Promising Insecticidal Properties of Essential Oils from Artemisia aragonensis Lam. and Artemisia negrei L. (Asteraceae) by Targeting Gamma-Aminobutyric Acid and Ryanodine Receptor Proteins: In Vitro and In Silico Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
2.1.2. Animal Material
2.2. Experimental Methods
2.2.1. Extraction of Volatile Compounds
2.2.2. Calculation of Yield
2.2.3. Identification of Volatile Compounds in EOs by GC-MS
2.2.4. Biological Test for Volatile Activity (Toxicity)
- a.
- Contact toxicity tests
- b.
- Inhalation tests (Fumigant Toxicity)
2.3. Theoretical Methods
Statistical Analysis
3. Results and Discussion
3.1. Yields of EOs and Chemical Composition
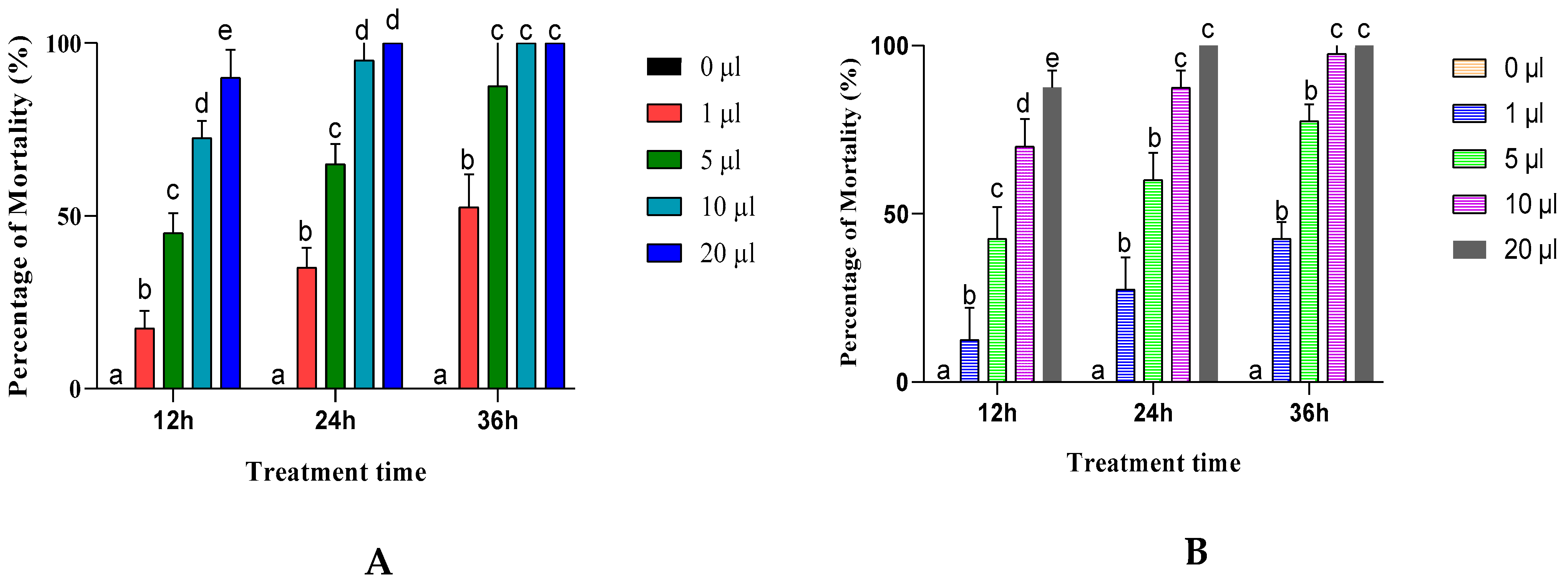
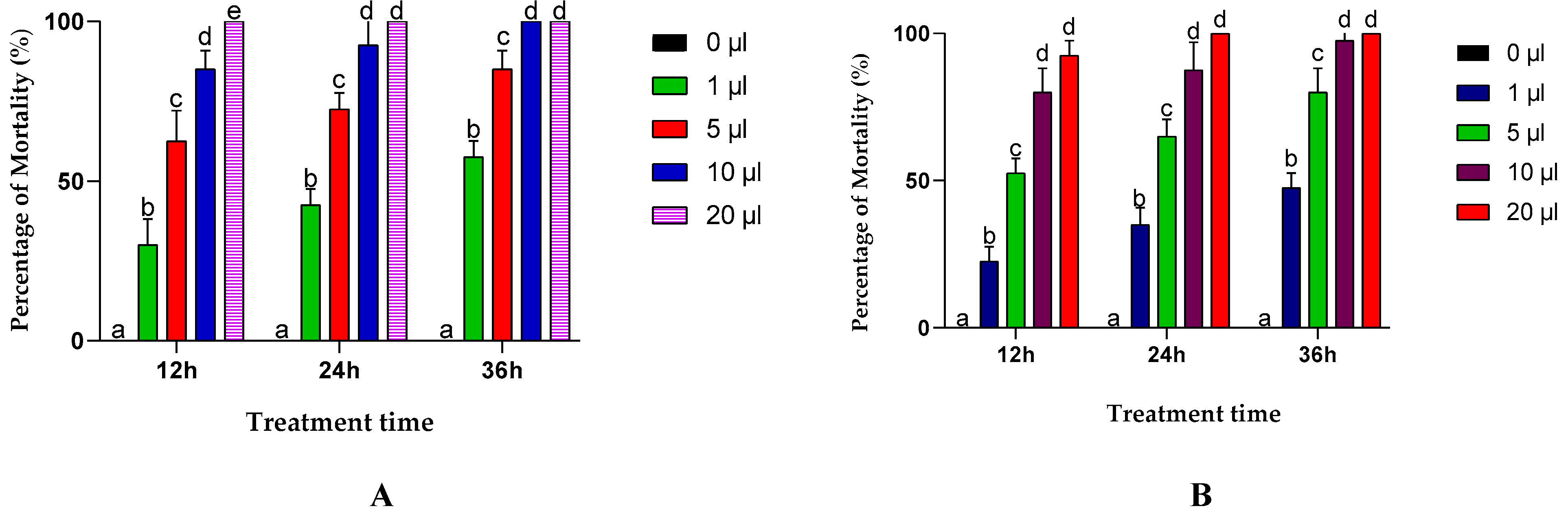
3.2. Inhalation Toxicity of EOs
3.3. Contact Toxicity of EOs from A. negrei and A. aragonensis
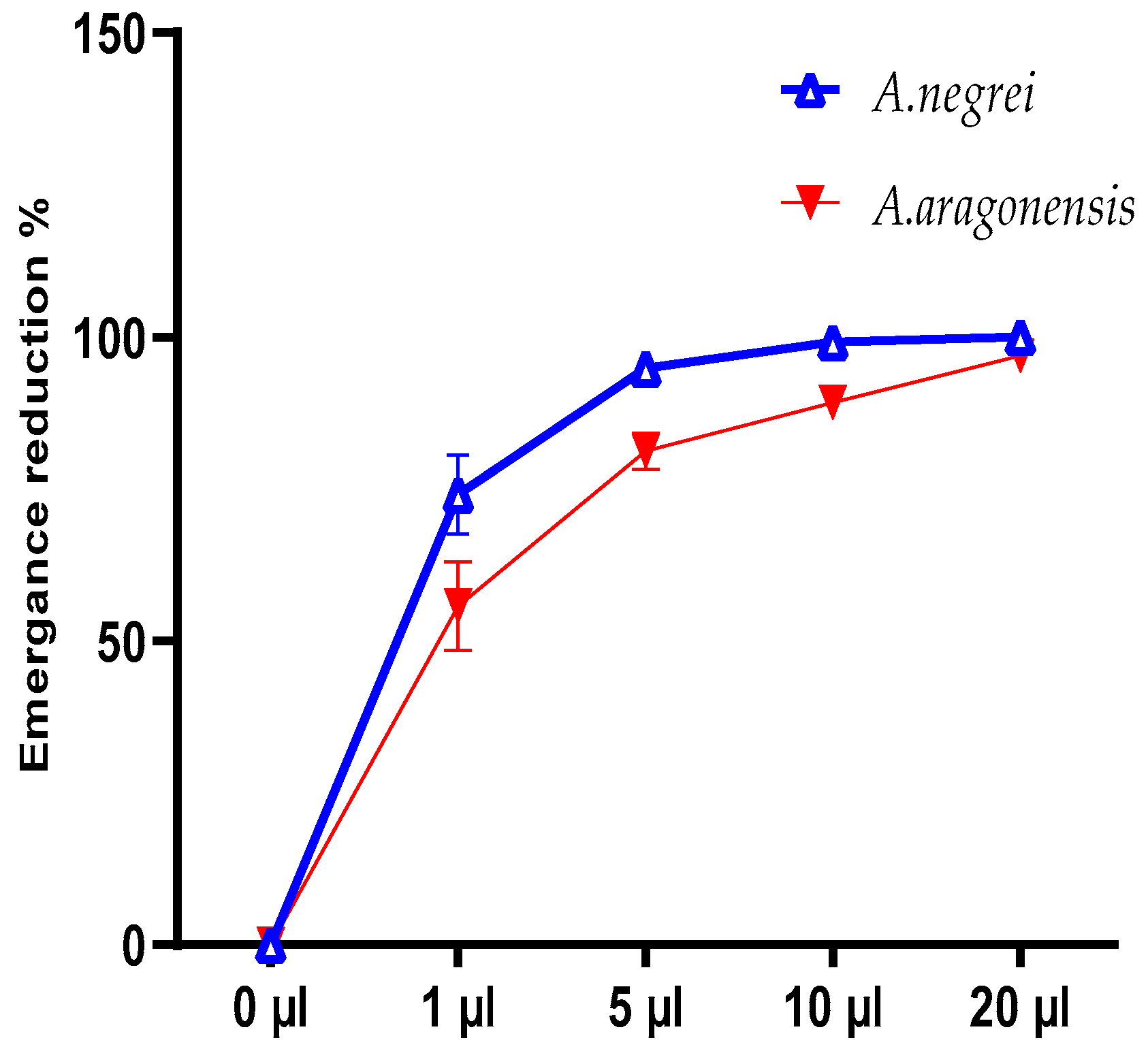
3.4. Effect of Direct Contact with EOs on C. maculatus Fab Fecundity and Emergence
4. Theoretical Calculations
4.1. Molecular Docking Study
4.2. ADME/Tox Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musolin, D.L.; Kirichenko, N.I.; Karpun, N.N.; Aksenenko, E.V.; Golub, V.B.; Kerchev, I.A.; Mandelshtam, M.Y.; Vasaitis, R.; Volkovitsh, M.G.; Zhuravleva, E.N.; et al. Invasive insect pests of forests and urban trees in Russia: Origin, pathways, damage, and management. Forests 2022, 13, 521. [Google Scholar] [CrossRef]
- Haggblade, S.; Diarra, A.; Traoré, A. Regulating agricultural intensification: Lessons from West Africa’s rapidly growing pesticide markets. Dev. Policy Rev. 2022, 40, e12545. [Google Scholar] [CrossRef]
- GM, E.L.; Reyes-Lagunes, I.; Scott, R.L. MMPI-2 for Mexico: Translation and adaptation. J. Pers. Assess. 1994, 63, 105–116. [Google Scholar]
- García-Oviedo, J.A. Elabora IPN frijol instantáneo altamente nutritivo. Nota Periodís. El Univers. Ed. 2007, 3, 43. [Google Scholar]
- Chávez-Díaz, G.; Valdés-Estrada, M.E.; Hernández-Reyes, M.C.; Gutiérrez-Ochoa, M.; Valladares-Cisneros, M.G. Aceites esenciales para controlar Acanthoscelides obtectus (Say) y Sitophilus zeamais (Motschulsky) plagas de granos almacenados. Rev. Mex. Agroecosistemas 2016, 3, 99–107. [Google Scholar]
- D’souza, C.; Taghian, M.; Lamb, P. An empirical study on the influence of environmental labels on consumers. Corp. Commun. Int. J. 2006, 11, 162–173. [Google Scholar] [CrossRef]
- Faham, S.; Hileman, R.E.; Fromm, J.R.; Linhardt, R.J.; Rees, D.C. Heparin structure and interactions with basic fibroblast growth factor. Science 1996, 271, 1116–1120. [Google Scholar] [CrossRef]
- Caswell, G.H. A review of the work done in the entomology section of the Institute for Agricultural Research on the pests of stored grain. Samaru Misc. Pap. 1980, 99, 12. [Google Scholar]
- Marannino, P.; Santiago-Álvarez, C.; de Lillo, E.; Quesada-Moraga, E. A new bioassay method reveals pathogenicity of Metarhizium anisopliae and Beauveria bassiana against early stages of Capnodis tenebrionis (Coleoptera; Buprestidae). J. Invertebr. Pathol. 2006, 93, 210–213. [Google Scholar] [CrossRef]
- Ramdani, A.; Ibriz, H.; Essahat, A. Le traitement des semences au thiaméthoxame contrôle le Sitone (Sitona lineatus (L.)) sur Fèverole au Maroc. Afr. Mediterr. Agric. J.-Al Awamia 2022, 135, 179–191. [Google Scholar]
- Iturralde-García, R.D.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J.; Riudavets, J.; Del Toro-Sánchez, C.L.; Rueda-Puente, E.O.; Martínez-Cruz, O.; Wong-Corral, F.J. Effect of controlled atmospheres on the insect Callosobruchus maculatus Fab. in stored chickpea. J. Stored Prod. Res. 2016, 69, 78–85. [Google Scholar] [CrossRef]
- Abdelkader, M.; Btissam, B.M.; Laila, P.N.; Jamal, P.I. Dénombrement Des Populations Naturelles De Rhizobium Du Pois Chiche (Cicer Arietinum) Dans Différents Sols Du Maroc. Eur. Sci. J. 2017, 13, 273–286. [Google Scholar] [CrossRef][Green Version]
- Lofty, H.M.; El-Aleem, A.E.-A.A.A.; Monir, H.H. Determination of insecticides malathion and lambda-cyhalothrin residues in zucchini by gas chromatography. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 255–260. [Google Scholar] [CrossRef][Green Version]
- Shayeghi, M.; Khoobdel, M.; Vatandoost, H. Determination of organophosphorus insecticides (malathion and diazinon) residue in the drinking water. Pak. J. Biol. Sci. PJBS 2007, 10, 2900–2904. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Lima, J.G.; Santos, J.P.; Cruz, C.D. Resistance to DDT and pyrethroids in Brazilian populations of Sitophilus zeamais Motsch.(Coleoptera: Curculionidae). J. Stored Prod. Res. 1995, 31, 145–150. [Google Scholar] [CrossRef]
- Kendall, C.; Silva, S.R.; Kelly, V.J. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol. Process. 2001, 15, 1301–1346. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Adler, C.; Bouda, H.; Fontem, D.A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Ribeiro, P.D.; Iribarne, O.O.; Jaureguy, L.; Navarro, D.; Bogazzi, E. Variable sex-specific mortality due to shorebird predation on a fiddler crab. Can. J. Zool. 2003, 81, 1209–1221. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Amiteye, S. Efficacy of mixing vegetable oils with pirimiphos-methyl against the maize weevil, Sitophilus zeamais Motschulsky in stored maize. J. Stored Prod. Res. 2005, 41, 57–66. [Google Scholar] [CrossRef]
- Harouna, M.A.; Baoua, I.; Lawali, S.; Tamò, M.; Amadou, L.; Mahamane, S.; Pittendrigh, B. Essai comparatif de l’utilisation des extraits du Neem et du virus entomopathogène MaviNPV dans la gestion des insectes ravageurs du niébé en milieu paysan au Niger. Int. J. Biol. Chem. Sci. 2019, 13, 950–961. [Google Scholar] [CrossRef]
- Poirié, M. Évolution et spécificité des interactions insectes hôtes–insectes parasitoïdes. Comptes Rendus Biol. 2019, 342, 265–267. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid. Based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef] [PubMed]
- Bourhia, M.; Laasri, F.E.; Aghmih, K.; Ullah, R.; Alqahtani, A.S.; Mahmood, H.M.; El-Mzibri, M.; Said, G.; Khlil, N.; Benbacer, L. Phytochemical composition, antioxidant activity, antiproliferative effect and acute toxicity study of Bryonia dioica roots used in North African alternative medicine. Int. J. Agric. Biol. 2020, 23, 597–602. [Google Scholar]
- Ebadollahi, A.; Nouri-Ganbalani, G.; Hoseini, S.A.; Sadeghi, G.R. Insecticidal activity of essential oils of five aromatic plants against Callosobruchus maculatus F.(Coleoptera: Bruchidae) under laboratory conditions. J. Essent. Oil Bear. Plants 2012, 15, 256–262. [Google Scholar] [CrossRef]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, anti-obesity, nutritional and other beneficial effects of different chili pepper: A review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Fusani, P.; Ronga, D.; Carminati, D.; Mandrioli, M.; Manicardi, G.C.; Giannì, S.; Tava, A. Composition and biological activity of essential oils from Artemisia roxburghiana Besser and Elsholtzia fruticosa Rehder cultivated in Italy. Ind. Crops Prod. 2022, 187, 115317. [Google Scholar] [CrossRef]
- Malhotra, A.; Rawat, A.; Prakash, O.; Kumar, R.; Srivastava, R.M.; Kumar, S. Chemical composition and pesticide activity of essential oils from Artemisia annua L. harvested in the rainy and winter seasons. Biochem. Syst. Ecol. 2023, 107, 104601. [Google Scholar] [CrossRef]
- Bachrouch, O.; Ferjani, N.; Haouel, S.; Jemâa, J.M.B. Major compounds and insecticidal activities of two Tunisian Artemisia essential oils toward two major coleopteran pests. Ind. Crops Prod. 2015, 65, 127–133. [Google Scholar] [CrossRef]
- Liu, C.H.; Mishra, A.K.; Tan, R.X.; Tang, C.; Yang, H.; Shen, Y.F. Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour. Technol. 2006, 97, 1969–1973. [Google Scholar] [CrossRef]
- Aljaiyash, A.; Kasrati, A.; Jamali, C.A.; Chaouch, A. Effect of cultivation on chemical composition and bioactivities of essential oils from Artemisia herba-alba Asso grown in Morocco. Biochem. Syst. Ecol. 2018, 81, 74–79. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef]
- Yuchi, Z.; Yuen, S.M.; Lau, K.; Underhill, A.Q.; Cornea, R.L.; Van Petegem, F. Crystal structures of ryanodine receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant. Nat. Commun. 2015, 6, 7947. [Google Scholar] [CrossRef]
- Lafraxo, S.; El Moussaoui, A.; Bin Jardan, Y.A.; El Barnossi, A.; Chebaibi, M.; Baammi, S.; Akka, A.A.; Chebbac, K.; Akhazzane, M.; Chelouati, T.; et al. GC-MS Profiling, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Essential Oil of Juniperus thurifera Bark. Evid. Based Complement. Altern. Med. 2022, 2022, e6305672. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- de Andrade Dutra, K.; de Oliveira, J.V.; Navarro, D.M.D.A.F.; Santos, J.P.O. Control of Callosobruchus maculatus (FABR.)(Coleoptera: Chrysomelidae: Bruchinae) in Vigna unguiculata (L.) WALP. with essential oils from four Citrus spp. plants. J. Stored Prod. Res. 2016, 68, 25–32. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar]
- Wekesa, I.; Onek, L.A.; Deng, A.L.; Hasanali, A.; Othira, J.O. Toxicity and repellant potency of Hyptis spicigera extracts on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Stored Prod. Postharvest Res. 2011, 2, 113–119. [Google Scholar]
- Bhat, M.A.; Tüzün, B.; Alsaif, N.A.; Khan, A.A.; Naglah, A.M. Synthesis, characterization, molecular modeling against EGFR target and ADME/T analysis of novel purine derivatives of sulfonamides. J. Mol. Struct. 2022, 1257, 132600. [Google Scholar] [CrossRef]
- Schrödinger. 4: Protein Preparation Wizard; Epik Schrödinger LLC: New York, NY, USA, 2016. [Google Scholar]
- Schrödinger. 2: LigPrep; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Shahzadi, I.; Zahoor, A.F.; Tüzün, B.; Mansha, A.; Anjum, M.N.; Rasul, A.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Repositioning of acefylline as anti-cancer drug: Synthesis, anticancer and computational studies of azomethines derived from acefylline tethered 4-amino-3-mercapto-1, 2, 4-triazole. PLoS ONE 2022, 17, e0278027. [Google Scholar] [CrossRef]
- Oh, E.; Wang, W.; Park, K.-H.; Park, C.; Cho, Y.; Lee, J.; Kang, E.; Kang, H. (+)-Usnic acid and its salts, inhibitors of SARS-CoV-2, identified by using in silico methods and in vitro assay. Sci. Rep. 2022, 12, 13118. [Google Scholar] [CrossRef]
- Diass, K.; Brahmi, F.; Mokhtari, O.; Abdellaoui, S.; Hammouti, B. Biological and pharmaceutical properties of essential oils of Rosmarinus officinalis L. and Lavandula officinalis L. Mater. Today Proc. 2021, 45, 7768–7773. [Google Scholar] [CrossRef]
- Zouirech, O.; Alajmi, R.; El Jeddab, H.; Allali, A.; Bourhia, M.; El Moussaoui, A.; El Barnossi, A.; Ahmed, A.M.; Giesy, J.P.; Aboul-Soud, M.A.M.; et al. Chemical Composition and Evaluation of Antifungal and Insecticidal Activities of Essential Oils Extracted from Jambosa caryophyllus (Thunb.) Nied: Clove Buds. Evid. Based Complement. Altern. Med. 2022, 2022, 4675016. [Google Scholar] [CrossRef] [PubMed]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 243–259. [Google Scholar]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A Review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Milutinović, M.; Savić, J. When Scent Becomes a Weapon—Plant Essential Oils as Potent Bioinsecticides. Sustainability 2022, 14, 6847. [Google Scholar] [CrossRef]
- Ketoh, G.K.; Koumaglo, H.K.; Glitho, I.A. Inhibition of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) development with essential oil extracted from Cymbopogon schoenanthus L. Spreng. (Poaceae), and the wasp Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2005, 41, 363–371. [Google Scholar] [CrossRef]
- Mssillou, I.; Saghrouchni, H.; Saber, M.; Zannou, A.J.; Balahbib, A.; Bouyahya, A.; Allali, A.; Lyoussi, B.; Derwich, E. Efficacy and role of essential oils as bio-insecticide against the pulse beetle Callosobruchus maculatus (F.) in post-harvest crops. Ind. Crops Prod. 2022, 189, 115786. [Google Scholar] [CrossRef]
- Pourya, M.; Sadeghi, A.; Ghobari, H.; Taning, C.N.T.; Smagghe, G. Bioactivity of Pistacia atlantica desf. Subsp. Kurdica (Zohary) Rech. F. and Pistacia khinjuk stocks essential oils against Callosobruchus maculatus (F, 1775) (Coloeptera: Bruchidae) under laboratory conditions. J. Stored Prod. Res. 2018, 77, 96–105. [Google Scholar] [CrossRef]
- Khelfane-Goucem, K.; Lardjane, N.; Medjdoub-Bensaad, F. Fumigant and repellent activity of Rutaceae and Lamiaceae essential oils against Acanthoscelides obtectus Say. Afr. J. Agric. Res. 2016, 11, 1499–1503. [Google Scholar]
- Agil, R.; Hosseinian, F.S. Bioactivity of alkylresorcinols. Bioact. Mol. Plant Foods 2012, 72, 131–162. [Google Scholar]
- Guèye, M.T.; Seck, D.; Wathelet, J.; Lognay, G. Lutte contre les ravageurs des stocks de céréales et de légumineuses au Sénégal et en Afrique occidentale: Synthèse bibliographique. Biotechnol. Agron. Soc. Environ. 2011, 15, 183–194. [Google Scholar]
- Aimad, A.; Bourhia, M.; Hana, H.; Sanae, R.; Salamatullah, A.M.; Soufan, W.; Rihan, H.Z.; Ouahmane, L.; Youness, E.A.; Noureddine, E.; et al. Essential Oils from Artemisia herba alba Asso., Maticaria Recutita L., and Dittrichia Viscosa L. (Asteraceae): A Promising Source of Eco-Friendly Agents to Control Callosobruchus maculatus Fab. Warehouse Pest. J. Chem. 2022, 2022, 2373460. [Google Scholar] [CrossRef]
- Sahaf, B.Z.; Moharramipour, S. Fumigant toxicity of Carum copticum and Vitex pseudo-negundo essential oils against eggs, larvae and adults of Callosobruchus maculatus. J. Pest Sci. 2008, 81, 213–220. [Google Scholar] [CrossRef]
- Abd-Elhady, H. Insecticidal activity and chemical composition of essential oil from Artemisia judaica L. against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Plant Prot. Res. 2012, 52, 347–352. [Google Scholar] [CrossRef]
- Titouhi, F.; Amri, M.; Messaoud, C.; Haouel, S.; Youssfi, S.; Cherif, A.; Ben Jemâa, J.M. Protective effects of three Artemisia essential oils against Callosobruchus maculatus and Bruchus rufimanus (Coleoptera: Chrysomelidae) and the extended side-effects on their natural enemies. J. Stored Prod. Res. 2017, 72, 11–20. [Google Scholar] [CrossRef]
- Manzoomi, N.; Ganbalani, G.N.; Dastjerdi, H.R.; Fathi, S.A.A. Fumigant toxicity of essential oils of Lavandula officinalis, Artemisia dracunculus and Heracleum persicum on the adults of Callosobruchus maculatus (Coleoptera: Bruchidae). Munn. Entomol. Zool. 2010, 5, 118–122. [Google Scholar]
- Seri-Kouassi, B.P.; Kanko, C.; Aboua, L.R.N.; Bekon, K.A.; Glitho, A.I.; Koukoua, G.; N’Guessan, Y.T. Action des huiles essentielles de deux plantes aromatiques de Côte-d’Ivoire sur Callosobruchus maculatus F. du niébé. Comptes Rendus Chim. 2004, 7, 1043–1046. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Chiasson, H.; Beloin, N. Les huiles essentielles, des biopesticides «Nouveau genre». Bull. Soc. D’Entomol. Qué. 2007, 14, 3–6. [Google Scholar]
- Abdelfattah, E.M.; Aimad, A.; Bourhia, M.; Chebbac, K.; Salamatullah, A.M.; Soufan, W.; Nafidi, H.-A.; Aboul-Soud, M.A.M.; Ouahmane, L.; Bari, A. Insecticidal and Antifungal Activities of Chemically-Characterized Essential Oils from the Leaves of Withania frutescens L. Life 2022, 12, 88. [Google Scholar] [CrossRef]
- Aimad, A.; Youness, E.A.; Sanae, R.; El Moussaoui, A. Chemical Composition and Antifungal, Insecticidal and Repellent Activity of Essential Oils from Origanum compactum Benth. Used in the Mediterranean Diet. Front. Plant Sci. 2022, 13, 798259. [Google Scholar] [CrossRef]
- Chalkha, M.; Akhazzane, M.; Moussaid, F.Z.; Daoui, O.; Nakkabi, A.; Bakhouch, M.; Chtita, S.; Elkhattabi, S.; Housseini, A.I.; El Yazidi, M. Design, synthesis, characterization, in vitro screening, molecular docking, 3D-QSAR, and ADME-Tox investigations of novel pyrazole derivatives as antimicrobial agents. New J. Chem. 2022, 46, 2747–2760. [Google Scholar] [CrossRef]
- Chalkha, M.; Nakkabi, A.; Ben Hadda, T.; Berredjem, M.; El Moussaoui, A.; Bakhouch, M.; Saadi, M.; El Ammari, L.; Almalki, F.A.; Laaroussi, H.; et al. Crystallographic study, biological assessment and POM/Docking studies of pyrazoles-sulfonamide hybrids (PSH): Identification of a combined Antibacterial/Antiviral pharmacophore sites leading to in-silico screening the anti-COVID-19 activity. J. Mol. Struct. 2022, 1267, 133605. [Google Scholar] [CrossRef] [PubMed]
- Tapera, M.; Kekeçmuhammed, H.; Tüzün, B.; Sarıpınar, E.; Koçyiğit, Ü.M.; Yıldırım, E.; Doğan, M.; Zorlu, Y. Synthesis, carbonic anhydrase inhibitory activity, anticancer activity and molecular docking studies of new imidazolyl hydrazone derivatives. J. Mol. Struct. 2022, 1269, 133816. [Google Scholar] [CrossRef]
- Majumdar, D.; Philip, J.E.; Tüzün, B.; Frontera, A.; Gomila, R.M.; Roy, S.; Bankura, K. Unravelling the Synthetic Mimic, Spectroscopic Insights, and Supramolecular Crystal Engineering of an Innovative Heteronuclear Pb (II)-Salen Cocrystal: An Integrated DFT, QTAIM/NCI Plot, NLO, Molecular Docking/PLIP, and Antibacterial Appraisal. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4320–4339. [Google Scholar] [CrossRef]
- Koçyiğit, M.; Doğan, M.; Muğlu, H.; Taslimi, P.; Tüzün, B.; Yakan, H.; Bal, H.; Güzel, E.; Gülçin, I. Determination of biological studies and molecular docking calculations of isatin-thiosemicarbazone hybrid compounds. J. Mol. Struct. 2022, 1264, 133249. [Google Scholar] [CrossRef]
- Poustforoosh, A.; Hashemipour, H.; Tüzün, B.; Azadpour, M.; Faramarz, S.; Pardakhty, A.; Mehrabani, M.; Nematollahi, M.H. The Impact of D614G Mutation of SARS-CoV-2 on the Efficacy of Anti-viral Drugs: A Comparative Molecular Docking and Molecular Dynamics Study. Curr. Microbiol. 2022, 79, 241. [Google Scholar] [CrossRef]
- Mermer, A.; Bulbul, M.V.; Kalender, S.M.; Keskin, I.; Tuzun, B.; Eyupoglu, O.E. Benzotriazole-oxadiazole hybrid Compounds: Synthesis, anticancer Activity, molecular docking and ADME profiling studies. J. Mol. Liq. 2022, 359, 119264. [Google Scholar] [CrossRef]
- Kafa, A.H.T.; Tüzün, G.; Güney, E.; Aslan, R.; Sayın, K.; Tüzün, B. Synthesis, computational analyses, antibacterial and antibiofilm properties of nicotinamide derivatives. Struct. Chem. 2022, 33, 1189–1197. [Google Scholar] [CrossRef]
- Kekeçmuhammed, H.; Tapera, M.; Tüzün, B.; Akkoç, S.; Zorlu, Y.; Sarıpınar, E. Synthesis, Molecular Docking and Antiproliferative Activity Studies of a Thiazole-Based Compound Linked to Hydrazone Moiety. ChemistrySelect 2022, 7, e202201502. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]

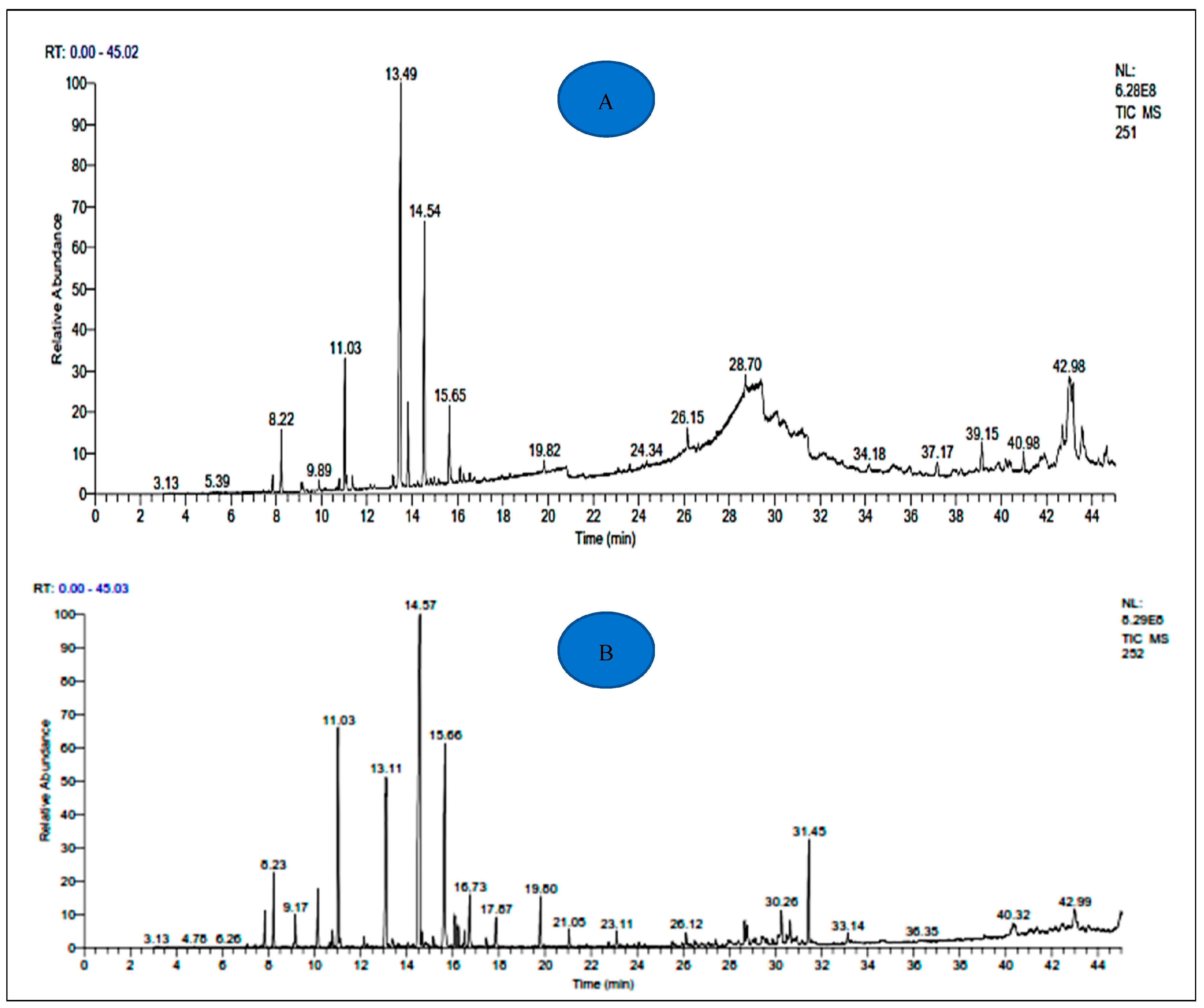

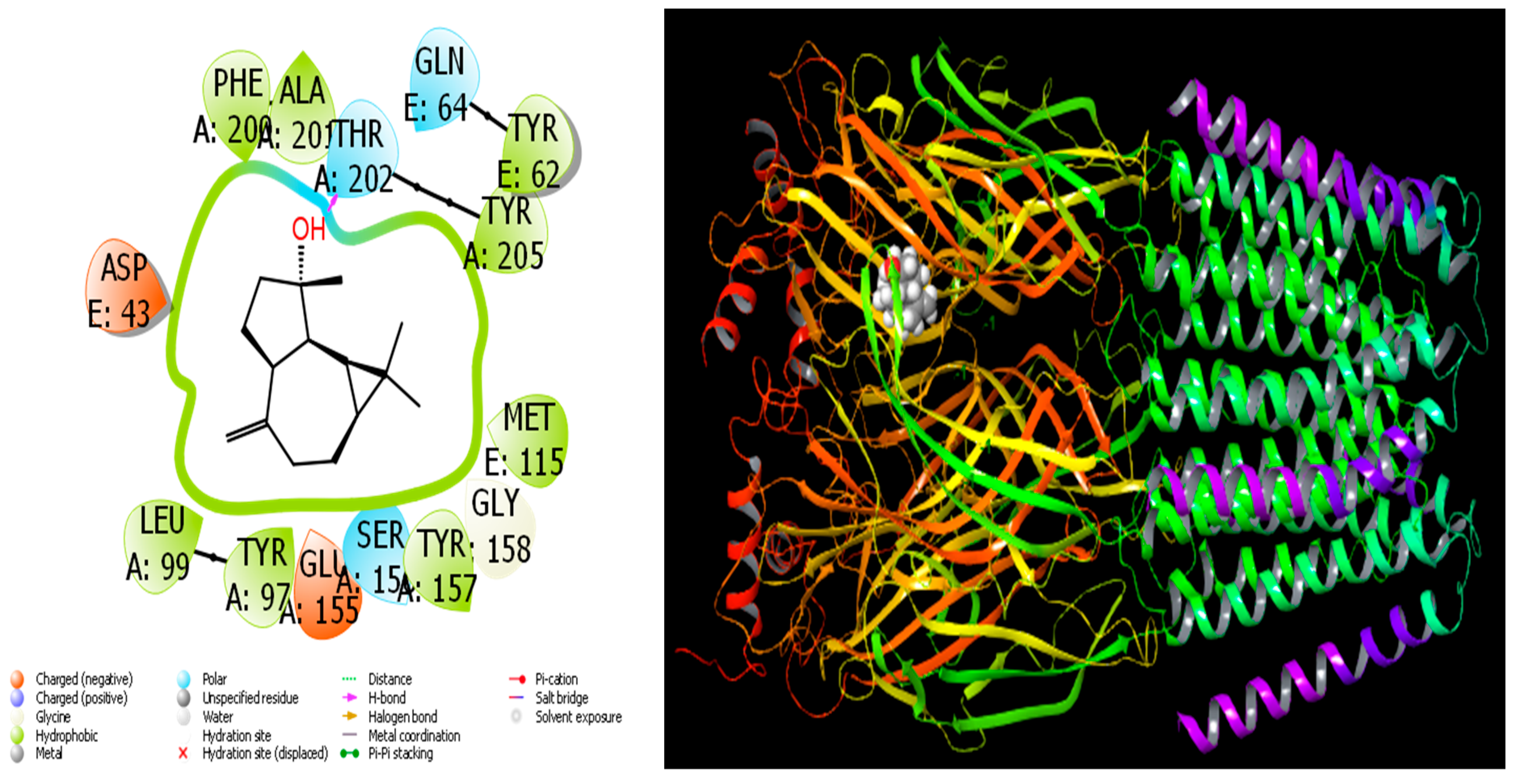
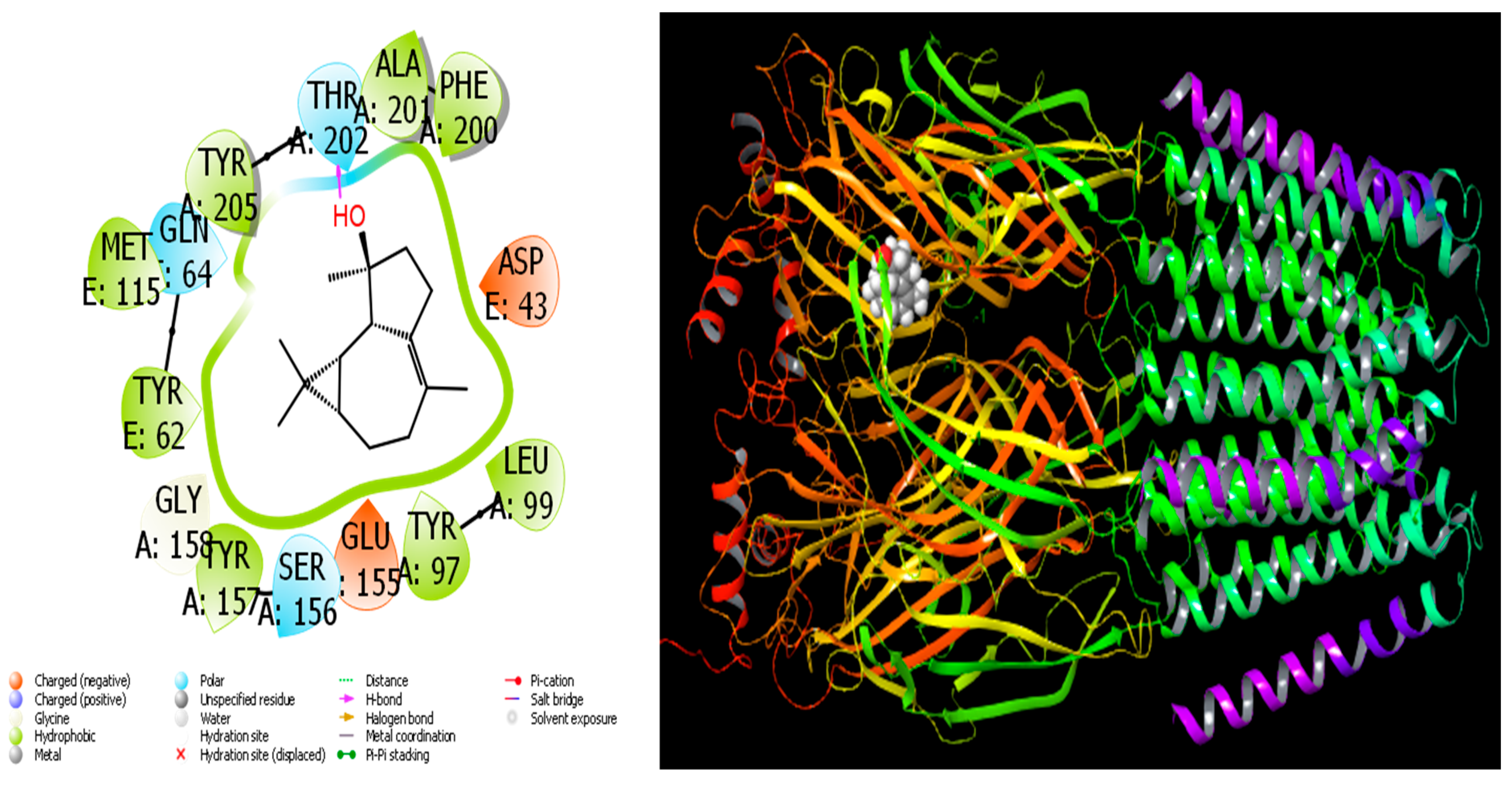
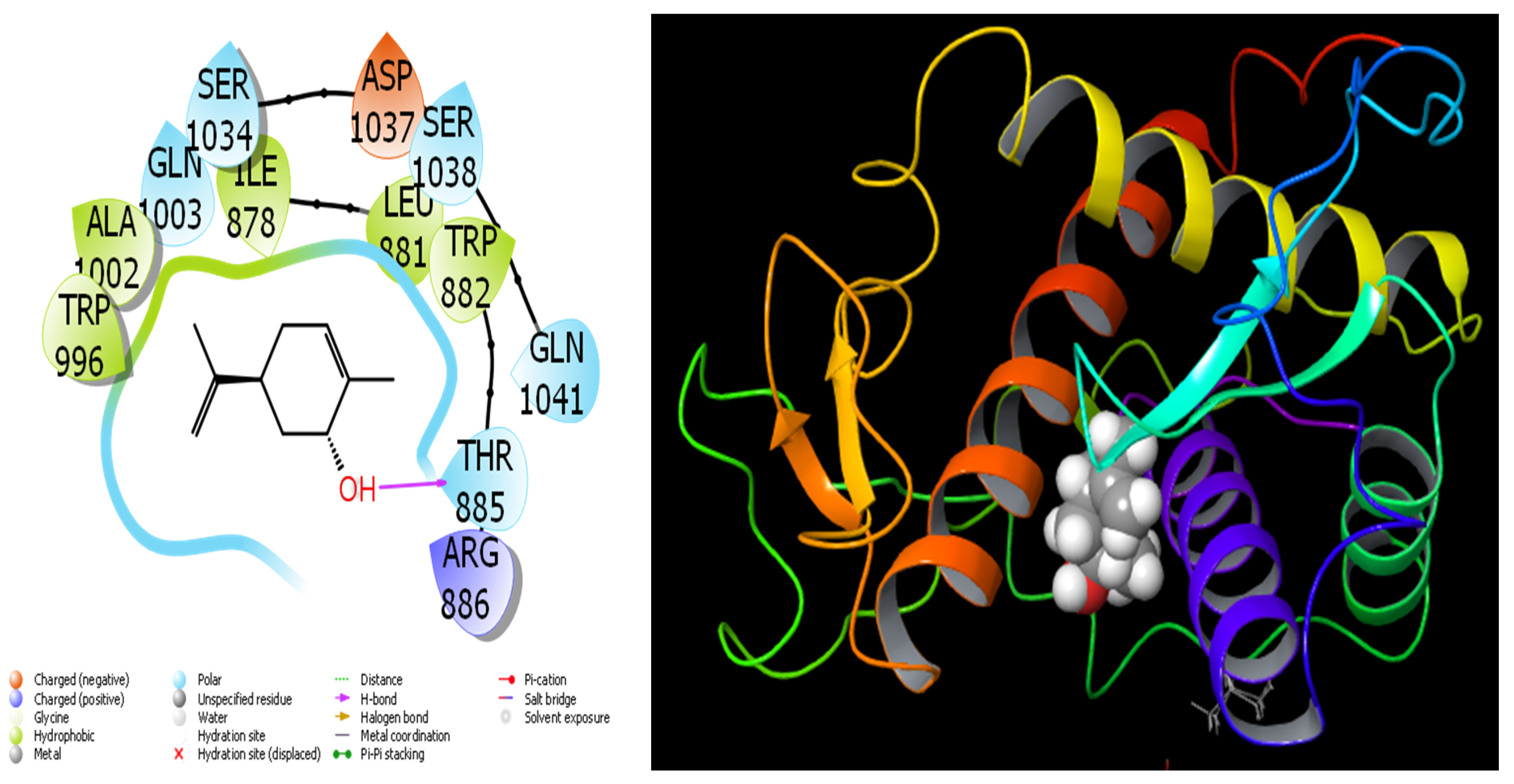
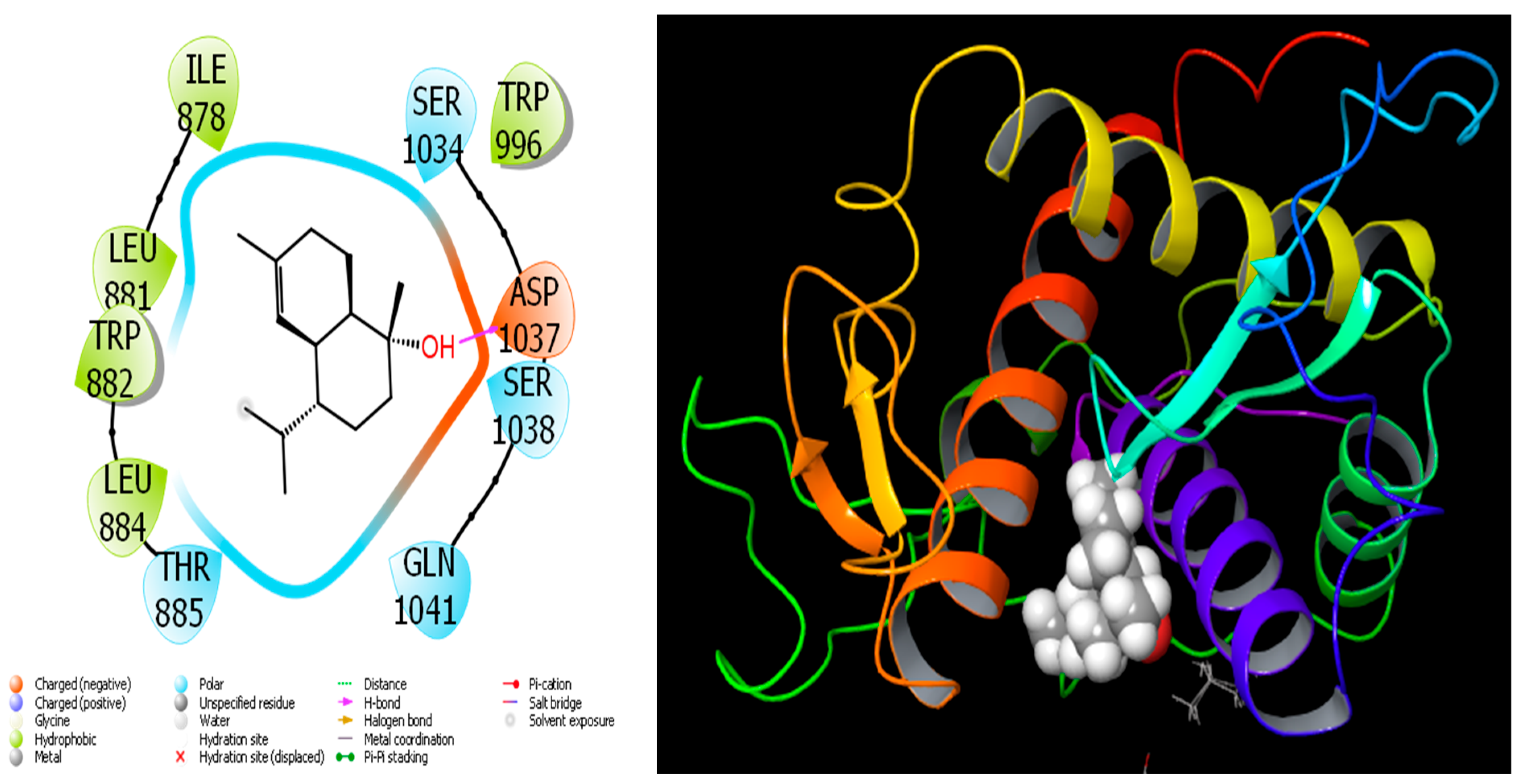
| Retention Index | Compound Name | A. aragonensis | A. negrei | ||||
|---|---|---|---|---|---|---|---|
| Peak | RT | Area (%) | Peak | RT | Area (%) | ||
| 933 | α-Pinene | 1 | 7.84 | 0.61 | 1 | 7.84 | 1.53 |
| 949 | Camphene | 2 | 8.22 | 2.38 | 2 | 8.23 | 3.10 |
| 980 | β-Pinene | 3 | 9.17 | 0.29 | 3 | 9.17 | 1.43 |
| 990 | Myrcene | 4 | 9.89 | 0.37 | - | - | - |
| 999 | Yamogi alcohol | - | - | - | 4 | 10.15 | 2.75 |
| 1026 | Cymene | 5 | 10.78 | 0.44 | 5 | 10.78 | 0.69 |
| 1029 | Limonene | 7 | 11.03 | 0.50 | - | - | - |
| 1032 | 1,8-Cineole | 6 | 11.11 | 5.60 | 6 | 11.03 | 10.88 |
| 1017 | α-Terpinene | - | - | - | 7 | 12.15 | 0.48 |
| 1086 | Fenchone | 8 | 11.36 | 0.50 | - | 13.11 | 10.20 |
| 1073 | Artemisia alcohol | 9 | 13.16 | 0.50 | 8 | - | - |
| 1102 | α-Thujone | 11 | 13.49 | 3.63 | 9 | 13.39 | 0.51 |
| 1114 | β-thujone | 10 | 13.82 | 29.02 | - | - | - |
| 1139 | Trans-pinocarveol | - | - | - | 11 | 14.65 | 0.52 |
| 1146 | Camphor | 12 | 14.54 | 14.68 | 10 | 14.57 | 24.97 |
| 1164 | Pinocarvone | - | - | - | 12 | 15.16 | 0.44 |
| 1169 | Borneol | 13 | 15.65 | 3.85 | 13 | 15.66 | 13.20 |
| 1082 | Terpinen-4-ol | - | - | - | 14 | 16.09 | 1.39 |
| 1173 | Artemisia acetate | - | - | - | 15 | 16.24 | 1.0 |
| 1133 | A-Terpineol | 15 | 16.54 | 0.35 | 16 | 16.51 | 0.69 |
| 1198 | Myrtenol | - | - | - | 17 | 16.73 | 2.73 |
| 1216 | Trans-Carveol | - | - | - | 18 | 17.46 | 0.42 |
| 1237 | Pulegone | - | - | - | 19 | 17.87 | 1.44 |
| 1288 | Bornyl acetate | 16 | 19.85 | 0.51 | 20 | 19.80 | 2.33 |
| 1298 | Geranyl formate | 17 | 20.81 | 0.88 | - | - | - |
| 1326 | Myrtenyl acetate | - | - | - | 21 | 21.05 | 0.83 |
| 1276 | α-Copaene | 18 | 26.15 | 1.00 | 22 | 23.11 | 0.75 |
| 1434 | Coumarin | 25 | 40.19 | 0.65 | - | - | - |
| 1479 | γ-Muurolene | 22 | 31.44 | 1.17 | - | - | - |
| 1485 | Germacrene D | - | - | - | 23 | 26.12 | 0.71 |
| 1513 | Cycloisolongifol-5-ol | 20 | - | 2.88 | - | - | - |
| 1578 | Spathulenol | - | - | - | 24 | 28.66 | 1.26 |
| 1586 | Caryophyllene oxide | - | - | - | 25 | 28.77 | 1.26 |
| 1624 | Isospathulenol | - | - | - | 26 | 30.12 | 0.50 |
| 1632 | γ-Eudesmo | - | - | - | 27 | 30.26 | 2.20 |
| 1633 | α-Acoreno | 21 | - | 1.00 | - | - | - |
| 1640 | Cadinol | - | - | - | 28 | 30.50 | 0.51 |
| 1641 | Aromadendrene epoxide | 19 | - | 1.20 | - | - | - |
| 1650 | β-Eudesmo | - | - | - | 29 | 30.64 | 1.30 |
| 1658 | Bisabolol oxyde B | - | - | - | 30 | 30.93 | 0.45 |
| 1667 | Limonen-4-ol | 14 | - | 0.56 | - | - | - |
| 1685 | Bisabolone oxide A | - | - | 31 | 31.45 | 5.63 | |
| 1718 | Curcuphenol | 23 | - | 1.09 | - | - | - |
| 1749 | α-Bisabolol oxide A | - | - | - | 32 | 33.14 | 0.56 |
| 1819 | Trihydroxy benzaIdehyde | 26 | 40.99 | 1.05 | - | - | - |
| 1829 | Isopropyltetradecanoate | 27 | 41.77 | 0.56 | - | - | - |
| 1845 | Isotorquatone | 28 | 41.91 | 1.43 | - | - | - |
| 1855 | Lanceol acetate | 29 | 42.56 | 0.95 | - | - | - |
| 1864 | thujopsenic acid | 30 | 42.69 | 1.54 | - | - | - |
| 1875 | Hexadecanol | 34 | 44.64 | 1.01 | - | - | - |
| 1960 | Palmitic acid | 24 | - | 1.72 | - | - | |
| 2125 | Octadecanoic acid, ethylester | 33 | 44.56 | 0.60 | - | - | - |
| 2500 | Pentacosane | 32 | 43.57 | 3.07 | 34 | 40.32 | 1.63 |
| 2800 | Octacosane | 31 | 43.02 | 14.02 | 33 | 42.99 | 1.33 |
| Monoterpene (C10) | 68.28% | 77.37% | |||||
| Sesquiterpene (C15) | 11.61% | 15.13% | |||||
| Other compounds | 25.02% | 7.12% | |||||
| Total compounds identified | 99.91% | 99.62% | |||||
| RT: retention time (min) | |||||||
| Plant | Test | Treatment (h) | df | Slope + SD | LC50 | LC95 | Intercept + SD | p Value | X2 |
|---|---|---|---|---|---|---|---|---|---|
| Artemisia negrei | Inhalation test | 12 | 3 | 0.14 + 0.01 | 7.88 | 16.36 | −1.13 + 0.09 | 0.0001 | 33.87 |
| 24 | 3 | 0.30 + 0.03 | 3.81 | 9.39 | −1.13 + 0.11 | 0.0001 | 29.23 | ||
| 36 | 3 | 0.47 + 0.04 | 2.1 | 5.62 | −0.98 + 0.12 | 0.0001 | 44.53 | ||
| Contact test | 12 | 3 | 0.24 + 0.02 | 4.67 | 11.55 | −1.11 + 0.11 | 0 | 29.13 | |
| 24 | 3 | 0.27 + 0.02 | 3.46 | 9.56 | −0.93 + 0.11 | 0 | 42.11 | ||
| 36 | 3 | 0.43 + 0.04 | 2.08 | 5.92 | −0.89 + 0.11 | 0 | 54.33 | ||
| Artemisia aragonensis | Inhalation test | 12 | 3 | 0.14 + 0.01 | 8.64 | 20.39 | −1.21 + 0.1 | 0 | 35.15 |
| 24 | 3 | 0.26 + 0.02 | 4.69 | 11.14 | −1.12 + 0.11 | 0 | 24.01 | ||
| 36 | 3 | 0.34 + 0.03 | 2.97 | 7.79 | −1.01 + 0.11 | 0 | 38.23 | ||
| Contact test | 12 | 3 | 0.15 + 0.01 | 6.61 | 17.33 | −1.01 + 0.1 | 0 | 45.57 | |
| 24 | 3 | 0.24 + 0.02 | 4.26 | 11.03 | −1.04 + 0.11 | 0 | 33.71 | ||
| 36 | 3 | 0.34 + 0.03 | 2.74 | 7.58 | −0.93 + 0.11 | 0 | 45.94 |
| Protein 4COF | Docking SCORE | Glide Ligand Efficiency | Glide Hbond | Glide Evdw | Glide Ecoul | Glide Emodel | Glide Energy | Glide Einternal | Glide Posenum |
| Spathulenol | −7.64 | −0.48 | −0.50 | −8.81 | −18.77 | −32.99 | −27.58 | 0.74 | 221 |
| Isospathulenol | −7.36 | −0.46 | −0.44 | −19.87 | −10.75 | −41.89 | −30.62 | 0.01 | 213 |
| Cadinol | −6.60 | −0.41 | −0.32 | −24.65 | −5.26 | −36.95 | −29.91 | 0.58 | 113 |
| Borneol | −6.59 | −0.60 | −0.32 | −18.00 | −11.89 | −41.00 | −29.89 | 0.14 | 114 |
| Trans-pinocarveol | −6.56 | −0.60 | −0.33 | −19.04 | −12.29 | −43.22 | −31.32 | 0.02 | 185 |
| Coumarin | −6.55 | −0.60 | −0.44 | −22.80 | −7.05 | −41.95 | −29.85 | 0.00 | 37 |
| Limonen-4-ol | −6.53 | −0.59 | −0.45 | −18.74 | −10.21 | −39.54 | −28.95 | 0.79 | 324 |
| Cycloisolongifol-5-ol | −6.37 | −0.40 | −0.32 | −20.31 | −5.71 | −28.92 | −26.02 | 0.01 | 202 |
| Camphor | −6.26 | −0.57 | −0.26 | −20.06 | −4.93 | −34.05 | −24.99 | 0.00 | 133 |
| Terpinen-4-ol | −6.25 | −0.57 | −0.42 | −18.60 | −10.17 | −36.07 | −28.77 | 6.33 | 237 |
| α-Copaene | −6.20 | −0.41 | 0.00 | −24.42 | 0.61 | −31.19 | −23.81 | 2.73 | 50 |
| Myrtenyl acetate | −6.15 | −0.44 | −0.32 | −26.10 | −6.03 | −44.97 | −32.12 | 0.70 | 346 |
| Bornyl acetate | −5.99 | −0.43 | 0.00 | −23.33 | −4.28 | −38.08 | −27.61 | 0.08 | 374 |
| Curcuphenol | −5.90 | −0.37 | −0.32 | −23.09 | −8.36 | −40.48 | −31.45 | 5.62 | 398 |
| β-Thujone | −5.86 | −0.53 | −0.32 | −19.93 | −5.61 | −34.50 | −25.54 | 0.59 | 58 |
| A-thujone | −5.86 | −0.53 | −0.32 | −19.93 | −5.61 | −34.50 | −25.54 | 0.59 | 58 |
| ϒ-Muurolene | −5.80 | −0.39 | 0.00 | −23.74 | −0.71 | −32.54 | −24.45 | 0.27 | 226 |
| Aromadendrene epoxide | −5.78 | −0.36 | −0.08 | −20.24 | −3.64 | −28.47 | −23.89 | 0.00 | 4 |
| A-Pinene | −5.77 | −0.58 | 0.00 | −18.68 | −1.32 | −27.08 | −20.00 | 0.00 | 265 |
| Caryophyllene oxide | −5.76 | −0.36 | 0.00 | −31.88 | −0.19 | −42.42 | −32.07 | 0.00 | 274 |
| Myrtenol | −5.67 | −0.52 | −0.25 | −24.80 | −4.41 | −39.11 | −29.21 | 0.71 | 128 |
| A-Terpineol | −5.62 | −0.51 | −0.36 | −16.49 | −10.19 | −34.04 | −26.67 | 4.07 | 263 |
| Germacrene D | −5.60 | −0.37 | 0.00 | −24.59 | −1.70 | −34.22 | −26.29 | 0.58 | 372 |
| Trans-Carveol | −5.32 | −0.48 | −0.32 | −15.05 | −11.57 | −32.71 | −26.62 | 4.50 | 242 |
| Fenchone | −5.29 | −0.48 | −0.31 | −19.68 | −4.97 | −32.76 | −24.65 | 0.00 | 121 |
| Pinocarvone | −5.26 | −0.48 | 0.00 | −23.95 | −4.39 | −37.18 | −28.34 | 0.00 | 141 |
| Pulegone | −5.22 | −0.47 | −0.31 | −19.54 | −4.82 | −32.38 | −24.36 | 0.00 | 146 |
| 1,8-Cineole | −4.95 | −0.45 | 0.00 | −24.41 | −2.39 | −35.37 | −26.80 | 0.00 | 329 |
| Camphene | −4.85 | −0.49 | 0.00 | −23.07 | −1.31 | −31.40 | −24.39 | 0.00 | 71 |
| Isotorquatone | −4.76 | −0.24 | −0.37 | −29.54 | −1.86 | −33.91 | −31.40 | 5.61 | 77 |
| β-Pinene | −4.73 | −0.47 | 0.00 | −22.79 | −1.95 | −31.69 | −24.74 | 0.00 | 31 |
| α-Terpinene | −4.68 | −0.47 | 0.00 | −20.56 | −2.70 | −29.57 | −23.25 | 0.77 | 267 |
| Artemisia acetate | −4.61 | −0.33 | −0.32 | −25.47 | −4.37 | −36.90 | −29.84 | 3.19 | 345 |
| Cymene | −4.58 | −0.46 | 0.00 | −21.09 | −2.63 | −30.45 | −23.71 | 0.10 | 208 |
| Limonene | −3.52 | −0.35 | 0.00 | −18.29 | −1.07 | −23.56 | −19.36 | 0.01 | 217 |
| α−Bisabolol oxide A | −3.31 | −0.19 | 0.00 | −7.17 | 0.81 | −8.92 | −6.36 | 10.04 | 308 |
| Artemisia alcohol | −3.30 | −0.30 | −0.32 | −19.43 | −9.21 | −32.79 | −28.64 | 3.14 | 150 |
| Geranyl formate | −2.54 | −0.20 | −0.32 | −23.39 | −4.89 | −31.95 | −28.28 | 2.94 | 60 |
| Lanceol acetate | −2.18 | −0.11 | −0.26 | −24.61 | −3.95 | −31.66 | −28.55 | 3.01 | 15 |
| Pentacosane | −2.04 | −0.08 | 0.00 | −34.54 | 0.59 | −35.76 | −33.95 | 4.85 | 92 |
| Octacosane | −1.49 | −0.05 | 0.00 | −34.64 | −0.61 | −35.73 | −35.24 | 5.51 | 370 |
| Myrcene | −0.91 | −0.09 | 0.00 | −18.36 | −0.84 | −20.21 | −19.20 | 0.32 | 183 |
| Isopropyltetradecanoate | −0.89 | −0.05 | 0.00 | −34.59 | −2.90 | −36.29 | −37.48 | 6.39 | 61 |
| Hexadecanol | −0.15 | −0.01 | −0.32 | −28.60 | −9.66 | −34.36 | −38.26 | 8.48 | 231 |
| Palmitic acid | 1.37 | 0.08 | −0.41 | −22.79 | −2.21 | −18.58 | −25.00 | 5.13 | 304 |
| Protein 5C30 | Docking Score | Glide Ligand Efficiency | Glide Hbond | Glide Evdw | Glide Ecoul | Glide Emodel | Glide Energy | Glide Einternal | Glide Posenum |
| Trans-Carveol | −5.41 | −0.49 | −0.31 | −18.16 | −2.84 | −25.04 | −21.00 | 5.60 | 144 |
| Cadinol | −5.15 | −0.32 | −0.15 | −21.51 | −2.82 | −32.50 | −24.33 | 0.17 | 4 |
| Spathulenol | −4.63 | −0.29 | −0.15 | −11.75 | −7.05 | −24.57 | −18.80 | 0.24 | 156 |
| Terpinen-4-ol | −4.18 | −0.38 | −0.32 | −11.96 | −4.96 | −21.42 | −16.91 | 0.15 | 19 |
| A-Terpineol | −3.96 | −0.36 | −0.32 | −10.97 | −4.73 | −19.72 | −15.69 | 0.14 | 9 |
| Pulegone | −3.92 | −0.36 | −0.32 | −11.50 | −3.73 | −19.04 | −15.23 | 0.00 | 367 |
| A-Terpinene | −3.90 | −0.39 | 0.00 | −15.14 | −1.43 | −20.48 | −16.57 | 0.00 | 169 |
| Cymene | −3.82 | −0.38 | 0.00 | −15.39 | −1.09 | −20.43 | −16.47 | 0.04 | 334 |
| Coumarin | −3.72 | −0.34 | −0.14 | −13.52 | −3.99 | −22.11 | −17.51 | 0.00 | 182 |
| Isospathulenol | −3.72 | −0.23 | −0.22 | −12.82 | −5.44 | −23.12 | −18.26 | 0.38 | 1 |
| β-thujone | −3.68 | −0.33 | 0.00 | −12.70 | −1.52 | −16.85 | −14.22 | 1.63 | 177 |
| α-thujone | −3.68 | −0.33 | 0.00 | −12.70 | −1.52 | −16.85 | −14.22 | 1.63 | 177 |
| Limonen-4-ol | −3.66 | −0.33 | −0.32 | −10.60 | −4.32 | −18.64 | −14.93 | 0.54 | 80 |
| Myrtenyl acetate | −3.49 | −0.25 | −0.32 | −16.70 | −3.80 | −25.03 | −20.50 | 0.46 | 69 |
| α−Bisabolol oxide A | −3.48 | −0.20 | −0.16 | −6.09 | −8.34 | 46.67 | −14.42 | 122.68 | 62 |
| Trans−pinocarveol | −3.33 | −0.30 | 0.00 | −13.42 | −1.44 | −17.50 | −14.86 | 0.91 | 24 |
| Caryophyllene oxide | −3.30 | −0.21 | 0.00 | −18.81 | −0.87 | −24.00 | −19.68 | 0.00 | 18 |
| Germacrene D | −3.25 | −0.22 | 0.00 | −15.89 | −0.05 | −19.30 | −15.93 | 0.00 | 272 |
| A-Copaene | −3.22 | −0.21 | 0.00 | −14.54 | −0.34 | −17.92 | −14.88 | 0.10 | 116 |
| Aromadendrene epoxide | −3.20 | −0.20 | 0.00 | −13.95 | −0.94 | −17.99 | −14.89 | 0.00 | 319 |
| Isotorquatone | −3.17 | −0.16 | −0.32 | −18.36 | −3.50 | −25.48 | −21.86 | 3.15 | 351 |
| ϒ-Muurolene | −3.15 | −0.21 | 0.00 | −12.28 | −0.45 | −15.32 | −12.73 | 0.00 | 4 |
| A-Pinene | −3.12 | −0.31 | 0.00 | −12.83 | −0.21 | −15.46 | −13.05 | 0.00 | 4 |
| Β-Pinene | −3.09 | −0.31 | 0.00 | −12.48 | −0.25 | −15.14 | −12.73 | 0.00 | 4 |
| Curcuphenol | −2.76 | −0.17 | −0.16 | −15.98 | −5.48 | −23.66 | −21.46 | 3.59 | 353 |
| Limonene | −2.57 | −0.26 | 0.00 | −10.85 | −1.60 | −14.42 | −12.44 | 0.00 | 296 |
| Pentacosane | −2.56 | −0.10 | 0.00 | −32.03 | −0.29 | −35.80 | −32.33 | 2.58 | 197 |
| Octacosane | −2.43 | −0.09 | 0.00 | −31.31 | −0.39 | −34.23 | −31.71 | 4.08 | 340 |
| Artemisia alcohol | −2.28 | −0.21 | −0.16 | −11.36 | −5.96 | −19.41 | −17.32 | 1.13 | 158 |
| Artemisia acetate | −2.28 | −0.16 | −0.32 | −13.10 | −2.90 | −18.43 | −16.00 | 0.82 | 388 |
| Geranyl formate | −1.67 | −0.13 | −0.16 | −14.63 | −2.49 | −17.68 | −17.12 | 3.23 | 193 |
| Lanceol acetate | −1.58 | −0.08 | 0.00 | −24.42 | −1.96 | −26.06 | −26.38 | 5.75 | 333 |
| Myrcene | −0.66 | −0.07 | 0.00 | −12.16 | −1.86 | −13.53 | −14.02 | 2.18 | 255 |
| Palmitic acid | −0.12 | −0.01 | −0.47 | −19.15 | −1.71 | −18.88 | −20.86 | 4.60 | 66 |
| Isopropyltetradecanoate | 0.72 | 0.04 | −0.32 | −26.07 | −1.95 | −25.52 | −28.02 | 2.26 | 106 |
| Hexadecanol | 2.28 | 0.13 | −0.48 | −19.07 | −6.44 | −20.10 | −25.51 | 2.98 | 246 |
| Spathulenol | Isospathulenol | Trans-Carveol | Cadinol | Reference Range | |
|---|---|---|---|---|---|
| mol_MW | 220 | 220 | 152 | 222 | 130–725 |
| dipole (D) | 2.239 | 2.134 | 1.652 | 2.073 | 1.0–12.5 |
| SASA | 460 | 468 | 395 | 480 | 300–1000 |
| FOSA | 398 | 426 | 293 | 429 | 0–750 |
| FISA | 32 | 36 | 40 | 34 | 7–330 |
| PISA | 30 | 5 | 62 | 18 | 0–450 |
| WPSA | 0 | 0 | 0 | 0 | 0–175 |
| volume (A3) | 819 | 826 | 640 | 845 | 500–2000 |
| donorHB | 1 | 1 | 1 | 1 | 0–6 |
| accptHB | 0.75 | 0.75 | 1.7 | 0.75 | 2.0–20.0 |
| glob (Sphere = 1) | 0.9 | 0.9 | 0.9 | 0.9 | 0.75–0.95 |
| QPpolrz (A3) | 26.3 | 26.3 | 18.8 | 26.6 | 13.0–70.0 |
| QPlogPC16 | 6.8 | 6.7 | 5.2 | 6.8 | 4.0–18.0 |
| QPlogPoct | 9.8 | 9.8 | 7.6 | 9.8 | 8.0–35.0 |
| QPlogPw | 3.2 | 3.1 | 4.1 | 3.0 | 4.0–45.0 |
| QPlogPo/w | 3.9 | 3.8 | 2.3 | 4.0 | −2.0–6.5 |
| QPlogS | −4.1 | −4.3 | −2.4 | −4.4 | −6.5–0.5 |
| CIQPlogS | −2.9 | −3.0 | −1.7 | −3.4 | −6.5–0.5 |
| QPlogHERG | −3.0 | −3.0 | −3.3 | −3.2 | * |
| QPPCaco (nm/s) | 4918 | 4524 | 4175 | 4736 | ** |
| QPlogBB | 0.3 | 0.2 | 0.1 | 0.2 | −3.0–1.2 |
| QPPMDCK (nm/s) | 2767 | 2528 | 2318 | 2657 | ** |
| QPlogKp | −1.9 | −2.1 | −1.8 | −1.9 | Kp in cm/hr |
| IP (ev) | 9.8 | 9.1 | 9.6 | 9.5 | 7.9–10.5 |
| EA (eV) | −1.1 | −1.1 | −0.9 | −1.0 | −0.9–1.7 |
| #metab | 3 | 5 | 5 | 4 | 1–8 |
| QPlogKhsa | 0.6 | 0.7 | −0.1 | 0.7 | −1.5–1.5 |
| Human Oral Absorption | 3 | 3 | 3 | 3 | - |
| Percent Human Oral Absorption | 100 | 100 | 100 | 100 | *** |
| PSA | 20 | 20 | 21 | 19 | 7–200 |
| RuleOfFive | 0 | 0 | 0 | 0 | Maximum is 4 |
| RuleOfThree | 0 | 0 | 0 | 0 | Maximum is 3 |
| Jm | 0.2 | 0.1 | 6.9 | 0.1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebbac, K.; Benziane Ouaritini, Z.; Allali, A.; Tüzün, B.; Zouirech, O.; Chalkha, M.; El Moussaoui, A.; Lafraxo, S.; Nafidi, H.-A.; Bin Jardan, Y.A.; et al. Promising Insecticidal Properties of Essential Oils from Artemisia aragonensis Lam. and Artemisia negrei L. (Asteraceae) by Targeting Gamma-Aminobutyric Acid and Ryanodine Receptor Proteins: In Vitro and In Silico Approaches. Separations 2023, 10, 329. https://doi.org/10.3390/separations10060329
Chebbac K, Benziane Ouaritini Z, Allali A, Tüzün B, Zouirech O, Chalkha M, El Moussaoui A, Lafraxo S, Nafidi H-A, Bin Jardan YA, et al. Promising Insecticidal Properties of Essential Oils from Artemisia aragonensis Lam. and Artemisia negrei L. (Asteraceae) by Targeting Gamma-Aminobutyric Acid and Ryanodine Receptor Proteins: In Vitro and In Silico Approaches. Separations. 2023; 10(6):329. https://doi.org/10.3390/separations10060329
Chicago/Turabian StyleChebbac, Khalid, Zineb Benziane Ouaritini, Aimad Allali, Burak Tüzün, Otmane Zouirech, Mohammed Chalkha, Abdelfattah El Moussaoui, Soufyane Lafraxo, Hiba-Allah Nafidi, Yousef A. Bin Jardan, and et al. 2023. "Promising Insecticidal Properties of Essential Oils from Artemisia aragonensis Lam. and Artemisia negrei L. (Asteraceae) by Targeting Gamma-Aminobutyric Acid and Ryanodine Receptor Proteins: In Vitro and In Silico Approaches" Separations 10, no. 6: 329. https://doi.org/10.3390/separations10060329
APA StyleChebbac, K., Benziane Ouaritini, Z., Allali, A., Tüzün, B., Zouirech, O., Chalkha, M., El Moussaoui, A., Lafraxo, S., Nafidi, H.-A., Bin Jardan, Y. A., Bourhia, M., & Guemmouh, R. (2023). Promising Insecticidal Properties of Essential Oils from Artemisia aragonensis Lam. and Artemisia negrei L. (Asteraceae) by Targeting Gamma-Aminobutyric Acid and Ryanodine Receptor Proteins: In Vitro and In Silico Approaches. Separations, 10(6), 329. https://doi.org/10.3390/separations10060329










