Abstract
Artemisia negrei (A. negrei) and Artemisia aragonensis (A. aragonensis) are in the family Asteraceae, which has been used in traditional medicine. The use of plant-derived insecticides has become a promising strategy to reduce the harmful effects of synthetic insecticides and overcome the bio-resistance of pest insects to insecticides. In this regard, the purpose of the current study was to determine the chemical composition and evaluate insecticidal effects of essential oils (EOs) extracted from A. negrei (EON) and A. aragonensis (EOA). Notably, all chemical constituents present in the EOs were identified through GC-MS analysis, whilst the insecticidal properties against Callosobruchus maculatus Fab. (C. maculatus) were investigated by use of in vitro an in silico approaches. The obtained results showed that both tested EOs present a significant insecticidal effect against C. maculatus, which increased significantly upon the dose used in both contact and inhalation tests. The lethal concentrations (LC50) for the inhalation test were found to be 2.1 and 2.97 μL/L, while in the contact test they were 2.08 and 2.74 μL/L of air for EON and EOA, respectively. At 5 μL/L of air, the spawn reduction rate was 88.53 % and 77.41%, while the emergence reduction rate was 94.86% and 81.22% by EON and EOA, respectively. With increasing doses of up to 20 μL/L of air, the reduction in individual emergence reached 100% by the two oils tested after 36 h of treatment. In addition, Molecular docking (MD) simulations supported the in vitro findings and indicated that certain identified components in EOA and EON exhibited stronger hydrogen bonding interactions with the target receptors. Interestingly, the prediction of ADMET properties indicates that the molecules investigated have great pharmacokinetic profiles with no side effects. Taken together, our findings suggest that EOA and EON may exert both potential contact and inhalation insecticidal actions and could be used as an alternative tool for the control of this major insect pest of stored products.
1. Introduction
Over the years, the emergence of serious pests (especially insects, pathogens and weeds) affecting stored products has increased. They have become the main cause of economic and food security problems [1,2]. Pests are among the issues that affect the quality of stored grain and attack a wide range of legume foods. For instance, in Latin America, between 30 and 40% of maize production is lost during storage [3]. In Mexico, weevils are responsible for 30–40% of the losses of stored black beans and chickpeas [4].
Callosobruchus maculatus Fab. (Coleoptera: Chrysomelidae), which is known as the cowpea and chickpea weevil, is one of the most severe pests of stored grain in the tropics [5,6]. It is also considered a primary pest because it attacks cereals (sorghum, wheat, rice and some industrialized dry products) both in the field and in storage, as well as other leguminous seeds, such as the lentil, broad bean and horse bean [7,8]. The life cycle of C. maculatus (Fab.) is characterized by a high reproductive capacity, which leads to a large population growth within a relatively short period of time [9]. Notably, C. maculatus (Fab.) feeds intensively on the grain, resulting in grain losses [10,11].
Chickpea (Cicer arietinum L.) is one of the main legumes grown in Morocco and is mainly consumed locally [12]. The increasing demand for food, in line with population growth, requires the development and improvement of new grain-handling techniques during storage. The control of C. maculatus (Fab.) in stored chickpea grains has been commonly carried out on a large scale through the use of synthetic protective insecticides and synthetic fumigants in silos and storage warehouses, mainly via the application of aluminum phosphate. Another type of control consists of cleaning the walls, floors and ceilings of storage sites with Malathion or phenothrin at doses of 1–2 mL/L water [13,14]. The control of C. maculatus (Fab.) is mainly based on the application of synthetic insecticides to stored grain, which are mainly organophosphorus compounds and pyrethroids. Although the use of synthetic insecticides is effective, this control measure can lead to undesirable effects, such as the poisoning of applicators, toxic residues in grain, increased storage costs and the development of resistant populations [15,16,17,18,19]. Therefore, the use of residual insecticides is becoming less and less desirable due to the resistance of key insects [20,21].
To overcome the problem of acquired resistance developed by insect pests and find novel alternatives to chemical insecticides, the development of new bio-insecticides from natural sources can be considered a better approach [22]. This approach may offer several advantages, including low toxicity, biodegradable products and lower costs. Medicinal plants have played a key role in pharmacological research and the development of drugs [23]. Moreover, several research studies undertaken have proven the effectiveness of essential oils of plants, such as insecticides against insect pests [24]. Chemical substances isolated from plants are widely used as starting materials in medicine for drug discovery and for the construction of new synthetic molecules with potential biological benefits [25].
The genus Artemisia is well known for its richness in bioactive substances, which have shown potential applications in numerous fields [26,27]. The EOs extracted from Artemisia species have received extensive attention for their insecticidal properties and have proven effective against a variety of insect pests [27,28,29]. These EOs are composed of complex mixtures of highly volatile lipophilic organic substances, such as terpenes (monoterpenes, sesquiterpenes and diterpenes), aromatic compounds and aliphatic compounds, with numerous functional groups [30]. Furthermore, several reports have indicated that the primary components present in EOs from Artemisia species are the key factors contributing to their insecticidal effects on various insect pests [28,30]. Therefore, the objective of this work was to chemically characterize EOs from Artemisia negrei (EON) and Artemisia aragonensis (EOA) along with the testing of their insecticidal activities against C. maculatus. In addition, molecular docking (MD) studies were carried out to ascertain the mode of action of the compounds found in EOs of Artemisia plants with the crystal structures of the human gamma-aminobutyric acid (GABA) receptor (PDB ID: 4COF) [31] and the ryanodine receptor protein 1 (PDB ID: 5C30) [32]. The ADME/T analysis (absorption, distribution, metabolism, excretion and toxicity) of the molecules was also carried out.
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
A. negrei and A. aragonensis were collected in October 2021, Morocco. Next, the plants were identified and were deposited in the herbarium of the Faculty of Sciences Dhar el-Mahraz Morocco, under the registration numbers BPRN/04/18 and AHA001T7621 for A. negrei and A. aragonensis, respectively (Figure 1).

Figure 1.
(A) photograph of A. negrei; (B) photograph of A. aragonensis.
2.1.2. Animal Material
For the rearing of chickpea weevils (C. maculatus), the grain of the chickpea variety Cicer arietinum L. was used as a substrate. It should be emphasized that the grain was cleaned and stored at −4 °C for 48 h to avoid the possible contamination of the grain with eggs and larvae.
Briefly, 1 kg of chickpea grains was placed individually in 2 L plastic containers (17 cm high and 10 cm in diameter). Subsequently, 50 females and 50 males of the F1 generation of C. maculatus, which were obtained from contaminated grains, were placed into the chickpea grains. The grains and insects were kept at an ambient temperature of 27 °C, a relative humidity (RH) of 50% and a photoperiod of 13:11.
2.2. Experimental Methods
2.2.1. Extraction of Volatile Compounds
The aerial parts of the plants used for testing were dried in the shade at 26 °C for 10 days before distillation. Importantly, the extraction of EOs from A. negrei and A. aragonensis was performed using a Clevenger apparatus [22,24]. The resulting EOs was stored in a 2 mL amber Eppendorf tube and cooled to −4 °C in the dark until further use.
2.2.2. Calculation of Yield
The extraction yield (Ye) was calculated using the initial mass of plant material (Mi) and the final mass of essential oil (Mf) as follows:
2.2.3. Identification of Volatile Compounds in EOs by GC-MS
The chemical constituents of the obtained EOs were identified using gas chromatography–mass spectrometry on a GCMS-TQ8040 NX Triple Quadrupole GC/MS/MS instrument from Shimadzu (Tokyo, Japan). Analyses were carried out through an apolar capillary column (RTxi-5 Sil MS-30 m × 0.25 mm ID × 0.25 µm) employing helium as a carrier gas. A detailed description of the analysis conditions is provided in our previous research [33]. The Kovats retention index (KRI) was calculated using a homologous series of n-alkanes and used to identify the compounds. Importantly, the identification of constituents was carried out by comparing the computed KRI values with those of NIST 98 collection and in the Adams database [34].
2.2.4. Biological Test for Volatile Activity (Toxicity)
- a.
- Contact toxicity tests
The contact toxicity against C. maculatus adults of EOA and EON was carried out by determining the mortality and emergence of this insect (F1), according to the methodology of De Andrade Dutra [35]. In this assay, 100 g of chickpea seeds were placed in a 1L glass bottle and mixed with the tested EOs at concentrations of 1, 5, 10 and 20 μL/L of air. Next, each bottle was infested with 5 pairs of C. maculatus (Fab.) aged 48 h. Simultaneously, 100 g of chickpeas infested with five pairs of insects without oils were used as control. Dead insects were counted daily until the end of the experiment. Four replicates were performed to measure insecticidal activity and expressed as a percentage of the average mortality of C. maculatus adults before being transformed into corrected mortality via Abbott’s formula [36,37].
Eggs laid by females were counted after 12 days from the start of experiment, whilst the emerged individuals were counted after 30 days. The reduction percentage in the number of eggs and adults emerging at each concentration of essential oil was calculated.
- b.
- Inhalation tests (Fumigant Toxicity)
Small cotton balls were threaded with a thread attached to the inside of each lid of 10 C. maculatus bruchids (5 males and 5 females), ranging in age from 0 to 48 h in 1 L glass jars with tightly closed lids, according to the De Andrade Dutra’s methodology [35].
Each essential oil was administered in amounts of 1, 5, 10 and 20 μL/L using a micropipette and was placed in the above cotton balls. Four replicates of each dose were carried out, and a comparison was made using a control sample of unscented cotton [35]. Regular monitoring was performed for 3 days and mortality data of adult bruchids were collected and recorded every 12 h. The percentage of mortality was evaluated and corrected with Abbott’s formula [36,37].
2.3. Theoretical Methods
Molecular docking (MD) calculations were conducted to compare and interpret the in vitro biological activity results. MD simulations were performed using the program developed by the Maestro Molecular modeling platform (version 12.8) by Schrödinger [38]. Several steps are involved in the computations. Each step is executed distinctively. In the first step, the protein preparation module [39] was utilized to prepare proteins. The proteins’ active sites were identified in this module. The investigated molecules are prepared in the following step.
The target molecules were first optimized using the Gaussian software, and then the LigPrep module was used to prepare the optimized structures for computations [40]. After preparation, the Glide ligand docking module [41] was employed to determine the likely contacts that can occur between the molecules and the target proteins. The OPLS4 method was employed in all calculations. Lastly, the ADME/Tox analysis is undertaken to assess the drug-like properties of the investigated molecules. The Schrödinger software’s Qik-prop module was utilized to predict the impacts and interactions of investigated molecules in human metabolism [42].
Statistical Analysis
The analyses were performed by use of SPSS for Windows® (version 21.0). Data were analyzed via the one-way analysis of variance (ANOVA). Fisher’s minimal significant difference (LSD) test was employed to distinguish between significant and non-significant means at α = 0.05. The probit approach was used to calculate the LC50 and LC95 fatal values as well as their confidence intervals.
3. Results and Discussion
3.1. Yields of EOs and Chemical Composition
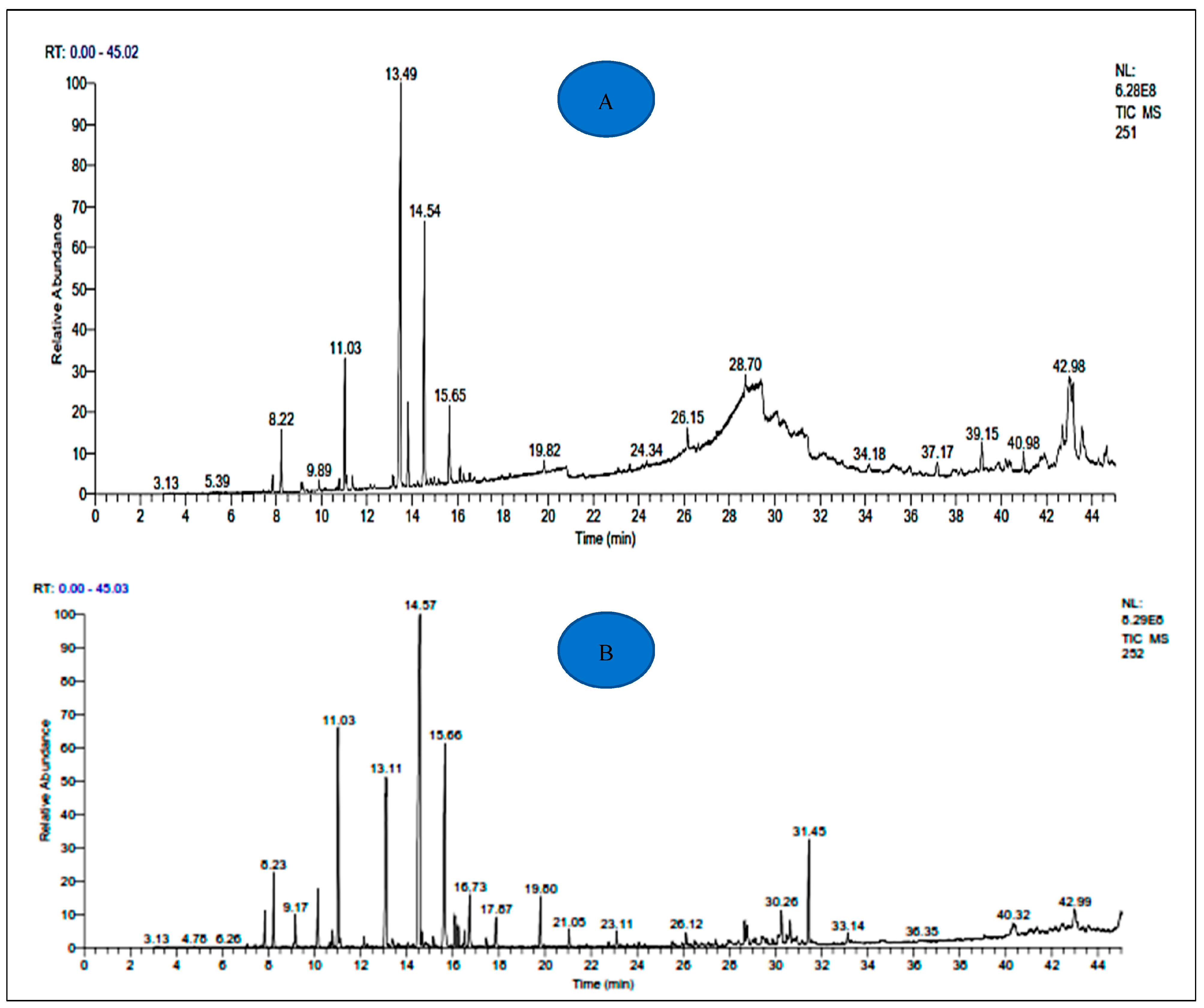
The yields of EOs of A. negrei and A. aragonensis were 1.19 and 1.17%, respectively. The chemical substances identified in the Eos of the two studied plants, as well as their percentages and Kovats retention index, are presented in Table 1. The results revealed the existence of 55 different and common potentially bioactive compounds in the analyzed EOs, including 34 components in the EOA and 34 components in the EON. The main compounds identified in EOA were β-thujone (29.02%), camphor (14.68%) and 1.8 cineol (5.60%), while for EON, Camphor (24.97%), Borneol (13.20%) and 1.8 Cineol (10.88%), were the main detected compounds (Table 1 and Figure 2). The phytochemical composition of the EOA and EON are comparable to the literature, which shows the richness of EOs from Artemisia in terpenoids [43,44]. The almost predominant components of the EOs of the two studied plants are essentially the same, but their compositions differ slightly. This variability can be explained by different factors, including the species, the type of clone, the organ concerned, the interaction with the environment (type of soil, climate, etc.) and the degree of maturity of the plant concerned as well as the influence of the geographical conditions [45,46,47].

Table 1.
Chemical constituents of the EOs from A. negrei and A. aragonensis.
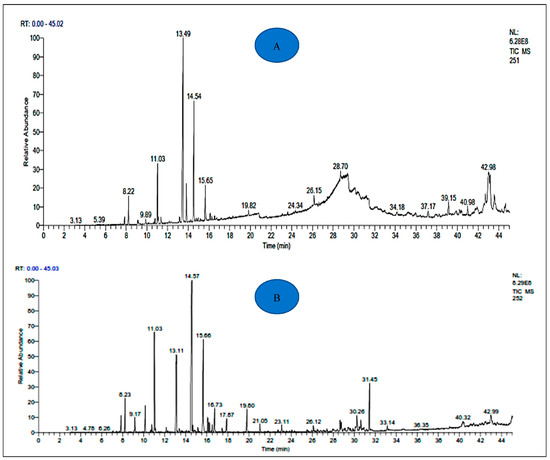
Figure 2.
Chromatogram of A. negrei (A) and A. aragonensis (B) essential oils.
3.2. Inhalation Toxicity of EOs
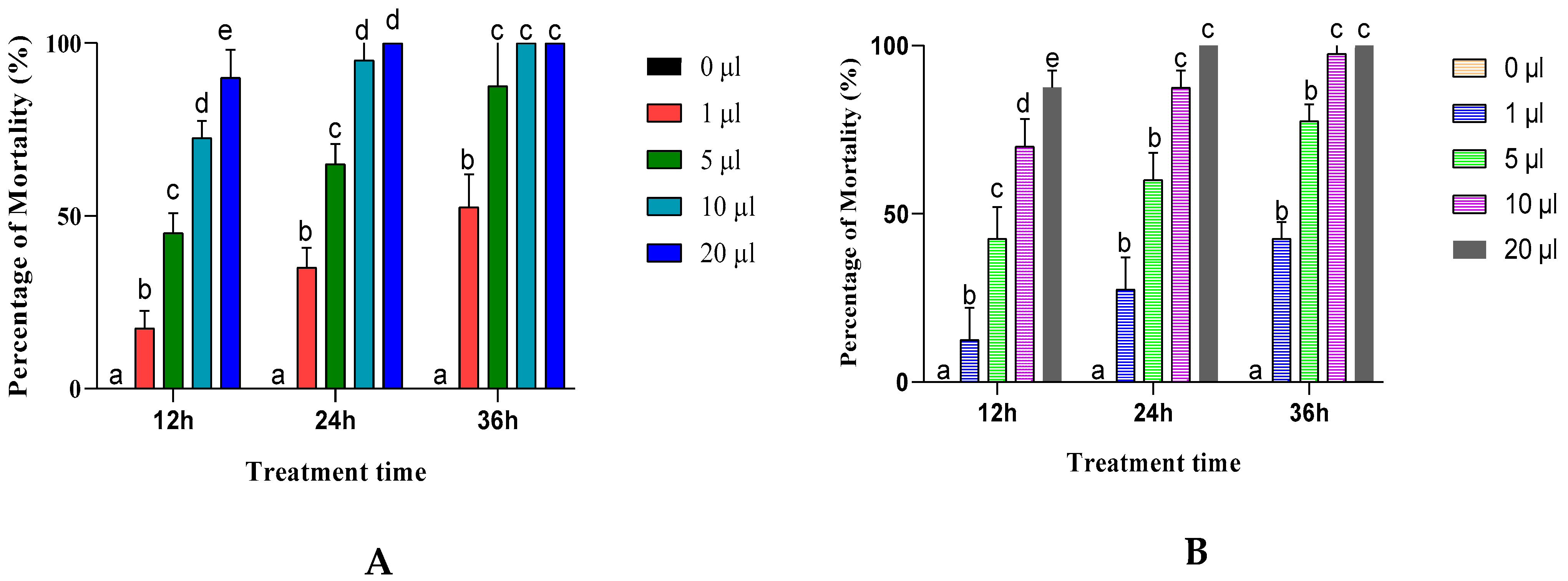
The effects of Artemisia negrei and Artemisia aragonenesis essential oils on adult insect mortality via inhalation were significant (p ≤ 0.0001) with increasing exposure time and dose of EO used (Figure 3).
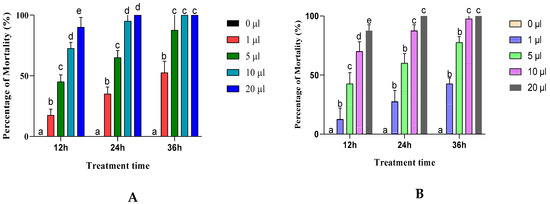
Figure 3.
C. maculatus mortality rate adults exposed to the essential oil inhalation tests for A. egeri (A) and A. aragonensis L. (B). Row values with the same letters (a, b, c, d or e) did not differ significantly (means ± SD, n = 4, one-way ANOVA; Tukey’s test, p ≤ 0.05).
The results indicate that exposing adult insects to a low dose (1 μL) of essential oils from A. egeri and A. aragonensis resulted in a reduction in mortality rate, causing only 52.5% and 42.5% of mortality, respectively, after 36 h of exposure, demonstrating their partial insecticidal effect.
For higher doses, and after 36 h of exposure, the two essential oils show a total insecticidal effect at a dose of 10 μL for A. egeri and 20 μL for A. aragonensis.
According to the results depicted in Table 2, it can be seen that EON (LC50 = 2.1 μL/L of air) is more toxic than EOA (2.74 μL/L of air) against C. maculatus beetles.

Table 2.
LC50 and LC95 values (μL/L of air) of Eos tested against C. maculatus insects after each 12 h post-contact and -inhalation.
Aromatic medicinal plants are thought to be a bio-insecticide capable of controlling a wide range of insects and pests in storage [48,49]. The insecticidal effect of the EOs against stored food pests by contact, ingestion and fumigation has been well demonstrated. In addition, much work has been carried out to improve methods and plant alternatives that can be used to enhance their insecticidal activity [43,50]. The obtained results showed that the EOs extracted from the two studied plants (A. negrei and A. aragonensis) were considered to be potent insecticides. Thus, our results agreed with previous scientific studies that reported the insecticidal effect of EOs from many aromatic and medicinal plants [22,51,52]. After subjecting adult insects to different doses of these two EOs, a significant mortality rate was recorded as a function of the dose and duration of exposure [53,54]. For comparative purposes, Chávez-Díaz et al. [5] observed that the EOs of Thymus vulgaris (Thyme), Origanum vulgare (Oregano) and Mentha spicata (Mint) caused the mortality of the genus Callosobruchus (Coleoptera: Bruchidae). Aimad et al. [55] demonstrated that the EOs of Dittrichia Viscosa, Maticaria Recutita and Artemisia herba alba Asso had toxic fumigation effects against this pest. Oils from Vitex pseudonegundo and Carum copticum also showed effects on the Callosobruchus genus at different stages of insect development [56].
3.3. Contact Toxicity of EOs from A. negrei and A. aragonensis
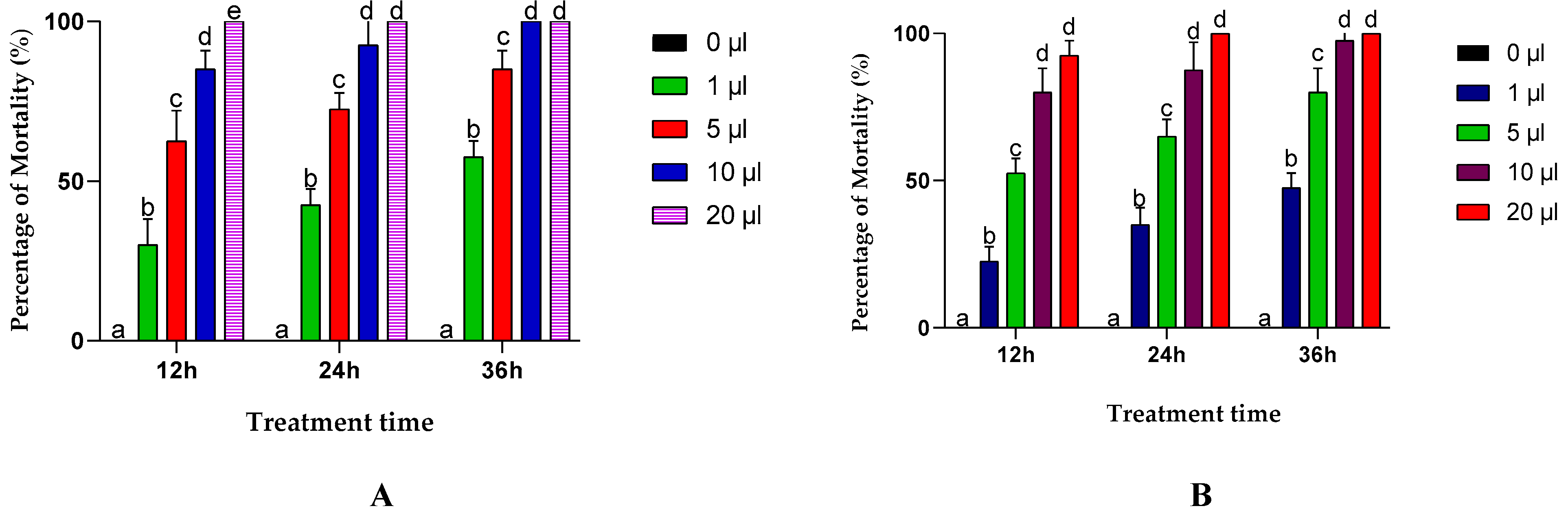
The results of this test showed that the mortality of the insects varied depending on several factors, such as the duration of exposure, the type of essential oil, the dose and the time of exposure. In general, the essential oils extracted from A. negrei and A. aragonensis were highly toxic to C. maculatus adults. EON exhibited total mortality of 92.5 % for a dose of 10 μL after 24 h, and 100 % mortality with the highest dose (20 μL) after only 12 h of treatment. For EOA, a total mortality of 87.5 % was recorded for the 10 μL dose after 24 h and a 100 % mortality for the 20 μL dose after one day of treatment (Figure 4).
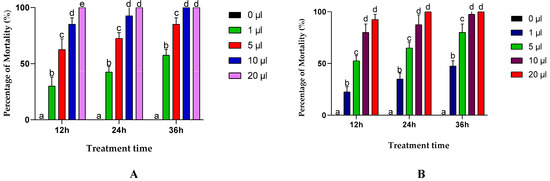
Figure 4.
Effects of the essential oil of the leaves of the A. negrie (A) and A. aragonensis Lam (B) in different concentrations on the adults of C. maculatus via contact test. Row values with the same letters (a, b, c, d or e) did not differ significantly (means ± SD, n = 4, one-way ANOVA; Tukey’s test, p ≤ 0.05).
The LC50 and LC95 lethal concentrations, which represent the concentrations required to cause 50% and 95% mortality of C. maculatus adults after the contact with EOs at different doses, respectively, and these were calculated using the Probit method and are presented in Table 2. In this way, it can be shown that EON is more toxic than EOA, with an LC50 of 2.08 and 2.74 μL/L of air at 36 h, respectively. These values show that both tested essential oils have potent insecticidal activity towards C. maculatus, and these results support previous research on the insecticidal effects of EOs from other species of the Artemisia genus, such as Artemisia herba alba, Artemisia judaica, Artemisia campestris, Artemisia absinthium and Artemisia dracunculus [55,57,58,59].
3.4. Effect of Direct Contact with EOs on C. maculatus Fab Fecundity and Emergence
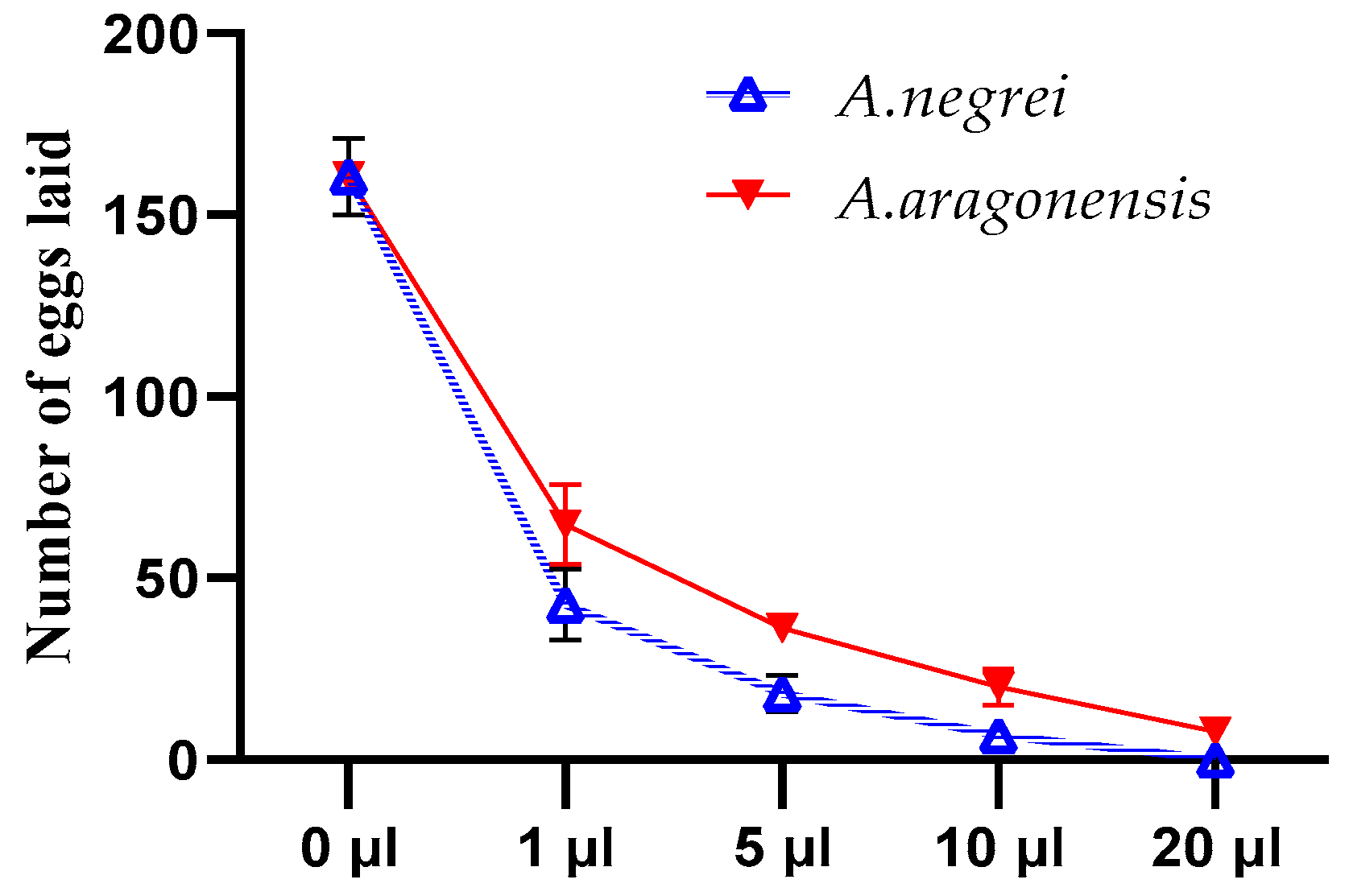
The fecundity and emergence of C. maculatus Fab were assessed using different doses of the EOs studied (Figure 5). From this figure, it can be seen that the quantity of eggs laid varied depending on the dose of EOs used for treatment. Notably, the quantity of eggs gradually decreased as the dose of essential oils increased.
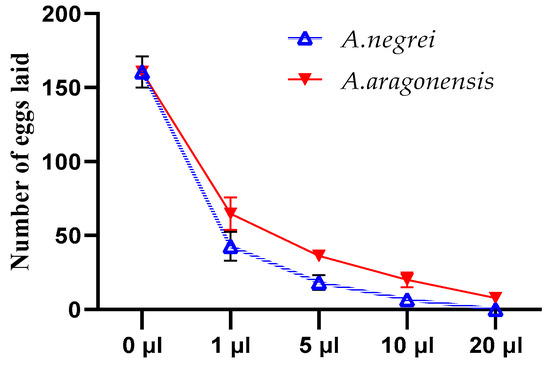
Figure 5.
Effects of essential oils (EOA and EON) on the number of eggs laid by C. maculatus Fab females.
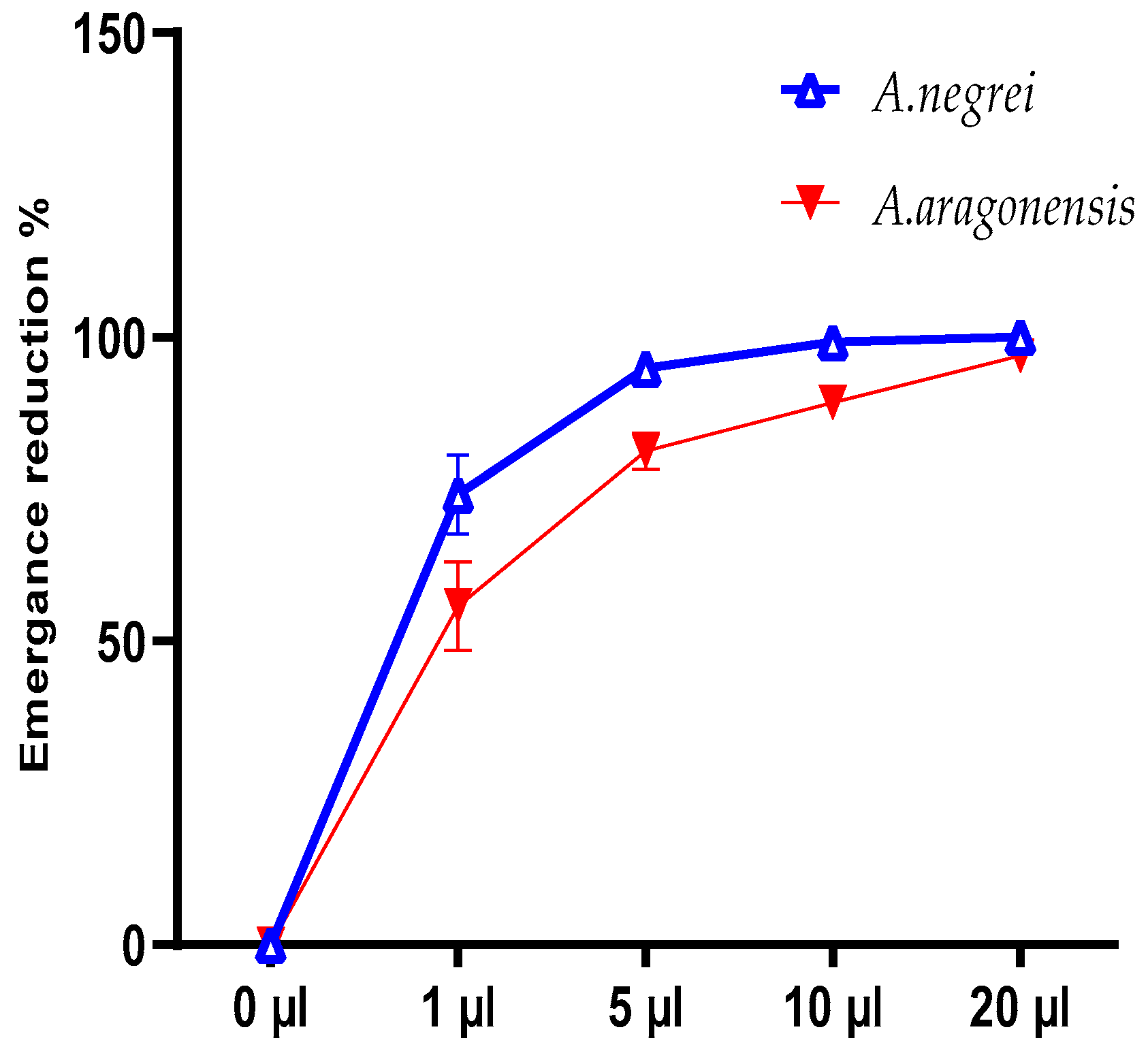
After completing the life cycle, the emergence of C. maculatus was evaluated at various doses of EOs (Figure 6). The rate of emergence reduction (%) was calculated to be 99.18% and 89.19% for EON and EOA, respectively, at a 10 μL dose. For the overdose (20 μL), we noted a complete disappearance of individuals in the presence of EON and a 96.94 % reduction in the emergence of EOA (Figure 6).
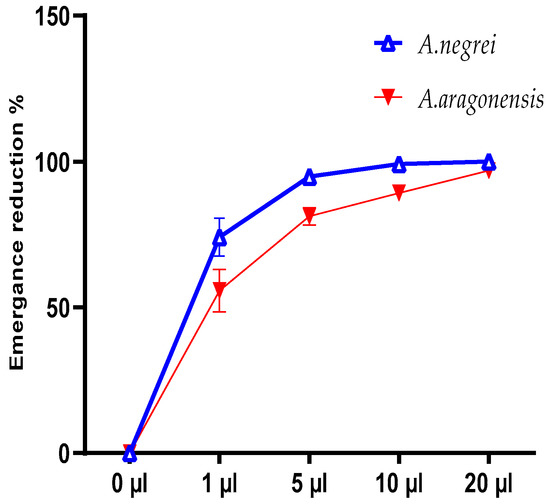
Figure 6.
Reduction rate of the emerging C. maculatus Fab. insect under the effect of EON and EOA.
In general, the work carried out by Seri-Kouassi et al. [60] showed that the toxicity of essential oils on insects is induced by the action of their main compounds—either they have a singular insecticidal efficacy or they are assembled. In other previous work [61], the insecticidal effect of essential oils has been attributed to the presence of terpene compounds that act as neurotoxic compounds in insects. Alternatively, Chiasson and Beloin [62] have proposed that essential oils may have a direct effect on the cuticle of insects and mites, especially those with soft bodies. These findings reinforce our obtained results in the current study and suggest that the insecticidal power of essential oils tested is due to the presence of thujone, camphor, artemisia alcohol, 1,8-cineole and borneol. On the other hand, another study [28] reported that the highest percentage of β-thujone, camphor, 1,8 cineole, borneol and camphene compounds in the essential oils of A. absinthium and A. herba alba gave them the best insecticidal potential against two basic stored insects. Our work indicates that the tested EOs were effective against C. maculatus Fab when used in both contact and inhalation assays. Importantly, the mortality rate of adults increases proportionally with the concentration of EOs. Many constituents of volatile EOs interact with the insects’ odor receptors, triggering various behaviors, such as flight, attraction and oviposition. Notably, the reduction in fecundity of C. maculatus females treated with the EO tested is not only related to the reduction of the oviposition period or the survival of the females but is also the result of vitellogenesis processes. The essential oils of Artemisia aragonensis and Artemisia negrei contain several compounds that may have insecticidal effects. In the literature, some compounds, such as β-thujone, camphor, borneol and 1,8 cineole, have been shown to have relevant effects on reducing fecundity and inhibiting adult emergence and larval mortality in some insects [63,64].
4. Theoretical Calculations
4.1. Molecular Docking Study
In the current investigation, the in vitro activity results of chemical molecules found in A. aragonensis Lam and A. negrei L. (Asteraceae), EOs were supported and validated by a variety of procedures utilizing theoretical approaches. Among them, MD simulation is a crucial tool frequently used for comparing and studying the activities of molecules [65,66]. In MD calculations, the most important thing in the activity comparisons of molecules for the inhibition of proteins is interaction, because molecules must interact with proteins to inhibit the proteins [67]. Consequently, the interaction of molecules with the target proteins is significant. Therefore, more interacting molecules have higher activity. Hydrogen bonds, polar and hydrophobic interactions, π–π and halogen are commonly the types of interactions that occur between screened molecules and proteins [68,69]. As these interactions increase, the activities of the molecules increase. Molecules with the highest activity of all interactions are given in Figure 7 and Figure 8. The docking score parameter is used for comparing the activities of the studied molecules in MD calculations. The most active molecule is the that with the parameter of the biggest negative numerical value. All calculated parameters are given in Table 3.
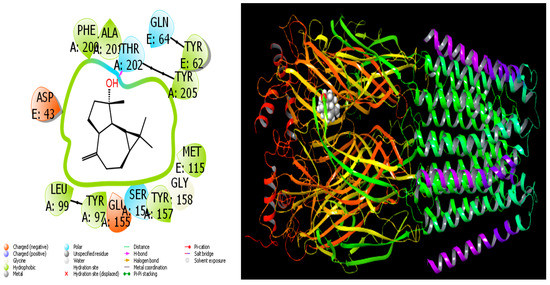
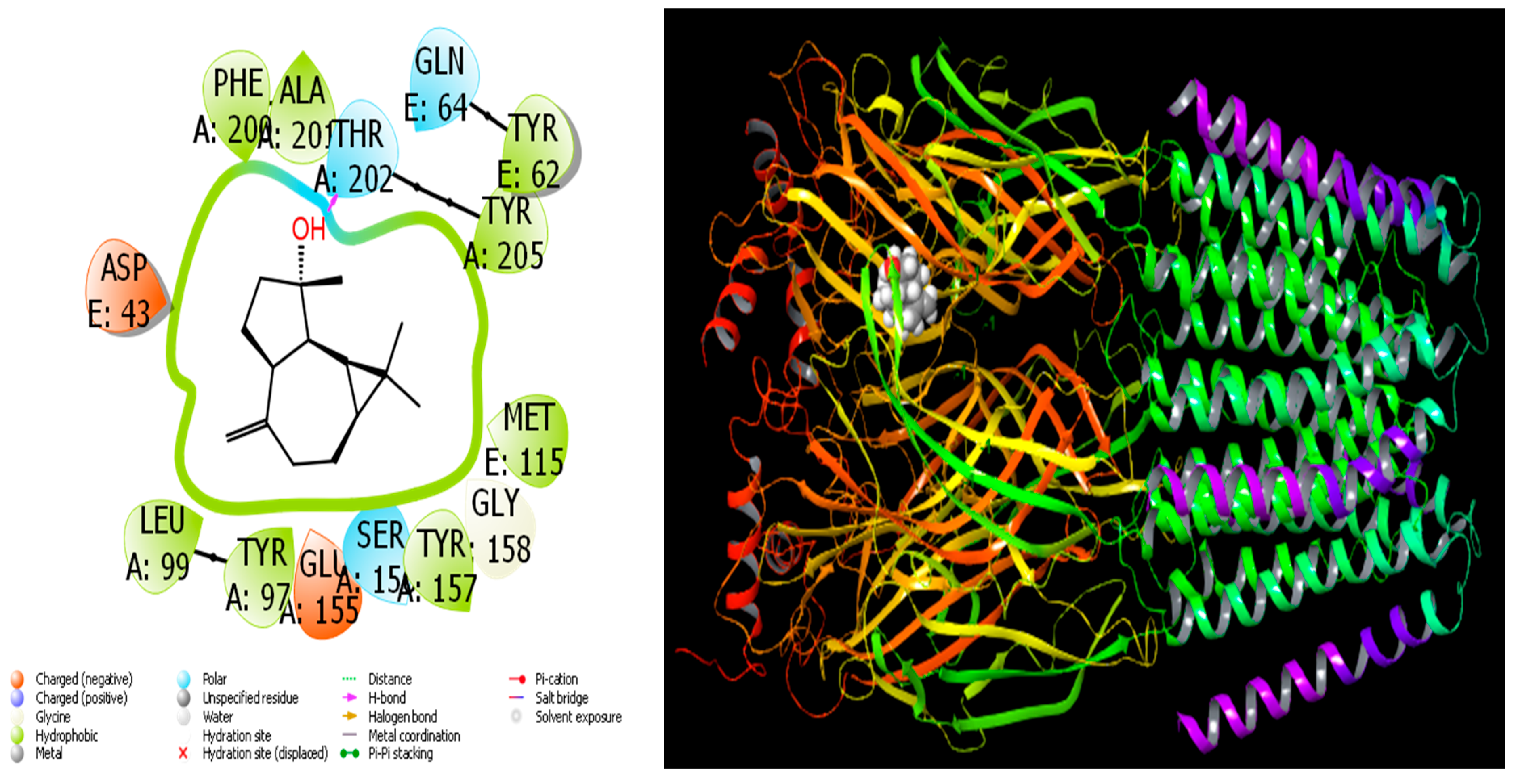
Figure 7.
Interactions of spathulenol with human GABA receptor proteins.
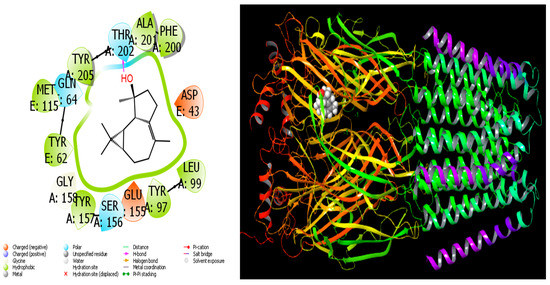
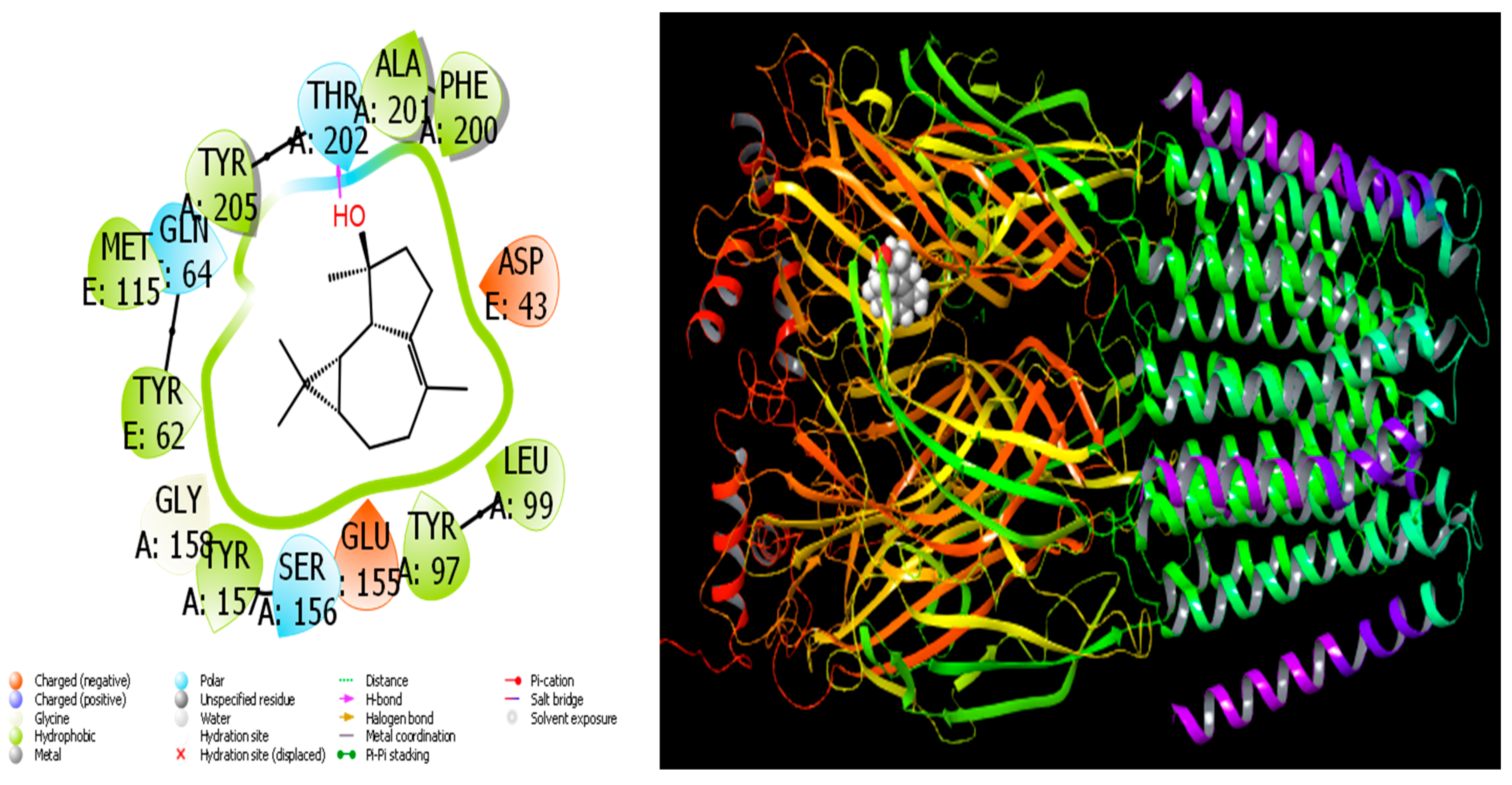
Figure 8.
Interactions of Isospathulenol with human GABA receptor proteins.

Table 3.
Numerical values of the docking parameters of the different molecules found in EO of Artemisia plants against the target proteins 4COF and 5C30.
Many parameters for chemical molecules found in EOs of A. aragonensis Lam and A. negrei L. are computed as a result of the interactions of the investigated molecules with the proteins. Each parameter examines different properties of molecules. Except for the docking score parameter, Glide ligand efficiency, Glide hbond, Glide evdw and Glide ecoul parameters are important parameters that look at how the chemical molecules interact with the target proteins [70]. Parameters such as Glide emodel, Glide energy, Glide einternal and Glide posenum are exposure-related parameters that result from molecule–protein interactions [71].
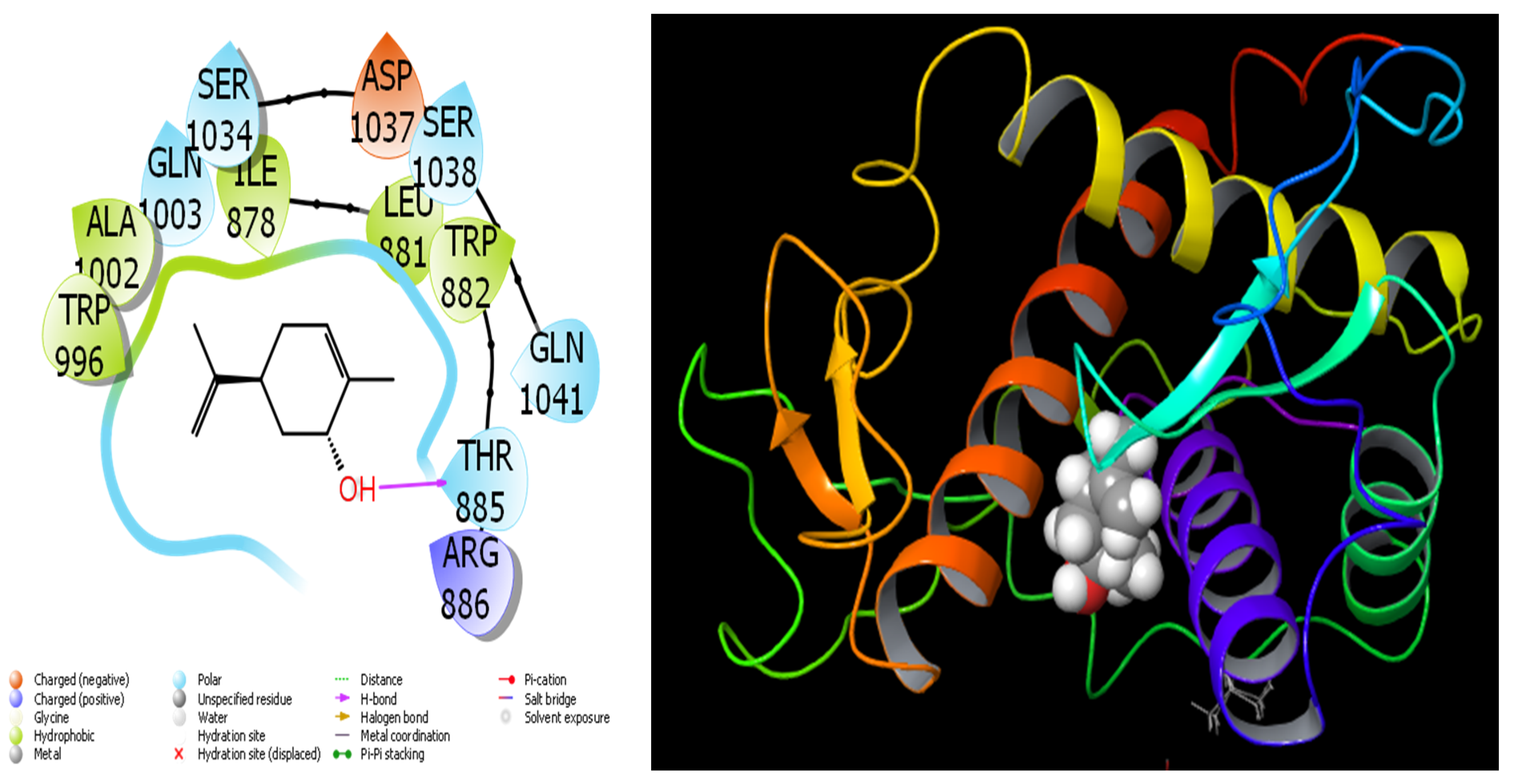
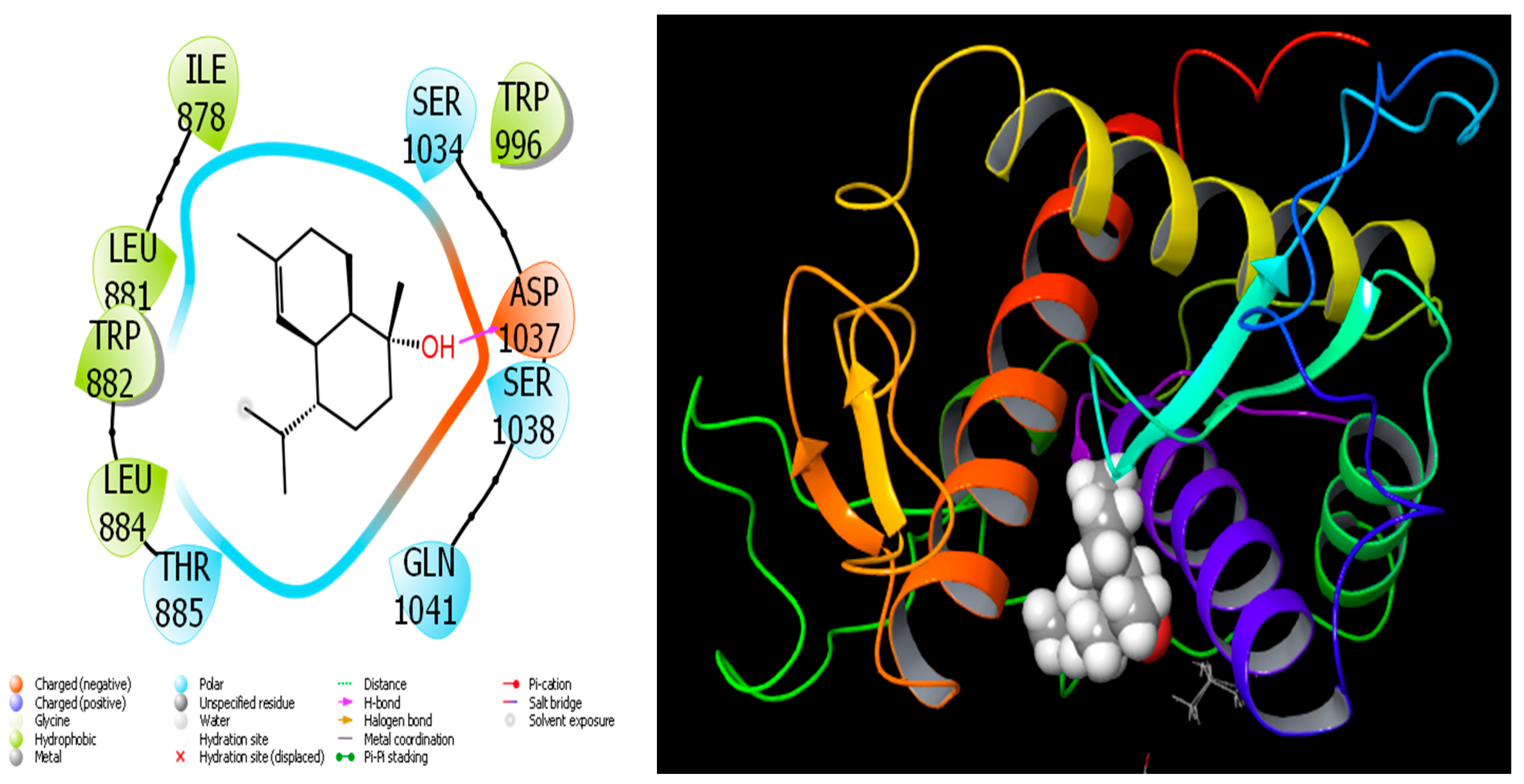
When we examine in detail the interaction between the spathulenol molecule and the human GABA receptor in Figure 7, the hydroxyl group in the spathulenol molecule creates a hydrogen bond interaction with the THR 202 protein. However, when the interaction between the Isospathulenol molecule and the human GABA receptor is examined in Figure 8, the hydroxyl group in the Isospathulenol molecule creates hydrogen bonds with the THR 202 protein. On the other hand, as a result of the interaction between the Trans-Carveol molecule and the Ryanodine Receptor 1 protein in Figure 9, a hydrogen bond occurs between the hydroxyl group in the Trans-Carveol molecule and the THR 885 protein. Finally, in the interaction between the Cadinol molecule and Ryanodine Receptor 1 protein in Figure 10, it is seen that the hydroxyl group in the Cadinol molecule forms a hydrogen bond interaction with the ASP 1037 protein.
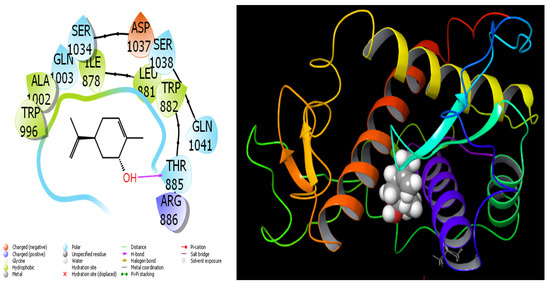
Figure 9.
Interactions of Trans-Carveol with Ryanodine Receptor 1 protein.
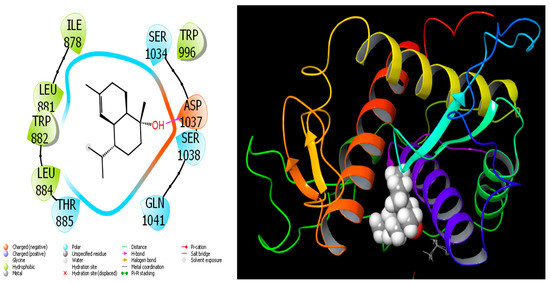
Figure 10.
Interactions of Cadinol with Ryanodine Receptor 1 protein.
4.2. ADME/Tox Properties
In this study, as a result of comparing the activities of chemical molecules found in EOs of A. aragonensis Lam and A.negrei L. (Asteraceae), it is not enough to find the molecule with the highest activity alone. Finding a molecule with high activity does not mean that the molecule will be a good drug, because it is of great importance to predict the movements of the molecule in human metabolism [72]. This is a significant event involving many processes, such as absorption, distribution, metabolism, excretion and toxicity by the human metabolism of molecules [73]. It is very important to examine each case individually and confirm drug feasibility. It should be predicted whether a molecule can be used as a drug in human metabolism by examining the movements of the molecules. For this purpose, the ADME/T analysis of the selected molecules was completed. All calculated ADME/T parameters are given in Table 4. The parameters in this table are divided into two classes, the first of which contains the parameters that examine the chemical properties of the molecules, and the second comprises the articles that examine the biological properties of the molecules.

Table 4.
ADMET properties of molecules.
All of the chemical molecules found in EOs of A. aragonensis Lam and A. negrei L. (Asteraceae) were studied; however, only the numerical values of the five most active molecules for the calculated parameters are given in Table 4. It examines many properties, such as the parameters of chemical properties, mole masses of molecules, dipole moment of molecules, total solvent accessible surface area of molecules, volume of molecules, hydrogen bond accepted and donated properties of molecules [72]. In addition to these, many parameters of the biological properties are examined, such as the oral absorption of molecules, absorption through the skin, the ability to pass through the intestinal–blood and brain–blood barriers and the number of likely metabolic reactions [73]. Apart from these, the numerical value of two parameters, such as RuleOfFive [74,75] and RuleOfThree [76], which determine whether molecules can be drugs, is expected to be zero. The RuleOfFive parameter is known as Lipinski’s rule of five and consists of four rules. On the other hand, the RuleOfThree parameter is known as Jorgensen’s rule of three and consists of three rules. In the light of the above information, it is seen that the numerical values of the ADME/T parameters of the chemical molecules found in EOs of A. aragonensis Lam and A. negrei L. are within the desired range for a molecule to be used as a drug.
5. Conclusions
In the current study, the chemical composition and insecticidal effects of essential oils derived from Artemisia plants were investigated, along with an in silico study to understand the interaction of these constituents with target proteins. The obtained results show that EOs from the A. negrei and A. aragonensis possess promising insecticidal effects against C. maculatus, which could be attributed to their components. Molecular docking studies were conducted to understand the binding affinities and modes of interactions between chemical components found in the EOs of two Artemisia plants and the target proteins. The results showed that some compounds had strong hydrogen interactions with the target receptors. In addition, some selected compounds were examined for their ADMET properties, and the results indicated that they had remarkable pharmacological properties and therapeutic potential. Interestingly, our results demonstrate the potential of the tested EOs as efficient insecticidal agents to control pest insects affecting stored products. However, further investigations are required to assess the effectiveness of these EOs against a wide range of insect pests. In addition, future studies aimed at isolating the active constituents of these plants would be interesting in order to develop new biopesticides that are non-toxic for the environment.
Author Contributions
All authors contributed to conceptualization, writing the original draft, formal analysis, investigations, funding acquisition, resources, project administration, reviewing and editing, data validation and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Researchers Supporting Project (RSP2023R457). King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP2023R457). The numerical calculations reported in this paper were fully/partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources). This work was supported by the Scientific Research Project Fund of Sivas Cumhuriyet University (CUBAP), Turkey under the project number RGD-020.
Conflicts of Interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
References
- Musolin, D.L.; Kirichenko, N.I.; Karpun, N.N.; Aksenenko, E.V.; Golub, V.B.; Kerchev, I.A.; Mandelshtam, M.Y.; Vasaitis, R.; Volkovitsh, M.G.; Zhuravleva, E.N.; et al. Invasive insect pests of forests and urban trees in Russia: Origin, pathways, damage, and management. Forests 2022, 13, 521. [Google Scholar] [CrossRef]
- Haggblade, S.; Diarra, A.; Traoré, A. Regulating agricultural intensification: Lessons from West Africa’s rapidly growing pesticide markets. Dev. Policy Rev. 2022, 40, e12545. [Google Scholar] [CrossRef]
- GM, E.L.; Reyes-Lagunes, I.; Scott, R.L. MMPI-2 for Mexico: Translation and adaptation. J. Pers. Assess. 1994, 63, 105–116. [Google Scholar]
- García-Oviedo, J.A. Elabora IPN frijol instantáneo altamente nutritivo. Nota Periodís. El Univers. Ed. 2007, 3, 43. [Google Scholar]
- Chávez-Díaz, G.; Valdés-Estrada, M.E.; Hernández-Reyes, M.C.; Gutiérrez-Ochoa, M.; Valladares-Cisneros, M.G. Aceites esenciales para controlar Acanthoscelides obtectus (Say) y Sitophilus zeamais (Motschulsky) plagas de granos almacenados. Rev. Mex. Agroecosistemas 2016, 3, 99–107. [Google Scholar]
- D’souza, C.; Taghian, M.; Lamb, P. An empirical study on the influence of environmental labels on consumers. Corp. Commun. Int. J. 2006, 11, 162–173. [Google Scholar] [CrossRef]
- Faham, S.; Hileman, R.E.; Fromm, J.R.; Linhardt, R.J.; Rees, D.C. Heparin structure and interactions with basic fibroblast growth factor. Science 1996, 271, 1116–1120. [Google Scholar] [CrossRef]
- Caswell, G.H. A review of the work done in the entomology section of the Institute for Agricultural Research on the pests of stored grain. Samaru Misc. Pap. 1980, 99, 12. [Google Scholar]
- Marannino, P.; Santiago-Álvarez, C.; de Lillo, E.; Quesada-Moraga, E. A new bioassay method reveals pathogenicity of Metarhizium anisopliae and Beauveria bassiana against early stages of Capnodis tenebrionis (Coleoptera; Buprestidae). J. Invertebr. Pathol. 2006, 93, 210–213. [Google Scholar] [CrossRef]
- Ramdani, A.; Ibriz, H.; Essahat, A. Le traitement des semences au thiaméthoxame contrôle le Sitone (Sitona lineatus (L.)) sur Fèverole au Maroc. Afr. Mediterr. Agric. J.-Al Awamia 2022, 135, 179–191. [Google Scholar]
- Iturralde-García, R.D.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J.; Riudavets, J.; Del Toro-Sánchez, C.L.; Rueda-Puente, E.O.; Martínez-Cruz, O.; Wong-Corral, F.J. Effect of controlled atmospheres on the insect Callosobruchus maculatus Fab. in stored chickpea. J. Stored Prod. Res. 2016, 69, 78–85. [Google Scholar] [CrossRef]
- Abdelkader, M.; Btissam, B.M.; Laila, P.N.; Jamal, P.I. Dénombrement Des Populations Naturelles De Rhizobium Du Pois Chiche (Cicer Arietinum) Dans Différents Sols Du Maroc. Eur. Sci. J. 2017, 13, 273–286. [Google Scholar] [CrossRef]
- Lofty, H.M.; El-Aleem, A.E.-A.A.A.; Monir, H.H. Determination of insecticides malathion and lambda-cyhalothrin residues in zucchini by gas chromatography. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 255–260. [Google Scholar] [CrossRef]
- Shayeghi, M.; Khoobdel, M.; Vatandoost, H. Determination of organophosphorus insecticides (malathion and diazinon) residue in the drinking water. Pak. J. Biol. Sci. PJBS 2007, 10, 2900–2904. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Lima, J.G.; Santos, J.P.; Cruz, C.D. Resistance to DDT and pyrethroids in Brazilian populations of Sitophilus zeamais Motsch.(Coleoptera: Curculionidae). J. Stored Prod. Res. 1995, 31, 145–150. [Google Scholar] [CrossRef]
- Kendall, C.; Silva, S.R.; Kelly, V.J. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrol. Process. 2001, 15, 1301–1346. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Adler, C.; Bouda, H.; Fontem, D.A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Ribeiro, P.D.; Iribarne, O.O.; Jaureguy, L.; Navarro, D.; Bogazzi, E. Variable sex-specific mortality due to shorebird predation on a fiddler crab. Can. J. Zool. 2003, 81, 1209–1221. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Amiteye, S. Efficacy of mixing vegetable oils with pirimiphos-methyl against the maize weevil, Sitophilus zeamais Motschulsky in stored maize. J. Stored Prod. Res. 2005, 41, 57–66. [Google Scholar] [CrossRef]
- Harouna, M.A.; Baoua, I.; Lawali, S.; Tamò, M.; Amadou, L.; Mahamane, S.; Pittendrigh, B. Essai comparatif de l’utilisation des extraits du Neem et du virus entomopathogène MaviNPV dans la gestion des insectes ravageurs du niébé en milieu paysan au Niger. Int. J. Biol. Chem. Sci. 2019, 13, 950–961. [Google Scholar] [CrossRef]
- Poirié, M. Évolution et spécificité des interactions insectes hôtes–insectes parasitoïdes. Comptes Rendus Biol. 2019, 342, 265–267. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid. Based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef] [PubMed]
- Bourhia, M.; Laasri, F.E.; Aghmih, K.; Ullah, R.; Alqahtani, A.S.; Mahmood, H.M.; El-Mzibri, M.; Said, G.; Khlil, N.; Benbacer, L. Phytochemical composition, antioxidant activity, antiproliferative effect and acute toxicity study of Bryonia dioica roots used in North African alternative medicine. Int. J. Agric. Biol. 2020, 23, 597–602. [Google Scholar]
- Ebadollahi, A.; Nouri-Ganbalani, G.; Hoseini, S.A.; Sadeghi, G.R. Insecticidal activity of essential oils of five aromatic plants against Callosobruchus maculatus F.(Coleoptera: Bruchidae) under laboratory conditions. J. Essent. Oil Bear. Plants 2012, 15, 256–262. [Google Scholar] [CrossRef]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, anti-obesity, nutritional and other beneficial effects of different chili pepper: A review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Fusani, P.; Ronga, D.; Carminati, D.; Mandrioli, M.; Manicardi, G.C.; Giannì, S.; Tava, A. Composition and biological activity of essential oils from Artemisia roxburghiana Besser and Elsholtzia fruticosa Rehder cultivated in Italy. Ind. Crops Prod. 2022, 187, 115317. [Google Scholar] [CrossRef]
- Malhotra, A.; Rawat, A.; Prakash, O.; Kumar, R.; Srivastava, R.M.; Kumar, S. Chemical composition and pesticide activity of essential oils from Artemisia annua L. harvested in the rainy and winter seasons. Biochem. Syst. Ecol. 2023, 107, 104601. [Google Scholar] [CrossRef]
- Bachrouch, O.; Ferjani, N.; Haouel, S.; Jemâa, J.M.B. Major compounds and insecticidal activities of two Tunisian Artemisia essential oils toward two major coleopteran pests. Ind. Crops Prod. 2015, 65, 127–133. [Google Scholar] [CrossRef]
- Liu, C.H.; Mishra, A.K.; Tan, R.X.; Tang, C.; Yang, H.; Shen, Y.F. Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour. Technol. 2006, 97, 1969–1973. [Google Scholar] [CrossRef]
- Aljaiyash, A.; Kasrati, A.; Jamali, C.A.; Chaouch, A. Effect of cultivation on chemical composition and bioactivities of essential oils from Artemisia herba-alba Asso grown in Morocco. Biochem. Syst. Ecol. 2018, 81, 74–79. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef]
- Yuchi, Z.; Yuen, S.M.; Lau, K.; Underhill, A.Q.; Cornea, R.L.; Van Petegem, F. Crystal structures of ryanodine receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant. Nat. Commun. 2015, 6, 7947. [Google Scholar] [CrossRef]
- Lafraxo, S.; El Moussaoui, A.; Bin Jardan, Y.A.; El Barnossi, A.; Chebaibi, M.; Baammi, S.; Akka, A.A.; Chebbac, K.; Akhazzane, M.; Chelouati, T.; et al. GC-MS Profiling, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Essential Oil of Juniperus thurifera Bark. Evid. Based Complement. Altern. Med. 2022, 2022, e6305672. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- de Andrade Dutra, K.; de Oliveira, J.V.; Navarro, D.M.D.A.F.; Santos, J.P.O. Control of Callosobruchus maculatus (FABR.)(Coleoptera: Chrysomelidae: Bruchinae) in Vigna unguiculata (L.) WALP. with essential oils from four Citrus spp. plants. J. Stored Prod. Res. 2016, 68, 25–32. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar]
- Wekesa, I.; Onek, L.A.; Deng, A.L.; Hasanali, A.; Othira, J.O. Toxicity and repellant potency of Hyptis spicigera extracts on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Stored Prod. Postharvest Res. 2011, 2, 113–119. [Google Scholar]
- Bhat, M.A.; Tüzün, B.; Alsaif, N.A.; Khan, A.A.; Naglah, A.M. Synthesis, characterization, molecular modeling against EGFR target and ADME/T analysis of novel purine derivatives of sulfonamides. J. Mol. Struct. 2022, 1257, 132600. [Google Scholar] [CrossRef]
- Schrödinger. 4: Protein Preparation Wizard; Epik Schrödinger LLC: New York, NY, USA, 2016. [Google Scholar]
- Schrödinger. 2: LigPrep; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Shahzadi, I.; Zahoor, A.F.; Tüzün, B.; Mansha, A.; Anjum, M.N.; Rasul, A.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Repositioning of acefylline as anti-cancer drug: Synthesis, anticancer and computational studies of azomethines derived from acefylline tethered 4-amino-3-mercapto-1, 2, 4-triazole. PLoS ONE 2022, 17, e0278027. [Google Scholar] [CrossRef]
- Oh, E.; Wang, W.; Park, K.-H.; Park, C.; Cho, Y.; Lee, J.; Kang, E.; Kang, H. (+)-Usnic acid and its salts, inhibitors of SARS-CoV-2, identified by using in silico methods and in vitro assay. Sci. Rep. 2022, 12, 13118. [Google Scholar] [CrossRef]
- Diass, K.; Brahmi, F.; Mokhtari, O.; Abdellaoui, S.; Hammouti, B. Biological and pharmaceutical properties of essential oils of Rosmarinus officinalis L. and Lavandula officinalis L. Mater. Today Proc. 2021, 45, 7768–7773. [Google Scholar] [CrossRef]
- Zouirech, O.; Alajmi, R.; El Jeddab, H.; Allali, A.; Bourhia, M.; El Moussaoui, A.; El Barnossi, A.; Ahmed, A.M.; Giesy, J.P.; Aboul-Soud, M.A.M.; et al. Chemical Composition and Evaluation of Antifungal and Insecticidal Activities of Essential Oils Extracted from Jambosa caryophyllus (Thunb.) Nied: Clove Buds. Evid. Based Complement. Altern. Med. 2022, 2022, 4675016. [Google Scholar] [CrossRef] [PubMed]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 243–259. [Google Scholar]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A Review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Milutinović, M.; Savić, J. When Scent Becomes a Weapon—Plant Essential Oils as Potent Bioinsecticides. Sustainability 2022, 14, 6847. [Google Scholar] [CrossRef]
- Ketoh, G.K.; Koumaglo, H.K.; Glitho, I.A. Inhibition of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) development with essential oil extracted from Cymbopogon schoenanthus L. Spreng. (Poaceae), and the wasp Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2005, 41, 363–371. [Google Scholar] [CrossRef]
- Mssillou, I.; Saghrouchni, H.; Saber, M.; Zannou, A.J.; Balahbib, A.; Bouyahya, A.; Allali, A.; Lyoussi, B.; Derwich, E. Efficacy and role of essential oils as bio-insecticide against the pulse beetle Callosobruchus maculatus (F.) in post-harvest crops. Ind. Crops Prod. 2022, 189, 115786. [Google Scholar] [CrossRef]
- Pourya, M.; Sadeghi, A.; Ghobari, H.; Taning, C.N.T.; Smagghe, G. Bioactivity of Pistacia atlantica desf. Subsp. Kurdica (Zohary) Rech. F. and Pistacia khinjuk stocks essential oils against Callosobruchus maculatus (F, 1775) (Coloeptera: Bruchidae) under laboratory conditions. J. Stored Prod. Res. 2018, 77, 96–105. [Google Scholar] [CrossRef]
- Khelfane-Goucem, K.; Lardjane, N.; Medjdoub-Bensaad, F. Fumigant and repellent activity of Rutaceae and Lamiaceae essential oils against Acanthoscelides obtectus Say. Afr. J. Agric. Res. 2016, 11, 1499–1503. [Google Scholar]
- Agil, R.; Hosseinian, F.S. Bioactivity of alkylresorcinols. Bioact. Mol. Plant Foods 2012, 72, 131–162. [Google Scholar]
- Guèye, M.T.; Seck, D.; Wathelet, J.; Lognay, G. Lutte contre les ravageurs des stocks de céréales et de légumineuses au Sénégal et en Afrique occidentale: Synthèse bibliographique. Biotechnol. Agron. Soc. Environ. 2011, 15, 183–194. [Google Scholar]
- Aimad, A.; Bourhia, M.; Hana, H.; Sanae, R.; Salamatullah, A.M.; Soufan, W.; Rihan, H.Z.; Ouahmane, L.; Youness, E.A.; Noureddine, E.; et al. Essential Oils from Artemisia herba alba Asso., Maticaria Recutita L., and Dittrichia Viscosa L. (Asteraceae): A Promising Source of Eco-Friendly Agents to Control Callosobruchus maculatus Fab. Warehouse Pest. J. Chem. 2022, 2022, 2373460. [Google Scholar] [CrossRef]
- Sahaf, B.Z.; Moharramipour, S. Fumigant toxicity of Carum copticum and Vitex pseudo-negundo essential oils against eggs, larvae and adults of Callosobruchus maculatus. J. Pest Sci. 2008, 81, 213–220. [Google Scholar] [CrossRef]
- Abd-Elhady, H. Insecticidal activity and chemical composition of essential oil from Artemisia judaica L. against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Plant Prot. Res. 2012, 52, 347–352. [Google Scholar] [CrossRef]
- Titouhi, F.; Amri, M.; Messaoud, C.; Haouel, S.; Youssfi, S.; Cherif, A.; Ben Jemâa, J.M. Protective effects of three Artemisia essential oils against Callosobruchus maculatus and Bruchus rufimanus (Coleoptera: Chrysomelidae) and the extended side-effects on their natural enemies. J. Stored Prod. Res. 2017, 72, 11–20. [Google Scholar] [CrossRef]
- Manzoomi, N.; Ganbalani, G.N.; Dastjerdi, H.R.; Fathi, S.A.A. Fumigant toxicity of essential oils of Lavandula officinalis, Artemisia dracunculus and Heracleum persicum on the adults of Callosobruchus maculatus (Coleoptera: Bruchidae). Munn. Entomol. Zool. 2010, 5, 118–122. [Google Scholar]
- Seri-Kouassi, B.P.; Kanko, C.; Aboua, L.R.N.; Bekon, K.A.; Glitho, A.I.; Koukoua, G.; N’Guessan, Y.T. Action des huiles essentielles de deux plantes aromatiques de Côte-d’Ivoire sur Callosobruchus maculatus F. du niébé. Comptes Rendus Chim. 2004, 7, 1043–1046. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Chiasson, H.; Beloin, N. Les huiles essentielles, des biopesticides «Nouveau genre». Bull. Soc. D’Entomol. Qué. 2007, 14, 3–6. [Google Scholar]
- Abdelfattah, E.M.; Aimad, A.; Bourhia, M.; Chebbac, K.; Salamatullah, A.M.; Soufan, W.; Nafidi, H.-A.; Aboul-Soud, M.A.M.; Ouahmane, L.; Bari, A. Insecticidal and Antifungal Activities of Chemically-Characterized Essential Oils from the Leaves of Withania frutescens L. Life 2022, 12, 88. [Google Scholar] [CrossRef]
- Aimad, A.; Youness, E.A.; Sanae, R.; El Moussaoui, A. Chemical Composition and Antifungal, Insecticidal and Repellent Activity of Essential Oils from Origanum compactum Benth. Used in the Mediterranean Diet. Front. Plant Sci. 2022, 13, 798259. [Google Scholar] [CrossRef]
- Chalkha, M.; Akhazzane, M.; Moussaid, F.Z.; Daoui, O.; Nakkabi, A.; Bakhouch, M.; Chtita, S.; Elkhattabi, S.; Housseini, A.I.; El Yazidi, M. Design, synthesis, characterization, in vitro screening, molecular docking, 3D-QSAR, and ADME-Tox investigations of novel pyrazole derivatives as antimicrobial agents. New J. Chem. 2022, 46, 2747–2760. [Google Scholar] [CrossRef]
- Chalkha, M.; Nakkabi, A.; Ben Hadda, T.; Berredjem, M.; El Moussaoui, A.; Bakhouch, M.; Saadi, M.; El Ammari, L.; Almalki, F.A.; Laaroussi, H.; et al. Crystallographic study, biological assessment and POM/Docking studies of pyrazoles-sulfonamide hybrids (PSH): Identification of a combined Antibacterial/Antiviral pharmacophore sites leading to in-silico screening the anti-COVID-19 activity. J. Mol. Struct. 2022, 1267, 133605. [Google Scholar] [CrossRef] [PubMed]
- Tapera, M.; Kekeçmuhammed, H.; Tüzün, B.; Sarıpınar, E.; Koçyiğit, Ü.M.; Yıldırım, E.; Doğan, M.; Zorlu, Y. Synthesis, carbonic anhydrase inhibitory activity, anticancer activity and molecular docking studies of new imidazolyl hydrazone derivatives. J. Mol. Struct. 2022, 1269, 133816. [Google Scholar] [CrossRef]
- Majumdar, D.; Philip, J.E.; Tüzün, B.; Frontera, A.; Gomila, R.M.; Roy, S.; Bankura, K. Unravelling the Synthetic Mimic, Spectroscopic Insights, and Supramolecular Crystal Engineering of an Innovative Heteronuclear Pb (II)-Salen Cocrystal: An Integrated DFT, QTAIM/NCI Plot, NLO, Molecular Docking/PLIP, and Antibacterial Appraisal. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4320–4339. [Google Scholar] [CrossRef]
- Koçyiğit, M.; Doğan, M.; Muğlu, H.; Taslimi, P.; Tüzün, B.; Yakan, H.; Bal, H.; Güzel, E.; Gülçin, I. Determination of biological studies and molecular docking calculations of isatin-thiosemicarbazone hybrid compounds. J. Mol. Struct. 2022, 1264, 133249. [Google Scholar] [CrossRef]
- Poustforoosh, A.; Hashemipour, H.; Tüzün, B.; Azadpour, M.; Faramarz, S.; Pardakhty, A.; Mehrabani, M.; Nematollahi, M.H. The Impact of D614G Mutation of SARS-CoV-2 on the Efficacy of Anti-viral Drugs: A Comparative Molecular Docking and Molecular Dynamics Study. Curr. Microbiol. 2022, 79, 241. [Google Scholar] [CrossRef]
- Mermer, A.; Bulbul, M.V.; Kalender, S.M.; Keskin, I.; Tuzun, B.; Eyupoglu, O.E. Benzotriazole-oxadiazole hybrid Compounds: Synthesis, anticancer Activity, molecular docking and ADME profiling studies. J. Mol. Liq. 2022, 359, 119264. [Google Scholar] [CrossRef]
- Kafa, A.H.T.; Tüzün, G.; Güney, E.; Aslan, R.; Sayın, K.; Tüzün, B. Synthesis, computational analyses, antibacterial and antibiofilm properties of nicotinamide derivatives. Struct. Chem. 2022, 33, 1189–1197. [Google Scholar] [CrossRef]
- Kekeçmuhammed, H.; Tapera, M.; Tüzün, B.; Akkoç, S.; Zorlu, Y.; Sarıpınar, E. Synthesis, Molecular Docking and Antiproliferative Activity Studies of a Thiazole-Based Compound Linked to Hydrazone Moiety. ChemistrySelect 2022, 7, e202201502. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).