Greenness Assessment of HPLC Analytical Methods with Common Detectors for Assay of Paracetamol and Related Materials in Drug Products and Biological Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. National Environmental Method Index (NEMI)
2.2. Analytical Eco-Scale Assessment (ESA) [40]
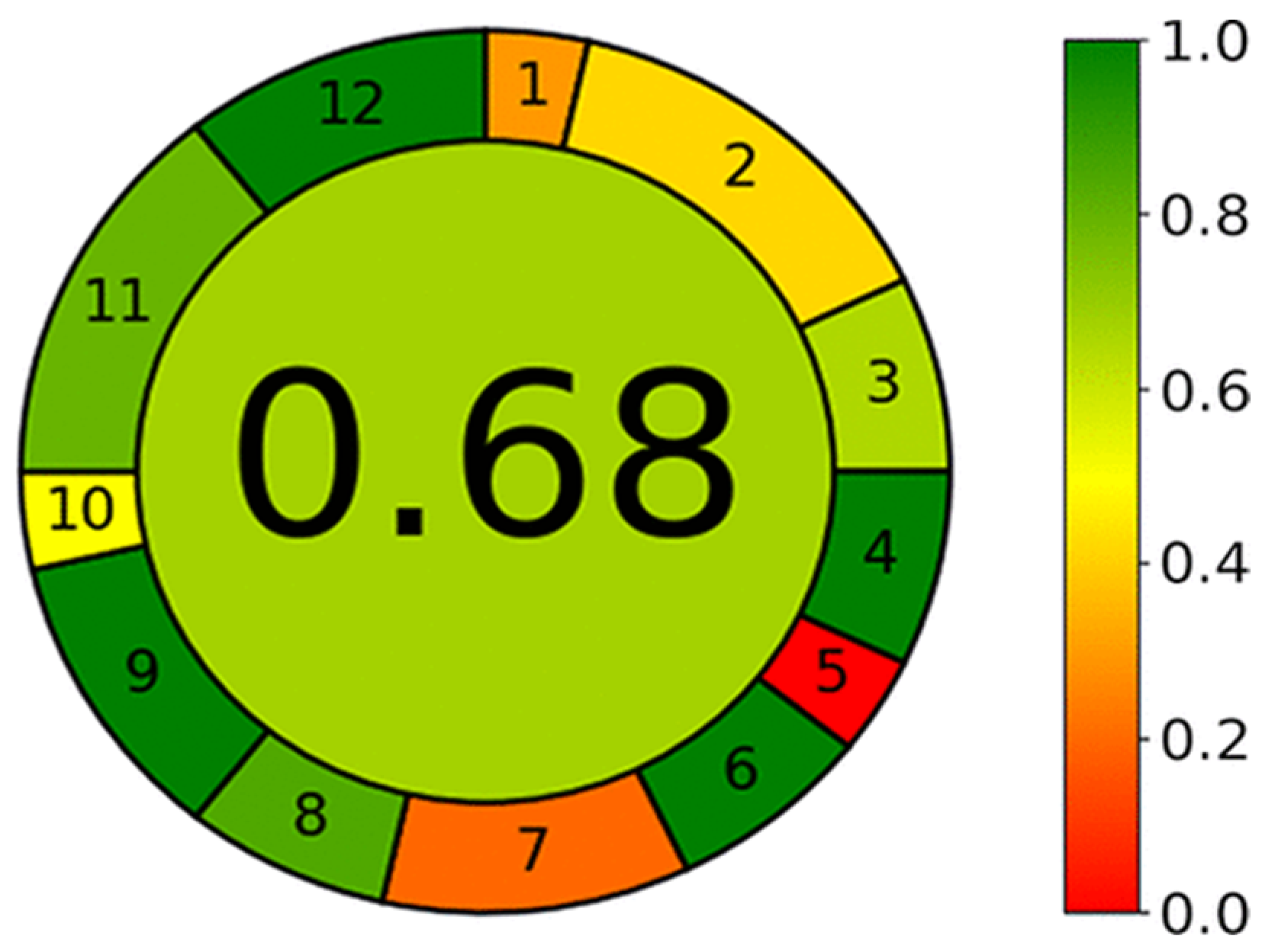
2.3. The Analytical Greenness Metric (AGREE) [41]
3. Results and Discussion
3.1. Paracetamol Assay in Drug Products and Raw Material
- -
- NEMI tool:
- -
- ESA tool:
- -
- AGREE tool:
3.2. Paracetamol Assay in Biological Fluids
- -
- NEMI tool:
- -
- ESA tool:
- -
- AGREE tool:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloukh, S.; Wazaify, M.; Matheson, C. Paracetamol: Unconventional uses of a well-known drug. Int. J. Pharm. Pract. 2021, 29, 527–540. [Google Scholar] [CrossRef]
- NHS Inform. Paracetamol—Tests & Treatments. Available online: https://www.nhsinform.scot/tests-and-treatments/medicines-and-medical-aids/types-of-medicine/paracetamol (accessed on 23 December 2022).
- McCrae, J.C.; Morrison, E.E.; MacIntyre, I.M.; Dear, J.W.; Webb, D.J. Long-term adverse effects of paracetamol—A review. Br. J. Clin. Pharmacol. 2018, 84, 2218–2230. [Google Scholar] [CrossRef]
- Ragab Ali, A.R. Pattern of Pediatric Toxicity in Saudi Arabia-Eastern Province (Incidence, Demographics and Predisposing Factors). Pediatr. Ther. 2015, 5, 220. [Google Scholar] [CrossRef]
- Almansori, M.A.; Alhammadi, H.I.; Almulhim, F.A. Paracetamol overdose: Analysis of a sample from a tertiary hospital in Eastern Saudi Arabia. Saudi J. Med. Med. Sci. 2015, 3, 209–212. [Google Scholar] [CrossRef]
- Dong, M.; Llanas, A. The Essence of Modern HPLC: Advantages, Limitations, Fundamentals, and Opportunities. LCGC N. Am. 2013, 31, 472. [Google Scholar]
- Lee, H. A brief note on high performance liquid chromatography (HPLC) and its applications. J. Chromatogr. Sep. Tech. 2022, 13, 466. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. TrAC 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Santos, L.H.; Paíga, P.; Araújo, A.N.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B. Development of a simple analytical method for the simultaneous determination of paracetamol, paracetamol-glucuronide and p-aminophenol in river water. J. Chromatogr. B 2013, 930, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, H.; Yue, Z.; Tian, S. HPLC determination of paracetamol in paracetamol suppositories. Chin. J. Pharm. Anal. 2004, 24, 417–419. [Google Scholar]
- Abdelaleem, E.A.; Naguib, I.A.; Hassan, E.S.; Ali, N.W. HPTLC and RP-HPLC methods for simultaneous determination of Paracetamol and Pamabrom in presence of their potential impurities. J. Pharm. Biomed. Anal. 2015, 114, 22–27. [Google Scholar] [CrossRef]
- Rao, R.N.; Narasaraju, A. Rapid separation and determination of process-related substances of paracetamol using reversed-phase HPLC with photo diode array as a detector. Anal. Sci. 2006, 22, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Hewala, I.I. High-performance liquid chromatographic and derivative difference spectrophotometric methods for the determination of acetaminophen and its degradation product in aged pharmaceutical formulations. Anal. Lett. 1994, 27, 561–582. [Google Scholar] [CrossRef]
- Kamberi, M.; Riley, C.M.; Ma, X.; Huang, C.-W.C. A validated, sensitive HPLC method for the determination of trace impurities in acetaminophen drug substance. J. Pharm. Biomed. Anal. 2004, 34, 123–128. [Google Scholar] [CrossRef]
- Primus, T.M.; Kohler, D.J.; Furcolow, C.A.; Goodall, M.J.; Johnston, J.J.; Savarie, P.J. Determination of acetaminophen residues in whole body brown treesnakes. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 897–909. [Google Scholar] [CrossRef]
- Călinescu, O.; Badea, I.A.; Vlădescu, L.; Meltzer, V.; Pincu, E. HPLC separation of acetaminophen and its impurities using a mixed-mode reversed-phase/cation exchange stationary phase. J. Chromatogr. Sci. 2012, 50, 335–342. [Google Scholar] [CrossRef]
- Monser, L.; Darghouth, F. Simultaneous LC determination of paracetamol and related compounds in pharmaceutical formulations using a carbon-based column. J. Pharm. Biomed. Anal. 2002, 27, 851–860. [Google Scholar] [CrossRef]
- Hazai, E.; Simon-Trompler, E.; Czira, G.; Vereczkey, L.; Monostory, K. New LC method using radioactivity detection for analysis of toxic metabolite of acetaminophen (Paracetamol). Chromatographia 2002, 56, S75–S78. [Google Scholar] [CrossRef]
- Azodi-Deilami, S.; Najafabadi, A.H.; Asadi, E.; Abdouss, M.; Kordestani, D. Magnetic molecularly imprinted polymer nanoparticles for the solid-phase extraction of paracetamol from plasma samples, followed its determination by HPLC. Microchim. Acta 2014, 181, 1823–1832. [Google Scholar] [CrossRef]
- Hewavitharana, A.K.; Lee, S.; Dawson, P.A.; Markovich, D.; Shaw, P.N. Development of an HPLC–MS/MS method for the selective determination of paracetamol metabolites in mouse urine. Anal. Biochem. 2008, 374, 106–111. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Watson, D.G.; Morton, N.S. A simple microanalytical technique for the determination of paracetamol and its main metabolites in blood spots. J. Pharm. Biomed. Anal. 2002, 29, 803–809. [Google Scholar] [CrossRef]
- Thatcher, N.J.; Murray, S. Analysis of the glutathione conjugate of paracetamol in human liver microsomal fraction by liquid chromatography mass spectrometry. Biomed. Chromatogr. 2001, 15, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidy, S.S.; Po, A.L.W.; McKiernan, P.J.; Glasgow, J.F.T.; Millership, J. Assay of paracetamol and its metabolites in urine, plasma and saliva of children with chronic liver disease. J. Pharm. Biomed. Anal. 1995, 13, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.F.; King, A.D.; van den Anker, J.N.; Wilkins, D.G. Simultaneous quantification of acetaminophen and five acetaminophen metabolites in human plasma and urine by high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry: Method validation and application to a neonatal pharmacokinetic study. J. Chromatogr. B 2015, 1007, 30–42. [Google Scholar]
- Abbasi, S.; Haeri, S.A.; Sajjadifar, S. Bio-dispersive liquid liquid microextraction based on nano rhamnolipid aggregates combined with molecularly imprinted-solid phase extraction for selective determination of paracetamol in human urine samples followed by HPLC. Microchem. J. 2019, 146, 106–114. [Google Scholar] [CrossRef]
- Tan, Q.-Y.; Zhu, R.-H.; Li, H.-D.; Wang, F.; Yan, M.; Dai, L.-B. Simultaneous quantitative determination of paracetamol and its glucuronide conjugate in human plasma and urine by liquid chromatography coupled to electrospray tandem mass spectrometry: Application to a clinical pharmacokinetic study. J. Chromatogr. B 2012, 893–894, 162–167. [Google Scholar] [CrossRef]
- Langlois, M.-H.; Vekris, A.; Bousses, C.; Mordelet, E.; Buhannic, N.; Séguard, C.; Couraud, P.-O.; Weksler, B.B.; Petry, K.G.; Gaudin, K. Development of a solvent-free analytical method for paracetamol quantitative determination in Blood Brain Barrier in vitro model. J. Chromatogr. B 2015, 988, 20–24. [Google Scholar] [CrossRef]
- Goicoechea, A.G.; De Alda, M.J.L.; Vila-Jato, J.L. A validated high-performance liquid chromatographic method for the determination of paracetamol and its major metabolites in urine. J. Liq. Chromatogr. Relat. Technol. 1995, 18, 3257–3268. [Google Scholar] [CrossRef]
- Lau, G.S.; Critchley, J. The estimation of paracetamol and its major metabolites in both plasma and urine by a single high-performance liquid chromatography assay. J. Pharm. Biomed. Anal. 1994, 12, 1563–1572. [Google Scholar] [CrossRef]
- Emara, S.; Masujima, T.; Hadad, G.; Kamal, M.; ZaaZaa, H.; Kawi, M.A. A rapid, sensitive, and environmentally friendly on-line solid phase extraction using protein-coated μ-bondapak cyanide silica precolumn for chromatographic determination of paracetamol in human serum. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1297–1311. [Google Scholar] [CrossRef]
- Modick, H.; Schütze, A.; Pälmke, C.; Weiss, T.; Brüning, T.; Koch, H.M. Rapid determination of N-acetyl-4-aminophenol (paracetamol) in urine by tandem mass spectrometry coupled with on-line clean-up by two dimensional turbulent flow/reversed phase liquid chromatography. J. Chromatogr. B 2013, 925, 33–39. [Google Scholar] [CrossRef]
- Vertzoni, M.V.; Archontaki, H.A.; Galanopoulou, P. Development and optimization of a reversed-phase high-performance liquid chromatographic method for the determination of acetaminophen and its major metabolites in rabbit plasma and urine after a toxic dose. J. Pharm. Biomed. Anal. 2003, 32, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Spooner, N.; Lad, R.; Barfield, M. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: Considerations for the validation of a quantitative bioanalytical method. Anal. Chem. 2009, 81, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Yoshida, H.; Nohta, H.; Yamaguchi, M. HPLC determination of acetaminophen in saliva based on precolumn fluorescence derivatization with 12-(3,5-Dichloro-2,4,6-triazinyl)-benzo [d] benzo [1′,2′-6,5] isoindolo [1,2-b][1,3] thiazolidine. Anal. Sci. 2005, 21, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Barfield, M.; Spooner, N.; Lad, R.; Parry, S.; Fowles, S. Application of dried blood spots combined with HPLC-MS/MS for the quantification of acetaminophen in toxicokinetic studies. J. Chromatogr. B 2008, 870, 32–37. [Google Scholar] [CrossRef]
- Cook, S.F.; King, A.D.; Chang, Y.; Murray, G.J.; Norris, H.-R.K.; Dart, R.C.; Green, J.L.; Curry, S.C.; Rollins, D.E.; Wilkins, D.G. Quantification of a biomarker of acetaminophen protein adducts in human serum by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: Clinical and animal model applications. J. Chromatogr. B 2015, 985, 131–141. [Google Scholar] [CrossRef]
- Klimek-Turek, A.; Sikora, M.; Rybicki, M.; Dzido, T.H. Frontally eluted components procedure with thin layer chromatography as a mode of sample preparation for high performance liquid chromatography quantitation of acetaminophen in biological matrix. J. Chromatogr. A 2016, 1436, 19–27. [Google Scholar] [CrossRef]
- Teffera, Y.; Abramson, F. Application of high-performance liquid chromatography/chemical reaction interface mass spectrometry for the analysis of conjugated metabolites: A demonstration using deuterated acetaminophen. Biol. Mass Spectrom. 1994, 23, 776–783. [Google Scholar] [CrossRef]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC 2013, 50, 78–84. [Google Scholar] [CrossRef]

| Study Number | Applied Instrument and Chromatographic Method | ESA | NEMI Pictogram | AGREE Pictogram |
|---|---|---|---|---|
| 1.1. [9] | HPLC/DAD The mobile phase: 10 mM ammonium acetate/acetic acid (pH 6) as solvent A and acetonitrile as solvent B, using a flow rate of 1.0 mL/min. | 33 |  |  |
| 1.2. [10] | HPLC/UV The mobile phase: a mixture of water-methanol (3:1) with a flow rate of 1.0 mL/min. | 18 | 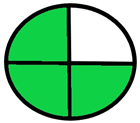 | 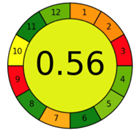 |
| 1.3. [11] | HPTLC The mobile phase:methanol:ethyl acetate:glacial acetic acid (8:0.8:0.6:0.2, v/v/v/v) at 1.0 mL/min. | 77 |  | 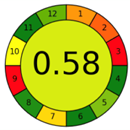 |
| 1.4. [12] | HPLC/DAD The mobile phase: potassium dihydrogen phosphate buffer (pH 3.0) and acetonitrile at 1.0 mL/min. LOD range (0.05–0.08 ug/mL) LOQ range (0.145–0.197 mg/mL) | 72 | 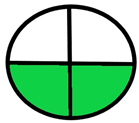 | 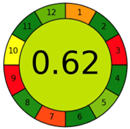 |
| 1.5. [13] | HPLC/UV The mobile phase: 99% formic acid, 0.2% v/v and 1% methanol at 1.0 mL/min. | 79 | 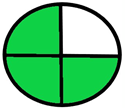 | 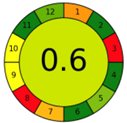 |
| 1.6. [14] | HPLC/UV The mobile phase: solvent A: 0.01 M phosphate buffer at pH 3.0 and solvent B: methanol at a flow rate of 1.0 mL/min. | 27 | 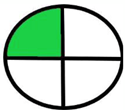 | 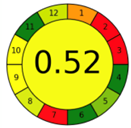 |
| 1.7. [15] | HPLC/UV The mobile phase: a 15:85 mixture of methanol, 50 mM potassium phosphate, monobasic (pH = 3.25) aqueous solution with a flow rate of 1.0 mL/min. LOD 0.034 mg/mL | 81 | 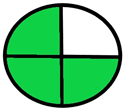 | 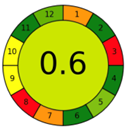 |
| 1.8. [16] | HPLC/UV The mobile phase: a mixture of phosphate buffer (pH = 4.88) and methanol at a flow rate of 1.0 mL/min. | 44 |  |  |
| 1.9. [17] | HPLC/UV The mobile phase: an isocratic mixture of 80/20 (v/v) acetonitrile/0.05 M potassium phosphate buffer (pH 5.5) with flow velocity of 1.0 mL/min. | 67 |  | 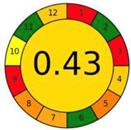 |
| Study Number | Applied Instrument and Chromatographic Method | ESA | NEMI Pictogram | AGREE Pictogram |
| 2.1. [18] | HPLC/UV The mobile phase: 40 mM ammonium acetate (pH 4.8): methanol [87:13 v/v] at a flow rate 1.0 mL/min. The sample type was human liver. | 74 | 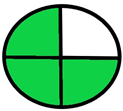 |  |
| 2.2. [19] | HPLC/UV The mobile phase: a mixture of methanol and acetic acid at a flow rate 1.0 mL/min. The sample type was human plasma LOD 0.17 mcg L−1 LOG 0.4 mcg L−1 | 31 | 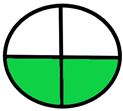 | 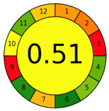 |
| 2.3. [20] | HPLC/MS The mobile phase: 0.1% (v/v) formic acid and acetonitrile at a flow rate of 0.2 mL/min. The sample type was mouse urine. LOD 0.66 mol/L | 84 | 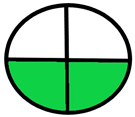 | 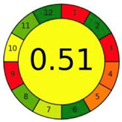 |
| 2.4. [21] | HPLC/UV The mobile phase: 20 mM ammonium formate buffer pH 3.5 (A) and methanol (B) (pH 3.5) at a flow rate of 0.8 mL/min. The sample type was blood spots. | 67 | 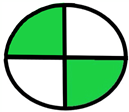 | 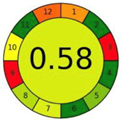 |
| 2.5. [22] | HPLC/UV The mobile phase: aqueous buffer solution and methanol at a flow rate of 1.0 mL/min. The sample type was human liver. | 58 | 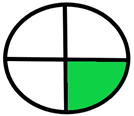 | 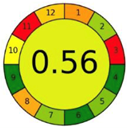 |
| 2.6. [23] | HPLC/UV The mobile phase: 35% water and 20% methanol at a flow rate 1.0 mL/min. The sample types were human plasma, urine and saliva. | 79 | 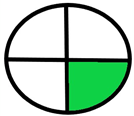 | 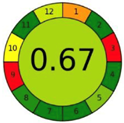 |
| 2.7. [24] | HPLC/MS The mobile phase: ammonium acetate, buffers, formate buffers and methanol at a flow rate of 0.25 mL/min. The sample types were human plasma and urine. | 66 | 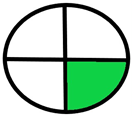 |  |
| 2.8. [25] | HPLC/UV The mobile phase consisted of water and methanol at a flow rate of 1.0 mL/min. The sample type was human urine LOQ 0.96 mcg/L−1. | 37 |  | 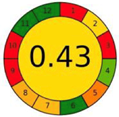 |
| 2.9. [26] | HPLC/MS The mobile phase: methanol-water containing 0.0875% formic acid at a flow rate of 1.5 mL/min. The sample types were human plasma and urine. | 72 | 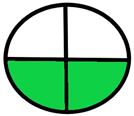 | 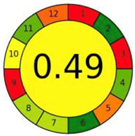 |
| 2.10. [27] | HPLC/UV The mobile phase: 0.3% methanol at a flow rate of 0.25 mL/min. The sample type was cell culture representing an in vitro model of blood–brain barrier. | 79 | 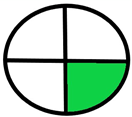 | 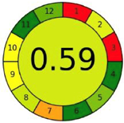 |
| 2.11. [28] | HPLC/UV The mobile phase: A gradient consisting of 0.1% formic acid and water at a flow rate of 0.25 mL/min. The sample type was human urine sample. | 75 | 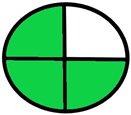 | 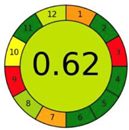 |
| 2.12. [29] | HPLC/UV The mobile phase: 0.1 M potassium dihydrogen orthophosphate, acetic acid and propane-2 at a flow rate of 1.5 mL/min. The sample types were human plasma and urine. | 65 |  | 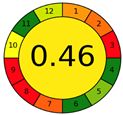 |
| 2.13. [30] | HPLC/MS The mobile phase: methanol and phosphate buffer (0.05 M) at a flow rate of 1.0 mL/min. The sample type was human serum. LOQ 7.41 ng/mL. | 67 | 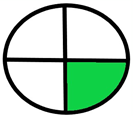 |  |
| 2.14. [31] | HPLC/MS The mobile phase: 75% water and 25% methanol at a flow rate of 1.0 mL/min. The sample type was human urine. LOQ 0.75 mcg/L. | 85 | 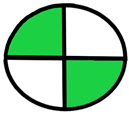 | 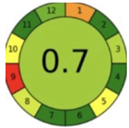 |
| 2.15. [32] | HPLC/UV The mobile phase: aqueous buffer solution of KH2PO4 (0.05 M) containing 1% CH3COOH (pH 6.5) and methanol at a flow rate of 1.5 mL/min. The sample types were rabbit plasma and urine. | 63 | 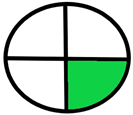 | 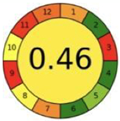 |
| 2.16. [33] | HPLC/MS The mobile phases: ammonium acetate (10 mM; adjusted to pH 10 with ammonia) and methanol at a flow rate 0.25 mL/min. The sample type was human dried blood spots. | 85 | 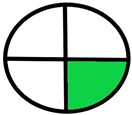 | 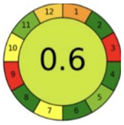 |
| 2.17. [34] | HPLC/MS The mobile phase: methanol degassed with ultra-sonication at flow rate of 1.0 mL/min The sample type was saliva. | 92 |  | 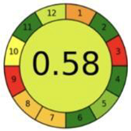 |
| 2.18. [35] | HPLC/MS The mobile phase: 10 mM ammonium formate containing 0.3% ammonia and methanol at a flow rate of 0.25 mL/min. The sample type was dog dried blood spots. | 91 | 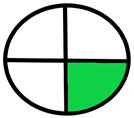 | 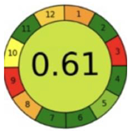 |
| 2.19. [36] | HPLC/MS The mobile phase: a gradient consisting of 0.1% formic acid in water and 0.1% in methanol at a flow rate of 0.25 mL/min. The sample type was human serum. | 86 | 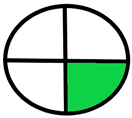 | 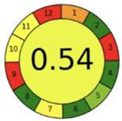 |
| 2.20. [37] | HPLC/MS The mobile phase: 25% methanol and 75% citrate-phosphate buffer (pH 3.0) at a flow rate of 1.0 mL/min. The sample type was serum. | 19 | 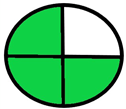 | 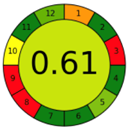 |
| 2.21. [38] | HPLC/MS The mobile phase: 0.1% trifluoroacetic acid in water (A), and methanol (B), at a flow rate of 1.0 mL/min. The sample type was urine–bile. | 83 |  | 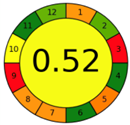 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naguib, I.A.; Majed, M.; Albogami, M.; Alshehri, M.; Bukhari, A.; Alshabani, H.; Alsalahat, I.; Abd-ElSalam, H.-A.H. Greenness Assessment of HPLC Analytical Methods with Common Detectors for Assay of Paracetamol and Related Materials in Drug Products and Biological Fluids. Separations 2023, 10, 283. https://doi.org/10.3390/separations10050283
Naguib IA, Majed M, Albogami M, Alshehri M, Bukhari A, Alshabani H, Alsalahat I, Abd-ElSalam H-AH. Greenness Assessment of HPLC Analytical Methods with Common Detectors for Assay of Paracetamol and Related Materials in Drug Products and Biological Fluids. Separations. 2023; 10(5):283. https://doi.org/10.3390/separations10050283
Chicago/Turabian StyleNaguib, Ibrahim A., Meral Majed, Maram Albogami, Maram Alshehri, Aseel Bukhari, Hadeel Alshabani, Izzeddin Alsalahat, and Heba-Alla H. Abd-ElSalam. 2023. "Greenness Assessment of HPLC Analytical Methods with Common Detectors for Assay of Paracetamol and Related Materials in Drug Products and Biological Fluids" Separations 10, no. 5: 283. https://doi.org/10.3390/separations10050283
APA StyleNaguib, I. A., Majed, M., Albogami, M., Alshehri, M., Bukhari, A., Alshabani, H., Alsalahat, I., & Abd-ElSalam, H.-A. H. (2023). Greenness Assessment of HPLC Analytical Methods with Common Detectors for Assay of Paracetamol and Related Materials in Drug Products and Biological Fluids. Separations, 10(5), 283. https://doi.org/10.3390/separations10050283







