Single-Step Surface Hydrophilization on Ultrafiltration Membrane with Enhanced Antifouling Property for Pome Wastewater Treatment
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Preparation and Characterization of Zwitterionic Polyethyleneimine (Z-PEI)
2.3. Preparation of Zwitterionic Membrane via Co-Deposition Method
2.4. Characterization of the Zwitterionic Membranes
2.4.1. Physicochemical Properties
Zeta Potential
Scanning Electron Microscopy (SEM)
Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
Water Contact Angle (WCA)
Membrane Surface Roughness
2.5. Performance Evaluation of the Zwitterionic Membrane
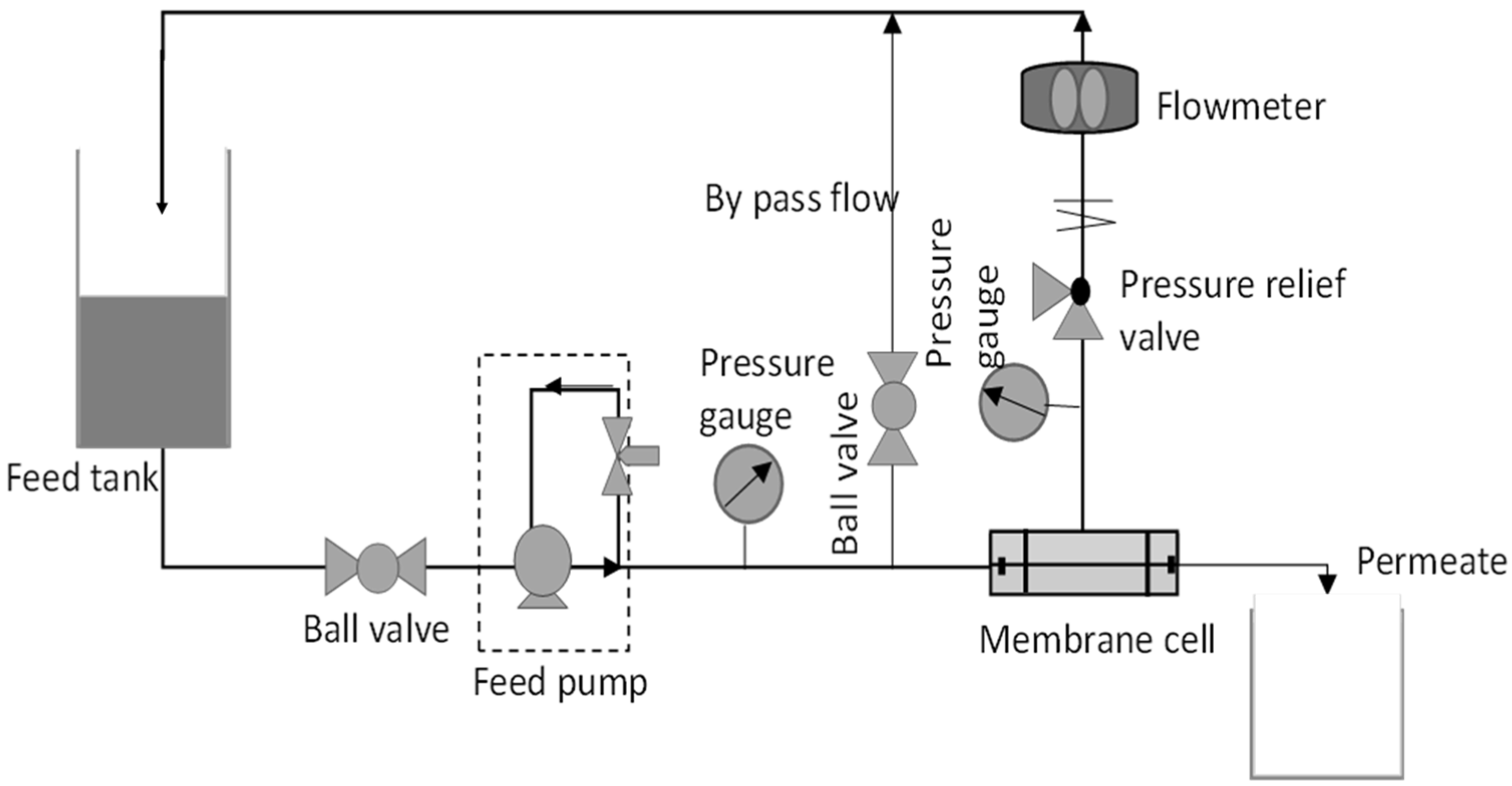
2.5.1. Water Flux
2.5.2. Rejection of Real Colored POME Wastewater
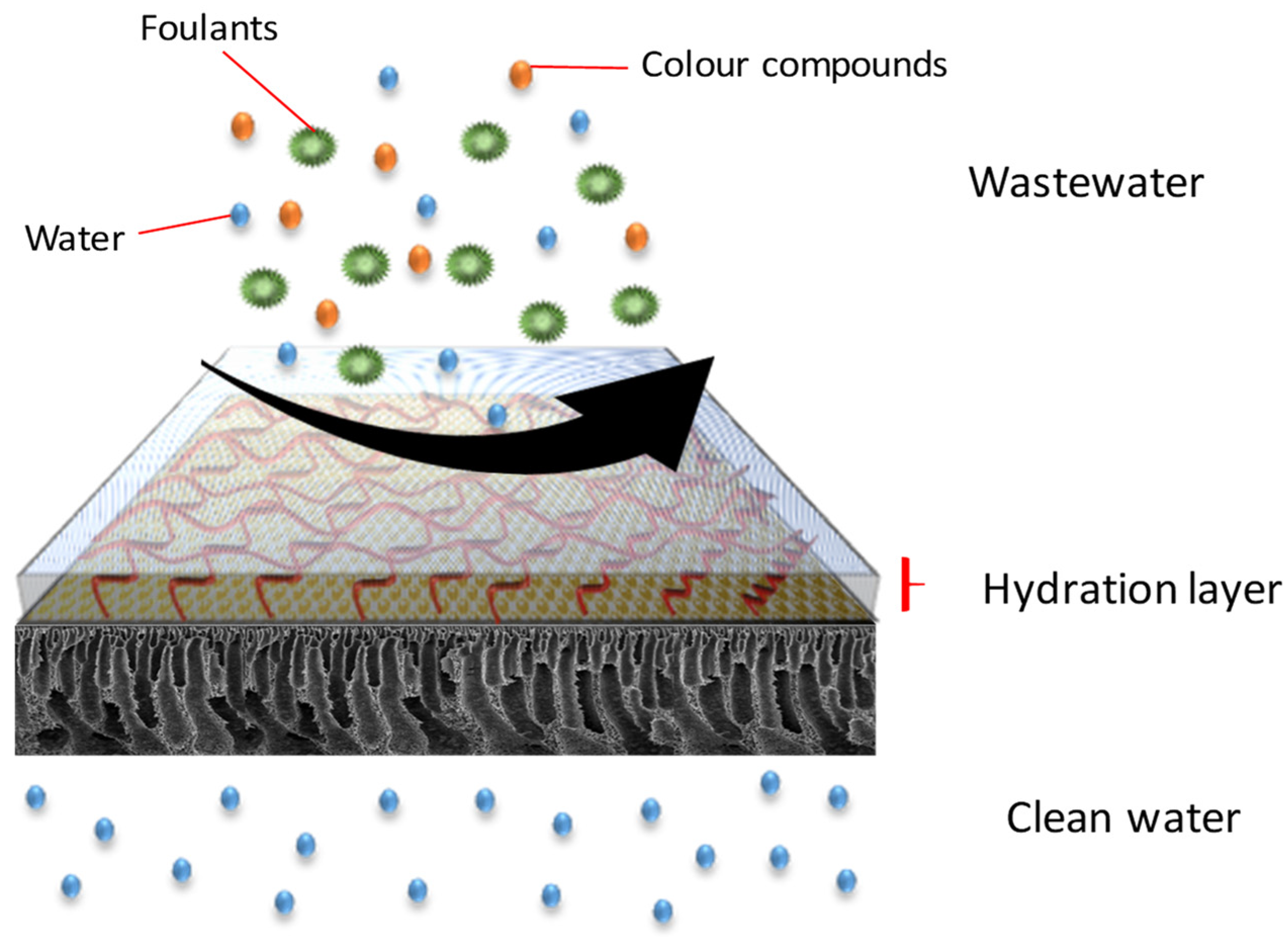
2.6. Antifouling Performances
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Surface Morphology
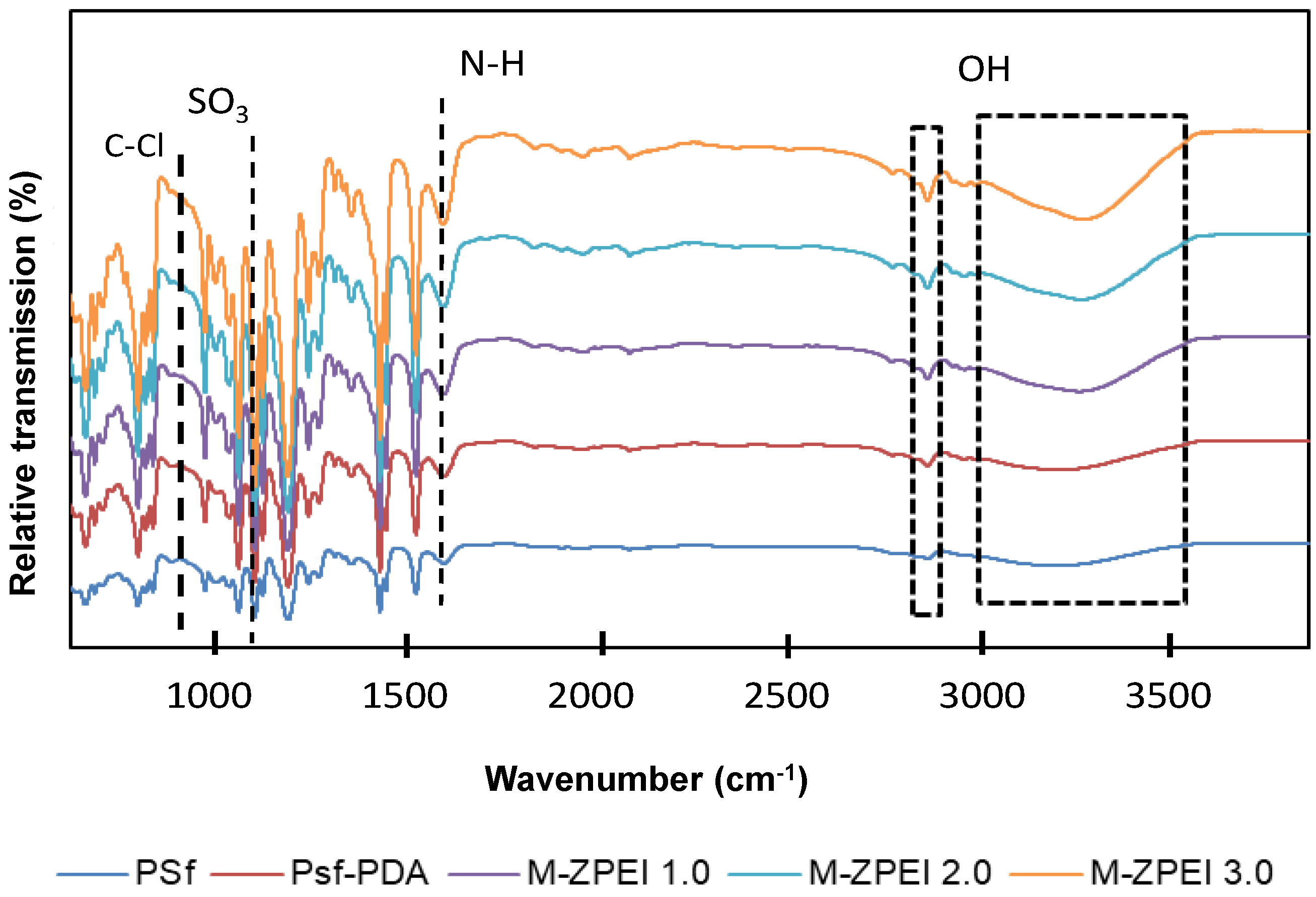
3.1.2. Surface Functional Group
3.1.3. Surface Charge
3.1.4. Membrane Hydrophilicity
3.2. Membrane Performance
3.2.1. Water Flux
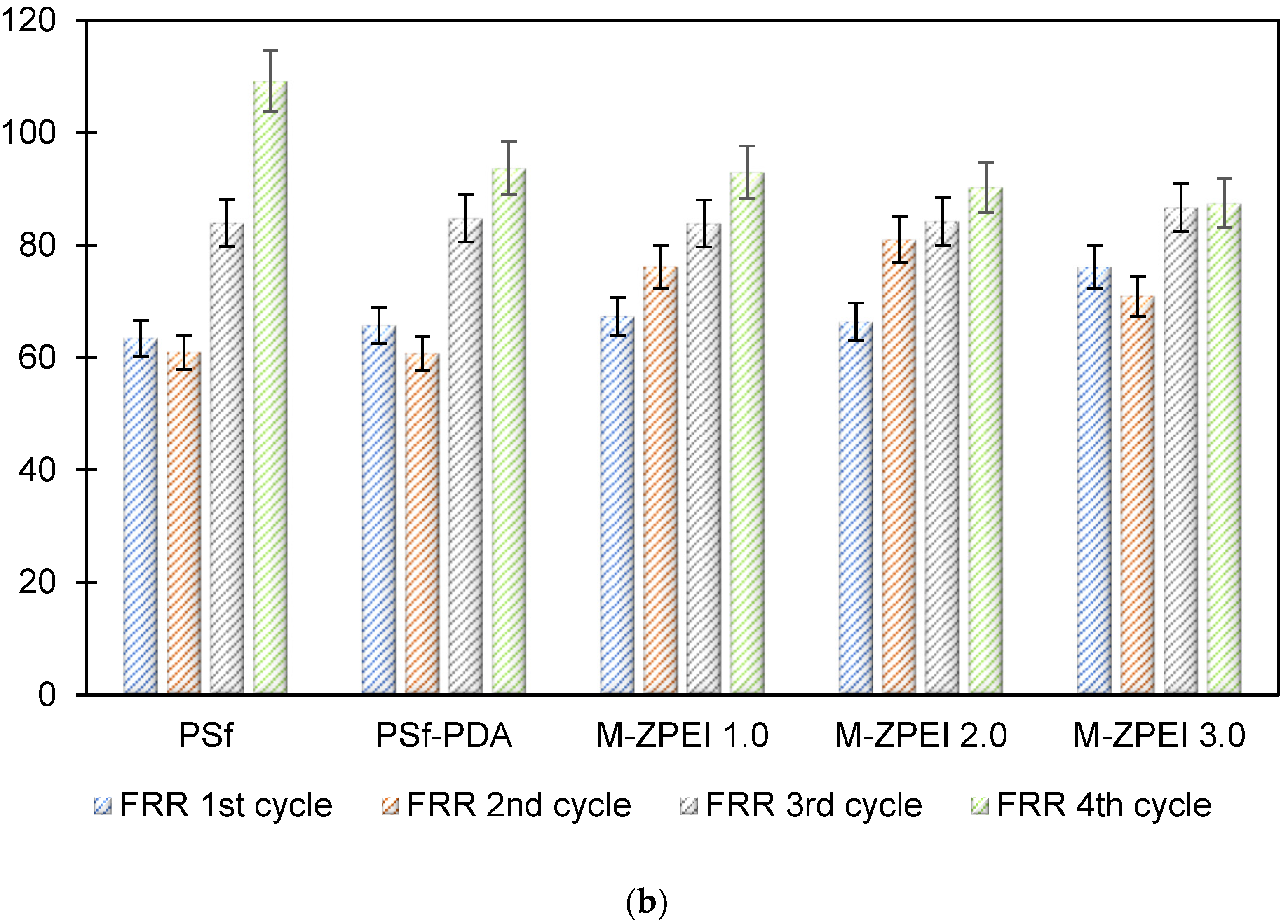
3.2.2. Flux Stability and Antifouling Performance
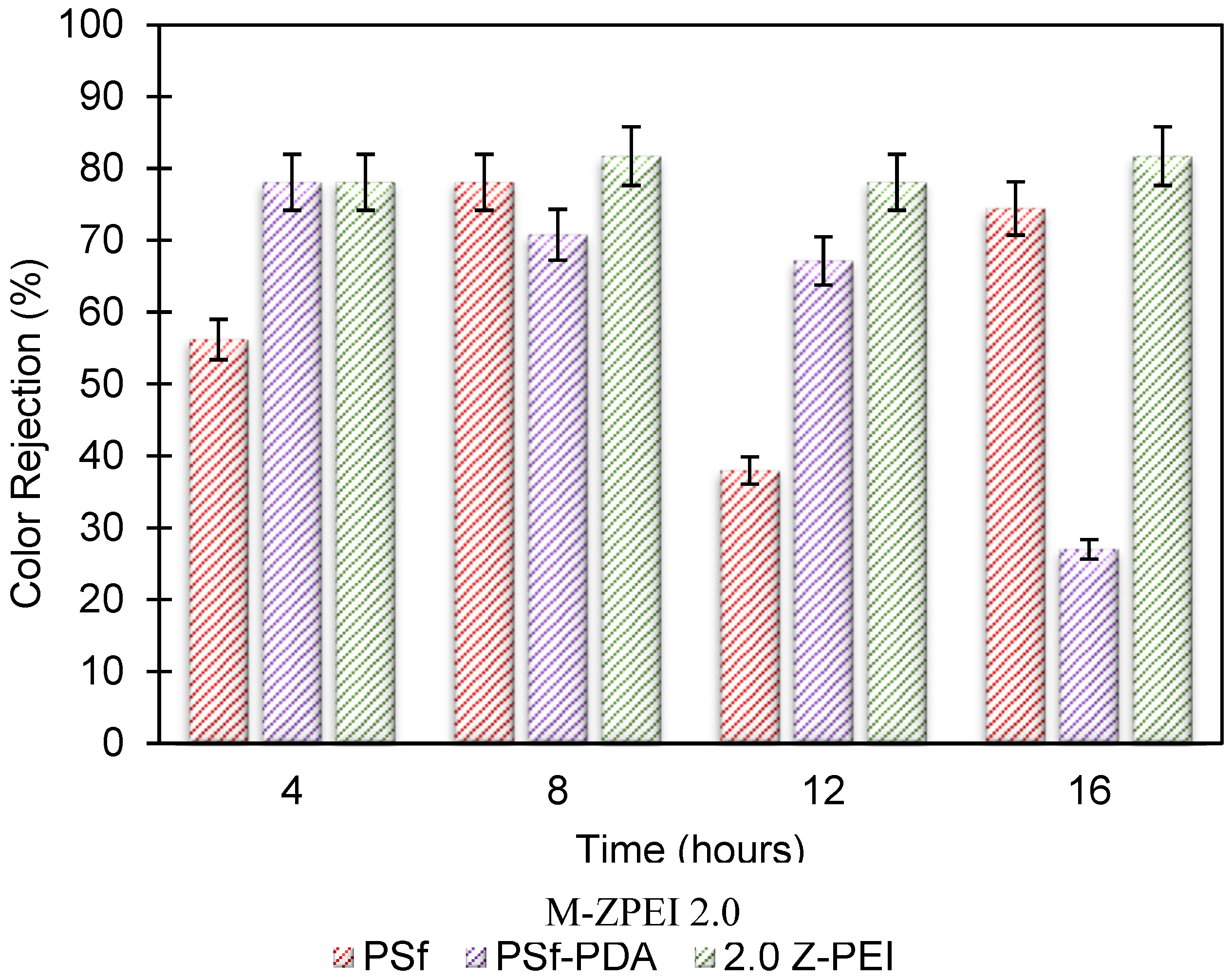
3.2.3. Rejection of Real POME Wastewater
4. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamimin, C.; Singkhala, A.; Kongjan, P.; Suraraksa, B.; Prasertsan, P.; Imai, T.; O-Thong, S. Two-stage thermophilic fermentation and mesophilic methanogen process for biohythane production from palm oil mill effluent. Int. J. Hydrogen Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Takriff, M.S.; Al-Rajabi, M.M.; Abdul, P.M.; Gunny, A.A.N.; Silvamany, H.; Jahim, J.M. Water reclamation from palm oil mill effluent (POME): Recent technologies, by-product recovery, and challenges. J. Water Process Eng. 2023, 52, 103488. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Kau, W.J.; Ismail, A.F. At-pomeAT-POME colour removal through photocatalytic submerged filtration using antifouling PVDF-TNT nanocomposite membrane. Sep. Purif. Technol. 2018, 191, 266–275. [Google Scholar] [CrossRef]
- Harby, N.F.A.; El-Batouti, M.; Elewa, M. Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review. Nanomaterials 2022, 12, 3637. [Google Scholar] [CrossRef]
- Batouti, M.E.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Application. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Ho, K.C.; Teow, Y.H.; Ang, W.L.; Mohammad, A.W. Novel GO/OMWCNTs mixed-matrix membrane with enhanced antifouling property for palm oil mill effluent treatment. Sep. Purif. Technol. 2017, 177, 337–349. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Mohanty, S.K.; Nayak, S.K. Antifouling, fouling release and antimicrobial materials for surface modification of reverse osmosis and nanofiltration membranes. J. Mater. Chem. A 2018, 6, 313–333. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhu, L.P.; Zhao, Y.F.; Zhu, B.K.; Xu, Y.Y. Anti-fouling and anti-bacterial polyethersulfone membranes quaternized from the additive of poly(2-dimethylamino ethyl methacrylate) grafted SiO2 nanoparticles. J. Mater. Chem. A 2014, 2, 15566–15574. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M.; Rabiee, M. Synthesis, characterization, and anti-fouling properties of cellulose acetate/polyethylene glycol-grafted nanodiamond nanocomposite membranes for humic acid removal from contaminated water. Iran. Polym. J. 2018, 27, 381–393. [Google Scholar] [CrossRef]
- Mondal, M.; De, I.S. Characterization and antifouling properties of polyethylene glycol doped PAN–CAP blend membrane. RSC Adv. 2015, 5, 38948–38963. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; With, G. With, Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Zen, F.; Angione, M.D.; Behan, J.A.; Cullen, R.J.; Duff, T.; Vasconcelos, J.M.; Scanlan, E.M.; Colavita, P.E. Modulation of Protein Fouling and Interfacial Properties at Carbon Surfaces via Immobilization of Glycans Using Aryldiazonium Chemistry. Sci. Rep. 2016, 6, 24840. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Zhao, D.; Qiu, G.; Li, X.; Wan, C.; Lu, K.; Chung, T.-S. Zwitterions coated hollow fiber membranes with enhanced antifouling properties for osmotic power generation from municipal wastewater. Water Res. 2016, 104, 389–396. [Google Scholar] [CrossRef]
- Zhu, J.; Su, Y.; Zhao, X.; Li, Y.; Zhang, R.; Fan, X.; Ma, Y.; Jiang, Z. Constructing a zwitterionic ultra fi ltration membrane surface via multisite anchorage for superior long-term antifouling properties. RSC Adv. 2015, 5, 40126–40134. [Google Scholar] [CrossRef]
- Lau, S.K.; Yong, W.F. Recent Progress of Zwitterionic Materials as Antifouling Membranes for Ultrafiltration, Nanofiltration, and Reverse Osmosis. Langmuir 2021, 3, 4390–4412. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, S.; Zhang, X.; Shi, Z.; Wei, Z.; Bao, J.; Zhao, W.; Zhao, C. Engineering of Tannic Acid Inspired Antifouling and Antibacterial Membranes through Co-deposition of Zwitterionic Polymers and Ag Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 11689–11697. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Xiao, T.; Zhao, W.; Li, J.; Zhao, C. Tannic acid-inspiration and post-crosslinking of zwitterionic polymer as a universal approach towards antifouling surface. Chem. Eng. J. 2018, 337, 122–132. [Google Scholar] [CrossRef]
- Yao, L.; He, C.; Chen, S.; Zhao, W.; Xie, Y.; Sun, S.; Nie, S.; Zhao, C. Codeposition of Polydopamine and Zwitterionic Polymer on Membrane Surface with Enhanced Stability and Antibiofouling Property. Langmuir 2019, 35, 1430–1439. [Google Scholar] [CrossRef]
- Tajuddin, M.H.; Yusof, N.; Abdullah, N.; Abidin, M.N.Z.; Salleh, W.N.W.; Ismail, A.F. Incorporation of layered double hydroxide nanofillers in polyamide nanofiltration membrane for high performance salt rejections. J. Taiwan Inst. Chem. Eng. 2019, 97, 1–11. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Subrahmanyam, K.S.; Kumar, P.; Govindaraj, A.; Rao, C.N.R. Reversible chemical storage of halogens in few-layer graphene. RSC Adv. 2012, 2, 1605–1608. [Google Scholar] [CrossRef]
- Tan, Y.H.; Goh, P.S.; Ismail, A.F.; Ng, B.C.; Lai, G.S. Decolourization of aerobically treated palm oil mill effluent (AT-POME) using polyvinylidene fluoride (PVDF) ultrafiltration membrane incorporated with coupled zinc-iron oxide nanoparticles. Chem. Eng. J. 2017, 308, 359–369. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring membrane surface charges: A novel study on electrostatic interactions during membrane fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Goh, P.S.; Hilal, N.; Ooi, B.S. Characterization methods of thin film composite nanofiltration membranes. Sep. Purif. Rev. 2015, 44, 135–156. [Google Scholar] [CrossRef]
- Yang, H.C.; Liao, K.J.; Huang, H.; Wu, Q.Y.; Wan, L.S.; Xu, Z.K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Ju, J.; Wang, C.; Wang, T.; Wang, Q. Preparation and characterization of pH-sensitive and antifouling poly(vinylidene fluoride) microfiltration membranes blended with poly(methyl methacrylate-2-hydroxyethyl methacrylate-acrylic acid). J. Colloid Interface Sci. 2014, 15, 175–180. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Xiao, W.; Luo, J.; Li, B.; Wang, L.; Xue, H.; Gao, J. Flexible PDA@ACNTs decorated polymer nanofiber composite with superhydrophilicity and underwater superoleophobicity for efficient separation of oil-in-water emulsion. J. Membr. Sci. 2020, 614, 118500. [Google Scholar] [CrossRef]





| Parameter | Unit | Value |
|---|---|---|
| pH | - | 8.20 |
| BOD | mg/L | 23 |
| COD | mg/L | 19 |
| Suspended Solid (SS) | mg/L | 101 |
| Oil & Grease (O&G) | mg/L | 29 |
| Total Nitrogen (TN) | mg/L | 20 |
| Ammonia Nitrogen (AN) | mg/L | 0 |
| Membrane Annotation | DA, mg/mL | Z-PEI, mg/mL | Ratio DA: Z-PEI |
|---|---|---|---|
| MPDA | 2 | 0 | Control * |
| 1.0-ZPEI | 2 | 2 | 1:1 |
| 2.0-ZPEI | 2 | 4 | 1:2 |
| 3.0-ZPEI | 2 | 6 | 1:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, N.; Yusof, N.; Dahim, M.A.; Hamid, M.F.; Jye, L.W.; Jaafar, J.; Aziz, F.; Wan Salleh, W.N.; Ismail, A.F.; Misdan, N. Single-Step Surface Hydrophilization on Ultrafiltration Membrane with Enhanced Antifouling Property for Pome Wastewater Treatment. Separations 2023, 10, 188. https://doi.org/10.3390/separations10030188
Abdullah N, Yusof N, Dahim MA, Hamid MF, Jye LW, Jaafar J, Aziz F, Wan Salleh WN, Ismail AF, Misdan N. Single-Step Surface Hydrophilization on Ultrafiltration Membrane with Enhanced Antifouling Property for Pome Wastewater Treatment. Separations. 2023; 10(3):188. https://doi.org/10.3390/separations10030188
Chicago/Turabian StyleAbdullah, Norfadhilatuladha, Norhaniza Yusof, Mohammed Abdullah Dahim, Muhammad Faris Hamid, Lau Woei Jye, Juhana Jaafar, Farhana Aziz, Wan Norhayati Wan Salleh, Ahmad Fauzi Ismail, and Nurasyikin Misdan. 2023. "Single-Step Surface Hydrophilization on Ultrafiltration Membrane with Enhanced Antifouling Property for Pome Wastewater Treatment" Separations 10, no. 3: 188. https://doi.org/10.3390/separations10030188
APA StyleAbdullah, N., Yusof, N., Dahim, M. A., Hamid, M. F., Jye, L. W., Jaafar, J., Aziz, F., Wan Salleh, W. N., Ismail, A. F., & Misdan, N. (2023). Single-Step Surface Hydrophilization on Ultrafiltration Membrane with Enhanced Antifouling Property for Pome Wastewater Treatment. Separations, 10(3), 188. https://doi.org/10.3390/separations10030188










