Fractionation of Marigold Waxy Extract Using Supercritical CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Supercritical Fluid Extraction
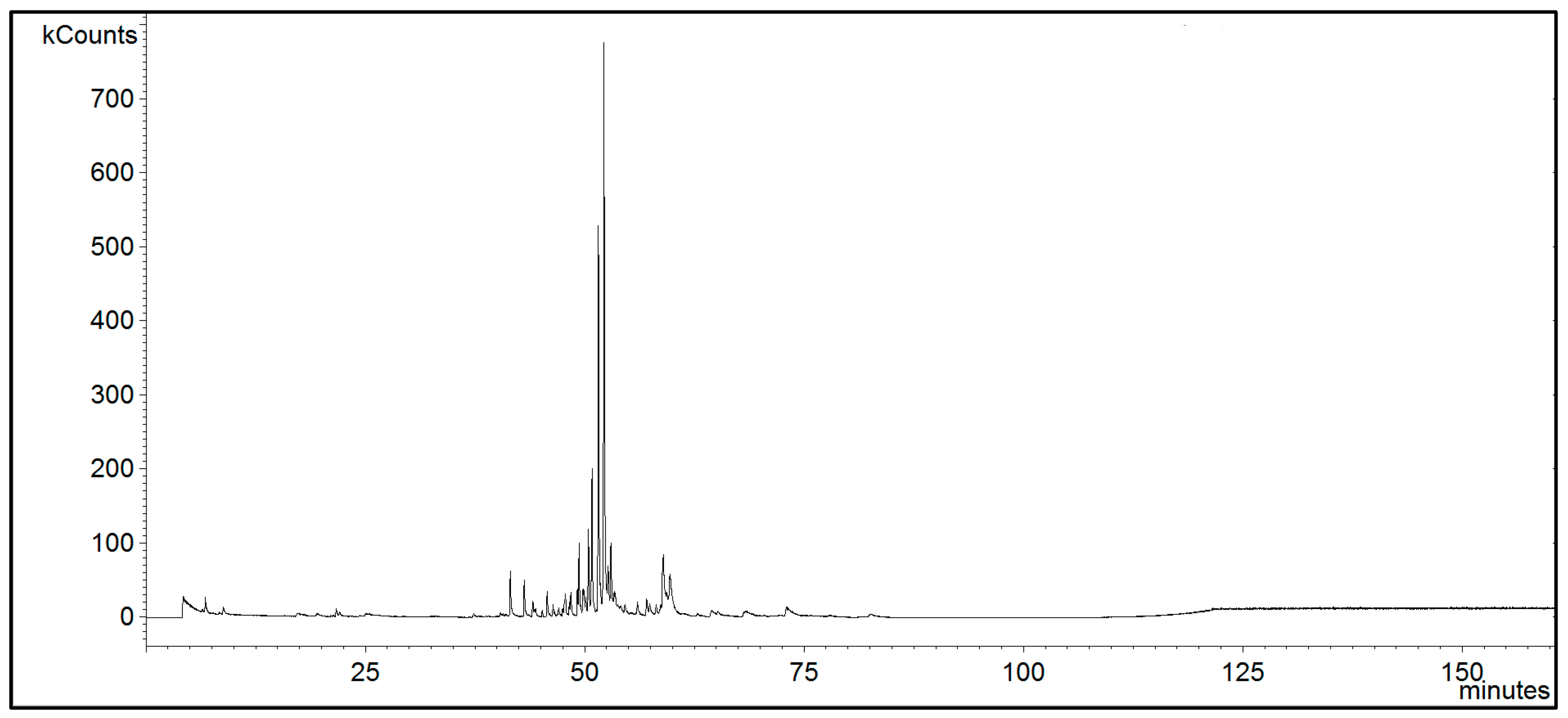
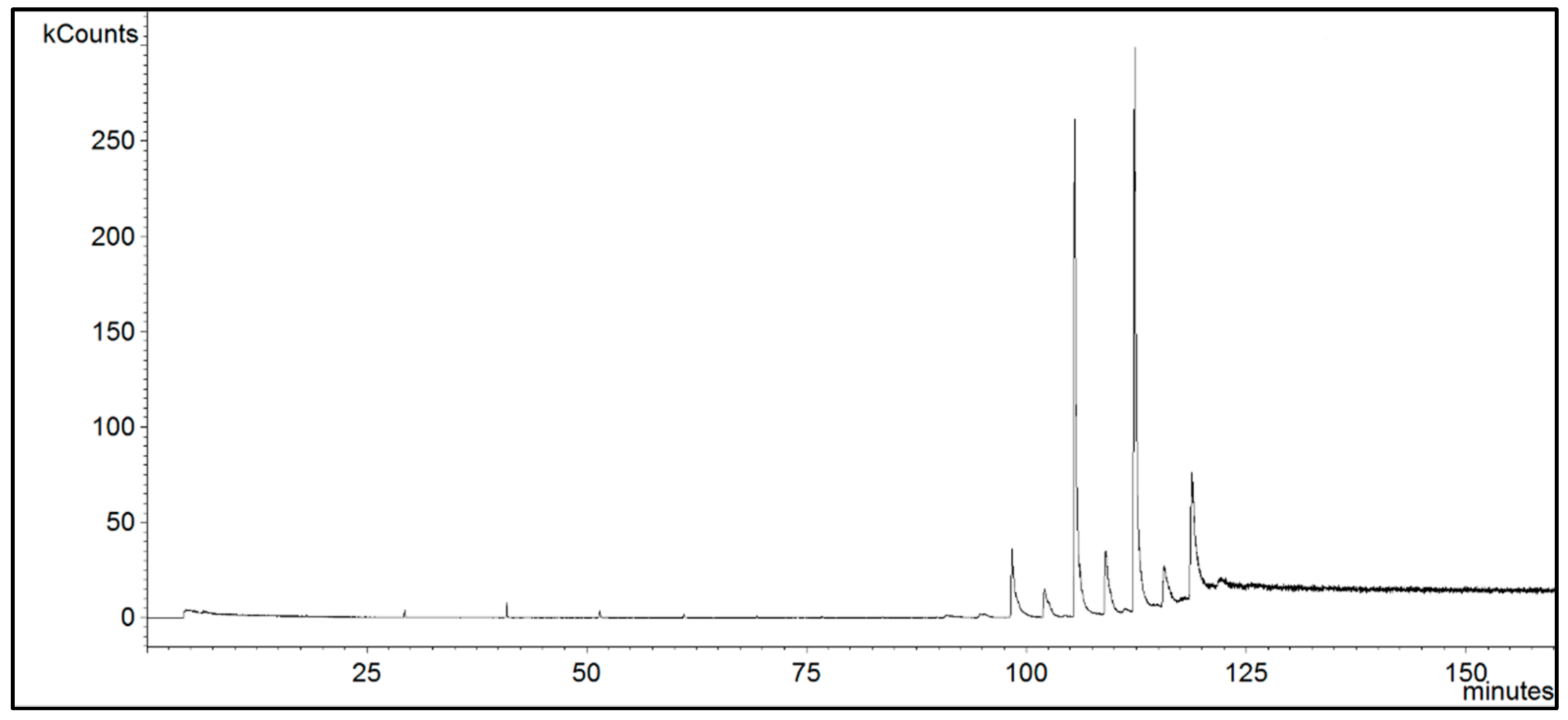
2.3. GC-MS Analysis
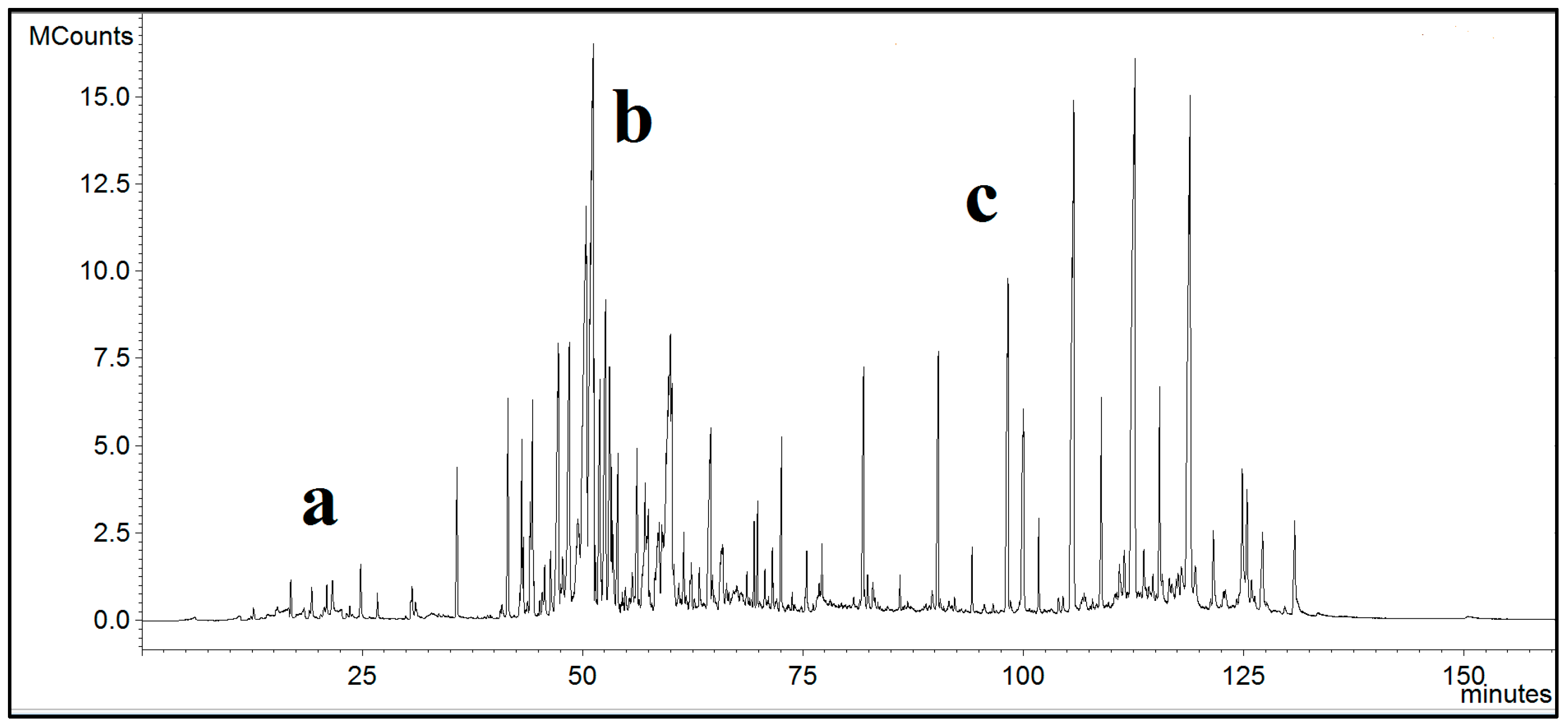
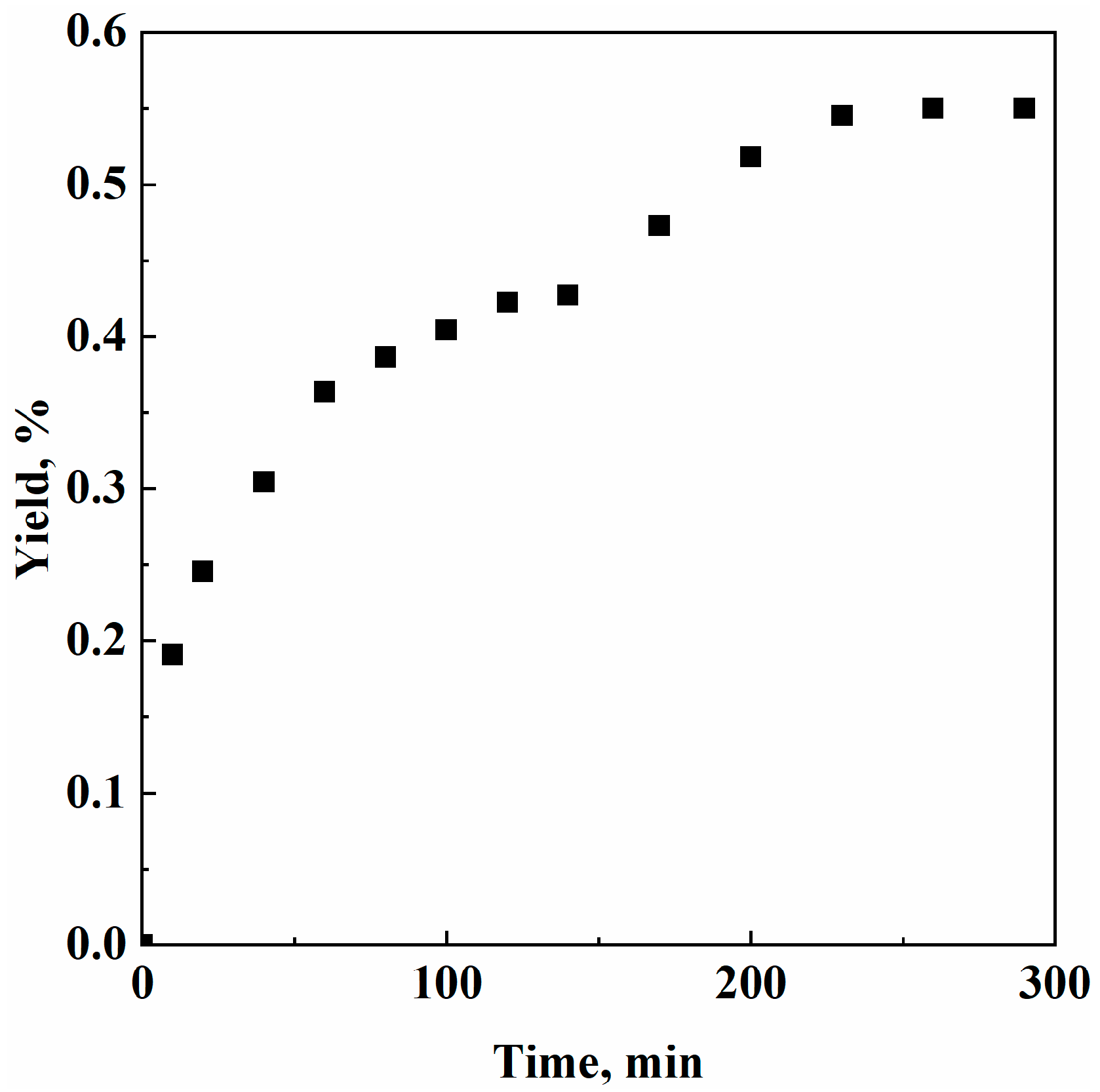
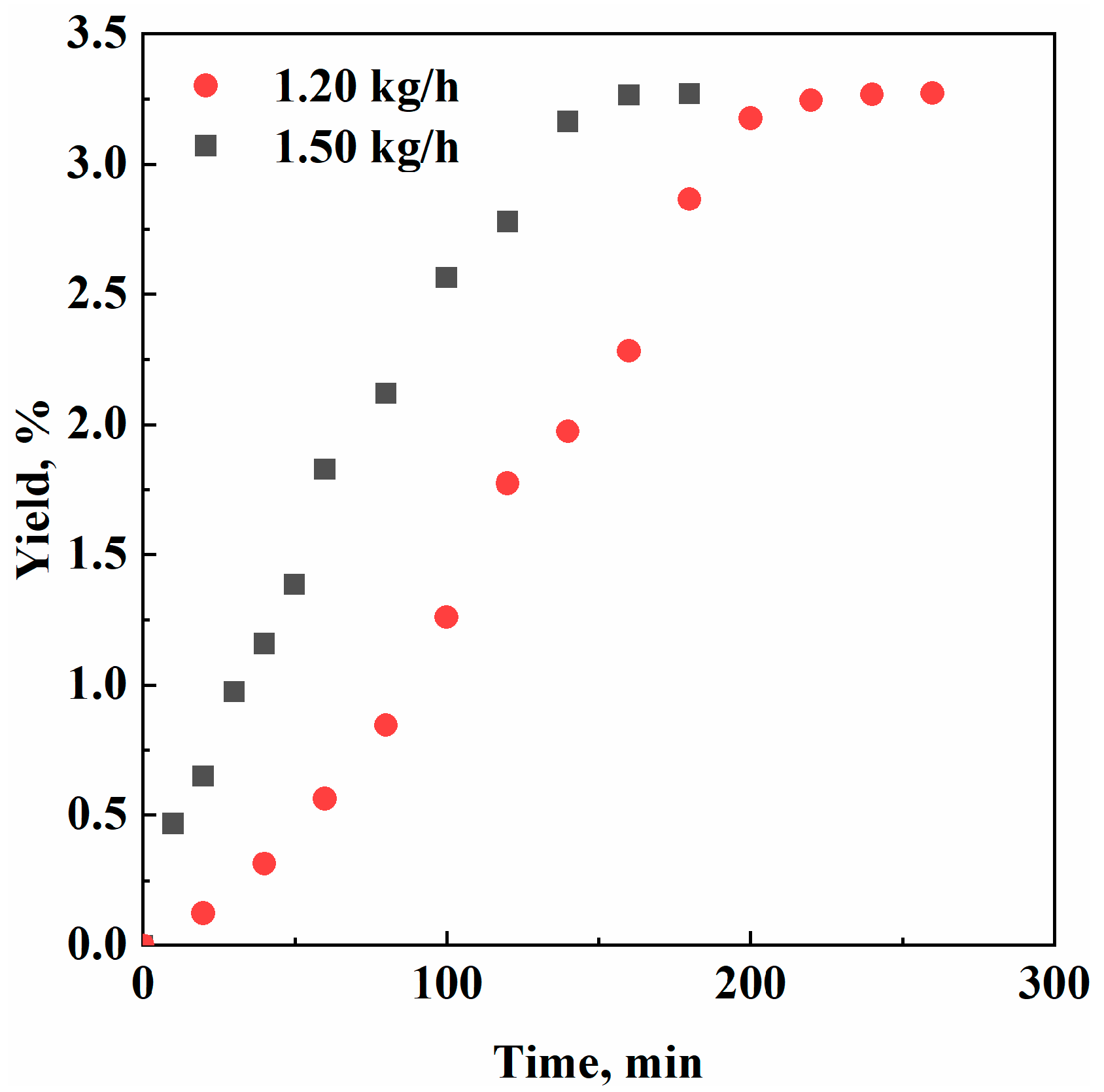
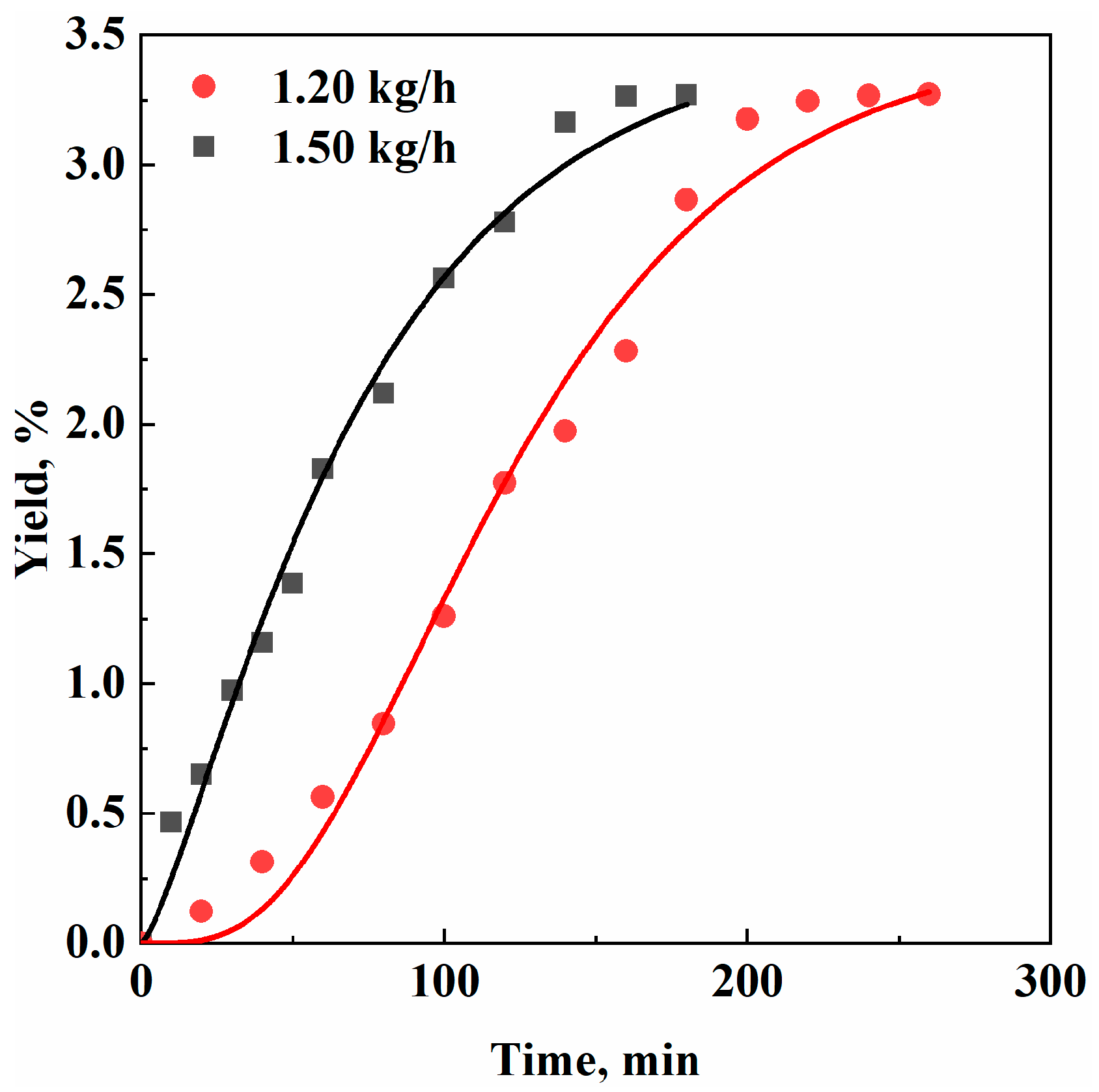
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muley, B.P.; Khadabadi, S.S.; Banarase, N.B. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): A Review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Jan, N.; Andrabi, K.I.; John, R. Calendula officinalis—An important medicinal plant with potential biological properties. Proc. Indian Natl. Sci. Acad. 2017, 93, 769–787. [Google Scholar] [CrossRef]
- Leach, M.J. Calendula officinalis and wound healing: A systematic review. Wounds Compend. Clin. Res. Pract. 2008, 8, 236–243. [Google Scholar]
- Aro, A.A.; Perez, M.O.; Vieira, C.P.; Esquisatto, M.A.M.; Rodrigues, R.A.F.; Gomes, L.; Pimentel, E.R. Effect of Calendula officinalis cream on achilles tendon healing. Anat. Rec. 2014, 298, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Varljen, J.; Lipták, A.; Wagner, H. Structural analysis of a rhamnoarabinogalactan and arabinogalactans with immuno-stimulating activity from Calendula officinalis. Phytochemistry 1989, 28, 2379–2383. [Google Scholar] [CrossRef]
- Hamad, M.N.; Mohammed, H.J.; Merdaw, M.A. Antibacterial activity of Calendula officinalis in vitro. Ibn Al-Haitham J. Pure Appl. Sci. 2017, 24. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef]
- Dhingra, G.; Dhakad, P.; Tanwar, S. Review on phytochemical constituents and pharmacological activities of plant Calendula officinalis Linn. Biol. Sci. 2022, 2, 216–228. [Google Scholar] [CrossRef]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV activity of extracts from Calendula officinalis flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef]
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A new extract of the plant Calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer 2006, 6, 119. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Ceylan, R.; Mahomoodally, M.F.; Jugreet, S.; Mollica, A.; Stefanucci, A. Chemical composition and biological activities of essential oils from Calendula officinalis L. flowers and leaves. Flavour Fragr. J. 2021, 36, 554–563. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakır, O.; Bensari, S.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A comparative bio-evaluation and chemical profiles of Calendula officinalis L. extracts prepared via different extraction techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Antioxidant potential of an extract of Calendula officinalis. Flowers in Vitro and in Vivo. Pharm. Biol. 2006, 44, 691–697. [Google Scholar] [CrossRef]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Vieira Fonseca, M.J. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Cordova, C.A.S.; Siqueira, I.R.; Netto, C.A.; Yunes, R.A.; Volpato, A.M.; Filho, V.C.; Curi-Pedrosa, R.; Creczynski-Pasa, T.B. Protective properties of butanolic extract of the Calendula officinalis L. (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox Rep. 2013, 7, 95–102. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Luca, S.V.; Kittl, T.; Minceva, M. Supercritical CO2 extraction of spices: A systematic study with focus on terpenes and piperamides from black pepper (Piper nigrum L.). Food Chem. 2023, 406, 135090. [Google Scholar] [CrossRef]
- Mouahid, A.; Claeys-Bruno, M.; Bombarda, I.; Amat, S.; Ciavarella, A.; Myotte, E.; Nisteron, J.-P.; Crampon, C.; Badens, E. Supercritical CO2 extraction of oil from Moroccan unroasted Argan Kernels: Effects of process parameters to produce cosmetic oil. J. CO2 Util. 2022, 59, 101952. [Google Scholar] [CrossRef]
- Melloul, S.; Zehioua, R.; Meniai, A.-H. Supercritical CO2 extraction of bioactive compounds from local Peganum Harmala plant seeds and optimization of the extraction yield and the antioxidant activities. Sustain. Chem. Pharm. 2022, 28, 100729. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Challenges in the production of pharmaceutical and food related compounds by SC-CO2 processing of vegetable matter. J. Supercrit. Fluids 2018, 134, 269–273. [Google Scholar] [CrossRef]
- Danielski, L.; Campos, L.M.A.S.; Bresciani, L.F.V.; Hense, H.; Yunes, R.A.; Ferreira, S.R.S. Marigold (Calendula officinalis L.) oleoresin: Solubility in SC-CO2 and composition profile. Chem. Eng. Process. Process Intensif. 2007, 46, 99–106. [Google Scholar] [CrossRef]
- Campos, L.M.A.S.; Michielin, E.M.Z.; Danielski, L.; Ferreira, S.R.S. Experimental data and modeling the supercritical fluid extraction of marigold (Calendula officinalis) oleoresin. J. Supercrit. Fluids 2005, 34, 163–170. [Google Scholar] [CrossRef]
- Baldino, L.; Adami, R.; Reverchon, E. Concentration of Ruta graveolens active compounds using SC-CO2 extraction coupled with fractional separation. J. Supercrit. Fluids 2018, 131, 82–86. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Senatore, F. Supercritical CO2 extraction and fractionation of lavender essential oil and waxes. J. Agric. Food Chem. 1995, 43, 1654–1658. [Google Scholar] [CrossRef]
- Reverchon, E. Fractional separation of SCF extracts from marjoram leaves: Mass transfer and optimization. J. Supercrit. Fluids 1992, 5, 256–261. [Google Scholar] [CrossRef]
- Crabps, N.; Marongiu, B.; Piras, A.; Pivetta, T.; Porcedda, S. Extraction, separation and isolation of volatiles and dyes from Calendula officinalis L. and Aloysia triphylla (L’Her.) Britton by supercritical CO2. J. Essent. Oil Res. 2003, 15, 350–355. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Rose concrete fractionation by supercritical CO2. J. Supercrit. Fluids 1996, 9, 199–204. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Gorgoglione, D. Supercritical CO2 fractionation of jasmine concrete. J. Supercrit. Fluids 1995, 8, 60–65. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Tuberose concrete fractionation by supercritical CO2. J. Agric. Food Chem. 1997, 45, 1356–1360. [Google Scholar] [CrossRef]
- Reverchon, E.; Donsì, G.; Sesti Osséo, L. Modeling of Supercritical Fluid Extraction from Herbaceous Matrices. Ind. Eng. Chem. Res. 1993, 32, 2721–2726. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Bayrak, A.; Akgül, A. Volatile oil composition of Turkish rose (Rosa damascena). J. Sci. Food Agric. 1994, 64, 441–448. [Google Scholar] [CrossRef]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative evaluation of antimicrobial activity and composition of rose oils from various geographic origins, in particular Bulgarian rose oil. Nat. Prod. Commun. 2008, 3, 1063–1067. [Google Scholar] [CrossRef]
- Ebrahimabadi, A.H.; Djafari-Bidgoli, Z.; Mazoochi, A.; Jookar-Kashi, F.; Batooli, H. Essential oils composition, antioxidant and antimicrobial activity of the leaves and flowers of Chaerophyllum macropodum Boiss. Food Control 2010, 21, 1173–1178. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, N.; Uma Kumari, K.; Singh, J.; Tripathi, P.; Meena, A.; Luqman, S.; Yadav, A.; Chanotiya, C.S.; Pandey, G.; et al. Diversity of essential oil-secretory cells and oil composition in flowers and buds of Magnolia sirindhorniae and its biological activities. Chem. Biodivers. 2020, 18, e2000750. [Google Scholar] [CrossRef]
- Shunying, Z.; Yang, Y.; Huaidong, Y.; Yue, Y.; Guolin, Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar] [CrossRef]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Kawka, E. A systematic approach to developing terpene extraction conditions utilizing supercritical carbon dioxide. Chromatogr. Today 2018. Available online: https://www.chromatographytoday.com/article/supercritical-fluid-sfcgreen-chromatography/45/cattis-consulting/pa-systematic-approach-to-developing-terpene-extraction-conditions-utilising-supercritical-carbon-dioxidep/2337 (accessed on 13 March 2018).
- Zanotti, A.; Baldino, L.; Scognamiglio, M.; Reverchon, E. Post-processing of a lavender flowers solvent extract using supercritical CO2 fractionation. J. Taiwan Inst. Chem. Eng. 2023. submitted. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- Chapman, D.G. Statistical problems in population dynamics. In Proceedings of the 4th Berkeley Symposium on Mathematical Statistics and Probability; Neyman, J., Ed.; University of California Press: Oakland, CA, USA, 1961. [Google Scholar]

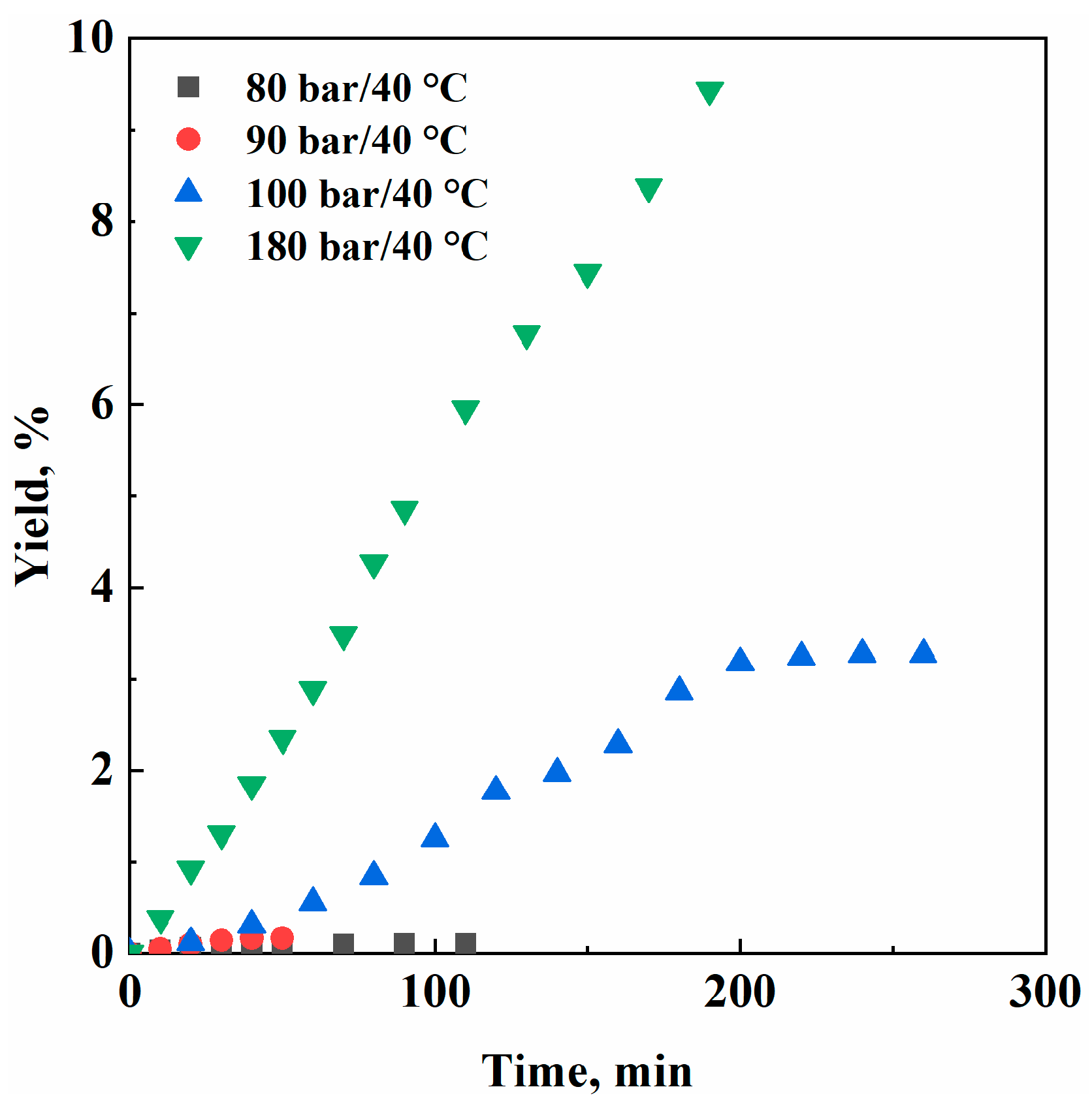
| Pressure (Bar) | SC-CO2 Density (g/cm3) | Extraction Yield (%) |
|---|---|---|
| 80 | 0.28 | 0.55 |
| 90 | 0.48 | 0.62 |
| 100 | 0.62 | 3.27 |
| 180 | 0.81 | 9.44 |
| Oil | Area, % |
|---|---|
| β-cubebene | 1.5 |
| α-gurjunene | 1.5 |
| β-caryophyllene | 1 |
| Aristolene | 1.5 |
| α-copaene | 1 |
| Germacrene | 3 |
| Cedrene | 2 |
| α-cubebene | 4.5 |
| α-muurolene | 6 |
| γ-cadinene | 16 |
| δ-cadinene | 25 |
| Cadina-1,4-diene | 7 |
| α-cadinene | 0.5 |
| Nerolidol | 3.5 |
| Viridiflorol | 1 |
| Oplopenon | 1 |
| Cubenol | 1 |
| τ-cadinol | 5.5 |
| Waxes | Area, % |
| Nonadecane | 5 |
| Eicosane | 1.5 |
| Heneicosane | 17 |
| Docosane | 2.5 |
| Tricosane | 30 |
| Pentacosane | 21 |
| 1-Hexacosanol | 2 |
| Heptacosane | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanotti, A.; Baldino, L.; Scognamiglio, M.; Reverchon, E. Fractionation of Marigold Waxy Extract Using Supercritical CO2. Separations 2023, 10, 298. https://doi.org/10.3390/separations10050298
Zanotti A, Baldino L, Scognamiglio M, Reverchon E. Fractionation of Marigold Waxy Extract Using Supercritical CO2. Separations. 2023; 10(5):298. https://doi.org/10.3390/separations10050298
Chicago/Turabian StyleZanotti, Alessandra, Lucia Baldino, Mariarosa Scognamiglio, and Ernesto Reverchon. 2023. "Fractionation of Marigold Waxy Extract Using Supercritical CO2" Separations 10, no. 5: 298. https://doi.org/10.3390/separations10050298
APA StyleZanotti, A., Baldino, L., Scognamiglio, M., & Reverchon, E. (2023). Fractionation of Marigold Waxy Extract Using Supercritical CO2. Separations, 10(5), 298. https://doi.org/10.3390/separations10050298











