Review of Artificial Nacre for Oil–Water Separation
Abstract
1. Introduction
2. Fabrication Methods of Nacre-Inspired Materials
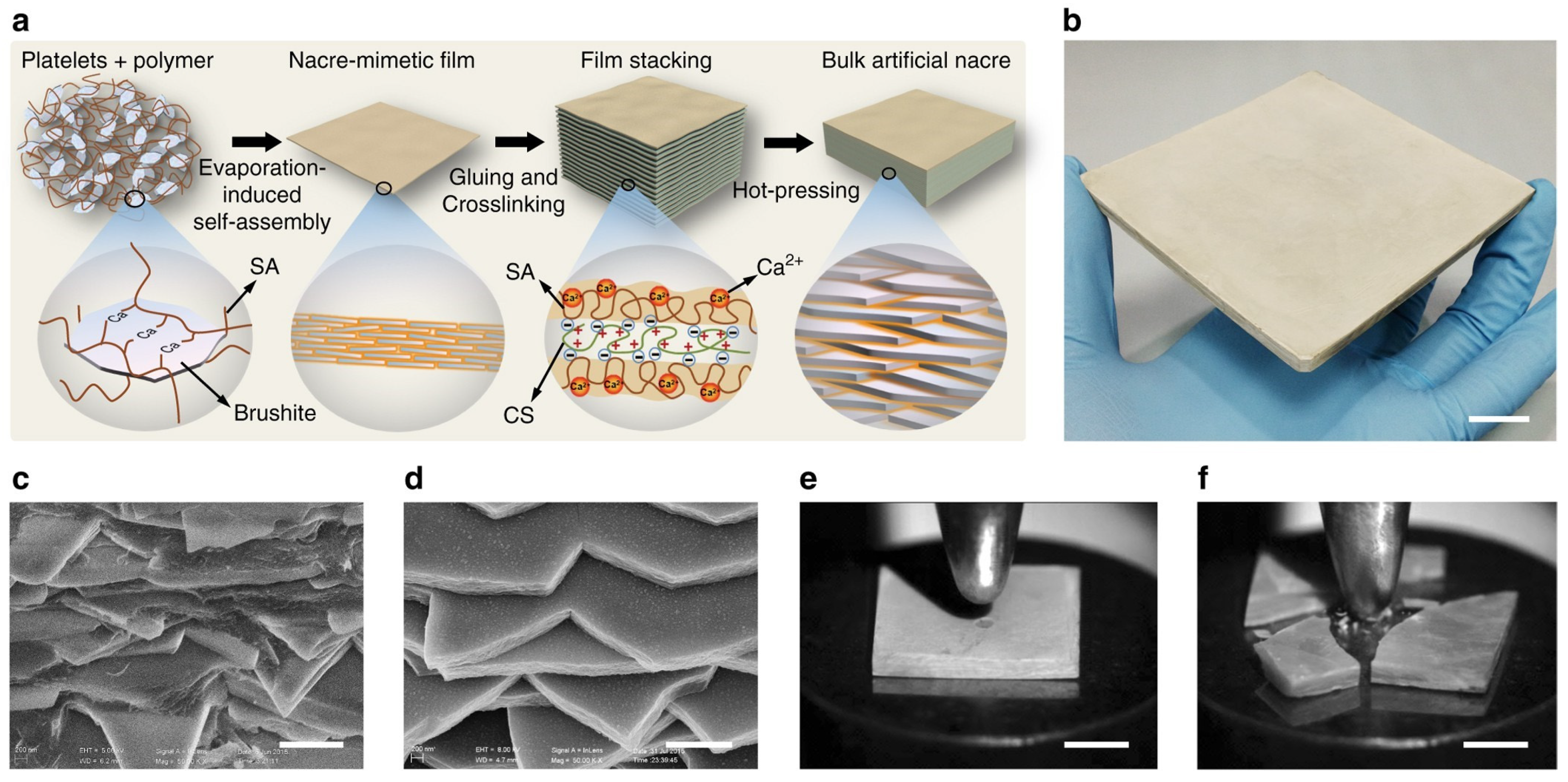
2.1. Self-Assembly
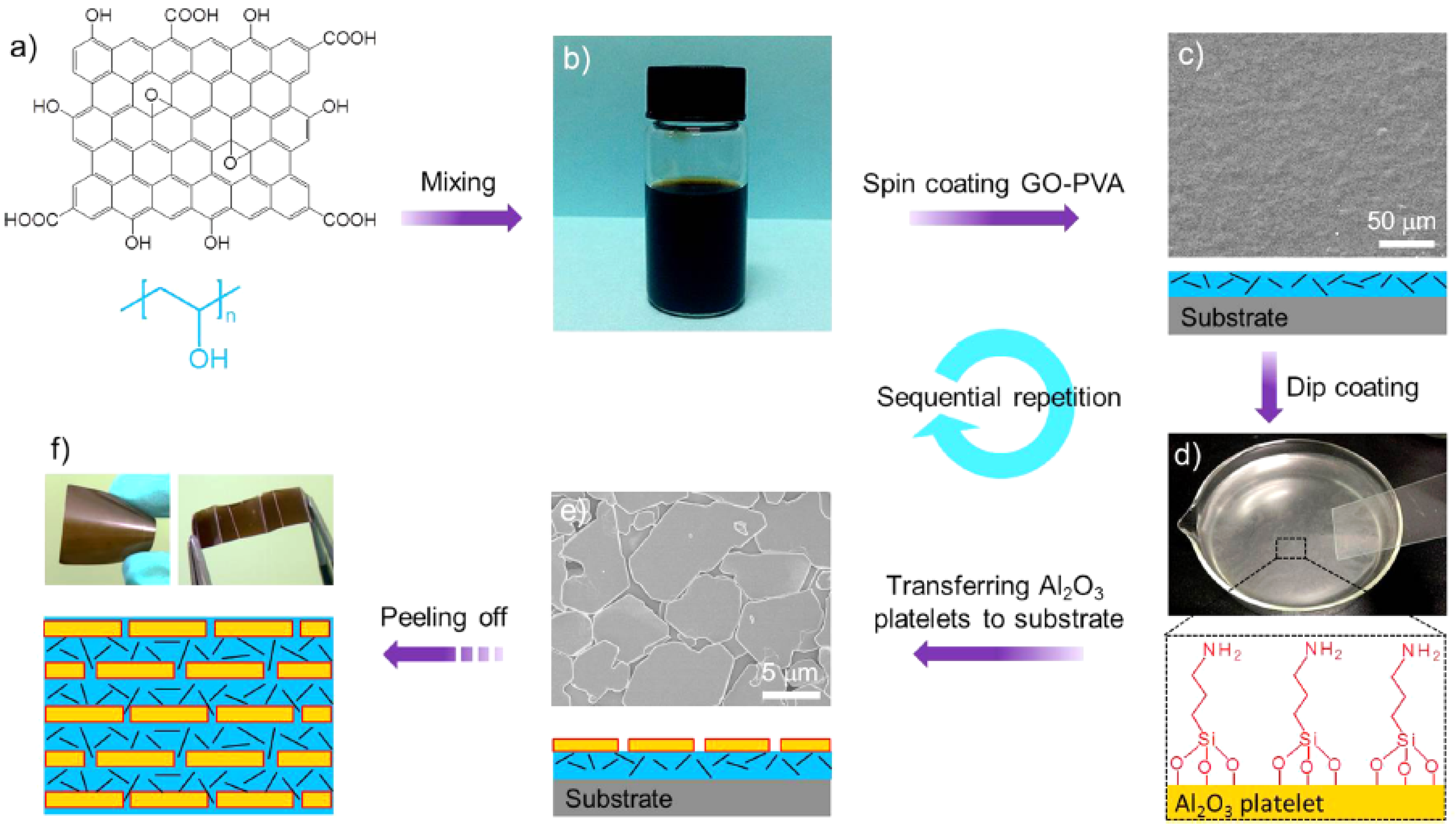
2.2. Layer-by-Layer
3. Antifouling Coating for Artificial Nacre Inspired by Natural Nacre
3.1. Chemical Antifouling Accessories
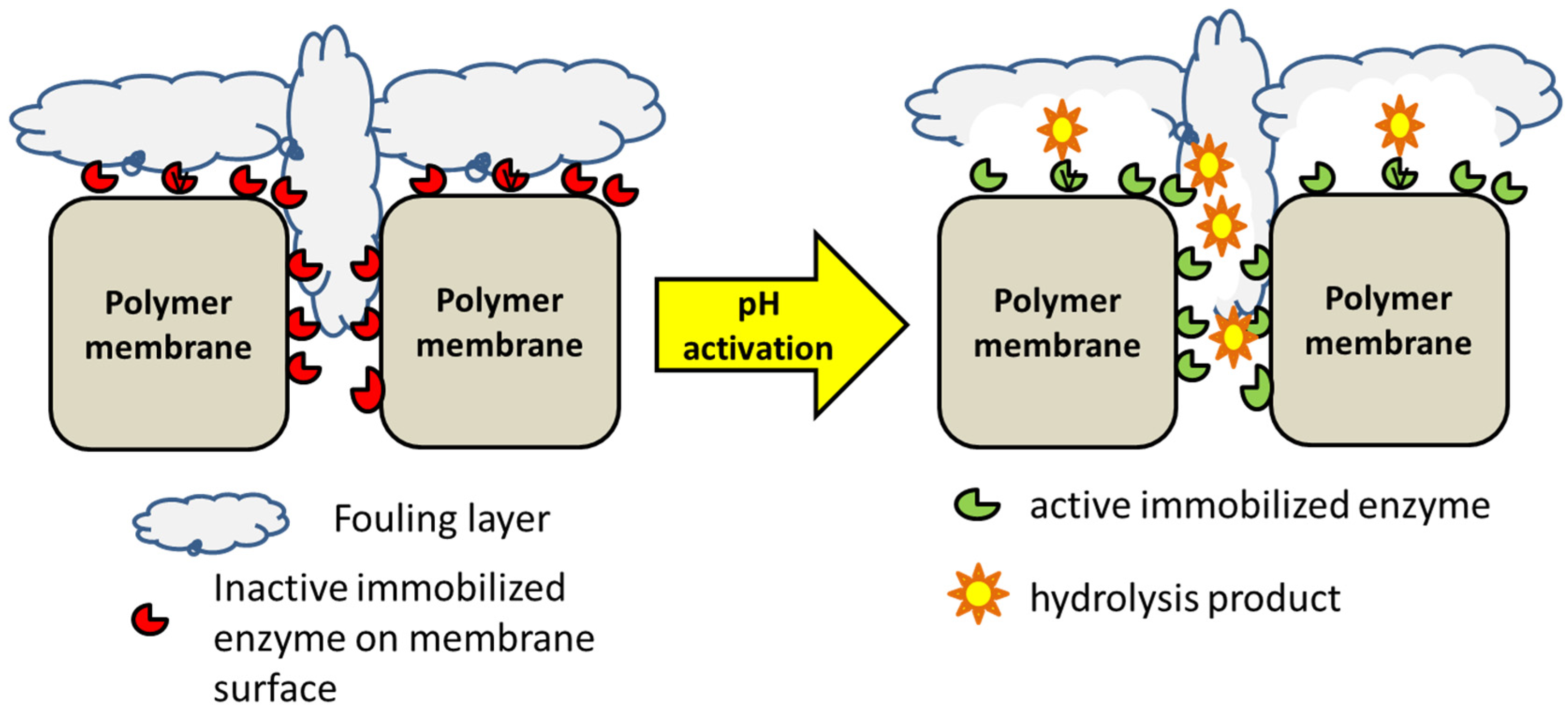
3.2. Enzymatic Antifouling Accessories
4. Oil–Water Separation
4.1. Hydrophilicity and Oleophobicity of Membranes
4.2. Performance of Membranes
4.3. Mechanical Properties
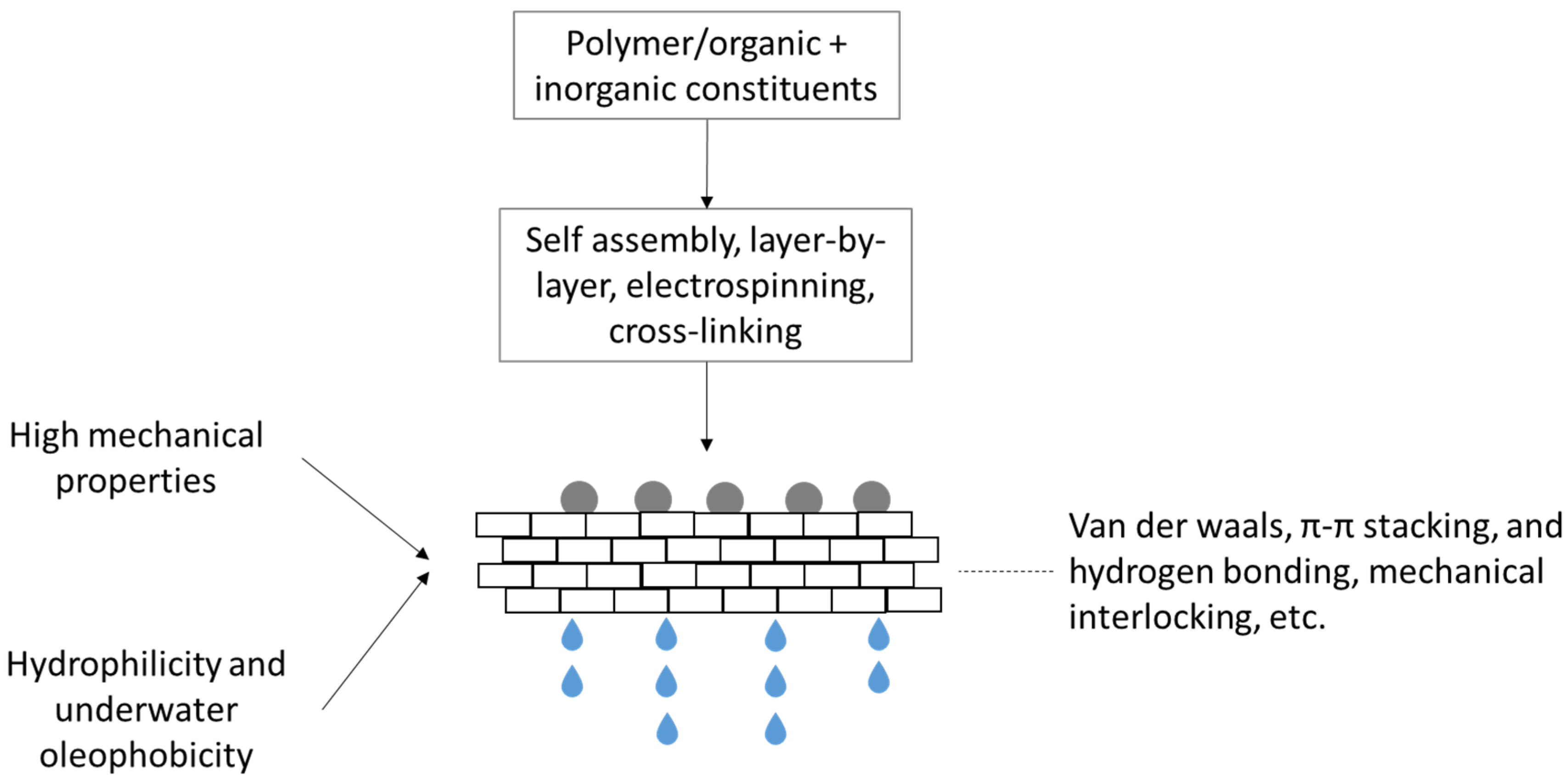
5. Interaction between Inorganic and Organic Constituents in the Membrane
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Niu, A.; Sun, X.; Lin, C. Trend in Research on Characterization, Environmental Impacts and Treatment of Oily Sludge: A Systematic Review. Molecules 2022, 22, 7795. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The Formation, Stabilization and Separation of Oil&Ndash;Water Emulsions: A Review. Processes 2022, 10, 738. [Google Scholar]
- Samuel, O.; Othman, M.H.D.; Kamaludin, R.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A.; Li, T.; Ismail, A.F.; Rahman, M.A.; et al. Oilfield-produced water treatment using conventional and membrane-based technologies for beneficial reuse: A critical review. J. Environ. Manag. 2022, 308, 114556. [Google Scholar] [CrossRef]
- Zolghadr, E.; Firouzjaei, M.D.; Amouzandeh, G.; LeClair, P.; Elliott, M. The Role of Membrane-Based Technologies in Environmental Treatment and Reuse of Produced Water. Front. Environ. Sci. 2021, 9, 71. [Google Scholar] [CrossRef]
- Medeiros, A.D.; Silva Junior, C.J.; Amorim, J.D.; Durval, I.J.; Costa, A.F.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Manusamy, Y.; Yong, T.J. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Faisal, W.; Hameed, B.; Almomani, F.; Abdullah, A. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. [Google Scholar] [CrossRef]
- Hizam, S.; Bilad, M.; Nordin, N.; Shamsuddin, N. Forward Osmosis for Produced Water Treatment: A Comprehensive Review. J. Penelit. Dan Pengkaj. Ilmu Pendidik. E-St. 2021, 5, 253–272. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Chu, W.; Chen, J.; Zhou, G. Research Progress and Prospects of Marine Oily Wastewater Treatment: A Review. Water 2019, 11, 2517. [Google Scholar] [CrossRef]
- Shabani, S.; Khorshidi, B.; Sadrzadeh, M. Chapter 9—Development of nanocomposite membranes by biomimicking nanomaterials. In Nanocomposite Membranes for Water and Gas Separation; Sadrzadeh, M., Mohammadi, T., Eds.; Elsevier: Amsterdan, The Netherlands, 2020; pp. 219–236. [Google Scholar] [CrossRef]
- Brock, L.; Sheng, J. Robust Fabrication of Polymeric Nanowire with Anodic Aluminum Oxide Templates. Micromachines 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Ma, Y.; Gorb, S.N. Compromise between mechanical and chemical protection mechanisms in the Mytilus edulis shell. J. Exp. Biol. 2019, 222, jeb201103. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, B.; Wang, Z.; Su, W.; Niu, S.; Han, Z.; Ren, L. Bioinspired Strategies for Excellent Mechanical Properties of Composites. J. Bionic Eng. 2022, 19, 1203–1228. [Google Scholar] [CrossRef]
- Grossman, M.; Bouville, F.; Masania, K.; Studart, A.R. Quantifying the role of mineral bridges on the fracture resistance of nacre-like composites. Proc. Natl. Acad. Sci. USA 2018, 115, 12698–12703. [Google Scholar] [CrossRef]
- Qiu, T.; Liang, L.; Hao, Y. Thickness effect on the mechanical properties of nacre in Hyriopsis cumingii under three-point bending. Eng. Fract. Mech. 2022, 276, 108869. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Liang, S.-M.; Ji, H.-M.; Li, X.-W. Distinctive Impact of Heat Treatment on the Mechanical Behavior of Nacreous and Crossed-Lamellar Structures in Biological Shells: Critical Role of Organic Matrix. ACS Biomater. Sci. Eng. 2022, 8, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yu, Y.; Wang, L. Learning from nature: Use material architecture to break the performance tradeoffs. Mater. Des. 2019, 168, 107650. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Q.; Tang, Z. Layered nanocomposites inspired by the structure and mechanical properties of nacre. Chem. Soc. Rev. 2012, 41, 1111–1129. [Google Scholar] [CrossRef]
- Wan, M.-c.; Qin, W.; Lei, C.; Li, Q.-h.; Meng, M.; Fang, M.; Song, W.; Chen, J.-h.; Tay, F.; Niu, L.-n. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Porter, M.; McKittrick, J. It’s tough to be strong: Advances in bioinspired structural ceramicbased materials. Am. Ceram. Soc. Bull. 2014, 95, 18–24. [Google Scholar]
- Zhang, Y.R.; Du, W.; Zhou, X.D.; Yu, H.Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Shu, Y.; Wan, P.; Zhao, H.; Dong, S.; Hao, W.; Yin, P. Genipin-enhanced nacre-inspired montmorillonite-chitosan film with superior mechanical and UV-blocking properties. Compos. Sci. Technol. 2019, 182, 107747. [Google Scholar] [CrossRef]
- Dai, J.; Wang, L.; Wang, Y.; Tian, S.; Tian, X.; Xie, A.; Zhang, R.; Yan, Y.; Pan, J. Robust Nacrelike Graphene Oxide–Calcium Carbonate Hybrid Mesh with Underwater Superoleophobic Property for Highly Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 4482–4493. [Google Scholar] [CrossRef]
- Sun, S.; Mao, L.B.; Lei, Z.; Yu, S.H.; Colfen, H. Hydrogels from Amorphous Calcium Carbonate and Polyacrylic Acid: Bio-Inspired Materials for “Mineral Plastics”. Angew. Chem. Int. Ed. Engl. 2016, 55, 11765–11769. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; You, T.; Liang, B.; Chen, H.; Yin, P. Bioinspired Ternary Artificial Nacre Graphene Oxide/Carboxyl Functionalized Single-Walled Carbon Nanotubes/Konjac Glucomannan with Enhanced Mechanical Properties. ACS Appl. Bio Mater. 2019, 2, 5544–5550. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Nakagawa, S.; Kim, C.; Yoshie, N. Fabrication of nacre-like polymer/clay nanocomposites with water-resistant and self-adhesion properties. J. Colloid Interface Sci. 2020, 564, 113–123. [Google Scholar] [CrossRef]
- Yao, H.-B.; Guan, Y.; Mao, L.-B.; Wang, Y.; Wang, X.-H.; Tao, D.-Q.; Yu, S.-H. A designed multiscale hierarchical assembly process to produce artificial nacre-like freestanding hybrid films with tunable optical properties. J. Mater. Chem. 2012, 22, 13005–13012. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, J.; Wang, J.; Zhu, Y.; Jiang, L. Bioinspired Hierarchical Alumina-Graphene Oxide-Poly(vinyl alcohol) Artificial Nacre with Optimized Strength and Toughness. ACS Appl. Mater. Interfaces 2015, 7, 9281–9286. [Google Scholar] [CrossRef]
- Chen, T.; Shi, P.; Zhang, J.; Li, Y.; Tian, X.; Lian, J.; Duan, T.; Zhu, W. Bioinspired enhancement of chitosan nanocomposite films via Mg-ACC crystallization, their robust, hydrophobic and biocompatible. Appl. Surf. Sci. 2018, 459, 129–137. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, H.; Zhang, Q.; Fan, Y.; Yue, Y.; Yin, P.; Guo, L. Ca2+ Enhanced Nacre-Inspired Montmorillonite-Alginate Film with Superior Mechanical, Transparent, Fire Retardancy, and Shape Memory Properties. ACS Appl. Mater. Interfaces 2016, 8, 28816–28823. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Ma, P.; Bai, H.; Chen, M.; Dong, W.; Xie, Y.; Deshmukh, Y.S. Superior Performance of Artificial Nacre Based on Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 4215–4222. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Zhang, D.; Yu, J.C.; Chang, Y.-W.; Yue, M.; Hou, Y.; Yang, M. Toward Record-High Stiffness in Polyurethane Nanocomposites Using Aramid Nanofibers. J. Phys. Chem. C 2015, 119, 27467–27477. [Google Scholar] [CrossRef]
- Teng, C.; Xie, D.; Wang, J.; Zhu, Y.; Jiang, L. A strong, underwater superoleophobic PNIPAM–clay nanocomposite hydrogel. J. Mater. Chem. A 2016, 4, 12884–12888. [Google Scholar] [CrossRef]
- Bers, A.V.; D’Souza, F.; Klijnstra, J.W.; Willemsen, P.R.; Wahl, M. Chemical defence in mussels: Antifouling effect of crude extracts of the periostracum of the blue mussel Mytilus edulis. Biofouling 2006, 22, 251–259. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Katti, K.S.; Katti, D.R. Effect of biopolymers on structure of hydroxyapatite and interfacial interactions in biomimetically synthesized hydroxyapatite/biopolymer nanocomposites. Ann. Biomed. Eng. 2008, 36, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.A.F. Biomimetic Approaches for Biomaterials Development; Wiley-VCH: Hoboken, NJ, USA, 2012. [Google Scholar]
- Eckert, A.; Abbasi, M.; Mang, T.; Saalwächter, K.; Walther, A. Structure, Mechanical Properties, and Dynamics of Polyethylenoxide/Nanoclay Nacre-Mimetic Nanocomposites. Macromolecules 2020, 53, 1716–1725. [Google Scholar] [CrossRef]
- Cheng, M.-m.; Huang, L.-j.; Wang, Y.-x.; Tang, J.-g.; Wang, Y.; Zhao, Y.-c.; Liu, G.-f.; Zhang, Y.; Kipper, M.; Belfiore, L.; et al. Recent developments in graphene-based/nanometal composite filter membranes. RSC Adv. 2017, 7, 47886–47897. [Google Scholar] [CrossRef]
- Song, P.; Xu, Z.; Wu, Y.; Cheng, Q.; Guo, Q.; Wang, H. Super-tough artificial nacre based on graphene oxide via synergistic interface interactions of π-π stacking and hydrogen bonding. Carbon 2017, 111, 807–812. [Google Scholar] [CrossRef]
- Kröger, R. Ion binding and nucleation. Nat. Mater. 2015, 14, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Chu, X.; Li, L.; Xu, X.; Tang, R. Controlled formation of calcium-phosphate-based hybrid mesocrystals by organic–inorganic co-assembly. Nanoscale 2010, 2, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Tsortos, A.; Nancollas, G.H. The Role of Polycarboxylic Acids in Calcium Phosphate Mineralization. J. Colloid Interface Sci. 2002, 250, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Sellinger, A.; Weiss, P.M.; Nguyen, A.; Lu, Y.; Assink, R.A.; Gong, W.; Brinker, C.J. Continuousself-assembly of organic–inorganic nanocompositecoatings that mimicnacre. Nature 1998, 394, 256–260. [Google Scholar] [CrossRef]
- Gao, H.L.; Chen, S.M.; Mao, L.B.; Song, Z.Q.; Yao, H.B.; Colfen, H.; Luo, X.S.; Zhang, F.; Pan, Z.; Meng, Y.F.; et al. Mass production of bulk artificial nacre with excellent mechanical properties. Nat. Commun. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, J.; Dang, B.; Sun, Q.; Li, H.; Zhai, T. Artificial Wooden Nacre: A High Specific Strength Engineering Material. ACS Nano. 2020, 14, 2036–2043. [Google Scholar] [CrossRef]
- Mao, L.-b.; Meng, Y.-F.; Meng, X.-S.; Yang, B.; Yang, Y.-L.; Lu, Y.-J.; Yang, Z.-Y.; Shang, L.-M.; Yu, S.-H. Matrix-Directed Mineralization for Bulk Structural Materials. J. Am. Chem. Soc. 2022, 144, 18175–18194. [Google Scholar] [CrossRef]
- Das, P.; Malho, J.-M.; Rahimi, K.; Schacher, F.H.; Wang, B.; Demco, D.E.; Walther, A. Nacre-mimetics with synthetic nanoclays up to ultrahigh aspect ratios. Nat. Commun. 2015, 6, 5967. [Google Scholar] [CrossRef]
- Du, G.; Mao, A.; Yu, J.; Hou, J.; Zhao, N.; Han, J.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Nacre-mimetic composite with intrinsic self-healing and shape-programming capability. Nat. Commun. 2019, 10, 800. [Google Scholar] [CrossRef]
- Finnemore, A.; Cunha, P.; Shean, T.; Vignolini, S.; Guldin, S.; Oyen, M.; Steiner, U. Biomimetic layer-by-layer assembly of artificial nacre. Nat. Commun. 2012, 3, 966. [Google Scholar] [CrossRef]
- Rianasari, I.; Benyettou, F.; Sharma, S.K.; Blanton, T.; Kirmizialtin, S.; Jagannathan, R. A Chemical Template for Synthesis of Molecular Sheets of Calcium Carbonate. Sci. Rep. 2016, 6, 25393. [Google Scholar] [CrossRef]
- Farhadi-Khouzani, M.; Schütz, C.; Durak, G.M.; Fornell, J.; Sort, J.; Salazar-Alvarez, G.; Bergström, L.; Gebauer, D. A CaCO3/nanocellulose-based bioinspired nacre-like material. J. Mater. Chem. A 2017, 5, 16128–16133. [Google Scholar] [CrossRef]
- Shu, Y.; Yin, P.; Liang, B.; Wang, H.; Guo, L. Artificial Nacre-Like Gold Nanoparticles–Layered Double Hydroxide–Poly(vinyl alcohol) Hybrid Film with Multifunctional Properties. Ind. Eng. Chem. Res. 2015, 54, 8940–8946. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Q.; Lin, L.; Jiang, L. Synergistic Toughening of Bioinspired Poly(vinyl alcohol) Clay Nanofibrillar Cellulose Artificial Nacre. ACS Nano. 2014, 8, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ye, L.; Yu, S.; Li, H.; Sun, R.; Xu, J.; Wong, C.P. Artificial nacre-like papers based on noncovalent functionalized boron nitride nanosheets with excellent mechanical and thermally conductive properties. Nanoscale 2015, 7, 6774–6781. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Gao, C. Bioinspired design and macroscopic assembly of poly(vinyl alcohol)-coated graphene into kilometers-long fibers. Nanoscale 2013, 5, 4370–4378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, H.; Liu, X.; Wang, M.; Meng, X.; Zhou, Q.; Xu, L. Nacre-like composite films with a conductive interconnected network consisting of graphene oxide, polyvinyl alcohol and single-walled carbon nanotubes. Mater. Des. 2019, 175, 107783. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Ren, L.; Yao, Y.; Wang, M.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Nacre-inspired polymer composites with high thermal conductivity and enhanced mechanical strength. Compos. Part A Appl. Sci. Manuf. 2019, 121, 92–99. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Chen, L.; Mu, C.; Qi, G.; Bian, F. Bio-inspired laminated graphite nanosheets/copper composites. RSC Adv. 2015, 5, 51342–51346. [Google Scholar] [CrossRef]
- Liu, S.; Ling, J.; Li, K.; Yao, F.; Oderinde, O.; Zhang, Z.; Fu, G. Hierarchical alginate biopolymer papers produced via lanthanide ion coordination. RSC Adv. 2016, 6, 63171–63177. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Liu, Q.; Kuang, J.; Zhou, D.; Ju, S.; Han, B.; Zhang, Z. High mechanical performance of layered graphene oxide/poly(vinyl alcohol) nanocomposite films. Small 2013, 9, 2466–2472. [Google Scholar] [CrossRef]
- Si, L.; Lu, Z.; Yao, C.; Ma, Q.; Zhao, Y.; Wang, Y.; Wang, D.; Jin, Z. Nacre-like nanocomposite film with excellent dielectric insulation properties and mechanical strength based on montmorillonite nanosheet and aramid nanofiber. J. Mater. Sci. 2020, 55, 5948–5960. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Chen, L.; Hu, Z.; Shi, Z.; Zhu, J. Nacre-like graphene paper reinforced by polybenzimidazole. RSC Adv. 2013, 3, 20353–20362. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Deng, Y.; Bhagia, S.; Meng, X.; Tao, Y.; Yong, Q.; Ragauskas, A.J. Robust galactomannan/graphene oxide film with ultra-flexible, gas barrier and self-clean properties. Compos. Part A Appl. Sci. Manuf. 2020, 131, 105780. [Google Scholar] [CrossRef]
- Yao, Y.; Zeng, X.; Wang, F.; Sun, R.; Xu, J.-b.; Wong, C.-P. Significant Enhancement of Thermal Conductivity in Bioinspired Freestanding Boron Nitride Papers Filled with Graphene Oxide. Chem. Mater. 2016, 28, 1049–1057. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Z.; Luan, Y.; Wang, L.; Zhao, D.; Xu, F.; Xiao, Y.; Guo, Z.; Wang, Z. Improved mechanical performance of graphene oxide based artificial nacre composites by regulating the micro-laminated structure and interface bonding. Compos. Sci. Technol. 2019, 179, 63–68. [Google Scholar] [CrossRef]
- Woo, J.Y.; Oh, J.H.; Jo, S.; Han, C.-S. Nacre-Mimetic Graphene Oxide/Cross-Linking Agent Composite Films with Superior Mechanical Properties. ACS Nano. 2019, 13, 4522–4529. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Ma, P.; Zhang, S.; Zhang, H.; Du, M.; Xie, Y.; Chen, M.; Dong, W.; Ming, W. Artificial Nacre from Supramolecular Assembly of Graphene Oxide. ACS Nano. 2018, 12, 6228–6235. [Google Scholar] [CrossRef]
- Yoo, S.C.; Park, Y.K.; Park, C.; Ryu, H.; Hong, S.H. Biomimetic Artificial Nacre: Boron Nitride Nanosheets/Gelatin Nanocomposites for Biomedical Applications. Adv. Funct. Mater. 2018, 28, 1805948. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, M.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Superstretchable Nacre-Mimetic Graphene/Poly(vinyl alcohol) Composite Film Based on Interfacial Architectural Engineering. ACS Nano. 2017, 11, 4777–4784. [Google Scholar] [CrossRef]
- Fang, B.; Peng, L.; Xu, Z.; Gao, C. Wet-Spinning of Continuous Montmorillonite-Graphene Fibers for Fire-Resistant Lightweight Conductors. ACS Nano. 2015, 9, 5214–5222. [Google Scholar] [CrossRef]
- HG, P.K.; Prabhakaran, S.; Xavior, A.; Kalainathan, S.; Lin, D.; Shukla, P.; Vasudevan, V.K. Enhanced surface and mechanical properties of bioinspired nanolaminate graphene-aluminum alloy nanocomposites through laser shock processing for engineering applications. Mater. Today Commun. 2018, 16, 81–89. [Google Scholar] [CrossRef]
- Han, M.; Shen, W. Nacre-inspired cellulose nanofiber/MXene flexible composite film with mechanical robustness for humidity sensing. Carbohydr. Polym. 2022, 298, 120109. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qiu, S.; Zhou, Y.; Zou, B.; Wang, J.; Jia, P.; Song, L. Nanolayered Graphene/Black Phosphorus Films for Fire-Retardant Coatings. ACS Appl. Nano. Mater. 2022, 5, 14841–14849. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Zeng, S.; Chen, P.; Xu, Y.; Nie, W.; Zhou, Y. Tunable mechanical properties of glass fiber/epoxy composites by incorporating bioinspired montmorillonite–carbon nanotube/epoxy interface layer around the fiber. Compos. Part B Eng. 2022, 242, 110092. [Google Scholar] [CrossRef]
- Theisen, B.F. Shell cleaning and deposit feeding in Mytilus edulis L. (Bivalvia). Ophelia 1972, 10, 49–55. [Google Scholar] [CrossRef]
- Xu, M.; Sun, T.; Tang, X.; Lu, K.; Jiang, Y.; Cao, S.; Wang, Y. Title: CO2 and HCl-induced seawater acidification impair the ingestion and digestion of blue mussel Mytilus edulis. Chemosphere 2020, 240, 124821. [Google Scholar] [CrossRef]
- Langton, R.W. Digestive rhythms in the mussel Mytilus edulis. Mar. Biol. 1977, 41, 53–58. [Google Scholar] [CrossRef]
- McHenery, J.G.; Allen, J.A.; Birkbeck, T.H. Effect of tidal submersion on lysozyme activity in Mytilus edulis and Tellina tenuis. Mar. Biol. 1983, 75, 57–61. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Schmidt, M.; Breite, D.; Thomas, I.; Went, M.; Prager, A.; Schulze, A. Polymer membranes for active degradation of complex fouling mixtures. J. Membr. Sci. 2018, 563, 481–491. [Google Scholar] [CrossRef]
- Wong, P.C.Y.; Lee, J.Y.; Teo, C.W. Application of dispersed and immobilized hydrolases for membrane fouling mitigation in anaerobic membrane bioreactors. J. Membr. Sci. 2015, 491, 99–109. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Konovalova, V.; Kharchenko, K.; Burban, A.; Knozowska, K.; Kujawski, W.; Kujawa, J. Improved antifouling properties of polyethersulfone membranes modified with α-amylase entrapped in Tetronic® micelles. J. Membr. Sci. 2019, 570–571, 436–444. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Taheri-Kafrani, A.; Asadnia, M.; Razmjou, A. Bienzymatic modification of polymeric membranes to mitigate biofouling. Sep. Purif. Technol. 2020, 237, 116464. [Google Scholar] [CrossRef]
- Cloete, W.; Hayward, S.; Swart, P.; Klumperman, B. Degradation of Proteins and Starch by Combined Immobilization of Protease, α-Amylase and β-Galactosidase on a Single Electrospun Nanofibrous Membrane. Molecules 2019, 24, 508. [Google Scholar] [CrossRef] [PubMed]
- Kolesnyk, I.; Konovalova, V.; Kharchenko, K.; Burban, A.; Kujawa, J.; Kujawski, W. Enhanced transport and antifouling properties of polyethersulfone membranes modified with α-amylase incorporated in chitosan-based polymeric micelles. J. Membr. Sci. 2020, 595, 117605. [Google Scholar] [CrossRef]
- Zhao, S.; Tao, Z.; Chen, L.; Han, M.; Zhao, B.; Tian, X.; Wang, L.; Meng, F. An antifouling catechol/chitosan-modified polyvinylidene fluoride membrane for sustainable oil-in-water emulsions separation. Front. Environ. Sci. Eng. 2020, 15, 63. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Multilayered chitosan/kaolin@calcium carbonate composite films with excellent chemical and thermal stabilities for oil/water filtration realized by a facile layer-by-layer assembly. Sep. Purif. Technol. 2022, 289, 120738. [Google Scholar] [CrossRef]
- Ding, J.; Wang, J.; Luo, X.; Xu, D.; Liu, Y.; Li, P.; Li, S.; Wu, R.; Gao, X.; Liang, H. A passive-active combined strategy for ultrafiltration membrane fouling control in continuous oily wastewater purification. Water Res. 2022, 226, 119219. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin Film Composite Membrane for Oily Waste Water Treatment: Recent Advances and Challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef]
- Guo, T.; Heng, L.; Wang, M.; Wang, J.; Jiang, L. Robust Underwater Oil-Repellent Material Inspired by Columnar Nacre. Adv. Mater. 2016, 28, 8505–8510. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, L.; Wu, L.; Zhang, J.; Wang, A. Durable Superhydrophobic/Superoleophilic Polyurethane Sponges Inspired by Mussel and Lotus Leaf for the Selective Removal of Organic Pollutants from Water. ChemPlusChem 2014, 79, 850–856. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Tao, L.; Li, K.; Xue, Z.; Feng, L.; Wei, Y. Mussel-Inspired Chemistry and Michael Addition Reaction for Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 4438–4442. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Mao, L.-B.; Jiang, Y.; Liu, M.-F.; Huang, Q.; Yu, Z.; Wang, S.; Yu, S.-H.; Lin, C.; et al. Seeded Mineralization Leads to Hierarchical CaCO3 Thin Coatings on Fibers for Oil/Water Separation Applications. Langmuir 2018, 34, 2942–2951. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Ke, Z.; Du, C.Z.; Zheng, L.; Wang, C.; Yuan, Z. Review: Porous Metal Filters and Membranes for Oil-Water Separation. Nano. Res. Lett. 2018, 13, 284. [Google Scholar] [CrossRef]
- Xu, L.-P.; Peng, J.; Liu, Y.; Wen, Y.; Zhang, X.; Jiang, L.; Wang, S. Nacre-Inspired Design of Mechanical Stable Coating with Underwater Superoleophobicity. ACS Nano. 2013, 7, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, J.; Shi, L.; Wang, X.; Guo, Z.; Liu, W. Underwater superoleophobic graphene oxide coated meshes for the separation of oil and water. Chem. Commun. 2014, 50, 5586–5589. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Dai, J.; Tian, S.; Xie, A.; Dai, X.; Pan, J. Coordination-driven interfacial cross-linked graphene oxide-alginate nacre mesh with underwater superoleophobicity for oil-water separation. Carbohydr. Polym. 2021, 251, 117097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, J.; Wei, Z.; Yang, M.; Yin, W.; Yu, K.; Yao, Y.; Lv, H.; He, X.; Leng, J. Electrospun silica/nafion hybrid products: Mechanical property improvement, wettability tuning and periodic structure adjustment. J. Mater. Chem. A 2014, 2, 16569–16576. [Google Scholar] [CrossRef]
- Meng, X.; Wang, M.; Heng, L.; Jiang, L. Underwater Mechanically Robust Oil-Repellent Materials: Combining Conflicting Properties Using a Heterostructure. Adv. Mater. 2018, 30, 1706634. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Cao, J.; Guan, J.; Xu, L.; Zhang, R.; He, M.; Zhang, Q.; Fan, L.; Jiang, Z. Synergy of the mechanical, antifouling and permeation properties of a carbon nanotube nanohybrid membrane for efficient oil/water separation. Nanoscale 2017, 9, 7508–7518. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Tolerant chitosan/carboxymethyl cellulose@calcium composite films on nylon fabric for high-flux water/oil separation. Carbohydr. Polym. 2022, 294, 119832. [Google Scholar] [CrossRef] [PubMed]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Polymer nanocomposites having a high filler content: Synthesis, structures, properties, and applications. Nanoscale 2019, 11, 4653–4682. [Google Scholar] [CrossRef]
- George, J.; Ishida, H. A review on the very high nanofiller-content nanocomposites: Their preparation methods and properties with high aspect ratio fillers. Prog. Polym. Sci. 2018, 86, 1–39. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, X.; Guo, J.; Sun, T.-Y.; Zhang, Y.; Chen, K. Directed Assembly of Large-Sized, Mechanically Robust, Nacre-Inspired Graphene Oxide/Sodium Alginate Nanocomposite Paper. Macromol. Mater. Eng. 2020, 305, 2000493. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Luan, Y.; Zheng, Y.; Yang, J.; Wang, L.; Guo, Z.; Wu, X. Bioinspired Nacre-like GO-based bulk with easy scale-up process and outstanding mechanical properties. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105829. [Google Scholar] [CrossRef]
- Magrini, T.; Bouville, F.; Lauria, A.; Le Ferrand, H.; Niebel, T.P.; Studart, A.R. Transparent and tough bulk composites inspired by nacre. Nat. Commun. 2019, 10, 2794. [Google Scholar] [CrossRef] [PubMed]



| Synthesis Method | Materials | Mechanical Properties | Reference | ||
|---|---|---|---|---|---|
| Yield Strength (MPa) | Hardness (MJ/m3) | Young’s Modulus (GPa) | |||
| Layer-by-layer | Gold nanoparticle/PVA | 122 | - | 8.5 | [54] |
| Layer-by-layer | Al2O3/GO/PVA | 143 ± 13 | 9.2 ± 2.7 | [29] | |
| Cross-linking of alginate with Ca ions | MMT/CaCl2 | 280 | 7.2 | [31] | |
| Evaporation-induced self-assembly | Clay platelet/nanofibrillar cellulose/PVA | 80–135 | 1.8 | [55] | |
| Spin-coating | Zeolite/CdSe-zeolite/PVA | 70 | [28] | ||
| Vacuum-assisted self-assembly | Noncovalent functionalized boron nitride nanosheets/PVA | 125.2 | 2.37 | [56] | |
| Continuous wet-spinning assembly | PVA-coated graphene | 161 | [57] | ||
| Film casting | Reduced GO (rGO)/PVA/single-walled carbon nanotubes (SWCNT) | 62.8 | 0.55 | [58] | |
| Hot press and curing | Ag-boron nitrite/epoxy | 80.3 | 0.35 | 23.4 | [59] |
| Combination of ball-milling and hot-rolling | Graphite nanosheets/Cu | 660 | 170 | [60] | |
| Lanthanide ion coordination | Sodium alginate biopolymers/lanthanide ions (Nd3+, Gd3+, Ce3+, and Yb3+) | 124.2 ± 5.2 | 8.2 ± 0.4 | 5.2 ± 0.2 | [61] |
| Vacuum-assisted self-assembly | GO/PVA | 360.7 | 42.2 | [62] | |
| Waterborne dispersion casting method | Nanoclay/polyethylene oxide (PEO) | 99.7 ± 10.3 | 24.3 ± 1.6 | [39] | |
| Vacuum-assisted self-assembly with ultrafiltration | MMT/aramid nanofiber | 126.5 | [63] | ||
| Solution-casting and in situ chemical reduction | Graphene/polybenzimidazole (PBI) | 77.7 | 6.33 | [64] | |
| Bottom-up assembly process based on laminating prefabricated two-dimensional nacre-mimetic films | Brushite (CaHPO4·2H2O) platelets/SA | 267 | [46] | ||
| Film casting and cured | Borate cross-linked galactomannan/GO | 135.54 | [65] | ||
| Film casting and cured | MMT/poly(3-mercaptopropyl)methylsiloxane (PMMS) | 64–110 | 5–12 | [27] | |
| Vacuum-assisted self-assembly | Boron nitride nanosheets (BNNSs) and graphene oxide (GO) | 16.3 | 6.5 | [66] | |
| Vacuum-assisted self-assembly | GO/carboxyl functionalized SWCNT/konjac glucomannan | 311.4 ± 9.2 | 11.1 ± 0.5 | [26] | |
| Stirring and ultrasonic treatment | GO-CNT/thermoplastic polyurethane | 209.8 | 5 | [67] | |
| Evaporation-based self-assembly | MMT/chitosan/genipin/NaOH | 226 | 5.1 | [23] | |
| Filtration and cross-linking | GO/p-diaminophenyl | 142.9 ± 6.4 | 4.7 ± 0.36 | [68] | |
| Filtration and vacuum drying | Polydopamine-capped graphene oxide (PDG)/2-ureido-4[1H]-pyrimidinone hexamethylene isocyanate (UPy-NCO) | 325.6 ± 17.8 | 11.1 | [69] | |
| Vacuum-assisted self-assembly | Boron nitride nanosheets/gelatin | 148.7 | 31 | [70] | |
| Modified bidirectional freezing technique | GO/PVA | 150.9 | 8.5 | [71] | |
| LBL assembly and the VAF method | Polyurethane/aramid nanofibers | 98.02 | 5.275 | [33] | |
| Wet-spinning | MMT nanoplatelets/graphene | 88–270 | [72] | ||
| Multi-step powder metallurgyroute and industrial extrusion process followed by laser shock peening | Graphene/aluminum alloy | 343 | 79.8 | [73] | |
| Film casting | Mg-amorphous calcium carbonate (ACC)/chitosan | 121.67 | 31.96 | [30] | |
| Vacuum-assisted self-assembly | 1D 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO)-oxidized cellulose nanofibers/MXene | 128.13 | 7 | [74] | |
| Vacuum-assisted self-assembly | PVA/hydroxylfunctionalized black phosphorus (BP-OH)/GO | 74.3 ± 3.5 | [75] | ||
| Vacuum-assisted resin infusion molding process | MMT-MWCNT/epoxy | 750 | 36 | [76] | |
| Synthesis Method | Materials | Mechanical Properties | Performance | Reference | |
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Young’s Modulus (GPa) | ||||
| Vacuum-assisted self-assembly | Clay/PNIPAM | 0.95 | 0.0147 | Act as oil repellent and could separate 99.9% hexane/water and crude oil/water completely. UOCA of 159°. | [34] |
| The mixture was shaken at 50 °C to form sponges | PU-polydopamine/Ag/dodecyl mercaptan | 0.218 ± 0.021 | Complete separation of n-octane/water. Could absorb various types of organic solutions with absorption capacity ranges from 18–43 g/g. The highest absorption capacity was for tetrachloromethane, while the lowest is for crude oil. Water contact angle of 155° and UOCA 0°, hence a superhydrophobic and superoleophilic material. | [94] | |
| Layer-by-layer self-assembly by dipping and washing | GO/CaCO3 | 25.4 ± 2.6 | Separation of solutions containing cyclohexane, toluene, diesel, hexane, and petroleum ether, with water flux reaches 179,640 Lm−2h−1 for cyclohexane and 120,000 Lm−2h−1 for diesel. UOCA of 155°. | [24] | |
| Spin/dip coating-seed mineralization | Chitosan/CaCO3-Pglu | 32.1 ± 9.0 | Separation of solutions containing cyclohexane, soybean oil, toluene, silicon oil, and engine oil, with oil concentrations after separation below 4 ppm for soybean oil and below 2 ppm for the rest. UOCA of 145.3 ± 1.6°. | [96] | |
| Film casting-evaporation | MMT/HEC | 129.3 ± 6.7 | 6.3 ± 0.36 | UOCA of various oils is higherthan 156.8° and adhesive force of less than 3.5 μN. | [93] |
| Dip coating-wash repeatedly | MMT/PDDA | 9.4 ± 2.4 | UOCA of various oils are higher than 160° and adhesive force of less than 4.7 ± 2.7 µN. | [98] | |
| Dip coating | GO | Separation efficiency of around 98 and 90% for light oil/water and heavy oil/water, respectively. UOCA above 150°. | [99] | ||
| Michael addition reaction | Polydopamine-n-dodecyl mercaptan/stainless steel mesh | Separation efficiency of 99.95% for hexane/water mixture, above 99.7% for petroleum ether and gasoline mixtures, and 98.12 ± 0.31% for diesel/water mixture. Water contact angle of 144° and UOCA 0°, hence a superhydrophobic and superoleophilic material. | [95] | ||
| Interfacial assembly and cross-linking | GO/sodium alginate/CaCl2 | 35.8 ± 4.9 | Separation efficiency of 99.6% for cyclohexane-water mixture with UOCA of 154 ± 1°. | [100] | |
| Electrospinning | Silica/nafion | Complete separation of carbon tetrachloride and benzene from water. Contact angle of 130°. | [101] | ||
| Solution casting | PAA/polyvinylidene fluoride (PVDF)-graphene nanosheet | 92.10 ± 10.01 | 21.28 ± 2.86 | UOCA of more than 150°. High mechanical stability after 5 h immersion in seawater (tensile strength and Young’s modulus changed to 84.7 ± 9.07 MPa and 12.96 ± 2.48 GPa, respectively). | [102] |
| Vacuum-assisted self-assembly | Polyethyleneimine (PEI)-CNT | 86 | 4.043 | UOAC of around 169.1°, 160.8°, 173.4°, 162.9°, and 168.9° for hexadecane, heptane, soybean oil, pump oil, and silicone oil, respectively. Oil–water separation performance: flux reached 1427 Lm−2h−1 and flux recovery ratio of 81.7%. | [103] |
| Facile and green layer-by-layer (LbL) assembly | Chitosan/carboxymethyl cellulose@CaCO3 | UOAC of 152°, 154°, 151.5°, 150.5°, and 151° for methanol, ethanol, chloroform, toluene, and n-hexane, respectively. Oil–water separation performance: flux reached 7532 Lm−2h−1 and efficiency can be maintained at 97.8% after 64 times usage. | [104] | ||
| Layer-by-layer self-assembly by dipping and rinsing | Chitosan/kaolin@CaCO3 | UOAC of > 152.5° for gasoline and diesel. Oil–water separation performance: the flux reached 48,520 Lm−2h−1 and efficiency can be maintained at 97.7% after 64 times usage. Separation efficiency of >98.4% for petroleum ether, kerosene, gasoline, diesel, xylene, and cyclohexane. | [89] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khayrani, A.C.; Sambudi, N.S.; Wijaya, H.; Buys, Y.F.; Radini, F.A.; Jusoh, N.; Kamal, N.A.; Suhaimi, H. Review of Artificial Nacre for Oil–Water Separation. Separations 2023, 10, 205. https://doi.org/10.3390/separations10030205
Khayrani AC, Sambudi NS, Wijaya H, Buys YF, Radini FA, Jusoh N, Kamal NA, Suhaimi H. Review of Artificial Nacre for Oil–Water Separation. Separations. 2023; 10(3):205. https://doi.org/10.3390/separations10030205
Chicago/Turabian StyleKhayrani, Apriliana Cahya, Nonni Soraya Sambudi, Hans Wijaya, Yose Fachmi Buys, Fitri Ayu Radini, Norwahyu Jusoh, Norashikin Ahmad Kamal, and Hazwani Suhaimi. 2023. "Review of Artificial Nacre for Oil–Water Separation" Separations 10, no. 3: 205. https://doi.org/10.3390/separations10030205
APA StyleKhayrani, A. C., Sambudi, N. S., Wijaya, H., Buys, Y. F., Radini, F. A., Jusoh, N., Kamal, N. A., & Suhaimi, H. (2023). Review of Artificial Nacre for Oil–Water Separation. Separations, 10(3), 205. https://doi.org/10.3390/separations10030205









