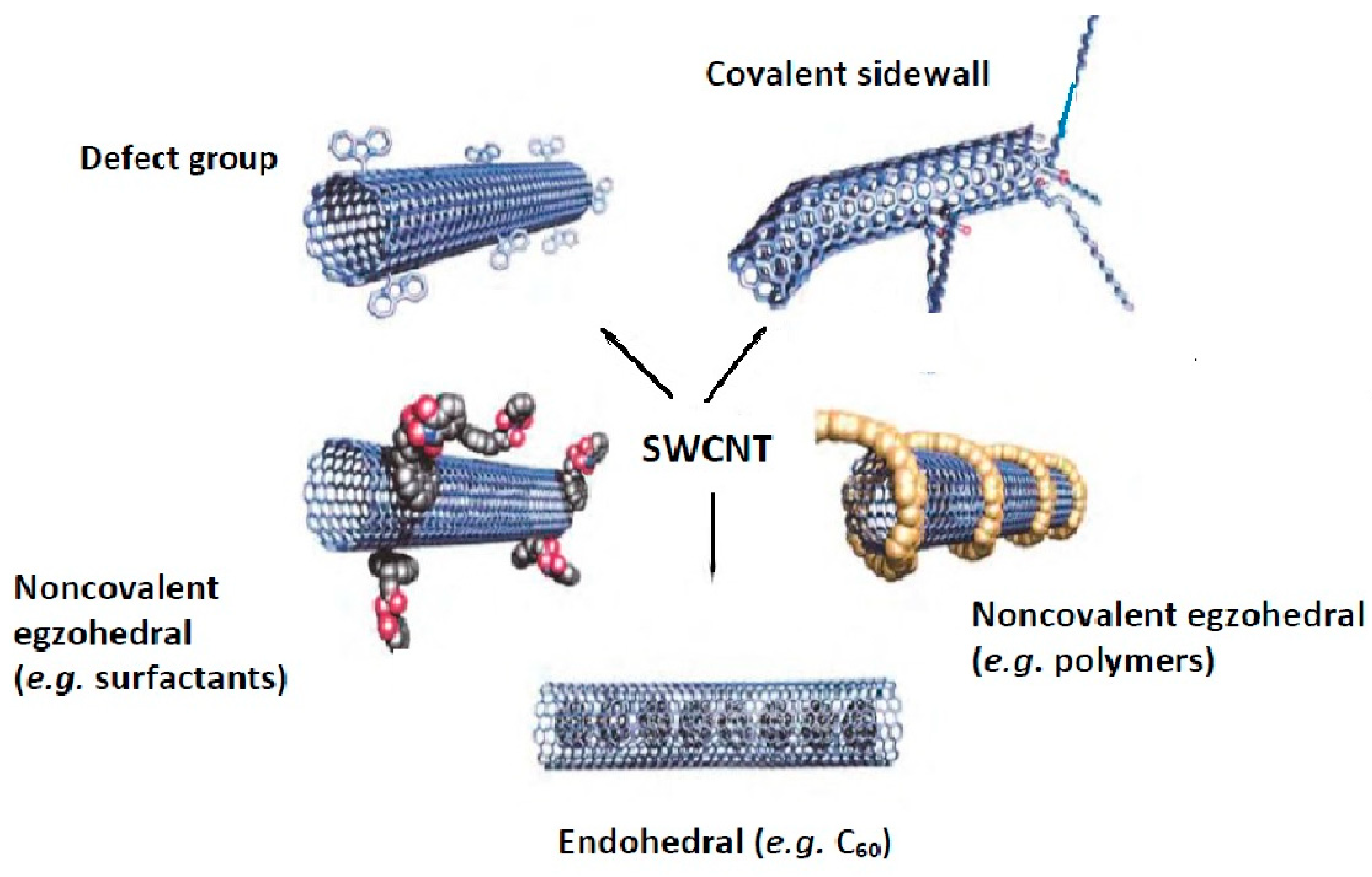
Similar to graphene-based nanomaterials, pristine CNTs can be modified by covalent, non-covalent, defect-group and endohedral functionalization using small molecules as well as doping with heteroatoms [23,24,25,26,27]. The functionalization of pristine carbon nanotubes (for the example of SWCTs) is schematically depicted in Figure 1 [23]. The functional groups increase their hydrophilicity and may also act as a stabilizer against the aggregation of individual nanotubes, caused by the strong van der Waals interactions. Moreover, the functionalization with magnetic nanoparticles facilitates their separation from the solution, together with adsorbed analytes, by applying an external magnetic field without filtration or centrifugation [2,28]. Thus, the modified CNTs with active groups, reagents, or materials are widely explored using more mutual interactions that can significantly improve their sorption performance.
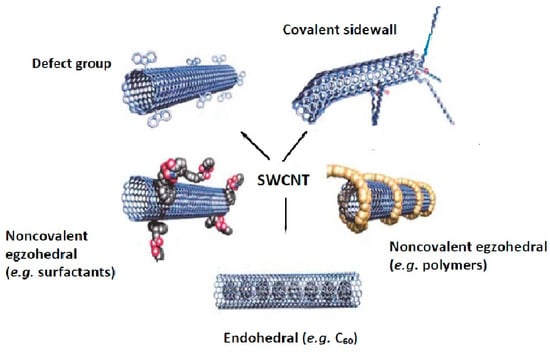
Figure 1.
The methods for functionalization of carbon nanotubes. Reprinted with permission from Ref. [23]. 2002 John Wiley and Son.
The application of carbon nanotubes is very helpful for preconcentration, separation, and speciation analysis of metal ions as well as for their removal from wastewaters [28,29]. The analysis of environmental samples is often difficult due to intereferences from the complex matrices and low content of the analytes. Thus, a separation/preconcentration procedure is very often necessary to increase the sensitivity and selectivity of the applied analytical methods. Solid-phase extraction (SPE) and its alternatives are commonly used for this purpose owing to their rapid phase separation, high preconcentration factor, simple procedure, and the ability to combine them with different detection techniques [30,31,32].
This review summarizes achievements made over the last five years in the use of carbon nanotubes in solid-phase extraction for preconcentration and separation of lead ions. Lead is one of the most toxic elements for humans, animals, and plants. Even its low dose can cause serious health problems for the kidney, brain, liver, and bones, as well as for the haematological and nervous systems [33,34]. The World Health Organization (WHO) has set lead a maximum guideline value of 0.010 mg/L and the European Commission (EC) has proposed lead limits of 0.005 mg/L [35]. Considering these low permissible levels, the development of novel sorption materials used in analytical procedures is of great importance for the sensitive and accurate determination of lead. This review highlights the CNTs nanocomposites that are promising sorbents for Pb(II) uptake. The optimal experimental conditions, calculated sorption capacity, and kinetic evaluation are presented.
2. Variations of SPE with CNTs
In the most proposed applications of CNTs for separation and preconcentration of Pb(II), conventional homemade packed columns or cartridges were used, where the sample loading step is performed by gravity flow or is pressure/vacuum-assisted. Dispersive solid phase extraction (DSPE) has been also evaluated as an alternative approach [36,37]. The sorbent is dispersed in a sample solution interacting directly with the target analytes. After the dispersion process is completed, the sorbent together with the retained analytes is separated by a mechanical process, such as centrifugation or filtration. Ultrasonic treatment was also used for the dispersion of sorbent, as it facilitates mass transfer [38,39]. The advantage of DSPE is the reduction in sample treatment time which allows more samples to be analyzed in a shorter period time. In magnetic dispersive solid phase extraction (MDSPE), the combination of CNTs with magnetic nanoparticles makes their separation much easier after the enrichment process by using an external magnet [19].
Recently, the miniaturization in dispersive microextraction using stir bar sorptive material with magnetic nanoparticles was presented [40]. The amount of sample is significantly reduced to a few microliters; therefore, it is very helpful for the analysis of low-availability samples, such as saline solutions.
Solid-phase microextraction (SPME) technique integrates a few operations in a simple step, such as sampling and analyte separation from sample matrices, its preconcentration, and sample collection [41]. The sample is exposed to fused silica fibre or stainless steel wires coated with a layer of carbon nanotubes that are directly exposed to the sample for a sufficiently long time. Several methods can be found in the literature to deposit CNTs in SPME fibres, such as physical attachment, chemical bonding, sol-gel method, electrochemical polymerization, or electrophoretic deposition [42,43].
In all SPE techniques, several factors influence the sorption process of metal ions on carbon nanotubes’ surfaces. The factors include pH, contact time, temperature, amount of CNTs, their surface charge, and the presence of other sample components. Sorption of Pb(II) is favoured when the pH of a sample solution is higher than a point of zero charge of the particle surface due to the electrostatic interaction. However, at a higher pH, precipitation of Pb(OH)2 may have significant participation in sorption. For this reason, the proposed pH for sorption of Pb(II) was mainly in the range of 5–7. The efficiency of sorption usually increases when the sorbent amount and extraction time are increasing, achieving equilibrium. The competitive effect from other matrix components may affect the sorption process of Pb(II), causing a decrease in its efficiency; thus, the high sorbent capacity as well as selectivity are favoured. In the separation/preconcentration procedures, a used eluent volume has also a significant effect on obtaining the highest analytical signal. Its low volume could not facilitate quantitative desorption, while a large volume dilutes the analyte and consequently the value of the enrichment factor (EF) is decreased. EF is one of the important parameters for evaluating the efficiency of a given SPE method.
3. CNTs for Separation and Enrichment of Pb(II)
3.1. Oxidized CNTs
Oxidative treatment of carbon nanotubes is a common way to introduce the oxygen functional groups, such as hydroxyl, carboxyl, and carbonyl, on the surface, and different oxidizing reagents, such as concentrated HNO3 and H2SO4, H2O2, and KMnO4 were used [44,45,46,47]. Thus, CNTs can adsorb heavy metal ions due to a partial negative charge on their surface. The amount of these groups depends on the used oxidant and increases in this order: HCl < NH4OH/H2O2 < H2SO4/H2O2 < refluxed HNO3 [46]. Double-oxidized multiwalled carbon nanotubes have also been proposed to increase the efficiency of heavy metals removal from wastewater [47]. First, MWCNTs are oxidized with concentrated HNO3 solution by refluxing at 120 °C for 4 h. In the second oxidation process, the mixture of HNO3 + H2SO4 (1:3, v/v) was used with sonication at 40–50 °C for 3 h. One of the main drawbacks of this procedure is the occurrence of nanotubes shortening by fragmentation and generation of defects in the graphitic network, particularly for oxidation with nitric acid. Bayazit and Inci reported that oxidation of SWCNTs with concentrated HNO3 under irradiation by UV-light increased their surface acidity more than ultrasonication [48]. The theoretical sorption capacity value of carbon nanotubes prepared by the UV-light method was 511.99 mg/g, while for ultrasonication it was 342.36 mg/g.
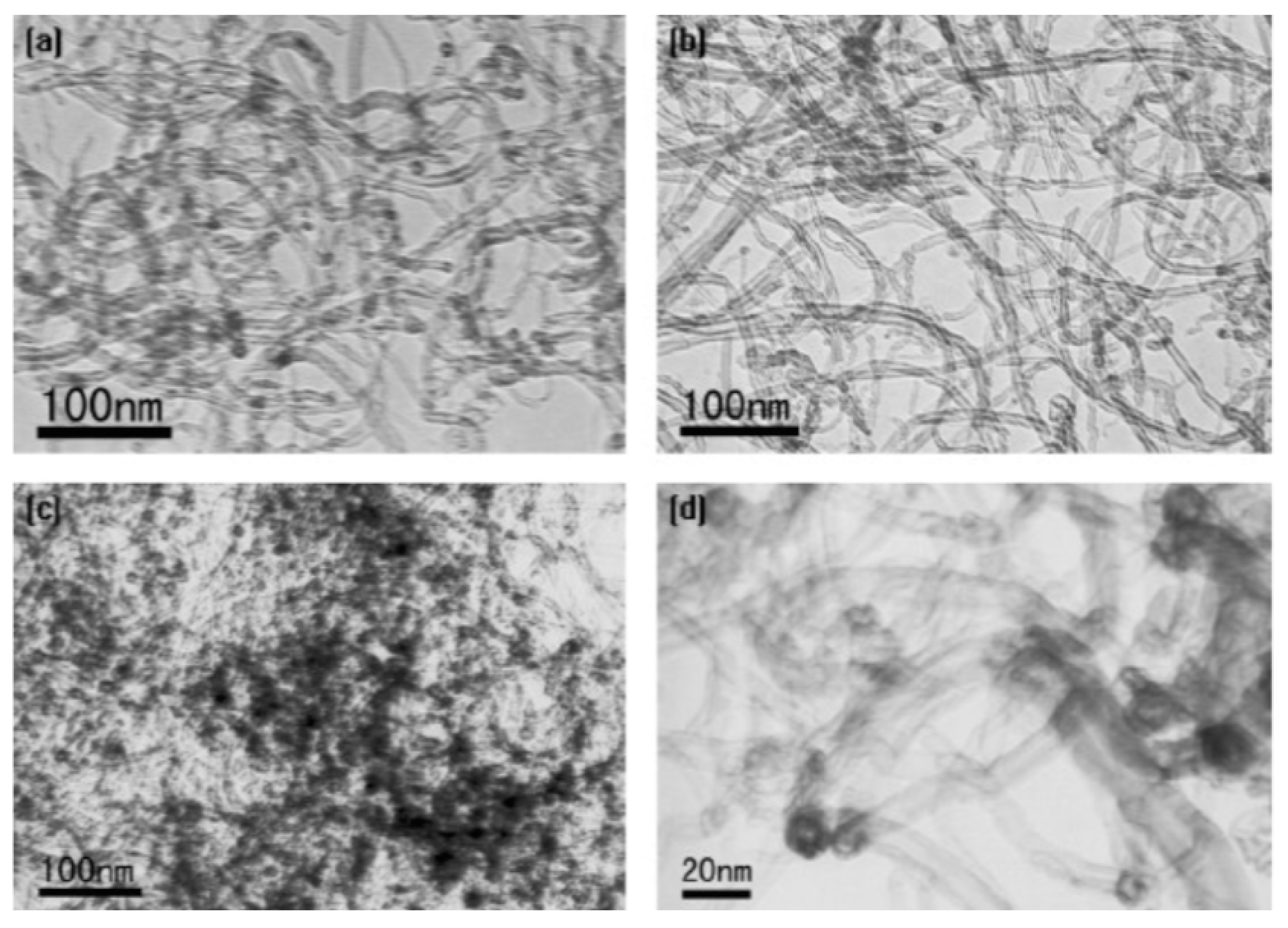
Wang et al. found that during the oxidation of CNTs with concentrated HNO3, the total amount of oxygen-containing functional groups increased quickly during 2 h of treatment to 1.6 and 2.7 mmol/g at 1 and 2 h treatment of acid, respectively [49]. Then, the increase was very slow from 6 h (2.8 mmol/g) to 10 h acid treatment (3.1 mmol/g). In addition, the amount of -OH and –C=O groups decreased after 2 h treatment, which was explained by the fact that these groups are further oxidized into carboxylic acid groups. Figure 2 shows the TEM images of pristine MWCNTs and MWCNTs acidified with nitric acid. Most of the contaminants present in pristine carbon nanotubes were removed after HNO3 treatment (Figure 2b) and Pb(II) adsorbed onto oxidized MWCNTs aggregated on their surface (Figure 2c), mostly on the caps and defective sites (Figure 2d).
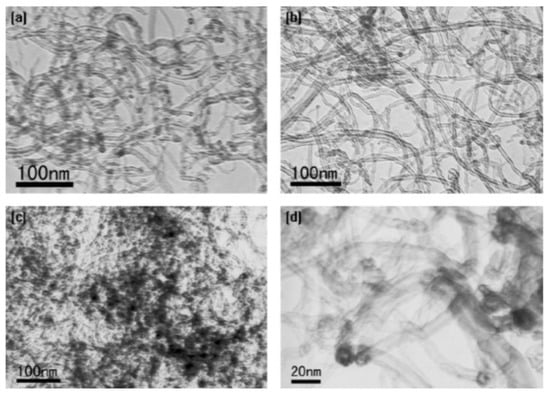
Figure 2.
TEM image of (a) pristine MWCNTs; (b) oxidized with concentrated HNO3; (c,d) oxidized MWCNTs after sorption of Pb(II). Reprinted with permission from Ref. [49]. 2007 Elsevier.
Several papers have reported the Pb(II) maximum sorption capacity calculated by applying Langmuir and Freundlich models or obtained experimentally using oxidized CNTs. However, as can be seen from Table 1, the reported values vary over a wide range. Part of the reason for this could be the differences in the oxidation condition of carbon nanotube surfaces, such as temperature and time. Other reasons may include differences in sorption pH, ionic strength, and experimental conditions, such as contact time or mixing rate. It has been stated that the average length of CNTs decreases significantly with the increase in oxidation time and temperature [50]. However, other studies have shown that for the oxidation time in the range of 0.5–7 days, the CNTs structure did not change considerably [51].

Table 1.
Examples of Pb(II) sorption onto oxidized CNTs.
Oxygen plasma treatment, as an alternative method in relatively mild conditions to oxidation using strong chemical reagents, has been proposed [51,58,59]. The presence of -C=O and -COOH groups on carbon nanotubes was confirmed by data obtained from Fourier Transform Infrared spectroscopy. Compared to the acid treatment, fewer acidic groups was generated by plasma oxidation, but they had less damage and were generated in a much shorter treatment time. The sorption capacity of MWCNTs for lead(II) was greatly enhanced after plasma oxidation from 9.79 ± 0.46 mg/g (raw nanotubes) up to 54.11 ± 2.67 mg/g [51]. Hosseini et al. summarized the modification of carbon nanotubes through different types of plasma, notably plasmas that operate at an ambient temperature and atmospheric pressure [58]. It is worth mentioning that changing the gases used during plasma processing, different chemical groups, for example with nitrogen, can be generated on the CNT surface [59].
Sellaoui et al. analyzed the adsorption mechanism of Pb(II) ions onto carbon nanotubes via an experimental data set and physical models [60]. They used commercially available MWCNTs, which contained hydroxyl (content of 2.36–2.60% mass fraction) and carboxylic groups (content: 1.47–1.63% mass fraction). The experimentally quantified maximum metal adsorption capacity was about 240 mg/g at pH 5 and 300 min contact time. The obtained results were used to interpret the role of surface functionalities via statistical physics calculations. Two models (homogenous and heterogeneous) were applied for the analysis of adsorption data based on the model fitting results. The first model assumed that the adsorbent has a unique type of functional group responsible for the adsorption of Pb(II) and that a single adsorption energy generation can be hypothesized between the metal ions and the adsorbent surface. The second model hypothesizes the presence of two types of functional group binding lead ions, involving two different adsorption energies. The analysis of the model parameters showed that each functional group can adsorb several ions simultaneously [54]. The determined values of adsorption energies for both the functional groups were in the typical range of physical adsorption.
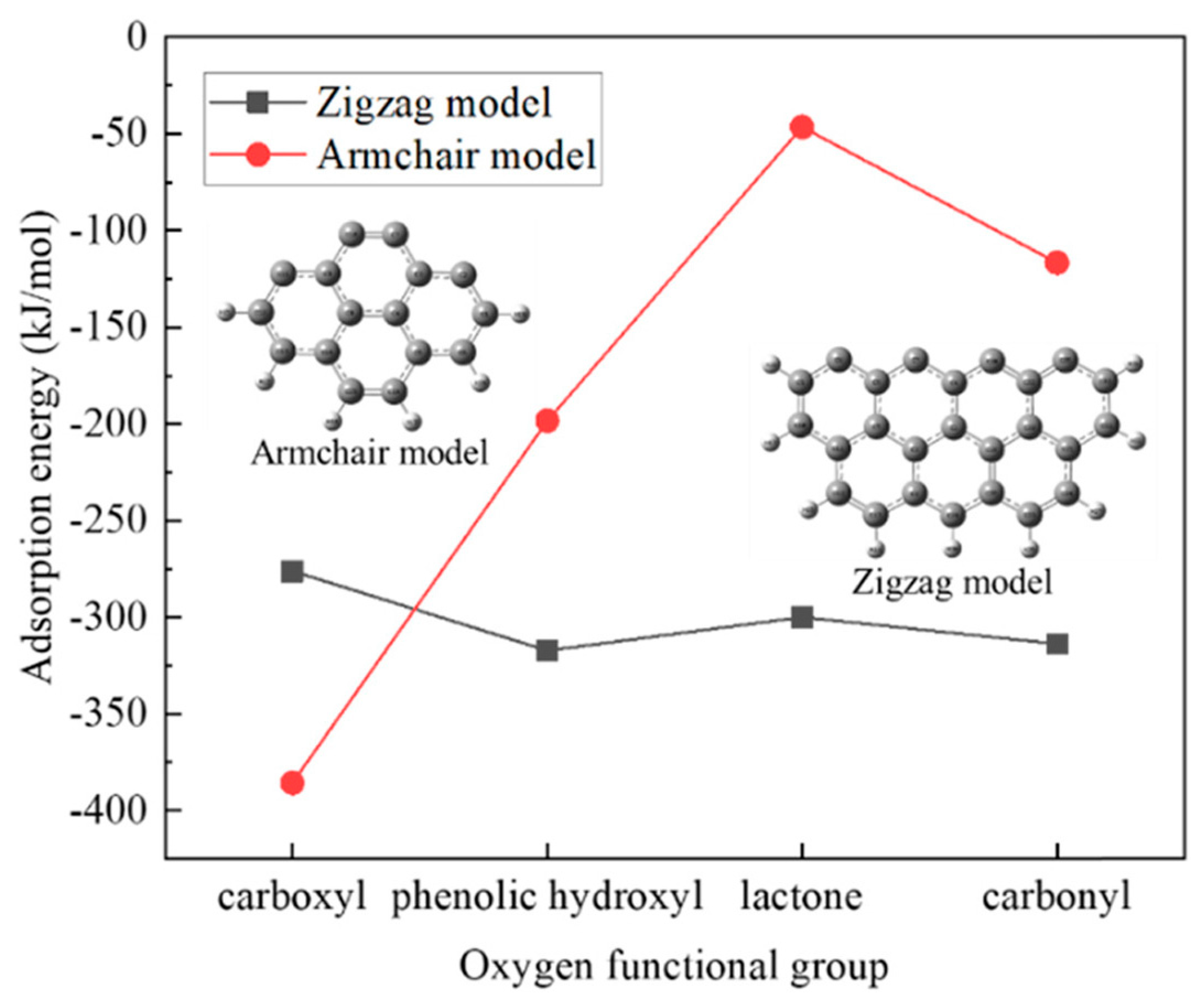
The adsorption mechanism of Pb2+ on a carbonaceous surface modified with oxygen functional groups was investigated by Xie et al. using the density functional theory method [61]. It was found that the adsorption of Pb(II) on the zigzag nanotube surface model (with seven benzene rings) was chemisorption with the adsorption energy in the range of −306.26 to −322.36 kJ/mol, while that on the armchair surface model with four benzene rings was physisorption (adsorption energy of −32.39 kJ/mol). The introduction of oxygen functional groups significantly enhanced the Pb(II) adsorption on the armchair surface. The physical sorption changed to chemisorption after introducing the oxygen functional groups, indicating the higher adsorption ability of the carbonaceous surfaces after modification. On the zigzag surface, however, the studied functional groups did not benefit the metal ions adsorption (Figure 3). Pb(II) tended to adsorb on the carbon atoms instead of moving to the oxygen atoms from the introduced functional groups for adsorption, which may suggest that the functional groups with oxygen promoted the Pb2+ adsorption by increasing the activity of their neighboring carbon atoms.
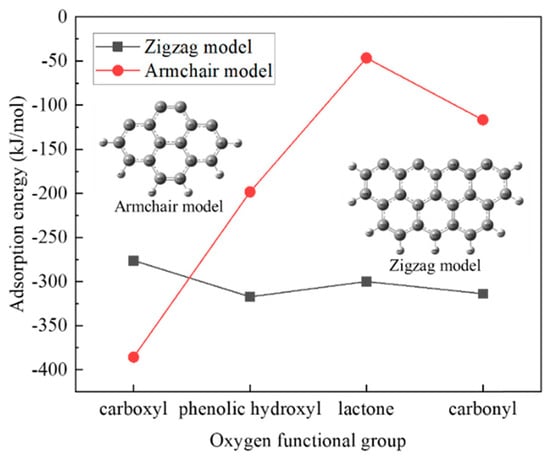
Figure 3.
The effects of oxygen functional groups on the adsorption energy for Pb(II) on the zigzag and armchair nanotube surface models. Reprinted with permission from Ref. [61]. 2021 Elsevier.
3.2. CNTs Modified with Organic Compounds
The modification of the surface of CNTs is one of the main research trends in the last years, as it is a way to improve their solubility as well as their sorption capacity and selectivity. Such modification increases the number of oxygen, nitrogen, sulfur, or other groups, and increases their dispersibility and the surface area [62,63]. Much research has been dedicated to the modification by forming covalent bonds between the carbon surface and the modifying reagent (covalent functionalization). The electrostatic and hydrophobic interactions, ion exchange, π–π electron binding, hydrogen bonding, and mesopore filling can be utilized for sorption of Pb(II) onto functionalized CNTs. In noncovalent functionalization, mainly hydrogen bonds, van der Waals, and hydrophobic interactions occur. The noncovalent functionalization of CNTs includes also coating with an appropriate ligand or surfactant, surface wrapping with polymer chains, and doping with metals or their oxides. The structure and original properties of CNTs are not changed after nonocovalent modification, but covalent functionalization is more stable and powerful.
The characterization of the presence of functional groups on the surface of CNTs and the efficiency of their functionalization can be carried out by various instruments, such as a transmission electron microscope (TEM), field emission scanning electron microscope (FESEM), energy dispersive X-ray (EDX), Raman spectroscope, thermogravimeter (TGA), Fourier transformed infrared spectroscope (FT-IR), and X-ray photoelectron spectroscope (XPS) [64]. The morphology and structure of carbon nanotubes are characterized by TEM and FESEM. The impurities and defects, such as amorphous carbon coatings and catalyst particles, could be observed as black dots on their surface inside the body. These techniques can allow the observing of the CNTs′ surface damage after chemical treatment. EDX measurements are used for quantitative measurements of the components on the CNTs′ surface. After the oxidation process, it is expected that EDX analysis shows more oxygen atoms as a result of grafting new oxygen-containing functional groups. FT-IR is used to analyze the chemical bonding and type of functional groups grafted onto the nanotubes.
Impregnation of magnetic MWCNTs with 1-(2-pyridazylo)2-naphtol (PAN) in vortex-assisted SPE was applied for preconcentration of Pb(II) at pH 5.5 from some herb and spice samples [65]. Elution was performed using 3 mL of 3 mol/L HNO3 in 10% acetone. The enrichment factor (EF), defined as the ratio of sample to eluent volume, was 10 in a very short time (1 min) [59]. The limit of detection was 16.6 µg/L with flame atomic absorption spectrometry (FAAS). The surface of oxidized carbon nanotubes was also modified with a cationic chelating agent, batophenanthroline [66]. Sorption was conducted at pH 9 in a borate buffer within 15 min. Due to the high preconcentration factor of 200, the proposed procedure for determination of Pb(II) in rice samples allowed researchers to obtain the limit of detection (LOD) as low as 0.25 µg/L using the simple FAAS detection in the range of 0.13 and 0.35 ng/mL.
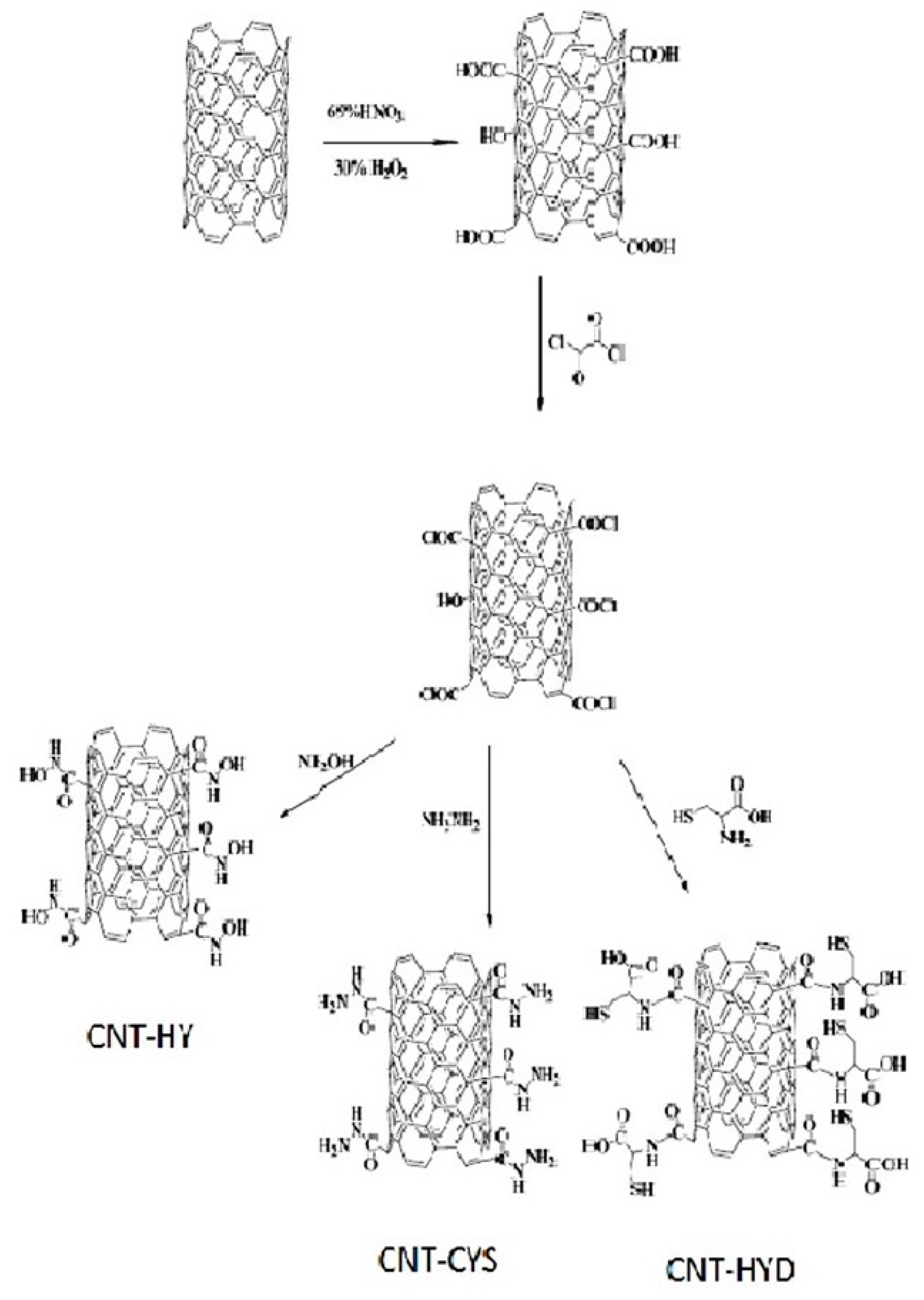
Hanbali et al. evaluated the potential of MWCNTs grafted with various reagents for Pb(II) removal [67]. Carbon nanotubes were first oxidized with concentrated nitric acid and then converted to acid chloride. In the next step, MWCNTs were functionalized separately with hydroxylamine (HY), cysteine (CYS), and hydrazine (HYD). Finally, they were mixed with iron oxides and suspended for 2 h to obtain the appropriate nanocomposites with magnetic properties (Figure 4). The efficiency of these adsorbents toward Pb(II) was studied as a function of adsorbent dose, pH, metal ion initial concentration, temperature, and contact time. The optimum adsorption conditions were found to be at a pH of 8.0 and the maximum removal was attained after 30 min at room temperature. However, the adsorption capacities derived from Langmuir isotherm seem relatively low, even in comparison with only oxidized MWCNTs (Table 1). For MWCNT-CYS and MWCNT-HYD, these values were 1.15, 3.31, and 9.09 mg/g, respectively. Moreover, after the third regeneration step by treatment with 0.1 M HCl solution and reuse cycles, a small reduction in their efficiency was observed [67].
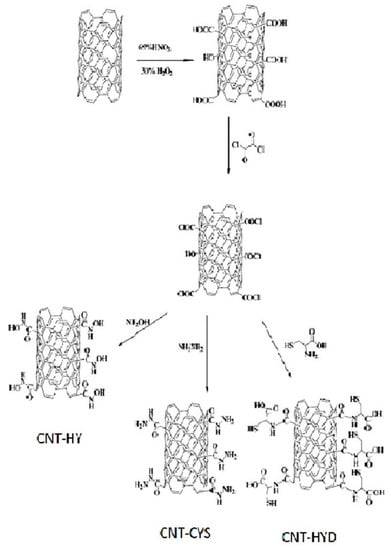
Figure 4.
Scheme for preparation of magnetic MWCNTs functionalized with hydroxylamine (HY), cysteine (CYS), and hydrazine (HYD). Reprinted with permission from Ref. [67]. 2020 Elsevier.
Other ligands which can form complexes with Pb(II) ions, such as 4′-(4-hydroxy-phenyl)-2,2′,6′,2′′-terpyridine [45], phenylenediamine [47], 5,7-dinitro-8-quinolinol [68], pyridine [69], sulfosalicylic acid [70], or hydroxamic acid derivatives [71], were covalently bonded on the carbon nanotube surface. According to the Langmuir adsorption model, the maximum adsorption capacity of Pb(II) after modification of MWCNTs with 5,7-dinitro-8- quinolinol was increased from 200 mg/g to 333.3 mg/g [68]. The adsorption rate of Pb(II) onto such modified carbon nanotubes reached equilibrium only after 1.5 h. The mean recovery of lead ions from the real wastewater samples collected from 4 different sites in the Suez Gulf in Egypt and spiked with 50 mg/L of Pb was 88.04%.
Bajaj et al. have proposed the flow injection system connected directly to the nebulizer of a FAAS spectrophotometer using MWCNTs modified with phenylenediamine for the preconcentration of lead ions before their determination in industrially contaminated water samples [55]. Such a methodology allows us to increase the sampling frequency up to 20 per hour. The preconcentration factors (PF), defined as the ratio of the slopes of the calibration curves with and without preconcentration step, were 94 and 73 for MWCNTs modified with phenylenediamine and for only oxidized MWCNTs, respectively. The linear relationship with the analyte concentration was obtained in the range of 3.80–260 μg/L with the LOD value equal to 1.2 μg/L.
MWCNTs functionalized with sulfosalicylic acid were obtained after oxidation and thiolation, followed by decoration with Fe3O4 nanoparticles [70]. Their high sorption capacity of 454.54 mg/g was used for lead preconcentration from water samples from electroplating industries.
Thiol-functionalized MWCNT was synthesised using 2-mercaptoethanol as a sulfur source [72]. The coordination between –SH groups and Pb(II) effectively enhances the adsorption performance of the obtained MWCNTs-SH nanoparticles. Their adsorption capacity at pH 5 was increased by approximately 30.5% in comparison to MWCNT-COOH. The maximum Pb(II) adsorption capacity derived from the Langmuir model was 144.9 mg/g. The surface of MWCNT-SH was negatively charged at pH > 1. This means that under these conditions the electrostatic repulsion increased, and the agglomeration phenomenon weakened. The chemical adsorption process was recognized as controlling the adsorption rate. To study the adsorption performance of MWCNT-SH in natural samples, various wastewaters with different concentrations of Pb(II) were used, such as copper smelting wastewater (lead concentration of 5.01 mg/L), tin tailings wastewater (54.07 mg/L), and antimony tailings wastewater (1.02 mg/L). The adsorption capacity of MWCNT-SH was 42.6, 239.7 and 9.4 mg/g, respectively, for these wastewaters.
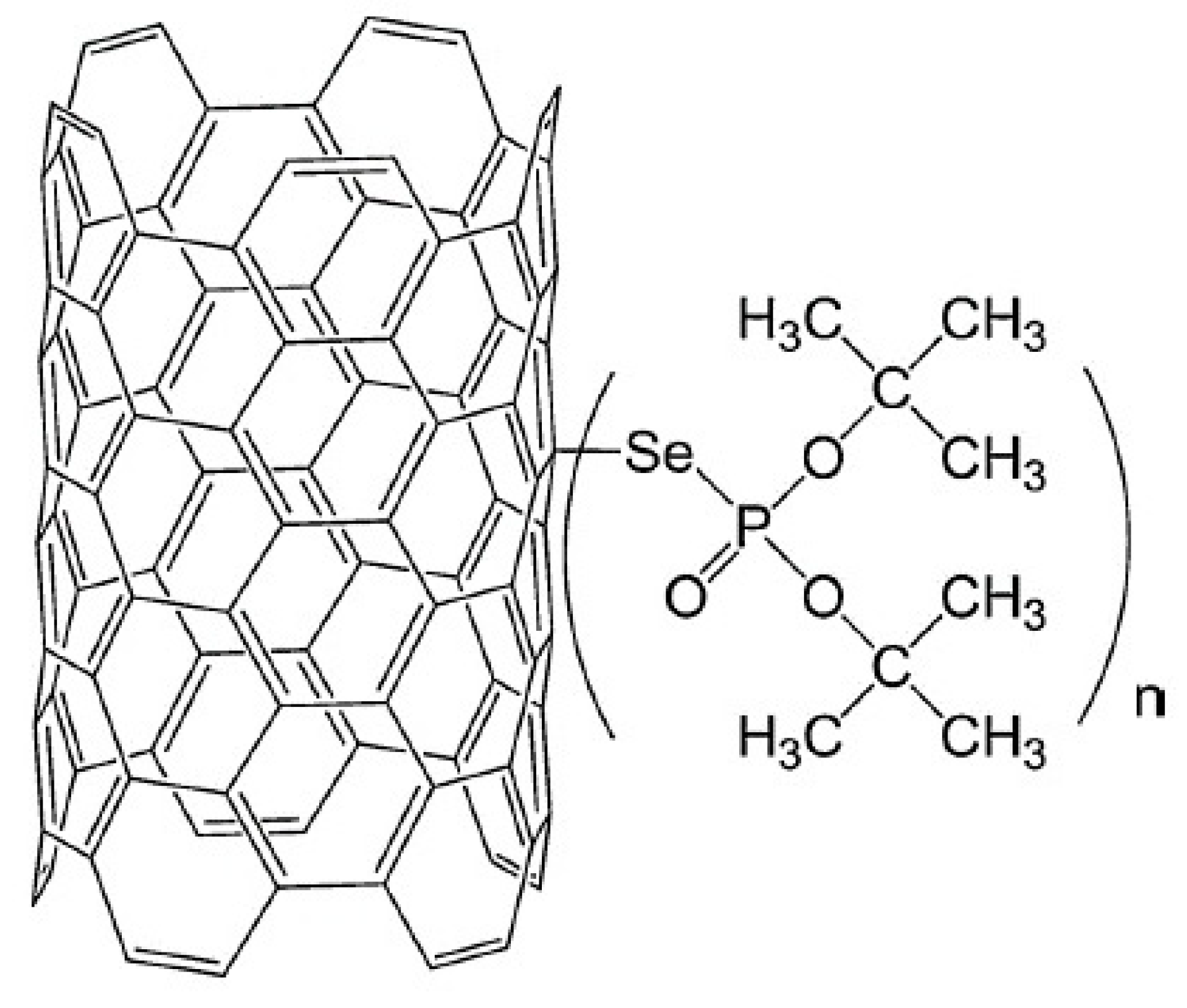
The characteristics of Pb(II) adsorption from an aqueous solution onto multiwalled carbon nanotubes functionalised by di-t-butyl selenophosphoryl groups connected by a selenium atom were investigated by Kończyk et al. [73]. The structure of this newly proposed adsorbent is presented in Figure 5. The authors reported that adsorbent enabled the highest Pb(II) removal efficiency at pH 5.0 within 60 min. The adsorption capacity increased as the temperature increased, and the maximum value of 156.25 mg/g was obtained at 313 K. The obtained experimental data were well-matched with the pseudo-second-order kinetic model. For further analysis of the adsorption kinetics, the diffusion model proposed by Weber and Morris was considered. That model states that when the plot q(t) = f(t0.5) is linear and passes through the origin (0,0), the intraparticle diffusion process dominates. In a case where the linearity is ensured but the plot does not pass through the origin, the adsorption may be limited by film diffusion. However, deviation from the origin was observed, which indicated faster mass transfer through the boundary layer on the adsorbent surface at the beginning of adsorption; then, the process was controlled by slower Pb(II) diffusion inside the particles. The competitive adsorption studies from the solution containing also other metal ions indicated high selectivity for Pb(II) with the affinity decreasing in the following order: Pb(II) >> Cd(II) > Zn(II), Cu(II), Ni(II), Co(II).
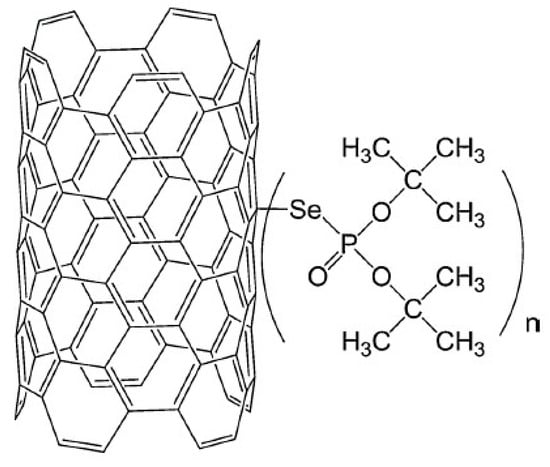
Figure 5.
MWCNTS functionalised by di-t-butyl selenophosphoryl groups. Reprinted with permission from Ref. [73]. 2007 Elsevier.
3.3. CNTs Decorated with Metal Oxides
CNTs decorated with metal oxide form a new class of hybrid nanomaterials that could potentially display not only the unique properties of nanoparticles and nanotubes but also additional novel physical and chemical properties due to their mutual interactions [68]. Thus, metal oxide nanoparticles supported on carbon nanotubes have been extensively studied and found to be effective adsorbents for the removal of heavy metal ions. The synthesis methodology for such materials has already been presented and discussed [74,75].
Apart from Fe2O3 or Fe3O4, present mainly in magnetic carbon nanotubes, various metal oxide nanoparticles have been decorated on the surface of CNTs to enhance their sorption properties and to broaden their applications [18,76]. These nanoparticles act also as a stabilizer against the aggregation of individual tubes, which is caused by strong van der Waals interactions. The addition of magnetic properties to CNTs, as was mentioned earlier, facilitates their collection after Pb(II) sorption from a sample solution. The silica structure was introduced to protect the magnetic cores against leaching, oxidation, and digestion in acidic media, as well as for improving their reusability [77].
For the preconcentration and separation of Pb(II), CNTs were successfully fitted with other metal oxides, such as NiO [78], MnO2 [79], or Al2O3 [80], as well as decorated with Au/Fe3O4 nanocomposites [81], thereby increasing their removal efficiencies. Egbosiuba et al. proposed nickel nanoparticles supported on MWCNTs, activated in an alkaline media, for the removal of Pb(II) from industrial wastewaters [82]. The proposed mechanism of Pb(II) sorption onto MWCNTs-KOH@NiNPs included electrostatic attraction, surface adsorption, ion exchange, and pore diffusion due to the incorporated nickel particles. The maximum adsorption capacity for this sorbent reached 480.95 mg/g. The obtained value of the maximum adsorption capacity for NiO/CNts (24.63 mg/g) is significantly lower than that reported earlier in the literature, e.g., for MnO2/CNTs (78.74 mg/g) [79] or Al2O3/CNTs (67.11 mg/g) [80]. In turn, the equilibrium in the studied NiO/CNts adsorption system is achieved relatively fast (10 min) in comparison to the other systems with metal oxides. Although Pb(II) adsorption by MnO2/CNTs occurred rapidly within the first 15 min of contact time, at least 2 h were needed to attain adsorption equilibrium [79].
3.4. CNTs Wrapped with Polymers
Functionalization of carbon nanotubes based on the polymer wrapping enhances their stability and it is easy to remove the unbound (free) polymer (e.g., by filtration, centrifugation, or precioitation) while leaving the wrapping polymer on the CNT surface [83,84]. Various interactions, including π−π, CH−π, and cation−π, were utilized for the polymer wrapping of CNTs. Electropolymerization using CNTs as the electrode enables homogeneous and stable wrapping by the polymers, even in the absence of a strong interaction between the wrapping polymer and CNT surfaces. Such a procedure was applied for polyaniline wrapping on MWCNTs and formed (PANI)/MWCNTs composite [85].
Oxidized MWCNTs wrapped with polypyrrole were prepared by in situ chemical oxidative polymerization and were used for the removal of Pb(II) ions in aqueous media [86]. The used instrument techniques (SEM, TEM, and EDX) confirmed the successful synthesis of PPy/o-MWCNT nanocomposite. Batch experiments showed a maximum sorption capacity of 408 mg/g for the prepared adsorbent. It should be noted that PPy/o-MWCNT nanocomposite also exhibited high affinity for Ni(II) and Cd(II) ions with the adsorption capacities of 409 and 392 mg/g, respectively. The maximum recovery of adsorbed lead ions (94%) was obtained for 0.2 M H2SO4 solution. The regenerated nanocomposite could be reused up to five removal/desorption cycles without considerable loss of efficiency.
Bankole et al. proposed the functionalization of CNTs with polyhydroxylbutyrate (PHB) [87]. This polymer is highly permeable to oxygen and biodegradable. Beyond its industrial applications as an environmentally friendly and more sustainable alternative to traditional plastics, it has been used for the development of formulations in the pharmaceutical industry. PHB-CNTs sorbent gave optimum Pb(II) sorption at pH of 5.7, a contact time of 10 min, and a maximum sorption capacity of 227.3 mg/g. A schematic diagram of Pb(II) sorption using CNTs-PHB sorbent is presented in Figure 6. Different adsorption isotherm models were taken into account to provide information on the distribution of lead ions in solid and liquid phases. The consistency of matching to the appropriate model increases in this order: Langmuir < Freundlich < Dubinin-Radukevich < Temkin. It should be mentioned that in most of the reported works concerning the use of carbon nanotubes for Pb(II) preconcentration and separation, the obtained results were best described by the Langmuir model, which reveals that sorption on the surface of CNT is homogeneous and monolayer.
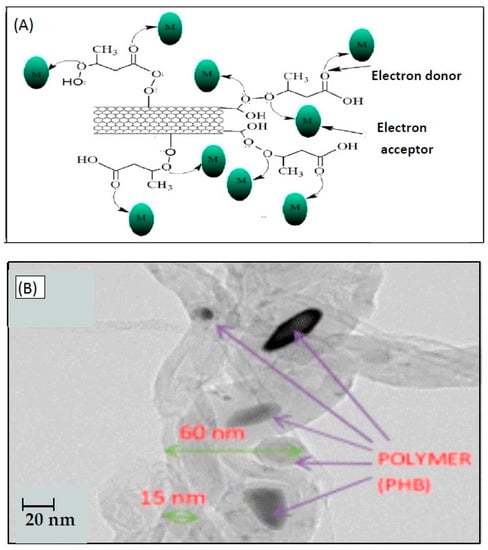
Figure 6.
(A) Schematic diagram of Pb(II) sorption using CNTs-PHB sorbent; (B) HRSEM image of CNTs-PHB sorbent. Reproduced with permission from MDPI [87].
Dehghani et al. fabricated MWCNTs/Fe3O4@polythiophene sorbent and used it for magnetic solid-phase microextraction of lead ions [88]. The factors affecting the separation and preconcentration procedure, such as sample pH (6.0), extraction time (3 min), and desorption conditions (0.75 mL of 0.5 M HNO3), were optimized. The value of the enrichment factor was calculated as 200 and the sorption capacity of 117.0 mg/g was obtained. The eluted Pb(II) by 750 μL of 0.5 M nitric acid was quantified by flame atomic absorption spectrometry. For a sample volume of 150 mL, the method showed linearity in the concentration ranges of 2.0–200.0 µg/L with an LOD value of 0.54 µg/L. The capability of the proposed method for its determination in several matrices (environmental waters, tea, rice, milk, and infant dry formula milk) was investigated. The recoveries of the spiked samples (at 10 mg/L Pb concentration) were in the range of 96.0–104.8%.
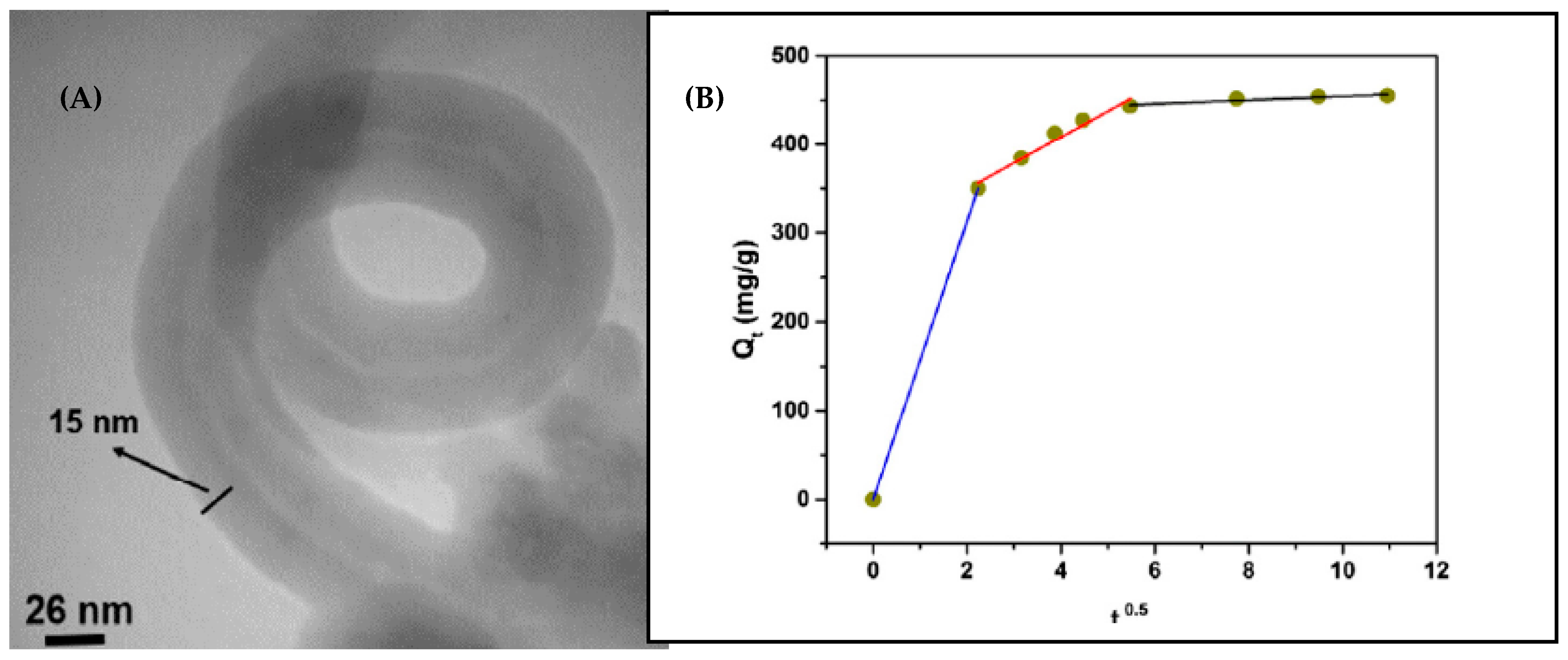
The adsorption performance of MWCNTs/polyrhodanine nanocomposite for the removal of Pb(II) from aqueous solution was also evaluated [89]. This conductive polymer possesses strong metal binding functional groups containing nitrogen, sulfur, and oxygen atoms. In order to prevent aggregation and adherence phenomena, carbon nanotubes were sonicated with polyvinylpyrrolidone prior to chemical oxidation polymerization. From the TEM image present in Figure 7, it can be seen that the average diameter of carbon nanotubes increased from 10–20 nm to around 60 nm after polymerization, which further proves that polyrhodanine was coated on MWCNTs (Figure 8a). The optimal Pb(II) ions adsorption occurred at pH of 4. The positive value of ΔHo indicated the endothermic nature of the adsorption process, which was supported by the increase in Pb(II) adsorption capacity with an increase in temperature. In addition, the calculated ΔHo of 56.19 kJ/mol confirmed the chemisorption process. It was found that the adsorption of Pb(II) ions onto MWCNTs/polyrhodanine fitted properly with both the Langmuir and Freundlich adsorption isotherms. The good correlation to the Freundlich adsorption model was explained as a result of heterogeneous distribution of adsorption sites on MWCNTs. The maximum adsorption capacity was 8118 mg/g and the required time to reach equilibrium was about 2 h. The experimental data were also fitted to the kinetic models. The pseudo-second-order kinetic model showed better correlation as the vast majority of the published papers related to adsorption of lead ions on CNTs. Considering the kinetic diffusion model, it was observed that the plot of q(t) = f(t0.5) shows three straight lines with different slopes and intercepts values (Figure 7b). It was explained that the first line depicted rapid adsorption by the external surface of the adsorbent, while the second line (rate-limiting step) represents intraparticle diffusion of Pb(II) ions onto MWCNTs/polyrhodanine, and finally the third linear portion corresponds to the saturation of the adsorbent surface [89].
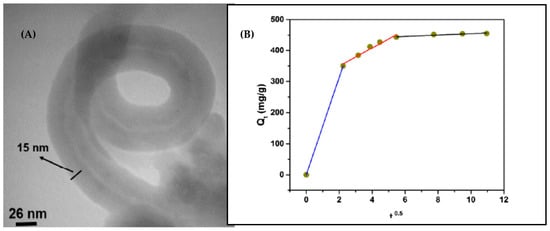
Figure 7.
(A) TEM image of MWCNTs/polyrhodanine nanocomposite; (B) Kinetic of Pb(II) adsorption by MWCNTs/polyrhodanine according to the intraparticle diffusion model. Reprinted with permission from Ref. [89] 2016 Elsevier.
3.5. Other Nanocomposities with CNTs
The novel polyethersulfone/ethylenediamine-functionalized multiwall carbon nanotubes (PES/MWCNTs-NH2) nanocomposite, synthesized through three steps—acidic treatment, acylation with SOCl2, and amine functionalization—was proposed as an efficient sorbent for Pb(II) removal from aqueous solutions [90]. The optimum condition was obtained at a neutral pH and contact time of 10 min. The equilibrium studies revealed that Pb(II) adsorption onto PES/MWCNTs-NH2 was performed on a heterogeneous surface with a non-uniform distribution of adsorption heat. The studies confirmed that the chemisorption process was favorable and controlled by film diffusion.
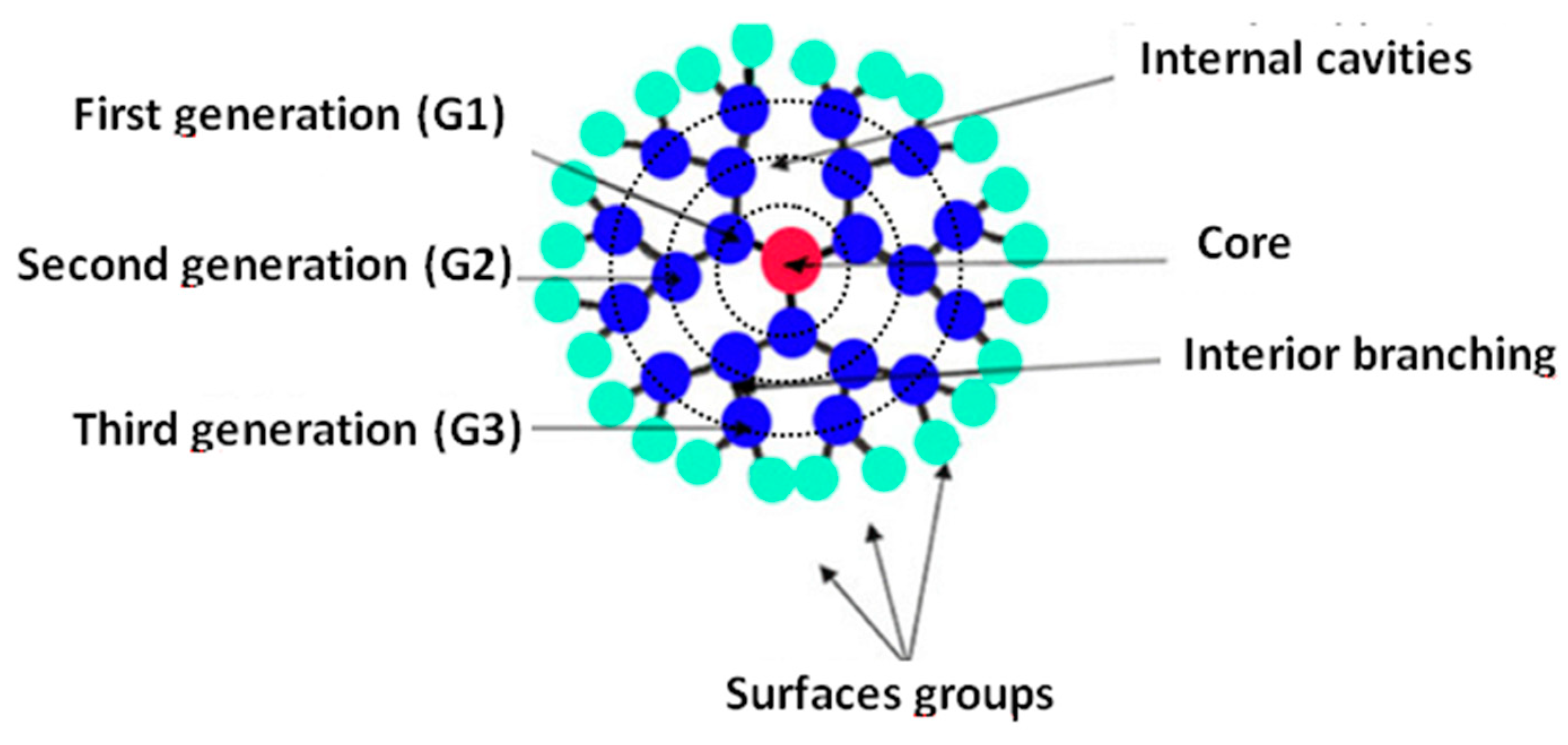
Dendrimers are a type of hyperbranched polymer similar to a tree structure. They posses three distinguishable architectural components, i.e., an interior core, interior layer (generations) composed of repeating units radially attached to the interior core, and exterior (terminal functionality) attached to the outermost interior generation [91]. They are composed of repeated structural units created by repeated and alternating reactions. The core is designated as generation zero (G0) and the subsequent layers with primary and tertiary amine functional groups are referred to as the following generations. The general scheme for these compounds is presented in Figure 8. The length of the molecule chains can be effectively regulated [92,93].
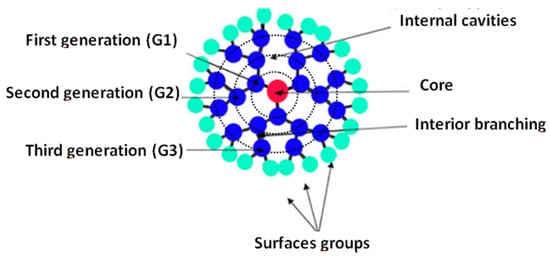
Figure 8.
General structure of dendrimers. Reprinted with permission from Ref. [91] 2010 Elsevier.
Figure 8.
General structure of dendrimers. Reprinted with permission from Ref. [91] 2010 Elsevier.
Dendrimers have a high efficiency for heavy metal removing via coordination and chelation linkages. To overcome the problem of their good aqueous solubility and difficulty in separating them from the solution, many researchers have grafted dendrimers to the surfaces of various nanomaterials. Hayati et al. reported the preparation of functionalized carbon nanotubes with polypropylene imine (PPI) dendrimer [94]. Raman analysis showed that the PPI dendrimer was covalently linked onto the surfaces of CNTs. This nanocomposite exhibited extremely high sorption capacity of 1750 mg/g at pH 7. The same research group modified carbon nanotubes with poly-amidoamine dendrimers which showed extremely high adsorption capacity for Pb(II) (4870 mg/g), achieved also at pH 7 [95]. The results showed that the Langmuir isotherm and pseudo-second-order kinetics are the most favourable models for Pb(II) ions adsorption onto these nanocomposities.
Zeolite, due to its interlaced structure with a negative charge that loosely binds exchangeable hydrated cations, was utilized for the preparation of the nanocomposite with magnetic carbon nanotubes [96]. The MWCNT-Fe3O4@Zeo nanocomposite was employed in ultrasonic-assisted SPE for the preconcentration of Pb(II). The optimal parameters affecting the preconcentration method were achieved using response surface methodology based on a central composite design: sample pH 8.3, preconcentration time 20 min, and eution with 1.8 M HNO3 solution. Under these conditions, the limit of detection was 0.023 µg/L using ICP OES. However, comparing this with the other nanocomposites containing carbon nanotubes described earlier, MWCNT-Fe3O4@Zeo exhibited relatively low adsorption capacity for lead ions, being 37.8 mg/g. It could be reused up to 11 cycles without evident decrease (>5%) in their sorption ablity [97].
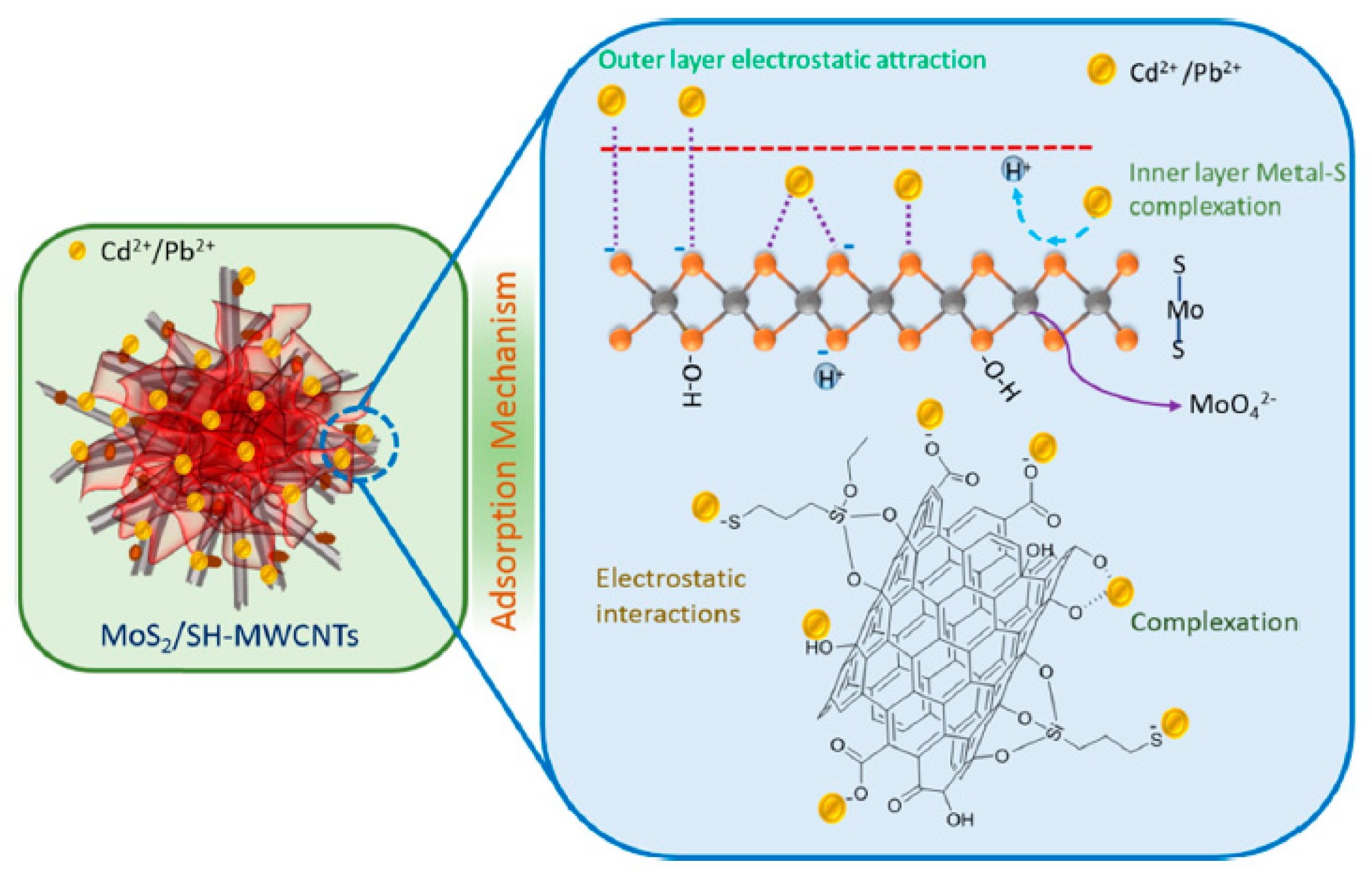
The MoS2/SH-MWCNT nanocomposite was synthesized by acid treatment and sulfurization of carbon nanotubes, followed by a facile hydrothermal reaction technique using sodium molybdate and diethyldithiocarbamate as MoS2 precursors [38]. It demonstrated at pH 6 the sorption capacity for Pb(II) equals 90 mg/g. The metal−sulfur complex formation was identified as the key contributor to adsorption, followed by electrostatic interactions between lead ions and the negatively charged functional groups (such as −COOH, −OH, and −SH) for multilayer sorption (Figure 9). Scanning electron microscopy-energy dispersive spectrometry and X-ray diffraction studies confirmed that this nanocomposite after the adsorption process is converted into a PbMoO4–xSx complex. The authors suggested that the obtained Pb-loaded MoS2/SH-MWCNT nanocomposite can be also further utilized in photocatalytic approaches, e.g., for the degradation of some organic pollutants.
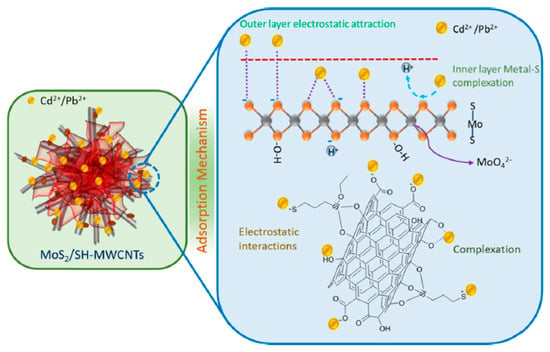
Figure 9.
Schematic diagram of Pb(II) sorption mechanism on MoS2/SH-MWCNTs. Reproduced with permission from MDPI [52].
The recent examples of the applications of carbon nanotubes for the preconcentration, separation, and removal of Pb(II) using solid phase extraction are presented in Table 2.

Table 2.
The recent examples of carbon tubes applications for preconcentration, separation and removal of Pb(II) using SPE technique.
4. Carbon Nanotube Membranes
Carbon nanotubes with a large specific surface area, chemical inertness, and outstanding water transport property have also received interest regarding the construction of new composite membranes for water treatment [97,98,99,100,101,102,103]. The stability of CNTs at high temperatures enables higher operating temperatures up to 673 K compared to conventional polymer membrane filters, which can operate up to 325 K [97]. They can also be reused after ultrasonication and autoclaving cleaning. Theoretical studies have demonstrated that in low diameter CNTs, the smallest SWCNTs reveal better water transport in comparison to MWCNTs of identical inner diameter, due to the lower hydrophobic contribution of the SWCNT walls [100]. Moreover, SWCNTs with small diameters allow the fabrication of highly permeable and very selective high-density membranes through the fabrication of vertically aligned CNT platforms, with water permeances that exceed those of conventional nano- and ultrafiltration membranes [101].
Based on the fabrication strategies, CNT-based membranes can be classified into three different types: vertically aligned (VA-CNT), CNT-based composite membranes (CNT-CPS), and buckypaper CNTs [98]. In VA-CNT membranes, the carbon nanotubes are perpendicularly arranged, yielding opened and aligned nanochannels, and a flux of water passes through the hollow CNT interior or between the bundles. Due to the vertically oriented water transportation and high packing density, they show the main advantage of allowing a high flux of water. VA-CNT membranes can be prepared by embedding the CNTs into a polymer matrix, or by growing them onto a substrate by the CVD method, to ensure the proper positioning. Their permeability and selectivity can be controlled by functionalization with suitable chemical groups.
CNT-based composite membranes consist of heterogeneous structures with randomly aligned CNTs embedded into a polymeric matrix, and the carbon nanotubes can be incorporated on the top layer of the membrane or used as an intermediate layer. The widely employed methods to synthesize these membranes include phase inversion, solution mixing, polymer grafting, in-situ polymerization, in-situ colloidal precipitation, and spray-assisted layer-by-layer and interfacial polymerization [98,102,104].
Buckypaper (BP) membranes consist of macroscopic paperlike structures based on randomly arranged CNTs that are held together by van der Waals forces and π–π interactions [105,106,107]. In BP membranes, the CNTs are selfassembled into a cohesive structure with a highly porous 3D network and high specific surface area, and retain the intrinsic physicochemical and thermal properties. The incorporation of polymers or other nanoparticles can improve the mechanical properties. They are obtained by applying ultrasonic energy to samples containing nanotubes and a suitable dispersant and then filtered onto a support membrane. Their major advantage is the simplicity of the fabrication metod as BP membranes do not require embedding or aligning the CNTs into a host matrix.
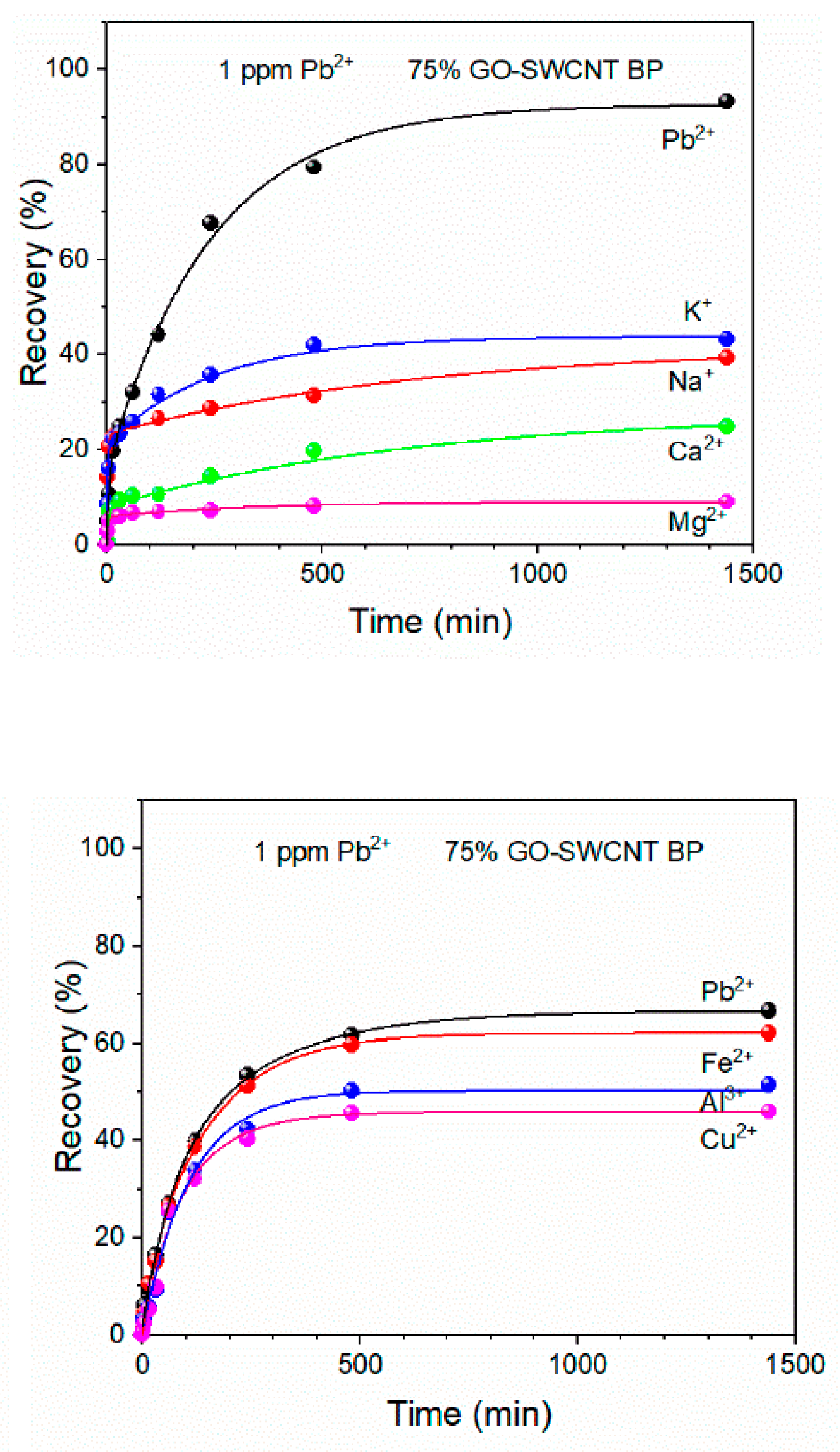
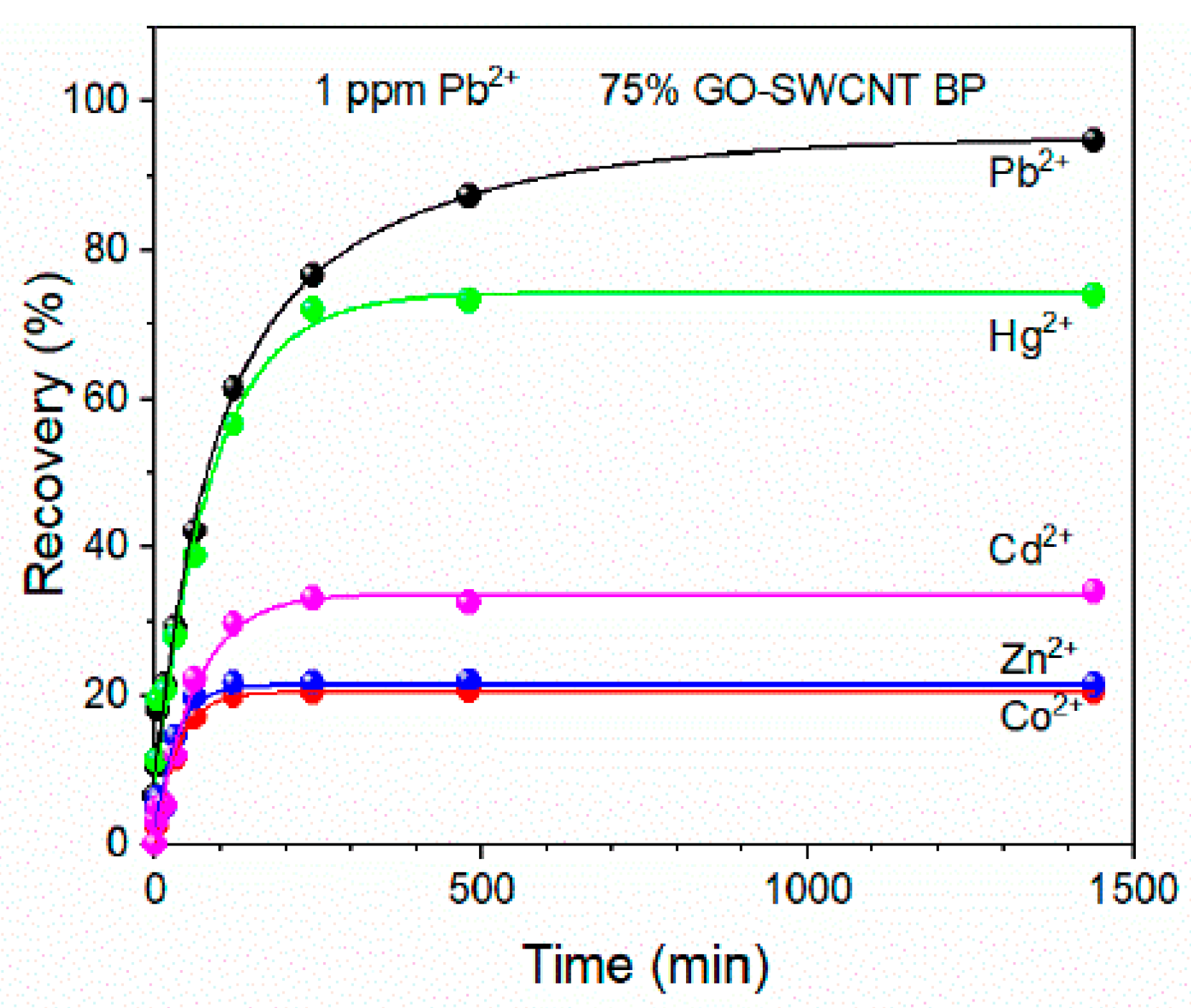
Recently, GO-SWCNT buckypaper has been proposed as a self-standing porous membrane for filtration and adsorption processes [105]. Commercially available SWCNTs-COOH were used with the average bundle lengths from 0.5 to 1.5 µm and diameters of 4–5 nm. The adsorption capacity and selectivity of Pb(II) were evaluated as a function of the increasing amount of graphene oxide present in the membrane. The obtained membranes containing 25%, 50%, and 75% of GO were flexible disks with an average thickness of 100 ± 2 µm and an average diameter of 38 ± 1 mm. All membranes were able to adsorb almost 100% of lead ions from 1 mg/L of its solution within 8 h and residual concentration was always lower than the WHO acceptable limit for drinking water (0.010 mg/L). The highest adsorption capacity (478.9 ± 25 mg/g) was determined for GO-SWCNT BP containing wt. 75% of graphene oxide at a pH of 6. The selectivity of that nanocomposite was confirmed by evaluating the recovery of Pb(II) in the presence of other metal ions in simulated wastewaters (Figure 10). The stability of GO-SWCNT BP after desorption of lead ions was evaluated by soaking the membranes in a 20% (v/v%) 2-mercaptoethanol aqueous solution for 24 h. Their regeneration indicated that Pb(II) recovery decreased to 93.42% after the fifth adsorption cycle. According to the estimation made by the authors, the production of GO-SWCNT BP membrane is cheaper than similar SWCNTs BP incorporating metal–organic frameworks (MOFs) [106].
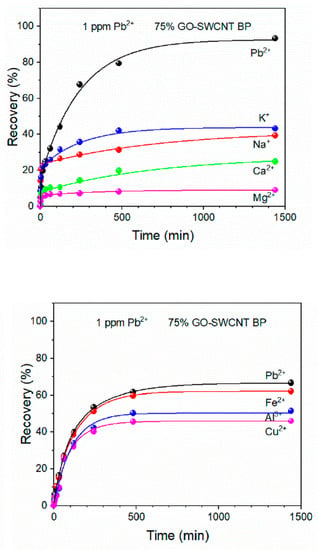
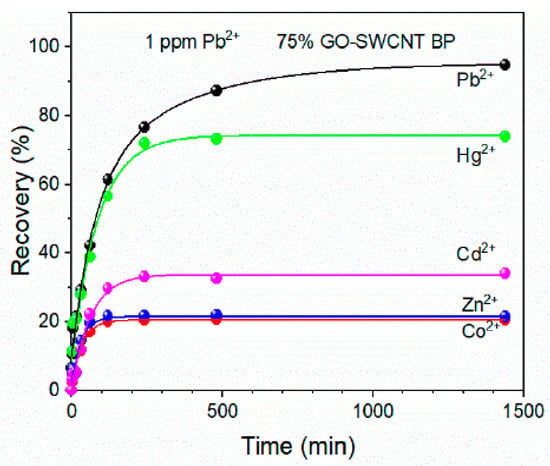
Figure 10.
Pb(II) recovery by 75% GO-SWCNT buckypaper membrane during soaking in 800 mL of various multielement metal ion solutions. The concentration of each metal ion in all mixtures was 1 mg/L. Reprinted with permission from Ref. [105] 2022 MDPI.
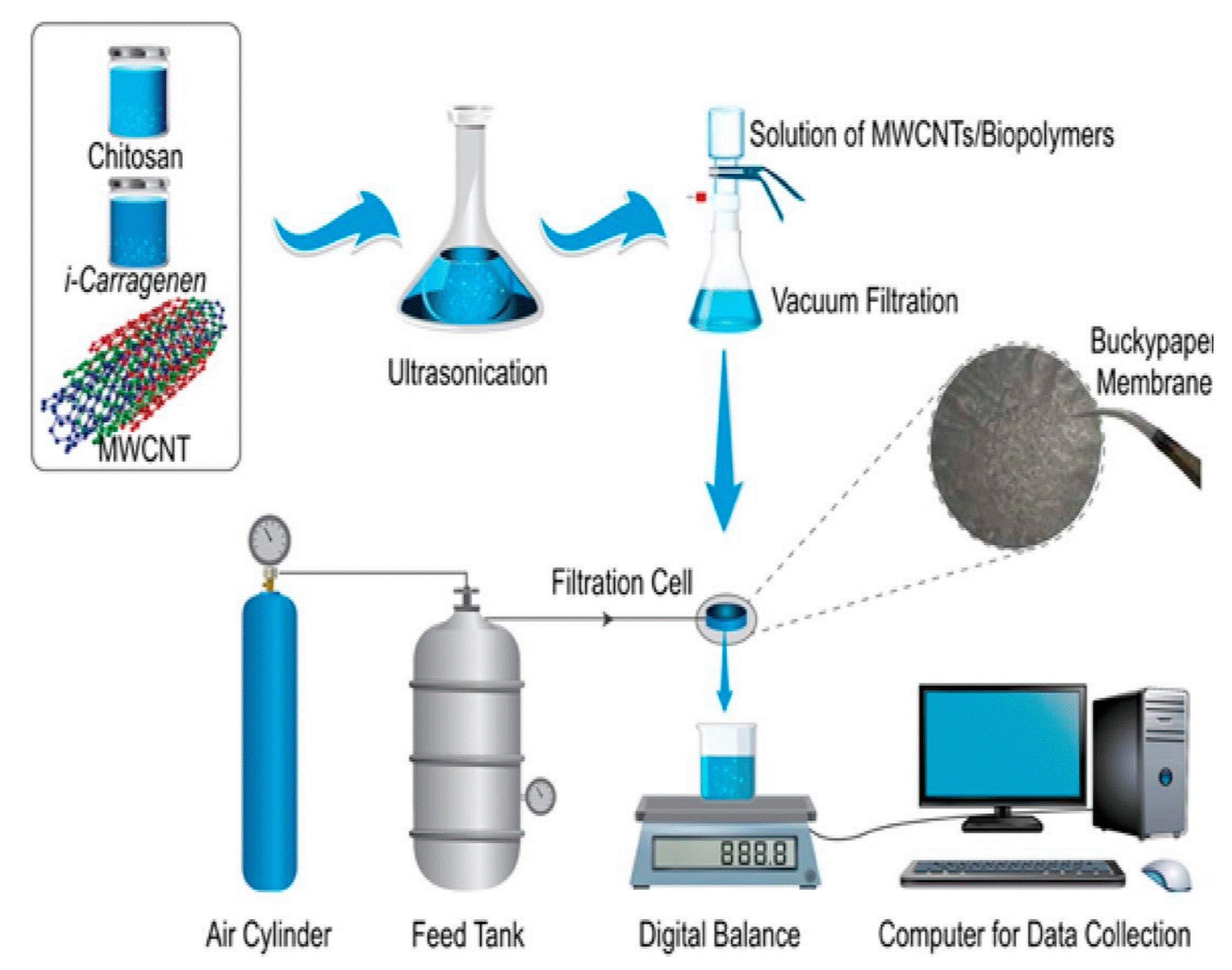
Alshahrami el al introduced a novel BP membrane fabricated using MWCNTs and a combination of two biopoymers—chitosan and carrageenan—using vacum filtration [106]. The scheme for their preparation is presented in Figure 11. The MWCNTs/chitosan membrane showed higher water permeabity and stronger mechanical properties, while MWCNTs/carrageenan membrane had the highest surface area according to BET analysis. The combination of these two biopolymers for membrane preparation exposed enhanced thermal stability and porosity, and demonstrated removal of Pb(II) with 91% efficiency at low pressure. However, these studies were conducted only for lead standard solutions in pure water at pH 7.
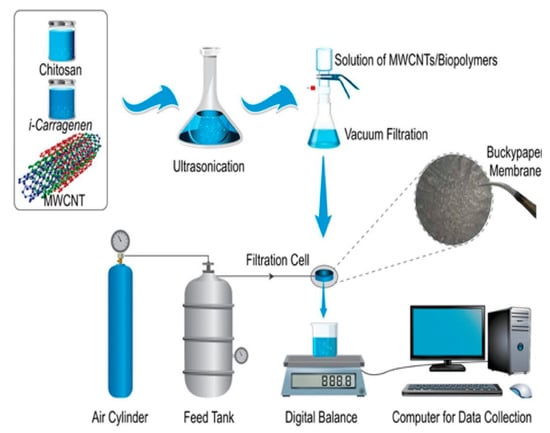
Figure 11.
The scheme for preparation of MWCNTs/biopolymers buckypaper and the dead-end filtration system. Reprinted with permission from Ref. [106] 2021 Elsevier.
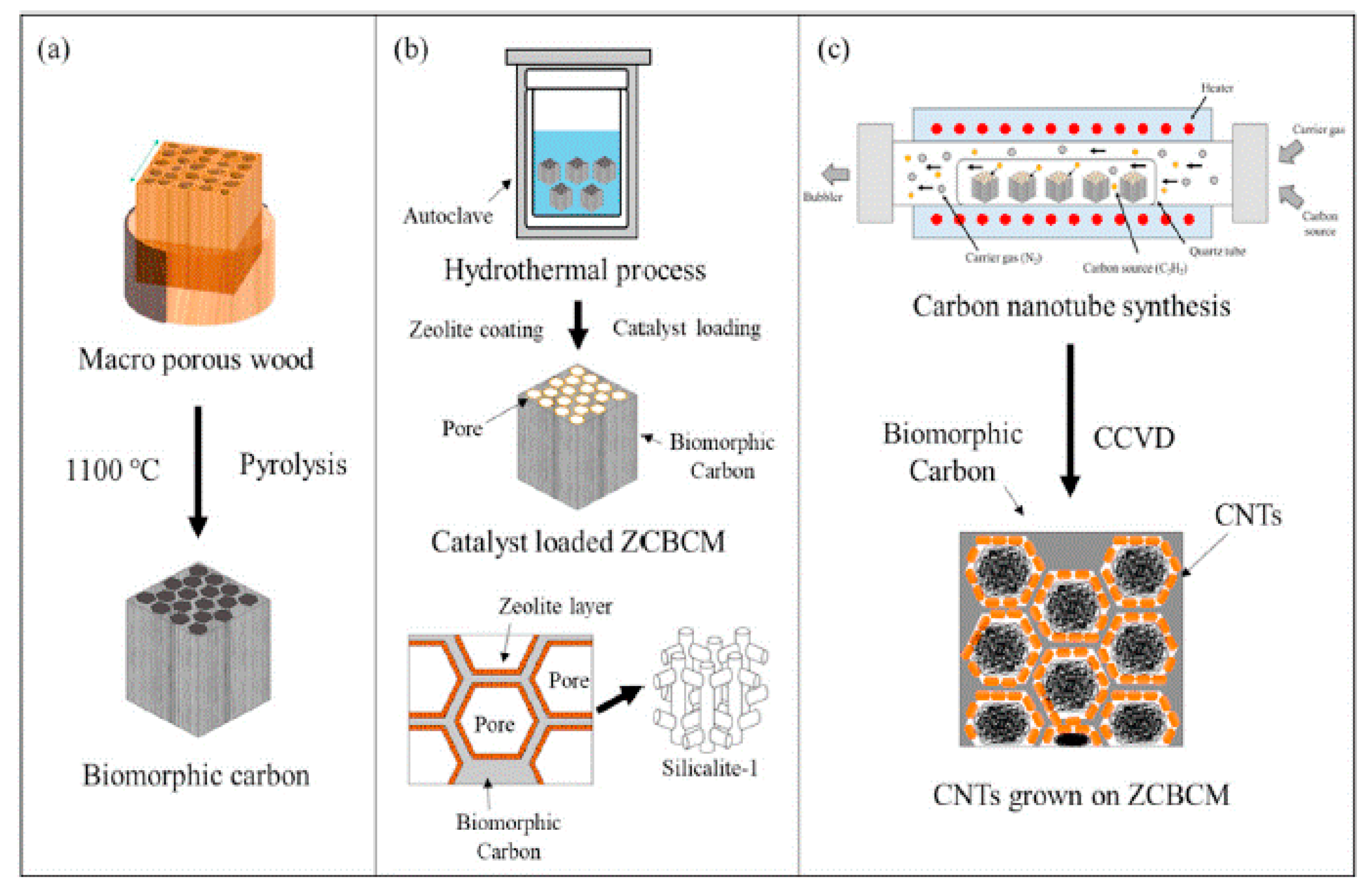
Kim et al. investigated carbon nanotube filtration technology using catalyst particles supported on silicalite-1–biomorphic carbon materials (BCMs) [97]. The scheme of their synthesis is shown in Figure 12. First, a hexagonal honeycomb-structured carbon substrate was developed through a carbonization reaction; then, the silicalite-1 template on BCMs was synthesized, and coated on BCMs using a hydrothermal method. Finally, after loading the catalytic metal ions into the template, a CNTs filter was fabricated through catalytic chemical vapor deposition. The prepared nanomaterial adsorbed lead ions from the mixture, which also contained Mn(II), Cd)II), Ni(II), and Cr(III) (all at 20 mg/L concentration), with the removal efficiency > 99.99%. The solution after the filtration process (lasting 60 min) contained less than 1 mg/L of Pb(II).
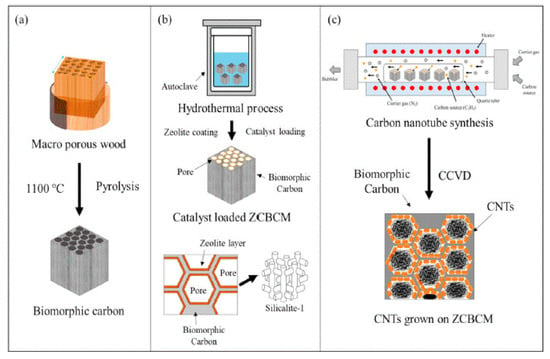
Figure 12.
Schematic of the synthesis of CNTs on BCMs: (a) pyrolysis, (b) template coating and catalyst loading, and (c) CNTs synthesis. Reprinted with permission from Ref. [97] 2021 Elsevier.
The noncovalent interaction of graphene oxide with oxidized multiwalled carbon nanotubes was utilized for preparation of the stable and highly durable membrane [108]. The suspension of GO and MWCNTs was prepared by ultrasonication of 100 mg of GO and 12.5 mg of CNTs in 100 mL of water for 60 min. Then, 1 mL of that suspension was passing through the Millipore nitrocellulose filter, washed, and dried. SEM images showed the highly wrinkled structure of GO and entangled CNTs stabilizing the membrane. The adsorption of metal ions was not only based on the electrostatic interaction but also on chelation by carboxyl and hydroxyl groups, and the membrane can be applied both under the flow conditions and vigorous shaking. Adsorption capacity for Pb(II), derived from the Langmuir model, was equal to 98.1 ± 0.83 mg/g. For other examined metal ions, these parameters were below 50 mg/g, and the affinities toward the GO/CNTs membranes followed the order: Pb(II) > Cu(II) > Cd(II) > Zn(II) > Ni(II) > Co(II). The same affinity order was obtained for the suspension of GO alone [109], oxidized-CNTs [110], and GO/cellulose membranes [111].
Musielak et al., combining a preconcentration method using graphene oxide/carbon nanotubes membranes and total-reflection X-ray fluorescence spectrometry (TXRF) for detection, proposed a procedure for the determination of Pb(II) at trace levels [112]. After passing a sample solution through the membrane, analyte was recovered by soaking the membrane in 0.75 µL of 2 mol/L nitric acid (in the presence of 2 μg/mL of yttrium as the internal standard) for 5 min and vortexed for 2 min. Then, 10 µL of that solution was transferred onto a siliconized quartz reflector for the subsequent TXRF analysis. The optimum value of sample pH was 5. The face centered central composite design was performed to study the influence of the flow rate and volume of the solution. The sample volume of 100 mL and the flow rate of 5 mL/min was chosen for the experiments, which led to recoveries higher than 90%. The enrichment factor of 133 was obtained, achieving the limits of detection of 0.12 ng/mL for W target X-ray tube with a measurement time of 2000 s, and a much lower LOD value for Mo target X-ray tube: it was 0.0008 ng/mL, with a very short measurement time of 600 s. To confirm the reliability and usefulness of the evaluated method, certified referenced materials (spring water and seawater) were examined with satisfactory agreement in comparison to the certified values. Additionally, the proposed analytical procedure for Pb(II) determination was checked for the requirements of green analytical chemistry basic principles, particularly through the reduction of reagents consumption, time of analysis, and amount of consumed energy. As it did not use any toxic reagents or organic solvents, only a small portion of the sample was required for the measurements and little energy was consumed. It was found to be in line with the requirements.
5. Conclusions
Carbon nanotubes have attracted significant interest for preconcentration and removal of Pb(II). They exhibit a huge surface area, good thermal and mechanical stability, and ease of surface modification. Similar to other carbon-based nanomaterials, they can be modified using polymers and metal oxides, as well as doping with heteroatoms. The functionalization with magnetic nanoparticles facilitates the separation of Pb ions loaded sorbent from the solution. In the described works, a particular focus was placed on the factors affecting the sorption process, such as adsorbent dose, pH, initial concentration, and contact time, which specifies the performance of carbon nanotubes as useful sorbents. The results decribed in this review indicated that CNT composites are promising adsorbents for Pb (II) uptake from aqueous solutions. The highest value of sorption capacity (8118 mg/g) was reported for the MWCNTs/polyrhodanine nanocomposite at a relatively low pH of 4 [89]. Electrostatic attraction, ion exchange, and surface complexation have been identified as the main mechanisms of lead sorption.
However, it should be noticed that besides the advantages of CNTs, such as high sorption capacity or selectivity, some drawbacks can also be found in their application in lead preconcentration and separation. The synthesis of several nanocomposities showing high sorption capacity is often time-consuming due to multistage processes and it requires appropriate high skills. Moreover, the reproducibility of their modification is very important, either from the same batch or batch-to-batch materials.
It should be added that other carbon-based nanomaterials from the graphene derivatives (graphene oxide, reduced graphene oxide, and their nanocomposites) were also applied as adsorbents for trace metal enrichment and removal [2,5,113]. The direct comparison of CNTs and graphene derivatives’ performance for these purposes is rather difficult. The major factors controlling the adsorption of metal ions on carbonaceous materials are the type and concentration of surface functional groups, and metal speciation forms in the solution depend on the pH [114].
Most of the proposed application of carbon nanotubes for Pb(II) enrichment and separation are present only on the laboratory scale in batch experiments. The commercialization of CNTs′ nanocomposite is rare due to the cost, reproducibility, and toxicity [115,116]. The large-scale production of these materials and the techniques for their purification are still under development. According to various reported papers, the regeneration of the CNTs and their reuses have been efficiently achieved, in some cases even up to 11 sorption/desorption cycles [97]. However, effective procedures for regeneration and preparation again suspensions without the aggregation of the CNTs should be developed to decrease the cost. The environmental impact of such nanomaterials should be also taken into consideration [117,118].
Future research on carbon nanotubes for preconcentration purposes in analytical procedures should concentrate on more selective functional groups that can strongly interact with lead ions in the presence of other heavy metal ions. Molecularly imprinted polymers (MIP), due to their excellent selectivity and specific separation, could be used as an example. Molecular imprinting has been widely recognized as a potential technique for the synthesis of tailor-made recognition materials through the formation of a polymer network around a template molecule. They are designed with the particular cavity in a suitable size and structure to remove a given analyte by the chemisorption or physicosorption process. Although covalent interactions used in adsorption processes can enhance the selectivity compared to non-covalent binding, further quantitative elution of an analyte can reveal some difficulties. The imprinted film prepared on the surface of CNTs can improve the access to the surface binding sites, increase the response kinetics, and enable the rebinding and extraction of the template molecules. Moreover, grafting such adsorbent with magnetic particles makes its separation much easier after the preconcentration step.
The application of natural components with several hydroxyl, amine, and carboxyl groups, such as chitosan, cellulose, β-cyclodextrin, and amino acids, in the synthesis of functionalized carbon nanotubes makes an attractive material in both an economic and an environmental perspective. The control of solution pH can change the surface charge of these functional groups. Thus, by choosing an appropriate condition, the electrostatic interaction with Pb(II) ions could be enhanced.
However, the main challenges for the application of CNTs in analytical chemistry is to obtain highly pure and well-characterized CNT materials. The commercially available CNTs have different diameters and lengths depending on the manufacturer, which undoubtedly affects their performances as well as comparison of the obtained results. Different conditions applied for oxidation of pristine nanotubes already affect the obtained results (Table 1).
Funding
This research received no external funding.
Data Availability Statement
Not available.
Conflicts of Interest
The author declares no conflict of interest.
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Li, W.K.; Shi, Y.P. Recent advances and applications of carbon nanotubes based composites in magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2019, 118, 652–665. [Google Scholar] [CrossRef]
- Vesali-Naseh, M.; Naseh, M.R.V.; Ameri, P. Adsorption of Pb(II) ions from aqueous solutions using carbon nanotubes: A systematic review. J. Cleaner Prod. 2021, 291, 125917. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Sinha Ray, S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar]
- Treviño, M.J.S.; Zarazùa, S.; Płotka-Wasylka, J. Nanosorbents as materials for extraction processes of environmental contaminants and others. Molecules 2022, 27, 1067. [Google Scholar] [CrossRef]
- Trivedi, M.; Reecha, K. Recent development and applications of carbon nanotubes. Chem. Sci. Rev. Lett. 2020, 9, 502–510. [Google Scholar]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Moghaddam, H.K.; Maraki, M.R.; Rajaei, A. Application of carbon nanotubes (CNT) on the computer science and electrical engineering: A review. Int. J. Reconfig. Embed. Syst. 2020, 9, 61–82. [Google Scholar]
- Rasheed, T.; Hassan, A.A.; Kausar, F.; Sher, F.; Bilal, M.; Iqbal, H.M.N. Carbon nanotubes assisted analytical detection-Sensing/delivery cues for environmental and biomedical monitoring. TrAC Trends Anal. Chem. 2020, 132, 116066. [Google Scholar] [CrossRef]
- Garg, A.; Chalak, H.D.; Belarbi, M.O.; Zenkour, A.M.; Sahoo, R. Estimation of carbon nanotubes and their applications as reinforcing composite materials-An engineering review. Compos. Struct. 2021, 272, 114234. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A.; Elshehy, E.A. Hierarchical inorganic–organic multi-shell nanospheres for intervention and treatment of lead-contaminated blond. Nanoscale 2013, 5, 7920–7927. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Khairy, M.; Shenashen, M.A.; Elshhehy, E.; Warkocki, W.; Sakai, M. Optical mesoscopic membrane sensor layouts for water-free and blood-free toxicants. Nano Res. 2015, 8, 3150–3163. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube-A review on synthesis, properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: A critical review. Int. J. Nanomed. 2020, 15, 7063–7078. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon nanotubes (CNTs) from synthesis to functionalized (CNTs) using conventional and new chemical approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Gacem, A.; Modi, S.; Yadav, V.K.; Islam, S.; Patel, A.; Dawane, V.; Jameel, M.; Inwati, G.K.; Piplode, S.; Solanki, V.S.; et al. Recent advances in methods for synthesis of carbon nanotubes and carbon nanocomposite and their emerging applications: A descriptive review. J. Nanomater. 2022, 2022, 7238602. [Google Scholar] [CrossRef]
- Pattanshetti, A.; Pradeep, N.; Chaitra, V.; Uma, V. Synthesis of multi-walled carbon nanotubes (MWCNTs) from plastic waste & analysis of garlic coated gelatin/MWCNTs nanocomposite films as food packaging material. Appl. Sci. 2020, 2, 730. [Google Scholar]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Tijani, J.O.; Ani, J.I.; Krikstolaityte, V.; Srinivasan, M.; Veksha, A.; Lisak, G. Taguchi optimization design of diameter-controlled synthesis of multi walled carbon nanotubes for the adsorption of Pb(II) and Ni(II) from chemical industry wastewater. Chemosphere 2021, 266, 128937. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi1, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Baghel, P.; Sakhiya, A.K.; Priyanka Kaushal, P. Ultrafast growth of carbon nanotubes using microwave irradiation: Characterization and its potential applications. Heliyon 2022, 8, e10943. [Google Scholar] [CrossRef]
- Kure, N.; Hamidon, N.M.; Azhari, S.; Mamat, N.S.; Yusoff, H.M.; Isa, B.M.; Yunusa, Z. Simple microwave-assisted synthesis of carbon nanotubes using polyethylene as carbon precursor. J. Nanomater. 2017, 2017, 2474267. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Dyachkova, T.P.; Rukhov, A.V.; Tkachev, A.G.; Tugolukov, E.N. Functionalization of carbon nanotubes: Methods, mechanisms and technological realization. Adv. Mater. Technol. 2018, 2, 18–41. [Google Scholar] [CrossRef]
- Aslam, M.M.; Kuo, H.W.; Den, W.; Usman, M.; Sultan, H.; Ashraf, M. Functionalized carbon nanotubes (CNTs) for water and wastewater treatment: Preparation to application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Chemical functionalization of carbon nanotubes with polymers: A brief overview. Macromol 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, H.; Teng, Y.; Xiong, Y.; Chen, Z.; You, L.; Xiao, D. Recent advances in magnetic carbon nanotubes: Synthesis, challenges and highlighted applications. J. Mater. Chem. B 2021, 9, 9076–9099. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibore, O.A. Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578. [Google Scholar] [CrossRef]
- Pyrzynska, K. Nanomaterials in speciation analysis of metals and metalloids. Talanta 2020, 212, 120784. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Nouri, N.; Khorram, P.; Duman, O.; Sibel, T.; Hassan, S. Overview of nanosorbents used in solid phase extraction techniques for the monitoring of emerging organic contaminants in water and wastewater samples. Trends Environ. Anal. Chem. 2020, 25, e00081. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Manna, K. Sources and toxicological effects of lead on human health. Indian J. Med. Spec. 2019, 10, 66–71. [Google Scholar]
- Tsaridou, C.; Karabelas, A.J. Drinking water standards and their implementation-A critical assessment. Water 2021, 13, 2918. [Google Scholar] [CrossRef]
- Büyüktiryaki, S.; Keçili, R.; Hussain, C.M. Functionalized nanomaterials in dispersive solid phase extraction: Advances & prospects. TrAC Trends Anal. Chem. 2020, 127, 115893. [Google Scholar]
- Ghorbani, M.; Aghamohammadhassan, M.; Ghorbani, H.; Ali Zabihi, A. Trends in sorbent development for dispersive micro-solid phase extraction. Microchem. J. 2020, 158, 105250. [Google Scholar]
- Gugushe, A.S.; Mpupa, A.; Nomngongo, P.N. Ultrasound-assisted magnetic solid phase extraction of lead and thallium in complex environmental samples using magnetic multiwalled carbon nanotubes/zeolite nanocomposite. Microchem. J. 2019, 149, 103960. [Google Scholar] [CrossRef]
- Krawczyk, M.; Jeszka-Skowron, M. Multiwalled carbon nanotubes as solid sorbent in dispersive micro solid-phase extraction for the sequential determination of cadmium and lead in water samples. Microchem. J. 2016, 126, 296–301. [Google Scholar] [CrossRef]
- Azorín, C.; Benedé, J.L.; Alberto Chisvert, A. New challenges in sample preparation: Miniaturized stir bar sorptive dispersive microextraction as a high-throughput and feasible approach for low-availability sample preparation. Anal. Chim. Acta 2023, 1238, 340627. [Google Scholar] [CrossRef]
- Song, X.Y.; Chen, J.; Shi, Y.P. Different configurations of carbon nanotubes reinforced solid-phase microextraction techniques and their applications in the environmental analysis. TrAC Trends Anal. Chem. 2017, 96, 263–275. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Ghaemi, F.; Amiri, A.; Yunus, R. Methods for coating solid-phase microextraction fibers with carbon nanotubes. TrAC Trends Anal. Chem. 2014, 59, 133–143. [Google Scholar]
- Domagała, K.; Borlaf, M.; Traber, J.; Kata, D.; Graule, T. Purification and functionalisation of multi-walled carbon nanotubes. Mater Lett. 2019, 253, 272–275. [Google Scholar] [CrossRef]
- Safo, I.A.; Liu, F.; Xie, K.; Wei Xia, W. Oxidation and stability of multi-walled carbon nanotubes in hydrogen peroxide solution. Mat. Chem. Phys. 2018, 214, 472–481. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Rodríguez, C.; Leiva, E. Enhanced heavy metal removal from acid mine drainage wastewater using double-oxidized multiwalled carbon nanotubes. Molecules 2020, 25, 111. [Google Scholar] [CrossRef]
- Bayazit, S.S.; Inci, I. Adsorption of Pb(II) ions from aqueous solutions by carbon nanotubes oxidized different methods. J. Ind. Eng. Chem. 2013, 19, 2064–2070. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhou, A.L.; Peng, F.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metalPb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466, 201–206. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lin, W.H.; Chang, Y.C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Yu, X.Y.; Luo, T.; Zhang, Y.X.; Jia, Y.; Zhu, B.J.; Fu, X.C.; Liu, J.H.; Huang, X.J. Adsorption of lead(II) on O2-plasma-oxidized multiwalled carbon nanotubes: Thermodynamics, kinetics, and desorption. ACS Appl. Mater. Interfaces 2011, 3, 2585–2593. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Jie, F.; Zhao, Z.; Zhou, K.; Liu, H. The selective adsorption performance and mechanism of multiwall magnetic carbon nanotubes for heavy metals in wastewater. Sci. Rep. 2021, 11, 16878. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Skelton, A.A.; Nyamori, V.O.; Jonnalagadda, S.B.; Martincigh, B.S. Experimental and DFT studies on the selective adsorption of Pb2+ and Zn2+ from aqueous solution by nitrogen-functionalized multiwalled carbon nanotubes. Sep. Purif. Technol. 2017, 188, 174–187. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Fosso-Kankeu, E.; Ray, S.S. Efficient removal of Pb(II) and Cd(II) from industrial mine water by a hierarchical MoS2/SH-MWCNT nanocomposite. ACS Omega 2019, 4, 13922–13935. [Google Scholar] [CrossRef]
- Bajaj, S.; Jain, V.; Sharma, N.; Tiwari, S.; Saxena, R. Efficient lead preconcentration using two chemically functionalized carbon nanotubes in hyphenated injection-flame atomic absorption spectrometry system. J. Chromatogr. A 2021, 1638, 461888. [Google Scholar] [CrossRef]
- Farghali, A.A.; Abdel Tawab, H.A.; Abdel Moaty, S.A.; Khaled, R. Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. J. Nanostruct. Chem. 2017, 7, 101–111. [Google Scholar] [CrossRef]
- Hong, C.E.; Lee, J.H.; Kalappa, P.; Advani, S.G. Effects of oxidative conditions on properties of multi-walled carbon nanotubes in polymer nanocomposites. Compos. Sci. Technol. 2007, 67, 1027–1034. [Google Scholar] [CrossRef]
- Hosseini, H.; Mohammad Ghaffarzade, M. Surface functionalization of carbon nanotubes via plasma discharge: A review. Inorg. Chem. Commun. 2022, 138, 10927. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Ogawa, D.; Nakamura, K. Nitrogen functionalization of MWCNTs in Ar-N2 dielectric barrier discharge–Gas ratio effect. Mater. Sci Eng. B 2020, 61, 114680. [Google Scholar] [CrossRef]
- Sellaoui, L.; Schnorr, C.E.; Dhaouadi, F.; Taamalli, S.; Louis, F.; El Bakali, A.; Dotto, G.L.; Silva, L.F.O.; Lamine, A.B.; Rtimi, S.; et al. Modeling the adsorption of divalent metallic cations onto multi-walled carbon nanotubes functionalized with COOH. J. Mol. Liq. 2022, 366, 120275. [Google Scholar] [CrossRef]
- Xie, N.; Wang, H.; You, C. Role of oxygen functional groups in Pb2+ adsorption from aqueous solution on carbonaceous surface: A density functional theory study. J. Hazard. Mater. 2021, 405, 124221. [Google Scholar] [CrossRef]
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid materials based on carbon nanotubes and nanofilters for environmental analysis. Front. Chem. 2020, 8, 546. [Google Scholar] [CrossRef]
- Atif, M.; Afzaal, I.; Naseer, H.; Abrar, M.; Bongiovann, R. Review-Surface modification of carbon nanotubes: A tool to control electrochemical performance. ECS J. Solid State Sci. Technol. 2020, 9, 041009. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Álvarez-Méndez, J.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal. Chim. Acta 2015, 853, 77–94. [Google Scholar] [CrossRef]
- Kamaş, D.; Karatepe, A.; Soylak, M. Vortex-assisted magnetic solid phase extraction of Pb and Cd in some herb samples on magnetic multiwalled carbon nanotubes. Turk. J. Chem. 2021, 45, 210–218. [Google Scholar] [CrossRef]
- Feist, B.; Sitko, R. Method for the determination of Pb, Cd, Zn, Mn and Fe in rice samples using carbon nanotubes and cationic complexes of batophenanthroline. Food Chem. 2018, 249, 38–44. [Google Scholar] [CrossRef]
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S.; Dagdag, O. Magnetic multiwalled carbon nanotube decorated with novel functionalities: Synthesis and application as adsorbents for lead removal from aqueous medium. Processes 2020, 8, 986. [Google Scholar] [CrossRef]
- Abdel Salam, E.T.; Abou El-Nour, K.M.; Awad, A.A.; Orabi, A.S. Carbon nanotubes modified with 5,7,-dinitro-8-quinolinol as potentially applicable tool for efficient removal of industrial wastewater pollutants. Arab. J. Chem. 2020, 13, 109–119. [Google Scholar] [CrossRef]
- Torkian, L.; Amini, M.M.; Gorji, T.; Sadeghi, O. A simple, rapid and sensitive method based on modified multiwalled carbon nanotubes for preconcentration and determination of lead ions in aqueous media in natural pHs. Arab. J. Chem. 2019, 12, 1315–1321. [Google Scholar] [CrossRef]
- Islam, A.; Zaidi, N.; Ahmad, H.; Kumar, S. Functionalized carbon nanotubes for dispersive solid phase extraction and atomic absorption spectroscopic determination of toxic metals ions. Int. J. Environ. Sci. Technol. 2018, 16, 707–718. [Google Scholar] [CrossRef]
- Al-Faiyz, Y.S.S.; Gouda, M. Multi-walled carbon nanotubes functionalized with hydroxamic acid derivatives for the removal of lead from wastewater: Kinetics, isotherm, and thermodynamic studiem. Polymers 2022, 14, 3870. [Google Scholar] [CrossRef]
- Qu, G.; Zhou, J.; Liang, S.; Li, Y.; Ning, P.; Pan, K.; Ji, W.; Tang, H. Thiol-functionalized multi-walled carbon nanotubes for effective removal of Pb(II) from aqueous solutions. Mat. Chem. Phys. 2022, 278, 125688. [Google Scholar] [CrossRef]
- Kończyk, J.; Żarska, S.; Ciesielski, W. Adsorptive removal of Pb(II) ions from aqueous solutions by multi-walled carbon nanotubes functionalised by selenophosphoryl groups: Kinetic, mechanism, and thermodynamic studies. Colloids Surf. A 2019, 575, 271–282. [Google Scholar] [CrossRef]
- Tawfik, A.S. Insights into carbon nanotube-metal oxide composite: Embedding in membranes. Int. J. Sci. Res. Environ. Sci. Toxicol. 2017, 2, 1–4. [Google Scholar]
- Long, H.; Guo, C.; Wei, G.; Jiang, L.; Yu, Y. Facile synthesis of various carbon nanotube/metal oxide nanocomposites with high quality. Vacuum 2019, 166, 147–150. [Google Scholar] [CrossRef]
- Mallakpour, S.; Elham Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Islam, A.; Chauhan, A.; Javed, H.; Rais, S.; Izhar Ahmad, I. Magnetic carbon nanotubes-silica binary composite for effective Pb(II) sequestration from industrial effluents: Multivariate process optimization. Clean-Soil Air Water 2021, 49, 2000401. [Google Scholar] [CrossRef]
- Navaei Diva, T.N.; Zare, K.; Faleshi, F.; Yousefi, M. Synthesis, characterization, and application of nickel oxide/CNT nanocomposites to remove Pb2+ from aqueous solution. J. Nanostruct. Chem. 2017, 7, 273–281. [Google Scholar] [CrossRef]
- Wang, S.G.; Gong, W.X.; Liu, X.W.; Yao, Y.W.; Gao, B.Y.; Yue, Q.Y. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 2007, 58, 17–23. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Horng, J.J. Adsorption behaviour of heavy metal ions by carbon nanotubes grown on microsize Al2O3 particles. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2007, 14, 77–84. [Google Scholar]
- Zondo, B.Z.; Sadare, O.O.; Simate, G.S.; Moothi, K. Removal of Pb2+ ions from synthetic wastewater using functionalized multiwalled carbon nanotubes decorated with green synthesized iron oxide-gold nanocomposite. Water SA 2022, 48, 304–316. [Google Scholar]
- Egbosiuba, T.C.; Egwunyenga, M.C.; Tijani, J.O.; Mustapha, S.; Abdulkareem, A.; Kovo, A.S.; Krikstolaityte, V.; Veksha, A.; Wagner, M.; Lisak, G. Activated multi-walled carbon nanotubes decorated with zero valent nickel nanoparticles for arsenic, cadmium and lead adsorption from wastewater in a batch and continuous flow modes. J. Hazard. Mater. 2022, 423, 126993. [Google Scholar] [CrossRef]
- Fujigaya, T.; Nakashima, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 2015, 16, 024802. [Google Scholar] [CrossRef]
- Fujigaya, T. Development of polymer-wrapping methods for functionalization of carbon materials. Polym. J. 2022, 55, 181–191. [Google Scholar] [CrossRef]
- Shao, D.; Chen, C.; Wang, X. Application of polyaniline and multiwalled carbon nanotubes magnetic composites for removal of Pb(II). Chem. Eng. J. 2012, 185, 144–150. [Google Scholar] [CrossRef]
- Kanthapazham, R.; Ayyavu, C.; Mahendiradas, D. Removal of Pb2+, Ni2+ and Cd2+ ions in aqueous media using functionalized MWCNT wrapped polypyrolle nanocomposite. Desalination Water Treat. 2016, 57, 16871–16885. [Google Scholar]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, I.O.; Abubakre, O.K.; Roos, W.D. Selected heavy metals removal from electroplating wastewater by purified and polyhydroxylbutyrate functionalized carbon nanotubes adsorbents. Sci. Rep. 2019, 9, 4475. [Google Scholar] [CrossRef]
- Dehghania, Z.; Dadfarniaa, S.; Shabania, A.M.H.; Ehrampoushb, M.H. Magnetic multi-walled carbon nanotubes modified with polythiophene as a sorbent for simultaneous solid phase microextraction of lead and cadmium from water and food samples. Anal. Bioanal. Chem. Res. 2020, 7, 509–523. [Google Scholar]
- Alizadeh, B.; Ghorbani, M.; Salehi, M.A. Application of polyrhodanine modified multi-walled carbon nanotubes for high efficiency removal of Pb(II) from aqueous solution. J. Mol. Liq. 2016, 220, 142–149. [Google Scholar] [CrossRef]
- Jamshidian, M.; Sadeghalvad, B.; Ghasemi, I. Fabrication of polyethersulfone/functionalized MWCNTs nanocomposite and investigation its efficiency as an adsorbent of Pb(II) Ions. Arab. J. Sci. Eng. 2021, 46, 6259–6273. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Ihsanullah; Baig, N.; Osman, A.M. Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Sep. Purif. Technol. 2018, 191, 400–423. [Google Scholar] [CrossRef]
- Guo, D.; Huang, S.; Zhu, Y. The adsorption of heavy metal ions by poly(amidoamine) dendrimer-functionalized nano- materials: A Review. Nanomaterials 2022, 12, 1831. [Google Scholar] [CrossRef]
- Hayati, B.; Malekia, A.; Najafib, F.; Shivaraju, R.R.; Puttaiahc, H.; Marzband, N.; Gordon McKay, G. PPI/CNT nanocomposite for novel high capacity removal of the toxic heavy metals, Hg, Pb and Ni from water. Desalination Water Treat. 2020, 198, 190–199. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Loiola, A.R.; Bessa, R.A.; Oliveira, C.P.; Freitas, A.D.L.; Soares, S.A.; Bohn, F.; Pergher, S.B.C. Magnetic zeolite composites: Classification, synthesis routes, and technological applications. J. Magn. Magn. Mater. 2022, 560, 1169651. [Google Scholar] [CrossRef]
- Kim, I.J.; Zhao, W.; Park, J.G.; Meng, Z. Carbon nanotube filter for heavy metal ion adsorption. Ceram. Int. 2021, 47, 33280–33285. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon nanotube membranes in water treatment applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water cleaning adsorptive membranes for efficient removal of heavy metals and metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Rizzuto, C.; Pugliese, G.; Bahattab, M.A.; Aljlil, S.A.; Drioli, E.; Tocci, E. Multiwalled carbon nanotube membranes for water purification. Sep. Purif. Technol. 2018, 193, 378. [Google Scholar] [CrossRef]
- Jue, M.L.; Buchsbaum, S.F.; Chen, C.; Park, S.J.; Meshot, E.R.; Wu, K.J.J.; Fornasiero, F. Ultra-permeable single-walled carbon nanotube membranes with exceptional performance at scale. Adv. Sci. 2020, 7, 2001670. [Google Scholar] [CrossRef]
- Arrora, B.; Attri, P. Carbon nanotubes (CNTs): A potential nanomaterial for water puriffiication. J. Compos. Sci. 2020, 4, 1. [Google Scholar] [CrossRef]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dum, L.F. Carbon nanotube membranes-Strategies and challenges towards scalable manufacturing and practical separation applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and water treatment application of carbon nanotubes (CNTs)-based composite membranes: A Review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nicoletta, F.P.; De Filpo, G. GO-SWCNT buckypapers as an enhanced technology for water decontamination from lead. Molecules 2022, 27, 4044. [Google Scholar] [CrossRef]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Sep. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C.; Troisi, A.; Ciambelli, P. Pb removal on carbon nanotubes membrane decorated by ZnO nanoparticles. Chem. Eng. Trans. 2017, 60, 277–282. [Google Scholar]
- Musielak, M.; Gagor, A.; Zawisza, B.; Talik, E.; Rafal Sitko, R. Graphene oxide/carbon nanotube membranes for highly efficient removal of metal ions from water. ACS Appl. Mater. Interfaces 2019, 11, 28582–28590. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef]
- Lasheen, M.R.; El-Sherif, I.Y.; Sabry, D.Y.; El-Wakee, S.T.; El-Shahat, M.F. Removal of heavy metals from aqueous solution by multiwalled carbon nanotubes: Equilibrium, isotherms, and kinetics. Desalination Water Treat. 2015, 53, 3521–3530. [Google Scholar] [CrossRef]
- Sitko, R.; Musielak, M.; Zawisza, B.; Talik, E.; Gagor, A. graphene oxide/cellulose membranes in adsorption of divalent metal ions. RSC Adv. 2016, 6, 96595–96605. [Google Scholar] [CrossRef]
- Musielak, M.; Kocot, K.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I.; Walczak, B.; Sitko, R. Ultratrace determination of metal ions using graphene oxide/carbon nanotubes loaded cellulose membranes and total-reflection X-ray fluorescence spectrometry: A green chemistry approach. Spectrochim. Acta Part A 2021, 177, 106069. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Romero, V.; de la Calle, I.; Lavilla, I.; Bendicho, C. Graphene-based nanocomposites in analytical extraction processes. TrAC Trends Anal. Chem. 2021, 142, 116303. [Google Scholar] [CrossRef]
- Kam, C.S.; Leung, T.L.; Liu, F.; Djusic, B.; Xie, M.H.; Chan, W.K.; Zhou, W.K.; Shih, K. Lead removal from water-dependence on the form of carbon and surface functionalization. RSC Adv. 2018, 8, 18355–183362. [Google Scholar] [CrossRef]
- Andersson, C.H.; Grennberg, H. Reproducibility and efficiency of carbon nanotube end-group generation and functionalization. Eur. J. Org. Chem. 2009, 26, 4421–4428. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J.Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Spaolonzi, M.P.; Duarte, E.D.V.; Oliveira, M.G.; Costa, H.P.S.; Ribeiro, M.C.; Silva, T.I.; Silva, M.C.G.; Vieira, M.G.A. Green-functionalized carbon nanotubes as adsorbents for the removal of emerging contaminants from aqueous media. J. Clean. Prod. 2022, 373, 133961. [Google Scholar] [CrossRef]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Lucha, A. Challenges in characterizing the environment al fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems. Environ. Sci. Nano 2018, 5, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).