Abstract
Because of its greater binding affinity and longer half-life than native glucagon-like peptide-1 (GLP-1), the GLP-1 receptor agonist lixisenatide is commonly used to treat type 2 diabetes mellitus. This study aimed to establish a simple and robust liquid chromatography–tandem mass spectrometry (LC–MS/MS) approach for lixisenatide for in vivo pharmacokinetic investigation. Methanol-based protein precipitation with formic acid was exploited for plasma sample extraction, using esomeprazole as the internal standard. Gradient elution with 0.1% formic acid in distilled water and acetonitrile was utilized for chromatographic separation. Mass spectrometry was used to monitor the MRM transition at m/z 810.8 → 129.2 for lixisenatide. In rat plasma, lixisenatide had a lower limit of quantification of 10 ng/mL. The LC–MS/MS was applied to describe the pharmacokinetics of lixisenatide in rats following intravenous and subcutaneous dosing. The average half-life of lixisenatide was 0.37 ± 0.06 h after intravenous injection. The estimated subcutaneous bioavailability of lixisenatide was 2.17%. This LC–MS/MS analysis might be relevant in future research to create novel dosage formulations of lixisenatide and other GLP-1 receptor agonists with optimal therapeutic effectiveness.
1. Introduction
Diabetes affects over half a billion people and is responsible for 1.5 million deaths annually [1]. Type 2 diabetes, a chronic illness defined by elevated blood glucose levels brought on by a confluence of insulin resistance and insufficient insulin secretion, accounts for more than 90% of all cases of diabetes [2]. In addition to lifestyle changes such as exercise, healthy diet, and weight loss, various diabetes medications, such as metformin, sulfonylureas, thiazolidinediones, insulin, dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists, have been widely used to manage type 2 diabetes [3,4,5].
Among others, GLP-1 receptor agonists represent a significant therapeutic breakthrough in the management of Type 2 diabetes [6]. GLP-1 is an endogenous peptide belonging to the incretin peptide family that has diverse effects on glycemic control [6,7]. GLP-1 receptor agonists stimulate the GLP-1 receptor to promote glucose-dependent insulin secretion and satiety, while suppressing unnecessary glucagon release and prolonging gastric emptying [6,7]. Thus, GLP-1 receptor agonists are also advantageous for patients with obesity, which is closely linked to type 2 diabetes. Another advantage of GLP-1 receptor agonists is their low risk of hypoglycemia, as GLP-1 does not decrease glucose below fasting levels [8].
Lixisenatide, a synthetic GLP-1 receptor agonist, received approval from the European Medicines Agency (EMA) in 2013 and from the U.S. Food and Drug Administration (FDA) in 2016 as a once-daily subcutaneous injection to enhance glycemic control in adults with type 2 diabetes [9,10]. Lixisenatide has a rapid onset of action and a half-life of approximately three hours [10]. The extended half-life of lixisenatide compared to intact GLP-1 is attributed to the C-terminal modification with six lysine residues and deletion of one proline in the exendin-4 backbone [8,11]. Structural change also improved binding affinity to the GLP-1 receptor; the affinity of lixisenatide is approximately four times higher than that of native human GLP-1 [8,11]. Furthermore, lixisenatide provides a substantial reduction in postprandial glucose and good gastrointestinal tolerability [6,12]. Thus, lixisenatide is promising as a replacement for prandial insulin [6]. In addition, the fixed-dose combination of insulin glargine plus lixisenatide has shown efficacy and safety by once-daily administration, suggesting its potential as an effective therapeutic approach for type 2 diabetes [13]. Efforts have also been made to develop novel lixisenatide formulations with sustained drug release rates [14].
The clinical pharmacokinetics of lixisenatide have been relatively well characterized by enzyme-linked immunosorbent assay (ELISA). Upon subcutaneous administration, lixisenatide is quickly absorbed into the bloodstream with a maximum concentration achieved at 1 to 3.5 h [9,15]. After absorption, lixisenatide undergoes proteolytic degradation and is excreted into the urine, with an average half-life (t1/2) of approximately 3 h [11]. Despite the relatively short t1/2, lixisenatide is administered once daily because of its high affinity to the GLP-1 receptor and inhibition of gastric emptying [6]. However, the absolute bioavailability of lixisenatide after subcutaneous administration in humans is still unknown. On the other hand, its pharmacokinetics is not significantly impacted by age, body weight, or gender, and it remains primarily unchanged in patients with mild to moderate renal impairment [6,11].
Nevertheless, robust analytical methods to determine lixisenatide concentrations in biological fluids are limited. Except for several initial nonclinical pharmacokinetic studies, ELISA or radioactivity methods have been primarily used to quantify lixisenatide in biological samples [10,14,16,17]. Clinical pharmacokinetics of lixisenatide in Phase 1 and 2 studies have been evaluated by ELISA [10]. Brain distribution studies of lixisenatide in animals were dependent on ELISA [16] or radioactivity assay of the radioisotope labeled lixisenatide [17]. New formulation development studies also relied on the Enzyme Immunoassay kit [14]. Although a liquid chromatography high-resolution mass spectrometry was developed to evaluate in vitro metabolism [18], no liquid chromatography–tandem mass spectrometry (LC–MS/MS) methods have been reported to be applicable for in vivo pharmacokinetic studies.
Therefore, the objective of this study was to establish an LC–MS/MS analysis of lixisenatide for pharmacokinetics investigation. The utility of the LC–MS/MS method was illustrated by in vivo pharmacokinetic studies of lixisenatide after intravenous and subcutaneous injection in rats. The LC–MS/MS analysis and pharmacokinetic results may be helpful for future lixisenatide and other GLP-1 receptor agonists research.
2. Materials and Methods
2.1. Materials
Lixisenatide acetate (99.8%) was purchased from ATK Chemical Co., Ltd. (Shanghai, China). The internal standard (IS) esomeprazole was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The chemical structures of lixisenatide and the IS are shown in Figure 1. Formic acid was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Methanol, acetonitrile, and distilled water, all high-performance liquid chromatography (HPLC)-grade, were the products of J.T. Baker, Inc. (Phillipsburg, NJ, USA).
Figure 1.
Structures of (A) lixisenatide and (B) esomeprazole (internal standard).
2.2. Preparation of Calibration Standard and Quality Control Samples
Lixisenatide acetate was dissolved in methanol to prepare the stock solution of lixisenatide at 1 mg/mL. The working standard solutions of lixisenatide were prepared from the stock solution by serial dilution with methanol, yielding 2000, 1000, 500, 100, 50, 20, and 10 ng/mL. The working solution of IS was also prepared by diluting the stock solution of esomeprazole (1 mg/mL) with methanol, yielding 5 ng/mL. Calibration standard samples were prepared by spiking 50 μL of blank rat plasma with 50 µL of the standard working solution of lixisenatide, 50 µL of IS working solution, 850 µL of methanol, and 0.5 µL of formic acid. The mixture was vortexed for 1 min, followed by centrifugation at 15,000 rpm for 10 min. The resulting supernatant 10 µL was injected into the LC–MS/MS. All sample preparation was conducted in Protein LoBind Tubes® (Eppendorf, Hamburg, Germany).
Quality control (QC) samples at four levels of high (1600 ng/mL), medium (800 ng/mL), low (40 ng/mL), and lower limit of quantification (LLOQ, 10 ng/mL) concentrations of lixisenatide were independently prepared using the blank rat plasma.
2.3. Sample Preparation
To prepare plasma samples, 50 µL of the obtained plasma samples were mixed with 50 µL of IS (5 ng/mL), 900 µL of methanol, and 0.5 µL of formic acid. The mixture was vortex mixed and centrifuged using the same method as for the preparation of calibration standard samples. The supernatant (10 µL) was injected into the LC–MS/MS.
2.4. LC–MS/MS Conditions
The liquid chromatography instrument used in this study included an Agilent 1260 HPLC (Agilent Technologies, Santa Clara, CA, USA). The analytical column BioZenTM 2.6 μm Peptide XB-C18 (100 × 2.1 mm, Phenomenex, Torrence, CA, USA) was employed, and gradient elution with a mobile phase containing 0.1% formic acid in distilled water (A) and 0.1% formic acid in acetonitrile (B) was utilized. The column temperature was maintained at 50 °C. The gradient elution profile was as follows; 0 min, A:B = 75:25 (v/v), 0.3 mL/min; 10 min, 50:50, 0.3 mL/min; 10.01 min, 75:25, 0.4 mL/min; 14.50 min, 75:25, 0.4 mL/min; 14.51 min, 75:25, 0.3 mL/min; 17.50 min, 75:25, 0.3 mL/min. The total run time was 17.5 min.
Mass spectrometry was operated using the electrospray ionization (ESI) source of the Agilent 6490 mass spectrometer (Agilent Technologies). Positive ions were monitored in the multiple reaction monitoring (MRM) mode with a dwell time of 200 ms. The observed MRM transitions were m/z 810.8 → 129.2 for lixisenatide and 346.1 → 198.0 for IS. The mass spectrometry settings for lixisenatide and IS are summarized in Table 1.

Table 1.
Multiple reaction monitoring (MRM) transitions and mass spectrometry settings.
2.5. Assay Validation
The LC–MS/MS method was validated for selectivity, linearity, sensitivity, accuracy and precision, extraction recovery, and stability according to “Bioanalytical Method Validation Guidance for Industry” [19]. Selectivity was assessed by analyzing blank plasma samples to determine the potential interference at the retention times of lixisenatide. Linearity was determined over the calibration range 10–2000 ng/mL in rat plasma. The lower limit of quantification (LLOQ) was established as the lowest concentration of the calibration range with acceptable accuracy and precision. Accuracy was defined as the relative percentage error (%) of the measured concentration of lixisenatide compared to the theoretical concentration using the QC samples, while precision was determined as the coefficient of variance (CV%) for each QC level. The extraction recovery was assessed by analyzing the peak responses of lixisenatide and IS in the plasma samples spiked before and after the extraction. The matrix effect was determined by comparing the peak responses of lixisenatide spiked into a blank matrix after extraction to those in the neat solution, i.e., distilled water. Carryover was evaluated by analyzing the blank samples following the upper limit of quantification (ULOQ) samples. The stability of lixisenatide in the rat plasma was evaluated under four different storage conditions, viz. short-term (room temperature for 4 h), autosampler (4 °C in the autosampler for 24 h), freeze/thaw (after three freeze/thaw cycles), and long-term (−20 °C for 7 days) storage conditions.
2.6. Animal Studies
The study involving male Sprague Dawley rats (7 weeks, 204–226 g) was conducted in accordance with the Ethics Committee for the Treatment of Laboratory Animals at Sungkyunkwan University (SKKUIACUC2021-09-01-2). The rats were obtained from DBL Co., Ltd. (Eumseong, Republic of Korea) and were randomly divided into two groups: intravenous (IV) injection and subcutaneous (SC) injection groups. Following an overnight fast, lixisenatide was administered either by IV injection via the penile vein at a dosage of 1 mg/kg (n = 5) dissolved in normal saline (1 mL/kg) or by SC injection at a dosage of 5 mg/kg (n = 5). Approximately 0.3 mL of venous blood samples were collected from the jugular vein at predetermined times: 0 (predose), 5, 10, 15, 30, 45 min, 1, 1.5, 2, 2.5, 3, and 4 h after drug administration. Blood samples were centrifuged at 15,000× g for 10 min to obtain plasma samples.
The volume of blood samples and the frequency of sampling should be based on the purpose of the scientific procedure and the total blood volume of the animal. It is common to collect blood samples frequently to characterize the absorption and distribution phases of the drug in pharmacokinetic studies. As a general guide, a maximum of 10% of total blood volume every 24 h is recommended from the animal [20]. With blood vessel catheterization, which we used in the present study, it is recommended that up to six samples are taken in a two-hour period or up to 20 samples over a 24 h period, depending on sample volume [20]. During the animal study, we also carefully monitored the animals and did not observe any signs of abnormality.
2.7. Pharmacokinetic Analysis
The obtained plasma concentration vs. time profiles of lixisenatide were analyzed to estimate the pharmacokinetic parameters of lixisenatide via non-compartmental analysis using Phoenix WinNonlin (Certara, Princeton, NJ, USA). The estimated pharmacokinetic parameters include half-life (t1/2), maximum plasma concentration (C0 or Cmax), the area under the plasma concentration vs. time curve from time zero to last observation (AUCall) and infinity (AUCinf), clearance (CL), and volume of distribution (Vss). The absolute bioavailability (BA) was calculated as the percentage of dose-normalized area under the plasma concentration vs. time curve (AUCinf) obtained after SC injection compared to that obtained after IV injection.
3. Results and Discussion
3.1. Development of LC–MS/MS Method for Lixisenatide
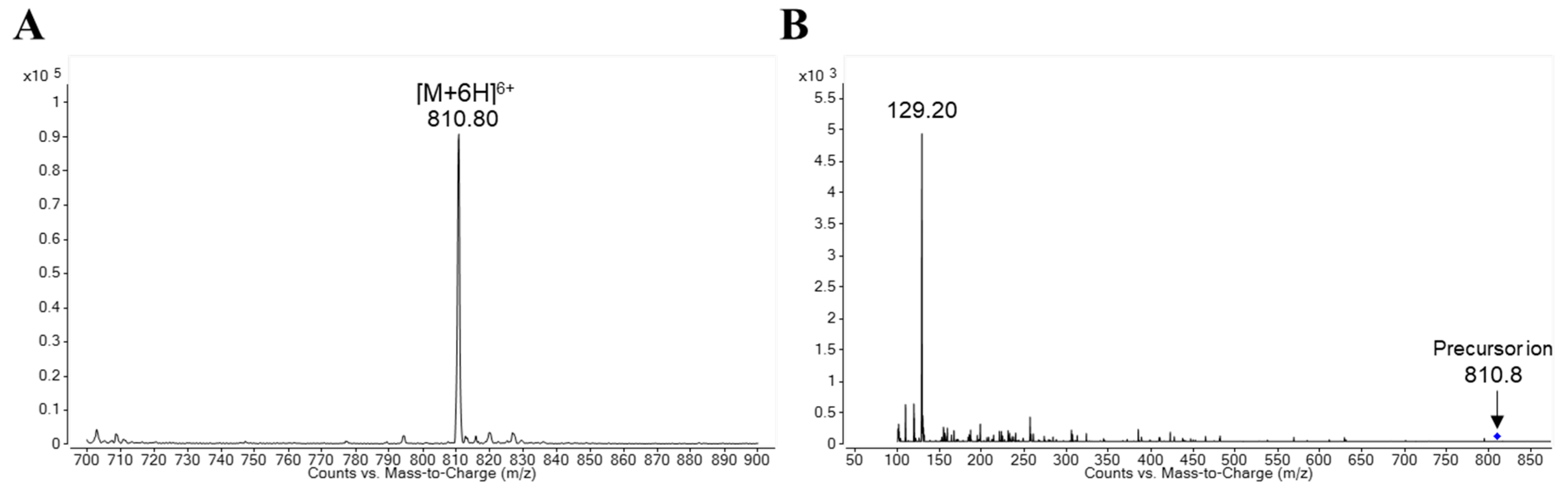
The Q1 mass scan spectrum of protonated lixisenatide is displayed in Figure 2A. The most pronounced precursor ion was observed at m/z 810.8 as [M + 6H]6+. Correspondingly, the product ion of protonated lixisenatide at m/z = 810.8 showed the dominant fragment ion at m/z 129.2 (Figure 2B). Thus, the MRM transition of m/z 810.9 → 129.2 was used for monitoring lixisenatide. Since lixisenatide is a synthetic peptide with 44 amino acids (m.w. = 4858), the selected fragment ion may also be a multiply charged peptide fragment of lixisenatide. However, additional studies are required to identify the fragmentation pattern of lixisenatide in mass spectrometry. The selected MRM transition for esomeprazole (IS) was m/z 346.1 → 198.0. The presently-used IS, esomeprazole, performed adequately as an internal standard for the analysis of lixisenatide. While the stable isotope-labeled analogs might have provided an ideal internal standard, a stable isotope-labeled lixisenatide is not commercially available.
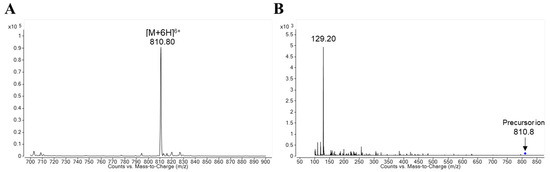
Figure 2.
Ion spectra of (A) protonated lixisenatide and (B) product ion of protonated lixisenatide ([M + 6H]6+, m/z = 810.8).
Methanol-based protein precipitation was utilized for the extraction of lixisenatide from plasma samples. Methanol demonstrated better sensitivity and recovery than acetonitrile as the precipitation solvent, and the addition of formic acid significantly improved the extraction recovery. Compared to methanol, acetonitrile provided poor recovery, which may be attributed to the coprecipitation of a partial peptide with plasma proteins [21]. Finally, the protein precipitation method with methanol and formic acid resulted in over 98.80% extraction recovery for both lixisenatide and IS from rat plasma. Solid phase extraction has been applied for the analysis of other GLP-1 receptor agonists, such as liraglutide and semaglutide, in previous studies [22,23,24]. However, protein precipitation is favorable due to its simplicity, rapidity, and cost-effectiveness compared to liquid-liquid extraction or solid phase extraction.
The chromatographic conditions were optimized to accomplish the optimal resolution for lixisenatide. Ultimately, the BioZenTM 2.6 μm Peptide XB-C18 (100 × 2.1 mm, Phenomenex, Torrence, CA, USA) column with a mobile phase consisting of 0.1% formic acid in distilled water and 0.1% formic acid in acetonitrile was chosen due to its high sensitivity and minimal endogenous interference. To avoid inconsistent carryover, a gradient elution profile was optimized whereby a gradual increase of the organic phase for 10 min was followed by a rapid return to the initial condition, resulting in a reproducible peak response.
3.2. Validation of the LC–MS/MS Method
3.2.1. Specificity, Linearity, and Sensitivity
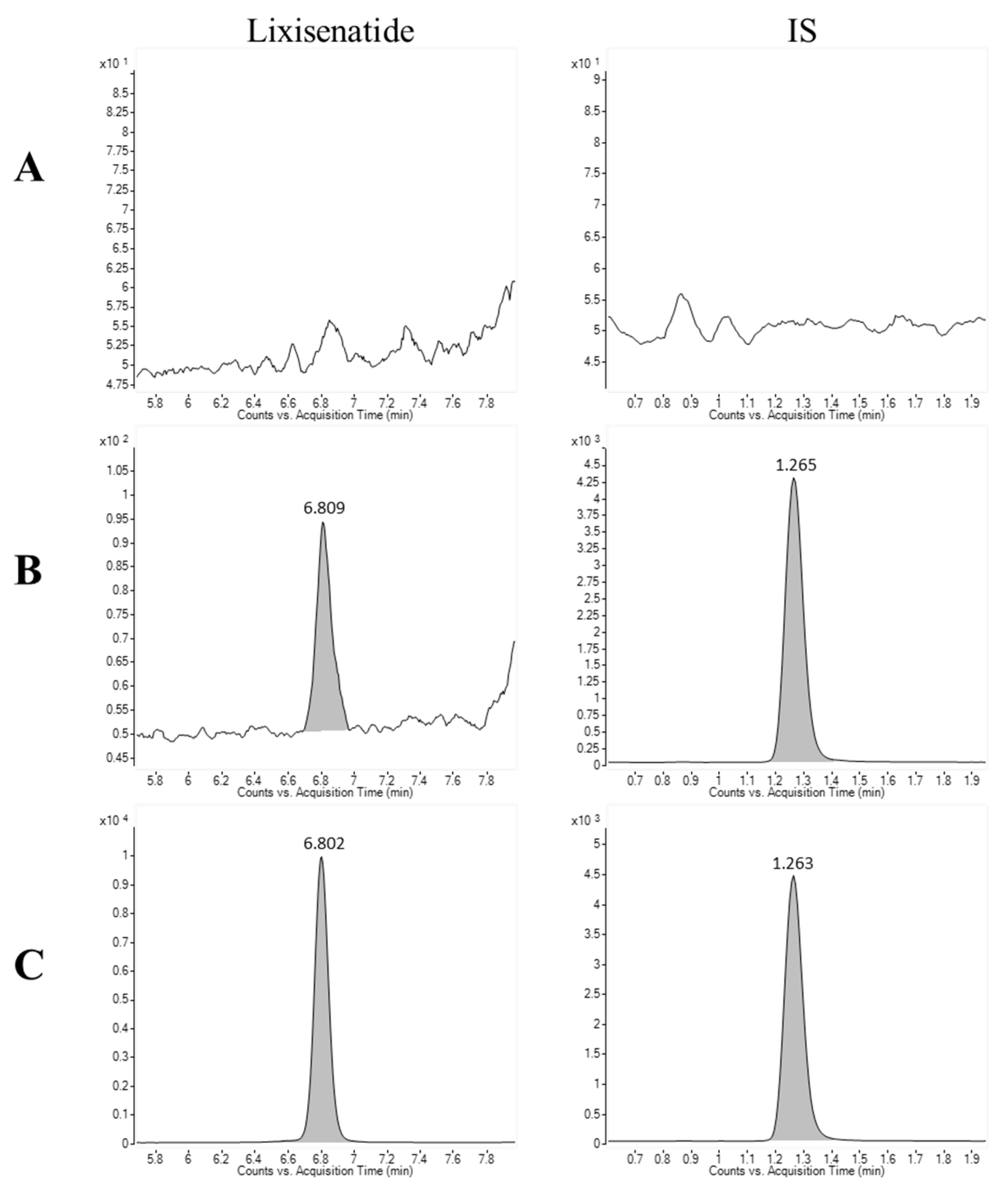
Figure 3 presents the representative multiple reaction monitoring (MRM) chromatograms of lixisenatide and the internal standard (IS) in rat plasma. The retention times for lixisenatide and IS were determined to be 6.8 and 1.26 min, respectively. There were no interfering endogenous or exogenous peaks at the retention times corresponding to lixisenatide and IS, when plasma spiked with lixisenatide at the lower limit of quantification (LLOQ = 10 ng/mL) and upper limit of quantification (ULOQ = 2000 ng/mL) was analyzed (Figure 3).
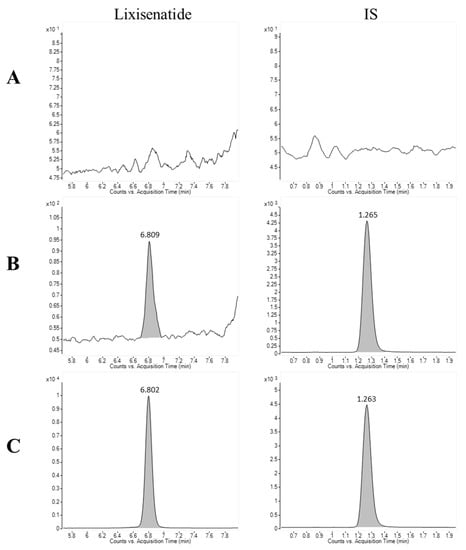
Figure 3.
Chromatograms of lixisenatide and the internal standard (IS) in (A) blank rat plasma, (B) blank rat plasma spiked with lixisenatide at the LLOQ concentration (10 ng/mL) and IS, and (C) blank rat plasma spiked at the ULOQ concentration (2000 ng/mL) and IS.
The calibration curve of lixisenatide was found to be linear over the concentration range of 10 to 2000 ng/mL, with a correlation coefficient greater than 0.999. The LLOQ for lixisenatide in rat plasma was determined to be 10 ng/mL, which was the lowest concentration in the calibration range. The signal-to-noise (S/N) ratio of the lixisenatide peak at LLOQ was calculated to be 84.2.
The clinical pharmacokinetics of lixisenatide were investigated utilizing the ELISA method with superior sensitivity. Nonetheless, when employed in pharmacokinetic investigations, ELISA technologies often have limitations. Because ELISA methods cannot tell if the epitope is present in the parent or metabolite and the methods have inherent cross-reactivity, they tend to overestimate the parent drug concentration. Furthermore, because ELISA is more susceptible to a strong matrix impact, it is challenging to apply it to another biological matrix for tissue distribution research [25,26].
For the study of peptides and protein drugs, LC–MS/MS methods have emerged as a feasible alternative to ELISA. As a preferable analytical technique, LC–MS/MS offers essential benefits, such as great specificity, reproducibility, and high throughput. Recent advances in LC–MS/MS have overcome its shortcomings in the bioanalysis of peptides and proteins, including inadequate ionization, considerable endogenous interference, and limited sensitivity [26].
Recently, LC–MS/MS methods for other GLP-1 receptor agonists, such as liraglutide and semaglutide, have been reported [21,22,23,24,25]. However, no LC–MS/MS methods for analysis of lixisenatide for pharmacokinetic studies have been reported. Although the sensitivity achieved in this study was 10 ng/mL, it was enough to determine the pharmacokinetics of lixisenatide in rats and comparable to those of previously used LC-MS methods in dog plasma (3.4 nM), rat plasma (2.6 nM), and pig plasma (4.1 nM) [10]. The present LC–MS/MS approach may also be relevant for further nonclinical and clinical studies to better understand the pharmacological activity or pharmacokinetics of novel formulations of lixisenatide.
3.2.2. Accuracy and Precision
Table 2 presents the evaluation of the accuracy and precision results of lixisenatide at high (1600 ng/mL), medium (800 ng/mL), low (40 ng/mL) QC, and LLOQ (10 ng/mL) concentrations for four consecutive days (inter-day, n = 4) with five replicates per day (intra-day, n = 5). The intra- and inter-day accuracy levels of lixisenatide measurement were found to be between 96.98% and 108.55%. The intra-day and inter-day precision levels were less than 8.36% and 6.89%, respectively. The achieved accuracy and precision satisfy the criteria of the FDA guidelines on bioanalytical method validation [19].

Table 2.
Accuracy and precision of lixisenatide measurement in rat plasma.
3.2.3. Extraction Recovery and Matrix Effect
To determine the extraction recovery of lixisenatide, the ratio of peak areas obtained from the standard solution spiked in pre-extraction and post-extraction was calculated. The obtained extraction recoveries of lixisenatide and the internal standard (IS) are summarized in Table 3. The results revealed an average extraction recovery in the rat plasma of 98.80–100.69% for lixisenatide and 99.67% for IS, indicating a highly efficient and reproducible extraction process.

Table 3.
Extraction recovery (%) of lixisenatide and IS in the rat plasma (mean ± SD).
The matrix effect was evaluated by comparing the peak responses of lixisenatide spiked into a blank matrix after extraction to those in the distilled water. The average matrix effect was 309.23 ± 42.10%, indicating an increase in response, i.e., ion enhancement, likely due to the presence of formic acid in the post-extraction matrix.
3.2.4. Carryover
During the method development process, inconsistent carryover peaks were observed in the chromatogram. Thus, a gradient elution profile was optimized to remove those inconsistent carryover peaks. After optimization of the chromatographic conditions, no significant carryover peaks were observed. The removal of the carryover was demonstrated by the absence of peaks at the retention time of lixisenatide in the blank samples following the injection of the upper limit of quantification (ULOQ) samples.
3.2.5. Stability
The results of the stability evaluation of lixisenatide in rat plasma are presented in Table 4. The evaluation was conducted under four different storage conditions, including short-term storage at room temperature for 4 h, storage in an autosampler at 4 °C for 24 h, three cycles of freeze/thaw, and long-term storage at −20 °C for 7 days. The average stability of lixisenatide was found to be in the range of 96.87% to 106.16%, with no significant deviations observed under the tested conditions. These findings demonstrate that lixisenatide is stable and suitable for regular analysis.

Table 4.
Stability of lixisenatide under different storage conditions (n = 3).
3.3. Pharmacokinetics of Lixisenatide in Rats
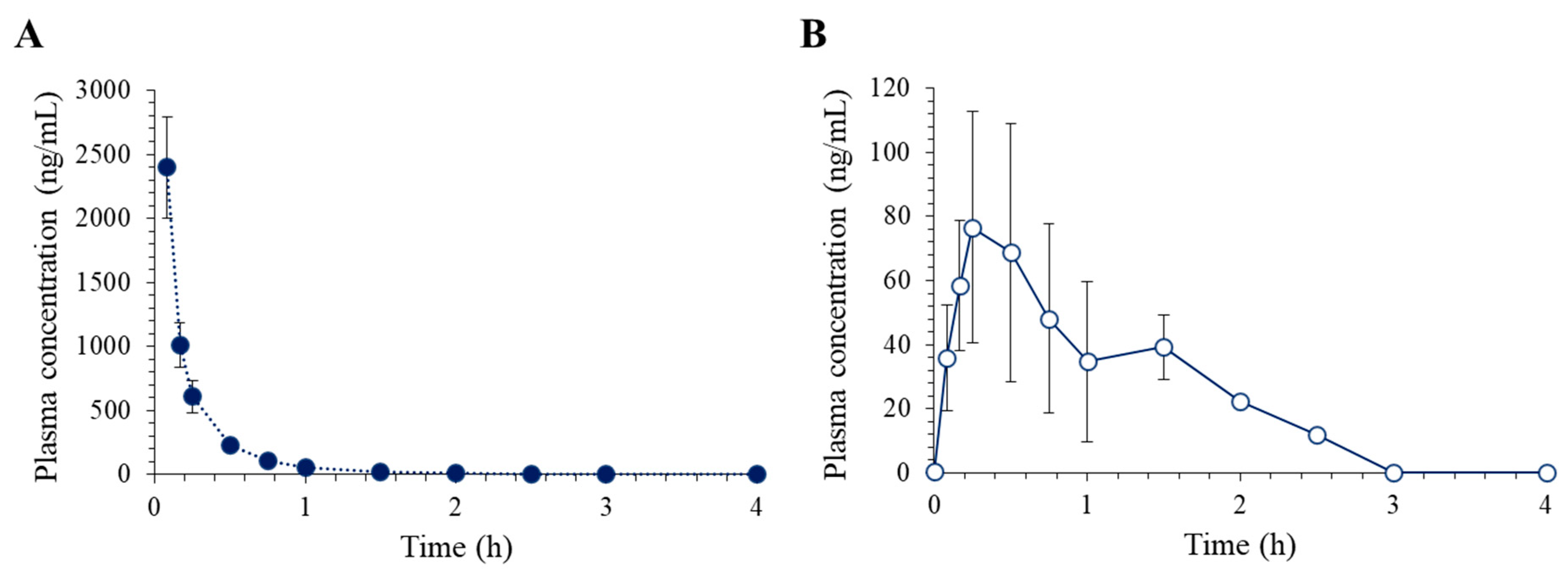
The LC–MS/MS assay developed in this study was employed to investigate the pharmacokinetics of lixisenatide in rats after IV and SC administrations. Figure 4 depicts the obtained time course of plasma concentration of lixisenatide, while the non-compartmental pharmacokinetic parameters are summarized in Table 5.
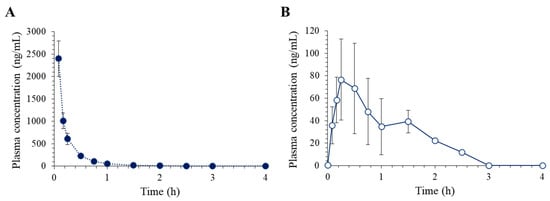
Figure 4.
Average plasma concentration vs. time profiles of lixisenatide following (A) intravenous injection at 1 mg/kg (n = 5) and (B) subcutaneous injection at 5 mg/kg (n = 5) in rats.

Table 5.
Pharmacokinetic parameters of lixisenatide following intravenous (IV, 1 mg/kg) and subcutaneous (SC, 5 mg/kg) injection in rats (mean ± SD, n = 5).
Following intravenous (IV) administration of lixisenatide, the plasma concentration of the drug decreased in a multiexponential fashion and was not detected after 2 h. The average half-life (t1/2) of lixisenatide was 0.37 ± 0.06 h. Our data also support the high systemic clearance (CL) of lixisenatide, i.e., 22.65 ± 3.45 mL/min/kg. The short half-life of lixisenatide may be associated with its extensive metabolism. Lixisenatide was intensively metabolized after 1 h of incubation in S9 fractions of liver and kidney from humans, dogs, and rabbits [9]. Twenty-eight metabolites of lixisenatide, comprising inactive degraded peptide products, were observed in human S9 fractions [9]. The volume of distribution (Vss) of lixisenatide was estimated to be 0.29 ± 0.08 L/kg, indicating lixisenatide is widely distributed within the body. Tissue distribution studies using the radioactive lixisenatide showed that lixisenatide was primarily distributed in the kidneys, followed by the thyroid, adrenals, salivary gland, lung, and liver [10]. Insignificant brain distribution of lixisenatide was also shown [10], even though its potential to cross the blood-brain barrier has been indicated [16]. On the other hand, following SC injection, the lixisenatide plasma concentration increased and reached its peak (Cmax) within 30 min. The t1/2 of lixisenatide after SC injection was 0.44 ± 0.08 h, which was comparable to that after IV injection. The estimated subcutaneous bioavailability of lixisenatide was 2.17% in rats (Table 5).
The obtained pharmacokinetic parameters of lixisenatide in rats are in good agreement with previous preclinical reports. The reported terminal half-life t1/2,z of lixisenatide in rats ranged from 29 to 48 min following IV and SC administration in rats [10]. The terminal half-life ranged between 0.5 and 6.5 h after IV administration in other animal species, including mice, rabbits, dogs, and pigs [9]. The reported absolute bioavailability after subcutaneous dosing was only 3% in rats, but higher in other species, i.e., ~90% in dogs, ~70% in pigs, 36–50% in db/db mice, and >30% in rabbits [10]. The subcutaneous bioavailability in humans has yet to be determined.
4. Conclusions
The present study provides a simple and robust analytical method for lixisenatide by using LC–MS/MS for in vivo pharmacokinetic studies. The LC–MS/MS method was fully validated and applied to examine the pharmacokinetics of lixisenatide in rats. In light of the diverse therapeutic efficiency of GLP-1 receptor agonists, there has been a growing interest in the development of novel formulations, including longer-acting versions or combinations with other medications, such as basal insulin. Compared to ELISA methods, LC–MS/MS methods have strengths, such as specificity, reproducibility, and high throughput, supporting their application in pharmacokinetic research. The established LC–MS/MS method may provide a valuable tool for further formulation development as well as tissue distribution studies of lixisenatide and GLP-1 receptor agonists, which require stringent pharmacokinetic assessments.
Author Contributions
Conceptualization, H.S.O., E.J.P., T.H.K., S.S. and B.S.S.; methodology, T.H.K., S.S. and B.S.S.; investigation, H.S.O., E.J.P., T.S.L., Y.A. and B.S.S.; writing—original draft preparation, H.S.O., E.J.P.; writing—review and editing, S.S. and B.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Trade, Industry and Energy of Korea (Grant No. 20012631) and National Research Foundation of Korea (NRF) (Grant No. NRF-2021R1A2B5B01001970).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee for the Treatment of Laboratory Animals at Sungkyunkwan University (SKKUIACUC2021-09-01-2, 11 November 2021).
Data Availability Statement
All data have been presented in the paper.
Conflicts of Interest
E.J.P. is an employee of D&D Pharmatech. No other potential conflicts of interest relevant to this paper exist.
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Prasad-Reddy, L.; Isaacs, D. A Clinical Review of GLP-1 Receptor Agonists: Efficacy and Safety in Diabetes and Beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S113–S124. [Google Scholar] [CrossRef] [PubMed]
- Amatya, R.; Min, K.A.; Shin, M.C. A Review of Glucoregulatory Hormones Potentially Applicable to the Treatment of Alzheimer’s Disease: Mechanism and Brain Delivery. J. Pharm. Investig. 2022, 52, 195–216. [Google Scholar] [CrossRef]
- Anderson, S.L.; Trujillo, J.M. Lixisenatide in Type 2 Diabetes: Latest Evidence and Clinical Usefulness. Adv. Chronic. Dis. 2016, 7, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R.; et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach: Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1ras in Type 2 Diabetes: Mechanisms That Underlie Cardiovascular Effects and Overview of Cardiovascular Outcome Data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef]
- European Medicines Agency. Lyxumia; Committee for Medicinal Products for Human Use, Ed.; European Medicines Agency: London, UK, 2012. [Google Scholar]
- U.S. Food and Drug Administration. Adlyxin (Lixisenatide) Injection, for Subcutaneous Use; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2016.
- Barnett, A.H. Lixisenatide: Evidence for Its Potential Use in the Treatment of Type 2 Diabetes. Core Evid. 2011, 6, 67–79. [Google Scholar] [CrossRef]
- Kapitza, C.; Forst, T.; Coester, H.-V.; Poitiers, F.; Ruus, P.; Hincelin-Méry, A. Pharmacodynamic Characteristics of Lixisenatide Once Daily Versus Liraglutide Once Daily in Patients with Type 2 Diabetes Insufficiently Controlled on Metformin. Diabetes Obes. Metab. 2013, 15, 642–649. [Google Scholar] [CrossRef]
- Giorgino, F.; Caruso, I.; Napoli, R. Titratable Fixed-Ratio Combination of Insulin Glargine Plus Lixisenatide: A Simplified Approach to Glycemic Control in Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2020, 170, 108478. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yang, X.; Li, Y.; Chen, Y.; Peng, X.; Yu, L.; Ding, J. Sustained Release Strategy Designed for Lixisenatide Delivery to Synchronously Treat Diabetes and Associated Complications. ACS Appl. Mater. Interfaces 2019, 11, 29604–29618. [Google Scholar] [CrossRef] [PubMed]
- Forst, T.; Pfutzner, A. Pharmacological Profile, Efficacy and Safety of Lixisenatide in Type 2 Diabetes Mellitus. Expert. Opin. Pharm. 2013, 14, 2281–2296. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.; Holscher, C. Drugs Developed to Treat Diabetes, Liraglutide and Lixisenatide, Cross the Blood Brain Barrier and Enhance Neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Salameh, T.S.; Rhea, E.M.; Talbot, K.; Banks, W.A. Brain Uptake Pharmacokinetics of Incretin Receptor Agonists Showing Promise as Alzheimer’s and Parkinson’s Disease Therapeutics. Biochem. Pharm. 2020, 180, 114187. [Google Scholar] [CrossRef]
- Esposito, S.; de Leonibus, M.L.; Ingenito, R.; Bianchi, E.; Orsatti, L.; Monteagudo, E. A Liquid Chromatography High-Resolution Mass Spectrometry in Vitro Assay to Assess Metabolism at the Injection Site of Subcutaneously Administered Therapeutic Peptides. J. Pharm. Biomed. Anal. 2018, 159, 449–458. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry; Center for Drug Evaluation and Research (CDER), Ed.; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- National Centre for the Replacement, Refinement & Reduction of Animals in Research, “Blood Sampling: Rat”. Available online: https://www.nc3rs.org.uk/3rs-resources/blood-sampling/blood-sampling-rat#summarybvc (accessed on 8 April 2023).
- Dong, S.; Gu, Y.; Wei, G.; Si, D.; Liu, C. Determination of Liraglutide in Rat Plasma by a Selective Liquid Chromatography-Tandem Mass Spectrometry Method: Application to a Pharmacokinetics Study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1091, 29–35. [Google Scholar] [CrossRef]
- Lee, T.S.; Park, E.J.; Choi, M.; Oh, H.S.; An, Y.; Kim, T.; Kim, T.H.; Shin, B.S.; Shin, S. Novel LC-MS/MS Analysis of the GLP-1 Analog Semaglutide with Its Application to Pharmacokinetics and Brain Distribution Studies in Rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2023, 1221, 123688. [Google Scholar] [CrossRef]
- Meng, X.; Xu, H.; Zhang, Z.; Fawcett, J.P.; Li, J.; Yang, Y.; Gu, J. Differential Mobility Spectrometry Tandem Mass Spectrometry with Multiple Ion Monitoring for the Bioanalysis of Liraglutide. Anal. Bioanal. Chem. 2017, 409, 4885–4891. [Google Scholar] [CrossRef]
- Shah, M.; Reddy, P.; Reddy, Y.; Bhawanishankar, M.; Raja, M.; Pillai, M. UHPLC-MS/MS Determination of GLP-1 Analogue, Liraglutide a Bioactive Peptide in Human Plasma. European J. Biomed. Pharm. Sci. 2017, 4, 304–311. [Google Scholar]
- Oh, H.S.; Choi, M.; Lee, T.S.; An, Y.; Park, E.J.; Kim, T.H.; Shin, S.; Shin, B.S. Pharmacokinetics and Brain Distribution of the Therapeutic Peptide Liraglutide by a Novel LC–MS/MS Analysis. J. Anal. Sci. Technol. 2023, 14, 19. [Google Scholar] [CrossRef]
- Pinho, A.R.; Fortuna, A.; Falcao, A.; Santos, A.C.; Seica, R.; Estevens, C.; Veiga, F.; Ribeiro, A.J. Comparison of ELISA and HPLC-MS Methods for the Determination of Exenatide in Biological and Biotechnology-Based Formulation Matrices. J. Pharm. Anal. 2019, 9, 143–155. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).